S-Scheme 2D/2D Heterojunction of ZnTiO3 Nanosheets/Bi2WO6 Nanosheets with Enhanced Photoelectrocatalytic Activity for Phenol Wastewater under Visible Light
Abstract
1. Introduction
2. Results and Discussion
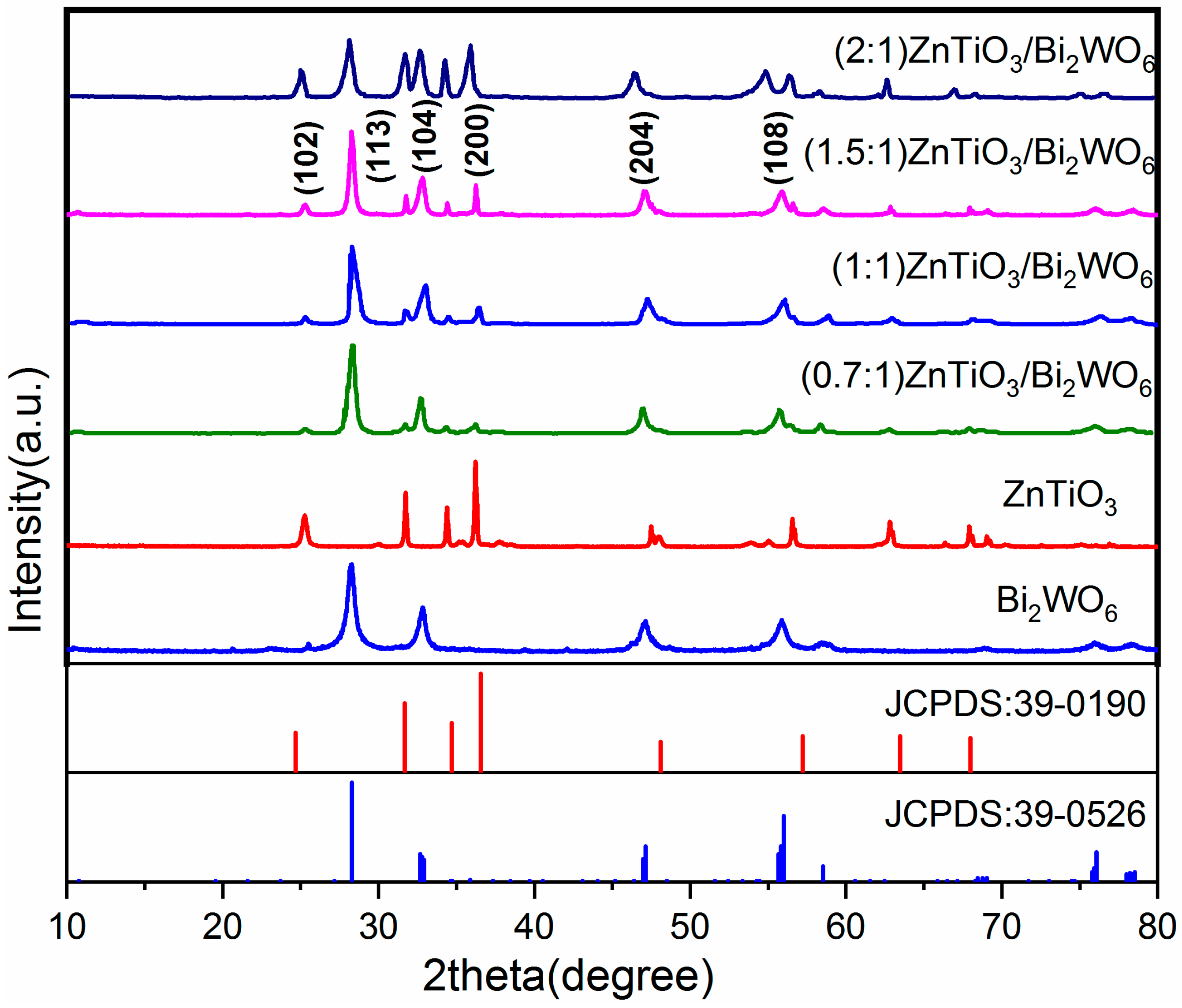
2.1. XRD Analysis
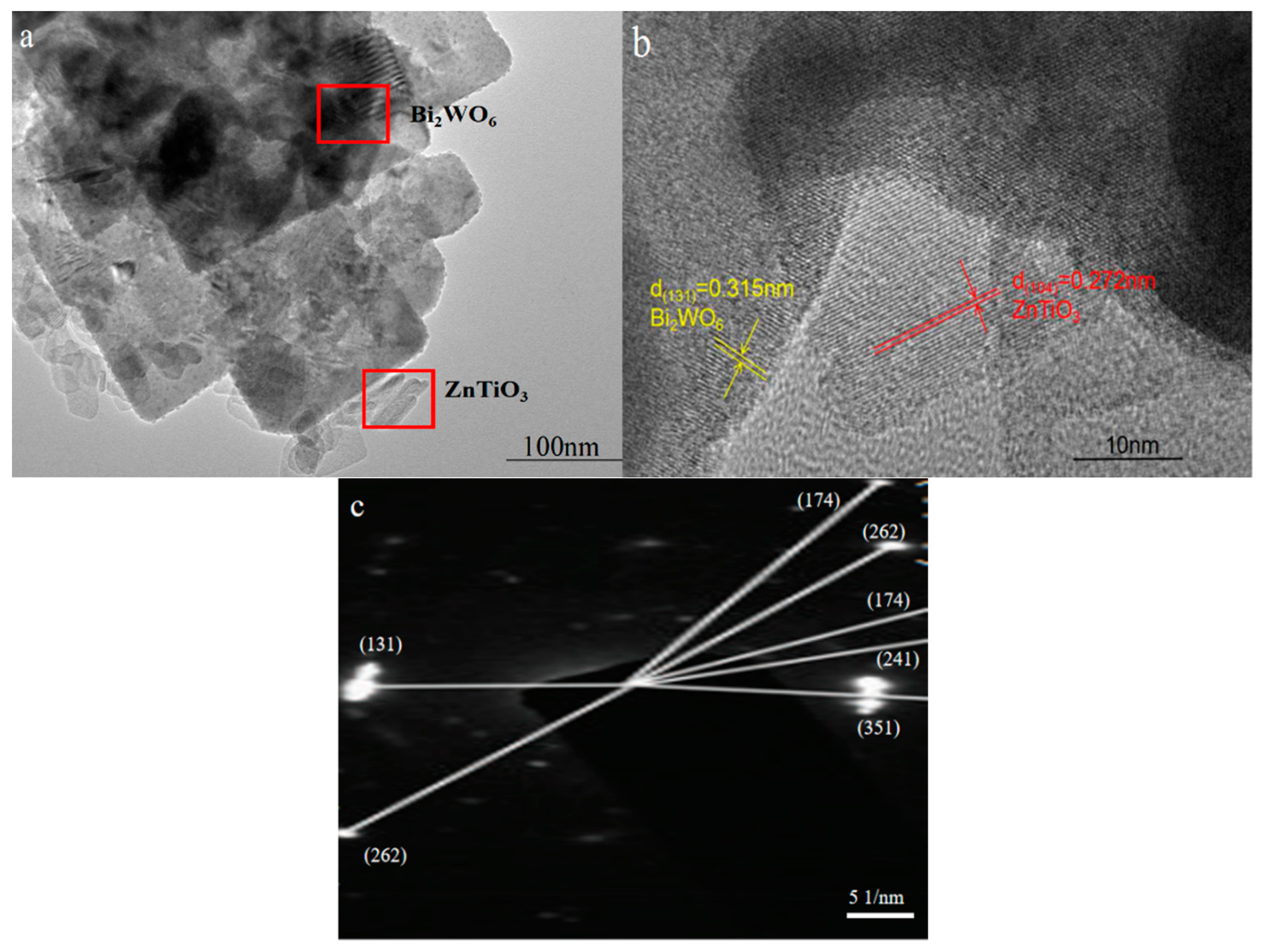
2.2. Morphology Analysis
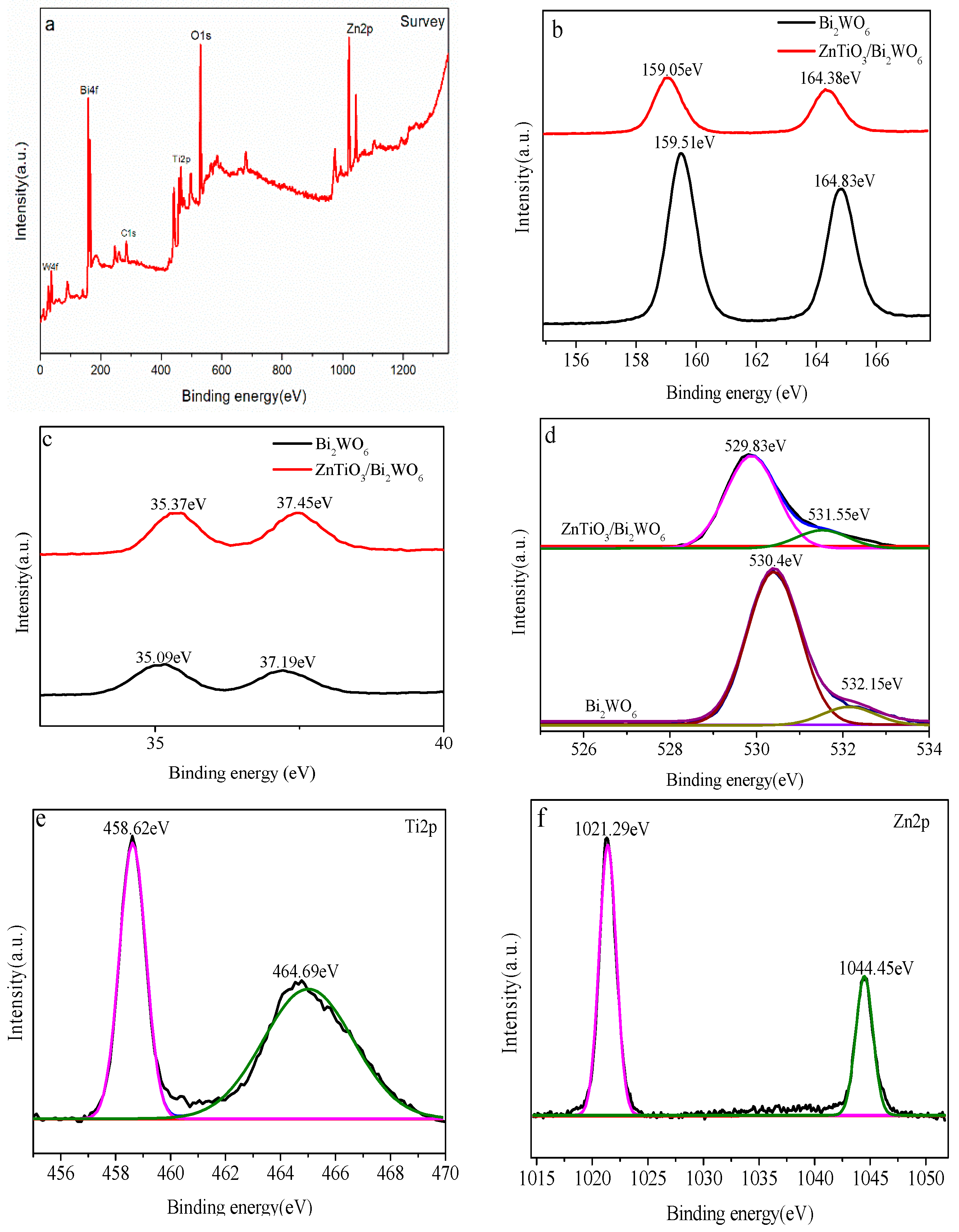
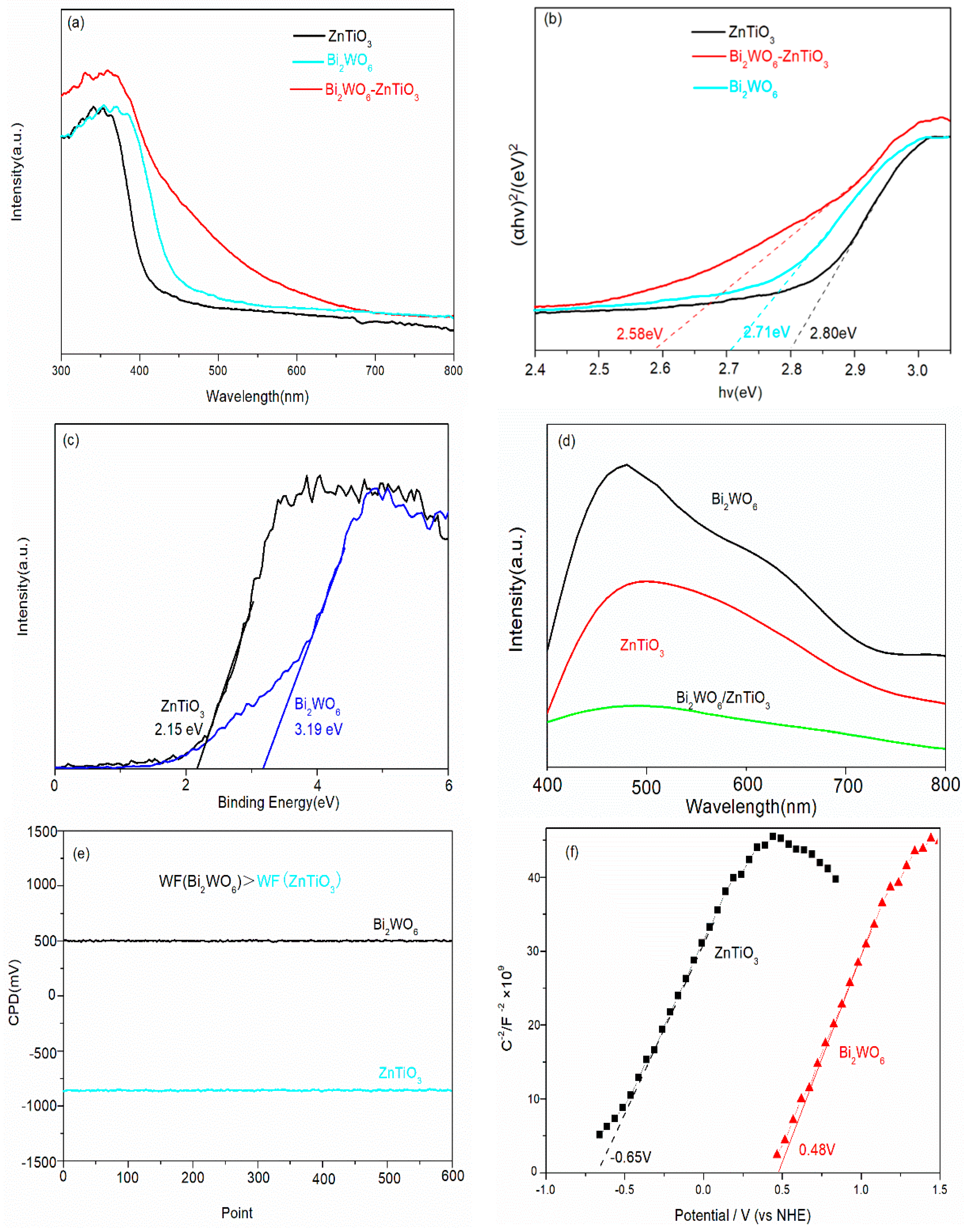
2.3. XPS Analysis
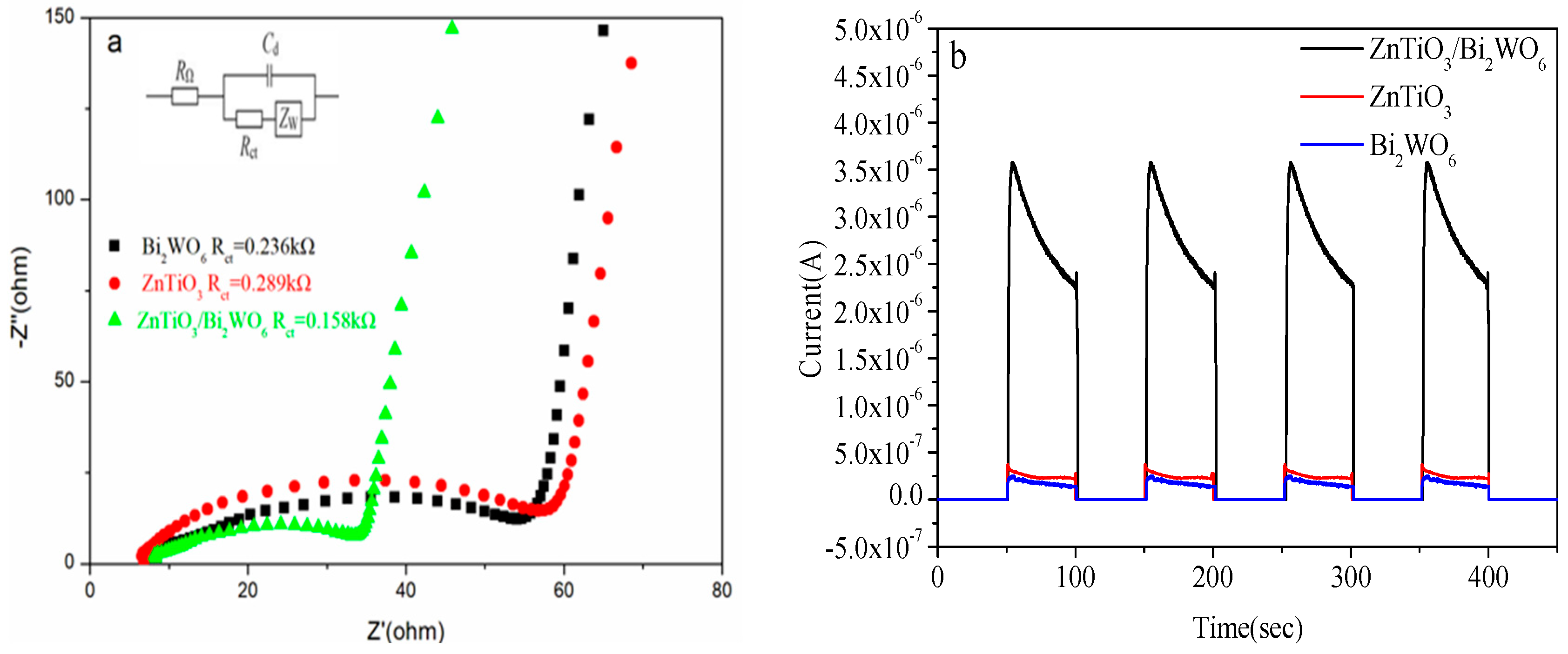
2.4. Electrochemical Analysis
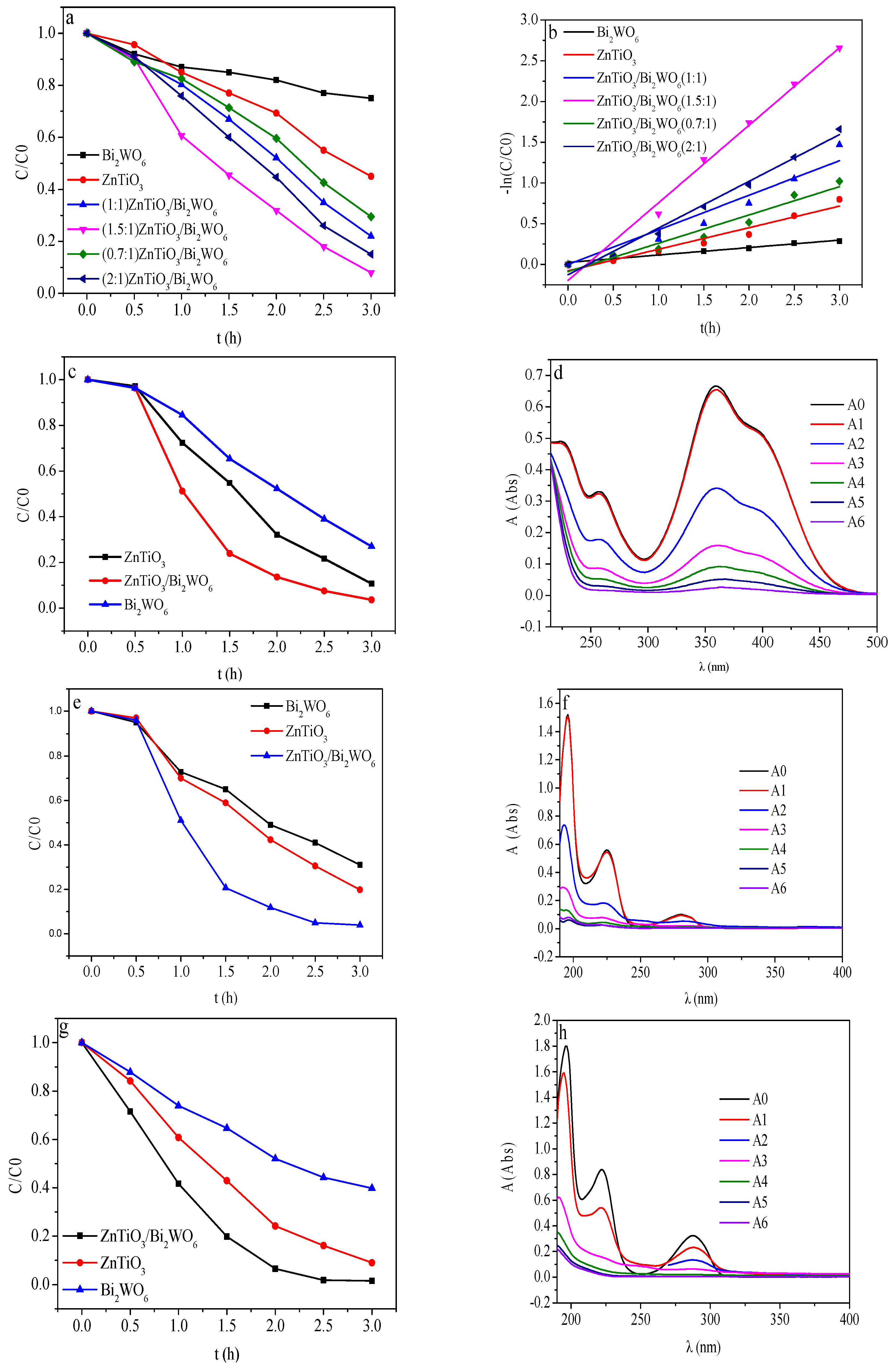
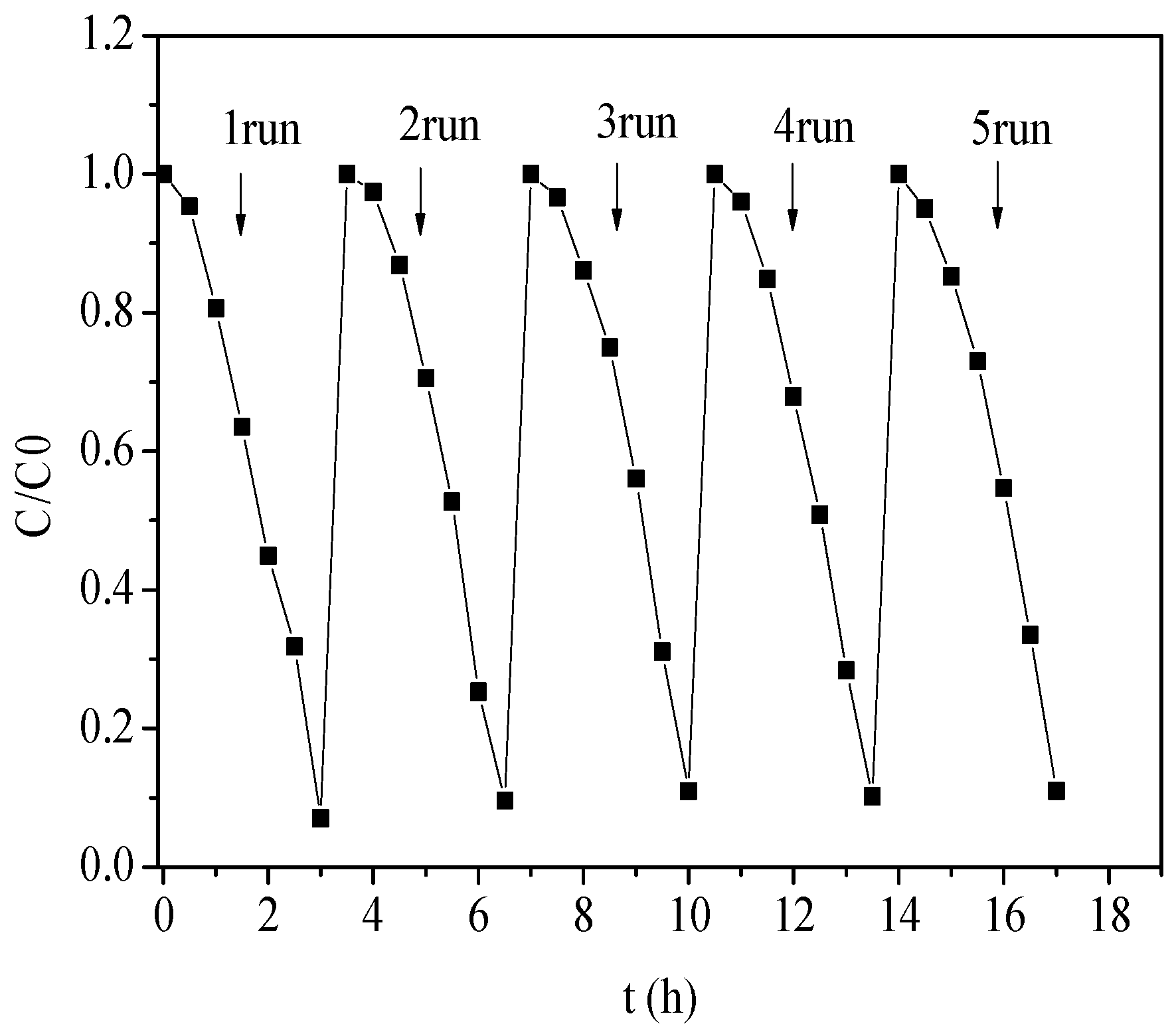
2.5. Photocatalytic Degradation of Phenolic Pollutants
| Photocatalyst | Synthesis Methods | Initial Pollutant Conc. | Light Source | Degradation (%) | Ref. |
|---|---|---|---|---|---|
| Bi7O9I3/rGO | Solvothermal | 10 mg/L | 500 W Xe lamp (cutoff filter: λ > 420 nm) | 78.3 | [40] |
| Au/BiOBr/graphene | Hydrothermal | 10 mg/L | 300 W xenon lamp (cutoff filter: λ > 400 nm) | 64 | [41] |
| Silica nanosheets (SNSs)—supported mixed phase | Hydrothermal and post-annealing | 20 mg/L | 300 W Xe lamp with a cut-off filter | 90 | [42] |
| GO/SmVO4 | Sonochemical | 1.0 × 10−4 mol dm−3 | 35 W LED lamp | 90 | [43] |
| TiO2-x/g-C3N4 nanorod arrays | Urea drop-calcined and NaBH4 reduction | 5 ppm | 300 W Xe lamp | 87 | [44] |
| g-CN@CuO | Calcination | 50 mg/L | 500 W Xe lamp with a cut-off filter | 87.8 | [45] |
| ZnTiO3/Bi2WO6 | Hydrothermal | 10 mg/L | 350 xenon lamp (λ ≥ 400 nm) | 93 | This work |
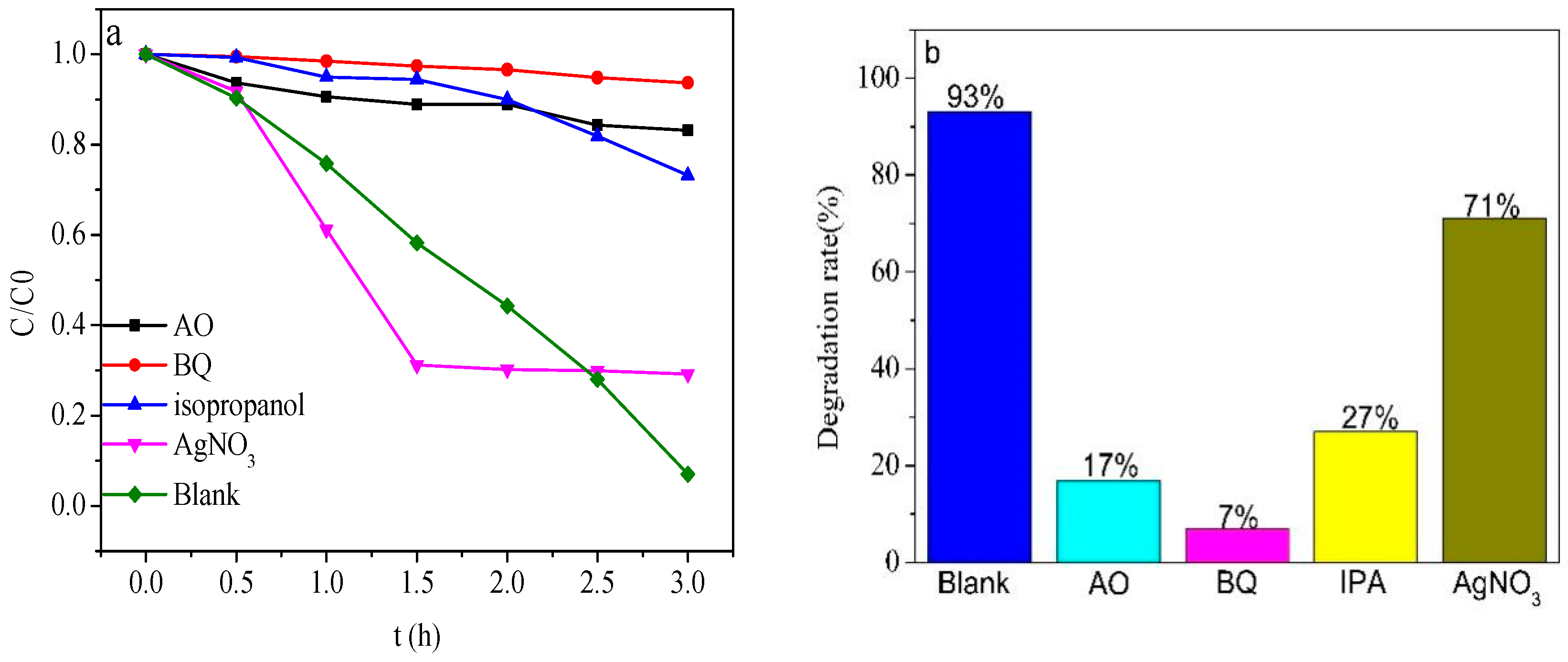
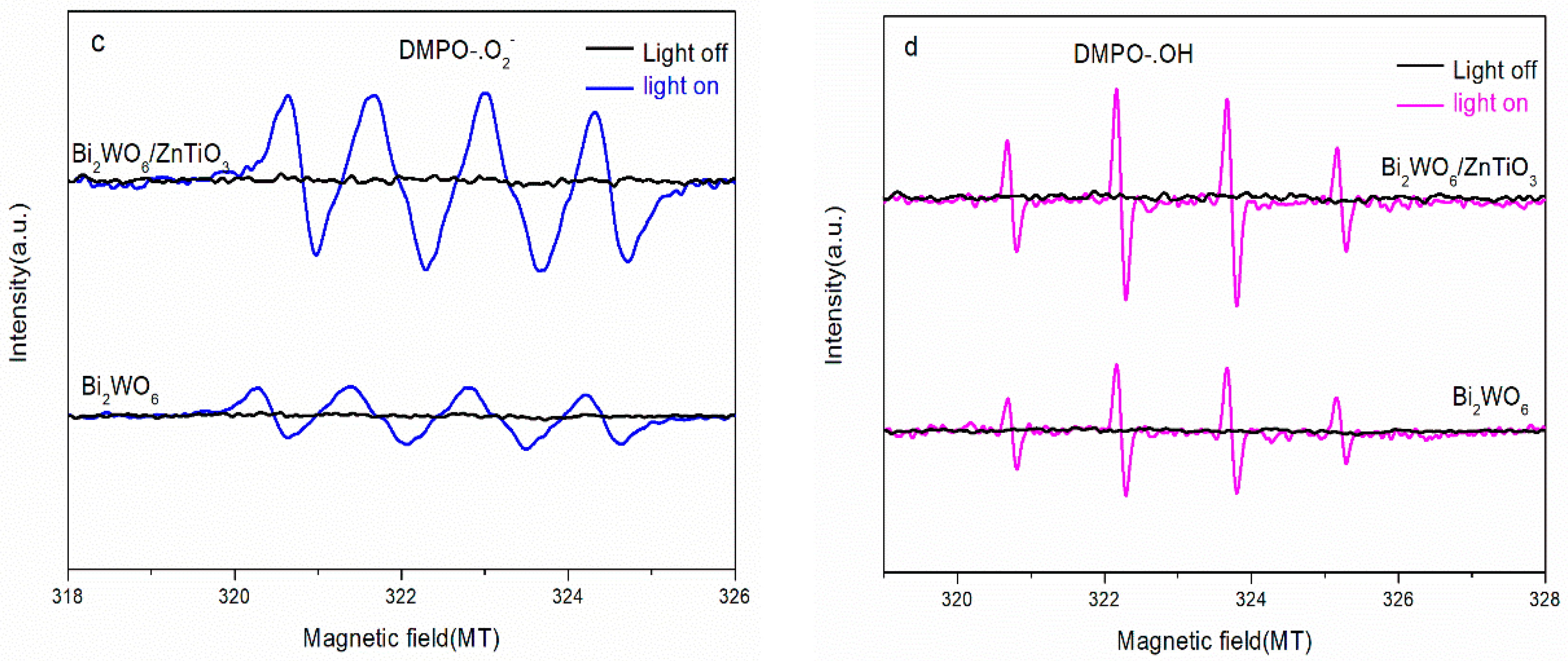
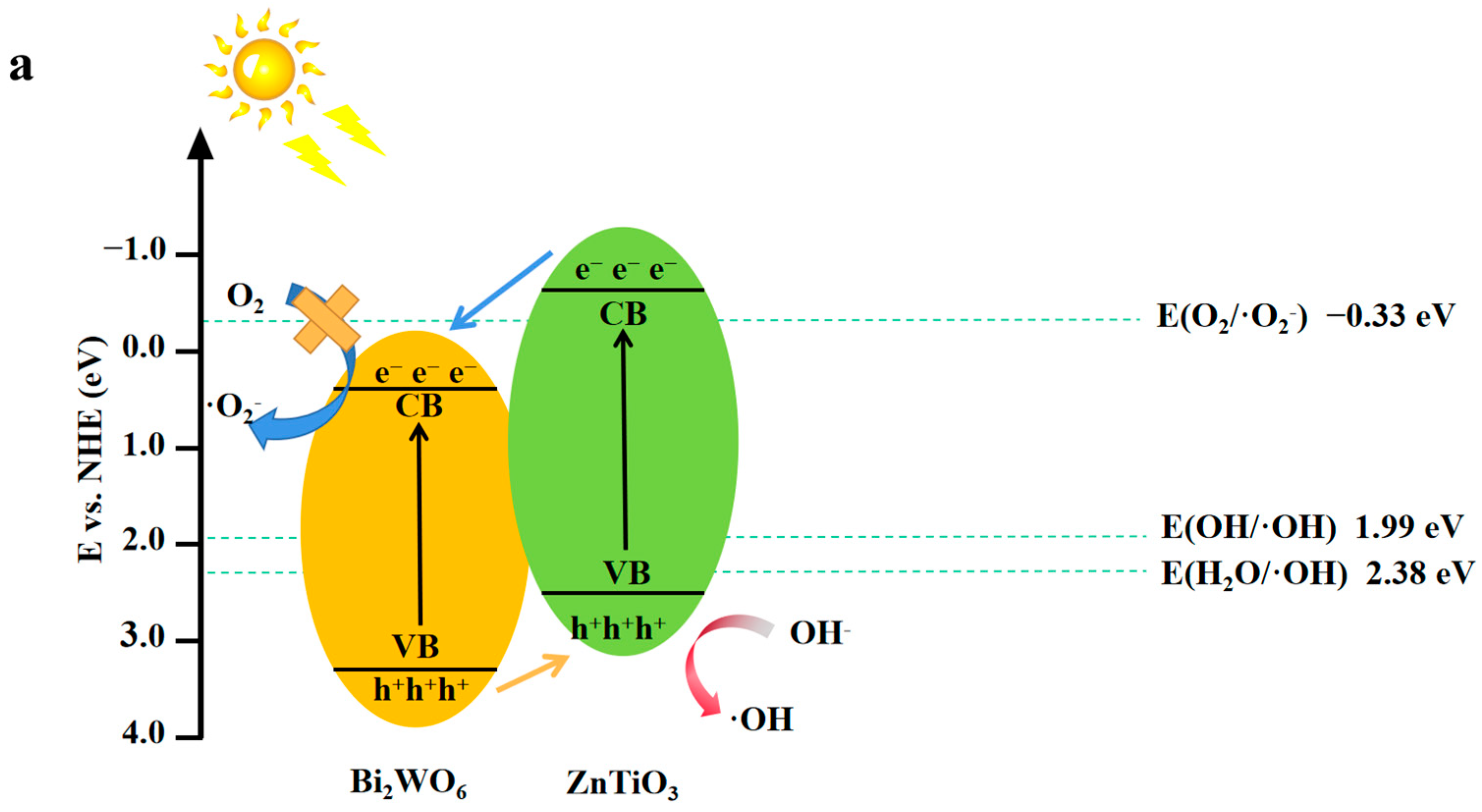
2.6. Possible Photoelectrocatalytic Mechanism
3. Experiments
3.1. Materials
3.2. Preparation of Bi2WO6 and ZnTiO3
3.3. Preparation of ZnTiO3/Bi2WO6
3.4. Photoelectrocatalytic Degradation of Phenolic Pollutants
3.5. Electrochemical Analysis
3.6. Characterization
4. Conclusions
