Exploring the Effect of Different Storage Conditions on the Aroma Profile of Bread by Using Arrow-SPME GC-MS and Chemometrics
Abstract
:1. Introduction
2. Results and Discussion
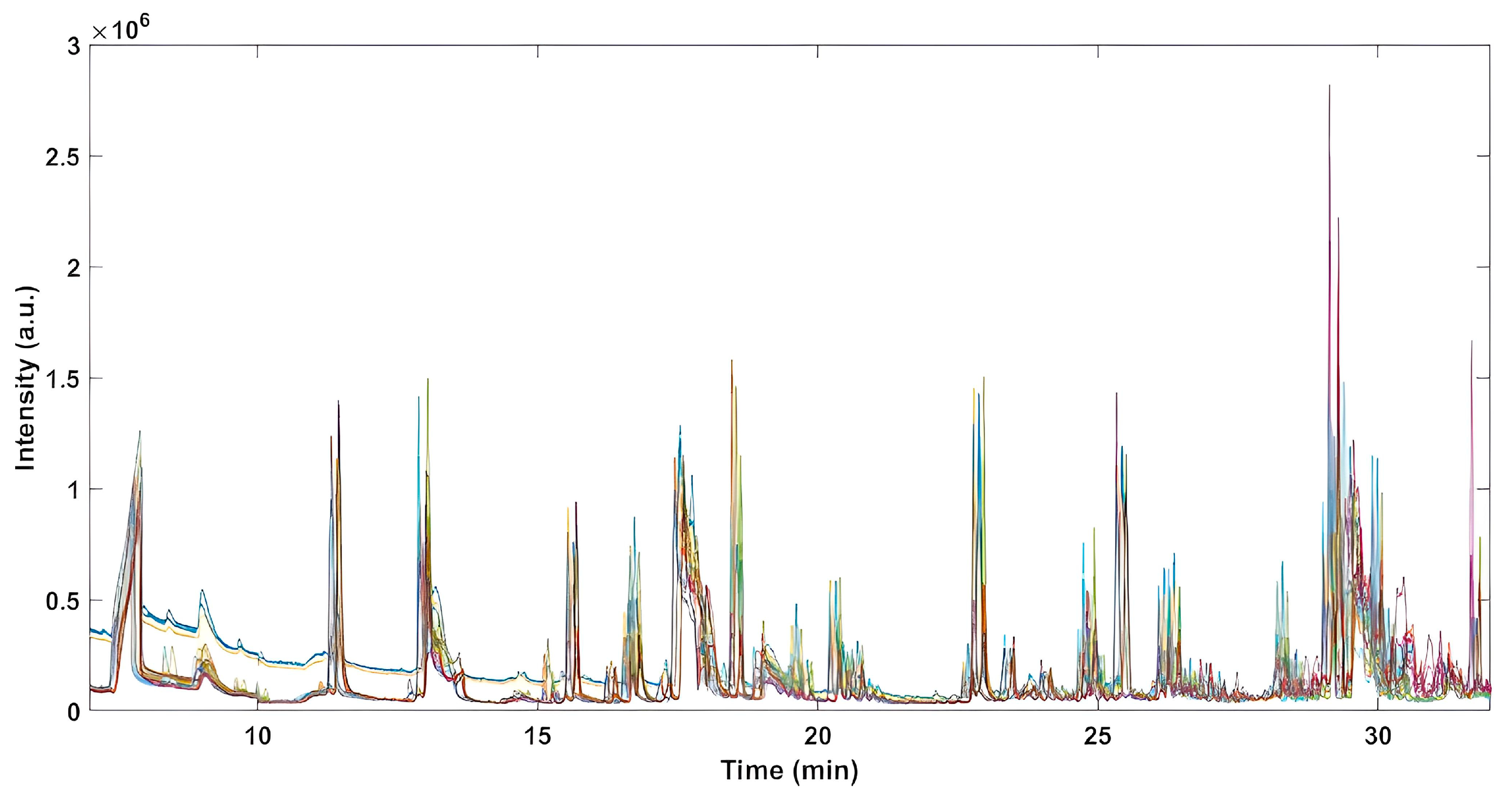
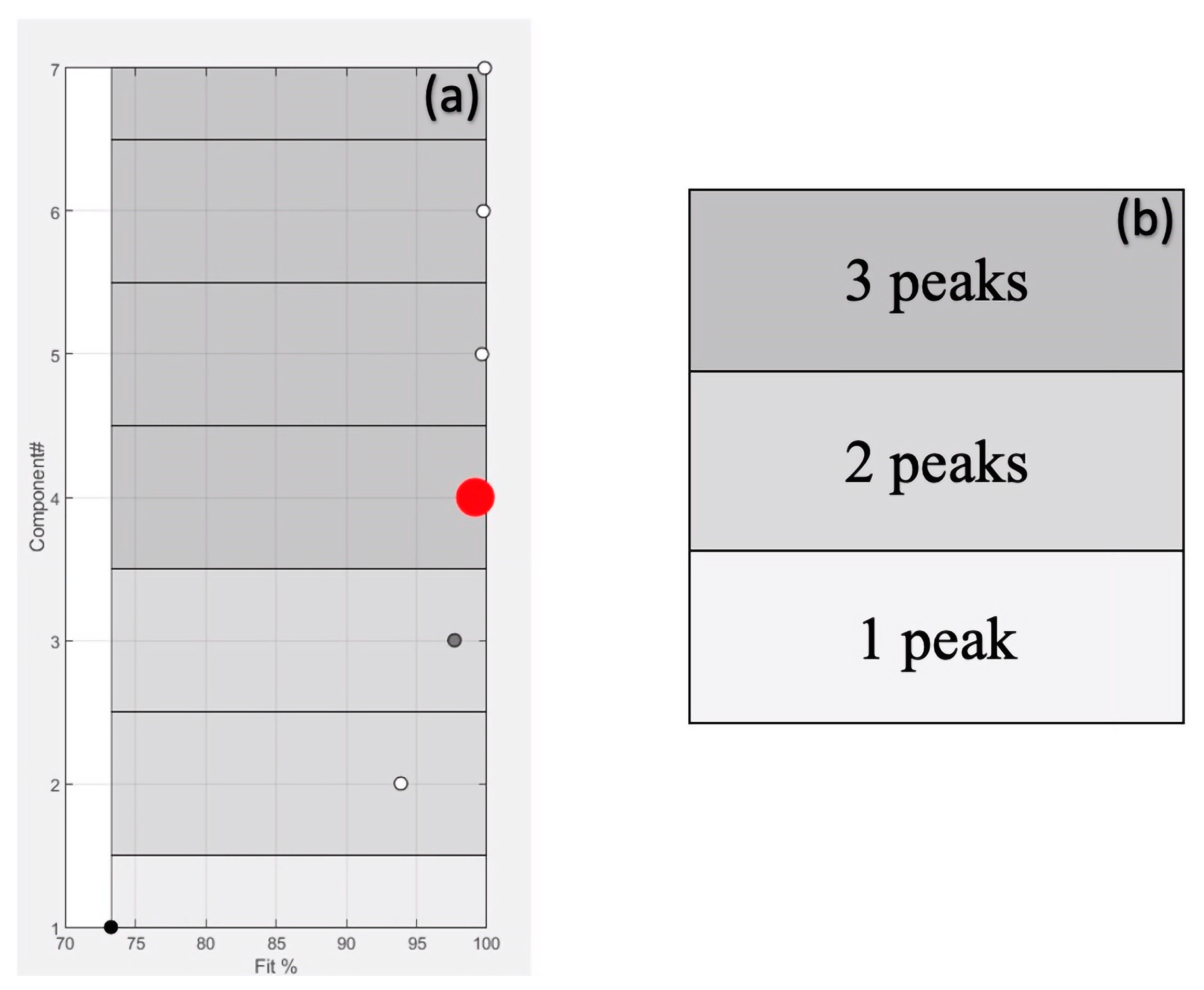
2.1. Peaks Deconvolution and Resolution
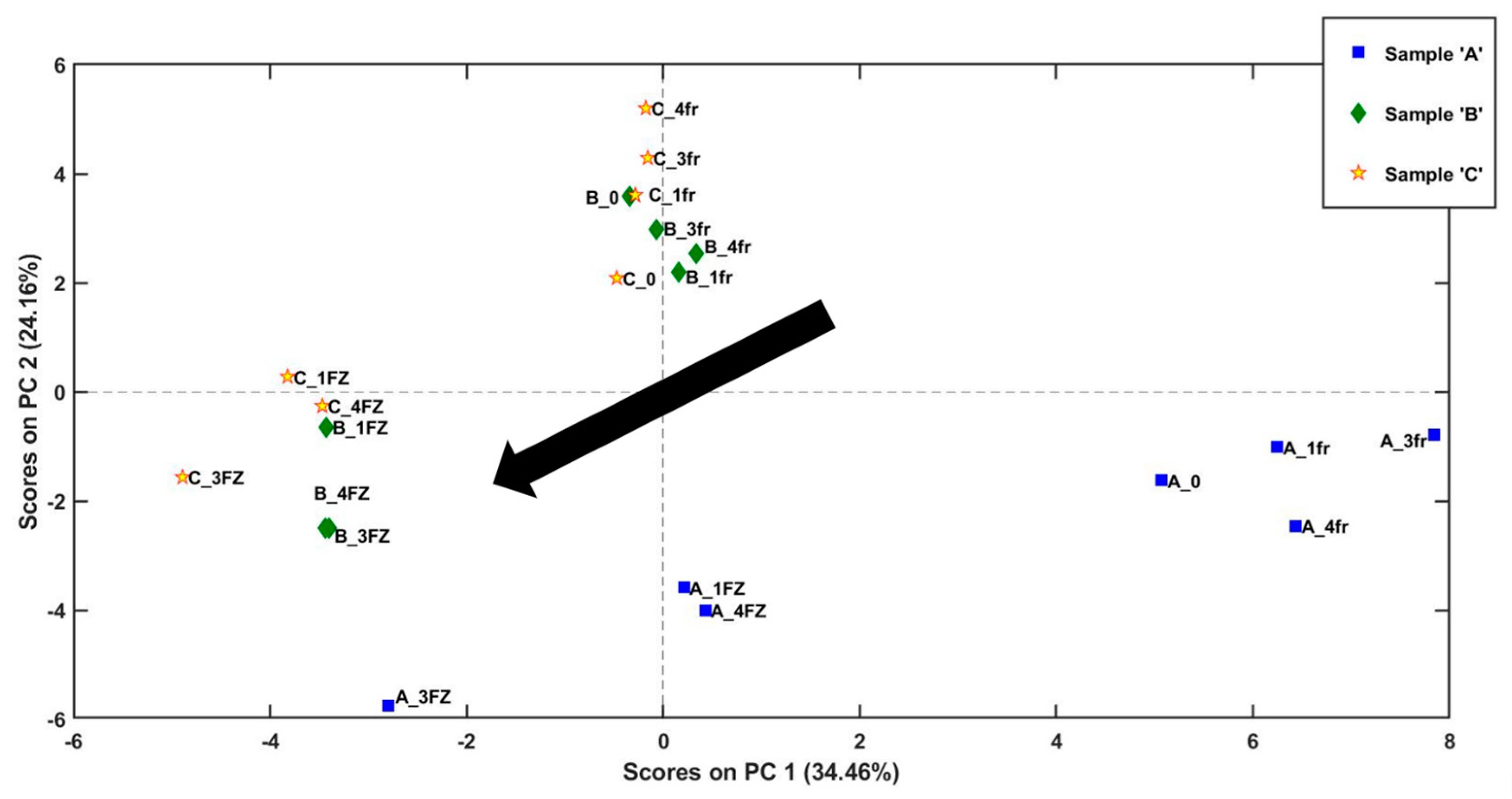
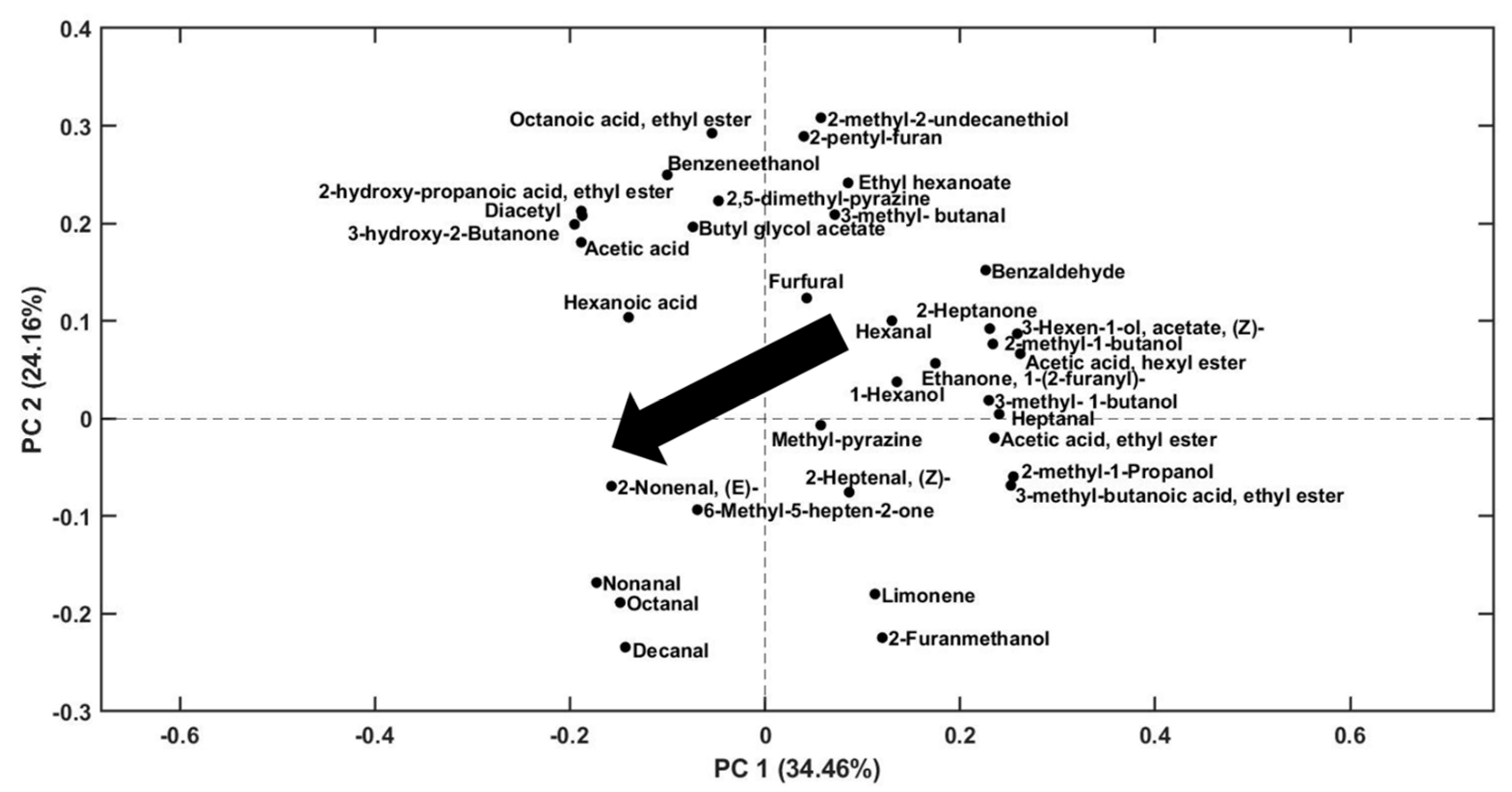
2.2. Evaluation of Volatile Compounds during Storage
3. Materials and Methods
3.1. Samples and Storage Conditions
3.2. SPME-Arrow-GC-MS
3.3. Data Analysis
3.4. Software
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- UNESCO. Mediterranean Diet. Available online: https://ich.unesco.org/en/RL/mediterranean-diet-00884 (accessed on 4 January 2023).
