Development and Characterization of New Green Propolis Extract Formulations as Promising Candidates to Substitute for Green Propolis Hydroalcoholic Extract
Abstract
:1. Introduction
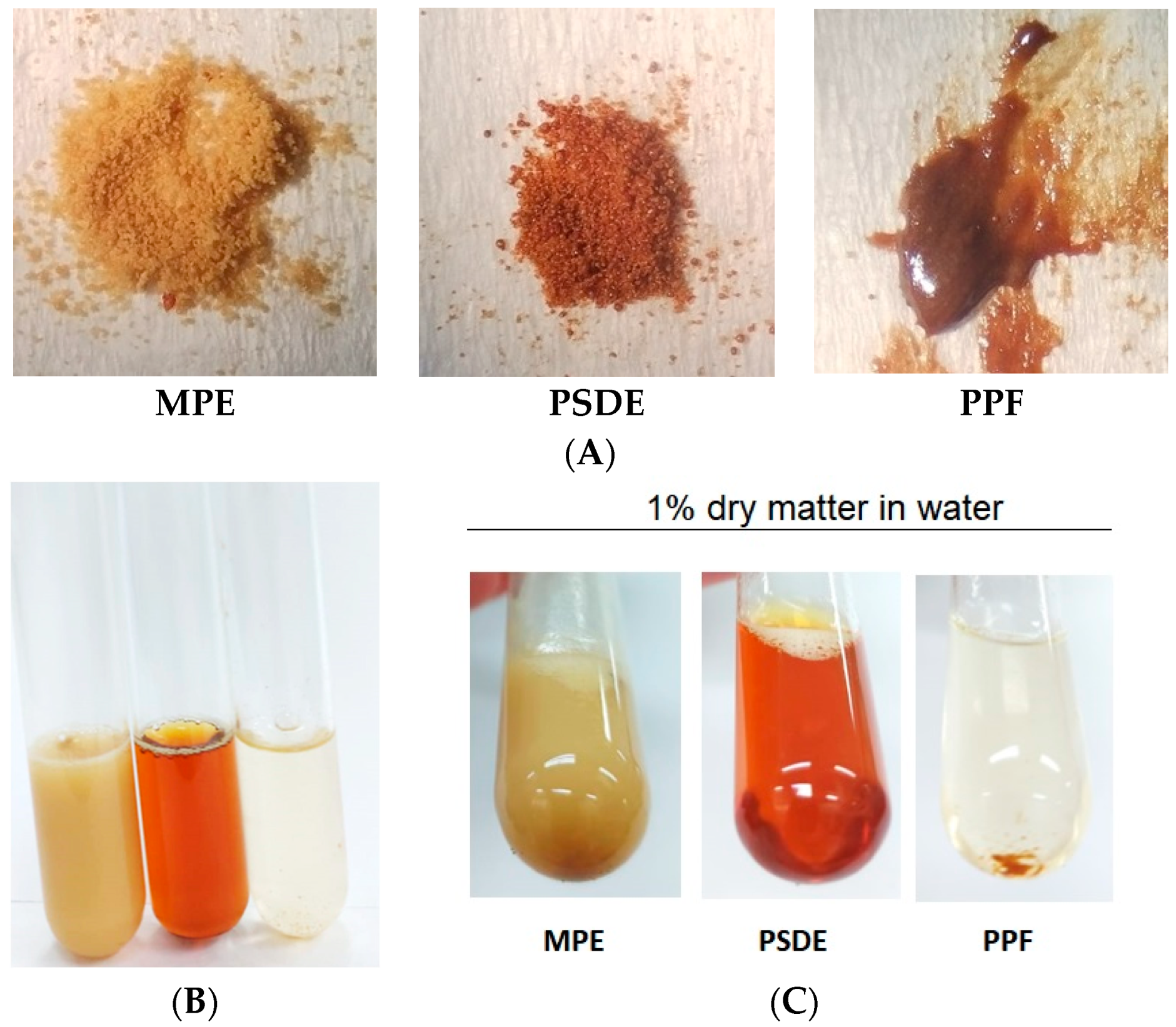
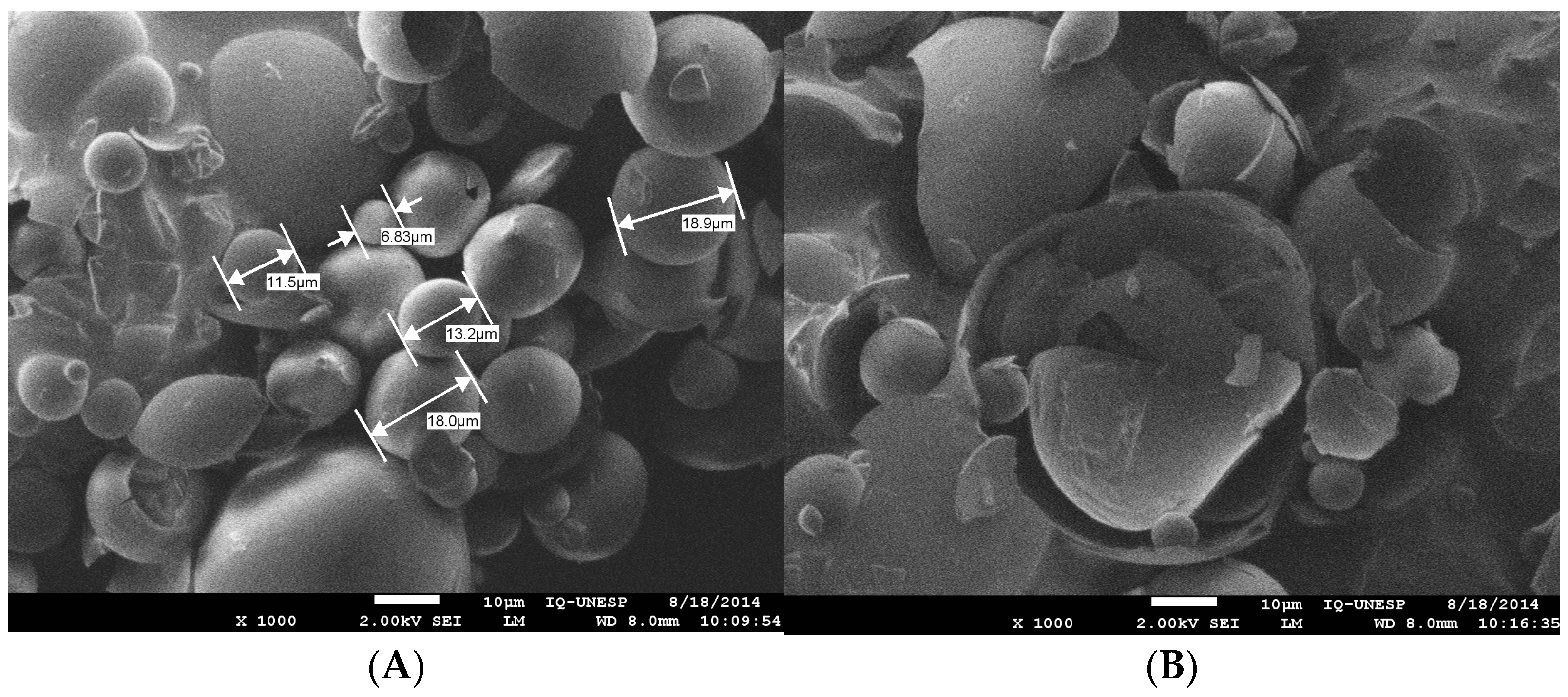
2. Results
2.1. Chemical Characterization of the Propolis Extracts
2.2. Antioxidant Activity
2.3. Antimicrobial Activity
3. Discussion
4. Materials and Methods
4.1. Preparation of Propolis Extracts
4.2. Chemical Markers
4.3. Chemical Characterization of the Propolis Extracts
4.3.1. HPLC Analysis
4.3.2. Determination of Total Phenol Content
4.3.3. Determination of Total Flavonoid Content
4.4. Antioxidant Evaluation
4.4.1. DPPH Free Radical Scavenging Method
4.4.2. Ferric Reducing Antioxidant Power Assay (FRAP)
4.5. Antimicrobial Activity Determination—Broth Microdilution Method
4.6. Statistical Analysis
5. Conclusions
6. Patents
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Nani, B.D.; Sardi, J.C.O.; Lazarini, J.G.; Silva, D.R.; Massariolli, A.P.; Cunha, T.M.; Alencar, S.M.; Franchin, M.; Rosalen, P.L. Anti-inflamatory and anti-Candida Effects of Brazilian Organic Propolis, a Promising Source of Bioactive Molecules and Functional Food. J. Agric. Food Chem. 2019, 68, 2861–2871. [Google Scholar] [CrossRef] [PubMed]
- Bankova, V. Chemical diversity of propolis and the problem of standardization. J. Ethnopharmacol. 2005, 100, 114–117. [Google Scholar] [CrossRef]
- Bankova, V. Recent trends and important developments in propolis research. Evid. Based Complement. Alternat. Med. 2005, 2, 29–32. [Google Scholar] [CrossRef] [PubMed]
- Berretta, A.A.; Arruda, C.; Miguel, F.G.; Baptista, N.; Nascimento, A.P.; Marquele-Oliveira, F.; Hori, J.I.; Barud, H.S.; Damaso, B.; Ramos, C.; et al. Functional Properties of Brazilian Propolis: From Chemical Composition Until the Market. In Superfood and Functional Food—An Overview of Their Processing and Utilization, 1st ed.; Waisundara, V.Y., Shiomi, N., Eds.; Intech: London, UK, 2017; p. 356. [Google Scholar]
- Salatino, A. Perspectives for Uses of Propolis in Therapy against Infectious Diseases. Molecules 2022, 27, 4594. [Google Scholar] [CrossRef] [PubMed]
- Berretta, A.A.; Nascimento, A.P.; Bueno, P.C.P.; Vaz, M.M.O.L.L.; Marchetti, J.M. Propolis Standardized Extract (EPP-AF®), an Innovative Chemically and Biologically Reproducible Pharmaceutical Compound for Treating Wounds. Int. J. Biol. Sci. 2012, 8, 512–521. [Google Scholar] [CrossRef] [PubMed]
