Production and Inhibition of Acrylamide during Coffee Processing: A Literature Review
Abstract
:1. Introduction
2. Formation, Hazards, and Determination of AA Content in Coffee
2.1. Pathway of AA Formation in Coffee
2.2. Potential Hazards of AA
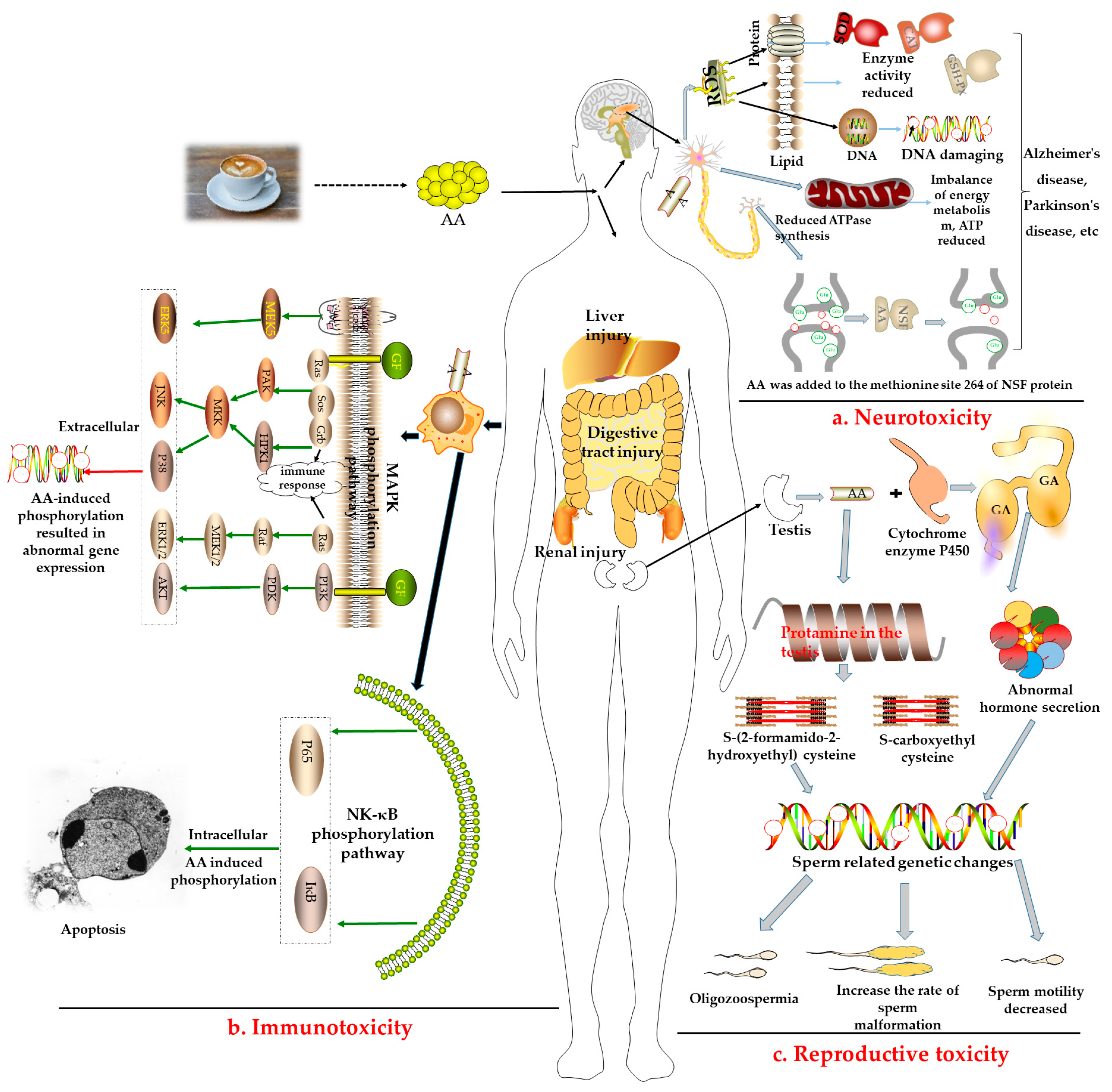
- a.
- Neurotoxicity: The central nervous system is an important site of active oxygen metabolism in the body. As described in Figure 2a, long-term intake of AA can induce reactive oxygen species (ROS) to constantly attack cell membrane lipids, proteins, and DNA, damage the main target organs, and induce diseases such as Alzheimer’s and Parkinson’s disease [42]. Some studies have reported that AA induced an increase in the levels of oxidative stress-related enzymes such as superoxide dismutase (SOD), glutathione peroxidase (GSH-Px), and catalase (CAT) in the peripheral blood and brain [43]. Furthermore, it induced the destruction of the structure or function of the peripheral nervous system, resulting in the weakening or disappearance of movement and sensation [44].
- b.
- Immunotoxicity: AA can also stimulate the immune system to produce immune responses and activate mitogen-activated protein kinase (MAPK), nuclear factor-κB (NF-κB), and other related pathways for defense, as shown in Figure 2b. Some studies have reported that treating human neuroblastoma cells with AA could activate the extracellular signal-regulated protein kinase (ERK) to induce the death signaling pathway, c-Junn terminal protein kinase (JNK), and p38 mitogen-activated protein kinase (p38 MAPK) pathways, and upregulate the expression of proapoptotic proteins, resulting in cell apoptosis [45,46].
- c.
