The SEB1741 Aptamer Is an Efficient Tool for Blocking CD4+ T Cell Activation Induced by Staphylococcal Enterotoxin B
Abstract
:1. Introduction
2. Results
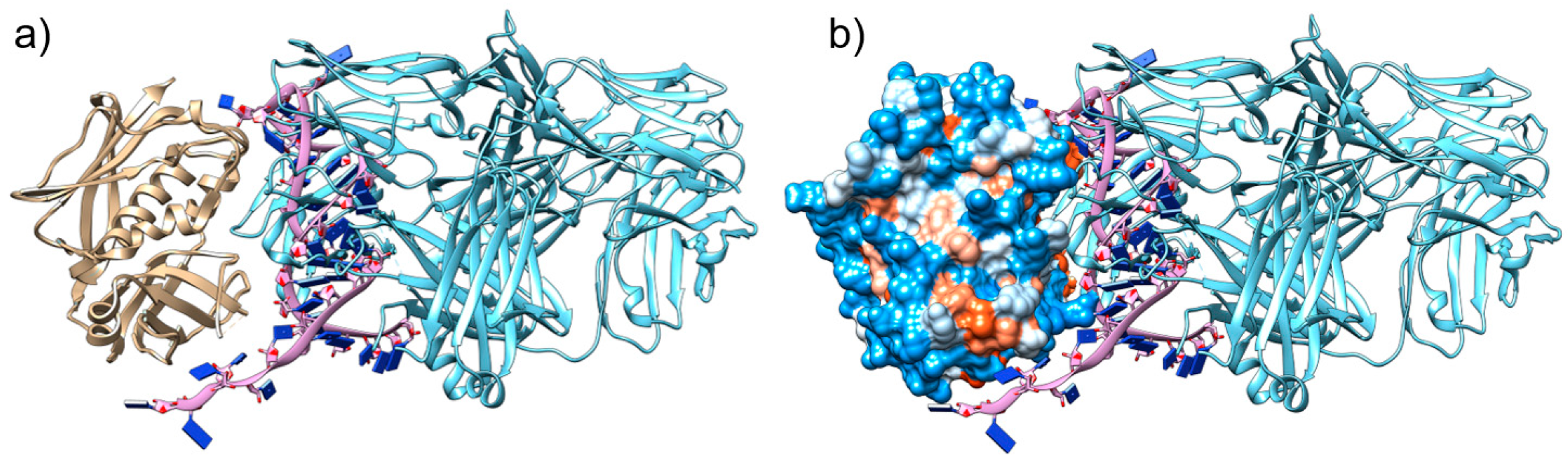
2.1. The Aptamer SEB1741 Interacts with the Active Site of SEB
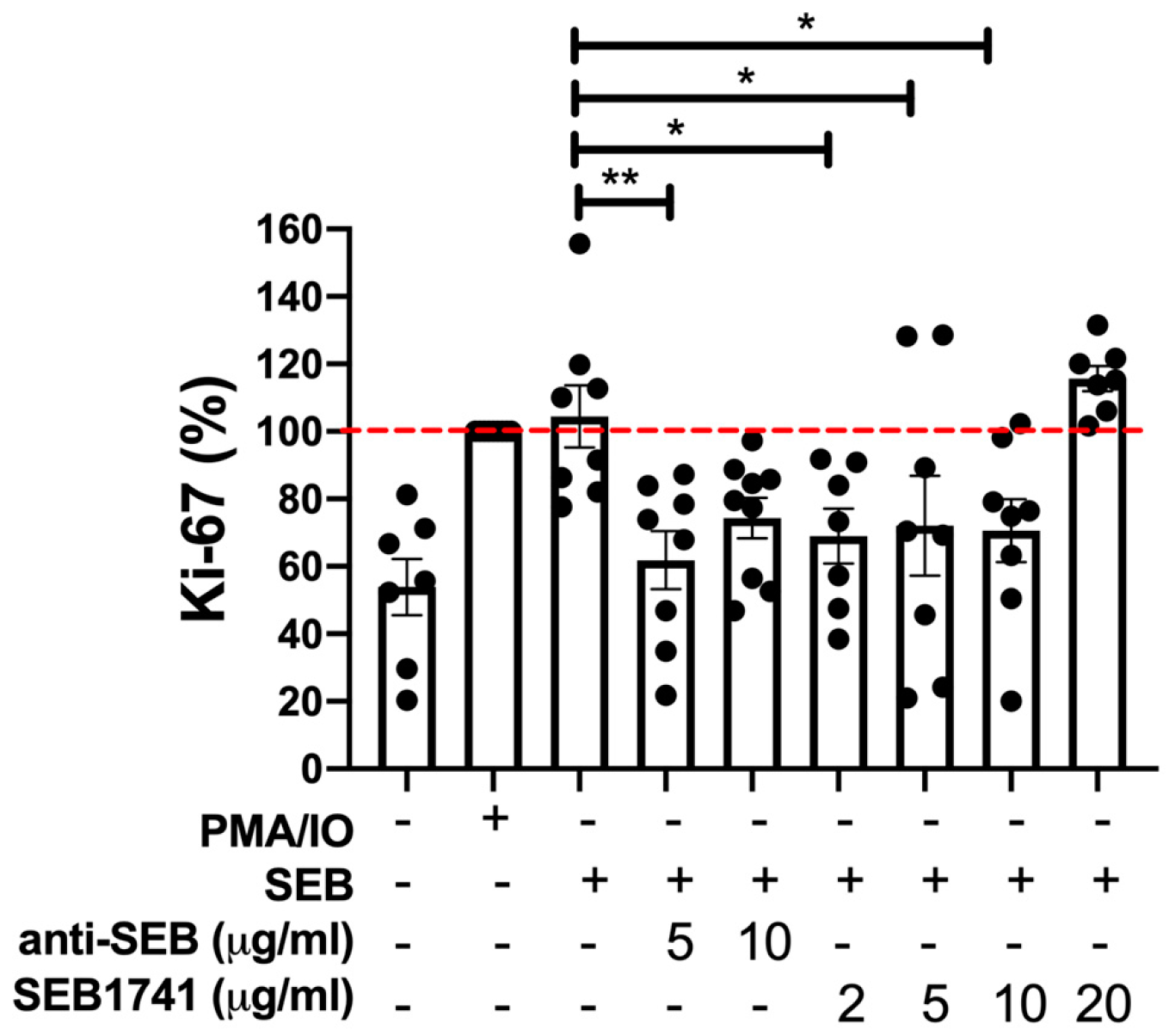
2.2. The SEB1741 Aptamer Inhibits the CD4+ T-Cell Proliferation Induced by SEB
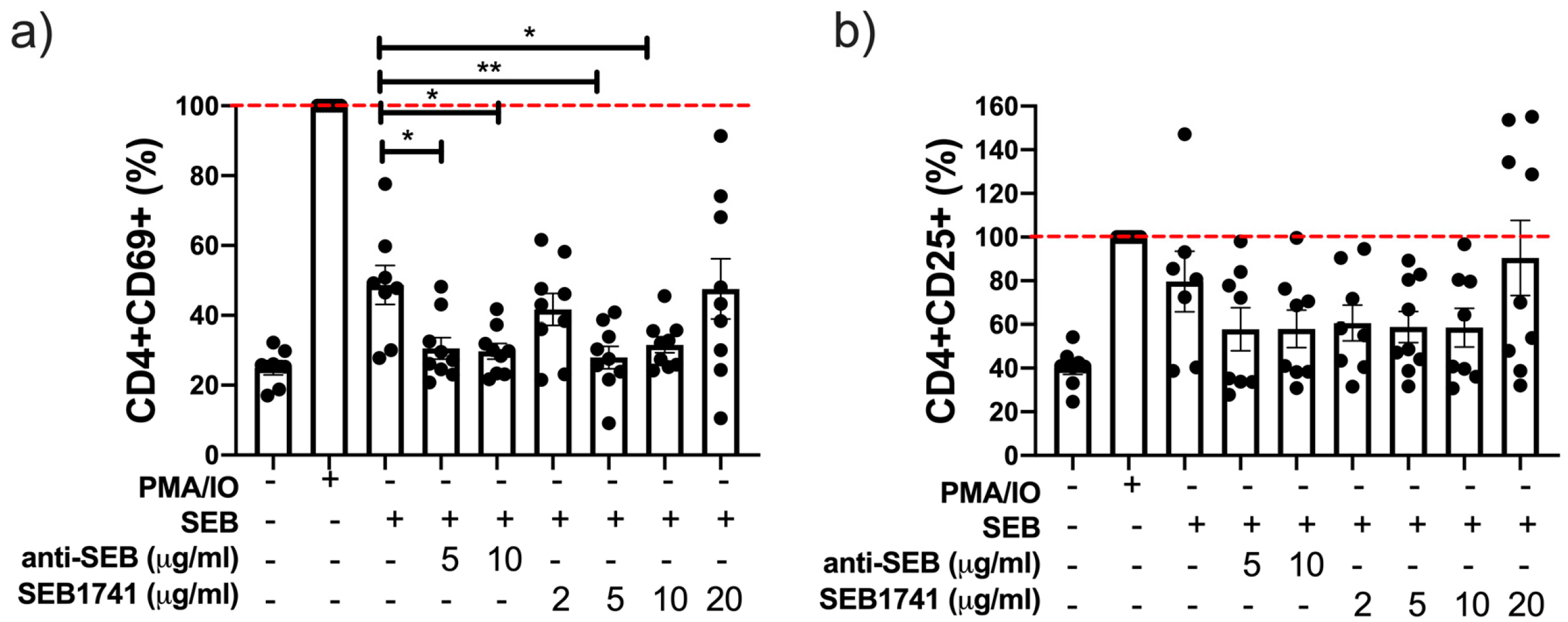
2.3. The SEB1741 Aptamer Decreases the CD4+ T-Cell Activation Induced by SEB
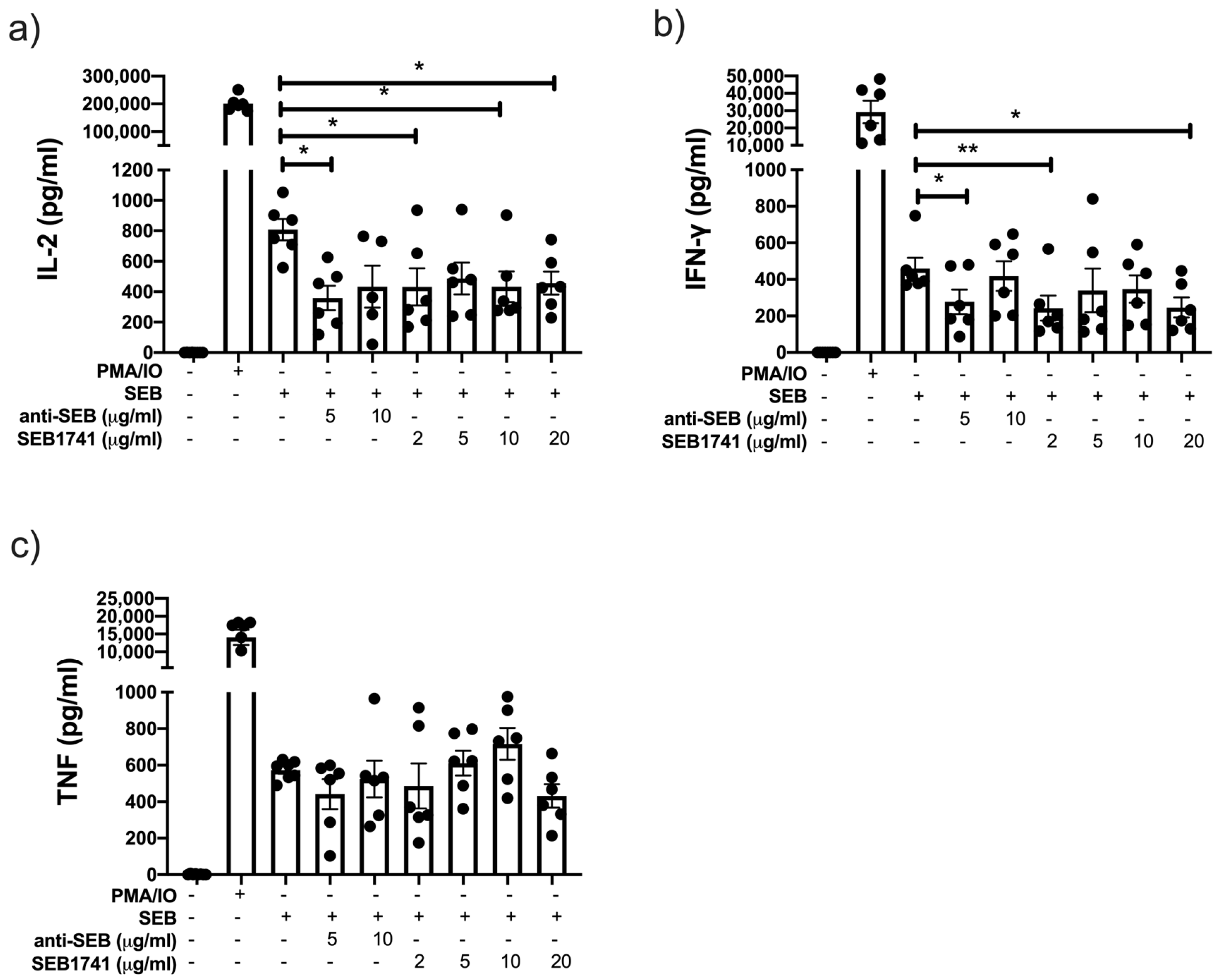
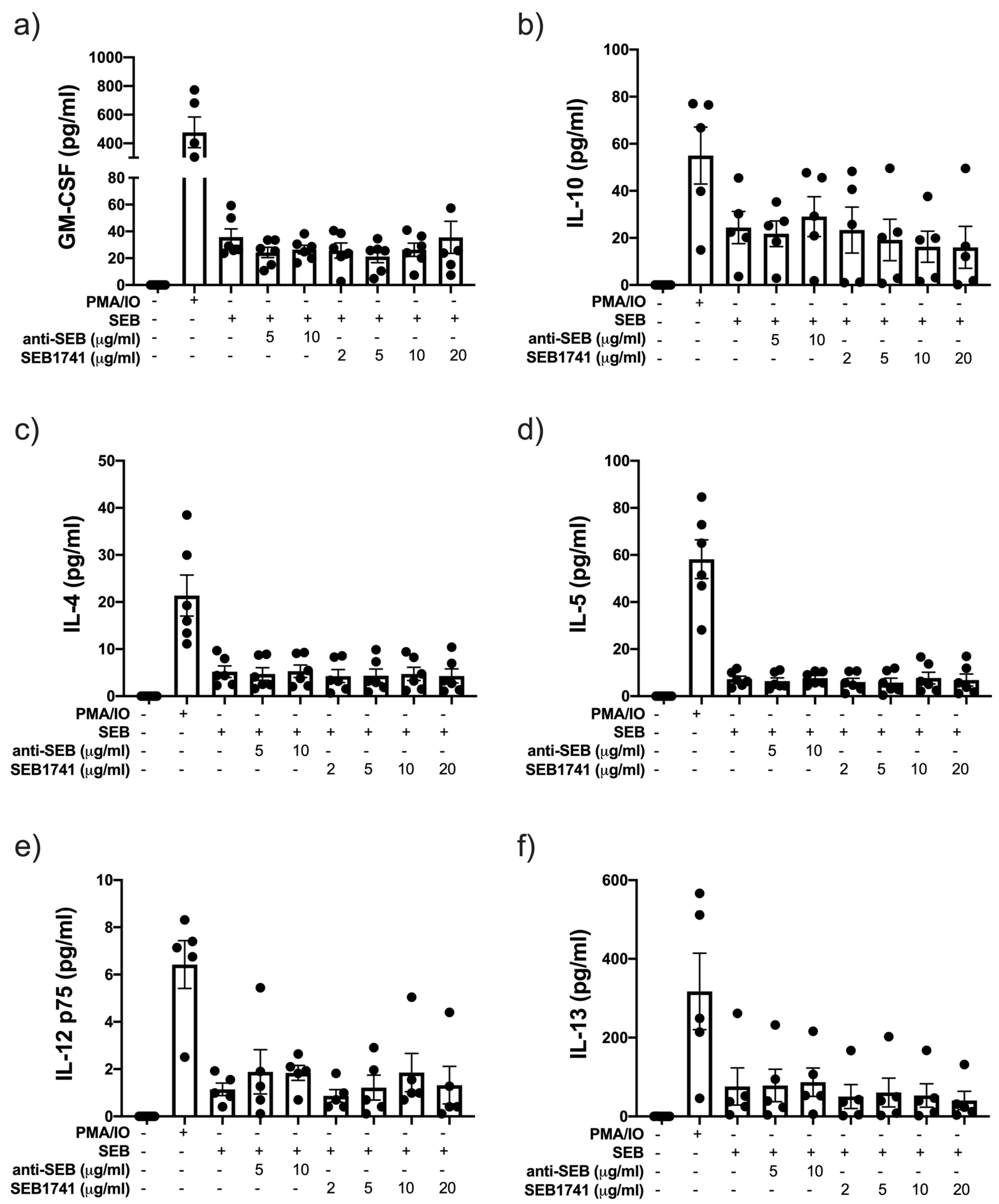
2.4. Low Concentration of the SEB1741 Aptamer Decreases the Release of the Proinflammatory Cytokines IL-2 and IFN-γ but Not That of TNF
2.5. SEB Induces the Release of GM-CSF and IL-10 but Not That of Another Anti-Inflammatory Cytokine
3. Materials and Methods
3.1. Ethical Approval
3.2. Peripheral Blood Mononuclear Cells (PBMCs)
3.3. Isolation and Purification of CD4+ T Cells
3.4. CD4+ T Cell Stimulation Assay with SEB
3.5. Flow Cytometry
3.6. Multiple Cytokine Assay in Supernatant Samples
3.7. Statistical Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
| Antibody | Company | Clone | Cat. Number |
|---|---|---|---|
| Brilliant Violet 421™ anti-human Ki-67 | BioLegend | Ki67 | B269687 |
| PE/Cyanine7 anti-human CD4 | BioLegend | OKT4 | B159354 |
| PE-A anti-human CD25 | BD Pharmingen | BC96 | 3235775 |
| Brilliant Violet 510™ anti-human CD69 | BioLegend | FN50 | B322621 |
References
- Douglas-Louis, R.; Lou, M.; Lee, B.; Minejima, E.; Bubeck-Wardenburg, J.; Wong-Beringer, A. Prognostic significance of early platelet dynamics in Staphylococcus aureus bacteremia. BMC Infect. Dis. 2023, 1, 82. [Google Scholar] [CrossRef] [PubMed]
