Silver-Promoted Radical Cascade Aryldifluoromethylation/Cyclization of 2-Allyloxybenzaldehydes for the Synthesis of 3-Aryldifluoromethyl-Containing Chroman-4-one Derivatives
Abstract
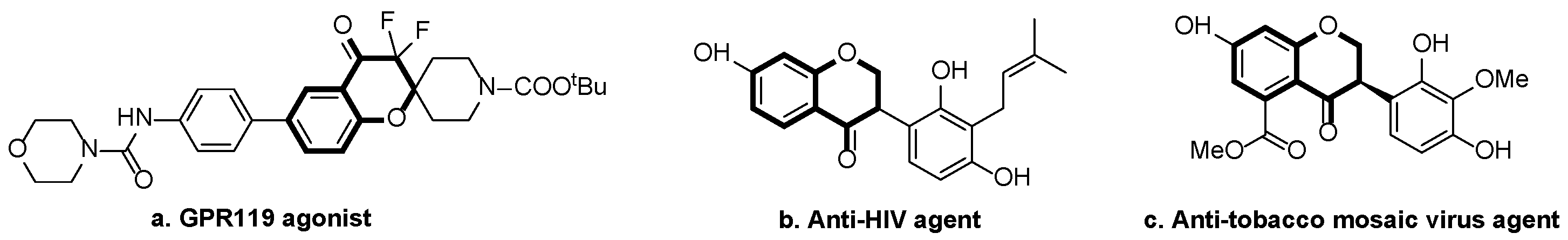
1. Introduction
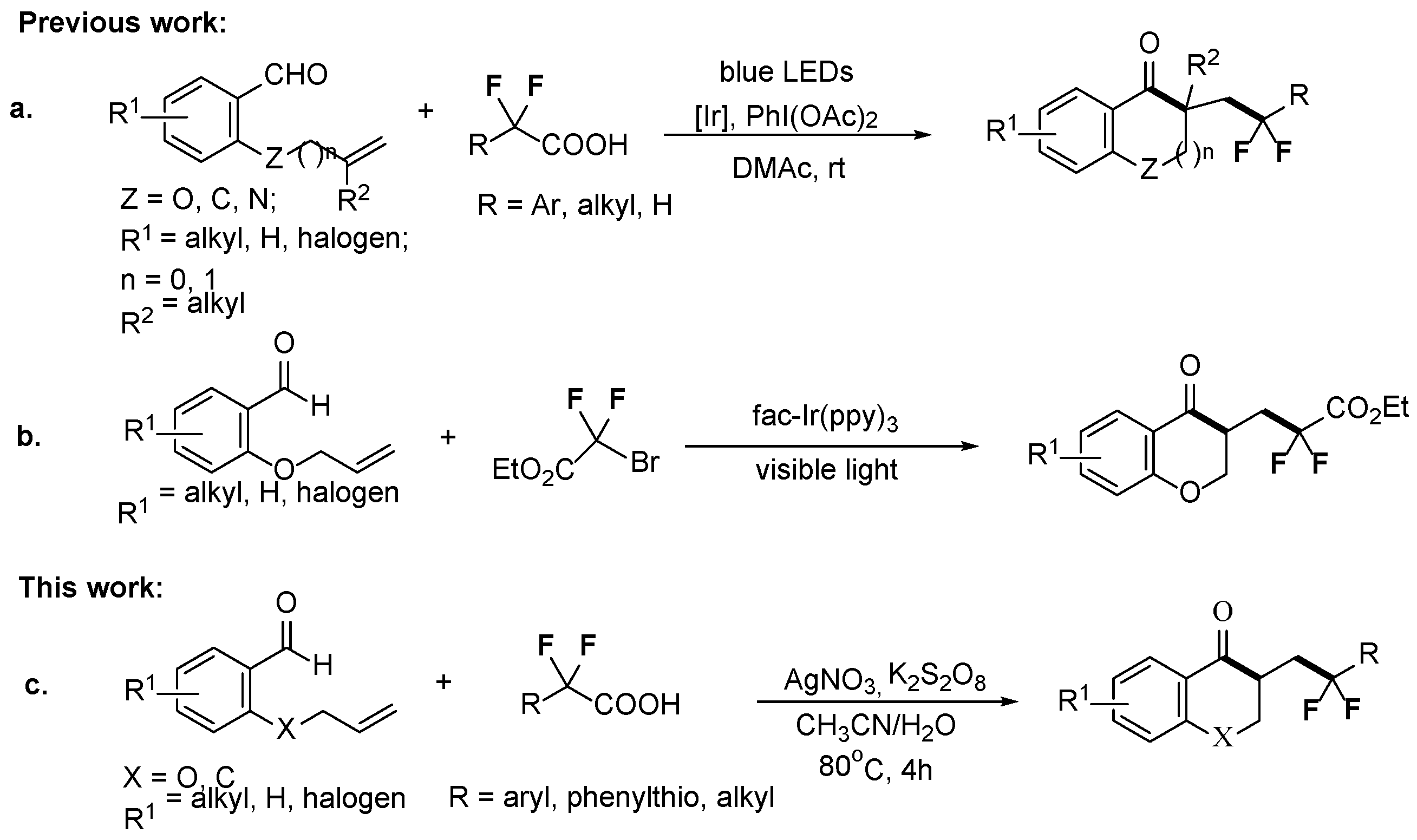
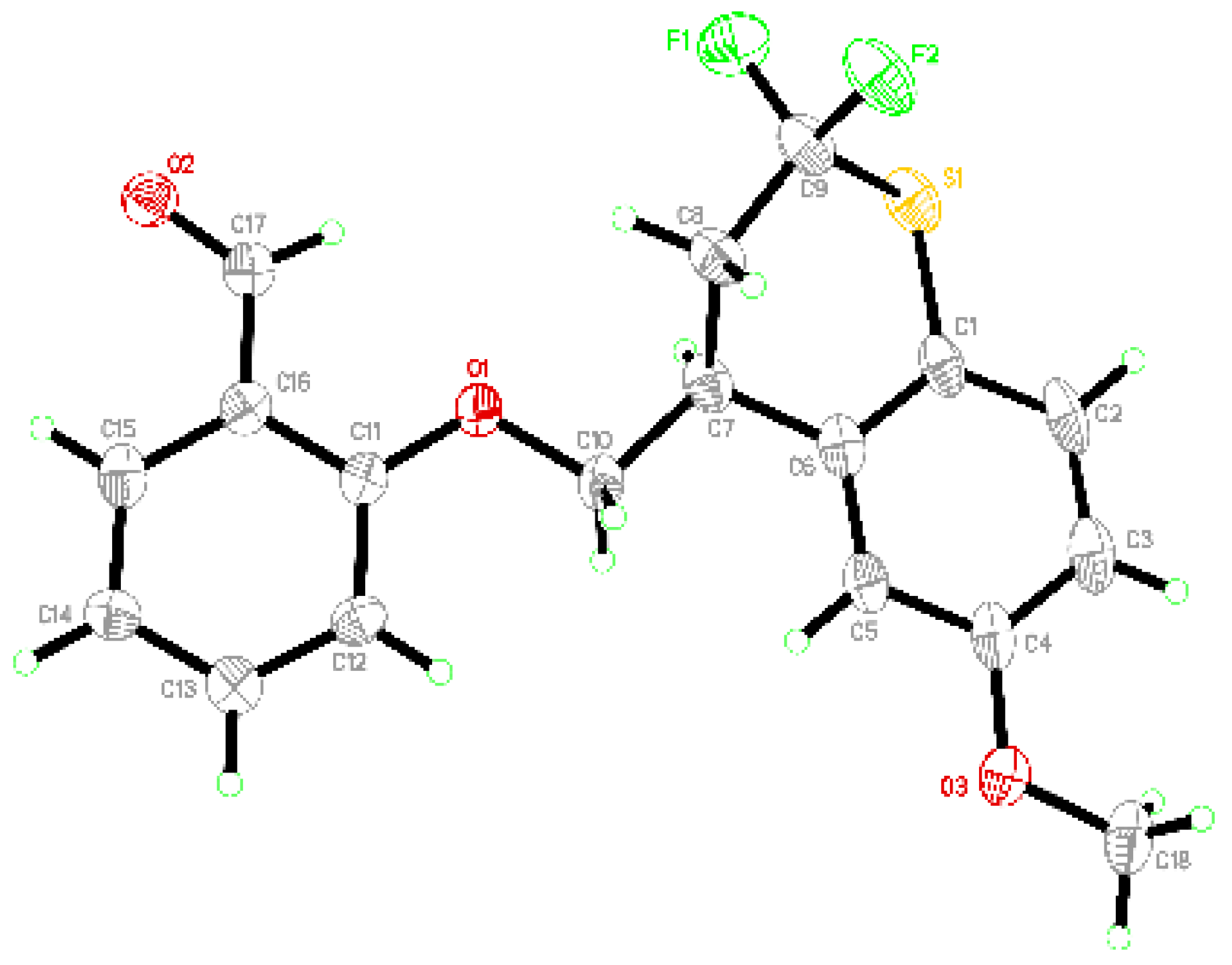
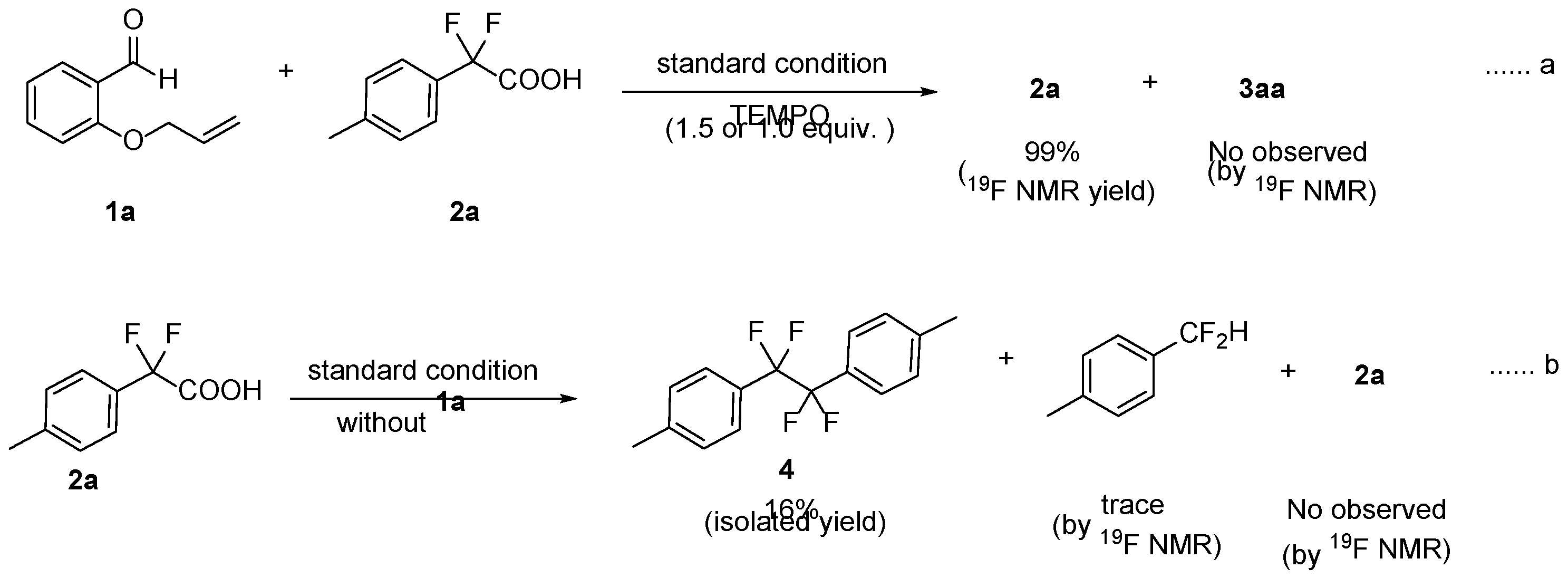
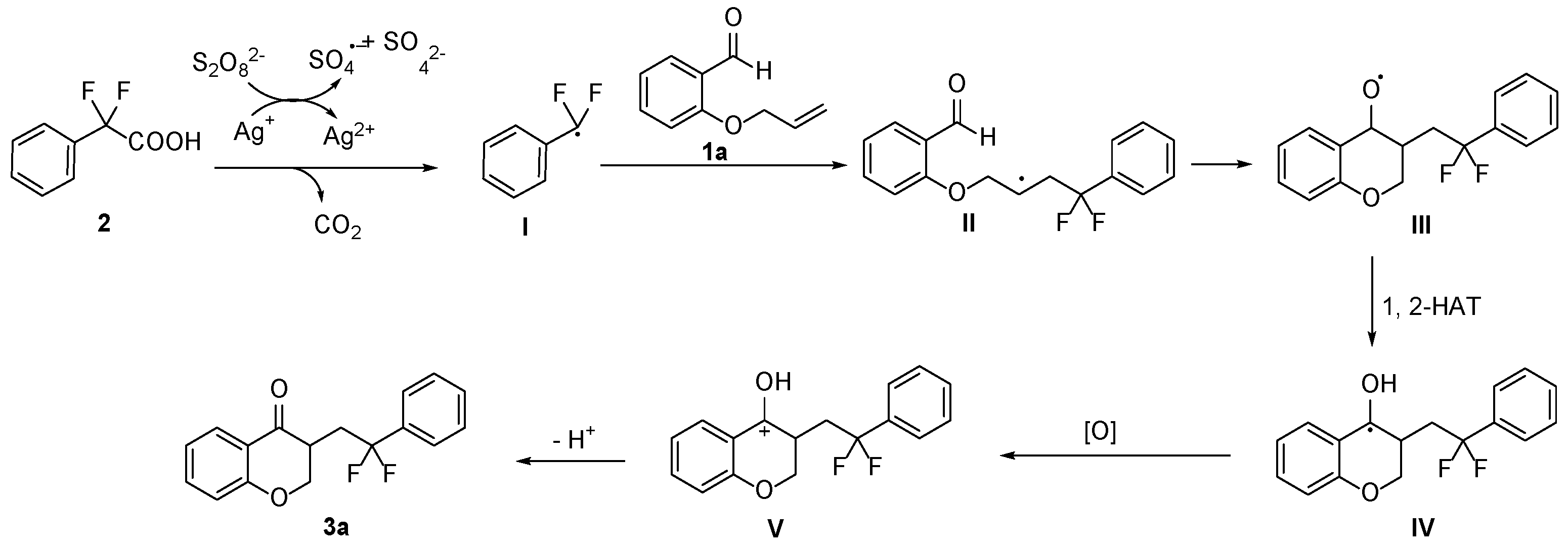
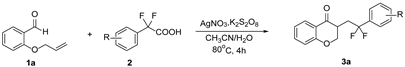
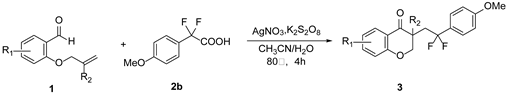
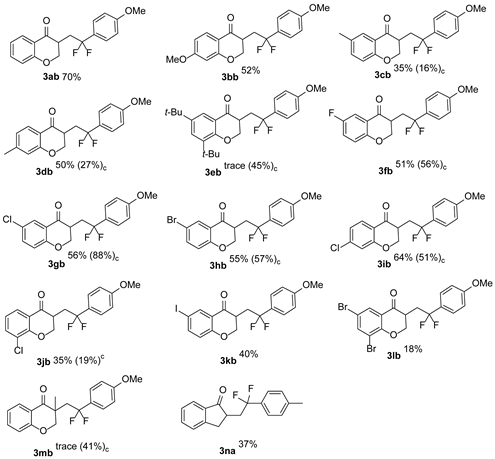
2. Results and Discussion
3. Conclusions
4. Materials and Methods
General Procedure for the Synthesis of Compounds 3
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Emami, S.; Ghanbarimasir, Z. Recent advances of chroman-4-one derivatives: Synthetic approaches and bioactivities. Eur. J. Med. Chem. 2015, 93, 539–563. [Google Scholar] [CrossRef]
- Fridén Saxin, M.; Seifert, T.; Landergren, M.R.; Suuronen, T.; Lahtela Kakkonen, M.; Jarho, E.M.; Luthman, K. Synthesis and evaluation of substituted chroman-4-one and chromone derivatives as sirtuin 2-selective inhibitors. J. Med. Chem. 2012, 55, 7104–7113. [Google Scholar] [CrossRef] [PubMed]
- Godlewski, G.; Offertaler, L.; Wagner, J.A.; Kunos, G. Receptors for acylethanolamides-GPR55 and GPR119. Prostag. Oth. Lipid Mediat. 2009, 89, 105–111. [Google Scholar] [CrossRef]
- McKee, T.C.; Bokesch, H.R.; McCormick, J.L.; Rashid, M.A.; Spielvogel, D.; Gustafson, K.R.; Alavanja, M.M.; Cardellina, J.H.; Boyd, M.R. Isolation and characterization of new anti-HIV and cytotoxic leads from plants, marine, and microbial organisms. J. Nat. Prod. 1997, 60, 431–438. [Google Scholar] [CrossRef]
