Simultaneous Quantification of Trehalose and Trehalose 6-Phosphate by Hydrophilic Interaction Chromatography/Electrospray Accurate Mass Spectrometry with Application in Non-Targeted Metabolomics
Abstract
:1. Introduction
2. Results and Discussion
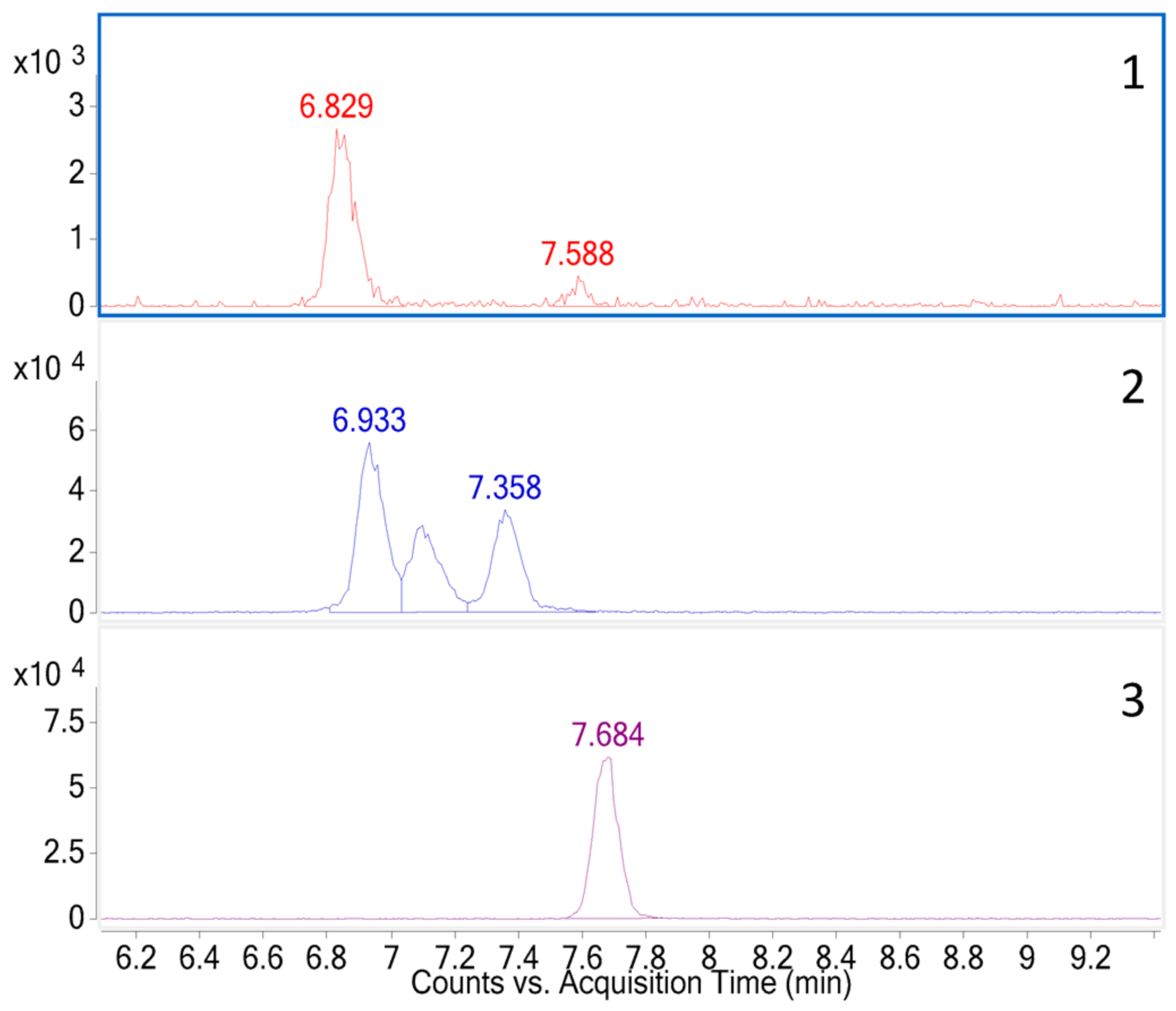
2.1. LC-HRMS Method Development and Validation
2.2. LC-HRMS Method Validation
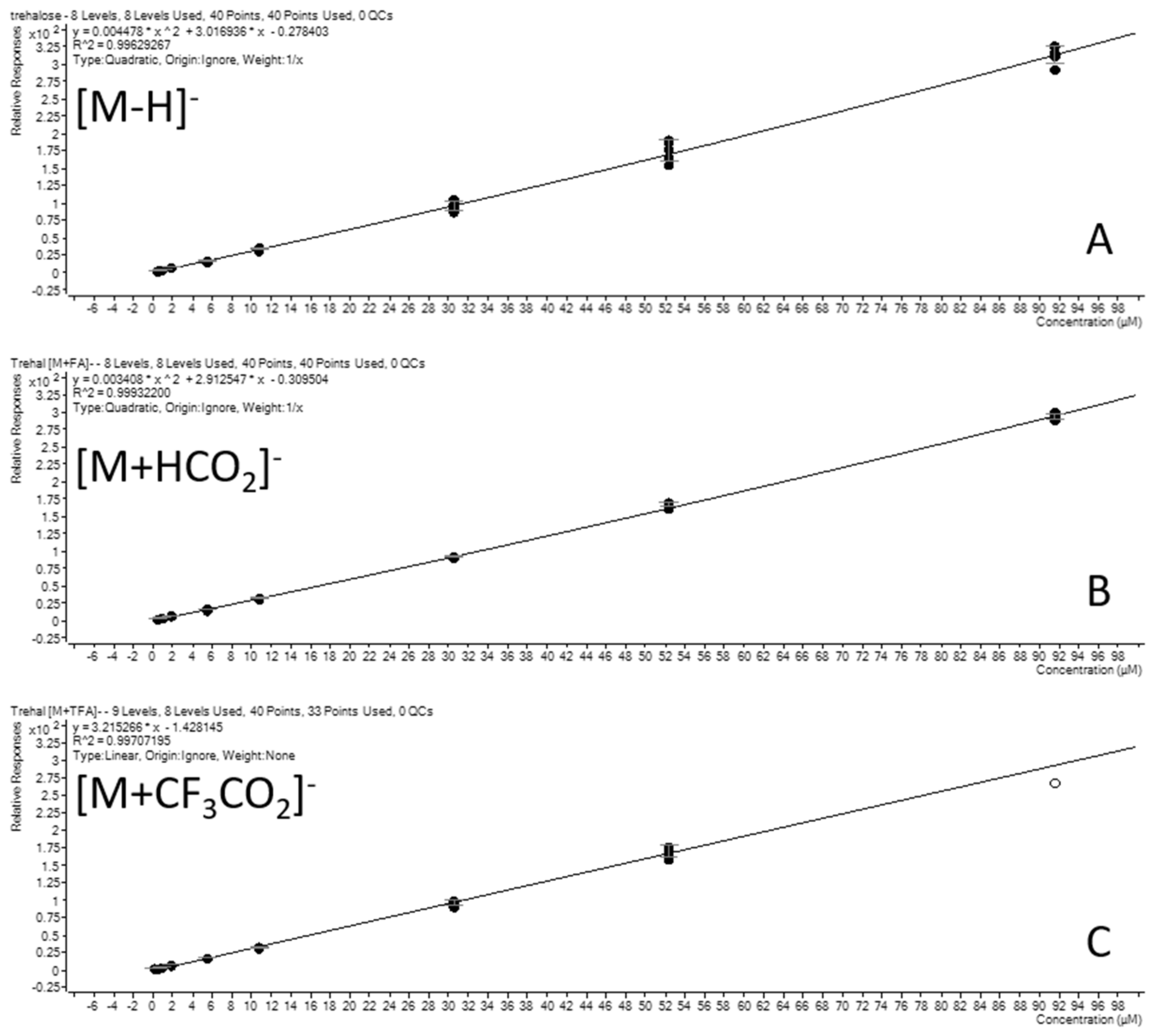
2.2.1. Linearity, Limit of Detection (LOD), and Limit of Quantification (LOQ)
2.2.2. Robustness, Method Precision, and Trueness
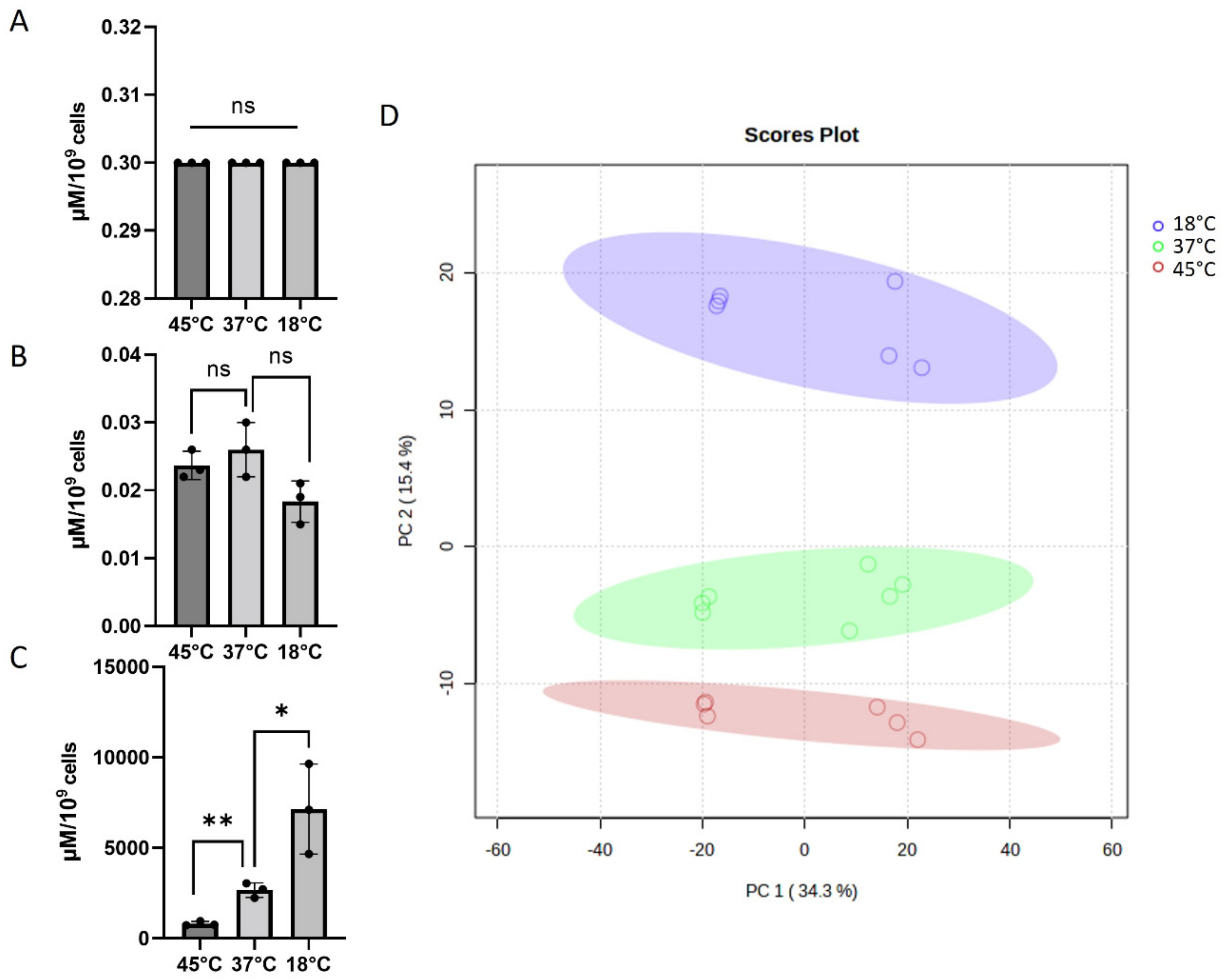
2.3. LC-HRMS Quantification and Metabolomic Analysis of Acinetobacter baumannii Samples
3. Experimentals
3.1. Chemicals
3.2. Biological Material
3.2.1. Yeasts
3.2.2. Acinetobacter baumannii
3.3. Internal Standard Preparation
3.4. Standard and QC Samples Preparation
3.5. Sample Preparation
3.6. LC-HRMS Instrumentation and Analytical Conditions
3.7. Validation
3.7.1. Selectivity
3.7.2. Linearity and LLOQ
3.7.3. Accuracy and Precision
3.7.4. Stability
3.8. Data and Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Empadinhas, N.; Da Costa, M.S. Osmoadaptation Mechanisms in Prokaryotes: Distribution of Compatible Solutes. Int. Microbiol. 2008, 11, 151–161. [Google Scholar] [CrossRef] [PubMed]
- Zeidler, S.; Hubloher, J.; Schabacker, K.; Lamosa, P.; Santos, H.; Müller, V. Trehalose, a Temperature- and Salt-Induced Solute with Implications in Pathobiology of Acinetobacter baumannii. Environ. Microbiol. 2017, 19, 5088–5099. [Google Scholar] [CrossRef] [PubMed]