- Howden, B.P.; Giulieri, S.G.; Lung, T.W.F.; Baines, S.L.; Sharkey, L.K.; Lee, J.Y.H.; Hachani, A.; Monk, I.R.; Stinear, T.P. Staphylococcus aureus host interactions and adaptation. Nat. Rev. Microbiol. 2023, 1, 1–16. [Google Scholar] [CrossRef]
- Francis, D.; Bhairaddy, A.; Joy, A.; Hari, G.V.; Francis, A. Secretory proteins in the orchestration of microbial virulence: The curious case of Staphylococcus aureus. Adv. Protein Chem. Struct. Biol. 2023, 133, 271–350. [Google Scholar]
- Lawrynowicz-Paciorek, M.; Kochman, M.; Piekarska, K.; Grochowska, A.; Windyga, B. The distribution of enterotoxin and enterotoxin-like genes in Staphylococcus aureus strains isolated from nasal carriers and food samples. Int. J. Food Microbiol. 2007, 117, 319–323. [Google Scholar] [CrossRef] [PubMed]
- Jang, J.H.; Kim, S.; Kim, S.G.; Lee, J.; Lee, D.G.; Jang, J.; Jeong, Y.S.; Song, D.H.; Min, J.K.; Park, J.G.; et al. A Sensitive Immunodetection Assay Using Antibodies Specific to Staphylococcal Enterotoxin B Produced by Baculovirus Expression. Biosensors 2022, 10, 787. [Google Scholar] [CrossRef] [PubMed]
- Purwanasari, H.N.; Permatasari, A.T.U.; Lestari, F.B.; Wasissa, M.; Zaini, K.; Salasia, S.I.O. Cellular immune response of Staphylococcus aureus enterotoxin B in Balb/c mice through intranasal infection. Vet. World. 2022, 7, 1765–1771. [Google Scholar] [CrossRef]
- Escamilla-Gutiérrez, A.; Córdova-Espinoza, M.G.; Sánchez-Monciváis, A.; Tecuatzi-Cadena, B.; Regalado-García, A.G.; Medina-Quero, K. In silico selection of aptamers for bacterial toxins detection. J. Biomol. Struct. Dyn. 2022, 1, 1–10. [Google Scholar] [CrossRef]
