Research Progress on Propylene Preparation by Propane Dehydrogenation
Abstract
:1. Introduction
2. Catalysts for Anaerobic Dehydrogenation Reaction
2.1. Platinum-Based Catalyst
2.1.1. Improvement of Support
2.1.2. Effect of Additives
2.2. Cr-Based Catalyst
Modification of Supports
2.3. Introduction of Several Propane Anaerobic Dehydrogenation Industrialization Technologies
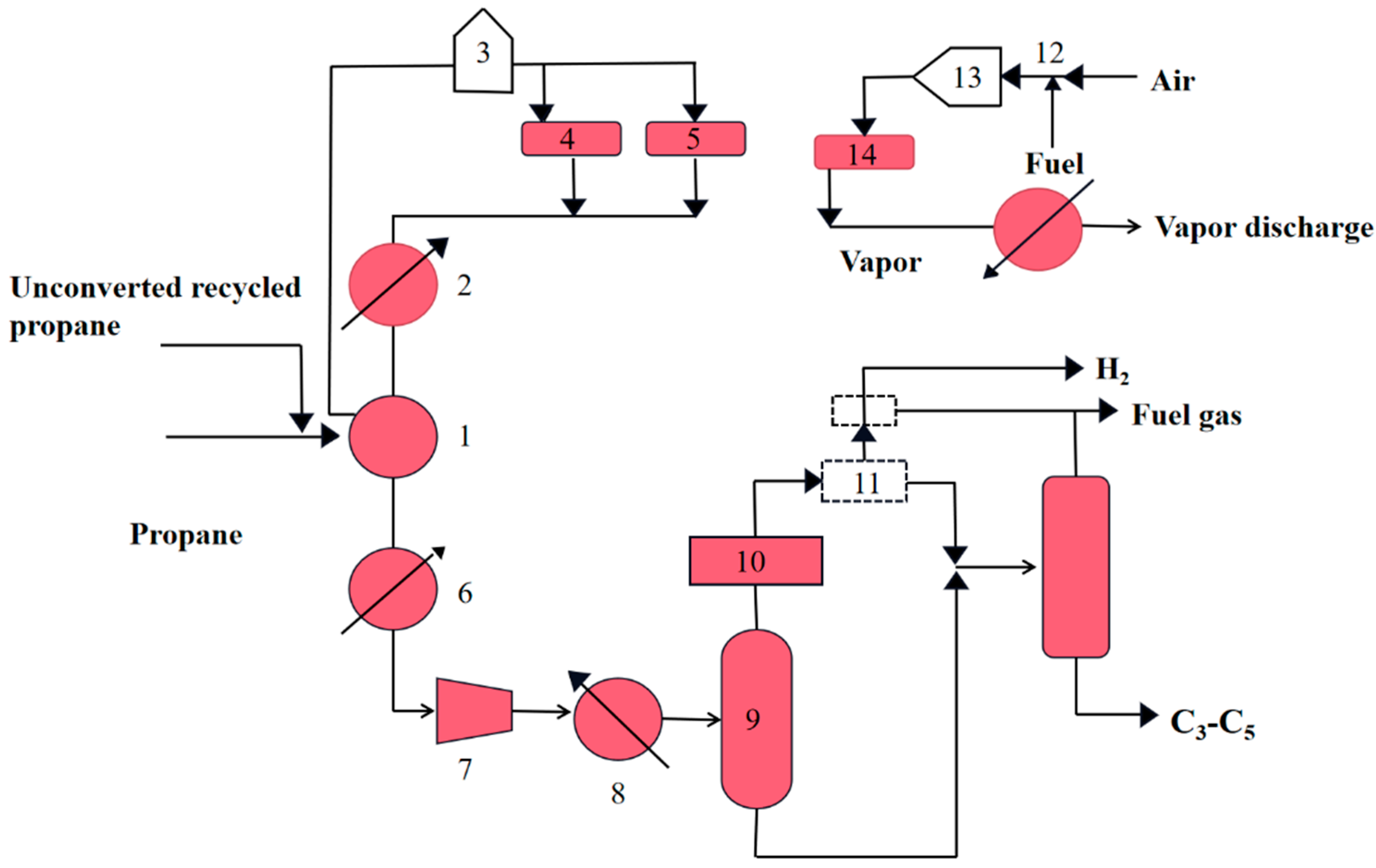
2.3.1. Catofin Process
2.3.2. Oleflex Process
3. Catalysts for Oxidative Dehydrogenation Reaction
3.1. Chromium-Based Catalysts
3.2. Vanadium-Based Catalysts
3.3. Gallium-Based Catalysts
4. The Process of Chemical Looping Oxidative Dehydrogenation
4.1. Monometallic Active Oxygen Carriers
4.2. Bimetallic or Polymetallic Composite Oxygen Carriers
5. Conclusions and Prospects
- (1)
- The current methods for propylene production are anaerobic and oxidative dehydrogenation. The anaerobic method has been used for many years, but is expensive, due to high equipment and catalyst costs. The oxidative dehydrogenation method is cheaper, but the extent of CO2 influence on the reaction is difficult to control at certain temperatures, and the reaction mechanism is still unclear, resulting in variable product yields.