- Hu, Q.F.; Niu, D.Y.; Zhou, B.; Ye, Y.Q.; Du, G.; Meng, C.Y.; Gao, X.M. Isoflavanones from the stem of cassia siamea and their anti-tobacco mosaic virus activities. Bull. Korean Chem. Soc. 2013, 34, 3013–3016. [Google Scholar] [CrossRef]
- Li, C.; Cao, Y.X.; Wang, R.; Wang, Y.N.; Lan, Q.; Wang, X.S. Cobalt-catalyzed difluoroalkylation of tertiary aryl ketones for facile synthesis of quaternary alkyl difluorides. Nat. Commun. 2018, 9, 4951. [Google Scholar] [CrossRef] [PubMed]
- Hirano, K.; Biju, A.T.; Piel, I.; Glorius, F. N-Heterocyclic carbene-catalyzed hydroacylation of unactivated double bonds. J. Am. Chem. Soc. 2009, 131, 14190–14191. [Google Scholar] [CrossRef]
- Hu, H.; Chen, X.; Sun, K.; Wang, J.; Liu, Y.; Liu, H.; Fan, L.; Yu, B.; Sun, Y.; Qu, L.; et al. Silver-Catalyzed radical cascade cyclization toward 1,5-/1,3-dicarbonyl heterocycles: An atom-/step-economical strategy leading to chromenopyridines and isoxazole-/pyrazole-containing chroman-4-ones. Org. Lett. 2018, 20, 6157–6160. [Google Scholar] [CrossRef]
- Li, K.; Chen, J.; Yang, C.; Zhang, K.; Pan, C.; Fan, B. Blue light promoted difluoroalkylation of aryl ketones: Synthesis of quaternary alkyl difluorides and tetrasubstituted monofluoroalkenes. Org. Lett. 2020, 22, 4261–4265. [Google Scholar] [CrossRef]
- Xie, L.-Y.; Peng, S.; Yang, L.-H.; Liu, X.-W. Metal-Free Synthesis of Carbamoylated Chroman-4-Ones via Cascade Radical Annulation of 2-(Allyloxy)arylaldehydes with Oxamic Acids. Molecules 2022, 27, 7049. [Google Scholar] [CrossRef]
- Sheng, J.; Liu, J.; Chen, L.; Zhang, L.; Zheng, L.; Wei, X. Silver-catalyzed cascade radical cyclization of 2-(allyloxy)arylaldehydes with cyclopropanols: Access to chroman-4-one derivatives. Org. Chem. Front. 2019, 6, 1471–1475. [Google Scholar] [CrossRef]