- Tuffs, S.W.; Goncheva, M.I.; Xu, S.X.; Craig, H.C.; Kasper, K.J.; Choi, J.; Flannagan, R.S.; Kerfoot, S.M.; Heinrichs, D.E.; McCormick, J.K. Superantigens promote Staphylococcus aureus bloodstream infection by eliciting pathogenic interferon-gamma production. Proc. Natl. Acad. Sci. USA 2022, 8, e2115987119. [Google Scholar] [CrossRef] [PubMed]
- Noli Truant, S.; De Marzi, M.C.; Sarratea, M.B.; Antonoglou, M.B.; Meo, A.P.; Iannantuono López, L.V.; Fernández Lynch, M.J.; Todone, M.; Malchiodi, E.L.; Fernández, M.M. egc Superantigens Impair Monocytes/Macrophages Inducing Cell Death and Inefficient Activation. Front. Immunol. 2020, 10, 3008. [Google Scholar] [CrossRef]
- Fraser, J.D.; Proft, T. The bacterial superantigen and superantigen-like proteins. Immunol. Rev. 2008, 1, 226–243. [Google Scholar] [CrossRef]
- Mihara, H.; Uchida, K.; Watanabe, Y.; Nanjo, S.; Sakumura, M.; Motoo, I.; Ando, T.; Minemura, M.; Muhammad, J.S.; Yamamoto, H.; et al. Colonic TRPV4 overexpression is related to constipation severity. BMC Gastroenterol. 2023, 23, 13. [Google Scholar] [CrossRef]
- Hu, H.; Liu, S.; Hon, K.; Psaltis, A.J.; Wormald, P.J.; Vreugde, S. Staphylococcal protein A modulates inflammation by inducing interferon signaling in human nasal epithelial cells. Inflamm. Res. 2023, 2, 251–262. [Google Scholar] [CrossRef]
- Lowy, F.D. Staphylococcus aureus Infections. N. Engl. J. Med. 1998, 8, 520–532. [Google Scholar] [CrossRef] [PubMed]
- Gottlieb, M.; Long, B.; Koyfman, A. The Evaluation and Management of Toxic Shock Syndrome in the Emergency Department: A Review of the Literature. J. Emerg. Med. 2018, 6, 807–814. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Gan, L.; Jiang, L.; Zhang, X.; Yang, X.; Chen, M.; Lan, X. Neutralization of Staphylococcal Enterotoxin B by an Aptamer Antagonist. Antimicrob. Agents Chemother. 2015, 4, 2072–2077. [Google Scholar] [CrossRef]