- Reproductive toxicity: AA has also been proven to exhibit reproductive toxicity (Figure 2c). After being catalyzed by the cytochrome P450 enzyme, AA is epoxidized to form glycidamide (GA). Then, AA and GA react with protamine in the testis to produce S-(2-formamido-2-hydroxyethyl) cysteine and S-carboxyethyl cysteine, eventually affecting fertility [47,48]. AA can damage the reproductive system by damaging normal Sertoli cells in male rats and the function of Leydig cells, as well as induce the abnormal secretion of testosterone and luteinizing hormone, resulting in abnormal sperm-related gene expression, decreasing the number of sperm to reduce the activity of sperm, and increasing the sperm deformity rate [49,50]. Further, AA can induce ovarian dysfunction in female Wistar rats by upregulating apoptosis-related genes [51].
- d.
- Other toxicities: AA can damage the liver, kidneys, lungs, bladder, and digestive tract and may even cause testicular mesothelioma, adrenal cortical adenoma, astrocytoma, and oral tumors [52]. At present, there are no studies on the harmful effects of AA in coffee on the human body; however, its toxic effects in food have long been confirmed via animal experiments or in vitro experiments using human cells. A AA toxicity test in rats in early 2005 revealed an LD50 of 107–203 mg/kg·bw and confirmed that AA has low toxicity [53]. Nevertheless, some studies have reported that the harmful effects of AA on the human body were mainly reflected as damage to human immune function, nervous system, genetic material, mitochondrial dysfunction, mutation, genotoxicity, as well as its potential carcinogenicity [54,55]. For example, the majority of GA-induced mutations in human tumors occurred at the A:T base pairs, with AT > TA and AT > GC mutations on specific TP53 codons [56]. In other words, DNA adducts provide a possible mechanistic basis for mutation types and mutational signatures occurring following GA treatment, a reactive metabolite of AA [57]. Thus, the European Regulation has advised a maximum AA content of 400 µg/kg in roasted coffee.
2.3. AA Detection
3. Control AA Production in Processing Stages
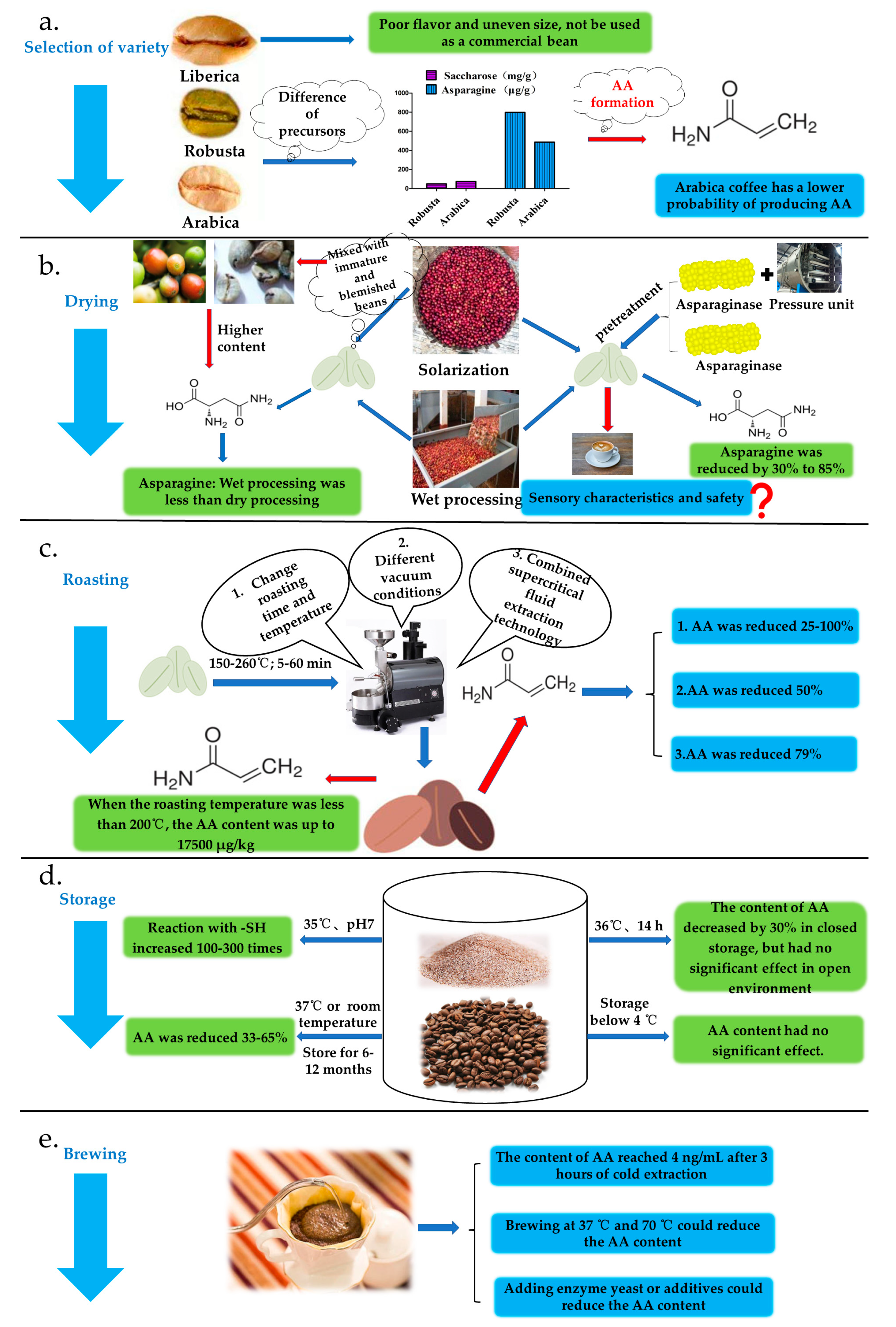
3.1. Variety Selection
3.2. Drying Process Stage
3.3. Roasting Stage
3.4. Storage Stage
3.5. Brewing Stage
4. Conclusions and Prospect
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Corrêa, C.L.O.; Das Merces Penha, E.; Dos Anjos, M.R.; Pacheco, S.; Freitas-Silva, O.; Luna, A.S.; Gottschalk, L.M.F. Use of asparaginase for acrylamide mitigation in coffee and its influence on the content of caffeine, chlorogenic acid, and caffeic acid. Food Chem. 2021, 338, 128045. [Google Scholar] [CrossRef]
- Hu, G.L.; Wang, X.; Zhang, L.; Qiu, M.H. The sources and mechanisms of bioactive ingredients in coffee. Food Funct. 2019, 10, 3113–3126. [Google Scholar] [CrossRef]
- Hu, G.; Peng, X.; Gao, Y.; Huang, Y.; Li, X.; Su, H.; Qiu, M. Effect of roasting degree of coffee beans on sensory evaluation: Research from the perspective of major chemical ingredients. Food Chem. 2020, 331, 127329. [Google Scholar] [CrossRef]
- Vignoli, J.A.; Viegas, M.C.; Bassoli, D.G.; Benassi, M.D.T. Roasting process affects differently the bioactive compounds and the antioxidant activity of arabica and robusta coffees. Food Res. Int. 2014, 61, 279–285. [Google Scholar] [CrossRef] [Green Version]
- Soares, C.M.D.; Alves, R.C.; Oliveira, M.B.P.P. Factors affecting acrylamide levels in coffee beverages. In Coffee in Health and Disease Prevention; Academic Press: Cambridge, MA, USA, 2015; pp. 217–224. [Google Scholar]
- Kučera, L.; Papoušek, R.; Kurka, O.; Barták, P.; Bednář, P. Study of composition of espresso coffee prepared from various roast degrees of Coffea arabica L. coffee beans. Food Chem. 2016, 199, 727–735. [Google Scholar] [CrossRef]
- Worku, M.; Astatkie, T.; Boeckx, P. Shade and postharvest processing effects on arabica coffee quality and biochemical composition in lowland and midland coffee-growing areas of southwestern Ethiopia. J. Food Compos. Anal. 2023, 115, 105027. [Google Scholar] [CrossRef]
- Tassew, A.A.; Yadessa, G.B.; Bote, A.D.; Obso, T.K. Influence of location, elevation gradients, processing methods, and soil quality on the physical and cup quality of coffee in the Kafa Biosphere Reserve of SW Ethiopia. Heliyon 2021, 7, e7790. [Google Scholar] [CrossRef]
