Synthesis of Graphene Oxide from Sugarcane Dry Leaves by Two-Stage Pyrolysis
Abstract
:1. Introduction
2. Results and Discussion
2.1. Mechanism of GO Synthesis by Two-Stage Pyrolysis
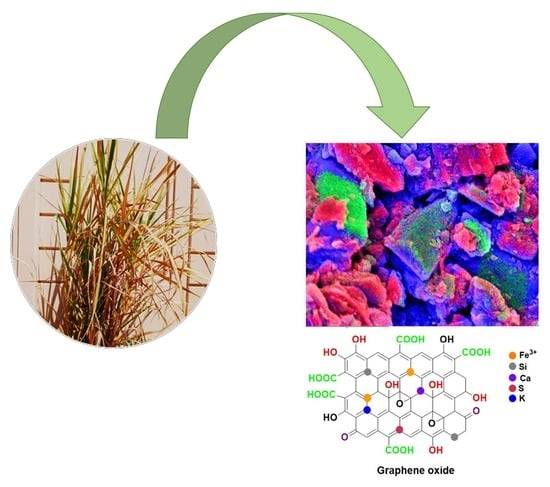
2.2. Characterization of Synthesized GO
3. Materials and Methods
3.1. Materials
3.2. Preparation of GO from Sugarcane Dry Leaves
3.3. Material Characterization
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Stobinksi, L.; Lesiak, B.; Malolepszy, A.; Mazurkiewicz, M.; Mierzwa, B.; Zemek, J.; Jiricek, P.; Bieloshapka, I. Graphene oxide and reduced graphene oxide studied by the XRD, TEM and electron spectroscopy methods. J. Electron. Spectrosc. Relat. Phenom. 2014, 195, 145–154. [Google Scholar] [CrossRef]
- Aragaw, B.A. Reduced graphene oxide-intercalated graphene oxide nano-hybrid for enhanced photoelectrochemical water reduction. J. Nanostruct. Chem. 2020, 10, 9–18. [Google Scholar] [CrossRef] [Green Version]
- Asghar, F.; Shakoor, B.; Fatima, S.; Munir, S.; Razzaq, H.; Naheed, S.; Butler, I.S. Fabrication and prospective applications of graphene oxide-modified nanocomposites for wastewater remediation. RSC Adv. 2022, 12, 11750–11768. [Google Scholar] [CrossRef]
- Zhu, Y.; Murali, S.; Cai, W.; Li, X.; Suk, J.W.; Potts, J.R.; Ruoff, R.S. Graphene and graphene oxide: Synthesis, properties, and applications. Adv. Mater. 2010, 22, 3906–3924. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Yang, Q.; Yang, Y.; Lv, W.; Wen, Y.; Hou, P.; Wang, M.; Cheng, H. Self-assembled free-standing graphite oxide membrane. Adv. Mater. 2009, 21, 3007–3011. [Google Scholar] [CrossRef]
- Lujaniene, G.; Novikau, R.; Joel, E.F.; Karalevičiūtė, K.; Šemukčuk, S.; Mažeika, K.; Talaikis, M.; Pakštas, V.; Tumenas, S.; Mažeika, J.; et al. Preparation of graphene oxide-maghemite-chitosan composites for the adsorption of europium ions from aqueous solutions. Molecules 2022, 27, 8035. [Google Scholar] [CrossRef] [PubMed]
- Goswami, S.; Banerjee, P.; Datta, S.; Mukhopadhayay, A.; Das, P. Graphene oxide nanoplatelets synthesized with carbonized agro-waste biomass as green precursor and its application for the treatment of dye rich wastewater. Process Saf. Environ. Prot. 2017, 106, 163–172. [Google Scholar] [CrossRef]
- Banerjee, P.; Sau, S.; Das, P.; Mukhopadhayay, A. Optimization and modelling of synthetic azo dye wastewater treatment using Graphene oxide nanoplatelets: Characterization toxicity evaluation and optimization using Artificial Neural Network. Ecotoxicol. Environ. Saf. 2015, 119, 47–57. [Google Scholar] [CrossRef] [PubMed]
- Rajan, P.; Agarwal, S.; Sinha, A.; Rao, T.R.; Balakrishnan, J.; Thakur, A.D. A low-cost non-explosive synthesis of graphene oxide for scalable applications. Sci. Rep. 2018, 8, 12007. [Google Scholar] [CrossRef] [PubMed]
- Kumar, P.; Som, S.; Pandey, M.K.; Das, S.; Chanda, A.; Singh, J. Investigations on optical properties of ZnO decorated graphene oxide (ZnO@GO) and reduced graphene oxide (ZnO@r-GO). J. Alloys Compd. 2018, 744, 64–74. [Google Scholar] [CrossRef]