- (2)
- In contrast, chemical looping oxidative dehydrogenation resolves the drawbacks of the previous methods. Lattice oxygen release can be controlled by appropriate bimetallic or polymetallic oxides, replacing molecular oxygen. This effectively controls the reaction rate of propane dehydrogenation to produce propylene, and improves the conversion of propane with high selectivity for propylene, compared to oxygen-free dehydrogenation and gas oxidant methods.
- (3)
- The future of chemical looping oxidative dehydrogenation for industrial applications requires the development of multi-component coupled composite oxygen carriers with high oxygen loading, extended cycle life, and high propylene yield.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bai, P.T.; Manokaran, V.; Saiprasad, P.S.; Srinath, S. Studies on Heat and Mass Transfer Limitations in Oxidative Dehydrogenation of Ethane Over Cr2O3/Al2O3 Catalyst. Procedia Eng. 2015, 127, 1338–1345. [Google Scholar] [CrossRef]
- Zeeshan, N. Light alkane dehydrogenation to light olefin technologies: A comprehensive review. Rev. Chem. Eng. 2015, 31, 413–436. [Google Scholar]
- Wang, H.M.; Chen, Y.; Yan, X.; Lang, W.Z.; Guo, Y.J. Cr doped mesoporous silica spheres for propane dehydrogenation in the presence of CO2: Effect of Cr adding time in sol-gel process. Micropor. Mesopor. Mat. 2019, 284, 69–77. [Google Scholar] [CrossRef]
- Marktus, A.; Fateme, R.; Abbas, J.; Mark, F. Oxidative dehydrogenation of propane to propylene with carbon dioxide. Appl. Catal. B-Environ. 2017, 52, 429–445. [Google Scholar]
- Chen, S.; Chang, X.; Sun, G.D.; Zhang, T.T.; Xu, Y.Y.; Wang, Y.; Pei, C.L.; Gong, J.L. Propane dehydrogenation: Catalyst development, new chemistry, and emerging technologies. Chem. Soc. Rev. 2021, 50, 3315. [Google Scholar] [CrossRef]
- Khadzhiev, S.N.; Usachev, N.Y.; Gerzeliev, I.M.; Belanova, E.P.; Kalinin, V.P.; Kharlamov, V.V.; Kazakov, A.V.; Kanaev, S.A.; Starostina, T.S.; Popov, A.Y. Oxidative dehydrogenation of ethane to ethylene in a system with circulating microspherical metal oxide oxygen carrier: 1. Synthesis and study of the catalytic system. Pet. Chem. 2015, 55, 651–654. [Google Scholar] [CrossRef]
- Darvishi, A.; Davand, R.; Khorasheh, F.; Fattahi, M. Modeling-based optimization of a fixed-bed industrial reactor for oxidative dehydrogenation of propane. Chin. J. Chem. Eng. 2016, 24, 612–622. [Google Scholar] [CrossRef]
- Zhai, Z.; Wang, X.; Licht, R.; Bell, A.T. Selective oxidation and oxidative dehydrogenation of hydrocarbons on bismuth vanadium molybdenum oxide. J. Catal. 2015, 325, 87–100. [Google Scholar] [CrossRef]
- Zea, L.C.H. Oxidative dehydrogenation of propane on Pd-Mo/gamma-Al2O3 catalyst: A kinetic study. Aust. J. Basic Appl. Sci. 2015, 9, 78–83. [Google Scholar]
- Zhao, D.; Tian, X.X.; Dmitry, E. In situ formation of ZnOx species for efficientpropane dehydrogenation. Nature 2021, 599, 234–238. [Google Scholar] [CrossRef]
