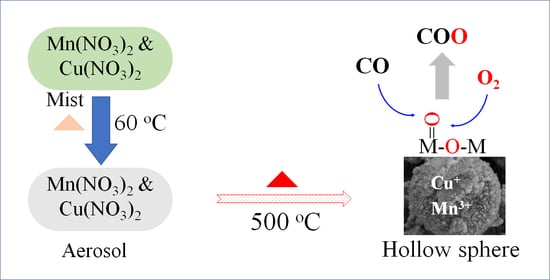
DRIFTS-MS Investigation of Low-Temperature CO Oxidation on Cu-Doped Manganese Oxide Prepared Using Nitrate Aerosol Decomposition
Abstract
:1. Introduction
2. Results and Discussion
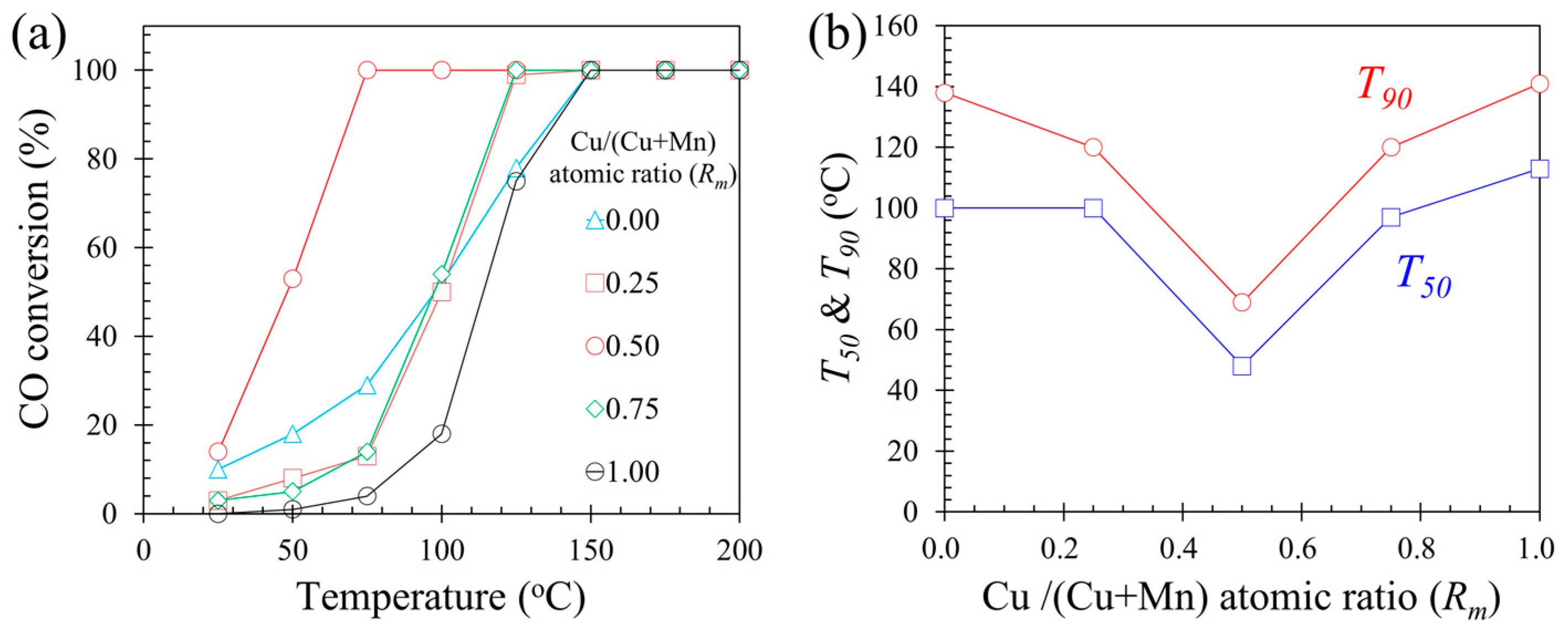
2.1. Catalyst Activity
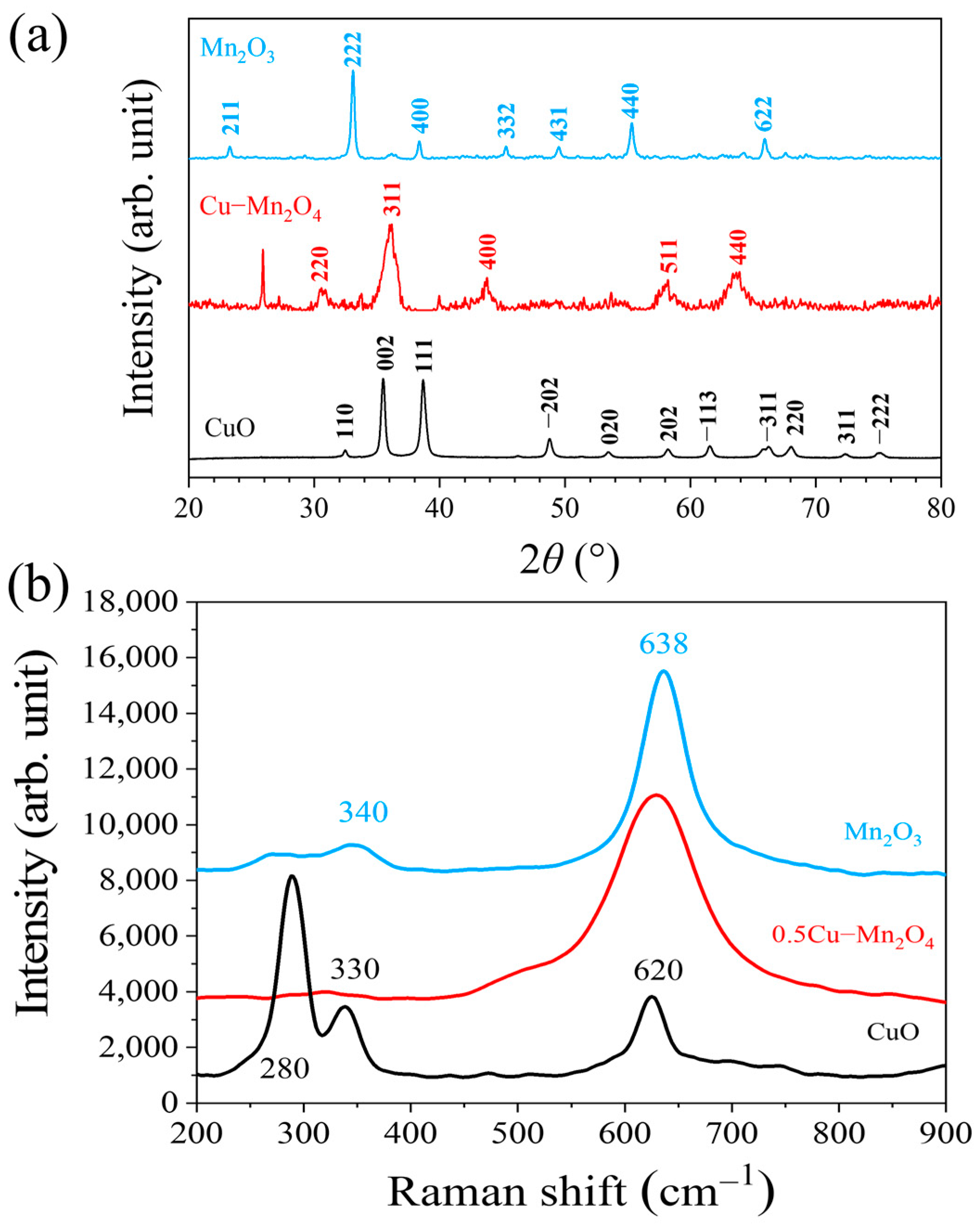
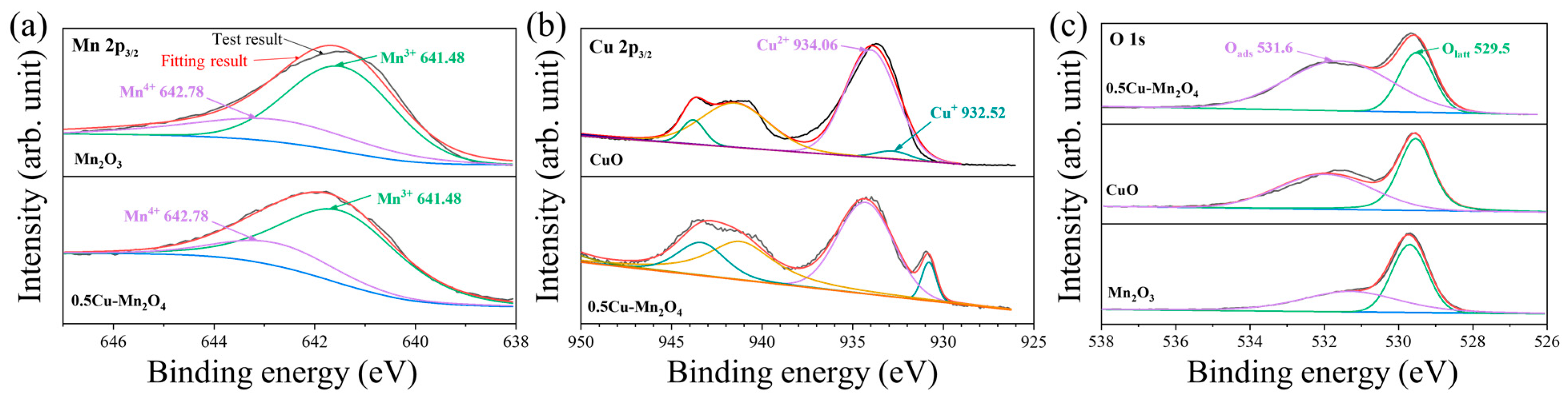
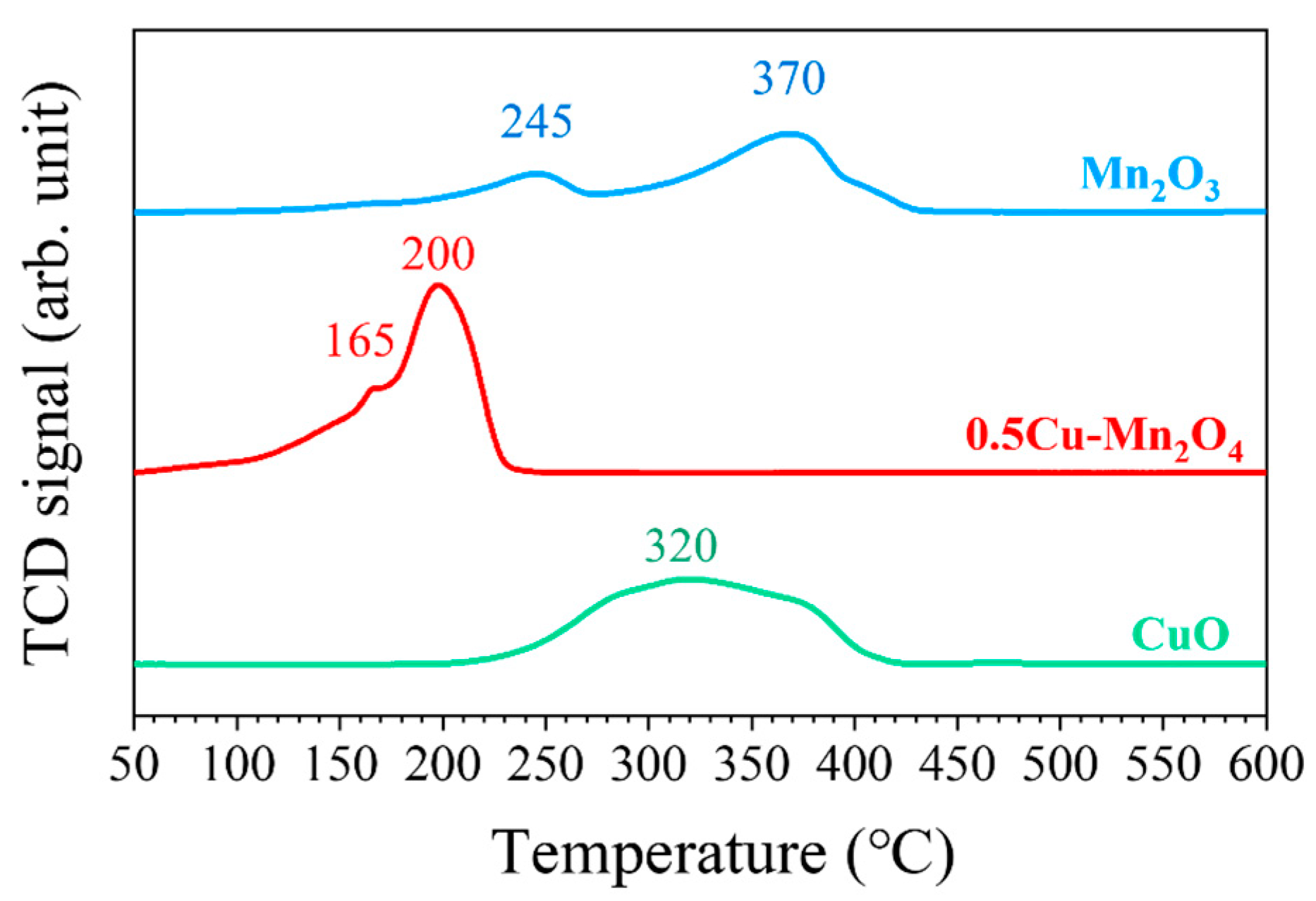
2.2. Structural Properties
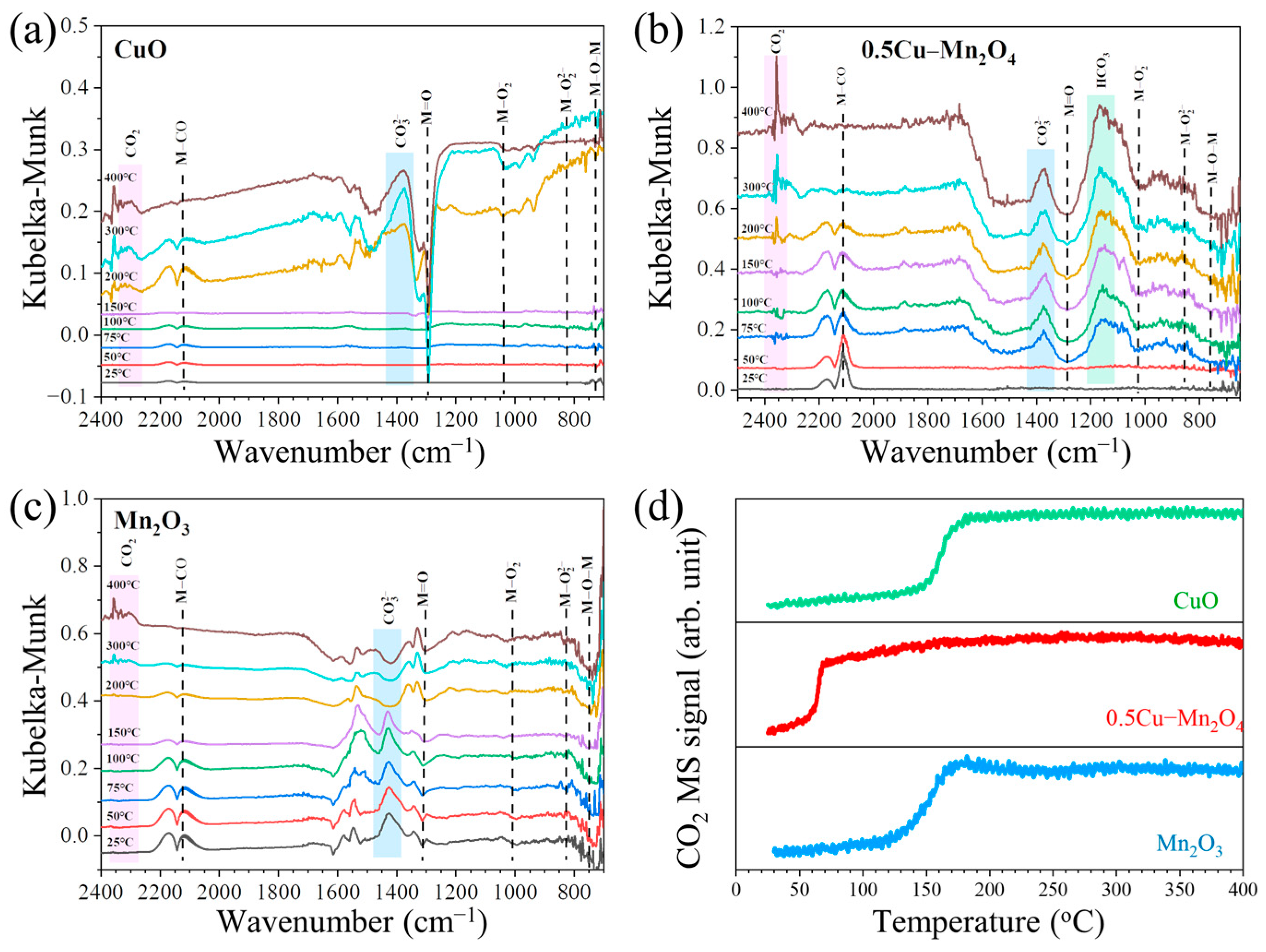
