A Chitosan-Based Flocculation Method for Efficient Recovery of High-Purity B-Phycoerythrin from a Low Concentration of Phycobilin in Wastewater
Abstract
1. Introduction
2. Results and Discussion
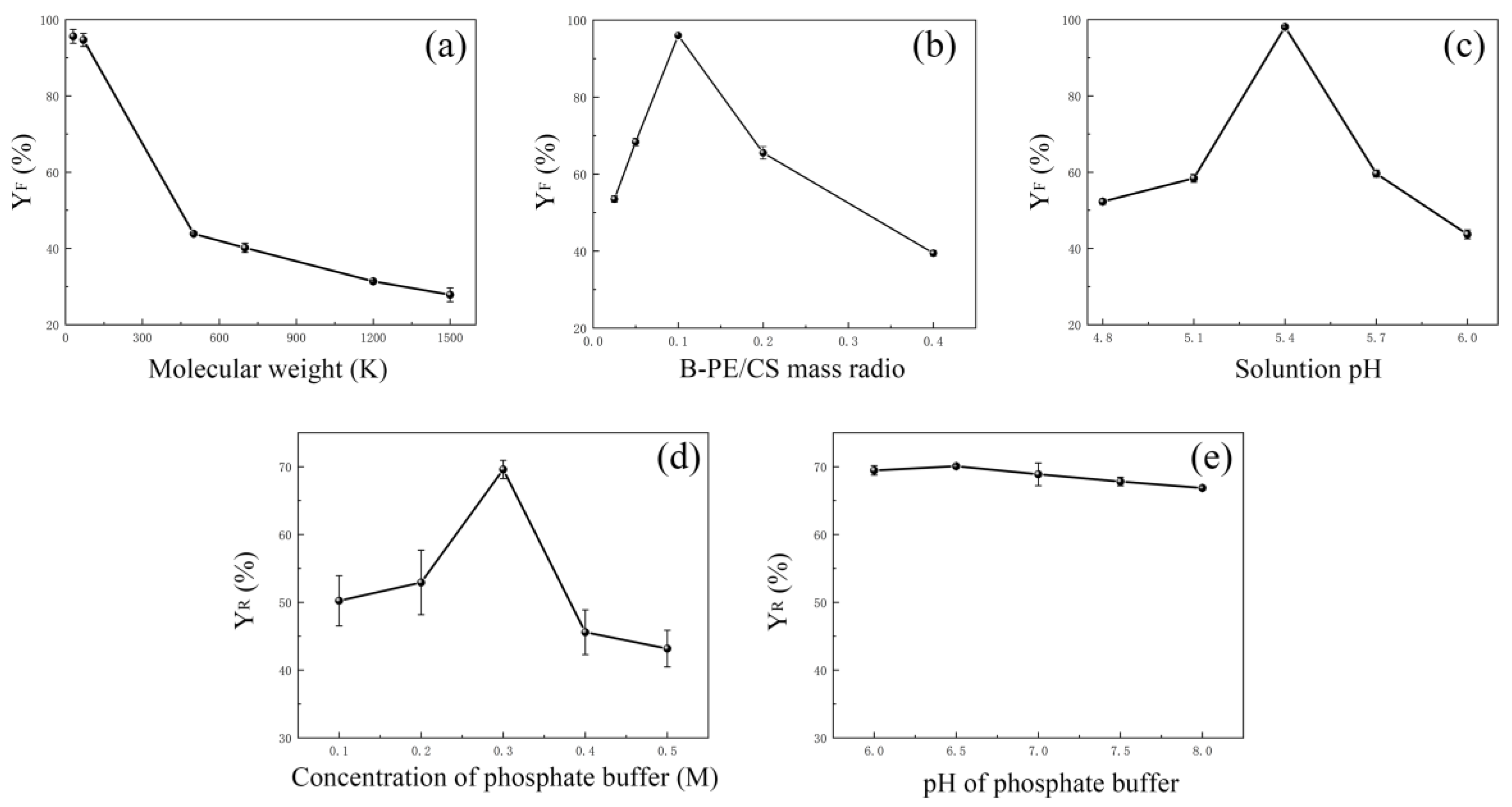
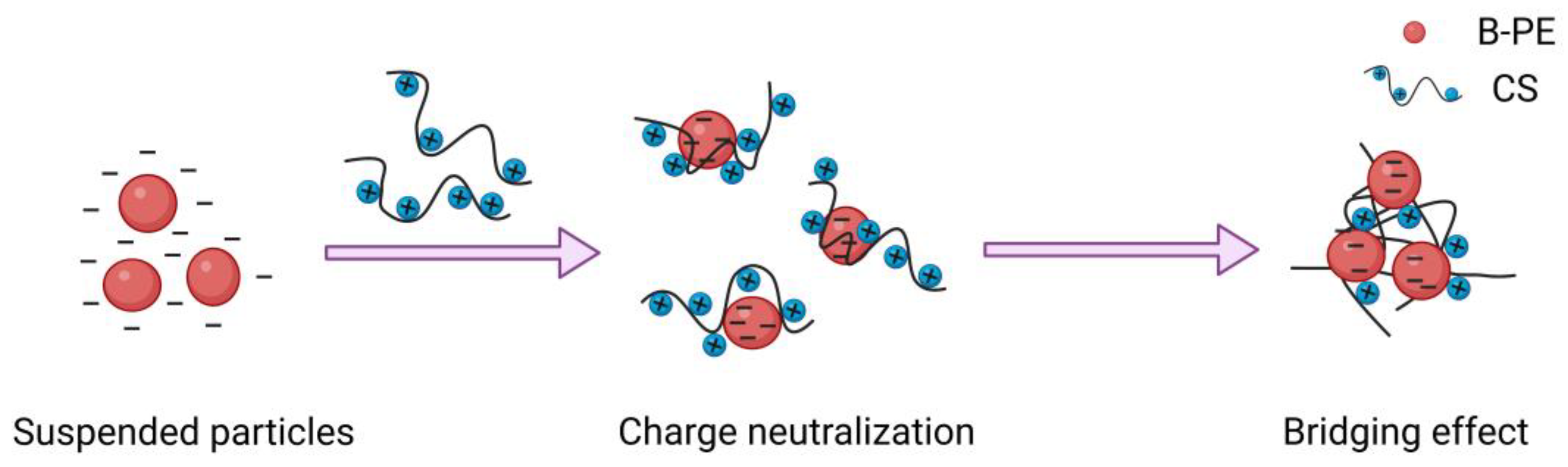
2.1. Formation of B-PE/CS Complexes
2.1.1. Effect of MW of CS
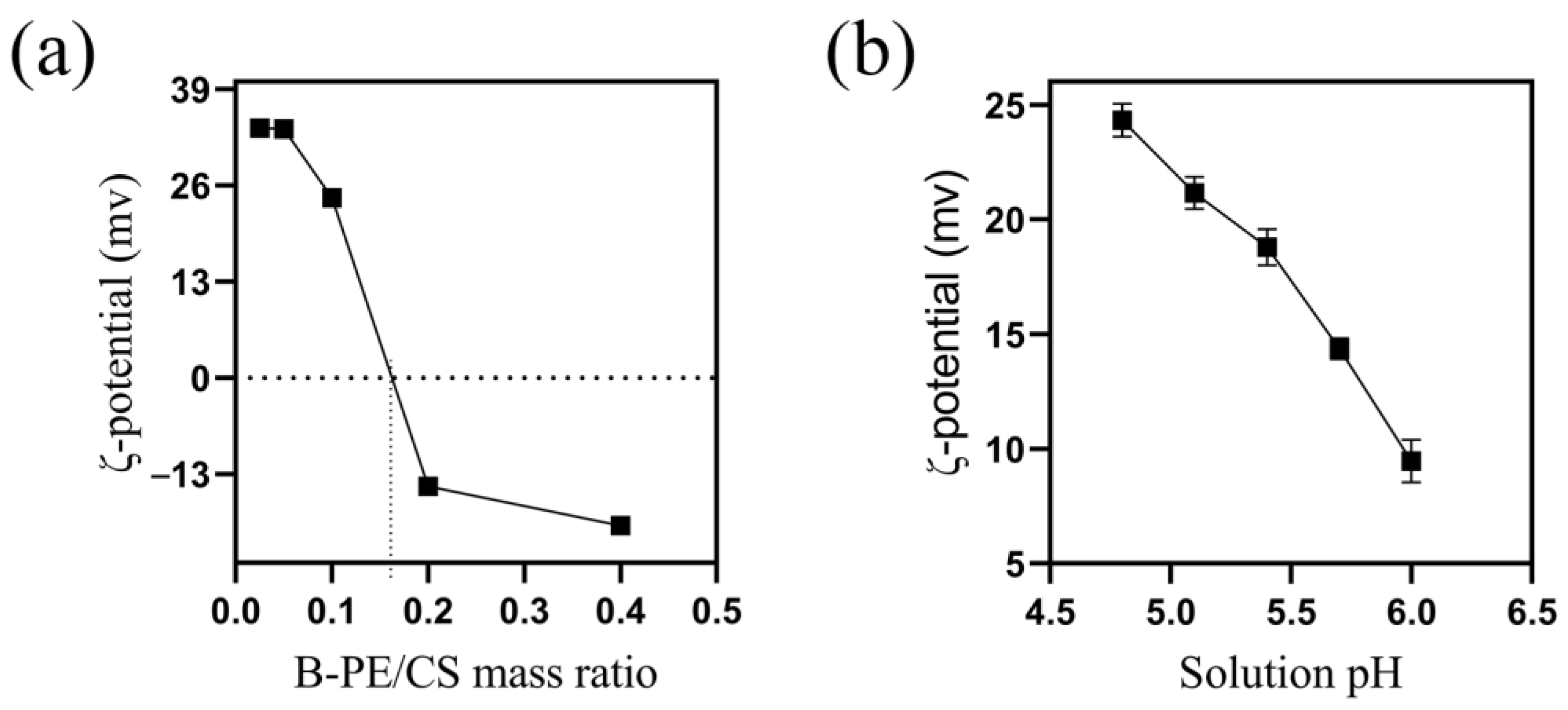
2.1.2. Effect of B-PE/CS Mass Ratio
2.1.3. Effect of Solution pH
2.2. Dissociation of B-PE/CS Complexes
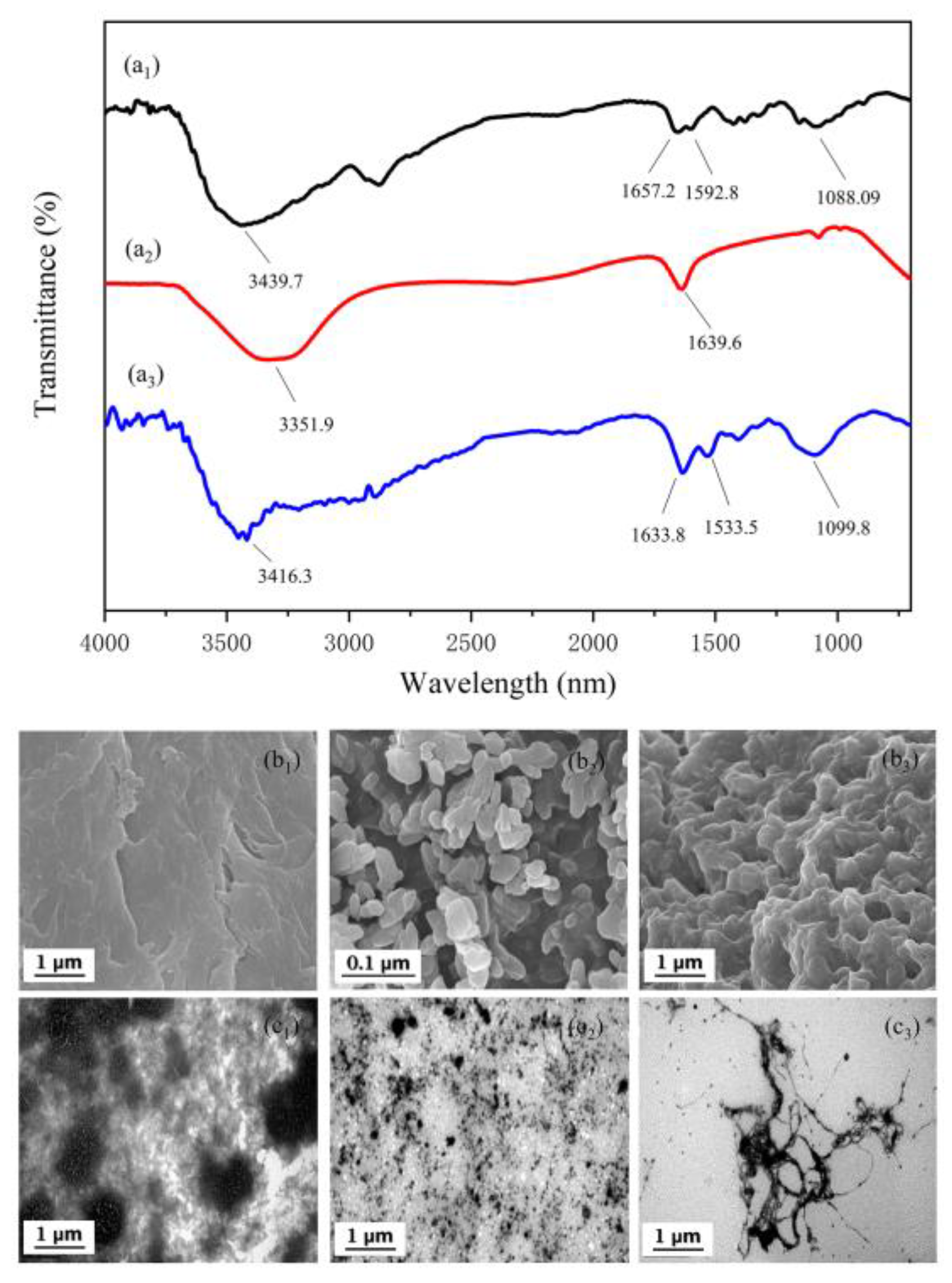
2.3. Analysis of B-PE Precipitation
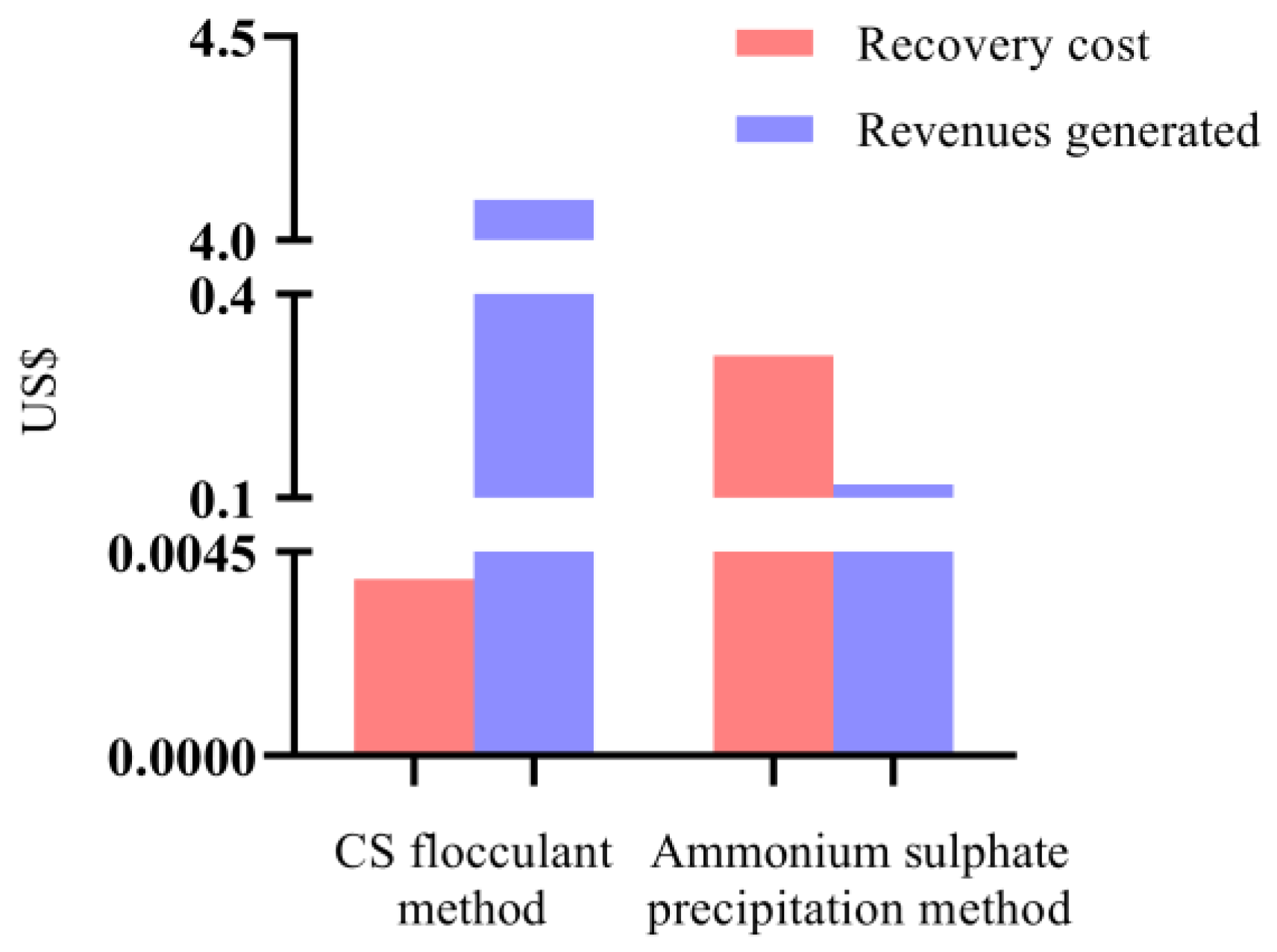
2.4. Economic Evaluation
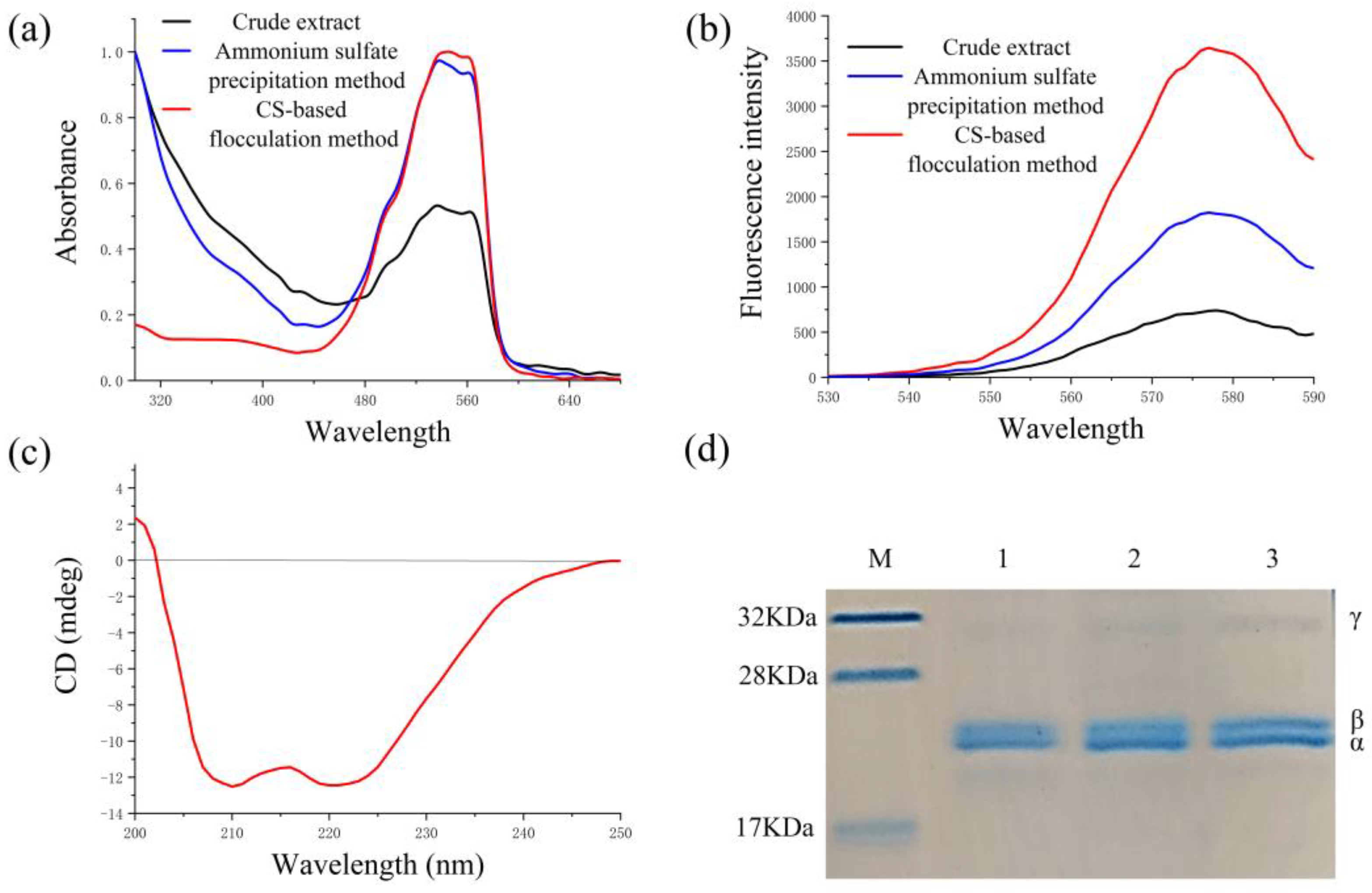
2.5. Properties of the Recovered B-PE
2.6. Analysis of B-PE Binding with CS
3. Materials and Methods
3.1. Materials
3.2. Preparation of the B-PE Crude Extract
3.3. Recovery of B-PE
3.3.1. Flocculation Experiments
3.3.2. Dissociation Experiments
3.3.3. Ammonium Sulfate Precipitation
3.3.4. Analytical Methods
3.4. Economic Evaluation
3.5. Properties of the Recovered B-PE
3.6. Study of Flocculation Mechanism
3.7. Statistical Analyses
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Zhang, Y.D.; Zhang, L.Q.; Hu, J.N.; Wang, Z.W.; Meng, D.M.; Li, H.; Zhou, Z.K.; Yang, R. The structural characterization and color stabilization of the pigment protein-phycoerythrin glycosylated with oligochitosan. Food Hydrocoll. 2023, 136, 108241. [Google Scholar] [CrossRef]
- Munier, M.; Jubeau, S.; Wijaya, A.; Morançais, M.; Dumay, J.; Marchal, L.; Jaouen, P.; Fleurence, J. Physicochemical factors affecting the stability of two pigments: R-phycoerythrin of Grateloupia turuturu and B-phycoerythrin of Porphyridium cruentum. Food Chem. 2014, 150, 400–407. [Google Scholar] [CrossRef] [PubMed]
- Meng, D.M.; Zhang, L.Q.; Wang, Q.E.; Zhang, Y.D.; Sun, Y.F.; Zhang, H.L.; Wang, Z.W.; Zhou, Z.K.; Yang, R. Self-Assembly of Phycoerythrin with Oligochitosan by Electrostatic Interaction for Stabilization of Phycoerythrin. J. Agric. Food Chem. 2021, 69, 12818–12827. [Google Scholar] [CrossRef] [PubMed]
- Marcati, A.; Ursu, A.V.; Laroche, C.; Soanen, N.; Marchal, L.; Jubeau, S.; Djelveh, G.; Michaud, P. Extraction and fractionation of polysaccharides and B-phycoerythrin from the microalga Porphyridium cruentum by membrane technology. Algal Res. 2014, 5, 258–263. [Google Scholar] [CrossRef]