- Mudili, V.; Makam, S.S.; Sundararaj, N.; Siddaiah, C.; Gupta, V.K.; Rao, P.V.L. Retraction Note: A novel IgY-Aptamer hybrid system for cost-effective detection of SEB and its evaluation on food and clinical samples. Sci. Rep. 2022, 1, 10939. [Google Scholar] [CrossRef] [PubMed]
- Moltajaei, M.H.; Pourzare Mehrbani, S.; Motahari, P.; Rezapour, R. Clinicopathological and prognostic value of Ki-67 expression in oral malignant melanoma: A systematic review and meta-analysis. J. Dent. Res. Dent. Clin. Dent. Prospects. 2022, 3, 140–146. [Google Scholar] [CrossRef]
- Di Rosa, F.; Cossarizza, A.; Hayday, A.C. To Ki or Not to Ki: Re-Evaluating the Use and Potentials of Ki-67 for T Cell Analysis. Front. Immunol. 2021, 12, 653974. [Google Scholar] [CrossRef]
- Chávez-Galán, L.; Illescas-Eugenio, J.; Alvarez-Sekely, M.; Baez-Saldaña, R.; Chávez, R.; Lascurain, R. Tuberculosis patients display a high proportion of CD8+ T cells with a high cytotoxic potential. Microbiol. Immunol. 2019, 8, 316–327. [Google Scholar] [CrossRef]
- Zhang, Y.; Su, J. Interleukin-2 family cytokines: An overview of genes, expression, signaling and functional roles in teleost. Dev. Comp. Immunol. 2023, 141, 104645. [Google Scholar] [CrossRef]
- Villalba, N.; Ma, Y.; Gahan, S.A.; Joly-Amado, A.; Spence, S.; Yang, X.; Nash, K.; Yuan, S.Y. Lung infection by P. aeruginosa induces neuroinflammation and blood-brain barrier dysfunction in mice. bioRxiv 2023, 1, 31. [Google Scholar]
- Spaulding, A.R.; Salgado-Pabón, W.; Kohler, P.L.; Horswill, A.R.; Leung, D.Y.M.; Schlievert, P.M. Staphylococcal and Streptococcal Superantigen Exotoxins. Clin. Microbiol. Rev. 2013, 3, 422–447. [Google Scholar] [CrossRef] [PubMed]