- In this paper, 2D/2D heterojunctions of ZnTiO3 nanosheets/Bi2WO6 nanosheets were prepared for the first time by combining a hydrothermal method and a two-step calcination method, and two types of phenolic pollutants were selected. The effects of photocatalysts on electron-absorbing and electron-donating phenolic pollutants were discussed. It was confirmed that the photocatalyst had an obvious degradation effect on the electron-donating phenolic pollutants. This was because the electron-donating group could accelerate the oxidation of the ortho hydroxyl, making the benzene ring easier to decompose.
- Compared with pure Bi2WO6 (25%), the degradation rate of phenol by the ZnTiO3/Bi2WO6 photocatalyst could reach 93%, and the kinetic rate was increased by 3.6 times. The main reasons for the performance improvement were as follows: (1) 2D/2D ZnTiO3/Bi2WO6 heterojunction shortened the charge-transfer path and reduced the resistance of photogenerated electrons and holes to the surface; (2) the S-scheme heterojunction mechanism was constructed at the ZnTiO3/Bi2WO6 interface, which maintained a higher oxidation potential and reduction potential and realized the spatial separation of photogenerated carriers; and (3) the photoelectric coupling effect of the applied electric field further promoted the separation of the photogenerated carrier and improved the active free radical·OH and·O2−. This work provides a new strategy for the degradation of phenolic wastewater.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Antony, R.P.; Mathews, T. Kinetics of dye destruction using electrochemically synthesized sunlight active N-doped and C and N Co-doped TiO2 nanotubular photocatalysts, Energy Environ. Focus 2013, 2, 139–148. [Google Scholar]
- Ochiai, T.; Fujishima, A. Photoelectrochemical properties of TiO2 photocatalyst and its applications for environmental purification. J. Photochem. Photobiol. C 2012, 13, 247–262. [Google Scholar] [CrossRef]
- Zhang, H.; Lv, X. P25-Graphene Composite as a High Performance Photocatalyst. ACS Nano 2010, 4, 380–386. [Google Scholar] [CrossRef]
- Zhou, W.; Fu, H. ChemInform Abstract: Mesoporous TiO2: Preparation, Doping, and as a Composite for Photocatalysis. ChemCatChem 2013, 5, 885–894. [Google Scholar] [CrossRef]