- Zhang, H.J.; Wan, H.; Zhao, Y.; Wang, W.Q. Effect of chlorine elimination from Pt-Sn catalyst on the behavior of hydrocarbon reconstruction in propane dehydrogenation. Catal. Today 2019, 330, 85–91. [Google Scholar] [CrossRef]
- Elbadawi, A.H.; Osman, M.S.; Razzak, S.A.; Hossain, M.M. VOx-Nb/La-γAl2O3 catalysts for oxidative dehydrogenation of ethane to ethylene. J. Taiwan Inst. Chem. Eng. 2016, 61, 106–116. [Google Scholar] [CrossRef]
- Nawaz, Z.; Baksh, F.; Zhu, J.; Wei, F. Dehydrogenation of C3-C4 paraffin’s to corresponding olefins over slit-SAPO-34 supported Pt-Sn-based novel catalyst. J. Ind. Eng. Chem. 2013, 19, 540–546. [Google Scholar] [CrossRef]
- Cavani, F.; Trifir ò, F. The oxidative dehydrogenation of ethane and propane as an alternative way for the production of light olefins. Catal. Today 1995, 24, 307–313. [Google Scholar] [CrossRef]
- Sanfilippo, D. Dehydrogenation of Paraffins; Key Technology for Petrochemicals and Fuels. Cattech 2000, 4, 56–73. [Google Scholar] [CrossRef]
- Cavani, F.; Ballarini, N.; Cericola, A. Oxidative dehydrogenation of ethane and propane: How far from commercial implementation? Catal. Today 2007, 127, 113–131. [Google Scholar] [CrossRef]
- Chang, C.D. Methanol Conversion to Light Olefins. Catal. Rev. 1984, 26, 323–345. [Google Scholar] [CrossRef]
- Bricker, J.C. Advanced catalytic dehydrogenation technologies. Top. Catal. 2012, 55, 1309–1314. [Google Scholar] [CrossRef]
- Sattler, J.J.H.B.; Ruiz-Martinez, J.; Santillan-Jimenez, E.; Weckhuysen, B.M. Catalytic dehydrogenation of light alkanes on metals and metal oxides. Chem. Rev. 2014, 114, 10613–10653. [Google Scholar] [CrossRef]
- Chen, J.Q.; Bozzano, A.; Glover, B.; Fuglerud, T.; Kvisle, S. Recent advancements in ethylene and propylene production using the UOP/Hydro MTO process. Cataly. Today 2005, 106, 103–107. [Google Scholar] [CrossRef]
- Vora, B.V. Development of Dehydrogenation Catalysts and Processes. Top. Catal. 2012, 55, 1297–1308. [Google Scholar] [CrossRef]
- Yoshimur, Y.; Kijima, N.; Hayakawa, T.; Murata, K.; Suzuki, K.; Mizukami, F.; Matano, K.; Konishi, T.; Oikawa, T.; Saito, M.; et al. Catalytic cracking of naphtha to light olefins. Catal. Surv. Jpn. 2000, 4, 157–167. [Google Scholar] [CrossRef]
- Hereijgers, B.P.C.; Bleken, F.; Nilsen, M.H.; Svelle, S.; Lillerud, K.P.; Weckhuysen, B.M.; Olsbye, U. Product shape selectivity dominates the Methanol-to-Olefins (MTO) reaction over H-SAPO-34 catalysts. J. Catal. 2009, 264, 77–87. [Google Scholar] [CrossRef]
- Steinfeldt, N.; Muller, D.; Berndt, H. VOx species on alumina at vanadia loadings and high calcination temperature and their role in the ODP reaction. Appl. Catal. A-Gen. 2004, 272, 201–213. [Google Scholar] [CrossRef]
- Heracleous, E.; Machli, M.; Lemonidou, A.A.; Vasalos, I.A. Oxidative dehydrogenation of ethane and propane over vanadia and molybdena supported catalysts. J. Mol. Catal. A-Chem. 2005, 232, 29–39. [Google Scholar] [CrossRef]
- Skorodumova, N.V.; Simak, S.I.; Lundqvist, B.I.; Abrikosov, I.A.; Johansson, B. Quantum origin of the oxygen storage capability of ceria. Phys. Rev. Lett. 2002, 89, 166601. [Google Scholar] [CrossRef]