- Bîrluţiu, V.; Criştiu, O.; Baicu, M.; Bîrluţiu, R.M. The Management of Staphylococcal Toxic Shock Syndrome. A Case Report. J. Crit. Care Med. 2016, 2, 85–88. [Google Scholar] [CrossRef]
- Chuang, Y.-Y.; Huang, Y.-C.; Lin, T.-Y. Toxic Shock Syndrome in Children. Pediatr. Drugs. 2005, 1, 11–25. [Google Scholar] [CrossRef] [PubMed]
- Visvanathan, K.; Charles, A.; Bannan, J.; Pugach, P.; Kashfi, K.; Zabriskie, J.B. Inhibition of Bacterial Superantigens by Peptides and Antibodies. Infect. Immun. 2001, 2, 875–884. [Google Scholar] [CrossRef]
- Shepherd, F.R.; Davies, K.; Miners, K.L.; Llewellyn-Lacey, S.; Kollnberger, S.; Redman, J.E.; Grant, M.M.; Ladell, K.; Price, D.A.; McLaren, J.E. The superantigens SpeC and TSST-1 specifically activate TRBV12-3/12-4+ memory T cells. Commun Biol. 2023, 1, 78. [Google Scholar] [CrossRef]
- Rha, M.S.; Kim, S.W.; Chang, D.Y.; Lee, J.K.; Kim, J.; Park, S.H.; Khalmuratova, R.; Lim, H.S.; Eun, K.M.; Hong, S.N.; et al. Superantigen-related TH2 CD4+ T cells in nonasthmatic chronic rhinosinusitis with nasal polyps. J. Allergy Clin. Immunol. 2020, 5, 1378–1388. [Google Scholar] [CrossRef]
- Deacy, A.M.; Gan, S.K.; Derrick, J.P. Superantigen Recognition and Interactions: Functions, Mechanisms and Applications. Front. Immunol. 2021, 12, 731845. [Google Scholar] [CrossRef]
- Gresham, H.D.; Lowrance, J.H.; Caver, T.E.; Wilson, B.S.; Cheung, A.L.; Lindberg, F.P. Survival of Staphylococcus aureus inside neutrophils contributes to infection. J. Immunol. 2000, 164, 3713–3722. [Google Scholar] [CrossRef]
- Larkin, E.A.; Stiles, B.G.; Ulrich, R.G. Inhibition of Toxic Shock by Human Monoclonal Antibodies against Staphylococcal Enterotoxin B. PLoS ONE 2010, 10, e13253. [Google Scholar]