- Marcano, D.C.; Kosynkin, D.V.; Berlin, J.M.; Sinitskii, A.; Sun, Z.; Slesarev, A.; Alemany, L.B.; Lu, W.; Tour, J.M. Improved synthesis of graphene oxide. ACS Nano 2010, 8, 4806–4814. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Yao, B.; Li, C.; Shi, G. An improved Hummers method for eco-friendly synthesis of graphene oxide. Carbon 2013, 64, 225–229. [Google Scholar] [CrossRef]
- Brodie, B.C. On the atomic weight of graphite. Phil. Trans. R. Soc. 1859, 149, 249–259. [Google Scholar] [CrossRef] [Green Version]
- Illevich, L.V.; Tkachenko, T.B.; Samarov, A.V.; Burtsev, A.A.; Sozinov, S.A.; Hitsova, L.M.; Popova, A.N.; Barnakov, C.N.; Kozlov, A.P. Fabrication and physicochemical characterization of graphene oxide derived from thermally expanded graphite. Russ. Chem. Bull. 2018, 67, 986–990. [Google Scholar] [CrossRef]
- Hummers, W.S.; Offeman, R.E. Preparation of graphitic oxide. J. Am. Chem. Soc. 1958, 86, 1339. [Google Scholar] [CrossRef]
- Alam, S.N.; Sharma, N.; Kumar, L. Synthesis of graphene oxide (GO) by Modified Hummers method and its thermal reduction to obtain reduced graphene oxide (rGO). Graphene 2017, 6, 1–18. [Google Scholar] [CrossRef] [Green Version]
- Sierra, U.; Álvarez, P.; Blanco, C.; Granda, M.; Santamaría, R.; Menéndez, R. Cokes of different origin as precursors of graphene oxide. Fuel 2016, 166, 400–403. [Google Scholar] [CrossRef]
- Hu, B.B.; Wang, K.; Wu, L.; Yu, S.H.; Antonietti, M.; Titrici, M.M. Engineering carbon materials from the hydrothermal carbonization process of biomass. Adv. Mater. 2010, 22, 813–828. [Google Scholar] [CrossRef]
- Thangaraj, B.; Chuangchote, S.; Wongyao, N.; Solomon, P.R.; Roongraung, K.; Chaiworn, W.; Surareungchai, W. Flexible sodium-ion batteries using electrodes from Samanea saman tree leaf-derived carbon quantum dots decorated with SnO2 and NaVO3. Clean Energy 2021, 5, 354–374. [Google Scholar] [CrossRef]
- Shams, S.S.; Zhang, L.S.; Hu, R.; Zhang, R.; Zhu, J. Green synthesis and characterization of graphene nanosheets. Mater. Lett. 2015, 161, 476–479. [Google Scholar] [CrossRef]
- Zheng, S.; Zhang, J.; Deng, H.; Du, Y.; Shi, X. Chitin derived nitrogen-doped porous carbons with ultrahigh specific surface area and tailored hierarchical porosity for high performance supercapacitors. J. Bioresour. Bioprod. 2021, 6, 142–151. [Google Scholar] [CrossRef]
- Zhang, Y.; Tian, X.; Zhang, Q.; Xie, H.; Wang, B.; Feng, Y. Hydrochar-embedded carboxymethyl cellulose-g-poly(acrylic acid) hydrogel as stable soil water retention and nutrient release agent for plant growth. J. Bioresour. Bioprod. 2022, 7, 116–127. [Google Scholar] [CrossRef]
- Yan, B.; Feng, L.; Zheng, J.; Zhang, Q.; Jiang, S.; Zhang, C.; Ding, Y.; Han, J.; Chen, W.; He, S. High performance supercapacitors based on wood-derived thick carbon electrodes synthesized via green activation process. Inorg. Chem. Front. 2022, 9, 6108–6123. [Google Scholar] [CrossRef]
- Supriyanto, G.; Rukman, N.K.; Nisa, A.K.; Jannatin, M.; Piere, B.; Abdullah, A.; Fahmi, M.Z.; Kusuma, H.S. Graphene oxide from Indonesian biomass: Synthesis and characterization. Bioresources 2018, 13, 4832–4840. [Google Scholar] [CrossRef]