- Toci, A.T.; Azevedo, D.A.; Farah, A. Effect of roasting speed on the volatile composition of coffees with different cup quality. Food Res. Int. 2020, 137, 109546. [Google Scholar] [CrossRef]
- Schouten, M.A.; Tappi, S.; Romani, S. Acrylamide in coffee: Formation and possible mitigation strategies-a review. Crit. Rev. Food Sci. Nutr. 2020, 60, 3807–3821. [Google Scholar] [CrossRef]
- Guenther, H.; Anklam, E.; Wenzl, T.; Stadler, R.H. Acrylamide in coffee: Review of progress in analysis, formation and level reduction. Food Addit. Contam. 2007, 24 (Suppl. S1), 60–70. [Google Scholar] [CrossRef]
- Chang, Y.; Zeng, X.Y.; Sung, W. Effect of chitooligosaccharide and different low molecular weight chitosans on the formation of acrylamide and 5-hydroxymethylfurfural and Maillard reaction products in glucose/fructose-asparagine model systems. LWT-Food Sci. Technol. 2020, 119, 108879. [Google Scholar] [CrossRef]
- Arribas-Lorenzo, G.; Morales, F.J. Dietary exposure to acrylamide from potato crisps to the Spanish population. Food Addit. Contam. Part A 2009, 26, 289–297. [Google Scholar] [CrossRef] [Green Version]
- Ecile, R.C.; Delphine, L.; Emilie, R.; Carole, P.; Thierry, S.E. Mitigation strategies of acrylamide, furans, heterocyclic amines and browning during the Maillard reaction in foods. Food Res. Int. 2016, 90, 76–154. [Google Scholar] [CrossRef]
- Boyaci-Gunduz, C.P. Acrylamide exposure of infants and toddlers through baby foods and current progress on regulations. Curr. Opin. Food Sci. 2022, 46, 100849. [Google Scholar] [CrossRef]
- Akgün, B.; Arıcı, M.; Çavuş, F.; Karataş, A.B.; Ekşi Karaağaç, H.; Uçurum, H.Ö. Application of L-asparaginase to produce high-quality Turkish coffee and the role of precursors in acrylamide formation. J. Food Process Preserv. 2021, 45, e15486. [Google Scholar] [CrossRef]
- Park, S.; Jo, A.; Lee, K. Effect of various roasting, extraction and drinking conditions on furan and 5-hydroxymethylfurfural levels in coffee. Food Chem. 2021, 358, 129806. [Google Scholar] [CrossRef]
- Grzelczyk, J.; Fiurasek, P.; Kakkar, A.; Budryn, G. Evaluation of the thermal stability of bioactive compounds in coffee beans and their fractions modified in the roasting process. Food Chem. 2022, 387, 132888. [Google Scholar] [CrossRef]
- Schouten, M.A.; Genovese, J.; Tappi, S.; Di Francesco, A.; Baraldi, E.; Cortese, M.; Caprioli, G.; Angeloni, S.; Vittori, S.; Rocculi, P.; et al. Effect of innovative pre-treatments on the mitigation of acrylamide formation in potato chips. Innov. Food Sci. Emerg. Technol. 2020, 64, 102397. [Google Scholar] [CrossRef]
- Alves, R.C.; Soares, C.; Casal, S.; Fernandes, J.O.; Oliveira, M.B.P.P. Acrylamide in espresso coffee: Influence of species, roast degree and brew length. Food Chem. 2010, 119, 929–934. [Google Scholar] [CrossRef]
- Schouten, M.A.; Tappi, S.; Angeloni, S.; Cortese, M.; Caprioli, G.; Vittori, S.; Romani, S. Acrylamide formation and antioxidant activity in coffee during roasting—A systematic study. Food Chem. 2021, 343, 128514. [Google Scholar] [CrossRef]
- Coreta-Gomes, F.M.; Lopes, G.R.; Passos, C.P.; Vaz, I.M.; Machado, F.; Geraldes, C.F.G.C.; Moreno, M.J.; Nyström, L.; Coimbra, M.A. In vitro hypocholesterolemic effect of coffee compounds. Nutrients 2020, 12, 437. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cong, S.; Dong, W.; Zhao, J.; Hu, R.; Long, Y.; Chi, X. Characterization of the lipid oxidation process of robusta green coffee beans and shelf life prediction during accelerated storage. Molecules 2020, 25, 1157. [Google Scholar] [CrossRef] [Green Version]
- Endo, Y.; Hayashi, C.; Yamanaka, T.; Takayose, K.; Yamaoka, M.; Tsuno, T.; Nakajima, S. Linolenic acid as the main source of acrolein formed during heating of vegetable oils. J. Am. Oil Chem. Soc. 2013, 90, 959–964. [Google Scholar] [CrossRef]
- Kocadağlı, T.; Gökmen, V. Formation of acrylamide in coffee. Curr. Opin. Food Sci. 2022, 45, 100842. [Google Scholar] [CrossRef]
- Sáez-Hernández, R.; Ruiz, P.; Mauri-Aucejo, A.R.; Yusa, V.; Cervera, M.L. Determination of acrylamide in toasts using digital image colorimetry by smartphone. Food Control 2022, 141, 109163. [Google Scholar] [CrossRef]