2.3. Operando DRIFTS-MS Spectra during CO Oxidation without Water
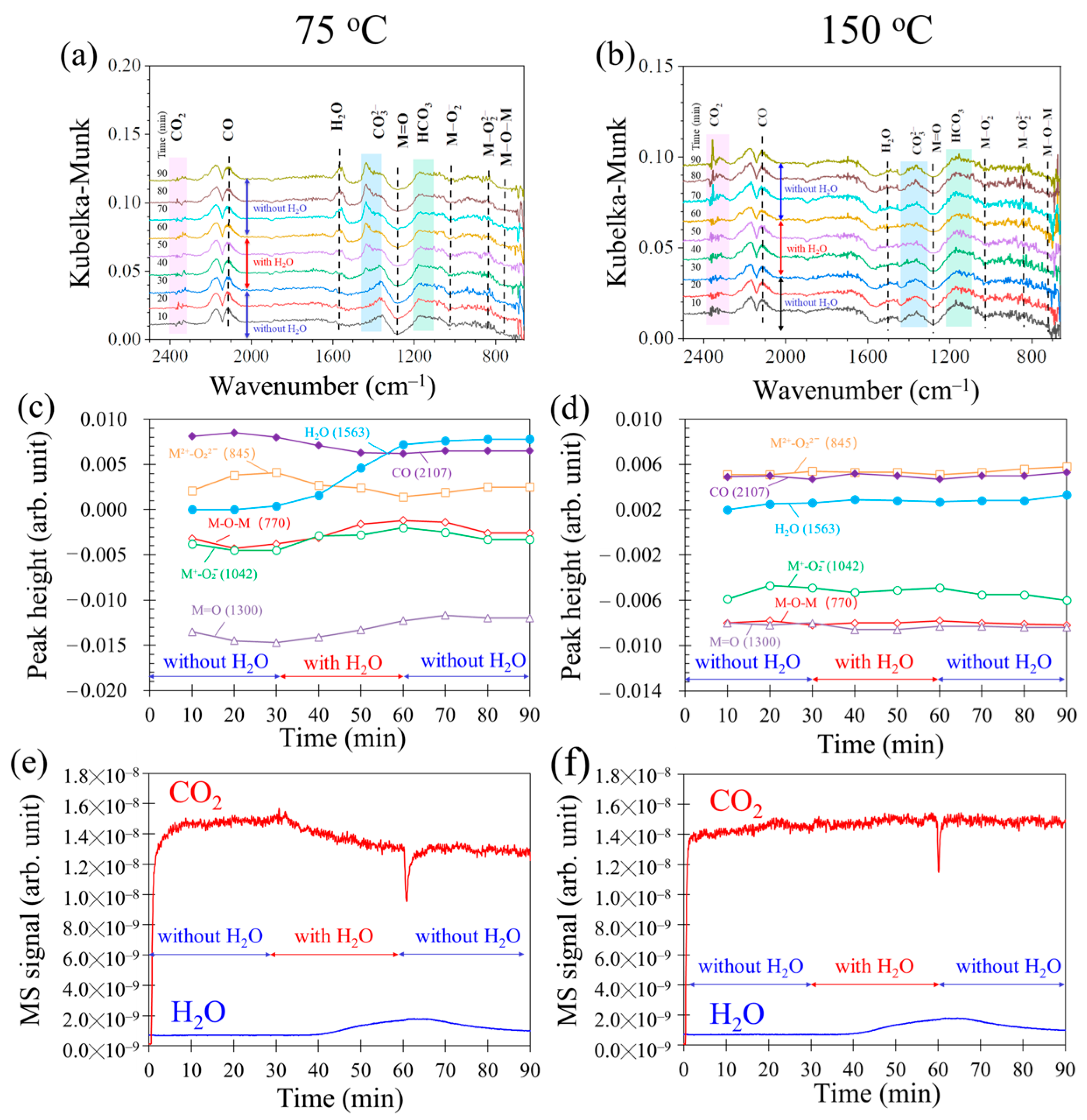
2.4. Operando DRIFTS-MS Spectra during CO Oxidation with Water
3. Materials and Methods
3.1. Catalyst Preparation
3.2. Evaluation of Catalyst Activity
3.3. Catalyst Characterization and Calculations
4. Conclusions
- (1)
- The catalyst 0.5Cu–Mn2O4 of 0.48 Cu/(Cu + Mn) atomic ratio had the best CO oxidation performance. T50 and T90 were as low as 48 and 69 °C, respectively.
