Spectroscopical and Molecular Studies of Four Manganese(I) PhotoCORMs with Bioinspired Ligands Containing Non-Coordinated Phenol Groups
Abstract
1. Introduction
2. Results and Discussion
2.1. Solid-State Characterization
2.1.1. X-ray Diffractometry
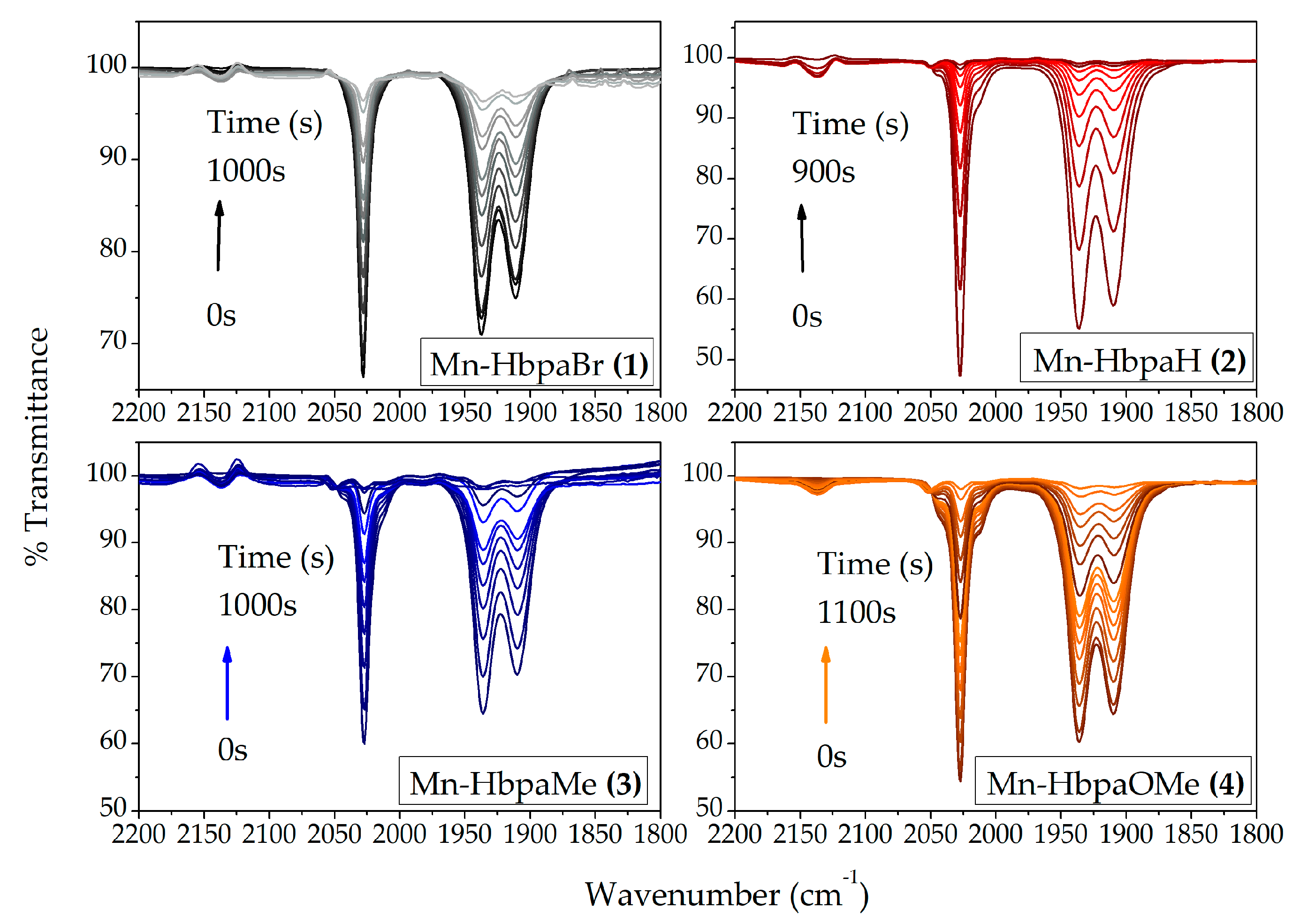
2.1.2. IR Spectroscopy
2.2. In-Solution Characterization
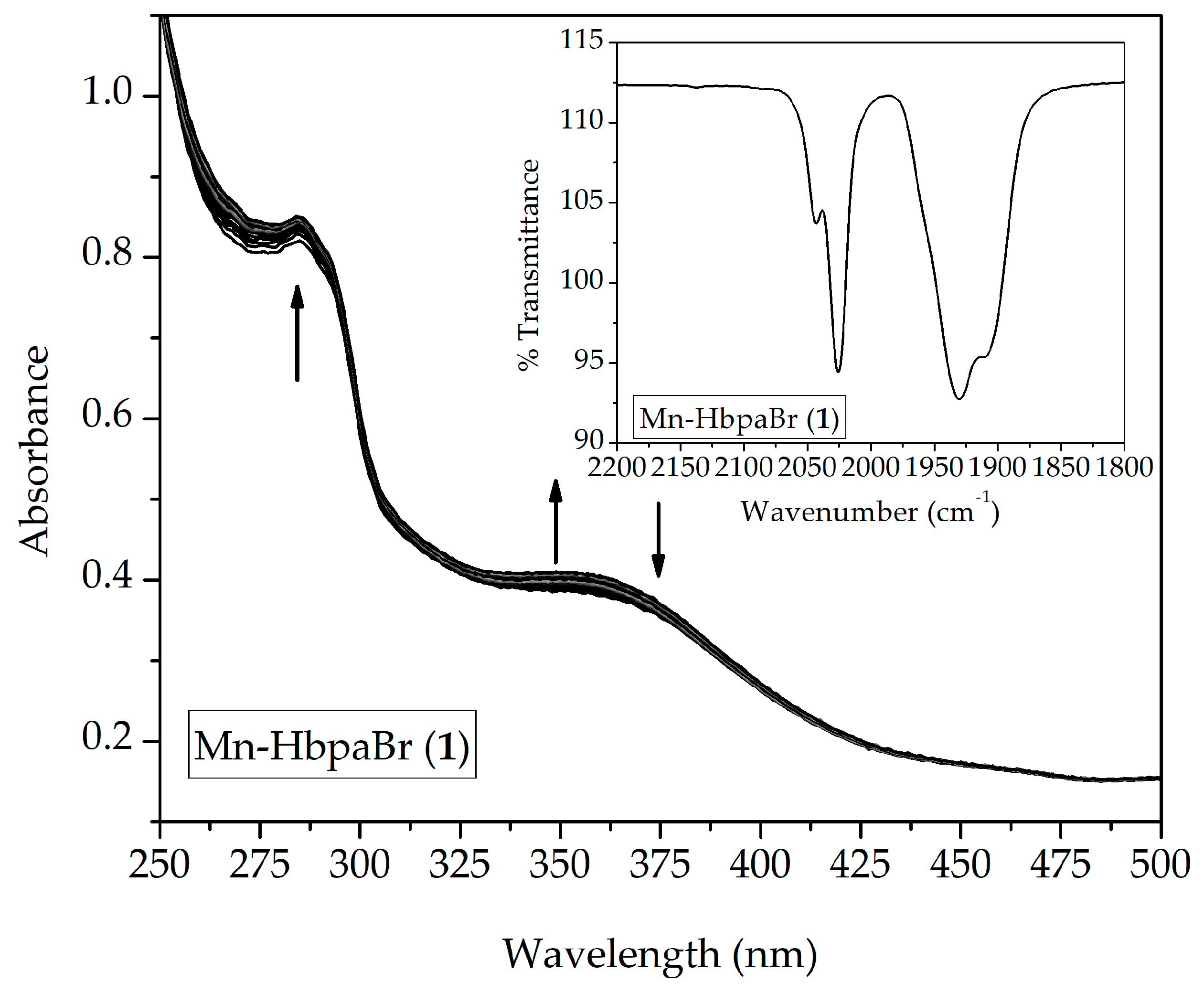
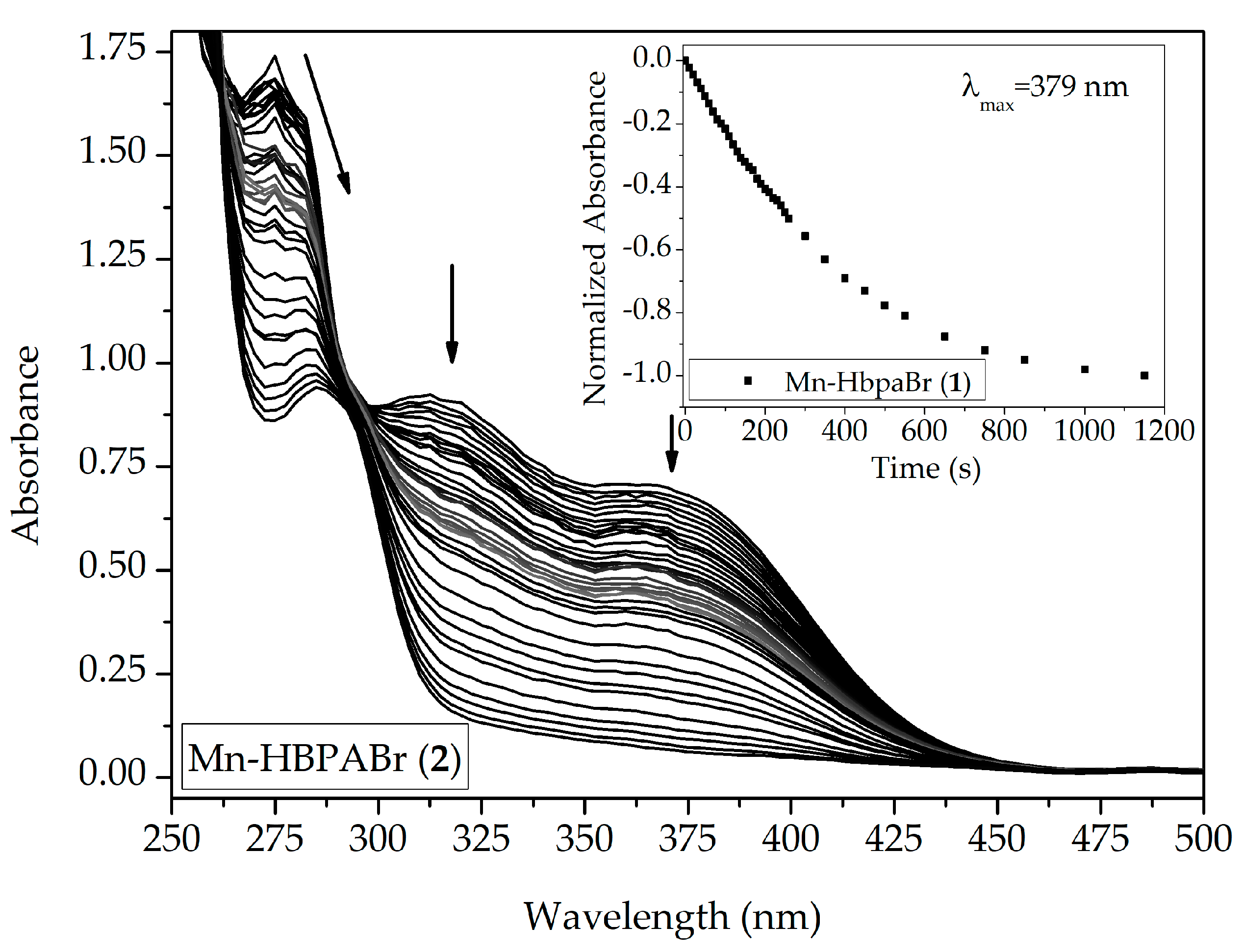
2.2.1. UV-Vis Spectroscopy
2.2.2. Electrochemistry
2.2.3. Mass Spectrometry (ESI-MS)
2.2.4. 1H NMR Spectroscopy
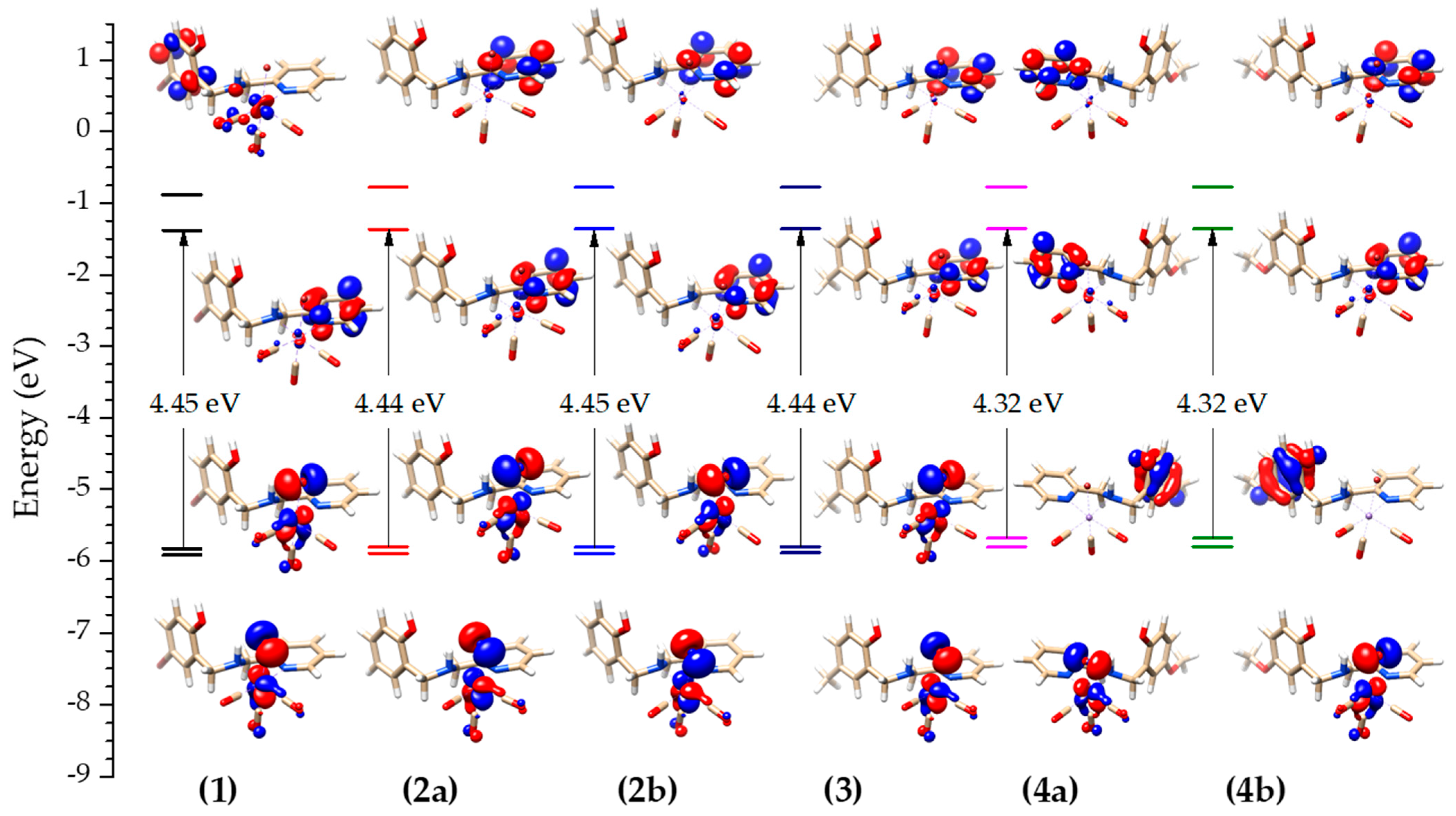
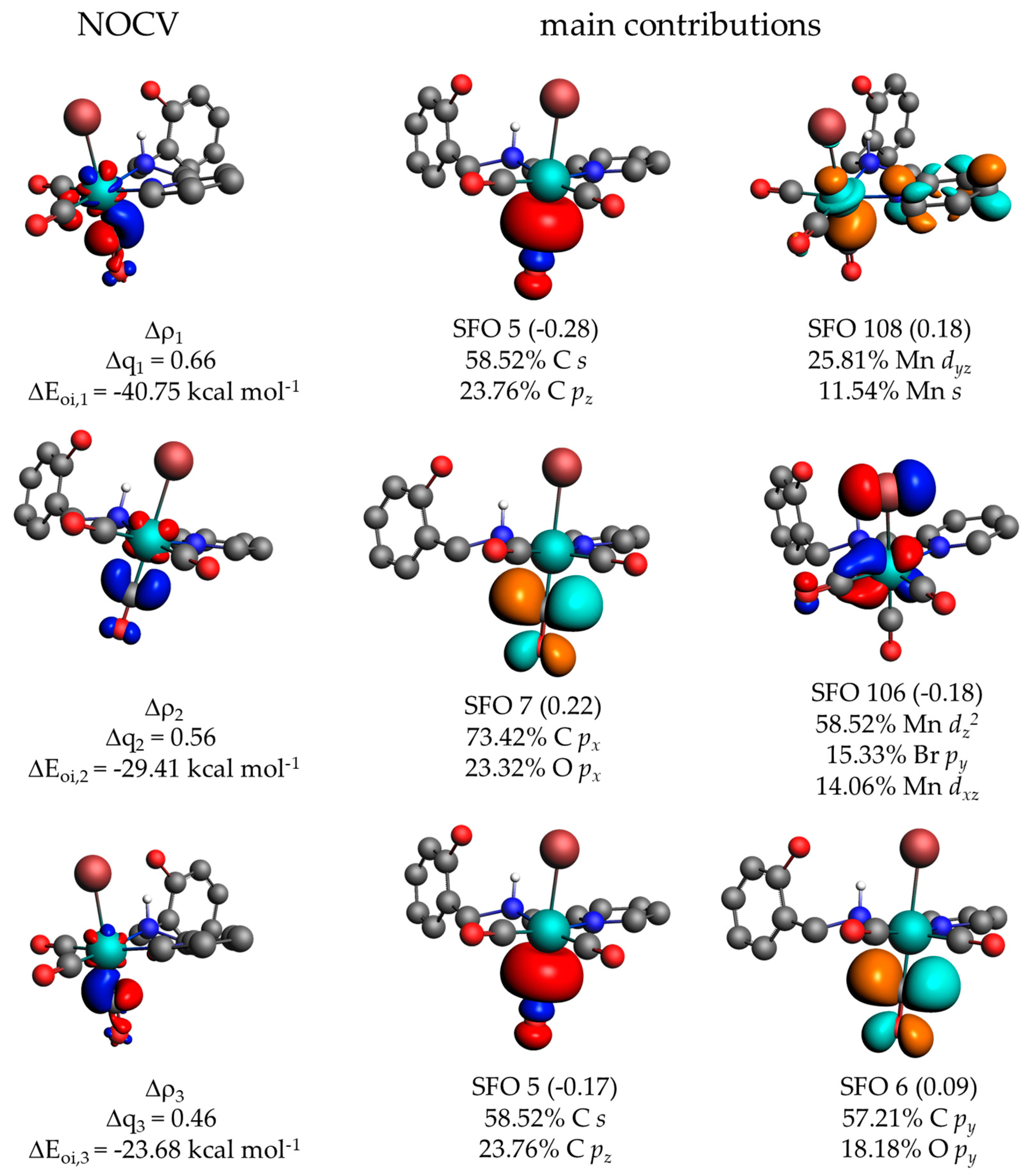
2.3. Computational Studies
2.4. Determination of Active Species in Solution
2.5. CO Release Assays
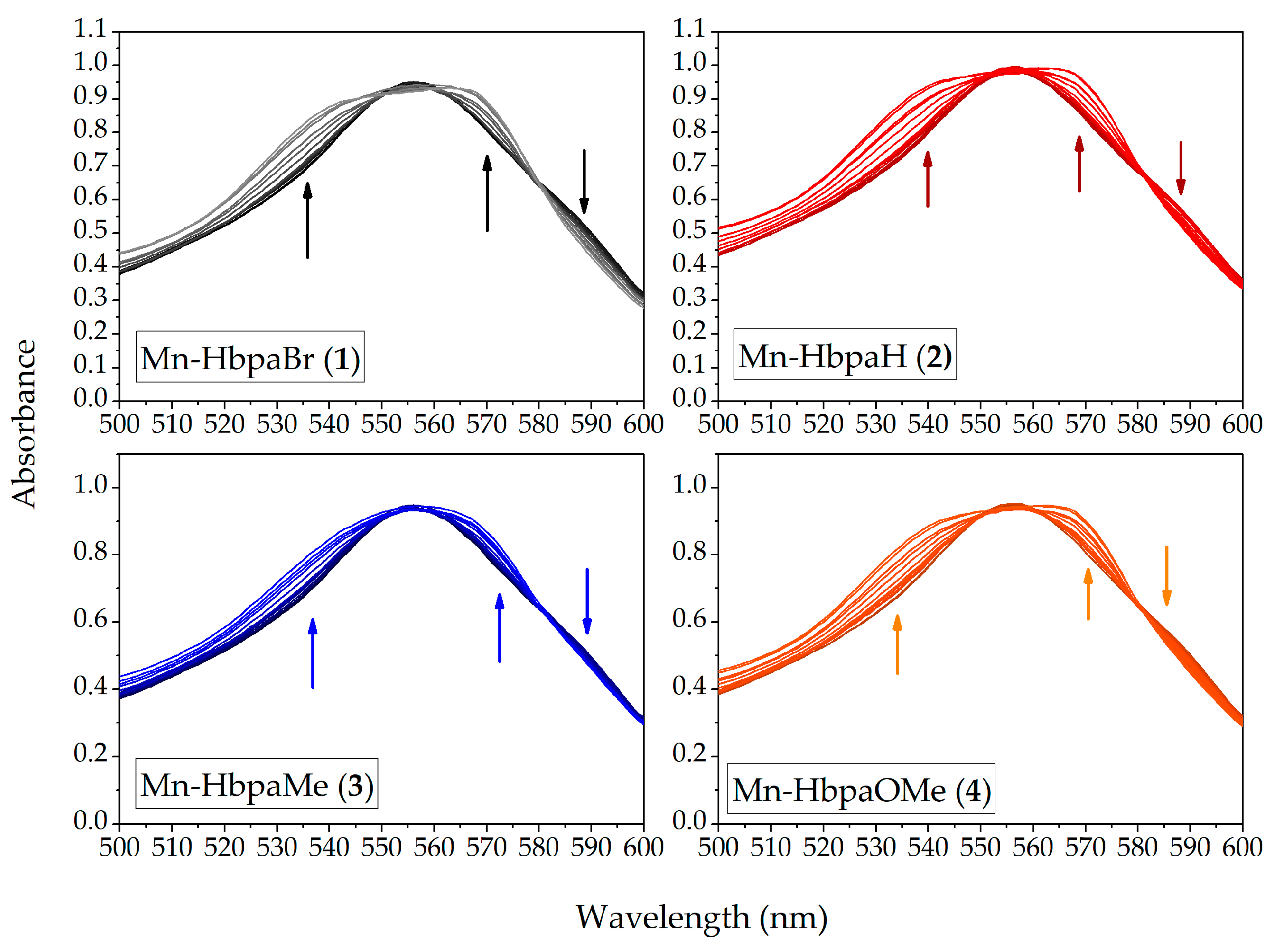
2.6. Myoglobin Assay
3. Materials and Methods
3.1. Single Crystal X-ray Diffraction
3.2. CO Release Assay
3.3. Myoglobin Assay
3.4. Computational Studies
3.5. Synthesis of the Compounds
3.5.1. N-(2-Pyridylmethyl)(2-hydroxy-5-bromobenzyl)amine—HbpaBr
3.5.2. N-(2-Pyridylmethyl)(2-hydroxybenzyl)amine—HbpaH
3.5.3. N-(2-Pyridylmethyl)(2-hydroxy-5-methylbenzyl)amine—HbpaMe
3.5.4. N-(2-Pyridylmethyl)(2-hydroxy-5-methoxybenzyl)amine—HbpaOMe
3.5.5. [MnBr(HbpaBr)(CO)3] (1)
3.5.6. [MnBr(Hbpa)(CO)3] (2)
3.5.7. [MnBr(HBPAMe)(CO)3] (3)
3.5.8. [MnBr(HBPAOMe)(CO)3] (4)
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kim, H.P.; Ryter, S.W.; Choi, A.M.K. CO as a Cellular Signaling Molecule. Annu. Rev. Pharmacol. Toxicol. 2006, 46, 411–449. [Google Scholar] [CrossRef]
- Morita, T.; Mitsialis, S.A.; Koike, H.; Liu, Y.; Kourembanas, S. Carbon Monoxide Controls the Proliferation of Hypoxic Vascular Smooth Muscle Cells. J. Biol. Chem. 1997, 272, 32804–32809. [Google Scholar] [CrossRef]
- Motterlini, R.; Foresti, R. Biological Signaling by Carbon Monoxide and Carbon Monoxide-Releasing Molecules. Am. J. Physiol. Cell Physiol. 2017, 312, C302–C313. [Google Scholar] [CrossRef]
- Otterbein, L.E.; Bach, F.H.; Alam, J.; Soares, M.; Tao Lu, H.; Wysk, M.; Davis, R.J.; Flavell, R.A.; Choi, A.M.K. Carbon Monoxide Has Anti-Inflammatory Effects Involving the Mitogen-Activated Protein Kinase Pathway. Nat. Med. 2000, 6, 422–428. [Google Scholar] [CrossRef]
- Brouard, S.; Otterbein, L.E.; Anrather, J.; Tobiasch, E.; Bach, F.H.; Choi, A.M.K.; Soares, M.P. Carbon Monoxide Generated by Heme Oxygenase 1 Suppresses Endothelial Cell Apoptosis. J. Exp. Med. 2000, 192, 1015–1026. [Google Scholar] [CrossRef] [PubMed]