- Martins, Z.E.; Erben, M.; Gallardo, A.E.; Silva, R.; Barbosa, I.; Pinho, O.; Ferreira, I.M.P.L.V.O. Effect of Spent Yeast Fortification on Physical Parameters, Volatiles and Sensorial Characteristics of Home-Made Bread. Int. J. Food Sci. Technol. 2015, 50, 1855–1863. [Google Scholar] [CrossRef]
- Angelino, D.; Cossu, M.; Marti, A.; Zanoletti, M.; Chiavaroli, L.; Brighenti, F.; Del Rio, D.; Martini, D. Bioaccessibility and Bioavailability of Phenolic Compounds in Bread: A Review. Food Funct. 2017, 8, 2368–2393. [Google Scholar] [CrossRef] [PubMed]
- Pico, J.; Bernal, J.; Gómez, M. Wheat Bread Aroma Compounds in Crumb and Crust: A Review. Food Res. Int. 2015, 75, 200–215. [Google Scholar] [CrossRef] [PubMed]
- Starowicz, M. Analysis of Volatiles in Food Products. Separations 2021, 8, 157. [Google Scholar] [CrossRef]
- Herrington, J.; Gómez-Ríos, G.; Myers, C.; Stidsen, G.; Bell, D. Hunting Molecules in Complex Matrices with SPME Arrows: A Review. Separations 2020, 7, 12. [Google Scholar] [CrossRef]
- Strani, L.; D’Alessandro, A.; Ballestrieri, D.; Durante, C.; Cocchi, M. Fast GC E-Nose and Chemometrics for the Rapid Assessment of Basil Aroma. Chemosensors 2022, 10, 105. [Google Scholar] [CrossRef]
- Maletti, L.; D’Eusanio, V.; Durante, C.; Marchetti, A.; Tassi, L. VOCs Analysis of Three Different Cultivars of Watermelon (Citrullus Lanatus L.) Whole Dietary Fiber. Molecules 2022, 27, 8747. [Google Scholar] [CrossRef]
- De Luca, L.; Aiello, A.; Pizzolongo, F.; Blaiotta, G.; Aponte, M.; Romano, R. Volatile Organic Compounds in Breads Prepared with Different Sourdoughs. Appl. Sci. 2021, 11, 1330. [Google Scholar] [CrossRef]
- Cho, I.H.; Peterson, D.G. Chemistry of Bread Aroma: A Review. Food Sci. Biotechnol. 2010, 19, 575–582. [Google Scholar] [CrossRef]
- Pico, J.; Martínez, M.M.; Bernal, J.; Gómez, M. Impact of Frozen Storage Time on the Volatile Profile of Wheat Bread Crumb. Food Chem. 2017, 232, 185–190. [Google Scholar] [CrossRef]
- Ronda, F.; Caballero, P.A.; Quilez, J.; Roos, Y.H. Staling of Frozen Partly and Fully Baked Breads. Study of the Combined Effect of Amylopectin Recrystallization and Water Content on Bread Firmness. J. Cereal Sci. 2011, 53, 97–103. [Google Scholar] [CrossRef]