- Hou, Y.; Li, X. Photoeletrocatalytic activity of a Cu2O loaded self-organized highly oriented TiO2 nanotube array electrode for 4-chlorophenol degradation. Environ. Sci. Technol. 2009, 43, 858–863. [Google Scholar] [CrossRef]
- Chen, X.; Liu, L.; Yu, P.Y.; Mao, S.S. Increasing Solar Absorption for Photocatalysis with Black Hydrogenated Titanium Dioxide Nanocrystals. Science 2011, 331, 746–750. [Google Scholar] [CrossRef]
- Luo, J.M.; Dong, G.; Zhu, Y. Switching of semiconducting behavior from n-type to p-type induced high photocatalytic NO removal activity in g-C3N4. Appl. Catal. B Environ. 2017, 214, 46–56. [Google Scholar] [CrossRef]
- Deng, F.; Zhang, Q.; Yang, L. Visible-light-responsive graphene-functionalized Bi-bridge Z-scheme black BiOCl/Bi2O3 heterojunction with oxygen vacancy and multiple charge transfer channels for effificient photocatalytic degradation of 2-nitrophenol and industrial wastewater treatment. Appl. Catal. B Environ. 2018, 238, 61–69. [Google Scholar] [CrossRef]
- Tada, H.; Mitsui, T.; Kiyonaga, T.; Akita, T.; Tanaka, K. All-solid-state Z-scheme in CdS-Au-TiO2 three-component nanojunction system. Nat. Mater. 2006, 5, 782–786. [Google Scholar] [CrossRef]
- Zhu, Y.; Xu, D.; Meng, M. Ultrasonic-assisted synthesis of amorphous Bi2S3 coupled (BiO)2CO3 catalyst with improved visible light-responsive photocatalytic activity. J. Mater. Sci. 2015, 50, 1594–1604. [Google Scholar] [CrossRef]
- Meng, X.C.; Li, Z.Z.; Zeng, H.M.; Chen, J.; Zhang, Z.S. MoS2 quantum dots-interspersed Bi2WO6 heterostructures for visible light-induced detoxifification and disinfection. Appl. Catal. B Environ. 2017, 210, 160–172. [Google Scholar] [CrossRef]
- Fu, J.W.; Xu, Q.L.; Low, J.X. Ultrathin 2D/2D WO3/g-C3N4 step-scheme H2-production photocatalyst. Appl. Catal. B Environ. 2019, 243, 556–565. [Google Scholar] [CrossRef]
- Bao, Y.J.; Song, S.Q.; Yao, G.J. S-Scheme Photocatalytic Systems. Sol. RRL 2021, 5, 2100118. [Google Scholar] [CrossRef]
- Zhang, L.Y.; Zhang, J.J.; Yu, H.G. Emerging S-Scheme Photocatalyst. Adv. Mater. 2022, 34, 2107668. [Google Scholar] [CrossRef] [PubMed]