- Sadrameli, S.M. Thermal/catalytic cracking of liquid hydrocarbons for the production of olefins: A state-of-the-art review II: Cataltic cracking review. Fuel 2016, 173, 285–297. [Google Scholar] [CrossRef]
- Botavina, M.; Agafonov, Y.A.; Gaidai, N.; Groppo, E.; Corberan, V.C.; Lapidus, A.; Martra, G. Towards efficient catalysts for the oxidative dehydrogenation of propane in the presence of CO2: Cr/SiO2 systems prepared by direct hydrothermal synthesis. Catal. Sci. Technol. 2016, 6, 840–850. [Google Scholar] [CrossRef]
- Koirala, R.; Buechel, R.; Pratsinis, S.E.; Baiker, A. Silica is preferred over various single and mixed oxides as support for CO2-assisted cobalt-catalyzed oxidative dehydrogenation of ethane. Appl. Catal. A Gen. 2016, 527, 96–108. [Google Scholar] [CrossRef]
- Ren, T.; Patel, M.; Blok, K. Olefins from conventional and heavy feedstocks: Energy use in steam cracking and alternative processes. Energy 2006, 31, 425–451. [Google Scholar] [CrossRef]
- Mukherjee, D.; Park, S.-E.; Reddy, B.M. CO2 as a soft oxidant for oxidative dehydrogenation reaction: An eco benign process for industry. J. CO2 Util. 2016, 16, 301–312. [Google Scholar] [CrossRef]
- Ajayi, B.P.; Rabindran Jermy, B.; Abussaud, B.A.; Al-Khattaf, S. Oxidative dehydrogenation of n-butane over bimetallic mesoporous and microporous zeolites with CO2 as mild oxidant. J. Porous. Mater. 2013, 20, 1257–1270. [Google Scholar] [CrossRef]
- U.S. Energy Information Administration (EIA). China Analysis Report; EIA: Washington, DC, USA, 2022. Available online: http://www.eia.gov/.pdf (accessed on 1 December 2022).
- Védrine, J. Heterogeneous Partial (amm) oxidation and oxidative dehydrogenation catalysis on mixed metal oxides. Catalysts 2016, 6, 22. [Google Scholar] [CrossRef]
- Elbadawi, A.H.; Ba-Shammakh, M.S.; Al-Ghamdi, S.; Razzak, S.A.; Hossain, M.M. Reduction kinetics and catalytic activity of VOx/γ-Al2O3-ZrO2 for gas phase oxygen free ODH of ethane. Chem. Eng. J. 2016, 284, 448–457. [Google Scholar] [CrossRef]
- Koc, S.N.; Dayioglu, K.; Ozdemir, H. Oxidative dehydrogenation of propane with K-MoO3/MgAl2O4 catalysts. J. Chem. Sci. 2016, 128, 67–71. [Google Scholar] [CrossRef]
- Kong, L.; Li, J.; Zhao, Z.; Liu, Q.; Sun, Q.; Liu, J.; Wei, Y. Oxidative dehydrogenation of ethane to ethylene over Mo-incorporated mesoporous SBA-16 catalysts: The effect of MoOx dispersion. Appl. Catal. A-Gen. 2016, 510, 84–97. [Google Scholar] [CrossRef]
- Yu, C.; Xu, H.; Chen, X. Preparation etcharacterization, catalytic performance of and PtZn-Sn/SBA-15 catalyst for propane dehydrogenation. J. Fuel. Chem. Technol. 2010, 38, 308–312. [Google Scholar] [CrossRef]
- Hien, N.; Jesper, J.; Bert, M. Role of Sn in the regeneration of Pt/γ-Al2O3 light alkane dehydrogenation catalysts. Chem. Soc. 2016, 6, 2257–2264. [Google Scholar]
- Antolini, E.; Colmati, F.; Gonzalez, E.R. Ethanol oxidation on carbon supported (Pt Sn) alloy/SnO2 and (Pt Sn Pd)alloy/SnO2 catalysts with a fixed Pt/SnO2 atomic ratio: Effect of the alloy phase characteristics. J. Power. Sources 2009, 193, 555–561. [Google Scholar] [CrossRef]