- Berretta, A.A.; Castro, P.A.; Cavalheiro, A.H.; Fortes, V.S.; Bom, V.P.; Nascimento, A.P.; Marquele-oliveira, F.; Pedrazzi, V.; Ramalho, L.N.Z.; Goldman, G.H. Evaluation of Mucoadhesive Gels with Propolis (EPP-AF) in Preclinical Treatment of Candidiasis Vulvo vaginal Infection. Evid. Based Complement. Alternat Med. 2013, 2013, 641480. [Google Scholar] [CrossRef] [PubMed]
- Hori, J.I.; Zamboni, D.S.; Carrão, D.B.; Goldman, G.H.; Berretta, A.A. The Inhibition of Inflammasome by Brazilian Propolis (EPP-AF). Evid. Based Complement. Alternat. Med. 2013, 2013, 418508. [Google Scholar] [CrossRef] [PubMed]
- Machado, J.L.; Assunção, A.K.M.; Silva, M.C.P.; Reis, A.S.; Costa, G.C.; Arruda, D.S.; Rocha, B.A.; Vaz, M.M.O.L.L.; Paes, A.M.A.; Guerra, R.N.M.; et al. Brazilian Green Propolis: Anti-Inflammatory Property by an Immunomodulatory Activity. Evid. Based Complement. Alternat. Med. 2012, 2012, 157652. [Google Scholar] [CrossRef] [PubMed]
- Barud, H.S.; Araújo Jr, A.M.; Saska, S.; Mestieri, L.B.; Campos, J.A.D.B.; Freitas, R.M.; Ferreira, N.U.; Nascimento, A.P.; Miguel, F.G.; Vaz, M.M.O.L.L.; et al. Antimicrobial Brazilian Propolis (EPP-AF) Containing Biocellulose Membranes as Promising Biomaterial for Skin Wound Healing. Evid. Based Complement. Alternat. Med. 2013, 2013, 703024. [Google Scholar] [CrossRef] [PubMed]
- De Castro, P.A.; Savoldi, M.; Bonatto, D.; Barros, M.H.; Goldman, M.H.S.; Berretta, A.A.; Goldman, G.H. Molecular Characterization of Propolis-Induced Cell Death in Saccharomyces cerevisiae. Eukaryot. Cell 2011, 10, 398–411. [Google Scholar] [CrossRef] [PubMed]
- Berretta, A.A.; Silveira, M.A.D.; Capcha, J.M.C.; De Jong, D. Propolis and its potential against SARS-CoV-2 infection mechanisms and COVID-19 disease Running title: Propolis against SARS-CoV-2 infection and COVID-19. Biomed. Pharmacother. 2020, 131, 110622. [Google Scholar] [CrossRef] [PubMed]
- Bachiega, T.F.; De Sousa, J.P.B.; Bastos, J.K.; Sforcin, J.M. Immunomodulatory/anti-inflammatory effects of Baccharis dracunculifolia leaves. Nat. Prod. Res. 2013, 27, 1646–1650. [Google Scholar] [CrossRef] [PubMed]