- Figueroa Campos, G.A.; Sagu, S.T.; Saravia Celis, P.; Rawel, H.M. Comparison of batch and continuous wet-processing of coffee: Changes in the main compounds in beans, by-products and wastewater. Foods 2020, 9, 1135. [Google Scholar] [CrossRef]
- Strocchi, G.; Rubiolo, P.; Cordero, C.; Bicchi, C.; Liberto, E. Acrylamide in coffee: What is known and what still needs to be explored. A review. Food Chem. 2022, 393, 133406. [Google Scholar] [CrossRef]
- Alamri, E.; Rozan, M.; Bayomy, H. A study of chemical Composition, Antioxidants, and volatile compounds in roasted Arabic coffee. Saudi J. Biol. Sci. 2022, 29, 3133–3139. [Google Scholar] [CrossRef]
- Barrios-Rodríguez, Y.F.; Gutiérrez-Guzmán, N.; Pedreschi, F.; Mariotti-Celis, M.S. Rational design of technologies for the mitigation of neo-formed contaminants in roasted coffee. Trends Food Sci. Technol. 2022, 120, 223–235. [Google Scholar] [CrossRef]
- Delatour, T.; Huertas-Perez, J.F.; Dubois, M.; Theurillat, X.; Desmarchelier, A.; Ernest, M.; Stadler, R.H. Thermal degradation of 2-furoic acid and furfuryl alcohol as pathways in the formation of furan and 2-methylfuran in food. Food Chem. 2020, 303, 125406. [Google Scholar] [CrossRef]
- Hamzalıoğlu, A.; Gökmen, V. 5-Hydroxymethylfurfural accumulation plays a critical role on acrylamide formation in coffee. Food Chem. 2020, 318, 126467. [Google Scholar] [CrossRef]
- Gökmen, V.; Kocadağlı, T.; Göncüoğlu, N.; Mogol, B.A. Model studies on the role of 5-hydroxymethyl-2-furfural in acrylamide formation from asparagine. Food Chem. 2012, 132, 168–174. [Google Scholar] [CrossRef]
- Anese, M.; Nicoli, M.C.; Verardo, G.; Munari, M.; Mirolo, G.; Bortolomeazzi, R. Effect of vacuum roasting on acrylamide formation and reduction in coffee beans. Food Chem. 2014, 145, 168–172. [Google Scholar] [CrossRef]
- Cai, Y.; Zhang, Z.; Jiang, S.; Yu, M.; Huang, C.; Qiu, R.; Zou, Y.; Zhang, Q.; Ou, S.; Zhou, H.; et al. Chlorogenic acid increased acrylamide formation through promotion of HMF formation and 3-aminopropionamide deamination. J. Hazard. Mater. 2014, 268, 1–5. [Google Scholar] [CrossRef]
- Schouten, M.A.; Fryganas, C.; Tappi, S.; Romani, S.; Fogliano, V. The use of kidney bean flour with intact cell walls reduces the formation of acrylamide in biscuits. Food Control 2022, 140, 109054. [Google Scholar] [CrossRef]
- Várady, M.; Tauchen, J.; Fraňková, A.; Klouček, P.; Popelka, P. Effect of method of processing specialty coffee beans (natural, washed, honey, fermentation, maceration) on bioactive and volatile compounds. LWT 2022, 172, 114245. [Google Scholar] [CrossRef]
- Verma, V.; Yadav, N. Acrylamide content in starch based commercial foods by using high performance liquid chromatography and its association with browning index. Curr. Res. Food Sci. 2022, 5, 464–470. [Google Scholar] [CrossRef]
- Pundir, C.S.; Yadav, N.; Chhillar, A.K. Occurrence, synthesis, toxicity and detection methods for acrylamide determination. Trends Food Sci. Technol. 2019, 85, 211–225. [Google Scholar] [CrossRef]
- Kumari, A.; Bhattacharya, B.; Agarwal, T.; Paul, V.; Chakkaravarthi, S. Integrated approach towards acrylamide reduction in potato-based snacks: A critical review. Food Res. Int. 2022, 156, 111172. [Google Scholar] [CrossRef]
- Zhao, T.; Guo, Y.; Ji, H.; Mao, G.; Feng, W.; Chen, Y.; Wu, X.; Yang, L. Short-term exposure to acrylamide exacerbated metabolic disorders and increased metabolic toxicity susceptibility on adult male mice with diabetes. Toxicol. Lett. 2022, 356, 41–53. [Google Scholar] [CrossRef]
- Wang, F.; Fan, B.; Chen, C.; Zhang, W. Acrylamide causes neurotoxicity by inhibiting glycolysis and causing the accumulation of carbonyl compounds in BV2 microglial cells. Food Chem. Toxicol. 2022, 163, 112982. [Google Scholar] [CrossRef]
- Hou, L.; Liu, S.; Zhao, C.; Fan, L.; Hu, H.; Yin, S. The combination of T-2 toxin and acrylamide synergistically induces hepatotoxicity and nephrotoxicity via the activation of oxidative stress and the mitochondrial pathway. Toxicon 2021, 189, 65–72. [Google Scholar] [CrossRef]
- Reshmitha, T.R.; Nisha, P. Lycopene mitigates acrylamide and glycidamide induced cellular toxicity via oxidative stress modulation in HepG2 cells. J. Funct. Food. 2021, 80, 104390. [Google Scholar] [CrossRef]