- Mei, Y.; Zhao, L.; Liu, Q.; Ruan, S.; Wang, L.; Li, P. Synthesis of sulfone-functionalized chroman-4-ones and chromans through visible-light-induced cascade radical cyclization under transition-metal-free conditions. Green Chem. 2020, 22, 2270–2278. [Google Scholar] [CrossRef]
- Pan, X.; Xia, H.; Wu, J. Recent advances in photoinduced trifluoromethylation and difluoroalkylation. Org. Chem. Front. 2016, 3, 1163–1185. [Google Scholar] [CrossRef]
- Hollingworth, C.; Gouverneur, V. Transition metal catalysis and nucleophilic fluorination. Chem. Commun. 2012, 48, 2929–2942. [Google Scholar] [CrossRef]
- Ni, C.; Hu, M.; Hu, J. Good partnership between sulfur and fluorine: Sulfur-Based fluorination and fluoroalkylation reagents for organic synthesis. Chem. Rev. 2015, 115, 765–825. [Google Scholar] [CrossRef]
- Hu, J.; Zhang, W.; Wang, F. Selective difluoromethylation and monofluoromethylation reactions. Chem. Commun. 2009, 7465–7478. [Google Scholar] [CrossRef]
- Tozer, M.J.; Herpin, T.F. Methods for the synthesis of gem-difluoromethylene compounds. Tetrahedron 1996, 52, 8619–8683. [Google Scholar] [CrossRef]
- Wang, J.; Sánchez Roselló, M.; Aceña, J.L.; Del Pozo, C.; Sorochinsky, A.E.; Fustero, S.; Soloshonok, V.A.; Liu, H. Fluorine in pharmaceutical industry: Fluorine-Containing drugs introduced to the market in the last decade (2001–2011). Chem. Rev. 2014, 114, 2432–2506. [Google Scholar] [CrossRef]
- Müller, K.; Faeh, C.; Diederich, F. Fluorine in pharmaceuticals: Looking beyond intuition. Science 2007, 317, 1881–1886. [Google Scholar] [CrossRef]
- Gillis, E.P.; Eastman, K.J.; Hill, M.D.; Donnelly, D.J.; Meanwell, N.A. Applications of fluorine in medicinal chemistry. J. Med. Chem. 2015, 58, 8315–8359. [Google Scholar] [CrossRef]
- Anderson, M.O.; Zhang, J.; Liu, Y.; Yao, C.J.; Phuan, P.W.; Verkman, A.S. Nanomolar potency and metabolically stable inhibitors of kidney urea transporter UT-B. J. Med. Chem. 2012, 55, 5942–5950. [Google Scholar] [CrossRef] [PubMed]