- Faiz, M.S.A.; Azurahanim, C.A.C.; Yazid, Y.; Suriani, A.B.; Ain, J.A.S.N. Preparation and characterization of graphene oxide from tea waste and it’s photocatalytic application of TiO2/graphene nanocomposite. Mater. Res. Express 2010, 7, 015613. [Google Scholar] [CrossRef]
- Yaqoob, A.A.; Serrá, A.; Ibrahim, M.N.M.; Yaakop, A.S. Self-assembled oil palm biomass-derived modified graphene oxide anode: An efficient medium for energy transportation and bioremediating Cd (II) via microbial fuel cells. Arab. J. Chem. 2021, 14, 103121. [Google Scholar] [CrossRef]
- Devi, M.; Rawat, S.; Sharma, S. A comprehensive review of the pyrolysis process: From carbon nanomaterial synthesis to waste treatment. Oxf. Open Mater. Sci. 2021, 1, 1–30. [Google Scholar] [CrossRef]
- De Silva, K.K.H.; Huang, H.-H.; Joshi, R.K.; Yoshimura, M. Chemical reduction of graphene oxide using green reductants. Carbon 2017, 119, 190–199. [Google Scholar] [CrossRef]
- Kumar, M.; Sabbarwal, S.; Mishra, P.K.; Upadhyay, S.N. Thermal degradation kinetics of sugarcane leaves (Saccharum officinarum L.) using thermo-gravimetric and differential scanning calorimetric studies. Bioresour. Technol. 2019, 279, 262–270. [Google Scholar] [CrossRef]
- Charusiria, W.; Vitidsant, T. Biofuel production via the pyrolysis of sugarcane (Saccharum officinarum L.) leaves: Characterization of the optimal conditions. Sustain. Chem. Pharm. 2018, 10, 71–78. [Google Scholar] [CrossRef]
- Ferreira-Leitão, V.; Perrone, C.C.; Rodrigues, J.; Franke, A.P.M.; Macrelli, S.; Zacch, G. An approach to the utilisation of CO2 as impregnating agent in steam pretreatment of sugar cane bagasse and leaves for ethanol production. Biotechnol. Biofuels 2010, 3, 7. [Google Scholar] [CrossRef] [Green Version]
- Kumar, M.; Upadhyay, S.N.; Mishra, P.K. Pyrolysis of sugarcane (Saccharum officinarum L.) leaves and characterization of products. ACS Omega 2022, 7, 28052–28064. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, R.; Sajjadi, B.; Chen, W.-Y.; Mattern, D.L.; Hammer, N.; Raman, V.; Dorris, A. Effect of pyrolysis temperature on physicochemical properties and acoustic-based amination of biochar for efficient CO2 adsorption. Front. Energy Res. 2020, 8, 85. [Google Scholar] [CrossRef]
- Bakshi, S.; Banik, C.; Laird, D.A. Estimating the organic oxygen content of biochar. Sci. Rep. 2020, 10, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Biryukova, G.P.; Shablygin, M.V.; Mikhailov, N.V.; Andrianov, K.A. Relationship between the conditions of pyrolysis and the structural and chemical transformation of cellulose hydrate. Polym. Sci. USSR 1973, 15, 1762–1766. [Google Scholar] [CrossRef]
- Ōtani, S.; Yamada, K.; Koitabashi, T.; Yokoyama, A. On the raw materials of MP carbon fiber. Carbon 1996, 4, 425–432. [Google Scholar] [CrossRef]
- Jenkins, G.M.; Kawamura, K. Polymeric Carbons: Carbon Fibre, Glass and Char; Cambridge University Press: Cambridge, UK, 1976; Available online: https://books.google.ae/books?id=wjc8AAAAIAAJ (accessed on 20 December 2022).