- Song, R.; Mahidhara, R.S.; Liu, F.; Ning, W.; Otterbein, L.E.; Choi, A.M.K. Carbon Monoxide Inhibits Human Airway Smooth Muscle Cell Proliferation via Mitogen-Activated Protein Kinase Pathway. Am. J. Respir. Cell. Mol. Biol. 2002, 27, 603–610. [Google Scholar] [CrossRef]
- Motterlini, R.; Gonzales, A.; Foresti, R.; Clark, J.E.; Green, C.J.; Winslow, R.M. Heme Oxygenase-1–Derived Carbon Monoxide Contributes to the Suppression of Acute Hypertensive Responses In Vivo. Circ. Res. 1998, 83, 568–577. [Google Scholar] [CrossRef]
- Hoetzel, A.; Schmidt, R.; Vallbracht, S.; Goebel, U.; Dolinay, T.; Kim, H.P.; Ifedigbo, E.; Ryter, S.W.; Choi, A.M.K. Carbon Monoxide Prevents Ventilator-Induced Lung Injury via Caveolin-1*. Crit. Care Med. 2009, 37, 1708–1715. [Google Scholar] [CrossRef] [PubMed]
- Dubuis, E.; Potier, M.; Wang, R.; Vandier, C. Continuous Inhalation of Carbon Monoxide Attenuates Hypoxic Pulmonary Hypertension Development Presumably through Activation of BK Channels. Cardiovasc. Res. 2005, 65, 751–761. [Google Scholar] [CrossRef] [PubMed]
- Motterlini, R.; Clark, J.E.; Foresti, R.; Sarathchandra, P.; Mann, B.E.; Green, C.J. Carbon Monoxide-Releasing Molecules: Characterization of Biochemical and Vascular Activities. Circ. Res. 2002, 90, e17–e24. [Google Scholar] [CrossRef]
- Romanski, S.; Stamellou, E.; Jaraba, J.T.; Storz, D.; Krämer, B.K.; Hafner, M.; Amslinger, S.; Schmalz, H.G.; Yard, B.A. Enzyme-Triggered CO-Releasing Molecules (ET-CORMs): Evaluation of Biological Activity in Relation to Their Structure. Free Radic. Biol. Med. 2013, 65, 78–88. [Google Scholar] [CrossRef] [PubMed]
- Ji, X.; De La Cruz, L.K.C.; Pan, Z.; Chittavong, V.; Wang, B. PH-Sensitive Metal-Free Carbon Monoxide Prodrugs with Tunable and Predictable Release Rates. Chem. Commun. 2017, 53, 9628–9631. [Google Scholar] [CrossRef] [PubMed]
- Gonzales, M.A.; Mascharak, P.K. Photoactive Metal Carbonyl Complexes as Potential Agents for Targeted CO Delivery. J. Inorg. Biochem. 2014, 133, 127–135. [Google Scholar] [CrossRef] [PubMed]
- Rimmer, R.D.; Richter, H.; Ford, P.C. A Photochemical Precursor for Carbon Monoxide Release in Aerated Aqueous Media. Inorg. Chem. 2010, 49, 1180–1185. [Google Scholar] [CrossRef]
- Velema, W.A.; Szymanski, W.; Feringa, B.L. Photopharmacology: Beyond Proof of Principle. J. Am. Chem. Soc. 2014, 136, 2178–2191. [Google Scholar] [CrossRef]