- (2)
- Cu could be successfully doped into Mn2O4 due to their nitrate precursors having closed thermal decomposition properties, which ensured the atomic ratio of Cu/(Cu + Mn) close to the atomic ratio in nitrate precursors.
- (3)
- The catalyst 0.5Cu–Mn2O4 had a hollow sphere morphology, and the sphere wall was composed of a large number of nanospheres (about 10 nm), yielding the largest specific surface area and the defects on the interfacing of the nanospheres.
- (4)
- The catalyst 0.5Cu–Mn2O4 had high Mn3+, Cu+, and Oads ratios, which facilitated oxygen vacancy formation, CO adsorption, and CO oxidation, respectively, which had a synergetic effect on CO oxidation.
- (5)
- Terminal-type oxygen (M=O) and bridge-type oxygen (M-O-M) on 0.5Cu–Mn2O4 were reactive at a low temperature, resulting in a good low-temperature CO oxidation performance.
- (6)
- Water could adsorb on 0.5Cu–Mn2O4 and inhibited M=O and M-O-M reaction with CO. Water could not inhibit O2 decomposition to M=O and M-O-M. The catalyst 0.5Cu–Mn2O4 had excellent water resistance at 150 °C at which the influence of water (up to 5%) on CO oxidation could be completely eliminated.
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Liu, S.; Liu, Y.; Yang, C.; Xie, D.; Zhang, X. Charcoal burning is associated with a higher risk of delayed neurological sequelae after domestic carbon monoxide poisoning in South China: A retrospective cohort study. Am. J. Emerg. Med. 2022, 60, 57–61. [Google Scholar] [CrossRef]
- Adach, W.; Błaszczyk, M.; Olas, B. Carbon monoxide and its donors—Chemical and biological properties. Chem. Biol. Interact. 2020, 318, 108973. [Google Scholar] [CrossRef]
- Yu, W.Z.; Wang, W.W.; Li, S.Q.; Fu, X.P.; Wang, X.; Wu, K.; Si, R.; Ma, C.; Jia, C.J.; Yan, C.H. Construction of active site in a sintered copper-ceria nanorod catalyst. J. Am. Chem. Soc. 2019, 141, 17548–17557. [Google Scholar] [CrossRef] [PubMed]
- Xiao, M.; Zhang, X.; Yang, Y.; Cui, X.; Chen, T.; Wang, Y. M (M = Mn, Co, Cu)-CeO2 catalysts to enhance their CO catalytic oxidation at a low temperature: Synergistic effects of the interaction between Ce3+-Mx+-Ce4+ and the oxygen vacancy defects. Fuel 2022, 323, 124379. [Google Scholar] [CrossRef]
- Xu, K.; Wang, Z.; He, C.; Ruan, S.; Liu, F.; Zhang, L. Flame in situ synthesis of metal-anchored CuO nanowires for CO catalytic oxidation and kinetic analysis. ACS Appl. Energy Mater. 2021, 4, 13226–13238. [Google Scholar] [CrossRef]
- Li, L.; Feng, X.; Nie, Y.; Chen, S.; Shi, F.; Xiong, K.; Ding, W.; Qi, X.; Hu, J.; Wei, Z.; et al. Insight into the effect of oxygen vacancy concentration on the catalytic performance of MnO2. ACS Catal. 2015, 5, 4825–4832. [Google Scholar] [CrossRef]
- Luo, M.F.; Zhong, Y.J.; Yuan, X.X.; Zheng, X.M. TPR and TPD studies of CuO/CeO2 catalysts for low temperature CO oxidation. Appl. Catal. A Gen. 1997, 162, 121–131. [Google Scholar] [CrossRef]
- Wintterlin, J.; Völkening, S.; Janssens, T.V.W.; Zambelli, T.; Ertl, G. Atomic and macroscopic reaction rates of a surface-catalyzed reaction. Science 1997, 278, 1931–1934. [Google Scholar] [CrossRef]
- Alavi, A.; Hu, P.; Deutsch, T.; Silvestrelli, P.L.; Hutter, J. CO oxidation on Pt(111): An ab initio density functional theory study. Phys. Rev. Lett. 1998, 80, 3650–3653. [Google Scholar] [CrossRef]
- Allian, A.D.; Takanabe, K.; Fujdala, K.L.; Hao, X.; Truex, T.J.; Cai, J.; Buda, C.; Neurock, M.; Iglesia, E. Chemisorption of CO and mechanism of CO oxidation on supported platinum nanoclusters. J. Am. Chem. Soc. 2011, 133, 4498–4517. [Google Scholar] [CrossRef]