- Wang, L.; Tian, C.; Wang, H.; Ma, Y.; Wang, B.; Fu, H. Mass Production of graphene via an in situ self-generating template route and its promoted activity as electrocatalytic support for methanol electroxidization. J. Phys. Chem. C 2010, 114, 8727–8733. [Google Scholar] [CrossRef]
- Kong, X.; Zhu, Y.; Lei, H.; Wang, C.; Zhao, Y.; Huo, E.; Lin, X.; Zhang, Q.; Qian, M.; Mateo, W.; et al. Hydrogen evolution under large-current-density based on fluorine-doped cobalt-iron phosphides. Chem. Eng. J 2020, 399, 125831. [Google Scholar] [CrossRef]
- Dong, G.; van Baarle, D.W.; Frenken, J.W.M. Advances in Graphene Science; Aliofkhazraei, M., Ed.; IntechOpen: Rijeka, Croatia, 2013. [Google Scholar] [CrossRef]
- Kalita, G.; Tanemura, M. Chapter 3—Fundamentals of chemical vapor deposited graphene and emerging applications. In Graphene Materials; Kyzas, G.Z., Mitropoulos, A.C., Eds.; IntechOpen: Rijeka, Croatia, 2017. [Google Scholar] [CrossRef] [Green Version]
- Kudus, M.H.A.; Zakaria, M.R.; Akil, H.M.; Ullah, F.; Javed, F. Oxidation of graphene via a simplified Hummers’ method for graphene diamine colloid production. J. King Saud. Univ. Sci. 2020, 32, 910–913. [Google Scholar] [CrossRef]
- Dimiev, A.M.; Tour, J.M. Mechanism of graphene oxide formation. ACS Nano 2014, 8, 3060–3068. [Google Scholar] [CrossRef]
- Hong, Y.; Wang, Z.; Jin, X. Sulfuric acid intercalated graphite oxide for graphene preparation. Sci. Rep. 2013, 3, 1–6. [Google Scholar] [CrossRef] [Green Version]
- Liu, Z.; Xu, Z.; Xu, L.; Buyong, F.; Chay, T.C.; Li, Z.; Cai, Y.; Hu, B.; Zhu, Y.; Wang, X. Modified biochar: Synthesis and mechanism for removal of environmental heavy metals. Carbon Res. 2022, 1, 8. [Google Scholar] [CrossRef]
- Ikram, M.; Raza, A.; Imran, M.; UI-Hamid, A.; Shahbaz, A.; Ali, S. Hydrothermal synthesis of silver decorated reduced graphene oxide (rGO) nanoflakes with effective photocatalytic activity for wastewater treatment. Nanoscale Res. Lett. 2020, 15, 95. [Google Scholar] [CrossRef] [PubMed]
- Luo, D.; Zhang, F.; Ren, Z.; Ren, W.; Ren, W.; Yu, L.; Jiang, L.; Ren, B.; Wang, L.; Wang, Z.; et al. An improved method to synthesize nanoscale graphene oxide using much less acid. Mater. Today Phys. 2019, 9, 1–6. [Google Scholar] [CrossRef]
- Johra, F.T.; Lee, J.-Q.; Jung, W.-G. Facile and safe graphene preparation on solution based platform. J. Ind. Eng. Chem. 2014, 20, 2883–2887. [Google Scholar] [CrossRef]
- Huang, N.M.; Lim, H.N.; Chia, C.H.; Yarmo, M.A.; Muhamad, M.R. Simple room-temperature preparation of high-yield large-area graphene oxide. Int. J. Nanomed. 2011, 6, 3443–3448. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, C.; Zhai, J.; Dong, S. Bifunctional fluorescent carbon nanodots: Green synthesis via soy milk and application as metal-free electrocatalysts for oxygen reduction. Chem. Comm. 2012, 48, 9367–9369. [Google Scholar] [CrossRef] [PubMed]