- Tornaletti, S.; Pfeifer, G.P. UV Damage and Repair Mechanisms in Mammalian Cells. Bioessays 1996, 18, 221–228. [Google Scholar] [CrossRef] [PubMed]
- Wright, M.A.; Wright, J.A. PhotoCORMs: CO Release Moves into the Visible. Dalton Trans. 2016, 45, 6801–6811. [Google Scholar] [CrossRef]
- Kottelat, E.; Fabio, Z. Visible Light-Activated PhotoCORMs. Inorganics 2017, 5, 24. [Google Scholar] [CrossRef]
- Romão, C.C.; Vieira, H.L.A. Metal Carbonyl Prodrugs: CO Delivery and Beyond. In Bioorganometallic Chemistry; Jaouen, G., Salmain, M., Eds.; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2014; pp. 165–202. ISBN 978-3-527-67343-8. [Google Scholar]
- Chakraborty, I.; Carrington, S.J.; Mascharak, P.K. Design Strategies to Improve the Sensitivity of Photoactive Metal Carbonyl Complexes (PhotoCORMs) to Visible Light and Their Potential as CO-Donors to Biological Targets. Acc. Chem. Res. 2014, 47, 2603–2611. [Google Scholar] [CrossRef]
- Pordel, S.; White, J.K. Impact of Mn(I) PhotoCORM Ligand Set on Photochemical Intermediate Formation during Visible Light-Activated CO Release. Inorg. Chim. Acta 2020, 500, 119206. [Google Scholar] [CrossRef]
- Amorim, A.L.; Guerreiro, A.; Glitz, V.A.; Coimbra, D.F.; Bortoluzzi, A.J.; Caramori, G.F.; Braga, A.L.; Neves, A.; Bernardes, G.J.L.; Peralta, R.A. Synthesis, Characterization and Photoinduced CO-Release by Manganese(I) Complexes. New J. Chem. 2020, 44, 10892–10901. [Google Scholar] [CrossRef]
- Amorim, A.L.; Peterle, M.M.; Guerreiro, A.; Coimbra, D.F.; Heying, R.S.; Caramori, G.F.; Braga, A.L.; Bortoluzzi, A.J.; Neves, A.; Bernardes, G.J.L.; et al. Synthesis, Characterization and Biological Evaluation of New Manganese Metal Carbonyl Compounds That Contain Sulfur and Selenium Ligands as a Promising New Class of CORMs. Dalton Trans. 2019, 48, 5574–5584. [Google Scholar] [CrossRef]
- Neves, A.; Verani, C.N.; de Brito, M.A.; Vencato, I.; Mangrich, A.; Oliva, G.; Souza, D.D.H.F.; Batista, A.A. Copper(II) Complexes with (2-Hydroxybenzyl-2-Pyridylmethyl)Amine–Hbpa: Syntheses, Characterization and Crystal Structures of the Ligand and [Cu(II)(Hbpa)2](ClO4)2·2H2O. Inorg. Chim. Acta 1999, 290, 207–212. [Google Scholar] [CrossRef]
- Peralta, R.A.; Bortoluzzi, A.J.; de Souza, B.; Jovito, R.; Xavier, F.R.; Couto, R.A.A.; Casellato, A.; Nome, F.; Dick, A.; Gahan, L.R.; et al. Electronic Structure and Spectro-Structural Correlations of Fe III Zn II Biomimetics for Purple Acid Phosphatases: Relevance to DNA Cleavage and Cytotoxic Activity. Inorg. Chem. 2010, 49, 11421–11438. [Google Scholar] [CrossRef] [PubMed]