- Tang, Z.H.; Zhao, J.L.; Ju, B.; Li, W.J.; Wen, S.H.; Pu, Y.; Qin, S. One-step chromatographic procedure for purification of B-phycoerythrin from Porphyridium cruentum. Protein Express. Purif. 2016, 123, 70–74. [Google Scholar] [CrossRef]
- García, A.B.; Longo, E.; Murillo, M.C.; Bermejo, R. Using a B-Phycoerythrin Extract as a Natural Colorant: Application in Milk-Based Products. Molecules 2021, 26, 297. [Google Scholar] [CrossRef] [PubMed]
- Tran, T.; Lafarge, C.; Pradelles, R.; Perrier-Cornet, J.M.; Cayot, N.; Loupiac, C. Effect of high hydrostatic pressure on the structure of the soluble protein fraction in Porphyridium cruentum extracts. Innov. Food Sci. Emerg. Technol. 2019, 58, 102226. [Google Scholar] [CrossRef]
- Torres-Acosta, M.A.; Ruiz-Ruiz, F.; Aguilar-Yáñez, J.M.; Benavides, J.; Rito-Palomares, M. Economic analysis of pilot-scale production of B-phycoerythrin. Biotechnol. Progr. 2016, 32, 1472–1479. [Google Scholar] [CrossRef]
- Silva, L.A.; Kuhn, K.R.; Moraes, C.C.; Burkert, C.A.V.; Kalil, S.J. Experimental Design as a Tool for Optimization of C-phycocyanin Purification by Precipitation from Spirulina platensis. J. Brazil. Chem. Soc. 2009, 20, 5–12. [Google Scholar] [CrossRef]
- Porav, A.S.; Bocăneală, M.; Fălămaş, A.; Bogdan, D.F.; Barbu-Tudoran, L.; Hegeduş, A.; Dragoş, N. Sequential aqueous two-phase system for simultaneous purification of cyanobacterial phycobiliproteins. Bioresour. Technol. 2020, 315, 123794. [Google Scholar] [CrossRef]
- Vu, H.P.; Nguyen, L.N.; Vu, M.T.; Labeeuw, L.; Emmerton, B.; Commault, A.S.; Ralph, P.J.; Mahlia, T.M.I.; Nghiem, L.D. Harvesting Porphyridium purpureum using polyacrylamide polymers and alkaline bases and their impact on biomass quality. Sci. Total Environ. 2021, 755, 142412. [Google Scholar] [CrossRef] [PubMed]
- Gargouch, N.; Karkouch, I.; Elleuch, J.; Elkahoui, S.; Michaud, P.; Abdelkafi, S.; Laroche, C.; Fendri, I. Enhanced B-phycoerythrin production by the red microalga Porphyridium marinum: A powerful agent in industrial applications. Int. J. Biol. Macromol. 2018, 120, 2106–2114. [Google Scholar] [CrossRef] [PubMed]