- Middleton, W.J.; Bingham, E.M. α,α-DifIuoroarylacetic acids: Preparation from (diethylamino)sulfur trifluoride and α-oxoarylacetates. J. Org. Chem. 1980, 45, 2883–2887. [Google Scholar] [CrossRef]
- Umemoto, T.; Singh, R.P.; Xu, Y.; Saito, N. Discovery of 4-tert-butyl-2,6-dimethylphenylsulfur trifluoride as a deoxofluorinating agent with high thermal stability as well as unusual resistance to aqueous hydrolysis, and its diverse fluorination capabilities including deoxofluoro-arylsulfinylation with high stereoselectivity. J. Am. Chem. Soc. 2010, 132, 18199–18205. [Google Scholar]
- Lal, G.S.; Pez, G.P.; Pesaresi, R.J.; Prozonic, F.M.; Cheng, H. Bis(2-methoxyethyl)aminosulfur trifluoride: A new broad-spectrum deoxofluorinating agent with enhanced thermal stability. J. Org. Chem. 1999, 64, 7048–7054. [Google Scholar] [CrossRef]
- Xia, J.B.; Zhu, C.; Chen, C. Visible light-promoted metal-free C-H activation: Diarylketone-Catalyzed selective benzylic mono- and difluorination. J. Am. Chem. Soc. 2013, 135, 17494–17500. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Guo, W.H.; He, X.; Guo, Y.L.; Zhang, X.Y.; Zhang, X.G. Difluoromethylation of (hetero)aryl chlorides with chlorodifluoromethane catalyzed by nickel. Nat. Commun. 2018, 9, 1170. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Y.L.; Min, Q.Q.; Xu, C.; Wang, R.W.; Zhang, X.G. Nickel-Catalyzed difluoroalkylation of (hetero)arylborons with unactivated 1-bromo-1,1-difluoroalkanes. Angew. Chem. Int. Ed. 2016, 55, 5837–5841. [Google Scholar] [CrossRef] [PubMed]
- Min, Q.Q.; Yin, Z.S.; Feng, Z.; Guo, W.H.; Zhang, X.G. Highly selective gem-difluoroallylation of organoborons with bromodifluoromethylated alkenes catalyzed by palladium. J. Am. Chem. Soc. 2014, 136, 1230–1233. [Google Scholar] [CrossRef]