- Eroglu, A.; Russo, M.J.; Bieganski, R.; Fowler, A.; Cheley, S.; Bayley, H.; Toner, M. Intracellular Trehalose Improves the Survival of Cryopreserved Mammalian Cells. Nat. Biotechnol. 2000, 18, 163–167. [Google Scholar] [CrossRef] [PubMed]
- Wannet, W.J.B.; Hermans, J.H.M.; Van Der Drift, C.; Op Den Camp, H.J.M. HPLC Detection of Soluble Carbohydrates Involved in Mannitol and Trehalose Metabolism in the Edible Mushroom Agaricus Bisporus. J. Agric. Food Chem. 2000, 48, 287–291. [Google Scholar] [CrossRef]
- Parrou, J.L.; François, J. A Simplified Procedure for a Rapid and Reliable Assay of Both Glycogen and Trehalose in Whole Yeast Cells. Anal. Biochem. 1997, 248, 186–188. [Google Scholar] [CrossRef]
- Ikegami, T.; Horie, K.; Saad, N.; Hosoya, K.; Fiehn, O.; Tanaka, N. Highly Efficient Analysis of Underivatized Carbohydrates Using Monolithic-Silica-Based Capillary Hydrophilic Interaction (HILIC) HPLC. Anal. Bioanal. Chem. 2008, 391, 2533–2542. [Google Scholar] [CrossRef]
- Hanko, V.P.; Rohrer, J.S. Determination of Carbohydrates, Sugar Alcohols, and Glycols in Cell Cultures and Fermentation Broths Using High-Performance Anion-Exchange Chromatography with Pulsed Amperometric Detection. Anal. Biochem. 2000, 283, 192–199. [Google Scholar] [CrossRef]
- Wei, Y.; Ding, M.Y. Analysis of Carbohydrates in Drinks by High-Performance Liquid Chromatography with a Dynamically Modified Amino Column and Evaporative Light Scattering Detection. J. Chromatogr. A 2000, 904, 113–117. [Google Scholar] [CrossRef]
- Liu, Z.; Rochfort, S. A Fast Liquid Chromatography-Mass Spectrometry (LC-MS) Method for Quantification of Major Polar Metabolites in Plants. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2013, 912, 8–15. [Google Scholar] [CrossRef]
