Comparison of Radionuclide Impurities Activated during Irradiation of 18O-Enriched Water in Tantalum and Silver Targets during the Production of 18F in a Cyclotron
Abstract
:1. Introduction
2. Results
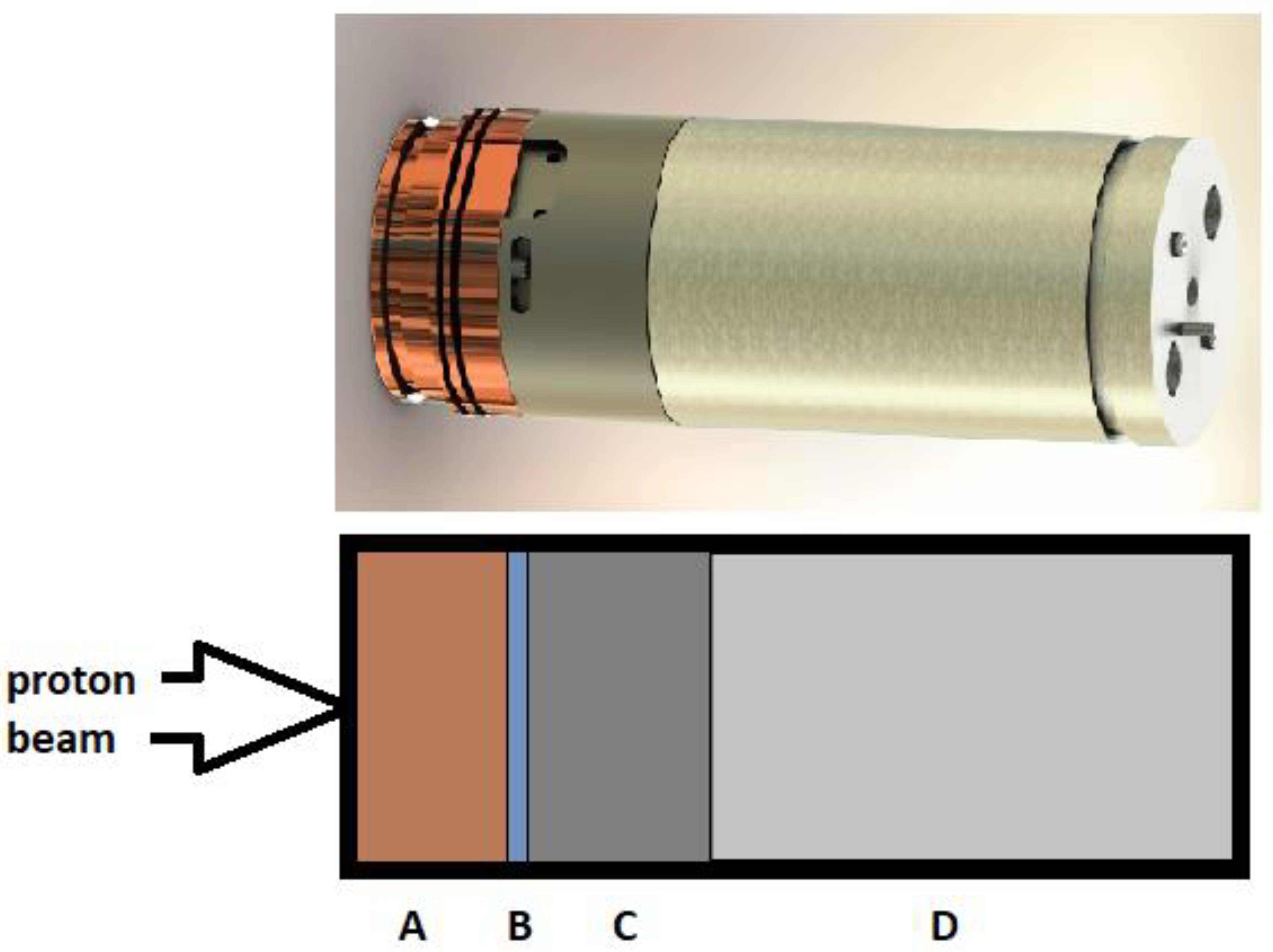
3. Materials and Methods
- Enrichment of 18O, typically >95%.