- Brückmann, N.E.; Wahl, M.; Reiß, G.J.; Kohns, M.; Wätjen, W.; Kunz, P.C. Polymer Conjugates of Photoinducible CO-Releasing Molecules. Eur. J. Inorg. Chem. 2011, 29, 4571–4577. [Google Scholar] [CrossRef]
- Liu, J.; Hoffmann, P.; Steinmetzer, J.; Askes, S.H.C.; Kupfer, S.; Görls, H.; Gräfe, S.; Neugebauer, U.; Gandra, U.R.; Schiller, A. Visible Light-Activated Biocompatible Photo-CORM for CO-Release with Colorimetric and Fluorometric Dual Turn-on Response. Polyhedron 2019, 172, 175–181. [Google Scholar] [CrossRef]
- Berends, H.-M.; Kurz, P. Investigation of Light-Triggered Carbon Monoxide Release from Two Manganese PhotoCORMs by IR, UV–Vis and EPR Spectroscopy. Inorg. Chim. Acta 2012, 380, 141–147. [Google Scholar] [CrossRef]
- Jiang, Q.; Xia, Y.; Barrett, J.; Mikhailovsky, A.; Wu, G.; Wang, D.; Shi, P.; Ford, P.C. Near-Infrared and Visible Photoactivation to Uncage Carbon Monoxide from an Aqueous-Soluble PhotoCORM. Inorg. Chem. 2019, 58, 11066–11075. [Google Scholar] [CrossRef]
- Gonzalez, M.A.; Carrington, S.J.; Fry, N.L.; Martinez, J.L.; Mascharak, P.K. Syntheses, Structures, and Properties of New Manganese Carbonyls as Photoactive CO-Releasing Molecules: Design Strategies That Lead to CO Photolability in the Visible Region. Inorg. Chem. 2012, 51, 11930–11940. [Google Scholar] [CrossRef]
- Pordel, S.; Pickens, R.N.; White, J.K. Release of CO and Production of 1O2 from a Mn-BODIPY Photoactivated CO Releasing Molecule with Visible Light. Organometallics 2021, 40, 2983–2994. [Google Scholar] [CrossRef]
- Weiss, V.C.; Farias, G.; Amorim, A.L.; Xavier, F.R.; Camargo, T.P.; Bregalda, M.B.; Haukka, M.; Nordlander, E.; de Souza, B.; Peralta, R.A. Luminescent PhotoCORMs: Enabling/Disabling CO Delivery upon Blue Light Irradiation. Inorg. Chem. 2020, 59, 13078–13090. [Google Scholar] [CrossRef]
- Waldvogel, S.R. Novel Anodic Concepts for the Selective Phenol Coupling Reaction. Pure Appl. Chem. 2010, 82, 1055–1063. [Google Scholar] [CrossRef]
- Weiss, V.; Amorim, A.; Xavier, F.; Bortoluzzi, A.; Neves, A.; Peralta, R. Light Response of Three Water-Soluble MnI PhotoCORMs: Spectroscopic Features and CO Release Investigation. J. Braz. Chem. Soc. 2019, 30, 2649–2659. [Google Scholar] [CrossRef]