- Kretschmer, P.M.; Bannister, A.M.; O’Brien, M.K.; MacManus-Spencer, L.A.; Paulick, M.G. A Liquid Chromatography–Tandem Mass Spectrometry Assay for the Detection and Quantification of Trehalose in Biological Samples. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2016, 1033–1034, 9–16. [Google Scholar] [CrossRef] [Green Version]
- Tolstikov, V.V.; Fiehn, O. Analysis of Highly Polar Compounds of Plant Origin: Combination of Hydrophilic Interaction Chromatography and Electrospray Ion Trap Mass Spectrometry. Anal. Biochem. 2002, 301, 298–307. [Google Scholar] [CrossRef] [Green Version]
- Delatte, T.L.; Selman, M.H.J.; Schluepmann, H.; Somsen, G.W.; Smeekens, S.C.M.; de Jong, G.J. Determination of Trehalose-6-Phosphate in Arabidopsis Seedlings by Successive Extractions Followed by Anion Exchange Chromatography-Mass Spectrometry. Anal. Biochem. 2009, 389, 12–17. [Google Scholar] [CrossRef]
- Mata, A.T.; Jorge, T.F.; Ferreira, J.; do Rosário Bronze, M.; Branco, D.; Fevereiro, P.; Araújo, S.; António, C. Analysis of Low Abundant Trehalose-6-Phosphate and Related Metabolites in Medicago Truncatula by Hydrophilic Interaction Liquid Chromatography–Triple Quadrupole Mass Spectrometry. J. Chromatogr. A 2016, 1477, 30–38. [Google Scholar] [CrossRef]
- Luo, X.T.; Cai, B.D.; Jiang, H.P.; Xiao, H.M.; Yuan, B.F.; Feng, Y.Q. Sensitive Analysis of Trehalose-6-Phosphate and Related Sugar Phosphates in Plant Tissues by Chemical Derivatization Combined with Hydrophilic Interaction Liquid Chromatography–Tandem Mass Spectrometry. J. Chromatogr. A 2019, 1592, 82–90. [Google Scholar] [CrossRef]