- Chen, F.; Hashmi, A.S.K. Silver-Catalyzed decarboxylative alkynylation of α,α-difluoroarylacetic acids with ethynylbenziodoxolone reagents. Org. Lett. 2016, 18, 2880–2882. [Google Scholar] [CrossRef]
- Yuan, J.-W.; Zhang, M.-Y.; Liu, Y.; Hu, W.-Y.; Yang, L.-R.; Xiao, Y.-M.; Diao, X.-Q.; Zhang, S.-R.; Mao, J. Transition-metal-free radical difluorobenzylation/cyclization of unactivated alkenes: Access to ArCF2-substituted ring-fused quinazolinones. Org. Biomol. Chem. 2022, 20, 9722–9733. [Google Scholar] [CrossRef]
- Zhao, H.; Ma, G.; Xie, X.; Wang, Y.; Hao, J.; Wan, W. Pd(Ⅱ)-Catalyzed decarboxylative meta-C-H difluoromethylation. Chem. Commun. 2019, 55, 3927–3930. [Google Scholar] [CrossRef] [PubMed]
- Hong, G.; Yuan, J.; Fu, J.; Pan, G.; Wang, Z.; Yang, L.; Xiao, Y.; Mao, P.; Zhang, X. Transition-metal-free decarboxylative C3-difluoroarylmethylation of quinoxalin-2(1H)-ones with α,α-difluoroarylacetic acids. Org. Chem. Front. 2019, 6, 1173–1182. [Google Scholar] [CrossRef]
- Wille, U. Radical cascades initiated by intermolecular radical addition to alkynes and related triple bond systems. Chem. Rev. 2013, 113, 813–853. [Google Scholar] [CrossRef]
- Zhou, Y.; Xiong, Z.; Qiu, J.; Kong, L.; Zhu, G. Visible light photocatalytic acyldifluoroalkylation of unactivated alkenes for the direct synthesis of gem-difluorinated ketones. Org. Chem. Front. 2019, 6, 1022–1026. [Google Scholar] [CrossRef]
- Zhou, N.; Wu, M.; Zhang, M.; Zhou, X. Visible-light-induced difluoroacetylation of O-(allyloxy) aryl-aldehydes: Access to difluoroacetylated chroman-4-ones. Asian J. Org. Chem. 2019, 8, 828–831. [Google Scholar] [CrossRef]