- Seručnik, M.; Vicente, F.A.; Brečko, Ž.; Coutinho, J.A.P.; Ventura, S.P.M.; Žnidaršič-Plazl, P. Development of a Microfluidic Platform for R-Phycoerythrin Purification Using an Aqueous Micellar Two-Phase System. ACS Sustain. Chem. Eng. 2020, 8, 17097–17105. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.F.; Wang, Q.F.; Hou, Y.H. Efficient Purification of R-phycoerythrin from Marine Algae (Porphyra yezoensis) Based on a Deep Eutectic Solvents Aqueous Two-Phase System. Mar. Drugs 2020, 18, 618. [Google Scholar] [CrossRef]
- Kovaleski, G.; Kholany, M.; Dias, L.M.S.; Correia, S.F.H.; Ferreira, R.A.S.; Coutinho, J.A.P.; Ventura, S.P.M. Extraction and purification of phycobiliproteins from algae and their applications. Front. Chem. 2022, 10, 1065355. [Google Scholar] [CrossRef]
- Patel, S.N.; Sonani, R.R.; Jakharia, K.; Bhastana, B.; Patel, H.M.; Chaubey, M.G.; Singh, N.K.; Madamwar, D. Antioxidant activity and associated structural attributes of Halomicronema phycoerythrin. Int. J. Biol. Macromol. 2018, 111, 359–369. [Google Scholar] [CrossRef]
- Arakawa, T.; Ejima, D.; Akuta, T. Protein aggregation under high concentration/density state during chromatographic and ultrafiltration processes. Int. J. Biol. Macromol. 2017, 95, 1153–1158. [Google Scholar] [CrossRef]
- Lapointe, M.; Barbeau, B. Understanding the roles and characterizing the intrinsic properties of synthetic vs. natural polymers to improve clarification through interparticle Bridging: A review. Sep. Purif. Technol. 2020, 231, 115893. [Google Scholar] [CrossRef]
- Ways, T.M.M.; Lau, W.M.; Khutoryanskiy, V.V. Chitosan and Its Derivatives for Application in Mucoadhesive Drug Delivery Systems. Polymers 2018, 10, 267. [Google Scholar] [CrossRef]
- Wang, W.Q.; Meng, Q.Y.; Li, Q.; Liu, J.B.; Zhou, M.; Jin, Z.; Zhao, K. Chitosan Derivatives and Their Application in Biomedicine. Int. J. Mol. Sci. 2020, 21, 487. [Google Scholar] [CrossRef]
- Huq, T.; Khan, A.; Brown, D.; Dhayagude, N.; He, Z.; Ni, Y. Sources, production and commercial applications of fungal chitosan: A review. J. Bioresour. Bioprod. 2022, 7, 85–98. [Google Scholar] [CrossRef]
- Pillai, C.K.S.; Paul, W.; Sharma, C.P. Chitin and chitosan polymers: Chemistry, solubility and fiber formation. Prog. Polym. Sci. 2009, 34, 641–678. [Google Scholar] [CrossRef]