- Beserra, F.P.; Gushiken, L.F.S.; Hussni, M.F.; Ribeiro, V.P.; Bonamin, F.; Jackson, C.J.; Pellizzon, C.H.; Bastos, J.K. Artepillin C as an outstanding phenolic compound of Brazilian green propolis for disease treatment: A review on pharmacological aspects. Phytother. Res. 2020, 35, 2274–2286. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, J.C.; Reis, M.B.; Coelho, G.D.P.; Gastaldello, G.H.; Peti, A.P.F.; Rodrigues, D.M.; Bastos, J.K.; Campo, V.L.; Sorgi, C.A.; Faccioli, L.H.; et al. Baccharin and p-coumaric acid from green propolis mitigate inflammation by modulating the production of cytokines and eicosanoids. J. Ethnopharmacol. 2021, 278, 114255. [Google Scholar] [CrossRef] [PubMed]
- De Sousa, J.P.B.; Filho, A.A.S.; Bueno, P.C.P.; Gregório, L.E.; Furtado, N.A.J.C.; Jorge, R.F.; Bastos, J.K. A validated reverse-phase HPLC analytical method for the quantification of phenolic compounds in Baccharis dracunculifolia. Phytochem. Anal. 2008, 20, 24–32. [Google Scholar] [CrossRef]
- Rocha, B.A.; Bueno, P.C.P.; Vaz, M.M.O.L.L.; Nascimento, A.P.; Ferreira, N.U.; Moreno, G.P.; Rodrigues, M.R.; Costa-Machado, A.R.M.; Barizon, E.A.; Campos, J.C.L.; et al. Evaluation of a Propolis Water Extract Using a Reliable RP-HPLC Methodology and In Vitro and In Vivo Efficacy and Safety Characterisation. Evid. Based. Complement. Alternat. Med. 2013, 2013, 670451. [Google Scholar] [CrossRef] [PubMed]
- Diniz, D.P.; Lorencini, D.A.; Berretta, A.A.; Cintra, M.A.C.T.; Lia, E.N.; Jordão, A.A., Jr.; Coelho, E.B. Antioxidant Effect of Standardized Extract of Propolis (EPP-AF®) in Healthy Volunteers: A “Before and After” Clinical Study. Evid. Based Complement. Alternat. Med. 2020, 2020, 7538232. [Google Scholar] [CrossRef]
- Batista, M.A.C.; Braga, D.C.A.; Moura, S.A.L.; Souza, G.H.B.; Santos, O.D.H.; Cardoso, L.M. Salt-dependent hypertension and inflammation: Targeting the gut–brain axis and the immune system with Brazilian green propolis. Inflammopharmacology 2020, 28, 1163–1182. [Google Scholar] [CrossRef] [PubMed]
- Paulino, N.; Teixeira, C.; Martins, R.; Scremin, A.; Dirschi, V.M.; Vollmar, A.M.; Abreu, S.R.L.; De Castro, S.L.; Marcucci, M.C. Evaluation of Analgesic and Anti-Inflammatory Effects of a Brazilian Green Propolis. Plant. Med. 2006, 72, 899–906. [Google Scholar] [CrossRef]
- Xu, X.; Yang, B.; Wang, D.; Zhu, Y.; Miao, X.; Yang, W. The Chemical Composition of Brazilian Green Propolis and Its Protective Effects on Mouse Aortic Endothelial Cells against Inflammatory Injury. Molecules 2020, 25, 4612. [Google Scholar] [CrossRef]