- Arán-Ais, R.M.; Vidal-Iglesias, F.J.; Farias, M.J.S.; Solla-Gullón, J.; Montiel, V.; Herrero, E.; Feliu, J.M. Understanding CO oxidation reaction on platinum nanoparticles. J. Electroanal. Chem. 2017, 793, 126–136. [Google Scholar] [CrossRef]
- L’vov, B.V.; Galwey, A.K. Catalytic oxidation of CO on platinum: Thermochemical approach. J. Therm. Anal. Calorim. 2013, 111, 145–154. [Google Scholar] [CrossRef]
- Kim, J.; Noh, M.C.; Doh, W.H.; Park, J.Y. In situ observation of competitive CO and O2 adsorption on the Pt(111) surface using near-ambient pressure scanning tunneling microscopy. J. Phys. Chem. C 2018, 122, 6246–6254. [Google Scholar] [CrossRef]
- Vogel, D.; Spiel, C.; Suchorski, Y.; Trinchero, A.; Schlögl, R.; Grönbeck, H.; Rupprechter, G. Local catalytic ignition during CO oxidation on low-index Pt and Pd surfaces: A combined PEEM, MS, and DFT study. Angew. Chem. Int. Edit. 2012, 51, 10041–10044. [Google Scholar] [CrossRef] [PubMed]
- Newton, M.A.; Ferri, D.; Smolentsev, G.; Marchionni, V.; Nachtegaal, M. Room- temperature carbon monoxide oxidation by oxygen over Pt/Al2O3 mediated by reactive platinum carbonates. Nat. Commun. 2015, 6, 8675. [Google Scholar] [CrossRef]
- An, N.; Yuan, X.; Pan, B.; Li, Q.; Li, S.; Zhang, W. Design of a highly active Pt/Al2O3 catalyst for low-temperature CO oxidation. RSC Adv. 2014, 4, 38250–38257. [Google Scholar] [CrossRef]
- Dong, C.; Zong, X.; Jiang, W.; Niu, L.; Liu, Z.; Qu, D.; Wang, X.; Sun, Z. Recent advances of ceria-based materials in the oxidation of carbon monoxide. Small Struct. 2021, 2, 2000081. [Google Scholar] [CrossRef]
- Jiang, D.; Wan, G.; García Vargas, C.E.; Li, L.; Pereira-Hernández, X.I.; Wang, C.; Wang, Y. Elucidation of the active sites in single-atom Pd1/CeO2 catalysts for low-temperature CO oxidation. ACS Catal. 2020, 10, 11356–11364. [Google Scholar] [CrossRef]
- Wang, J.; Chen, H.; Hu, Z.; Yao, M.; Li, Y. A review on the Pd-based three-way catalyst. Catal. Rev. 2015, 57, 79–144. [Google Scholar] [CrossRef]
- Lambert, C.K. Current state of the art and future needs for automotive exhaust catalysis. Nat. Catal. 2019, 2, 554–557. [Google Scholar] [CrossRef]
- Landon, P.; Ferguson, J.; Solsona, B.E.; Garcia, T.; Al-Sayari, S.; Carley, A.F.; Herzing, A.A.; Kiely, C.J.; Makkee, M.; Moulijn, J.A.; et al. Selective oxidation of CO in the presence of H2, H2O and CO2 utilising Au/α-Fe2O3 catalysts for use in fuel cells. J. Mater. Chem. 2006, 16, 199–208. [Google Scholar] [CrossRef]
- Wang, M.; Ma, P.; Wu, Z.; Chu, S.; Zheng, Y.; Zhou, Z.; Weng, W. Evolution of Pd chemical states and effects of C3H6 and H2O on the CO oxidation over Pd/CeO2 catalyst. Appl. Surf. Sci. 2022, 599, 153897. [Google Scholar] [CrossRef]
- Haruta, M.; Yamada, N.; Kobayashi, T.; Iijima, S. Gold catalysts prepared by coprecipitation for low-temperature oxidation of hydrogen and of carbon monoxide. J. Catal. 1989, 115, 301–309. [Google Scholar] [CrossRef]
- Qiao, B.; Wang, A.; Yang, X.; Allard, L.F.; Jiang, Z.; Cui, Y.; Liu, J.; Li, J.; Zhang, T. Single-atom catalysis of CO oxidation using Pt1/FeOx. Nat. Chem. 2011, 3, 634–641. [Google Scholar] [CrossRef]
- Kang, Y.; Sun, M.; Li, A. Studies of the catalytic oxidation of CO over Ag/CeO2 catalyst. Catal. Lett. 2012, 142, 1498–1504. [Google Scholar] [CrossRef]
- Huang, M.; Zhao, X.L.; Li, F.; Zhang, L.L.; Zhang, Y.X. Facile synthesis of ultrathin manganese dioxide nanosheets arrays on nickel foam as advanced binder-free supercapacitor electrodes. J. Power Sources 2015, 277, 36–43. [Google Scholar] [CrossRef]