- Macczak, P.; Kaczmarek, H.; Ziegler-Borowska, M. Recent Achievements in Polymer Bio-Based Flocculants for Water Treatmentn. Materials 2020, 13, 3951. [Google Scholar] [CrossRef] [PubMed]
- Jiménez-Gómez, C.P.; Cecilia, J.A. Chitosan: A Natural Biopolymer with a Wide and Varied Range of Applications. Molecules 2020, 25, 3981. [Google Scholar] [CrossRef] [PubMed]
- Demir, I.; Blockx, J.; Dague, E.; Guiraud, P.; Thielemans, W.; Muylaert, K.; Formosa-Dague, C. Nanoscale Evidence Unravels Microalgae Flocculation Mechanism Induced by Chitosan. ACS Appl. Bio. Mater. 2020, 3, 8446–8459. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.Y.; Lu, J.; Zhou, Y.; Zhou, Y.B. Multifunctional antibacterial materials for the control of hazardous microbes and chemicals: A review. ACS EST Water. 2021, 1, 479–497. [Google Scholar] [CrossRef]
- El-Gaayda, J.; Titchou, F.E.; Oukhrib, R.; Yap, P.S.; Liu, T.Q.; Hamdani, M.; Akbour, R.A. Natural flocculants for the treatment of wastewaters containing dyes or heavy metals: A state-of-the-art review. J. Environ. Chem. Eng. 2021, 9, 106060. [Google Scholar] [CrossRef]
- Zeng, D.F.; Hu, D.; Cheng, J. Experimental study on chitosan composite flocculant for treating papermaking wastewater. J. Water Chem. Technol. 2012, 34, 35–41. [Google Scholar] [CrossRef]
- Usman, A.; Zia, K.M.; Zuber, M.; Tabasum, S.; Rehman, S.; Zia, F. Chitin and chitosan based polyurethanes: A review of recent advances and prospective biomedical applications. Int. J. Biol. Macromol. 2016, 86, 630–645. [Google Scholar] [CrossRef]
- Mittal, H.; Ray, S.S.; Kaith, B.S.; Bhatia, J.K.; Sukriti; Sharma, J.; Alhassan, S.M. Recent progress in the structural modification of chitosan for applications in diversified biomedical fields. Eur. Polym. J. 2018, 109, 402–434. [Google Scholar] [CrossRef]
- Veeman, D.; Sai, M.S.; Sureshkumar, P.; Jagadeesha, T.; Natrayan, L.; Ravichandran, M.; Mammo, W.D. Additive Manufacturing of Biopolymers for Tissue Engineeringand Regenerative Medicine: An Overview, Potential Applications, Advancements, and Trends. Int. J. Polym. Sci. 2021, 2021, 4907027. [Google Scholar] [CrossRef]
- Wang, S.P.; Sun, C.K.; Guan, S.; Li, W.F.; Xu, J.Q.; Ge, D.; Zhuang, M.L.; Liu, T.Q.; Ma, X.H. Chitosan/gelatin porous scaffolds assembled with conductive poly(3,4-ethylenedioxythiophene) nanoparticles for neural tissue engineering. J. Mater. Chem. B. 2017, 5, 4774–4788. [Google Scholar] [CrossRef] [PubMed]