- Xu, Q.L.; Zhang, L.Y.; Cheng, B. S-Scheme Heterojunction Photocatalyst. Chem 2020, 6, 1543–1559. [Google Scholar] [CrossRef]
- Wang, J.; Wang, G.H.; Cheng, B. Sulfur-doped g-C3N4/TiO2 S-scheme heterojunction photocatalyst for Congo Red photodegradation. Chin. J. Catal. 2021, 42, 56–68. [Google Scholar] [CrossRef]
- Chen, R.F.; Zhou, W.; Qu, W.W.; Wang, Y.J. A novel hierarchical nanostructured S-scheme RGO/Bi2MoO6/Bi2WO6 heterojunction: Excellent photocatalytic degradation activity for pollutants. Appl. Surf. Sci. 2022, 588, 152788. [Google Scholar] [CrossRef]
- Tang, D.Y.; Xu, D.S.; Luo, Z.P. Highly Dispersion Cu2O QDs Decorated Bi2WO6 S-Scheme Heterojunction for Enhanced Photocatalytic Water Oxidation. Nanomaterials 2022, 12, 2455. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.F.; Xia, Z.L.; Yang, Q. Review on g-C3N4-based S-scheme heterojunction photocatalysts. J. Mater. Sci. Technol. 2022, 125, 128–144. [Google Scholar] [CrossRef]
- Han, X.X.; Lu, B.J.; Huang, X. Novel p- and n-type S-scheme heterojunction photocatalyst for boosted CO2 photoreduction activity. Appl. Catal. B Environ. 2022, 316, 121587. [Google Scholar] [CrossRef]
- Reddy, K.H.; Martha, S.; Parida, K.M. Erratic charge transfer dynamics of Au/ZnTiO3 nanocomposites under UV and visible light irradiation and their related photocatalytic activities. Nanoscale 2018, 10, 18540–18554. [Google Scholar] [CrossRef] [PubMed]
- Anwer, H.; Mahmood, A.; Lee, J. Photocatalysts for degradation of dyes in industrial effluents: Opportunities and challenges. Nano Res. 2019, 12, 955–972. [Google Scholar] [CrossRef]
- Chen, F.; Yu, C.; Wei, L. Fabrication and characterization of ZnTiO3/Zn2Ti3O8/ZnO ternary photocatalyst for synergetic removal of aqueous organic pollutants and Cr(VI) ions. Sci. Total Environ. 2019, 706, 136026. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Li, D.; Chai, Y. Rational design and preparation of nanoheterostructures based on zinc titanate for solar-driven photocatalytic conversion of CO2 to valuable fuels. Appl. Catal. B-Environ. 2019, 5, 117800. [Google Scholar] [CrossRef]