- Chen, G.; Karauzum, H.; Long, H.; Carranza, D.; Holtsberg, F.W.; Howell, K.A.; Abaandou, L.; Zhang, B.; Jarvik, N.; Ye, W.; et al. Potent Neutralization of Staphylococcal Enterotoxin B In Vivo by Antibodies that Block Binding to the T-Cell Receptor. J. Mol. Biol. 2019, 431, 4354–4367. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Song, Z.; Ge, S.; Zhang, J.; Xu, L.; Yang, F.; Lu, D.; Luo, P.; Gu, J.; Zou, Q.; et al. Determining the immunological characteristics of a novel human monoclonal antibody developed against staphylococcal enterotoxin B. Hum. Vaccines Immunother. 2020, 7, 1708–1718. [Google Scholar] [CrossRef]
- Bai, G.; Ge, Y.; Su, Y.; Chen, S.; Zeng, X.; Lu, H.; Ma, B. Computational Construction of a Single-Chain Bi-Paratopic Antibody Allosterically Inhibiting TCR-Staphylococcal Enterotoxin B Binding. Front. Immunol. 2021, 12, 732938. [Google Scholar] [CrossRef]
- MacIntyre, J.L.; Varshney, A.K.; Wang, X.; Gatto, S.; Friedman, C.; Liu, Y.; Kerns, K.; Kovalenko, O.; Adkins, K.; Zollner, R.; et al. Optimization of experimental conditions for functional in vitro characterization of humanized antibodies specific for staphylococcal enterotoxin B. Int. Immunopharmacol. 2015, 28, 354–358. [Google Scholar] [CrossRef] [PubMed]
- Verreault, D.; Ennis, J.; Whaley, K.; Killeen, S.Z.; Karauzum, H.; Aman, M.J.; Holtsberg, R.; Doyle-Meyers, L.; Didier, P.J.; Zeitlin, L.; et al. Effective Treatment of Staphylococcal Enterotoxin B Aerosol Intoxication in Rhesus Macaques by Using Two Parenterally Administered High-Affinity Monoclonal Antibodies. Antimicrob. Agents. Chemother. 2019, 63, e02049-18. [Google Scholar] [CrossRef]
- Hu, N.; Qiao, C.; Wang, J.; Wang, Z.; Li, X.; Zhou, L.; Wu, J.; Zhang, D.; Feng, J.; Shen, B.; et al. Identification of a novel protective human monoclonal antibody, LXY8, that targets the key neutralizing epitopes of staphylococcal enterotoxin B. Biochem. Biophys. Res. Commun. 2021, 549, 120–127. [Google Scholar] [CrossRef] [PubMed]