- Rong, S.; Li, K.; Zhang, P.; Liu, F.; Zhang, J. Potassium associated manganese vacancy in birnessite-type manganese dioxide for airborne formaldehyde oxidation. Catal. Sci. Technol. 2018, 8, 1799–1812. [Google Scholar] [CrossRef]
- Abad, A.; Adánez, J.; García-Labiano, F.; de Diego, L.F.; Gayán, P.; Celaya, J. Mapping of the range of operational conditions for Cu-, Fe-, and Ni-based oxygen carriers in chemical-looping combustion. Chem. Eng. Sci. 2007, 62, 533–549. [Google Scholar] [CrossRef]
- Xu, L.; Edland, R.; Li, Z.; Leion, H.; Zhao, D.; Cai, N. Cu-modified manganese ore as an oxygen carrier for chemical looping combustion. Energy Fuels 2014, 28, 7085–7092. [Google Scholar] [CrossRef]
- Wang, K.; Yu, Q.; van Sint Annaland, M.; Wu, T.; Qin, Q. Improvement of the oxygen uncoupling properties of copper-based composite oxygen carriers for chemical looping air separation. Energy Fuels 2020, 34, 3449–3457. [Google Scholar] [CrossRef]
- Zhang, X.; Li, H.; Yang, Y.; Zhang, T.; Wen, X.; Liu, N.; Wang, D. Facile synthesis of new efficient Cu/MnO2 catalysts from used battery for CO oxidation. J. Environ. Chem. Eng. 2017, 5, 5179–5186. [Google Scholar] [CrossRef]
- Gao, J.; Jia, C.; Zhang, L.; Wang, H.; Yang, Y.; Hung, S.F.; Hsu, Y.Y.; Liu, B. Tuning chemical bonding of MnO2 through transition-metal doping for enhanced CO oxidation. J. Catal. 2016, 341, 82–90. [Google Scholar] [CrossRef]
- Kong, M.; Li, Y.; Chen, X.; Tian, T.; Fang, P.; Zheng, F.; Zhao, X. Tuning the relative concentration ratio of bulk defects to surface defects in TiO2 nanocrystals leads to high photocatalytic efficiency. J. Am. Chem. Soc. 2011, 133, 16414–16417. [Google Scholar] [CrossRef]
- Zhu, L.; Wang, J.; Rong, S.; Wang, H.; Zhang, P. Cerium modified birnessite-type MnO2 for gaseous formaldehyde oxidation at low temperature. Appl. Catal. B Environ. 2017, 211, 212–221. [Google Scholar] [CrossRef]
- Dong, C.; Qu, Z.; Jiang, X.; Ren, Y. Tuning oxygen vacancy concentration of MnO2 through metal doping for improved toluene oxidation. J. Haz. Mat. 2020, 391, 122181. [Google Scholar] [CrossRef]
- Wang, Q.; Li, Z.; Banares, M.A.; Weng, L.T.; Gu, Q.; Price, J.; Han, W.; Yeung, K.L. A novel approach to high-performance aliovalent-substituted catalysts-2D bimetallic MOF-derived CeCuO(x) microsheets. Small 2019, 15, 1903525. [Google Scholar] [CrossRef]
- Yang, Y.; Si, W.; Peng, Y.; Wang, Y.; Liu, H.; Su, Z.; Li, J. Defect engineering on CuMn2O4 spinel surface: A new path to high-performance oxidation catalysts. Environ. Sci. Technol. 2022, 56, 16249–16258. [Google Scholar] [CrossRef]
- Tang, W.; Wu, X.; Li, S.; Shan, X.; Liu, G.; Chen, Y. Co-nanocasting synthesis of mesoporous Cu–Mn composite oxides and their promoted catalytic activities for gaseous benzene removal. Appl. Catal. B Environ. 2015, 162, 110–121. [Google Scholar] [CrossRef]
- Zhang, X.; Tian, P.; Tu, W.; Zhang, Z.; Xu, J.; Han, Y.F. Tuning the dynamic interfacial structure of copper–ceria catalysts by indium oxide during CO Oxidation. ACS Catal. 2018, 8, 5261–5275. [Google Scholar] [CrossRef]
- Zhang, C.; Xie, A.; Zhang, W.; Chang, J.; Liu, C.; Gu, L.; Duo, X.; Pan, F.; Luo, S. CuMn2O4 spinel anchored on graphene nanosheets as a novel electrode material for supercapacitor. J. Energy Storage 2021, 34, 102181. [Google Scholar] [CrossRef]
- Yuan, X.; Qing, M.; Meng, L.; Zhao, H. One-step synthesis of nanostructured Cu–Mn/TiO2 via flame spray pyrolysis: Application to catalytic combustion of CO and CH4. Energ. Fuels 2020, 34, 14447–14457. [Google Scholar] [CrossRef]