- Yu, C.; Chen, F.; Zhou, W. A facile phase transformation strategy for fabrication of novel Z-scheme ternary heterojunctions with efficient photocatalytic properties. Nanoscale 2019, 11, 7720–7733. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Tan, G.; Yang, W. Synthesis and Photocatalytic Properties of N/Bi2WO6 Flower-like Crystallites Self-Assembled from Nanoflflakes. J. Clust. Sci. 2013, 24, 1139–1149. [Google Scholar] [CrossRef]
- Chi, Y.; Yuan, Q.; Hou, S.; Zhao, Z. Synthesis and characterization of mesoporous ZnTiO3 rods via a polyvinylpyrrolidone assisted sol–gel method. Ceram. Int. 2016, 42, 5094–5099. [Google Scholar] [CrossRef]
- Meng, X.C.; Zhang, Z.S. Synthesis and characterization of plasmonic and magnetically separable Ag/AgCl-Bi2WO6@ Fe3O4@SiO2 core-shell composites for visible light-induced water detoxification. J. Colloid Interface Sci. 2017, 485, 296–307. [Google Scholar] [CrossRef] [PubMed]
- Raveendra, R.S.; Prashantha, P.A.; Krishnac, R.H. Synthesis, structural characterization of nano ZnTiO3 ceramic: An effective azo dye adsorbent and antibacterial agent. J. Asian Ceram. Soc. 2014, 2, 357–365. [Google Scholar] [CrossRef]
- Hu, J.; Chen, D.; Mo, Z. Z-Scheme 2D/2D Heterojunction of Black Phosphorus/ Monolayer Bi2WO6 Nanosheets with Enhanced Photocatalytic Activities. Angew. Chem. Int. Ed. 2019, 58, 2073–2077. [Google Scholar] [CrossRef]
- Xu, J.; Yue, J.; Niu, J. Fabrication of Bi2WO6 quantum dots/ultrathin nanosheets 0D/2D homojunctions with enhanced photocatalytic activity under visible light irradiation. Chin. J. Catal. 2018, 39, 1910–1918. [Google Scholar] [CrossRef]
- Zhu, Z.L.; Ren, Y.; Li, Q. One-pot electrodeposition synthesis of Bi2WO6/graphene composites for photocatalytic applications under visible light irradiation. Ceram. Int. 2018, 44, 3511–3516. [Google Scholar] [CrossRef]
- Wang, R.S.; Li, B.; Xiao, Y. Optimizing Pd and Au-Pd decorated Bi2WO6 ultrathin nanosheets for photocatalytic selective oxidation of aromatic alcohols. J. Catal. 2018, 364, 154–165. [Google Scholar] [CrossRef]