- Okuno, T.; Matsuoka, M.; Sumizawa, T.; Igisu, H. Involvement of the extracellular signal-regulated protein kinase pathway in phosphorylation of p53 protein and exerting cytotoxicity in human neuroblastoma cells (SH-SY5Y) exposed to acrylamide. Arch. Toxicol. 2006, 80, 146–153. [Google Scholar] [CrossRef]
- Matoso, V.; Bargi-Souza, P.; Ivanski, F.; Romano, M.A.; Romano, R.M. Acrylamide: A review about its toxic effects in the light of Developmental Origin of Health and Disease (DOHaD) concept. Food Chem. 2019, 283, 422–430. [Google Scholar] [CrossRef]
- Hansen, S.H.; Olsen, A.K.; Søderlund, E.J.; Brunborg, G. In vitro investigations of glycidamide-induced DNA lesions in mouse male germ cells and in mouse and human lymphocytes. Mutat. Res. Genet. Toxicol. Environ. Mutagen. 2010, 696, 55–61. [Google Scholar] [CrossRef]
- Zhang, H.; Shan, L.; Aniagu, S.; Jiang, Y.; Chen, T. Paternal acrylamide exposure induces transgenerational effects on sperm parameters and learning capability in mice. Food Chem. Toxicol. 2022, 161, 112817. [Google Scholar] [CrossRef]
- Ma, Y.; Shi, J.; Zheng, M.; Liu, J.; Tian, S.; He, X.; Zhang, D.; Li, G.; Zhu, J. Toxicological effects of acrylamide on the reproductive system of weaning male rats. Toxicol. Ind. Health 2011, 27, 617–627. [Google Scholar] [CrossRef]
- ALKarim, S.; ElAssouli, S.; Ali, S.; Ayuob, N.; ElAssouli, Z. Effects of low dose acrylamide on the rat reproductive organs structure, fertility and gene integrity. Asian Pac. J. Reprod. 2015, 4, 179–187. [Google Scholar] [CrossRef]
- Firouzabadi, A.M.; Imani, M.; Zakizadeh, F.; Ghaderi, N.; Zare, F.; Yadegari, M.; Pourentezari, M.; Fesahat, F. Evaluating effect of acrylamide and ascorbic acid on oxidative stress and apoptosis in ovarian tissue of wistar rat. Toxicol. Rep. 2022, 9, 1580–1585. [Google Scholar] [CrossRef]
- Hashem, M.M.; Abo-EL-Sooud, K.; Abd El-Hakim, Y.M.; Abdel-hamid Badr, Y.; El-Metwally, A.E.; Bahy-EL-Dien, A. The impact of long-term oral exposure to low doses of acrylamide on the hematological indicators, immune functions, and splenic tissue architecture in rats. Int. Immunopharmacol. 2022, 105, 108568. [Google Scholar] [CrossRef] [PubMed]
- Dybing, E.; Farmer, P.B.; Andersen, M.; Fennell, T.R.; Lalljie, S.P.D.; Müller, D.J.G.; Olin, S.; Petersen, B.J.; Schlatter, J.; Scholz, G.; et al. Human exposure and internal dose assessments of acrylamide in food. Food Chem. Toxicol. 2005, 43, 365–410. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.; Yucai, L.; Lu, L.; Hui, L.; Yong, P.; Haiyang, Y. Acrylamide induces ferroptosis in HSC-T6 cells by causing antioxidant imbalance of the XCT-GSH-GPX4 signaling and mitochondrial dysfunction. Toxicol. Lett. 2022, 368, 24–32. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, Y.; Zhang, X.; Jiao, Y.; Duan, L.; Dai, L.; Yan, H. Chronic acrylamide exposure resulted in dopaminergic neuron loss, neuroinflammation and motor impairment in rats. Toxicol. Appl. Pharmacol. 2022, 451, 116190. [Google Scholar] [CrossRef] [PubMed]
- Hölzl-Armstrong, L.; Kucab, J.E.; Moody, S.; Zwart, E.P.; Loutkotová, L.; Duffy, V.; Luijten, M.; Gamboa Da Costa, G.; Stratton, M.R.; Phillips, D.H.; et al. Mutagenicity of acrylamide and glycidamide in human TP53 knock-in (Hupki) mouse embryo fibroblasts. Arch. Toxicol. 2020, 94, 4173–4196. [Google Scholar] [CrossRef]
- Benford, D.; Bignami, M.; Chipman, J.K.; Ramos Bordajandi, L. Assessment of the genotoxicity of acrylamide. EFSA J. 2022, 20, e07293. [Google Scholar] [CrossRef]
- Xian, Y.; Wu, Y.; Dong, H.; Chen, L.; Zhang, C.; Hou, X.; Zeng, X.; Bai, W.; Guo, X. Modified QuEChERS purification and Fe3O4 nanoparticle decoloration for robust analysis of 14 heterocyclic aromatic amines and acrylamide in coffee products using UHPLC-MS/MS. Food Chem. 2019, 285, 77–85. [Google Scholar] [CrossRef]
- Akgün, B.; Arıcı, M. Evaluation of acrylamide and selected parameters in some Turkish coffee brands from the Turkish market. Food Addit. Contaminants. Part A Chem. Anal. Control. Expo. Risk Assess. 2019, 36, 548–560. [Google Scholar] [CrossRef]
- Singh, G.; Brady, B.; Koerner, T.; Becalski, A.; Zhao, T.; Feng, S.; Godefroy, S.B.; Huet, A.; Delahaut, P. Development of a highly sensitive competitive indirect enzyme-linked immunosorbent assay for detection of acrylamide in foods and water. Food Anal. Meth. 2014, 7, 1298–1304. [Google Scholar] [CrossRef]