- Chemically pure enriched water 18O, above 99.99%.
- Target volume of 2.5 mL.
- A beam of protons with an energy of 11 MeV.
- Beam current of 60 μA.
- Irradiation time of 90 min.
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- O’Donnell, R.G.; Vintro, L.L.; Duffy, G.J.; Mitchell, P.I. Measurement of the residual radioactivity induced in the front foil of a target assembly in a modern medical cyclotron. Appl. Radiat. Isot. 2004, 60, 539–542. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Serrano, J.J.; Diez de Los Rios, A. Predicting induced activity in the Havar foils of the (18)F production targets of a PET cyclotron and derived radiological risk. Health Phys. 2014, 107, 103–110. [Google Scholar] [CrossRef]
- Długosz-Lisiecka, M.; Jakubowska, T.; Zawada, A. High-Level Radioactive Wastes from 18F and 11C Isotopes Production. J. Hazard. Toxic Radioact. Waste 2021, 25, 04020072. [Google Scholar] [CrossRef]
- Berridge, M.S.; Kjellström, R. Designs and use of silver [18O]water targets for [18F]fluoride production. Appl. Radiat. Isot. 1999, 50, 699–705. [Google Scholar] [CrossRef]
- Jacobson, O.; Kiesewetter, D.O.; Chen, X. Fluorine-18 radiochemistry, labeling strategies and synthetic routes. Bioconjug. Chem. 2015, 26, 1–18. [Google Scholar] [CrossRef] [Green Version]
- Helmeke, H.-J.; Harms, T.; Knapp, W.H. A water target with beam sweep for routine fluorine-18 production. Appl. Radiat. Isot. 2001, 54, 753–759. [Google Scholar] [CrossRef]
- Zeisler, S.K.; Becker, D.W.; Pavan, R.A.; Moschel, R.; Rühle, H. A water-cooled spherical niobium target for the production of [18F]fluoride. Appl. Radiat. Isot. 2000, 53, 449–453. [Google Scholar] [CrossRef]
- Siikanen, J.; Ohlsson, T.; Medema, J.; Van-Essen, J.; Sandell, A. A niobium water target for routine production of [18F]Fluoride with a MC 17 cyclotron. Appl. Radiat. Isot. 2013, 72, 133–136. [Google Scholar] [CrossRef]
- Długosz-Lisiecka, M.; Jakubowska, T.; Zbrojewska, M. Formation of 107Cd radionuclide impurities during 18F production. Appl. Radiat. Isot. 2019, 147, 48–53. [Google Scholar] [CrossRef]
- Kambali, I. Comprehensive theoretical studies on stable and radioactive isotopes produced by proton irradiation of titanium target. J. Phys. Conf. Ser. 2020, 1572, 012013. [Google Scholar] [CrossRef]