- Rende, U.; Niittylä, T.; Moritz, T. Two-Step Derivatization for Determination of Sugar Phosphates in Plants by Combined Reversed Phase Chromatography/Tandem Mass Spectrometry. Plant Methods 2019, 15, 127. [Google Scholar] [CrossRef] [Green Version]
- Nguyen, H.P.; Schug, K.A. The Advantages of ESI-MS Detection in Conjunction with HILIC Mode Separations: Fundamentals and Applications. J. Sep. Sci. 2008, 31, 1465–1480. [Google Scholar] [CrossRef]
- Trivedi, D.K.; Jones, H.; Shah, A.; Iles, R.K. Development of Zwitterionic Hydrophilic Liquid Chromatography (ZIC®HILIC-MS) Metabolomics Method for Shotgun Analysis of Human Urine. J. Chromatogr. Sep. Tech. 2012, 3, 1000144. [Google Scholar] [CrossRef] [Green Version]
- Feith, A.; Teleki, A.; Graf, M.; Favilli, L.; Takors, R. Hilic-Enabled13c Metabolomics Strategies: Comparing Quantitative Precision and Spectral Accuracy of Qtof High- and Qqq Low-Resolution Mass Spectrometry. Metabolites 2019, 9, 63. [Google Scholar] [CrossRef] [Green Version]
- Acket, S.; Degournay, A.; Merlier, F.; Thomasset, B. 13C Labeling Analysis of Sugars by High Resolution-Mass Spectrometry for Metabolic Flux Analysis. Anal. Biochem. 2017, 527, 45–48. [Google Scholar] [CrossRef]
- Tomás, S.; Erwin, M.; Franck, J.Z.; Martin, M.; Yuemei, P.; Van Loosdrecht, M.C.M.; Weissbrodt, D.G. Turnover of the Extracellular Polymeric Matrix of Granules Performing Biological Phosphate Removal. Appl. Microbiol. Biotechnol. 2023, 107, 1997–2009. [Google Scholar] [CrossRef]