- Yang, M.L.; Zhu, Y.A.; Zhou, X.G. First-principles calculations of propane dehydrogenation over PtSn catalysts. ACS Catal. 2012, 2, 1247–1258. [Google Scholar] [CrossRef]
- Vu, B.K.; Song, M.B.; Ahn, I.Y. Location andstructure of coke generated over Pt-Sn/Al2O3 in propanedehydrogenation. J. Ind. Eng. Chem. 2011, 17, 71–76. [Google Scholar] [CrossRef]
- Hauser, A.W.; Gomes, J.; Bajdich, M. Subnanometer-sized Pt/Sn alloy cluster catalysts for thedehydrogenation of linear alkanes. Phys. Chem. ChemPhys. 2013, 15, 20727–20734. [Google Scholar] [CrossRef]
- Kumar, M.S.; Chen, D.; Walmesley, J.C. Dehydrogenation of propane over Pt-SBA-15: Effect of Pt particle size. Catal Commun. 2008, 9, 747–750. [Google Scholar] [CrossRef]
- Nykanen, L.; Honkala, K. Selectivity in Propene Dehydrogenation on Pt and Pt3Sn Surfaces from First Principles. ACS Catal. 2013, 3, 3026–3030. [Google Scholar] [CrossRef]
- Kikuchi, I.; Haibara, Y.; Ohshima, M. Dehydrogenation of n-butane to butadiene over Pt-Sn/MgO-Al2O3. J. Jpn. Petrol. Inst. 2012, 55, 33–39. [Google Scholar] [CrossRef]
- Kobayashi, S.; Kaneko, S.; Ohshima, M. Effect of iron oxide on isobutene dehydrogenation over Pt/Fe2O3-Al2O3 catalyst. Appl. Catal. A-Gen. 2012, 417–418, 306–311. [Google Scholar] [CrossRef]
- Vu, B.K.; Song, M.B.; Ahn, I.Y. Propane dehydrogenation over Pt-Sn/Rare-earth-doped Al2O3: Influence of La, Ce, or Y on the formation and stabilityof Pt-Sn alloys. Catal. Today 2011, 164, 214–220. [Google Scholar] [CrossRef]
- Chen, F.Q.; Huang, X.L.; Guo, K.Q.; Yang, L.; Sun, H.R.; Xia, W.; Zhang, Z.G.; Yang, Q.W.; Yang, Y.W.; Zhao, D.; et al. Molecular Sieving of Propylene from Propane in Metal-Organic Framework-Derived Ultramicroporous Carbon Adsorbents. ACS Appl. Mater. Inter. 2023, 14, 30443–30453. [Google Scholar] [CrossRef]
- Li, W.; Yu, S.Y.; Meitzner, G.D. Structure andproperties of cobalt-exchanged H-ZSM5 catalysts fordehydrogenation and dehydrocyclization of alkanes. J. Phys. Chem. B. 2001, 105, 1176–1184. [Google Scholar] [CrossRef]
- Zhou, J.; Zhang, Y.; Liu, H.; Xiong, C.; Hu, P.; Wang, H.; Chen, S.W.; Ji, H.B. Enhanced performance for propane dehydrogenation through Pt clusters alloying with copper in zeolite. Nano Res. 2023. [CrossRef]
- Xia, K.; Wan, Z.L.; Pei, P.L. The influences of Mg/Al molar ratio on the properties of PtIn/Mg(Al)O-x catalysts for propane dehydrogenation reaction. Chem. Eng. J. 2015, 9, 1068–1079. [Google Scholar] [CrossRef]
- Zhang, Y.W.; Zhou, Y.M.; Shi, J.J. Propane dehydrogenation over Pt Sn Na/La-doped Al2O3 catalyst: Effect of La content. Fuel. Process. Technol. 2013, 11, 94–104. [Google Scholar] [CrossRef]
- Bocanegra, S.A.; Castro, A.A.; Scelza, O.A.; de Miguel, S.R. Characterization and catalytic behavior in the n-butane dehydrogenation of trimetallic In Pt Sn/MgAl2O4 catalysts. Appl. Catal. A Gen. 2007, 333, 49–56. [Google Scholar] [CrossRef]