- Asnaashari, M.; Kenari, R.E.; Farahmandfar, R.; Abnous, K.; Taghdisi, S.M. An electrochemical biosensor based on hemoglobin-oligonucleotides-modified electrode for detection of acrylamide in potato fries. Food Chem. 2019, 271, 54–61. [Google Scholar] [CrossRef]
- Madihah, K.Y.K.; Zaibunnisa, A.H.; Norashikin, S.; Rozita, O.; Misnawi, J. Optimization of roasting conditions for high-quality robusta coffee. APCBEE Procedia 2012, 4, 209–214. [Google Scholar] [CrossRef] [Green Version]
- Quan, Y.; Chen, M.; Zhan, Y.; Zhang, G. Development of an enhanced chemiluminescence ELISA for the rapid detection of acrylamide in food products. J. Agric. Food Chem. 2011, 59, 6895–6899. [Google Scholar] [CrossRef] [PubMed]
- Cheng, J.; Zhang, S.; Wang, S.; Wang, P.; Su, X.; Xie, J. Rapid and sensitive detection of acrylamide in fried food using dispersive solid-phase extraction combined with surface-enhanced Raman spectroscopy. Food Chem. 2019, 276, 157–163. [Google Scholar] [CrossRef] [PubMed]
- Kocadagli, T.; Goncuoglu, N.; Hamzalioglu, A.; Gokmen, V. In depth study of acrylamide formation in coffee during roasting: Role of sucrose decomposition and lipid oxidation. Food Funct. 2012, 3, 970–975. [Google Scholar] [CrossRef]
- Dongxiang, Z.; Chunyang, L.; Yongming, Z. Determenation of acrylamide in instant coffee by inhibitory reduction spectrophotmetry. Chem. World 2020, 61, 507–511. [Google Scholar] [CrossRef]
- Bagdonaite, K.; Murkovic, M. Factors affecting the formation of acrylamide in coffee. Czech. J. Food Sci. 2004, 22, S22–S24. [Google Scholar] [CrossRef]
- Senyuva, H.Z.; Gokmen, V. Study of acrylamide in coffee using an improved liquid chromatography mass spectrometry method: Investigation of colour changes and acrylamide formation in coffee during roasting. Food Addit. Contam. 2005, 22, 214–220. [Google Scholar] [CrossRef]
- Lachenmeier, D.W.; Schwarz, S.; Teipel, J.; Hegmanns, M.; Kuballa, T.; Walch, S.G.; Breitling-Utzmann, C.M. Potential antagonistic effects of acrylamide mitigation during coffee roasting on furfuryl alcohol, furan and 5-hydroxymethylfurfural. Toxics 2019, 7, 1. [Google Scholar] [CrossRef] [Green Version]
- Akıllıoglu, H.G.; Gökmen, V. Mitigation of acrylamide and hydroxymethyl furfural in instant coffee by yeast fermentation. Food Res. Int. 2014, 61, 252–256. [Google Scholar] [CrossRef]
- Troise, A.D.; Fogliano, V. Quantitation of acrylamide in foods by high-resolution mass spectrometry. In Acrylamide in Food; WSPC: Hong Kong, China, 2014; pp. 74–79. [Google Scholar]
- Wang, J.; Cai, Z.; Zhang, N.; Hu, Z.; Zhang, J.; Ying, I.; Zhao, Y.; Feng, L.; Zhang, J.; Wu, P. A novel single step solid-phase extraction combined with bromine derivatization method for rapid determination of acrylamide in coffee and its products by stable isotope dilution ultra-performance liquid chromatography tandem triple quadrupole electrospray ionization mass spectrometry. Food Chem. 2022, 388, 132977. [Google Scholar] [CrossRef]
- Esposito, F.; Fasano, E.; De Vivo, A.; Velotto, S.; Sarghini, F.; Cirillo, T. Processing effects on acrylamide content in roasted coffee production. Food Chem. 2020, 319, 126550. [Google Scholar] [CrossRef] [PubMed]
- Kyaw, E.M.; Budiastra, I.W.; Sutrisno; Samsudin. Estimation of moisture content in Liberica coffee by using near infrared spectroscopy. IOP Conf. Ser. Earth Environ. Sci. 2020, 542, 12013. [Google Scholar] [CrossRef]
- Rubayiza, A.B.; Meurens, M. Chemical discrimination of arabica and robusta coffees by fourier transform raman spectroscopy. J. Agric. Food Chem. 2005, 53, 4654–4659. [Google Scholar] [CrossRef]
- Bagdonaite, K.; Derler, K.; Murkovic, M. Determination of acrylamide during roasting of coffee. J. Agric. Food Chem. 2008, 56, 6081–6086. [Google Scholar] [CrossRef]
- Pedreschi, F.; Mariotti, M.S.; Granby, K. Current issues in dietary acrylamide: Formation, mitigation and risk assessment. J. Sci. Food Agric. 2014, 94, 9–20. [Google Scholar] [CrossRef]
- De Abreu, H.M.C.; Nonile, P.M.; Shimizu, M.M.; Yamamoto, P.Y.; Silva, E.A.; Colombo, C.A.; Mazzafera, P. Infl uence of air temperature on proteinase activity and beverage quality in Coffea arabica. Braz. J. Bot. 2012, 35, 357–376. [Google Scholar] [CrossRef] [Green Version]