- Mochizuki, S.; Ogata, Y.; Hatano, K.; Abe, J.; Ito, K.; Ito, Y.; Nishino, M.; Miyahara, H.; Ishigure, N. Measurement of the Induced Radionuclides in Production of Radiopharmaceuticals for Positron Emission Tomography (PET). J. Nucl. Sci. Technol. 2006, 43, 348–353. [Google Scholar] [CrossRef]
- Guarino, P.; Rizzo, S.; Tomarchio, E.; Greco, D. Gamma-ray spectrometric characterization of waste activated target components in a PET cyclotron, Cyclotrons and their applications. In Proceedings of the 18th International Conference, Cyclotrons 2007, Giardini Naxos, Italy, 1–5 October 2007. [Google Scholar]
- Ferguson, D.; Orr, P.; Gillanders, J.; Corrigan, G.; Marshall, C. Measurement of long lived radioactive impurities retained in the disposable cassettes on the Tracerlab MX system during the production of [18F]FDG. Appl. Radiat. Isot. 2011, 69, 1479–1485. [Google Scholar] [CrossRef]
- Bowden, L.; Vintró, L.; Mitchell, P.J.; O’Donnell, R.G.; Seymour, A.M.; Duffy, G.J. Radionuclide impurities in proton-irradiated [18O]H2O for the production of 18F−: Activities and distribution in the [18F]FDG synthesis process. Appl. Radiat. Isot. 2009, 67, 248–255. [Google Scholar] [CrossRef]
- Köhler, M.; Degering, D.; Zessin, J.; Füchtner, F.; Konheiser, J. Radionuclide impurities in [18F]F− and [18F]FDG for positron emission tomography. Appl. Radiat. Isot. 2013, 81, 268–271. [Google Scholar] [CrossRef]
- Długosz-Lisiecka, M.; Biegała, M.; Jakubowska, T. Activation of medical accelerator components and radioactive waste classification based on low beam energy model Clinac 2300. Radiat. Phys. Chem. 2023, 205, 110730. [Google Scholar] [CrossRef]
- Jakubowska, T.; Długosz-Lisiecka, M. Estimation of effective dose using two ICRP criteria, applied to radiation protection of personnel in an unshielded PET cyclotron facility. Radiat. Phys. Chem. 2020, 171, 108688. [Google Scholar] [CrossRef]
- Francis, F.; Wuest, F. Advances in [18F]Trifluoromethylation Chemistry for PET Imaging. Molecules 2021, 26, 6478. [Google Scholar] [CrossRef]
- Sublet, J.-C.; Dzysiuk, N.; Fleming, M.; van der Marck, S. TENDL: Complete Nuclear Data Library for Innovative Nuclear Science and Technology. Nucl. Data Sheets 2019, 155, 1–55. [Google Scholar] [CrossRef]