- Silveira, M.A.D.; Teles, F.; Berretta, A.A.; Sanches, T.R.; Rodrigues, C.E.; Seguro, A.C.; Andrade, L. Effects of Brazilian green propolis on proteinuria and renal function in patients with chronic kidney disease: A randomized, double-blind, placebo-controlled trial. BMC Nephrol. 2019, 20, 140. [Google Scholar] [CrossRef]
- Anvarifard, P.; Anbari, M.; Ostadrahimi, A.; Ardalan, M.; Ghoreishi, Z. A comprehensive insight into the molecular and cellular mechanisms of the effects of Propolis on preserving renal function: A systematic review. Nutr. Metab. 2022, 19, 6. [Google Scholar] [CrossRef] [PubMed]
- Galeotti, F.; Maccari, F.; Fachini, A.; Volpi, N. Chemical Composition and Antioxidant Activity of Propolis Prepared in Different Forms and in Different Solvents Useful for Finished Products. Foods 2018, 7, 41. [Google Scholar] [CrossRef] [PubMed]
- Funari, C.S.; Carneiro, R.L.; Egeness, M.J.; Leme, G.M.; Cavalheiro, A.J.; Hilder, E.F. On track for a truly green propolis–fingerprinting propolis samples from seven countries by means of a fully green approach. ACS Sustain. Chem. Eng. 2016, 4, 7110–7117. [Google Scholar] [CrossRef]
- Funari, C.S.; Sutton, A.T.; Carneiro, R.L.; Fraige, K.; Cavalheiro, A.J.; Bolzani, V.S.; Hilder, E.F.; Arrua, R.D. Natural deep eutectic solvents and aqueous solutions as an alternative extraction media for propolis. Food Res. Int. 2019, 125, 108559. [Google Scholar] [CrossRef] [PubMed]
- Mani, F.; Damasceno, H.C.R.; Novelli, E.L.B.; Martins, E.A.M.; Sforcin, J.M. Propolis: Effect of different concentrations, extracts and intake period on seric biochemical variables. J. Ethnopharmacol. 2006, 105, 95–98. [Google Scholar] [CrossRef]
- Bachiega, T.F. Produção de citocinas pró- e anti-inflamatórias por macrófagos estimulados in vitro com própolis, alecrim-do-campo, capim-limão e cravo-da-índia. Master’s Thesis, Faculty of Medicine of Botucatu-FMB, Botucatu, SP, Brazil, 2011. [Google Scholar]
- Asgharpour, F.; Moghadamnia, A.A.; Motallebnejad, M.; Nouri, H.R. Propolis attenuates lipopolysaccharide-induced inflammatory responses through intracellular ROS and NO levels along with downregulation of IL-1β and IL-6 expressions in murine RAW 264.7 macrophages. J. Food Biochem. 2019, 43, 12926. [Google Scholar] [CrossRef]