- He, W.; Sun, Y.; Jiang, G. Activation of amorphous Bi2WO6 with synchronous Bi metal and Bi2O3 coupling: Photocatalysis mechanism and reaction pathway. Appl. Catal. B Environ. 2018, 232, 340–347. [Google Scholar] [CrossRef]
- Ranjith, K.S.; Uyar, T. Conscientious Design of Zn-S/Ti N Layer by Transformation of ZnTiO3 on Electrospun ZnTiO3@TiO2 Nanofifibers: Stability and Reusable Photocatalytic Performance under Visible Irradiation. ACS Sustain. Chem. Eng. 2018, 6, 12980–12992. [Google Scholar] [CrossRef]
- Qian, W.; Greaney, P. Low-Temperature Nitrogen Doping in Ammonia Solution for Production of N-Doped TiO2-Hybridized Graphene as a Highly Efficient Photocatalyst for Water Treatment. ACS Sustain. Chem. Eng. 2014, 2, 1802–1810. [Google Scholar] [CrossRef]
- Long, G.Y.; Ding, G.Y. Fabrication of mediator-free g-C3N4/Bi2WO6 Z-scheme with enhanced photocatalytic reduction dechlorination performance of 2,4-DCP. Appl. Surf. Sci. 2018, 455, 1010–1018. [Google Scholar] [CrossRef]
- Zhang, Z.; Shen, Q.H.; Cissoko, N. Catalytic dechlorination of 2,4-dichlorophenol by Pd/Fe bimetallic nanoparticles in the presence of humic acid. J. Hazard. Mater. 2010, 182, 252–258. [Google Scholar] [CrossRef] [PubMed]
- Arellano-Gonzalez, M.A.; Texier, A.C.; Lartundo-Rojas, L.; Gonzalez, I. Electrochemical dechlorination of 2-chlorophenol on Pd/Ti, Ni/Ti and Pd-Ni Alloy/Ti electrodes. J. Electrochem. Soc. 2015, 162, 223–230. [Google Scholar] [CrossRef]
- Gawande, S.B.; Gawande, K.B.; Thakare, S.R. Photocatalytic degradation of phenol over novel rod shaped graphene@BiPO4 nanocomposite. J. Phys. Chem. Solids 2015, 85, 132–137. [Google Scholar] [CrossRef]
- Yu, X.; Wang, L.; Feng, L.J. Preparation of Au/BiOBr/Graphene composite and its photocatalytic performance in phenol degradation under visible light. J. Fuel. Chem. Tech. 2016, 44, 937–942. [Google Scholar] [CrossRef]
- Wang, L.; Wang, X.; Yin, J. Silica induced oxygen vacancies in supported mixed-phase TiO2 for photocatalytic degradation of phenol under visible light irradiation. Catal. Commun. 2016, 87, 98–101. [Google Scholar] [CrossRef]