- Madni, A.; Kousar, R.; Naeem, N.; Wahid, F. Recent advancements in applications of chitosan-based biomaterials for skin tissue engineering. J. Bioresour. Bioprod. 2021, 6, 11–25. [Google Scholar] [CrossRef]
- Li, J.; Song, X.Y.; Pan, J.F.; Zhong, L.; Jiao, S.F.; Ma, Q.M. Adsorption and flocculation of bentonite by chitosan with varying degree of deacetylation and molecular weight. Int. J. Biol. Macromol. 2013, 62, 4–12. [Google Scholar] [CrossRef] [PubMed]
- Roussy, J.; Voorenb, M.V.; Dempsey, B.A.; Guibala, E. Influence of chitosan characteristics on the coagulation and the flocculation of bentonite suspensions. Water Res. 2005, 39, 3247–3258. [Google Scholar] [CrossRef] [PubMed]
- Kong, C.; Fodjo, E.K.; Li, D.; Cai, Y.Q.; Huang, D.M.; Wang, Y.; Shen, X.S. Chitosan-based adsorption and freeze deproteinization: Improved extraction and purification of synthetic colorants from protein-rich food samples. Food Chem. 2015, 188, 240–247. [Google Scholar] [CrossRef] [PubMed]
- Song, Z.Y.; Hu, Y.D.; Qi, L.K.; Xu, T.T.; Yang, Y.S.; Xu, Z.C.; Lai, X.P.; Wang, X.L.; Zhang, D.Y.; Li, S.J. An effective and recyclable deproteinization method for polysaccharide from oyster by magnetic chitosan microspheres. Carbohyd. Polym. 2018, 195, 558–565. [Google Scholar] [CrossRef] [PubMed]
- Ding, L.; Huang, Y.; Cai, X.X.; Wang, S.Y. Impact of pH, ionic strength and chitosan charge density on chitosan/casein complexation and phase behavior. Carbohyd. Polym. 2019, 208, 133–141. [Google Scholar] [CrossRef]
- Básaca-Loya, G.A.; Burboa, M.G.; Álvarez, M.E.; Gutiérrez-Millán, L.E.; Goycoolea, F.M.; Valdez, M.A. Interfacial Properties of B Phycoerythrin Extracted from the Red Microalga Rhodosorus Marinus at Hexadecane-Water and Air-Water Interfaces. Sci. Adv. Mater. 2011, 3, 259–268. [Google Scholar] [CrossRef]
- Huang, G.Q.; Sun, Y.T.; Xiao, J.X.; Yang, J. Complex coacervation of soybean protein isolate and chitosan. Food Chem. 2012, 135, 534–539. [Google Scholar] [CrossRef]
- Liang, S.Q.; Zhang, T.; Fu, X.D.; Zhu, C.L.; Mou, H.J. Partially degraded chitosan-based flocculation to achieve effective deodorization of oyster (Crassostrea gigas) hydrolysates. Carbohyd. Polym. 2020, 234, 115948. [Google Scholar] [CrossRef] [PubMed]