- Sachs, U.; Schaper, G.; Winkler, D.; Kratzert, D.; Kurz, P. Light- or Oxidation-Triggered CO Release from [MnI(CO)3(κ3-L)] Complexes: Reaction Intermediates and a New Synthetic Route to [MnIII/IV2(μ-O)2(L)2] Compounds. Dalton Trans. 2016, 45, 17464–17473. [Google Scholar] [CrossRef]
- Sheldrick, G.M. Crystal Structure Refinement with SHELXL. Acta Crystallogr. C Struct. Chem. 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Bischof, C.; Joshi, T.; Dimri, A.; Spiccia, L.; Schatzschneider, U. Synthesis, Spectroscopic Properties, and Photoinduced CO-Release Studies of Functionalized Ruthenium(II) Polypyridyl Complexes: Versatile Building Blocks for Development of CORM–Peptide Nucleic Acid Bioconjugates. Inorg. Chem. 2013, 52, 9297–9308. [Google Scholar] [CrossRef]
- Atkin, A.J.; Lynam, J.M.; Moulton, B.E.; Sawle, P.; Motterlini, R.; Boyle, N.M.; Pryce, M.T.; Fairlamb, I.J.S. Modification of the Deoxy-Myoglobin/Carbonmonoxy-Myoglobin UV-Vis Assay for Reliable Determination of CO-Release Rates from Organometallic Carbonyl Complexes. Dalton Trans. 2011, 40, 5755. [Google Scholar] [CrossRef] [PubMed]
- Neese, F. The ORCA Program System. WIREs Comput. Mol. Sci. 2012, 2, 73–78. [Google Scholar] [CrossRef]
- Neese, F. Software Update: The ORCA Program System—Version 5.0. WIREs Comput. Mol. Sci. 2022, 12, e1606. [Google Scholar] [CrossRef]
- Becke, A.D. Density-functional Thermochemistry. III. The Role of Exact Exchange. J. Chem. Phys. 1993, 98, 5648–5652. [Google Scholar] [CrossRef]
- Stephens, P.J.; Devlin, F.J.; Chabalowski, C.F.; Frisch, M.J. Ab Initio Calculation of Vibrational Absorption and Circular Dichroism Spectra Using Density Functional Force Fields. J. Phys. Chem. 1994, 98, 11623–11627. [Google Scholar] [CrossRef]
- Weigend, F.; Ahlrichs, R. Balanced Basis Sets of Split Valence, Triple Zeta Valence and Quadruple Zeta Valence Quality for H to Rn: Design and Assessment of Accuracy. Phys. Chem. Chem. Phys. 2005, 7, 3297. [Google Scholar] [CrossRef]
- Caldeweyher, E.; Bannwarth, C.; Grimme, S. Extension of the D3 Dispersion Coefficient Model. J. Chem. Phys. 2017, 147, 034112. [Google Scholar] [CrossRef] [PubMed]
- Caldeweyher, E.; Ehlert, S.; Hansen, A.; Neugebauer, H.; Spicher, S.; Bannwarth, C.; Grimme, S. A Generally Applicable Atomic-Charge Dependent London Dispersion Correction. J. Chem. Phys. 2019, 150, 154122. [Google Scholar] [CrossRef] [PubMed]