- Marquiafável, F.S.; Nascimento, A.P.; Barud, H.S.; Marquele-Oliveira, F.; Freitas, L.A.P.; Bastos, J.K.; Berretta, A.A. Development and characterization of a novel standardized propolis dry extract obtained by factorial design with high artepillin C content. J. Pharm. Technol. Drug Res. 2015, 4, 1. [Google Scholar] [CrossRef]
- Brazilian Ministry of Agriculture. Instruction Normative to Propolis Extracts, Brazil. n. 3/2001. Available online: http://iberpharm.com.br/www/arquivos/IN03-19-01-2001.pdf (accessed on 15 February 2023).
- Šuran, J.; Cepanec, I.; Mašek, T.; Radić, B.; Radić, S.; Gajger, I.T.; Vlainić, J. Propolis Extract and Its Bioactive Compounds—From Traditional to Modern Extraction Technologies. Molecules 2021, 26, 2930. [Google Scholar] [CrossRef]
- Xue, M.; Shi, H.; Zhang, J.; Liu, Q.Q.; Guan, J.; Zhang, J.Y.; Ma, Q. Stability and degradation of caffeoylquinic acids under different storage conditions studied by high-performance liquid chromatography with photo diode array detection and high-performance liquid chromatography with electrospray ionization collision-induced dissociation tandem mass spectrometry. Molecules 2016, 21, 948. [Google Scholar] [PubMed]
- Magaña, A.A.; Kamimura, N.; Soumyanath, A.; Stevens, J.F.; Maier, C.S. Caffeoylquinic acids: Chemistry, biosynthesis, occurrence, analytical challenges, and bioactivity. Plant J. 2021, 107, 1299–1319. [Google Scholar] [CrossRef] [PubMed]
- Arruda, C.; Ribeiro, V.P.; Almeida, M.O.; Mejía, J.A.A.; Casoti, R.; Bastos, J.K. Effect of light, oxygen and temperature on the stability of artepillin C and p-coumaric acid from Brazilian green propolis. J. Pharm. Biomed. Anal. 2020, 178, 112922. [Google Scholar] [CrossRef]
- Jurasekova, Z.; Domingo, C.; Garcia-Ramos, J.V.; Sanchez-Cortes, S. Effect of pH on the chemical modification of quercetin and structurally related flavonoids characterized by optical (UV-visible and Raman) spectroscopy. Phys. Chem. Chem. Phys. 2014, 16, 25. [Google Scholar] [CrossRef] [PubMed]
- Kung, Y.C.; Chua, L.S.; Yam, M.F.; Soo, J. Alkaline hydrolysis and discrimination of propolis at different pH values using high throughput 2D IR spectroscopy and LC-MS/MS. Bioresour. Technol. Rep. 2022, 18, 101120. [Google Scholar] [CrossRef]