- Huang, H.L.; Du, J.Y.; Li, Q.L.; Gao, F.; Ma, C.L. Visible-light-promoted cascade radical cyclization: Synthesis of chroman-4-ones and dihydroquinolin-4-ones. J. Org. Chem. 2020, 85, 3963–3972. [Google Scholar] [CrossRef]
- Wang, P.; Du, P.; Sun, Q.; Zhang, J.; Deng, H.; Jiang, H. Silver-catalyzed decarboxylative radical allylation of α,α-difluoroarylacetic acids for the construction of CF2-allyl bonds. Org. Biomol. Chem. 2021, 19, 2023–2029. [Google Scholar] [CrossRef]
- Liu, X.; Wang, Z.; Cheng, X.; Li, C. Silver-Catalyzed decarboxylative alkynylation of aliphatic carboxylic acids in aqueous solution. J. Am. Chem. Soc. 2012, 134, 14330–14333. [Google Scholar] [CrossRef] [PubMed]
- Hu, F.; Shao, X.; Zhu, D.; Lu, L.; Shen, Q. Silver-Catalyzed decarboxylative trifluoromethylthiolation of aliphatic carboxylic acids in aqueous emulsion. Angew. Chem. Int. Ed. 2014, 53, 6105–6109. [Google Scholar] [CrossRef]
- Zhu, Y.; Li, X.; Wang, X.; Huang, X.; Shen, T.; Zhang, Y.; Sun, X.; Zou, M.; Song, S.; Jiao, N. Silver-Catalyzed decarboxylative azidation of aliphatic carboxylic acids. Org. Lett. 2015, 17, 4702–4705. [Google Scholar] [CrossRef]
- Du, P.; Sun, Q.; Li, H.; Zhang, J.; Deng, H.; Jiang, H. Silver-catalyzed radical cascade arylthiodifluoromethylation/cyclization of isonitriles for the synthesis of 6-phenanthridinyldifluoromethyl aryl thioethers. Chem. Asian J. 2022, 17, e202200088. [Google Scholar] [CrossRef] [PubMed]
- Hu, H.; Chen, X.; Sun, K.; Wang, J.; Liu, Y.; Liu, H.; Yu, B.; Sun, Y.; Qu, L.; Zhao, Y. Silver-catalyzed decarboxylative cascade radical cyclization of tert-carboxylic acids and o-(allyloxy)arylaldehydes towards chroman-4-one derivatives. Org. Chem. Front. 2018, 5, 2925–2929. [Google Scholar] [CrossRef]