- Marenich, A.V.; Cramer, C.J.; Truhlar, D.G. Universal Solvation Model Based on Solute Electron Density and on a Continuum Model of the Solvent Defined by the Bulk Dielectric Constant and Atomic Surface Tensions. J. Phys. Chem. B 2009, 113, 6378–6396. [Google Scholar] [CrossRef] [PubMed]
- Pettersen, E.F.; Goddard, T.D.; Huang, C.C.; Couch, G.S.; Greenblatt, D.M.; Meng, E.C.; Ferrin, T.E. UCSF Chimera A Visualization System for Exploratory Research and Analysis. J. Comput. Chem. 2004, 25, 1605–1612. [Google Scholar] [CrossRef]
- Mitoraj, M.P.; Michalak, A.; Ziegler, T. A Combined Charge and Energy Decomposition Scheme for Bond Analysis. J. Chem. Theory Comput. 2009, 5, 962–975. [Google Scholar] [CrossRef]
- te Velde, G.; Bickelhaupt, F.M.; Baerends, E.J.; Fonseca Guerra, C.; van Gisbergen, S.J.A.; Snijders, J.G.; Ziegler, T. Chemistry with ADF. J. Comput. Chem. 2001, 22, 931–967. [Google Scholar] [CrossRef]
- van Lenthe, E.; Baerends, E.J.; Snijders, J.G. Relativistic Regular Two-component Hamiltonians. J. Chem. Phys. 1993, 99, 4597–4610. [Google Scholar] [CrossRef]
- van Lenthe, E.; Baerends, E.J. Optimized Slater-Type Basis Sets for the Elements 1–118. J. Comput. Chem. 2003, 24, 1142–1156. [Google Scholar] [CrossRef]
- Grimme, S.; Antony, J.; Ehrlich, S.; Krieg, H. A Consistent and Accurate Ab Initio Parametrization of Density Functional Dispersion Correction (DFT-D) for the 94 Elements H-Pu. J. Chem. Phys. 2010, 132, 154104. [Google Scholar] [CrossRef] [PubMed]
- Grimme, S.; Ehrlich, S.; Goerigk, L. Effect of the Damping Function in Dispersion Corrected Density Functional Theory. J. Comput. Chem. 2011, 32, 1456–1465. [Google Scholar] [CrossRef] [PubMed]
- Neves, A.; Tomkowicz, Z.; Couto, R.A.A.; Bombazar, C.C.; Amorim, S.M.; Bortoluzzi, A.J.; Peralta, R.A. Trinuclear CuII Complex Containing a New Pentadentate Ligand: Structure, Magnetism, Physicochemical Properties and Catecholase Activity. Inorg. Chim. Acta 2022, 533, 120804. [Google Scholar] [CrossRef]

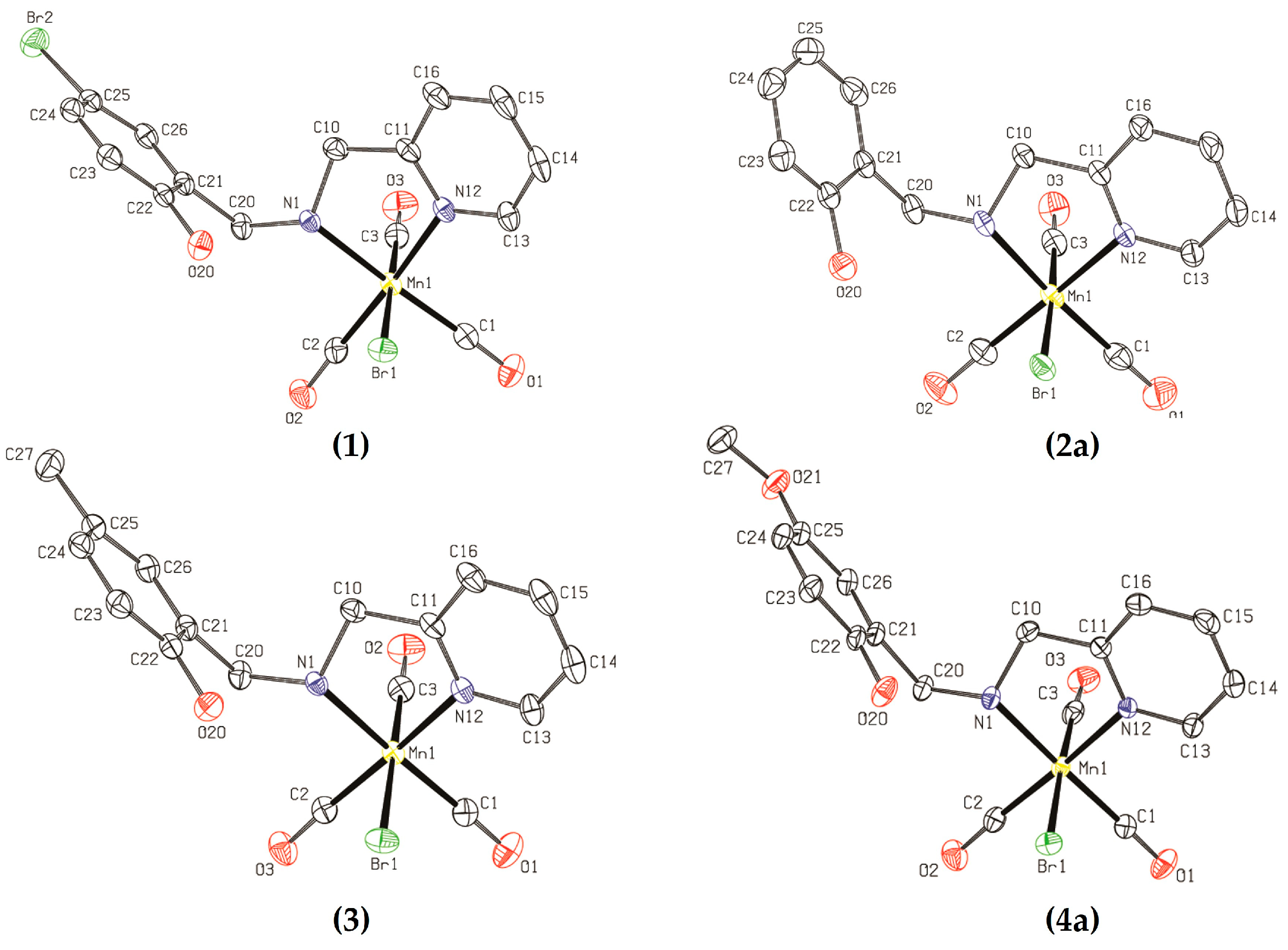



| Bond | Compound | |||
|---|---|---|---|---|
| (1) | (2a) | (3) | (4a) | |
| Mn–C(1) | 1.815(2) | 1.816(2) | 1.8214(17) | 1.814(3) |
| Mn–C(2) | 1.811(2) | 1.8119(19) | 1.8154(17) | 1.816(3) |
| Mn–C(3) | 1.802(2) | 1.7948(19) | 1.7973(17) | 1.798(3) |
| Mn–N(sp3) | 2.0856(16) | 2.0886(15) | 2.0860(14) | 2.089(2) |
| Mn–N(sp2) | 2.0590(15) | 2.0525(14) | 2.0610(14) | 2.052(2) |