- Shandilya, P.; Mittal, D.; Soni, M. Fabrication of fuorine doped graphene and SmVO4 based dispersed and adsorptive photocatalyst for abatement of phenolic compounds from water and bacterial disinfection. J. Clean Prod. 2018, 203, 386–399. [Google Scholar] [CrossRef]
- Qi, F.; An, W.J.; Wang, H. Combing oxygen vacancies on TiO2 nanorod arrays with g-C3N4 nanosheets for enhancing photoelectrochemical degradation of phenol. Mater. Sci. Semicond. Process 2020, 109, 104954. [Google Scholar] [CrossRef]
- Baishnisha, A.; Divakaran, K.; Balakumar, V. Synthesis of highly efcient g-CN@CuO nanocomposite for photocatalytic degradation of phenol under visible light. J. Alloy Compd. 2021, 886, 161167. [Google Scholar] [CrossRef]
- Chen, W.; Liu, T.Y.; Huang, T. In situ fabrication of novel Z-scheme Bi2WO6 quantum dots/g-C3N4 ultrathin nanosheets heterostructures with improved photocatalytic activity. Appl. Surf. Sci. 2015, 355, 379–387. [Google Scholar] [CrossRef]
- Jiang, D.L.; Wan, X. Enhanced photocatalytic activity of graphitic carbon nitride/carbon nanotube/Bi2WO6 ternary Z-scheme heterojunction with carbon nanotube as efficient electron mediator. J. Colloid Interface Sci. 2018, 512, 693–700. [Google Scholar] [CrossRef]
- Guo, H.P.; Hayakawa, T.; Nogami, M.; Hao, Z. Zinc titanium glycolate acetate hydrate and its transformation to zinc titanate microrods: Synthesis, characterization and photocatalytic properties. Rsc Adv. 2015, 5, 88590–88601. [Google Scholar]
- Li, X.B.; Xiong, J.; Huang, J.T. Novel g-C3N4/ h′ ZnTiO3-a′ TiO2 direct Z-scheme heterojunction with significantly enhanced visible-light photocatalytic activity. J. Alloy. Compd. 2019, 774, 768–778. [Google Scholar] [CrossRef]
- Ruan, X.; Hu, Y.Y. Effectively enhanced photodegradation of Bisphenol A by in-situ g-C3N4-Zn/Bi2WO6 heterojunctions and mechanism study. Chemosphere 2020, 246, 125782. [Google Scholar] [CrossRef] [PubMed]