- Silveira, M.A.D.; De Jong, D.; Berretta, A.A.; Galvão, E.B.S.; Ribeiro, J.C.; Cerqueira-Silva, T.; Amorim, T.C.; Conceição, L.F.M.R.; Gomes, M.M.D.; Teixeira, M.B.; et al. Efficacy of Brazilian green propolis (EPP-AF®) as an adjunct treatment for hospitalized COVID-19 patients: A randomized, controlled clinical trial. Biomed. Pharmacother. 2021, 138, 111526. [Google Scholar] [CrossRef] [PubMed]
- Kurek-Górecka, A.; Rzepecka-Stojko, A.; Górecki, M.; Stojko, J.; Sosada, M.; Świerczek-Zięba, G. Structure and antioxidant activity of polyphenols derived from propolis. Molecules 2014, 19, 78–101. [Google Scholar] [CrossRef] [PubMed]
- Benbettaieb, N.; Nyagaya, J.; Seuvre, A.M.; Debeaufort, F. Antioxidant activity and release kinetics of caffeic and p-coumaric acids from hydrocolloid-based active films for healthy packaged food. J. Agric. Food Chem. 2018, 66, 6906–6916. [Google Scholar] [CrossRef] [PubMed]
- Nakajima, Y.; Tsuruma, K.; Shimazawa, M.; Mishima, S.; Hara, H. Comparison of bee products based on assays of antioxidant capacities. BMC Complement. Altern. Med. 2009, 9, 4. [Google Scholar] [CrossRef]
- Izuta, H.; Narahara, Y.; Shimazawa, M.; Mishima, S.; Kondo, S.I.; Hara, H. 1, 1-diphenyl-2-picrylhydrazyl radical scavenging activity of bee products and their constituents determined by ESR. Biol. Pharm. Bull. 2009, 32, 1947–1951. [Google Scholar] [CrossRef] [PubMed]
- Castelo-Branco, V.N.; Torres, A.G. Total antioxidant capacity of edible vegetable oils: Chemical determinants and associations with oil quality. Rev. de Nutr. 2011, 24, 173–187. [Google Scholar] [CrossRef]
- Sucupira, N.R.; Da Silva, A.B.; Pereira, G.; Da Costa, J.N. Methods for Measuring Antioxidant Activity of Fruits. J. Health Sci. 2012, 14, 263–269. [Google Scholar]
- Bittencourt, M.L.F.; Ribeiro, P.R.; Franco, R.L.P.; Hilhorst, H.W.M.; Castro, R.D.; Fernandez, L.G. Metabolite profiling, antioxidant and antibacterial activities of Brazilian propolis: Use of correlation and multivariate analyses to identify potential bioactive compounds. Food Res. Int. 2015, 76, 449–457. [Google Scholar] [CrossRef] [PubMed]
- Sawaya, A.C.H.S.; Cunha, I.B.S.; Marcucci, M.C. Analytical methods applied to diverse types of Brazilian propolis. Chem. Cent. J. 2011, 5, 27. [Google Scholar] [CrossRef] [PubMed]
- Drago, L.; Mombelli, B.; De Vecchi, E.; Fassina, M.C.; Tocalli, L.; Gismondo, M.R. In Vitro Antimicrobial Activity of Propolis Dry Extract. Antimicrob. Chemother. 2000, 12, 390–395. [Google Scholar]