- Cabrera, F.; Ardissone, D.; Gorriz, O.F. Dehydrogenation of propane on chromia/alumina catalysts promoted by tin. Catal. Today 2007, 133–135, 800–804. [Google Scholar] [CrossRef]
- Kim, T.H.; Kang, H.H.; Baek, M.S. Dehydrogenation of propane to propylene with lattice oxygen over CrOy/Al2O3-ZrO2 catalysts. Mol. Catal. 2017, 43, 1–7. [Google Scholar] [CrossRef]
- Koirala, R.; Buechel, R.; Krumeich, F.; Pratsinis, S.E.; Baiker, A. Oxidative Dehydrogenation of Ethane with CO2 over Flame-Made Ga-Loaded TiO2. ACS Catal. 2015, 5, 690–702. [Google Scholar] [CrossRef]
- Liu, H.; Zhang, Z.; Li, H.; Huang, Q. Intrinsic kinetics of oxidative dehydrogenation of propane in the presence of CO2 over Cr/MSU-1 catalyst. J. Nat. Gas Chem. 2011, 20, 311–317. [Google Scholar] [CrossRef]
- Michorczyk, P.; Ogonowski, J.; Kustrowski, P. Chromium oxide supported on MUM-41 as a highly active and selective catalyst for dehydrogenation of propane with CO2. Appl. Catal. A-Gen. 2008, 349, 62–69. [Google Scholar] [CrossRef]
- Zhang, X.Z.; Yue, Y.H.; Gao, Z. Chromium oxide supported on mesoporous SBA-15 as propane dehydrogenation and oxidaive dehydrogenation. Catal. Chem. 2002, 83, 19–25. [Google Scholar]
- Frank, B.; Dinse, A.; Ovsitser, O.; Kondratenko, E.V.; Schomäcker, R. Mass and heat transfer effects on the oxidative dehydrogenation of propane (ODP) over a low loaded VOx/Al2O3 catalyst. Appl. Catal. A Gen. 2007, 323, 66–76. [Google Scholar] [CrossRef]
- Elbadawi, A.H.; Ba-Shammakh, M.S.; Al-Ghamdi, S.; Razzak, S.A.; Hossain, M.M.; de Lasa, H.I. A fluidizable VOx/γ -Al2O3-ZrO2 catalyst for the ODH of ethane to ethylene operating in a gas phase oxygen free environment. Chem. Eng. Sci. 2016, 145, 59–70. [Google Scholar] [CrossRef]
- Khan, M.Y.; Al-Ghamdi, S.; Razzak, S.A.; Hossain, M.M.; deLasa, H. Fluidized bed oxidativede hydrogenation of ethane to ethylene over VOx/Ce-γ Al2O3 catalysts: Reduction kinetics and catalyst activity. Mol. Catal. 2017, 443, 78–91. [Google Scholar] [CrossRef]
- Rostom, S.; de Lasa, H.I. Propane Oxidative Dehydrogenation Using Consecutive Feed Injections and Fluidizable VOx/γ Al2O3 and VOx/ZrO2 -γ Al2O3 Catalysts. Ind. Eng. Chem. Res. 2017, 56, 13109–13124. [Google Scholar] [CrossRef]
- Elfadly, A.M.; Badawi, A.M.; Yehia, F.Z.; Mohamed, Y.A.; Betiha, M.A.; Rabie, A.M. Selective nano alumina supported vanadium oxide catalysts for oxidative dehydrogenation of ethylbenzene to styrene using CO2 as soft oxidant. Egypt. J. Pet. 2013, 22, 373–380. [Google Scholar] [CrossRef]
- Klose, F.; Wolff, T.; Lorenz, H.; Seidelmorgenstern, A.; Suchorski, Y.; Piorkowska, M.; Weiss, H. Active species on γ-alumina-supported vanadia catalysts: Nature and reducibility. J. Catal. 2007, 247, 176–193. [Google Scholar] [CrossRef]
- Rischard, J.; Antinori, C.; Maier, L.; Deutschmann, O. Oxidative dehydrogenation of n-butane to butadiene with Mo-V-MgO catalysts in a two-zone fluidized bed reactor. Appl. Catal. A Gen. 2016, 511, 23–30. [Google Scholar] [CrossRef]