- Lantz, I.; Ternité, R.; Wilkens, J.; Hoenicke, K.; Guenther, H.; van der Stegen, G.H.D. Studies on acrylamide levels in roasting, storage and brewing of coffee. Mol. Nutr. Food Res. 2006, 50, 1039–1046. [Google Scholar] [CrossRef]
- Worku, M.; Astatkie, T.; Boeckx, P. Effect of growing conditions and postharvest processing on arabica coffee bean physical quality features and defects. Heliyon 2022, 8, e9201. [Google Scholar] [CrossRef] [PubMed]
- Dias, E.C.; Borém, F.M.; Pereira, R.G.F.A.; Guerreiro, M.C. Amino acid profiles in unripe Arabica coffee fruits processed using wet and dry methods. Eur. Food Res. Technol. 2012, 234, 25–32. [Google Scholar] [CrossRef]
- Hendriksen, H.V.; Kornbrust, B.A.; Østergaard, P.R.; Stringer, M.A. Evaluating the potential for enzymatic acrylamide mitigation in a range of food products using an asparaginase from aspergillus oryzae. J. Agric. Food Chem. 2009, 57, 4168–4176. [Google Scholar] [CrossRef]
- Porto, A.C.V.; Freitas-Silva, O.; Souza, E.F.D.; Gottschalk, L.M.F. Effect of asparaginase enzyme in the reduction of asparagine in green coffee. Beverages 2019, 5, 32. [Google Scholar] [CrossRef] [Green Version]
- Anese, M. Acrylamide in coffee and coffee substitutes. In Acrylamide in Food; WSPC: Hong Kong, China, 2015; pp. 181–195. [Google Scholar]
- Bertuzzi, T.; Rastelli, S.; Mulazzi, A.; Pietri, A. Survey on acrylamide in roasted coffee and barley and in potato crisps sold in Italy by a LC-MS/MS method. Food Addit. Contam. Part B Surveill. 2017, 10, 292–299. [Google Scholar] [CrossRef] [PubMed]
- Teixeira, S.L.; Santos, J.R.; Almeida, P.J.; Rodrigues, J.A. Fan assisted extraction and HPLC-DAD-MS/MS identification of volatile carbonyl compounds as chemical descriptors of healthy and defective roasted coffee beans. Food Control 2022, 138, 109014. [Google Scholar] [CrossRef]
- Caporaso, N.; Whitworth, M.B.; Fisk, I.D. Prediction of coffee aroma from single roasted coffee beans by hyperspectral imaging. Food Chem. 2022, 371, 131159. [Google Scholar] [CrossRef]
- Summa, C.A.; de la Calle, B.; Brohee, M.; Stadler, R.H.; Anklam, E. Impact of the roasting degree of coffee on the in vitro radical scavenging capacity and content of acrylamide. LWT—Food Sci. Technol. 2007, 40, 1849–1854. [Google Scholar] [CrossRef]
- Bertuzzi, T.; Martinelli, E.; Mulazzi, A.; Rastelli, S. Acrylamide determination during an industrial roasting process of coffee and the influence of asparagine and low molecular weight sugars. Food Chem. 2020, 303, 125372. [Google Scholar] [CrossRef]
- Rattanarat, P.; Chindapan, N.; Devahastin, S. Comparative evaluation of acrylamide and polycyclic aromatic hydrocarbons contents in Robusta coffee beans roasted by hot air and superheated steam. Food Chem. 2021, 341, 128266. [Google Scholar] [CrossRef]
- Banchero, M.; Pellegrino, G.; Manna, L. Supercritical fluid extraction as a potential mitigation strategy for the reduction of acrylamide level in coffee. J. Food Eng. 2013, 115, 292–297. [Google Scholar] [CrossRef]
- Pattnayak, B.C.; Mohapatra, S. A smartphone-assisted ultrasensitive detection of acrylamide in thermally processed snacks using CQD@Au NP integrated FRET sensor. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2023, 286, 122009. [Google Scholar] [CrossRef]
- Michalak, J.; Gujska, E.; Czarnowska, M.; Klepacka, J.; Nowak, F. Effect of storage on acrylamide and 5-hydroxymethylfurfural contents in selected processed plant products with long shelf-life. Plant Food Hum. Nutr. 2016, 71, 115–122. [Google Scholar] [CrossRef] [Green Version]
- Andrzejewski, D.; Roach, J.A.G.; Gay, M.L.; Musser, S.M. Analysis of coffee for the presence of acrylamide by LC-MS/MS. J. Agric. Food Chem. 2004, 52, 1996–2002. [Google Scholar] [CrossRef]
- Hoenicke, K.; Gatermann, R. Studies on the stability of acrylamide in food during storage. J. AOAC Int. 2005, 88, 268–273. [Google Scholar] [CrossRef] [Green Version]
- Pastoriza, S.; Rufián-Henares, J.Á.; Morales, F.J. Reactivity of acrylamide with coffee melanoidins in model systems. LWT—Food Sci. Technol. 2012, 45, 198–203. [Google Scholar] [CrossRef] [Green Version]
- Baum, M.; Böhm, N.; Görlitz, J.; Lantz, I.; Merz, K.H.; Ternité, R.; Eisenbrand, G. Fate of 14C-acrylamide in roasted and ground coffee during storage. Mol. Nutr. Food Res. 2008, 52, 600–608. [Google Scholar] [CrossRef] [PubMed]