- Marengo, M.; Lodi, F.; Magi, S.; Cicoria, G.; Pancaldi, D.; Boschi, S. Assessment of radionuclid icimpurities in2-[18F]fluoro-2-deoxy-d-glucose ([18F]FDG) routine production. Appl. Radiat. Isot. 2008, 66, 295–302. [Google Scholar] [CrossRef]
- Satyamurthy, N.; Amarasekera, B. Phelps Tantalum [18O]Water Target for the Production of [18F]Fluoride with High Reactivity for the Preparation of 2-Deoxy-2-[18F]Fluoro-D-Glucose. Mol. Imaging Biol. 2002, 4, 65–70. [Google Scholar] [CrossRef]
- Kambali, I.; Suryanto, H. Recoiled and sputtered radioactive impurities in 11 MeV proton-based F-18 production; recoiled and sputtered radioactive impurities in 11 MeV proton-based F-18 production. Int. J. Technol. 2019, 10, 300. [Google Scholar] [CrossRef] [Green Version]
- Üncü, Y.A.; Özdoğan, H.; Şekerci, M.; Kaplan, A. Investigation of the production routes of Palladium-103 and Iodine-125 radioisotopes. Radiat. Phys. Chem. 2023, 204, 110658. [Google Scholar] [CrossRef]
- Deilami-Nezhad, L.; Moghaddam-Banaem, L.; Sadeghi, M.; Asgari, M. Production and purification of Scandium-47: A potential radioisotope for cancer theranostics. Appl. Radiat. Isot. 2016, 118, 124–130. [Google Scholar] [CrossRef] [PubMed]
- Yiğit, M.; Bostan, S.N. Study on cross section calculations for (n,p) nuclear reactions of cadmium isotopes. Appl. Radiat. Isot. 2019, 154, 108868. [Google Scholar] [CrossRef]
| Nuclear Reaction | Threshold Energy [MeV] | Maximum Cross-Section [mbarn]/Energy [MeV] | Decay Mode | Half-Life |
|---|---|---|---|---|
| 107Ag(p,n)107Cd | 2.219 | 519.354/11 | EC, β+ | 6.5 h |
| 107Ag(p,2n)106Cd | 10.22 | 95.313/11 | Stable | - |
| 107Ag(p,a)104Pa | 0 | 1.792/11 | Stable | - |
| 107Ag(p,d)106Ag | 7.38 | 56,952/11 | EC, β+ | 23.96 min |
| 107Ag(n,g)108Ag | 0 | 852/thermal neutrons | EC, β+ | 2.382 min |
| 107Ag(n,p)107Pd | 0 | 24.43/11 | β− | 6.5 × 106 y |
| 109Ag(p,n)109Cd | 1.0071 | 394/9 | EC, β+ | 462 d |
| 109Ag(p,2n)108Cd | 8.398 | 428/11 | Stable | - |
| 109Ag(p,a)106Pa | 0 | 394/11 | Stable | - |
| 109Ag(p,d)108Ag | 7.024 | 0.001/11 | EC, β+ | 2.38 min |
| 109Ag(n,g)110Ag | 0 | 1,784,480/thermal neutrons | Stable | - |
| 109Ag(n,p)109Pd | 0.334 | 12.61/11 | β− | 13.59 h |
| Nuclear Reaction | Threshold Energy | Maximum Cross-Section [mbarn]/Energy [MeV] | Decay Mode | Half-Life |