- Demets, R.; Van Broekhoven, S.; Gheysen, L.; Van Loey, A.; Foubert, I. The Potential of Phaeodactylum as a Natural Source of Antioxidants for Fish Oil Stabilization. Foods 2022, 11, 1461. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.-B.; Murtada, K.; Pawliszyn, J. Determination of Selected Volatile Terpenes in Fish Samples via Solid Phase Microextraction Arrow Coupled with GC-MS. Talanta 2021, 221, 121446. [Google Scholar] [CrossRef] [PubMed]
- Nam, T.G.; Lee, J.-Y.; Kim, B.-K.; Song, N.-E.; Jang, H.W. Analyzing Volatiles in Brown Rice Vinegar by Headspace Solid-Phase Microextraction (SPME)–Arrow: Optimizing the Extraction Conditions and Comparisons with Conventional SPME. Int. J. Food Prop. 2019, 22, 1195–1204. [Google Scholar] [CrossRef]
- Šikuten, I.; Štambuk, P.; Karoglan Kontić, J.; Maletić, E.; Tomaz, I.; Preiner, D. Optimization of SPME-Arrow-GC/MS Method for Determination of Free and Bound Volatile Organic Compounds from Grape Skins. Molecules 2021, 26, 7409. [Google Scholar] [CrossRef]
- Stilo, F.; Cordero, C.; Sgorbini, B.; Bicchi, C.; Liberto, E. Highly Informative Fingerprinting of Extra-Virgin Olive Oil Volatiles: The Role of High Concentration-Capacity Sampling in Combination with Comprehensive Two-Dimensional Gas Chromatography. Separations 2019, 6, 34. [Google Scholar] [CrossRef]
- Pellacani, S.; Celli, S.; Cocchi, M.; Durante, C.; Mariani, M.; Marchetti, A.; Strani, L. Novel analytical method based on SPME-Arrow and deep learning for the characterization of the aroma profile of industrial bread. Talanta 2023. submitted. [Google Scholar]
- Amigo, J.M.; Skov, T.; Bro, R.; Coello, J.; Maspoch, S. Solving GC-MS Problems with PARAFAC2. TrAC Trends Anal. Chem. 2008, 27, 714–725. [Google Scholar] [CrossRef]
- Kamstrup-Nielsen, M.H.; Johnsen, L.G.; Bro, R. Core Consistency Diagnostic in PARAFAC2. J. Chemom. 2013, 27, 99–105. [Google Scholar] [CrossRef]
- Baccolo, G.; Quintanilla-Casas, B.; Vichi, S.; Augustijn, D.; Bro, R. From Untargeted Chemical Profiling to Peak Tables—A Fully Automated AI Driven Approach to Untargeted GC-MS. TrAC Trends Anal. Chem. 2021, 145, 116451. [Google Scholar] [CrossRef]
- Risum, A.B.; Bro, R. Using Deep Learning to Evaluate Peaks in Chromatographic Data. Talanta 2019, 204, 255–260. [Google Scholar] [CrossRef] [PubMed]
- Seitz, L.M.; Chung, O.K.; Rengarajan, R. Volatiles in Selected Commercial Breads. Cereal Chem. 1998, 75, 847–853. [Google Scholar] [CrossRef]
- Birch, A.N.; Petersen, M.A.; Hansen, Å.S. REVIEW: Aroma of Wheat Bread Crumb. Cereal Chem. 2014, 91, 105–114. [Google Scholar] [CrossRef]
- FoodB. Available online: https://foodb.ca/ (accessed on 27 March 2023).