- Cordoba, N.; Pataquiva, L.; Osorio, C.; Moreno, F.L.M.; Ruiz, R.Y. Effect of grinding, extraction time and type of coffee on the physicochemical and flavour characteristics of cold brew coffee. Sci. Rep. 2019, 9, 8440. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Basaran, B.; Aydin, F.; Kaban, G. The determination of acrylamide content in brewed coffee samples marketed in Turkey. Food Addit. Contam. Part A Chem. 2020, 37, 280–287. [Google Scholar] [CrossRef]
- Han, J.; Boo, H.; Chung, M. Effects of extraction conditions on acrylamide/furan content, antioxidant activity, and sensory properties of cold brew coffee. Food Sci. Biotechnol. 2020, 29, 1071–1080. [Google Scholar] [CrossRef]
- Cha, M. Enzymatic control of the acrylamide level in coffee. Eur. Food Res. Technol. 2013, 236, 567–571. [Google Scholar] [CrossRef]
- Bedade, D.K.; Sutar, Y.B.; Singhal, R.S. Chitosan coated calcium alginate beads for covalent immobilization of acrylamidase: Process parameters and removal of acrylamide from coffee. Food Chem. 2019, 275, 95–104. [Google Scholar] [CrossRef]
- Narita, Y.; Inouye, K. Decrease in the acrylamide content in canned coffee by heat treatment with the addition of cysteine. J. Agric. Food Chem. 2014, 62, 12218–12222. [Google Scholar] [CrossRef]

| No. | Coffee Samples | Methods | Treatment | Content (μg/kg) | Reference |
|---|---|---|---|---|---|
| 1 | Arabica, Robusta | GC-MS | 210 °C roasted 8–11 min | 0.87–2.92 μg/(30 mL espresso) | [20] |
| 2 | Arabica, Robusta | LC-MS/MS | Wet drying (5 degrees of roasting) | 400–1130 | [21] |
| 3 | Robusta | GC-FID | Wet drying (180–202 °C roasted) | 1000–17,500 | [62] |
| 4 | Arabica | LC-MS/MS | 220 °C roasted | 468 | [65] |
| 5 | Instant coffee | Inhibitory reduction spectrophotometry | Hot water dissolved | 888.3 | [66] |
| 6 | Arabica, Robusta | LC-MS | Wet drying (220–260 °C roasted) | 90–500 | [67] |
| 7 | Arabica | LC-APCI-MS | 150, 200, and 225 °C roasted | 50–500 | [68] |
| 8 | Arabica, Robusta | LC-MS/MS | Six ways to roast | 130–480 | [69] |
| 9 | Arabica | LC-MS/MS | Medium roast | 1020 | [70] |
| 10 | Instant coffee | LC-HRMS | Dissolve ultrapure water mixed with AA internal standard | 159 | [71] |
| 11 | Arabica | Stable isotope dilution and LC-MS/MS | Roasted | 22.2–326.4 | [72] |
| 12 | Robusta and Arabica | GC-MS | 200–245 °C | 159–484 | [73] |
| 13 | Instant coffee | LC-ESI-MS | Dissolve ultrapure water mixed with AA internal standard | 41–1049 | [74] |
| No. | Samples | Roasting Conditions (T: °C, t: min) | Treatment | Optimal Inhibition Conditions | Inhibition Ratio | Reference |
|---|---|---|---|---|---|---|
| 1 | Arabica | T: 5, 10, 15, 30 and 60 | 0.15 kPa | 200 °C roasted 10 min | 50% | [34] |
| 2 | Robusta | T: 200, t: 10 tradition, tradition-vacuum combined and vacuum | Different roasted temperatures and times | 210 °C roasted 40 min | 90.92% | [62] |
| 3 | Arabica | T: 220 | Different roasted times | 220 °C roasted 60 min | 100% | [65] |
| 4 | Turkey Arabica | T: 150–210 | Different roasted temperatures and times | 225 °C roasted 30 min | 43.48% | [68] |
| 5 | Robusta, Arabica | T: 15–40 | Different roasted temperatures and times | 260 °C roasted 15 min | 81.37% and 25.2% | [76] |
| 6 | Robusta, Arabica | T: 150, 200, 225 | Different roasted temperatures and times | 236 °C roasted 10 min | 42.86% and 57.14% | [88] |
| 7 | Robusta, Arabica | T: 5, 10, 15, 20, and 30 | Different roasted temperatures and times | 138 °C roasted 6 min | 97.23% and 92.34% | [89] |
| 8 | Robusta | T: 220–260 | Supercritical CO2 extraction | 100 °C, 200 Pa, 9.5% ethanol solution for 1035 min | 79% | [91] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Z.; Zhao, C.; Cao, C. Production and Inhibition of Acrylamide during Coffee Processing: A Literature Review. Molecules 2023, 28, 3476. https://doi.org/10.3390/molecules28083476
Li Z, Zhao C, Cao C. Production and Inhibition of Acrylamide during Coffee Processing: A Literature Review. Molecules. 2023; 28(8):3476. https://doi.org/10.3390/molecules28083476
Chicago/Turabian StyleLi, Zelin, Chunyan Zhao, and Changwei Cao. 2023. "Production and Inhibition of Acrylamide during Coffee Processing: A Literature Review" Molecules 28, no. 8: 3476. https://doi.org/10.3390/molecules28083476