- Li, B.R.; Cui, Y.H.; Feng, Y. Study of enhanced photocatalytic performance mechanisms towards a new binary-Bi heterojunction with spontaneously formed interfacial defects. Appl. Surf. Sci. 2020, 532, 147412. [Google Scholar] [CrossRef]



| Element | Weight % | Atomic % |
|---|---|---|
| O K | 46.25 | 90.12 |
| Ti K | 2.16 | 1.41 |
| Zn K | 1.30 | 0.62 |
| W M | 17.20 | 2.92 |
| Bi M | 33.09 | 4.93 |
| Totals | 100.00 | 100.00 |
| Photoelectrocatalysts | Degradation Rate | Slope | R2 | Kinetic Equation |
|---|---|---|---|---|
| Bi2WO6 | 25% | 0.0913 | 0.986 | Y = 0.0913X + 0.02486 |
| ZnTiO3 | 55% | 0.264 | 0.976 | Y = 0.264X − 0.07816 |
| ZnTiO3/Bi2WO6(1:1) | 77% | 0.424 | 0.989 | Y = 0.424X |
| ZnTiO3/Bi2WO6(1.5:1) | 93% | 0.9517 | 0.992 | Y = 0.9517X − 0.195 |
| ZnTiO3/Bi2WO6(0.7:1) | 71% | 0.3472 | 0.971 | Y = 0.3472X − 0.086 |
| ZnTiO3/Bi2WO6(2:1) | 81% | 0.5733 | 0.992 | Y = 0.5733X − 0.126 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zuo, C.; Tai, X.; Jiang, Z.; Liu, M.; Jiang, J.; Su, Q.; Yan, X. S-Scheme 2D/2D Heterojunction of ZnTiO3 Nanosheets/Bi2WO6 Nanosheets with Enhanced Photoelectrocatalytic Activity for Phenol Wastewater under Visible Light. Molecules 2023, 28, 3495. https://doi.org/10.3390/molecules28083495
Zuo C, Tai X, Jiang Z, Liu M, Jiang J, Su Q, Yan X. S-Scheme 2D/2D Heterojunction of ZnTiO3 Nanosheets/Bi2WO6 Nanosheets with Enhanced Photoelectrocatalytic Activity for Phenol Wastewater under Visible Light. Molecules. 2023; 28(8):3495. https://doi.org/10.3390/molecules28083495
Chicago/Turabian StyleZuo, Cheng, Xishi Tai, Zaiyong Jiang, Meifang Liu, Jinhe Jiang, Qian Su, and Xueyuan Yan. 2023. "S-Scheme 2D/2D Heterojunction of ZnTiO3 Nanosheets/Bi2WO6 Nanosheets with Enhanced Photoelectrocatalytic Activity for Phenol Wastewater under Visible Light" Molecules 28, no. 8: 3495. https://doi.org/10.3390/molecules28083495
APA StyleZuo, C., Tai, X., Jiang, Z., Liu, M., Jiang, J., Su, Q., & Yan, X. (2023). S-Scheme 2D/2D Heterojunction of ZnTiO3 Nanosheets/Bi2WO6 Nanosheets with Enhanced Photoelectrocatalytic Activity for Phenol Wastewater under Visible Light. Molecules, 28(8), 3495. https://doi.org/10.3390/molecules28083495