- Schwarz, O.; Habel, D.; Ovsitser, O.; Kondratenko, E.V.; Hess, C.; Schomäcker, R.; Schubert, H. Impact of preparation method on physico-chemical and catalytic properties of VOx /γ -Al2O3 materials. J. Mol. Catal. A Chem. 2008, 293, 45–52. [Google Scholar] [CrossRef]
- Botková, Š.; Capek, L.; Setnicka, M.; Bulánek, R.; Cicmanec, P.; Kalužov á, A.; Pastva, J.; Zukal, A. VOx species supported on Al2O3-SBA-15 prepared by the grafting of alumina onto SBA-15: Structure and activity in the oxidative dehydrogenation of ethane. React. Kinet Mech. Catal. 2016, 119, 319–333. [Google Scholar] [CrossRef]
- Rubio, O.; Herguido, J.; Men é ndez, M. Oxidative dehydrogenation of n-butane on V/MgO catalysts-kinetic study in anaerobic conditions. Chem. Eng. Sci. 2003, 58, 4619–4627. [Google Scholar] [CrossRef]
- Hossain, M.M. Kinetics of Oxidative Dehydrogenation of Propane to Propylene Using Lattice Oxygen of VOx/CaO/γAl2O3 Catalysts. Ind. Eng. Chem. Res. 2017, 56, 4309–4318. [Google Scholar] [CrossRef]
- Tan, S.; Gil, L.B.; Subramanian, N.; Sholl, D.S.; Nair, S.; Jones, C.W.; Moore, J.S.; Liu, Y.; Dixit, R.S.; Pendergast, J.G. Catalytic propane dehydrogenation over In2O3–Ga2O3 mixed oxides. Appl. Catal. A Gen. 2015, 498, 167–175. [Google Scholar] [CrossRef]
- Meriaudeau, P.; Naccahe, C. The role of Ga2O3 and proton acidlity on the dehydrogenation activity of Ga2O3-HZSM-5 catalysts-evidence of a bifunctional mechanism. J. Mol. A-Chem. 1990, 59, 31–36. [Google Scholar]
- Michorczyk, P.; Kuśtrowski, P.; Kolak, A. Ordered mesoporous Ga2O3 and Ga2O3-Al2O3 prepared by nanocasting as effective catalysts for propane dehydrogenation in the presence of CO2. Catal. Commun. 2013, 35, 95–100. [Google Scholar] [CrossRef]
- Wu, J.L.; Chen, M.; Liu, Y.M.; Cao, Y.; He, H.Y.; Fan, K.N. Sucrose-templated mesoporous β-Ga2O3 as a novel efficient catalyst for dehydrogenation of propane in the presence of CO2. Catal. Commun. 2012, 30, 61–65. [Google Scholar] [CrossRef]
- Xu, B.J.; Zheng, B.; Hua, W. High Si/Al Ratio HZSM-5 Supported Ga2O3: A highly stable catalyst for dehydrogenation of propane to propene in the presence of CO2. Stud. Surf. Sci. Catal. 2007, 170, 1072–1079. [Google Scholar]
- Xu, B.J.; Zheng, B.; Hua, W. Support effect in dehydrogenation of propane in the presence of CO2 over supported gallium oxide catalysts. J. Catal. 2006, 239, 470–477. [Google Scholar] [CrossRef]
- Ren, Y.J.; Zhang, F.; Hua, W.M. ZnO supported on high silica HZSM-5 as new catalysts for dehydrogenation of propane to propene in the presence of CO2. Catal. Today 2019, 148, 316–322. [Google Scholar] [CrossRef]
- Blank, J.H.; Beckers, J.; Collignon, P.F.; Rothenberg, G. Redox kinetics of ceria-based mixed oxides in selective hydrogen combustion. ChemPhysChem 2007, 8, 2490–2497. [Google Scholar] [CrossRef]
- Deo, G.; Wachs, I.E. Reactivity of Supported Vanadium Oxide Catalysts: The Partial Oxidation of Methanol. J. Catal. 1994, 146, 323–334. [Google Scholar] [CrossRef]
- Blasco, T.; Lopez-Nieto, J.M. Oxidative dyhydrogenation of short chain alkanes on supported vanadium oxide catalysts. Appl. Catal. A Gen. 1997, 157, 117–142. [Google Scholar] [CrossRef]