- Fonseca, L.C.; de Araújo, M.M.; de Moraes, A.C.M.; da Silva, D.S.; Ferreira, A.G.; Franqui, L.S.; Martinez, D.S.T.; Alves, O.L. Nanocomposites based on graphene oxide and mesoporous silica nanoparticles: Preparation, characterization and nanobiointeractions with red blood cells and human plasma proteins. Appl. Surf. Sci. 2018, 437, 110–121. [Google Scholar] [CrossRef]
- Sharma, N.; Sharma, V.; Vyas, R.; Kumari, M.; Kaushal, A.; Gupta, R.; Sharma, S.K.; Sachdev, K. A new sustainable green protocol for production of reduced graphene oxide and its gas sensing properties. J. Sci. Adv. Mater. Devices 2019, 4, 473–482. [Google Scholar] [CrossRef]
- Shen, J.; Shi, M.; Li, N.; Yan, B.; Ma, H.; Hu, Y.; Ye, M. Facile synthesis and application of Ag-chemically converted graphene nanocomposite. Nano Res. 2010, 3, 339–349. [Google Scholar] [CrossRef] [Green Version]
- Kumar, N.; Srivastava, V.C. Simple synthesis of large graphene oxide sheets via electrochemical method coupled with oxidation process. ACS Omega 2018, 3, 10233–10242. [Google Scholar] [CrossRef]
- Emiru, T.F.; Ayele, D.W. Controlled synthesis, characterization and reduction of graphene oxide: A convenient method for large scale production. Egypt. J. Basic Appl. Sci. 2017, 4, 74–79. [Google Scholar] [CrossRef] [Green Version]
- Guo, H.L.; Wang, X.F.; Qian, Q.Y.; Wang, F.B.; Xia, X.H. A green approach to the synthesis of graphene nanosheets. ACS Nano 2009, 3, 2653–2659. [Google Scholar] [CrossRef] [PubMed]
- Nazri, S.R.B.; Liu, W.-W.; Khe, C.-S.; Hidayah, N.M.S.; Teoh, Y.-B.; Voon, C.-H.; Lee, C.; Adelyn, P.Y.P. Synthesis, characterization and study of graphene oxide. AIP Conf. Proc. 2018, 5, 1–8. [Google Scholar] [CrossRef]
- Méndez-Lozano, N.; Pérez-Reynoso, F.; González-Gutiérrez, C. Eco-friendly approach for graphene oxide synthesis by Modified Hummers method. Materials 2022, 15, 7228. [Google Scholar] [CrossRef]
- Saxena, S.; Tyson, T.A.; Shukla, S.; Negusse, E.; Chen, H.; Bai, J. Investigation of structural and electronic properties of graphene oxide. Appl. Phy. Lett. 2011, 99, 013104. [Google Scholar] [CrossRef] [Green Version]
- Ramli, R.; Hidayat, R. Graphene Oxide Based on Biomass Waste: Synthesis and Applications; IntechOpen: Rijeka, Croatia, 2022. [Google Scholar] [CrossRef]
- Díez, N.; Śliwak, A.; Gryglewicz, S.; Grzyb, B.; Gryglewicz, G. Enhanced reduction of graphene oxide by high-pressure hydrothermal treatment. RSC Adv. 2015, 5, 81831–81837. [Google Scholar] [CrossRef]
- Pei, S.; Cheng, H.-M. The reduction of graphene oxide. Carbon 2012, 50, 3210–3228. [Google Scholar] [CrossRef]