- Guibal, E.; Vooren, M.V.; Dempsey, B.A.; Roussy, J. A Review of the Use of Chitosan for the Removal of Particulate and Dissolved Contaminants. Sep. Sci. Technol. 2006, 41, 2487–2514. [Google Scholar] [CrossRef]
- Roselet, F.; Vandamme, D.; Roselet, M.; Muylaert, K.; Abreu, P.C. Screening of commercial natural and synthetic cationic polymers for flocculation of freshwater and marine microalgae and effects of molecular weight and charge density. Algal Res. 2015, 10, 183–188. [Google Scholar] [CrossRef]
- Kayitmazer, A.B.; Koksal, A.F.; Iyilik, E.K. Complex coacervation of hyaluronic acid and chitosan: Effects of pH, ionic strength, charge density, chain length and the charge ratio. Soft Matter. 2015, 11, 8605–8612. [Google Scholar] [CrossRef]
- Duong-Ly, K.C.; Gabelli, S.B. Salting out of Proteins Using Ammonium Sulfate Precipitation. In Methods in Enzymology; Academic Press: Cambridge, MA, USA, 2014; Volume 541, pp. 85–94. [Google Scholar]
- Martins, M.; Fernandes, A.P.M.; Torres-Acosta, M.A.; Collén, P.N.; Abreu, M.H.; Ventura, S.P.M. Extraction of chlorophyll from wild and farmed Ulva spp. using aqueous solutions of ionic liquids. Sep. Purif. Technol. 2021, 254, 117589. [Google Scholar] [CrossRef]
- Bekasova, O.D.; Shubin, V.V.; Safenkova, I.V.; Kovalyov, L.I.; Kurganov, B.I. Structural changes in R-phycoerythrin upon CdS quantum dot synthesis in tunnel cavities of protein molecules. Int. J. Biol. Macromol. 2013, 62, 623–628. [Google Scholar] [CrossRef]
- Shang, L.; Wang, Y.Z.; Jiang, J.G.; Dong, S.J. pH-Dependent Protein Conformational Changes in Albumin: Gold Nanoparticle Bioconjugates: A Spectroscopic Study. Langmuir 2007, 23, 2714–2721. [Google Scholar] [CrossRef]
- Muniz, G.L.; Borges, A.C.; da Silva, T.C.F.; Batista, R.O.; de Castro, S.R. Chemically enhanced primary treatment of dairy wastewater using chitosan obtained from shrimp wastes: Optimization using a Doehlert matrix design. Environ. Technol. 2022, 43, 237–254. [Google Scholar] [CrossRef]
- Feizi, Z.H.; Kazzaz, A.E.; Kong, F.; Fatehi, P. Evolving a flocculation process for isolating lignosulfonate from solution. Sep. Purif. Technol. 2019, 222, 254–263. [Google Scholar] [CrossRef]