- Al-Ghamdi, S.; Moreira, J.; de Lasa, H. Kinetic Modeling of Propane Oxidative Dehydrogenation over VOx/γ-Al2O3 Catalysts in the Chemical Reactor Engineering Center Riser Reactor Simulator. Ind. Eng. Chem. Res. 2014, 53, 15317–15332. [Google Scholar] [CrossRef]
- Wu, T.W.; Yu, Q.B.; Hou, L.M.; Duan, W.J.; Wang, K.; Qin, Q. Selecting suitable oxygen carriers for chemical looping oxidative dehydrogenation of propane by thermodynamic method. J. Therm. Anal. Calorim. 2020, 140, 1837–1843. [Google Scholar] [CrossRef]
- Fukudome, K.; Ikenaga, N.O.; Miyake, T.; Suzuki, T. Oxidative dehydrogenation of alkanes over vanadium oxide prepared with V(t-BuO)3O and Si(OEt)4 in the presence of polyethyleneglycol. Catal. Today 2013, 203, 10–16. [Google Scholar] [CrossRef]
- Fukudome, K.; Ikenaga, N.; Miyake, T.; Suzuki, T. Oxidative dehydrogenation of propane using lattice oxygen of vanadium oxides on silica. Catal. Sci. Technol. 2011, 1, 987. [Google Scholar] [CrossRef]
- Gao, Y.; Neal, L.M.; Li, F. Li-Promoted LaxSr2-xFeO4-δ Core—Shell Redox Catalysts for Oxidative Dehydrogenation of Ethane under a Cyclic Redox Scheme. ACS Catal. 2016, 6, 7293–7302. [Google Scholar] [CrossRef]
- Fattahi, M.; Kazemeini, M.; Khorasheh, F.; Rashidi, A. An investigation of the oxidative dehydrogenation of propane kinetics over a vanadium-graphene catalyst aiming at minimizing of the COx species. Chem. Eng. J. 2014, 250, 14–24. [Google Scholar] [CrossRef]
- Argyle, M.; Bartholomew, C. Heterogeneous Catalyst Deactivation and Regeneration: A Review. Catalysts 2015, 5, 145–269. [Google Scholar] [CrossRef]
- Gao, Y.F.; Haeri, F.; He, F.; Li, F.X. Alkali Metal-Promoted LaxSr2−xFeO4−δ redox catalysts for chemical looping oxidative dehydrogenation of ethane. ACS Catal. 2018, 8, 1757–1766. [Google Scholar] [CrossRef]



| Projects | Process Technology | |
|---|---|---|
| Catofin Process | Oleflex Process | |
| Technology exporter | ABB Lummus | UOP |
| Reactor type | Fixed Bed | Moving Bed |
| Total number of reactors | 5 | 3~4 |
| Catalyst | CrOx/Al2O3 | Pt-Sn/Al2O3 |
| Cycle regeneration time | 15~30 min | 2~7 d |
| Temperature/°C | 600–700 | 550~620 |
| Pressure/Mpa | 0.3~0.5 | 2~3 |
| Diluent | - | H2 |
| Propane conversion | 48~65 | 80~88 |
| Propylene selectivity | 25 | 89~91 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zuo, C.; Su, Q. Research Progress on Propylene Preparation by Propane Dehydrogenation. Molecules 2023, 28, 3594. https://doi.org/10.3390/molecules28083594
Zuo C, Su Q. Research Progress on Propylene Preparation by Propane Dehydrogenation. Molecules. 2023; 28(8):3594. https://doi.org/10.3390/molecules28083594
Chicago/Turabian StyleZuo, Cheng, and Qian Su. 2023. "Research Progress on Propylene Preparation by Propane Dehydrogenation" Molecules 28, no. 8: 3594. https://doi.org/10.3390/molecules28083594
APA StyleZuo, C., & Su, Q. (2023). Research Progress on Propylene Preparation by Propane Dehydrogenation. Molecules, 28(8), 3594. https://doi.org/10.3390/molecules28083594