- Kudin, K.N.; Ozbas, B.; Schniepp, H.C.; Prud’homme, R.K.; Aksay, I.A.; Car, R. Raman spectra of graphene oxide and functionalized graphene sheets. Nano Lett. 2008, 8, 36–41. [Google Scholar] [CrossRef]
- Ganguly, A.; Sharma, S.; Papakonstantinou, P.; Hamilton, J. Probing the thermal deoxygenation of graphene oxide using high-resolution in situ X-ray-based spectroscopies. J. Phys. Chem. C 2011, 115, 17009–17019. [Google Scholar] [CrossRef] [Green Version]
- Wang, S.J.; Geng, Y.; Zheng, Q.; Kim, J.-K. Fabrication of highly conducting and transparent graphene films. Carbon 2010, 48, 1815–1823. [Google Scholar] [CrossRef]
- Hafiz, S.M.; Ritikos, R.; Whitcher, T.J.; Razib, N.M.; Bien, D.C.D.; Chanlek, N.; Nakajima, H.; Saisopa, T.; Songsirirritthigul, P.; Huang, N.M.; et al. A practical carbon dioxide gas sensor using room-temperature hydrogen plasma reduced graphene oxide. Sens. Actuators B Chem. 2014, 193, 692–700. [Google Scholar] [CrossRef]
- Aukstakojyte, R.; Gaidukevic, J.; Niaura, G.; Skapas, M.; Bukauskas, V.; Barkauskas, J. Structural control and electrical behavior of thermally reduced graphene oxide samples assisted with malonic acid and phosphorus pentoxide. Inorganics 2022, 10, 142. [Google Scholar] [CrossRef]
- Liu, Y.-Z.; Chen, C.-M.; Li, Y.-F.; Li, X.-M.; Kong, Q.-Q.; Wang, M.-Z. Crumpled reduced graphene oxide by flame-induced reduction of graphite oxide for supercapacitive energy storage. J. Mater. Chem. A 2014, 2, 5730–5737. [Google Scholar] [CrossRef]
- Qin, H.; Gong, T.; Cho, Y.; Lee, C.; Kim, T. A conductive copolymer of graphene oxide/poly(1-(3-aminopropyl)pyrrole) and the adsorption of metal ions. Polym. Chem. 2014, 5, 4466–4473. [Google Scholar] [CrossRef]
- Guerrero-Contreras, J.; Caballero-Briones, F. Graphene oxide powders with different oxidation degree, prepared by synthesis variations of the Hummers method. Mater. Chem. Phys. 2015, 153, 209–220. [Google Scholar] [CrossRef]
- Muniyalakshmi, M.; Sethuraman, K.; Silambarasan, D. Synthesis and characterization of graphene oxide nanosheets. Mater. Today Proc. 2020, 21, 408–410. [Google Scholar] [CrossRef]
- Díez-Betriu, X.; Álvarez-García, S.; Botas, C.; Álvarez, P.; Sánchez-Marcos, J.; Prieto, C.; Menèndez, R.; de Andrès, A. Raman spectroscopy for the study of reduction mechanisms and optimization of conductivity in graphene oxide thin films. J. Mater. Chem. C 2013, 1, 6905–6912. [Google Scholar] [CrossRef]
- Kumar, P.V.; Bardhan, N.M.; Tongay, S.; Wu, J.; Belcher, A.M.; Grossman, J.C. Scalable enhancement of graphene oxide properties by thermally driven phase transformation. Nat. Chem. 2014, 6, 152–158. [Google Scholar] [CrossRef]