- Apriyanto, A.; Compart, J.; Fettke, J. A Review of Starch, a Unique Biopolymer—Structure, Metabolism and in Planta Modifications. Plant Sci. 2022, 318, 111223. [Google Scholar] [CrossRef]
- Hebeda, R.E.; Zobel, H.F. (Eds.) Baked Goods Freshness: Technology, Evaluation, and Inhibition of Staling; Food Science and Technology (Dekker); Marcel Dekker: New York, NY, USA, 1996; ISBN 978-0-8247-9348-7. [Google Scholar]
- Birch, A.N.; Petersen, M.A.; Arneborg, N.; Hansen, Å.S. Influence of Commercial Baker’s Yeasts on Bread Aroma Profiles. Food Res. Int. 2013, 52, 160–166. [Google Scholar] [CrossRef]
- Amigo, J.M.; Popielarz, M.J.; Callejón, R.M.; Morales, M.L.; Troncoso, A.M.; Petersen, M.A.; Toldam-Andersen, T.B. Comprehensive Analysis of Chromatographic Data by Using PARAFAC2 and Principal Components Analysis. J. Chromatogr. A 2010, 1217, 4422–4429. [Google Scholar] [CrossRef]


| Analyte | Retention Time (min) | Match Factor (MF) |
|---|---|---|
| Diacetyl | 7.53 | 87.6 |
| Acetic acid | 7.87 | 98.1 |
| Acetic acid, ethyl ester | 8.42 | 89.4 |
| 2-methyl-1-propanol | 9.01 | 93.2 |
| 3-methyl-butanal | 9.70 | 88.3 |
| 2-butanone, 3-hydroxy- | 11.40 | 95.2 |
| 3-methyl-1-butanol | 12.99 | 93.3 |
| 2-methyl-1-butanol | 13.18 | 95.2 |
| Hexanal | 15.64 | 95 |
| Propanoic acid, 2-hydroxy-, ethyl ester | 16.33 | 93.2 |
| Methyl-pyrazine | 16.64 | 91.1 |
| Furfural | 16.76 | 97.2 |
| 2-furanmethanol | 18.03 | 92.3 |
| 3-methyl-butanoic acid, ethyl ester | 18.34 | 94.5 |
| 1-hexanol | 19.08 | 93.9 |
| 2-heptanone | 19.82 | 91.8 |
| Heptanal | 20.32 | 95.7 |
| Ethanone, 1-(2-furanyl)- | 20.47 | 95.8 |
| 2,5-dimethyl-pyrazine | 20.74 | 93.6 |
| 2-heptenal, (Z)- | 22.69 | 95.5 |
| Benzaldehyde | 22.89 | 97 |
| Hexanoic acid | 23.42 | 93.3 |
| 6-methyl-5-hepten-2-one | 24.082 | 95.5 |
| Ethyl hexanoate | 24.76 | 96.9 |
| 2-pentyl-furan, | 24.85 | 92.7 |
| Octanal | 24.91 | 89.2 |
| 3-hexen-1-ol, acetate, (Z)- | 25.03 | 98.3 |
| Acetic acid, hexyl ester | 25.33 | 92.6 |
| Limonene | 26.95 | 96.1 |
| 2-Octenal | 27.19 | 93.5 |
| Butyl glycol acetate | 28.29 | 90.3 |
| 2-methyl-2-undecanethiol | 29.13 | 91.2 |
| Nonanal | 29.22 | 88.4 |
| Benzeneethanol | 29.62 | 97.7 |
| 2-nonenal, (E)- | 31.19 | 90.1 |
| Octanoic acid, ethyl ester | 32.42 | 87.9 |
| Decanal | 32.73 | 88.8 |
| 2(3H)-furanone, dihydro-5-pentyl- | 36.46 | 90.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pellacani, S.; Cocchi, M.; Durante, C.; Strani, L. Exploring the Effect of Different Storage Conditions on the Aroma Profile of Bread by Using Arrow-SPME GC-MS and Chemometrics. Molecules 2023, 28, 3587. https://doi.org/10.3390/molecules28083587
Pellacani S, Cocchi M, Durante C, Strani L. Exploring the Effect of Different Storage Conditions on the Aroma Profile of Bread by Using Arrow-SPME GC-MS and Chemometrics. Molecules. 2023; 28(8):3587. https://doi.org/10.3390/molecules28083587
Chicago/Turabian StylePellacani, Samuele, Marina Cocchi, Caterina Durante, and Lorenzo Strani. 2023. "Exploring the Effect of Different Storage Conditions on the Aroma Profile of Bread by Using Arrow-SPME GC-MS and Chemometrics" Molecules 28, no. 8: 3587. https://doi.org/10.3390/molecules28083587