- Krakauer, T. Sulfasalazine Attenuates Staphylococcal Enterotoxin B-Induced Immune Responses. Toxins 2015, 2, 553–559. [Google Scholar] [CrossRef]
- Krakauer, T.; Buckley, M. Intranasal Rapamycin Rescues Mice from Staphylococcal Enterotoxin B-Induced Shock. Toxins 2012, 9, 718–728. [Google Scholar] [CrossRef]
- Fan, R.; Tao, X.; Zhai, X.; Zhu, Y.; Li, Y.; Chen, Y.; Dong, D.; Yang, S.; Lv, L. Application of aptamer-drug delivery system in the therapy of breast cancer. Biomed. Pharmacother. 2023, 161, 114444. [Google Scholar] [CrossRef]
- Egli, M.; Manoharan, M. Chemistry, structure and function of approved oligonucleotide therapeutics. Nucleic Acids Res. 2023, 51, 2529–2573. [Google Scholar] [CrossRef]
- Wu, M.; Gao, X.; Tang, Y.; Wu, W.; Zhou, J.; Shao, Y.; Hao, C.; Yang, Y.; Zhang, J. Cbl-b inhibited CD4+ T cell activation by regulating the expression of miR-99a/miR-125b. Int. Immunopharmacol. 2023, 115, 109677. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Son, M.J.; Ho, C.C.; Lee, S.H.; Kim, Y.; An, J.; Lee, S.K. Transcriptional inhibition of STAT1 functions in the nucleus alleviates Th1 and Th17 cell-mediated inflammatory diseases. Front. Immunol. 2022, 13, 1054472. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Kang, O.-H.; Kwon, D.-Y. Bisdemethoxycurcumin Reduces Methicillin-Resistant Staphylococcus aureus Expression of Virulence-Related Exoproteins and Inhibits the Biofilm Formation. Toxins 2021, 11, 804. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.-S.; Kim, K.-W.; Yoon, D.; Kim, G.-S.; Kwon, D.-Y.; Kang, O.-H.; Lee, D. Comparison of Antivirulence Activities of Black Ginseng against Methicillin-Resistant Staphylococcus aureus According to the Number of Repeated Steaming and Drying Cycles. Antibiotics 2021, 6, 617. [Google Scholar] [CrossRef]