|---|---|---|---|---|
| 181Ta(p,n)181W | 0.976 | 64.994/9 | EC. β+ | 121.2 d |
| 181Ta (p,2n)180W | 7.699 | 398.1/11 | Stable | - |
| 181Ta (p,a)178Hf | 8.86 | 0.0352/11 | Stable | - |
| 181Ta (p,d)180Ta | 5.38 | 0.000009/11 | Stable | - |
| 181Ta (n,g)182Ta | 0 | 1,784,480/thermal neutrons | EC. β+ | 114.74 d |
| 181Ta (n,p)181Hf | 0 | 0.493/thermal neutrons | β− | 42.39 d |
| Isotope | Half-Life | Gamma Radiation/Intensity |
|---|---|---|
| 52Mn | 5.591 d | 1434.09 (100%), 935.54 (94.5%), 744.23 (90%) |
| 55Co | 17.53 h | 931.3 (75%), 477.2 (20.2%), 1408.5 (16.9%) |
| 56Co | 77.23 d | 846.77 (99.9%), 1238.29 (66.46%), 2598.50 (16.97%), 1771.35 (15.41%), 1037.84 (14.05%) |
| 57Co | 271.74 d | 122.06 (85.60%), 136.47 (10.68%) |
| 95Tc | 20 h | 765.79 (93.82%) |
| 95mTc | 61 d | 204.117 (63.20%), 582.08 (29.96%), 835.149 (26.63%) |
| 96Tc | 4.28 d | 778.224 (99.76%), 849.93 (98%), 812.58 (82%), 1126.85 (15.2%) |
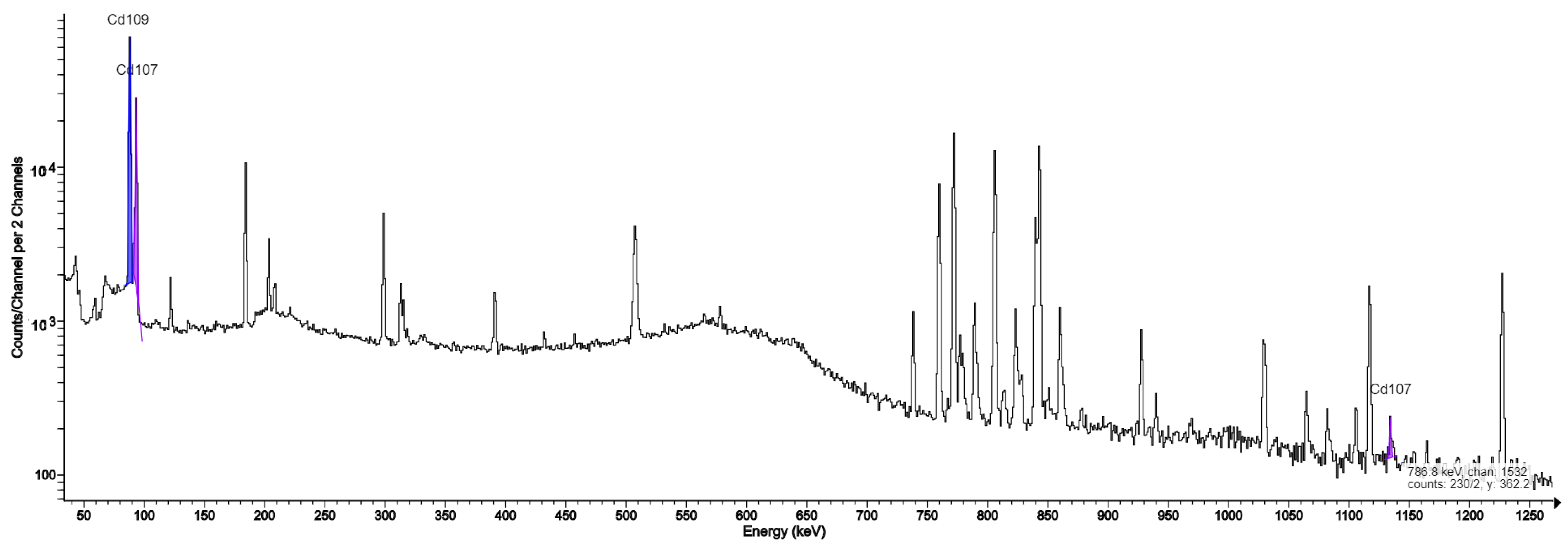
| 107Cd | 6.5 h | 93.12 (4.8%), 828.93 (0.17%) |
| 109Cd | 462.6 d | 88.03 (3.61%) |
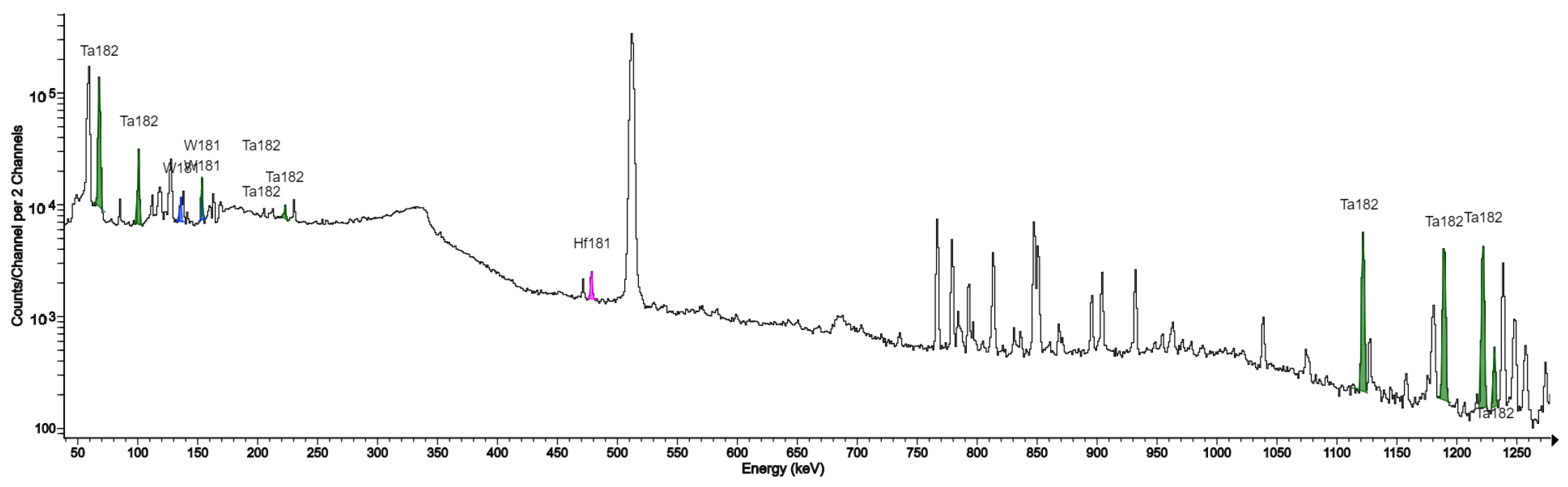
| 181W | 121.2 d | 152.32 (0.08%), 136.28 (0.03%) |
| 181Hf | 43.39 | 482.18 (80.5%), 133.02 (43.3%), 345.92 (15.12%) |
| 182Ta | 114.43 d | 67.749 (42.9%), 1121.3 (35.24%), 1221.4 (27.23 %), 1189.04 (16.49%) |
| 182Re | 64 h | 229.32 (25.8%), 67.85 (22.2%), 1121.3 (22.1%), 1221.1 (17.5%), 100.1 (16.5%), 1231.01 (14.9%), 169.15 (11.4 %) |
| 182mRe | 14.14 h | 67.75 (38.3%), 1121.4 (32%), 1221.5 (25%), 1189.2 (15.1%), 100.2 (14.4%) |
| 183Re | 70 d | 162.33 (25.1%), 46.48 (8.0%) |
| 184Re | 38 d | 903.28 (38.1%), 792.06 (37.7%), 111.21 (17.2%), 894.76 (15.7%) |
| 184mRe | 169 d | 104.73 (13.4%) |
| 186Re | 3.718 d | 137.15 (9.47%) |
| Autor | Point of Analysis | Target Foil/Target Body | Identified Importunities from Foil | Identified Importunities from Target Body |
|---|---|---|---|---|
| Ferguson [13] | Synthesis cassettes | Havar/silver | 51Cr, 52,54Mn, 56,57,58Co, 95m,96Tc, 182,183Re | 109Cd |
| Bowden [14] | Silver target body “in situ” in cyclotron, synthesis cassettes, sample of irradiated [18O]H2O, before and passing through the QMA column | Havar/silver | 51Cr, 52Mn, 56,57,58Co, 95m,96Tc, 183,184Re | 110mAg 109Cd |