- Buszewski, B.; Noga, S. Hydrophilic Interaction Liquid Chromatography (HILIC)-a Powerful Separation Technique. Anal. Bioanal. Chem. 2012, 402, 231–247. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jandera, P. Stationary and Mobile Phases in Hydrophilic Interaction Chromatography: A Review. Anal. Chim. Acta 2011, 692, 1–25. [Google Scholar] [CrossRef] [PubMed]
- Antonio, C.; Larson, T.; Gilday, A.; Graham, I.; Bergström, E.; Thomas-Oates, J. Hydrophilic Interaction Chromatography/Electrospray Mass Spectrometry Analysis of Carbohydrate-Related Metabolites from Arabidopsis Thaliana Leaf Tissue. Rapid Commun. Mass Spectrom. 2008, 22, 1399–1407. [Google Scholar] [CrossRef] [PubMed]
- Wakamatsu, A.; Morimoto, K.; Shimizu, M.; Kudoh, S. A Severe Peak Tailing of Phosphate Compounds Caused by Interaction with Stainless Steel Used for Liquid Chromatography and Electrospray Mass Spectrometry. J. Sep. Sci. 2005, 28, 1823–1830. [Google Scholar] [CrossRef] [PubMed]
- Kandror, O.; DeLeon, A.; Goldberg, A.L. Trehalose Synthesis Is Induced upon Exposure of Escherichia coli to Cold and Is Essential for Viability at Low Temperatures. Proc. Natl. Acad. Sci. USA 2002, 99, 9727–9732. [Google Scholar] [CrossRef] [Green Version]
- Gouffi, K.; Blanco, C. Is the Accumulation of Osmoprotectant the Unique Mechanism Involved in Bacterial Osmoprotection? Int. J. Food Microbiol. 2000, 55, 171–174. [Google Scholar] [CrossRef]
- Hayner, G.A.; Khetan, S.; Paulick, M.G. Quantification of the Disaccharide Trehalose from Biological Samples: A Comparison of Analytical Methods. ACS Omega 2017, 2, 5813–5823. [Google Scholar] [CrossRef] [Green Version]