- Jansen-Alves, C.; Maia, D.S.V.; Krumreich, F.D.; Crizel-Cardoso, M.M.; Fioravante, J.B.; Da Silva, W.P.; Borges, C.D.; Zambiazi, R.C. Propolis microparticles produced with pea protein: Characterization and evaluation of antioxidant and antimicrobial activities. Food Hydrocoll. 2019, 87, 703–711. [Google Scholar] [CrossRef]
- De Andrade, U.V.C.; Hartmann, W.; Funayama, S.; De Alencar, S.M.; Masson, M.L. Propolis obtained by means of alkaline hydrolysis and action on Staphylococcus aureus. Ars. Vet. 2009, 25, 151–154. [Google Scholar]
- Waterman, P.G.; Mole, S. Analysis of Phenolic Plant Metabolites, 1st ed.; Blackwell Publishing: Oxford, UK, 1994; p. 248. [Google Scholar]
- Funari, C.S.; Ferro, V.O. Análise de própolis. Food Sci. Technol. 2006, 26, 171–178. [Google Scholar] [CrossRef]
- Lee, S.E.; Hwang, H.J.; Ha, J.S.; Jeong, H.S.; Kim, J.H. Screening of medicinal plant extracts for antioxidant activity. Life Sci. 2003, 73, 167–179. [Google Scholar] [CrossRef] [PubMed]
- Benzie, I.F.F.; Strain, J.J. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: The FRAP assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef] [PubMed]
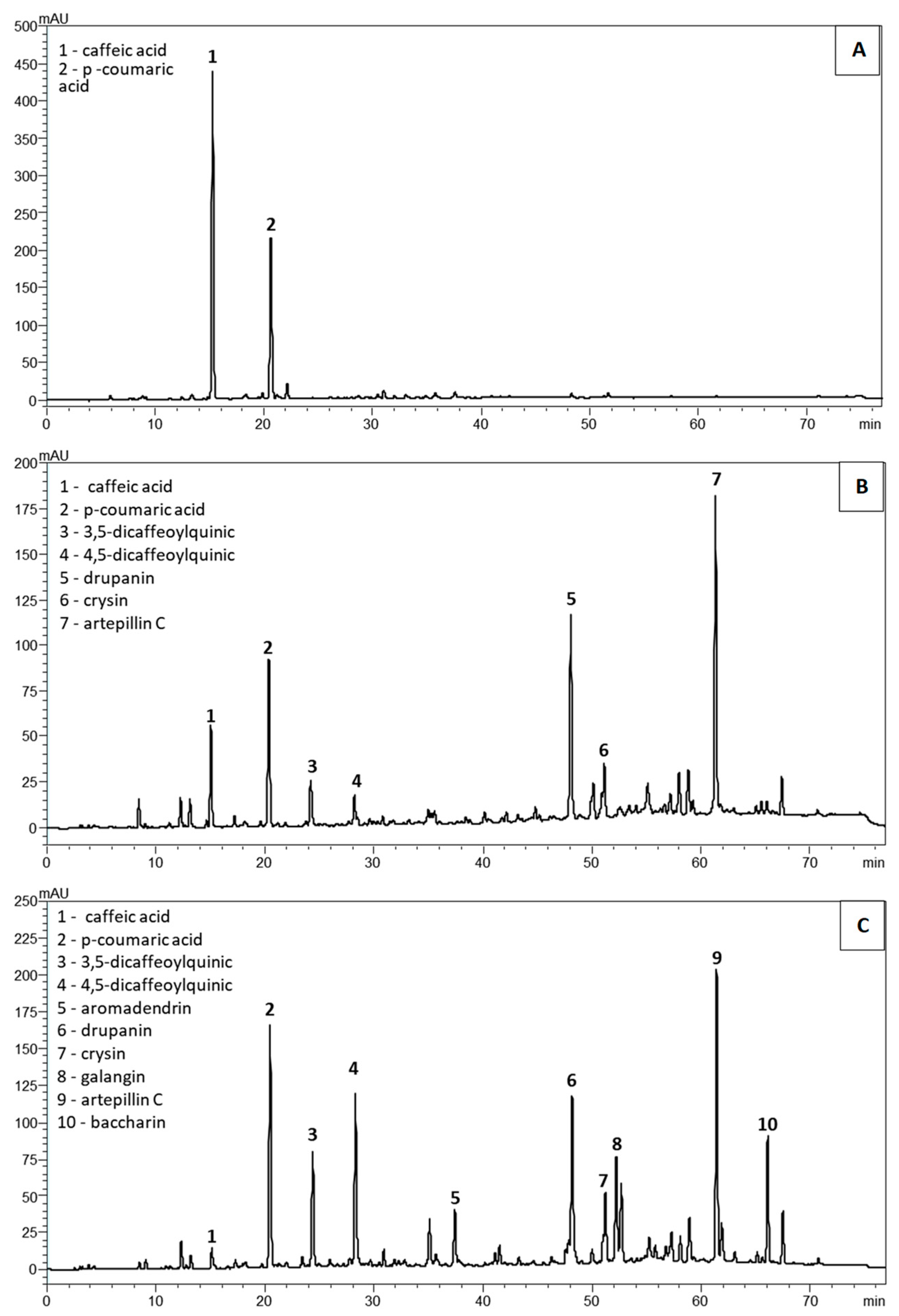
| Constituents | PPF | PSDE | MPE |
|---|---|---|---|
| mg/g | mg/g | mg/g | |
| Caffeic acid | 71.76 ± 7.4 | 3.52 ± 0.01 * | 0.79 ±0.004 ** |
| p-Coumaric acid | 21.70 ± 1.6 | 3.49 ± 0.007 * | 4.67 ± 0.03 ** |
| 3,5 Dicaffeoylquinic acid | - | 3.64 ± 0.01 | 6.62 ± 0.04 # |
| 4,5 Dicaffeoylquinic acid | - | 2.37 ± 0.02 | 11.84 ± 0.2 # |
| Aromadendrin-4′-O-methyl-ether | - | - | 2.62 ± 0.04 # |
| Drupanin | - | 9.48 ± 0.07 | 7.72 ± 0.04 # |
| Chrysin | - | 0.98 ± 0.02 | 1.22 ± 0.03 # |
| Galangin | - | - | 3.61 ± 0.09 # |
| Artepillin C | - | 22.49 ± 0.5 | 18.85 ± 0.4 # |
| Baccharin | - | - | 2.58 ± 0.07 # |
| Propolis Samples | Total Phenolics (Expressed as Gallic Acid) | Total Flavonoids (Expressed as Quercetin) | ||
|---|---|---|---|---|
| Content mg/g | RSD (%) | Content mg/g | RSD (%) | |
| PPF | 22.58 ± 0.50 * | 2.37 | 16.22 ± 0.60 * | 3.53 |
| PSDE | 61.62 ± 0.90 ** | 1.53 | 22.74 ± 0.30 | 1.17 |
| MPE | 49.45 ± 1.30 # | 2.60 | 23.17 ± 0.60 | 2.42 |
| Sample | DPPH IC50 | FRAP |
|---|---|---|
| (µg/mL) | (µmol FeII/mg) | |
| MPE | 10.07 ± 0.37 | 1.04 ± 0.02 # |
| PSDE | 12.81 ± 0.52 | 1.51 ± 0.05 ** |
| PPF | 4.44 ± 0.16 * | 4.33 ± 0.21 * |
| Caffeic acid | 1.11 ± 0.04 | 32.81 ± 1.72 |