- Cui, L.; Chen, H.; Liu, C.; Li, C. Silver-Catalyzed decarboxylative allylation of aliphatic carboxylic acids in aqueous solution. Org. Lett. 2016, 18, 2188–2191. [Google Scholar] [CrossRef] [PubMed]
- Jagdale, A.R.; Park, J.H.; Youn, S.W. Cyclization Reaction for the Synthesis of Polysubstituted Naphthalenes in the Presence of Au (I) Precatalysts. J. Org. Chem. 2011, 76, 7204–7215. [Google Scholar] [CrossRef]
- Mizuta, S.; Stenhagen, I.S.R.; O’Duill, M.; Wolstenhulme, J.; Kirjavainen, A.K.; Forsback, S.J.; Tredwell, M.; Sandford, G.; Moore, P.R.; Huiban, M.; et al. Catalytic decarboxylative fluorination for the synthesis of tri-and difluoromethyl arenes. Org. Lett. 2013, 15, 2648–2651. [Google Scholar] [CrossRef]
- Chang, Y.; Tewari, A.; Adi, A.; Bae, C. Direct nucleophilic fluorination of carbonyl groups of benzophenones and benzils with Deoxofluor. Tetrahedron 2008, 64, 9837–9842. [Google Scholar] [CrossRef]
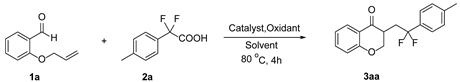 | ||||
|---|---|---|---|---|
| Entry | Catalyst (mol%) | Oxidant (equiv.) | Solvent | Yield (%) b |
| 1 | AgNO3 (20) | K2S2O8 (2.0) | CH3CN/H2O (1:1) | 56 |
| 2 | AgNO3 (20) | K2S2O8 (2.0) | DMSO/H2O (1:1) | 45 |
| 3 | AgNO3 (20) | K2S2O8 (2.0) | EtOH/H2O (1:1) | 5 |
| 4 | AgNO3 (20) | K2S2O8 (2.0) | H2O | 10 |
| 5 | AgNO3 (20) | K2S2O8 (2.0) | CH3CN | N.R. |
| 6 | AgNO3 (20) | K2S2O8 (2.0) | CH3CN/H2O (1:3) | 73 |
| 7 | AgNO3 (20) | Na2S2O8 (2.0) | CH3CN/H2O (1:3) | 66 |
| 8 | AgNO3 (20) | (NH4)2S2O8 (2.0) | CH3CN/H2O (1:3) | 63 |
| 9 | AgNO3 (20) | Selectfluor (2.0) | CH3CN/H2O (1:3) | 0 |
| 10 | \ | K2S2O8 (2.0) | CH3CN/H2O (1:3) | 49 |
| 11 | AgNO3 (20) | \ | CH3CN/H2O (1:3) | 0 |
| 12 | AgNO3 (30) | K2S2O8 (2.0) | CH3CN/H2O (1:3) | 67 |
| 13 | AgNO3 (10) | K2S2O8 (2.0) | CH3CN/H2O (1:3) | 63 |
| 14 c | AgNO3 (20) | K2S2O8 (2.0) | CH3CN/H2O (1:3) | 50 |