- Tsugawa, H.; Cajka, T.; Kind, T.; Ma, Y.; Higgins, B.; Ikeda, K.; Kanazawa, M.; Vandergheynst, J.; Fiehn, O.; Arita, M. MS-DIAL: Data-Independent MS/MS Deconvolution for Comprehensive Metabolome Analysis. Nat. Methods 2015, 12, 523–526. [Google Scholar] [CrossRef]
- Tao, Y.; Acket, S.; Beaumont, E.; Galez, H.; Duma, L.; Rossez, Y. Colistin Treatment Affects Lipid Composition of Acinetobacter baumannii. Antibiotics 2021, 10, 528. [Google Scholar] [CrossRef]
- Pang, Z.; Chong, J.; Li, S.; Xia, J. MetaboAnalystR 3.0: Toward an Optimized Workflow for Global Metabolomics. Metabolites 2020, 10, 186. [Google Scholar] [CrossRef]
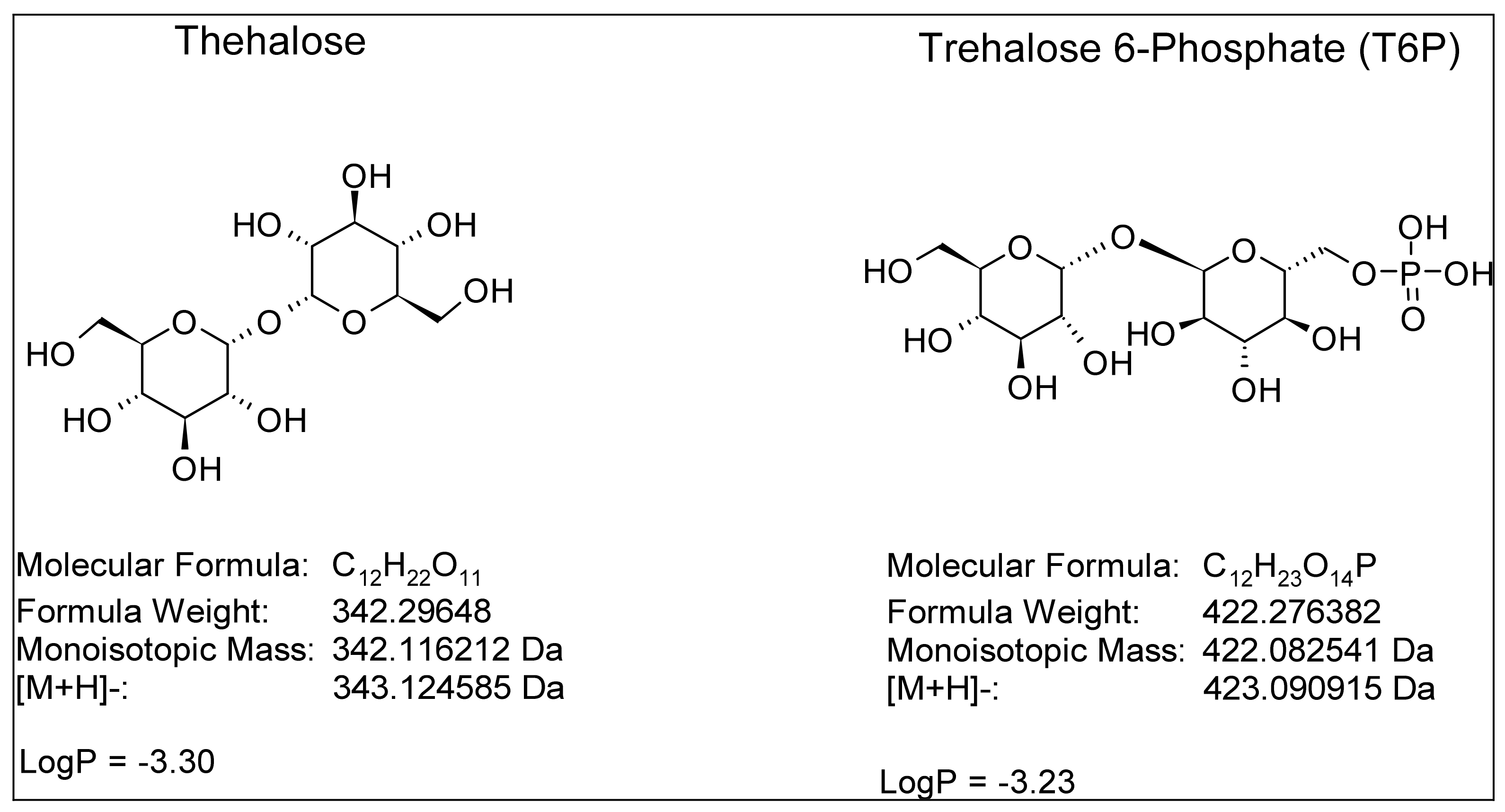

| Target | Formula | [M − H]− | [M + HCO2]− | [M + CF3CO2]− | RT (min) |
|---|---|---|---|---|---|
| Trehalose | C12H22O11 | 341.1089 | 387.1144 | 455.1018 | 7.6 |
| 13C12-trehalose | 13C12H22O11 | 353.1492 | 399.1547 | 467.1421 | 7.6 |
| T6P | C12H23O14P | 421.0753 | 467.0807 | 535.0681 | 10.3 |
| [Trehalose] (µM) | [M − H]− | [M + HCO2]− | [M + CF3CO2]− | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Area | S/N | RIS | Area | S/N | RIS | Area | S/N | RIS | |
| 0.5 | 8313 | 3 | 1.35 | 55,156 | 54 | 1.14 | 328,667 | 72 | 1.13 |
| 1 | 23,466 | Infinity | 2.37 | 148,429 | 90 | 2.23 | 683,028 | 142 | 2.31 |
| 2 | 45,477 | 24 | 5.39 | 303,672 | 77 | 5.52 | 1,124,959 | 166 | 5.56 |
| 30 | 656,765 | 123 | 86.33 | 4,166,579 | 563 | 89.82 | 4,647,415 | 268 | 89.92 |
| 90 | 1,607,254 | 229 | 292.1 | 10,082,234 | 768 | 299.14 | 4,716,700 | 23 | 267.23 |
| Id | Conc. Target (µM) | Average Conc. (µM) | Standard Deviation | RSD | LC Slow | LC Fast | Trueness | |
|---|---|---|---|---|---|---|---|---|
| QC2 | Trehalose | 22.45 | 20.88 | 0.54 | 2.60% | 8 | 5 | 6.98% |