| Mochizuki [11] | Target foil, separation and filtration column | Havar/titan | 48V, 51Cr, 52,54Mn, 56,57,58,60Co, 95m,96Tc, 183,184Re | 46Sc, 48V |
| Guarino [12] | Target window foil (havar), titanium vacuum window | Havar and titanium/- | H: 51Cr, 52,54Mn, 56,57,58Co, Ti: 46Sc, 48V | Not analyzed |
| Köhler [15] | Sample of irradiated [18O]H2O | Niob/- | 48V, 51Cr, 52,54Mn, 55,56,57,58Co, 57Ni, 89Zr, 92mNb, 93mMo, 95,96Tc, | Not analyzed |
| Marengo [20] | Synthesis cassettes, sample of collected [18O]H2O, purification filters | Havar/silver | 48V, 51Cr, 52,54,56Mn, 55,56,57,58,60Co, 57Ni, 95,95m,96,98Tc, 182,182m,183,184,186Re | 105Ag, 106mAg, 109Cd |
| Długosz-Lisiecka [9] | Synthesis cassettes, QMA separation filter, purification filters | Havar/silver | Not analyzed | 107Cd, 109Cd |
| in this work | QMA separation filter, purification filters | Havar/silver Havar/tantal | 52Mn, 55,56,57Co, 95,95m,96Tc, 182,182m,183,184,184m,186Re | Ag: 107Cd, 109Cd Ta: 181W, 181Hf, 182Ta |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jakubowska, T.; Długosz-Lisiecka, M.; Biegała, M. Comparison of Radionuclide Impurities Activated during Irradiation of 18O-Enriched Water in Tantalum and Silver Targets during the Production of 18F in a Cyclotron. Molecules 2023, 28, 3485. https://doi.org/10.3390/molecules28083485
Jakubowska T, Długosz-Lisiecka M, Biegała M. Comparison of Radionuclide Impurities Activated during Irradiation of 18O-Enriched Water in Tantalum and Silver Targets during the Production of 18F in a Cyclotron. Molecules. 2023; 28(8):3485. https://doi.org/10.3390/molecules28083485
Chicago/Turabian StyleJakubowska, Teresa, Magdalena Długosz-Lisiecka, and Michał Biegała. 2023. "Comparison of Radionuclide Impurities Activated during Irradiation of 18O-Enriched Water in Tantalum and Silver Targets during the Production of 18F in a Cyclotron" Molecules 28, no. 8: 3485. https://doi.org/10.3390/molecules28083485
APA StyleJakubowska, T., Długosz-Lisiecka, M., & Biegała, M. (2023). Comparison of Radionuclide Impurities Activated during Irradiation of 18O-Enriched Water in Tantalum and Silver Targets during the Production of 18F in a Cyclotron. Molecules, 28(8), 3485. https://doi.org/10.3390/molecules28083485