- Goldman, S.J.; Uniyal, S.; Ferguson, L.M.; Golan, D.E.; Burakoff, S.J.; Kiener, P.A. Differential Activation of Phosphotyrosine Protein Phosphatase Activity in a Murine T Cell Hybridoma by Monoclonal Antibodies to CD45. J. Biol. Chem. 1992, 267, 6197–6204. [Google Scholar] [CrossRef] [PubMed]
- Nimjee, S.M.; White, R.R.; Becker, R.C.; Sullenger, B.A. Aptamers as Therapeutics. Annu. Rev. Pharmacol. Toxicol. 2017, 1, 61–79. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chavez-Galan, L.; Ruiz, A.; Ramón-Luing, L.A.; Escamilla-Gutiérrez, A.; Sánchez-Monciváis, A.; Tecuatzi-Cadena, B.; Medina-Quero, K.; Córdova-Espinoza, M.G. The SEB1741 Aptamer Is an Efficient Tool for Blocking CD4+ T Cell Activation Induced by Staphylococcal Enterotoxin B. Molecules 2023, 28, 3480. https://doi.org/10.3390/molecules28083480
Chavez-Galan L, Ruiz A, Ramón-Luing LA, Escamilla-Gutiérrez A, Sánchez-Monciváis A, Tecuatzi-Cadena B, Medina-Quero K, Córdova-Espinoza MG. The SEB1741 Aptamer Is an Efficient Tool for Blocking CD4+ T Cell Activation Induced by Staphylococcal Enterotoxin B. Molecules. 2023; 28(8):3480. https://doi.org/10.3390/molecules28083480
Chicago/Turabian StyleChavez-Galan, Leslie, Andy Ruiz, Lucero A. Ramón-Luing, Alejandro Escamilla-Gutiérrez, Anahí Sánchez-Monciváis, Brenda Tecuatzi-Cadena, Karen Medina-Quero, and María Guadalupe Córdova-Espinoza. 2023. "The SEB1741 Aptamer Is an Efficient Tool for Blocking CD4+ T Cell Activation Induced by Staphylococcal Enterotoxin B" Molecules 28, no. 8: 3480. https://doi.org/10.3390/molecules28083480