| 15 d | AgNO3 (20) | K2S2O8 (2.0) | CH3CN/H2O (1:3) | 53 |
| 16 e | AgNO3 (20) | K2S2O8 (2.0) | CH3CN/H2O (1:3) | 68 |
| 17 | AgNO3 (20) | K2S2O8 (1.75) | CH3CN/H2O (1:3) | 70 |
| 18 | AgNO3 (20) | K2S2O8 (2.25) | CH3CN/H2O (1:3) | 64 |
| 19 | AgOAc (20) | K2S2O8 (2.0) | CH3CN/H2O (1:3) | 65 |
| 20 | Ag2O (20) | K2S2O8 (2.0) | CH3CN/H2O (1:3) | 68 |
| 21 | Ag2CO3 (20) | K2S2O8 (2.0) | CH3CN/H2O (1:3) | 65 |
| 22 f | \ | (NH4)2S2O8 (2.0) | DMSO | 32 |
 |
 |
 |
 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, Q.; Li, H.; Chen, X.; Hao, J.; Deng, H.; Jiang, H. Silver-Promoted Radical Cascade Aryldifluoromethylation/Cyclization of 2-Allyloxybenzaldehydes for the Synthesis of 3-Aryldifluoromethyl-Containing Chroman-4-one Derivatives. Molecules 2023, 28, 3578. https://doi.org/10.3390/molecules28083578
Sun Q, Li H, Chen X, Hao J, Deng H, Jiang H. Silver-Promoted Radical Cascade Aryldifluoromethylation/Cyclization of 2-Allyloxybenzaldehydes for the Synthesis of 3-Aryldifluoromethyl-Containing Chroman-4-one Derivatives. Molecules. 2023; 28(8):3578. https://doi.org/10.3390/molecules28083578
Chicago/Turabian StyleSun, Qianqian, Hongxiao Li, Xingyu Chen, Jian Hao, Hongmei Deng, and Haizhen Jiang. 2023. "Silver-Promoted Radical Cascade Aryldifluoromethylation/Cyclization of 2-Allyloxybenzaldehydes for the Synthesis of 3-Aryldifluoromethyl-Containing Chroman-4-one Derivatives" Molecules 28, no. 8: 3578. https://doi.org/10.3390/molecules28083578
APA StyleSun, Q., Li, H., Chen, X., Hao, J., Deng, H., & Jiang, H. (2023). Silver-Promoted Radical Cascade Aryldifluoromethylation/Cyclization of 2-Allyloxybenzaldehydes for the Synthesis of 3-Aryldifluoromethyl-Containing Chroman-4-one Derivatives. Molecules, 28(8), 3578. https://doi.org/10.3390/molecules28083578