- Fang, R.; Liu, F.; Liu, J.; Li, Y. Experimental and theoretical insights into the reaction mechanism of spinel CuMn2O4 with CO in chemical-looping combustion. Appl. Surf. Sci. 2021, 561, 150065. [Google Scholar] [CrossRef]
- Meng, M.; Liu, Y.; Sun, Z.; Zhang, L.; Wang, X. Synthesis of highly-dispersed CuO–CeO2 catalyst through a chemisorption-hydrolysis route for CO preferential oxidation in H2-rich stream. Int. J. Hydrogen Energ. 2012, 37, 14133–14142. [Google Scholar] [CrossRef]
- Chen, Y.; Liu, Y.; Mao, D.; Yu, J.; Zheng, Y.; Guo, X.; Ma, Z. Facile cyclodextrin-assisted synthesis of highly active CuO-CeO2/MCF catalyst for CO oxidation. J. Taiwan Inst. Chem. E 2020, 113, 16–26. [Google Scholar] [CrossRef]
- Xu, J.; Zhang, T.; Fang, S.; Li, J.; Wu, Z.; Wang, W.; Zhu, J.; Gao, E.; Yao, S. Exploring the roles of oxygen species in H2 oxidation at β-MnO2 surfaces using operando DRIFTS-MS. Comm. Chem. 2022, 5, 97. [Google Scholar] [CrossRef]
- Wu, J.; Su, T.; Jiang, Y.; Xie, X.; Qin, Z.; Ji, H. In situ DRIFTS study of O3 adsorption on CaO, γ-Al2O3, CuO, α-Fe2O3 and ZnO at room temperature for the catalytic ozonation of cinnamaldehyde. Appl. Surf. Sci. 2017, 412, 290–305. [Google Scholar] [CrossRef]
- Pu, Y.; Luo, Y.; Wei, X.; Sun, J.; Li, L.; Zou, W.; Dong, L. Synergistic effects of Cu2O-decorated CeO2 on photocatalytic CO2 reduction: Surface Lewis acid/base and oxygen defect. Appl. Catal. B Environ. 2019, 254, 580–586. [Google Scholar] [CrossRef]
- Che, M.; Tench, A.J. Characterization and reactivity of molecular oxygen species on oxide surfaces. Adv. Catal. 1983, 32, 1–148. [Google Scholar] [CrossRef]




| Sample | Specific Surface Area (m2/g) | Pore Volume (cm3/g) | Pore Size (nm) | Grain Size (nm) |
|---|---|---|---|---|
| Mn2O3 | 24.22 | 0.079 | 14.02 | 27.0 |
| 0.5Cu–Mn2O4 | 56.22 | 0.228 | 15.79 | 12.4 |
| CuO | 15.70 | 0.050 | 5.82 | 18.8 |
| Catalyst | Oads/(Oads + Olatt) (%) | Mn3+/(Mn3+ + Mn4+) (%) | Cu+/(Cu+ + Cu2+) (%) | Rm | Rb-ICP | Rs-XPS | OVD (%) |
|---|---|---|---|---|---|---|---|
| Mn2O3 | 49 | 51.8 | 0.00 | 0.00 | 0.00 | 4.35 | |
| 0.25Cu–MnxOy | 0.25 | 0.29 | |||||
| 0.5Cu–Mn2O4 | 72 | 81.7 | 9.8 | 0.5 | 0.48 | 0.45 | 16.46 |
| 0.75Cu–MnxOy | 0.75 | 0.94 | |||||
| CuO | 53 | 8.9 | 1.00 | 1.00 | 1.00 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gong, X.; Xu, J.; Zhang, T.; Sun, Y.; Fang, S.; Li, N.; Zhu, J.; Wu, Z.; Li, J.; Gao, E.; et al. DRIFTS-MS Investigation of Low-Temperature CO Oxidation on Cu-Doped Manganese Oxide Prepared Using Nitrate Aerosol Decomposition. Molecules 2023, 28, 3511. https://doi.org/10.3390/molecules28083511
Gong X, Xu J, Zhang T, Sun Y, Fang S, Li N, Zhu J, Wu Z, Li J, Gao E, et al. DRIFTS-MS Investigation of Low-Temperature CO Oxidation on Cu-Doped Manganese Oxide Prepared Using Nitrate Aerosol Decomposition. Molecules. 2023; 28(8):3511. https://doi.org/10.3390/molecules28083511
Chicago/Turabian StyleGong, Xingfan, Jiacheng Xu, Tiantian Zhang, Yan Sun, Shiyu Fang, Ning Li, Jiali Zhu, Zuliang Wu, Jing Li, Erhao Gao, and et al. 2023. "DRIFTS-MS Investigation of Low-Temperature CO Oxidation on Cu-Doped Manganese Oxide Prepared Using Nitrate Aerosol Decomposition" Molecules 28, no. 8: 3511. https://doi.org/10.3390/molecules28083511
APA StyleGong, X., Xu, J., Zhang, T., Sun, Y., Fang, S., Li, N., Zhu, J., Wu, Z., Li, J., Gao, E., Wang, W., & Yao, S. (2023). DRIFTS-MS Investigation of Low-Temperature CO Oxidation on Cu-Doped Manganese Oxide Prepared Using Nitrate Aerosol Decomposition. Molecules, 28(8), 3511. https://doi.org/10.3390/molecules28083511