| Mn–Br | 2.5254(4) | 2.5273(3) | 2.5258(3) | 2.5320(5) |
| Compound | EpMnI/II (V) vs. Fc/Fc+ | UV-Vis λmax, nm (ε, L mol−1 cm−1) |
|---|---|---|
| (1) | 0.681 | 284 (4127), 321sh (~2128), 379sh (~1600) |
| (2) | 0.741 | 273 (6524), 323sh (~3716), 379sh (~2900) |
| (3) | 0.667 | 281 (6021), 323sh (~3030), 379sh (~2400) |
| (4) | 0.643 | 295 (5926), 329sh (~2300), 379sh (~1800) |
| 1 ΔEint | ΔEPauli | 2 ΔEelstat | 2 ΔEdisp | 2 ΔEoi | 3 ΔEoi-σ | 3 ΔEoi-π | 3 ΔEoi-rest | |
| (1) | ||||||||
| 1-CO | −44.93 | 168.27 | −114.24 (53.6) | −2.59 (1.2) | −96.38 (45.2) | −40.42 (41.9) | −52.98 (55.0) | −2.98 (3.1) |
| 2-CO | −36.64 | 166.95 | −112.41 (55.2) | −2.38 (1.2) | −88.80 (43.6) | −37.74 (42.5) | −47.61 (53.6) | −3.45 (3.9) |
| 3-CO | −37.15 | 167.67 | −112.96 (55.2) | −2.07 (1.0) | −89.78 (43.8) | −38.31 (42.7) | −48.09 (53.6) | −3.38 (3.8) |
| (2a) | ||||||||
| 1-CO | −45.16 | 169.01 | −114.78 (53.6) | −2.55 (1.2) | −96.83 (45.2) | −40.75 (42.1) | −53.09 (54.8) | −2.99 (3.1) |
| 2-CO | −36.50 | 168.41 | −113.24 (55.3) | −2.37 (1.2) | −89.31 (43.6) | −38.16 (42.7) | −47.69 (53.4) | −3.46 (3.9) |
| 3-CO | −37.10 | 167.72 | −112.99 (55.2) | −2.10 (1.0) | −89.73 (43.8) | −38.11 (42.5) | −48.20 (53.7) | −3.42 (3.8) |
| (2b) | ||||||||
| 1-CO | −45.03 | 167.86 | −113.99 (53.5) | −2.60 (1.2) | −96.31 (45.2) | −40.25 (41.8) | −53.08 (55.1) | −2.98 (3.1) |
| 2-CO | −36.58 | 166.79 | −112.34 (55.2) | −2.36 (1.2) | −88.67 (43.6) | −37.78 (42.6) | −47.46 (53.5) | −3.43 (3.9) |
| 3-CO | −37.28 | 167.45 | −112.90 (55.1) | −2.07 (1.0) | −89.75 (43.8) | −38.05 (42.4) | −48.37 (53.9) | −3.33 (3.7) |
| (3) | ||||||||
| 1-CO | −45.06 | 167.73 | −114.64 (53.6) | −2.58 (1.2) | −96.73 (45.2) | −40.64 (42.0) | −53.10 (54.9) | −2.99 (3.1) |
| 2-CO | −36.58 | 166.11 | −112.81 (55.3) | −2.39 (1.2) | −89.07 (43.6) | −37.84 (42.5) | −47.75 (53.6) | −3.48 (3.9) |
| 3-CO | −37.14 | 166.72 | −113.01 (55.1) | −2.07 (1.0) | −89.76 (43.8) | −38.18 (42.5) | −48.13 (53.6) | −3.45 (3.8) |
| (4a) | ||||||||
| 1-CO | −45.06 | 169.66 | −113.91 (53.5) | −2.61 (1.2) | -96.27 (45.2) | −40.94 (42.5) | −53.09 (55.1) | −2.24 (3.8) |
| 2-CO | −36.57 | 167.39 | −112.03 (55.3) | −2.33 (1.1) | -88.32 (43.6) | −37.95 (43.0) | −47.40 (53.7) | −2.97 (4.3) |
| 3-CO | −37.43 | 167.46 | −112.46 (55.1) | −2.08 (1.0) | -89.61 (43.9) | −38.05 (42.5) | −48.33 (53.9) | −3.23 (3.9) |
| (4b) | ||||||||
| 1-CO | −45.01 | 168.89 | −115.05 (53.6) | −2.61 (1.2) | -97.01 (45.2) | −40.22 (41.5) | −53.06 (54.7) | −3.73 (2.3) |