- Barany, S.; Meszaros, R.; Kozakova, I.; Skvarla, I. Kinetics and mechanism of flocculation of bentonite and kaolin suspensions with polyelectrolytes and the strength of floccs. Colloid J. 2009, 71, 285–292. [Google Scholar] [CrossRef]
- Gregory, J.; Barany, S. Adsorption and flocculation by polymers and polymer mixtures. Adv. Colloid Interface Sci. 2011, 169, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Rêgo, T.V.; Cadaval, T.R.S.; Dotto, G.L.; Pinto, L.A.A. Statistical optimization, interaction analysis and desorption studies for the azo dyes adsorption onto chitosan films. J. Colloid. Interf. Sci. 2013, 411, 27–33. [Google Scholar] [CrossRef] [PubMed]
- Chang, A.K.T.; Frias, R.R.; Alvarez, L.V.; Bigol, U.G.; Guzman, J.P.M.D. Comparative antibacterial activity of commercial chitosan and chitosan extracted from Auricularia sp. Biocatal. Agric. Biotech. 2019, 17, 189–195. [Google Scholar] [CrossRef]
- Li, S.Q.; Qi, B.K.; Luo, J.Q.; Zhuang, Y.B.; Wan, Y.H. Ultrafast selective adsorption of pretreatment inhibitors from lignocellulosic hydrolysate with metal-organic frameworks: Performance and adsorption mechanisms. Sep. Purif. Technol. 2021, 275, 119183. [Google Scholar] [CrossRef]
- Song, Z.Z.; Zhang, W.J.; Gao, H.Y.; Wang, D.S. Comprehensive assessment of flocculation conditioning of dredged sediment using organic polymers: Dredged sediment dewaterability and release of pollutants. Sci. Total Environ. 2020, 739, 139884. [Google Scholar] [CrossRef]
- Guo, K.Y.; Gao, B.Y.; Yue, Q.Y.; Xu, X.; Li, R.H.; Shen, X. Characterization and performance of a novel lignin-based flocculant for the treatment of dye wastewater. Int. Biodeter. Biodegr. 2018, 133, 99–107. [Google Scholar] [CrossRef]
- Ma, J.Y.; Fu, K.; Jiang, L.Y.; Ding, L.; Guan, Q.Q.; Zhang, S.H.; Zhang, H.W.; Shi, J.; Fu, X. Flocculation performance of cationic polyacrylamide with high cationic degree in humic acid synthetic water treatment and effect of kaolin particles. Sep. Purif. Technol. 2017, 181, 201–212. [Google Scholar] [CrossRef]
- Bennett, A.; Bogorad, L. Complementary chromatic adaptation in a filamentous blue-green alga. J. Cell Biol. 1973, 58, 419–435. [Google Scholar] [CrossRef]