- Pantelic, R.S.; Meyer, J.C.; Kaiser, U.; Baumeister, W.; Plitzko, J.M. Graphene oxide: A substrate for optimizing preparations of frozen-hydrated samples. J. Struct. Biol. 2010, 170, 152–156. [Google Scholar] [CrossRef]
- Wilson, N.R.; Pandey, P.A.; Beanland, R.; Young, R.J.; Kinloch, I.A.; Gong, L.; Liu, Z.; Suenaga, K.; Rourke, J.P.; York, S.J.; et al. Graphene Oxide: Structural analysis and application as a highly transparent support for electron microscopy. ACS Nano 2009, 3, 2547–2556. [Google Scholar] [CrossRef]
- Kim, J.; Cote, L.J.; Huang, J. Two dimensional soft material: New faces of graphene oxide. Acc. Chem. Res. 2012, 45, 1356–1364. [Google Scholar] [CrossRef]
- Pei, S.; Wei, Q.; Huang, K.; Cheng, H.-M.; Ren, W. Green synthesis of graphene oxide by seconds timescale water electrolytic oxidation. Nat. Commun. 2018, 9, 145. [Google Scholar] [CrossRef] [Green Version]
- Handayani, M.; Nafi’ah, N.; Nugroho, A.; Rasyida, A.; Prasetyo, A.B.; Febriana, E.; Sulistiyono, E.; Firdiyono, F. The Development of Graphene/Silica Hybrid Composites: A review for their applications and challenges. Crystals 2021, 11, 1337. [Google Scholar] [CrossRef]
- Mokobia, K.; Ikhuoria, E.U.; Olugbemide, D.; Omorogbe, S.O. Production and characterization of biogas obtained from sugarcane leaves (Saccharum species). Int. J. Basic Appl. Sci. 2012, 1, 258–262. [Google Scholar]
- Shahriary, L.; Athawale, A.A. Graphene oxide synthesized by using modified Hummers approach. Int. J. Renew. Energy Environ. Eng. 2014, 2, 58–63. [Google Scholar]
- Chen, J.; Li, Y.; Huang, L.; Li, C.; Shi, G. High-yield preparation of graphene oxide from small graphite flakes via an improved Hummers method with a simple purification process. Carbon 2015, 81, 826–834. [Google Scholar] [CrossRef]
- Yu, H.; Zhang, B.; Bulin, C.; Li, R.; Xing, R. High-efficient synthesis of graphene oxide based on Improved Hummers method. Sci. Rep. 2016, 6, 36143. [Google Scholar] [CrossRef] [Green Version]







Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Thangaraj, B.; Mumtaz, F.; Abbas, Y.; Anjum, D.H.; Solomon, P.R.; Hassan, J. Synthesis of Graphene Oxide from Sugarcane Dry Leaves by Two-Stage Pyrolysis. Molecules 2023, 28, 3329. https://doi.org/10.3390/molecules28083329
Thangaraj B, Mumtaz F, Abbas Y, Anjum DH, Solomon PR, Hassan J. Synthesis of Graphene Oxide from Sugarcane Dry Leaves by Two-Stage Pyrolysis. Molecules. 2023; 28(8):3329. https://doi.org/10.3390/molecules28083329
Chicago/Turabian StyleThangaraj, Baskar, Fatima Mumtaz, Yawar Abbas, Dalaver H. Anjum, Pravin Raj Solomon, and Jamal Hassan. 2023. "Synthesis of Graphene Oxide from Sugarcane Dry Leaves by Two-Stage Pyrolysis" Molecules 28, no. 8: 3329. https://doi.org/10.3390/molecules28083329