| ρ-Coumaric acid | 117.86 ± 5.82 | 2.53 ± 0.04 |
| Artepillin C | 5.29 ± 0.25 | 4.53 ± 0.09 |
| MBC (mg Propolis Dry Matter/mL) (Mean ± Standard Deviation) | ||||
|---|---|---|---|---|
| Samples | S. aureus | S. aureus MRSA | S. epidermidis | K. pneumoniae |
| MPE | 3.44 ± 0.00 | 6.88 ± 0.02 ** | 10.31 ± 4.84 | 20.62 ± 9.67 |
| PSDE | 1.72 ± 0.00 * | 3.44 ± 0.00 | 6.89 ± 0.01 | 6.89 ± 0.01 |
| PPF | 3.44 ± 0.00 | 3.44 ± 0.00 | 3.44 ± 0.00 | 3.44 ± 0.00 |
| µg/mL | ||||
| Caffeic acid | >100 | >100 | >100 | >100 |
| p-Coumaric acid | >100 | >100 | >100 | >100 |
| Artepillin C | >100 | >100 | >100 | >100 |
| % v/v | ||||
| DMSO | 50 ± 0.00 | 50 ± 0.00 | >50 ± 0.00 | 50 ± 0.00 |
| Ethanol | 25 ± 0.00 | 25 ± 0.00 | 25 ± 0.00 | 18.75 ± 8.84 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Berretta, A.A.; Zamarrenho, L.G.; Correa, J.A.; De Lima, J.A.; Borini, G.B.; Ambrósio, S.R.; Barud, H.d.S.; Bastos, J.K.; De Jong, D. Development and Characterization of New Green Propolis Extract Formulations as Promising Candidates to Substitute for Green Propolis Hydroalcoholic Extract. Molecules 2023, 28, 3510. https://doi.org/10.3390/molecules28083510
Berretta AA, Zamarrenho LG, Correa JA, De Lima JA, Borini GB, Ambrósio SR, Barud HdS, Bastos JK, De Jong D. Development and Characterization of New Green Propolis Extract Formulations as Promising Candidates to Substitute for Green Propolis Hydroalcoholic Extract. Molecules. 2023; 28(8):3510. https://doi.org/10.3390/molecules28083510
Chicago/Turabian StyleBerretta, Andresa Aparecida, Luana Gonçalves Zamarrenho, Juliana Arcadepani Correa, Jéssica Aparecida De Lima, Giovanna Bonfante Borini, Sérgio Ricardo Ambrósio, Hernane da Silva Barud, Jairo Kenupp Bastos, and David De Jong. 2023. "Development and Characterization of New Green Propolis Extract Formulations as Promising Candidates to Substitute for Green Propolis Hydroalcoholic Extract" Molecules 28, no. 8: 3510. https://doi.org/10.3390/molecules28083510
APA StyleBerretta, A. A., Zamarrenho, L. G., Correa, J. A., De Lima, J. A., Borini, G. B., Ambrósio, S. R., Barud, H. d. S., Bastos, J. K., & De Jong, D. (2023). Development and Characterization of New Green Propolis Extract Formulations as Promising Candidates to Substitute for Green Propolis Hydroalcoholic Extract. Molecules, 28(8), 3510. https://doi.org/10.3390/molecules28083510