| 2-CO | −36.44 | 167.69 | −112.70 (55.3) | −2.37 (1.2) | -88.75 (43.5) | −37.74 (42.5) | −47.17 (53.1) | −3.84 (3.4) |
| 3-CO | −37.28 | 167.7 | −112.89 (55.1) | −2.09 (1.0) | -89.76 (43.8) | −37.96 (42.3) | −48.30 (53.8) | −3.50 (3.6) |
| Compound | kCO,old (10−3 s−1) | σp | kCO,new (10−3 s−1) | ε (L mol−1 cm−1) | t1/2 (s) | Φ |
|---|---|---|---|---|---|---|
| (1) | 2.36 ± 0.11 | 0.23 | 2.37 ± 0.09 | ~1600 | 264.85 ± 9.71 | 0.031 ± 0.0002 |
| (2) | 1.77 ± 0.02 | 0.00 | 1.90 ± 0.001 | ~2900 | 243.63 ± 10.98 | 0.019 ± 0.0002 |
| (3) | 1.60 ± 0.02 | −0.17 | 2.03 ± 0.04 | ~2400 | 266.60 ± 3.78 | 0.011 ± 0.0002 |
| (4) | 1.24 ± 0.04 | −0.27 | 2.07 ± 0.04 | ~1800 | 265.22 ± 1.18 | 0.017 ± 0.0001 |
| Compound | Amount of CO Released |
|---|---|
| (1) | 1.544 ± 0.033 |
| (2) | 1.827 ± 0.093 |
| (3) | 1.248 ± 0.068 |
| (4) | 1.591 ± 0.042 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Paqui, M.S.S.; Glitz, V.A.; Durigon, D.C.; Amorim, A.L.; Caramori, G.F.; Parreira, R.L.T.; Bortoluzzi, A.J.; Xavier, F.R.; Peralta, R.A. Spectroscopical and Molecular Studies of Four Manganese(I) PhotoCORMs with Bioinspired Ligands Containing Non-Coordinated Phenol Groups. Molecules 2023, 28, 3439. https://doi.org/10.3390/molecules28083439
Paqui MSS, Glitz VA, Durigon DC, Amorim AL, Caramori GF, Parreira RLT, Bortoluzzi AJ, Xavier FR, Peralta RA. Spectroscopical and Molecular Studies of Four Manganese(I) PhotoCORMs with Bioinspired Ligands Containing Non-Coordinated Phenol Groups. Molecules. 2023; 28(8):3439. https://doi.org/10.3390/molecules28083439
Chicago/Turabian StylePaqui, Matheus S. S., Vinícius A. Glitz, Daniele C. Durigon, André L. Amorim, Giovanni F. Caramori, Renato L. T. Parreira, Adailton J. Bortoluzzi, Fernando R. Xavier, and Rosely A. Peralta. 2023. "Spectroscopical and Molecular Studies of Four Manganese(I) PhotoCORMs with Bioinspired Ligands Containing Non-Coordinated Phenol Groups" Molecules 28, no. 8: 3439. https://doi.org/10.3390/molecules28083439
APA StylePaqui, M. S. S., Glitz, V. A., Durigon, D. C., Amorim, A. L., Caramori, G. F., Parreira, R. L. T., Bortoluzzi, A. J., Xavier, F. R., & Peralta, R. A. (2023). Spectroscopical and Molecular Studies of Four Manganese(I) PhotoCORMs with Bioinspired Ligands Containing Non-Coordinated Phenol Groups. Molecules, 28(8), 3439. https://doi.org/10.3390/molecules28083439