| QC3 | 7.84 | 7.12 | 0.11 | 1.50% | 0 | 5 | 9.18% | |
| QC5 | 2.57 | 2.36 | 0.03 | 1.29% | 9 | 0 | 8.23% | |
| QC2 | T6P | 7.84 | 7.29 | 0.25 | 3.36% | 0 | 5 | 8.23% |
| Ris | Intra-Day | Extra-Week | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Conc. (µM) | D0 | D1 | D6 r1 | D6 r2 | D6 r3 | Average | Standard Deviation | RSD | Average | Standard Deviation | RSD |
| 0.46 | 1.14 | 1.10 | 1.09 | 1.08 | 1.06 | 1.08 | 0.02 | 1.42% | 1.09 | 0.03 | 2.74% |
| 0.91 | 2.23 | 2.22 | 2.21 | 2.20 | 2.27 | 2.23 | 0.04 | 1.58% | 2.23 | 0.03 | 1.14% |
| 1.81 | 5.52 | 5.27 | 5.21 | 5.31 | 5.46 | 5.33 | 0.12 | 2.32% | 5.36 | 0.13 | 2.40% |
| 5.55 | 14.37 | 14.48 | 14.95 | 14.60 | 14.56 | 14.70 | 0.21 | 1.44% | 14.59 | 0.22 | 1.49% |
| 10.77 | 31.03 | 30.53 | 31.70 | 31.39 | 31.79 | 31.63 | 0.21 | 0.67% | 31.29 | 0.52 | 1.65% |
| 30.53 | 89.82 | 90.93 | 91.82 | 91.89 | 89.48 | 91.06 | 1.37 | 1.50% | 90.79 | 1.11 | 1.22% |
| 52.33 | 161.21 | 167.33 | 165.28 | 165.02 | 169.15 | 166.48 | 2.31 | 1.39% | 165.60 | 2.97 | 1.79% |
| 91.58 | 299.14 | 291.69 | 288.28 | 294.58 | 291.64 | 291.50 | 3.15 | 1.08% | 293.07 | 4.06 | 1.39% |
| Conc. (µM) | Area (External Cal.) | Intra-Day | Intra-Week | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| D0 | D2 | D4 n1 | D4 n2 | Average | Standard Deviation | RSD | Average | Standard Deviation | RSD | |
| 0.55 | 9080 | 10,476 | 10,590 | 9377 | 9984 | 858 | 8.59% | 9881 | 765 | 7.74% |
| 1.09 | 19,783 | 16,930 | 20,161 | 23,334 | 21,747 | 2244 | 10.32% | 20,052 | 2621 | 13.07% |
| 2.71 | 48,540 | 54,242 | 58,297 | 63,093 | 60,695 | 3391 | 5.59% | 56,043 | 6173 | 11.02% |
| 5.28 | 100,434 | 109,706 | 123,374 | 121,699 | 122,537 | 1185 | 0.97% | 113,803 | 10,793 | 9.48% |
| 10.01 | 204,196 | 195,637 | 230,506 | 255,039 | 242,773 | 17,347 | 7.15% | 221,345 | 26,921 | 12.16% |
| 27.75 | 530,722 | 540,559 | 636,781 | 640,689 | 638,735 | 2764 | 0.43% | 587,188 | 59,678 | 10.16% |
| 55.50 | 1,103,943 | 1,113,634 | 1,310,572 | 1,358,745 | 1,334,659 | 34,063 | 2.55% | 122,1723 | 131,940 | 10.80% |
| 111.00 | 3,187,728 | 3,669,272 | 3,897,902 | 3,983,834 | 3,940,868 | 60,763 | 1.54% | 3,684,684 | 356,911 | 9.69% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tao, Y.; Rossez, Y.; Bortolus, C.; Duma, L.; Dubar, F.; Merlier, F. Simultaneous Quantification of Trehalose and Trehalose 6-Phosphate by Hydrophilic Interaction Chromatography/Electrospray Accurate Mass Spectrometry with Application in Non-Targeted Metabolomics. Molecules 2023, 28, 3443. https://doi.org/10.3390/molecules28083443
Tao Y, Rossez Y, Bortolus C, Duma L, Dubar F, Merlier F. Simultaneous Quantification of Trehalose and Trehalose 6-Phosphate by Hydrophilic Interaction Chromatography/Electrospray Accurate Mass Spectrometry with Application in Non-Targeted Metabolomics. Molecules. 2023; 28(8):3443. https://doi.org/10.3390/molecules28083443
Chicago/Turabian StyleTao, Ye, Yannick Rossez, Clovis Bortolus, Luminita Duma, Faustine Dubar, and Franck Merlier. 2023. "Simultaneous Quantification of Trehalose and Trehalose 6-Phosphate by Hydrophilic Interaction Chromatography/Electrospray Accurate Mass Spectrometry with Application in Non-Targeted Metabolomics" Molecules 28, no. 8: 3443. https://doi.org/10.3390/molecules28083443