- Winayu, B.N.R.; Lai, K.T.; Hsueh, T.H.; Chu, H. Production of phycobiliprotein and carotenoid by efficient extraction from Thermosynechococcus sp. CL-1 cultivation in swine wastewater. Bioresour. Technol. 2021, 319, 124125. [Google Scholar] [CrossRef]
- Chittapun, S.; Jonjaroen, V.; Khumrangsee, K.; Charoenrat, T. C-phycocyanin extraction from two freshwater cyanobacteria by freeze thaw and pulsed electric field techniques to improve extraction efficiency and purity. Algal Res. 2020, 46, 101789. [Google Scholar] [CrossRef]
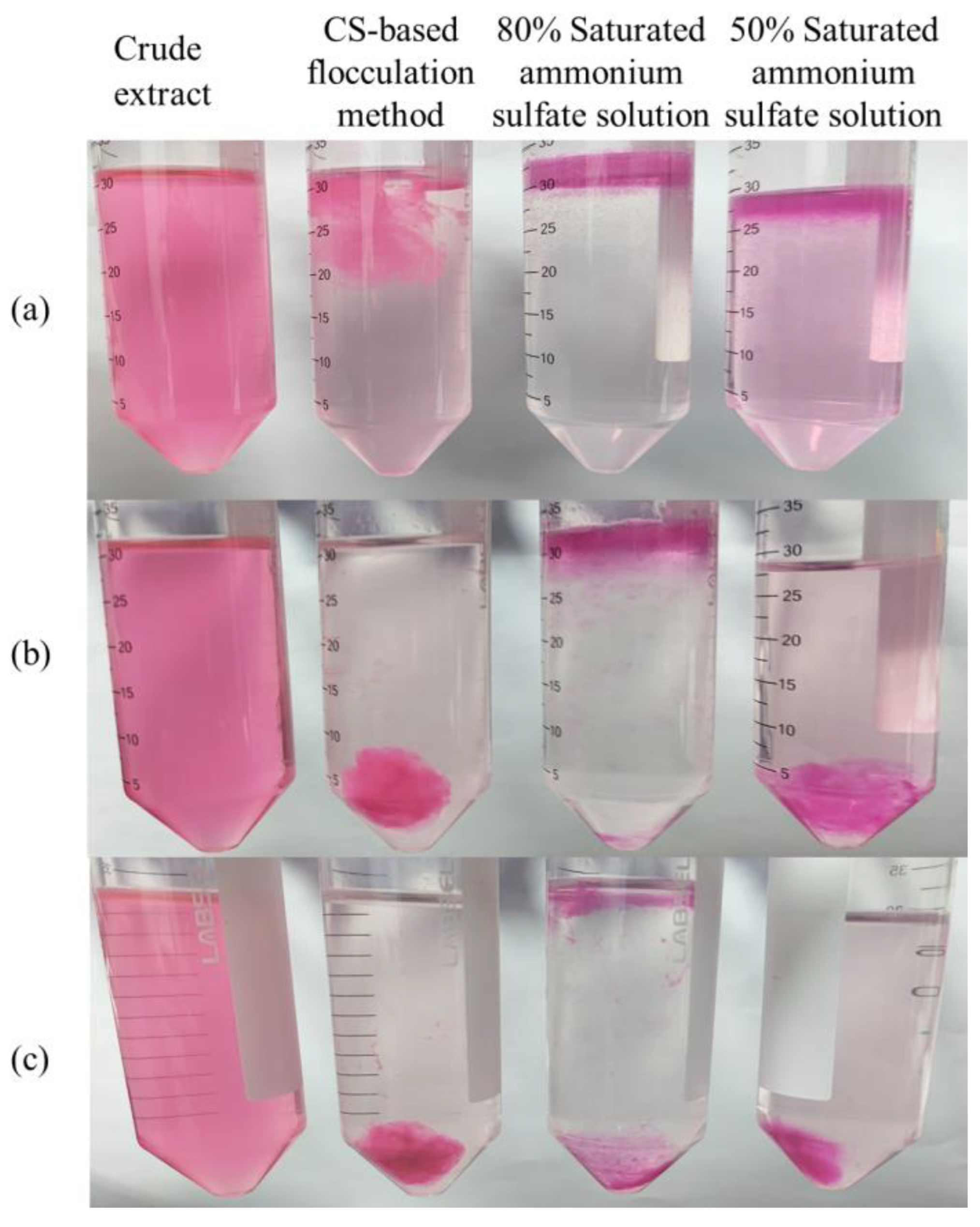

| Items | CS-Based Flocculation Method | 80% Saturated Ammonium Sulfate Solution | 50% Saturated Ammonium Sulfate Solution |
|---|---|---|---|
| Centrifugal speed (rpm) | 2000 | 8000 | 8000 |
| Centrifugal time (min) | 15 | 30 | 30 |
| Dialysis | No | Yes | Yes |
| Purity index | 3.20 ± 0.025 | 0.62 ± 0.021 | 0.83 ± 0.018 |
| Recovery rate (%) | 72.07 ± 1.37 | 45.10 ± 2.28 | 36.13 ± 2.44 |
| Item | CS-Based Flocculation Method | Ammonium Sulfate Precipitation Method |
|---|---|---|
| Material price (USD per kg) | 278.57 | 10.29 |
| Material consumption (kg) | 1.39 × 10−5 | 3.10 × 10−2 |
| Purity index | 3.20 ± 0.025 | 0.62 ± 0.021 |
| Total costs (USD) | 3.90 × 10−3 | 3.10 × 10−1 |
| Revenues generated (USD) | 4.10 | 0.12 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liang, Y.; Deng, L.; Feng, Z.; Ouyang, Q.; Wu, X.; Quan, W.; Zhu, Y.; Ye, H.; Wu, K.; Luo, H. A Chitosan-Based Flocculation Method for Efficient Recovery of High-Purity B-Phycoerythrin from a Low Concentration of Phycobilin in Wastewater. Molecules 2023, 28, 3600. https://doi.org/10.3390/molecules28083600
Liang Y, Deng L, Feng Z, Ouyang Q, Wu X, Quan W, Zhu Y, Ye H, Wu K, Luo H. A Chitosan-Based Flocculation Method for Efficient Recovery of High-Purity B-Phycoerythrin from a Low Concentration of Phycobilin in Wastewater. Molecules. 2023; 28(8):3600. https://doi.org/10.3390/molecules28083600
Chicago/Turabian StyleLiang, Yingye, Luming Deng, Zhenhui Feng, Qianqian Ouyang, Xia Wu, Weiyan Quan, Yuzhen Zhu, Hua Ye, Kefeng Wu, and Hui Luo. 2023. "A Chitosan-Based Flocculation Method for Efficient Recovery of High-Purity B-Phycoerythrin from a Low Concentration of Phycobilin in Wastewater" Molecules 28, no. 8: 3600. https://doi.org/10.3390/molecules28083600
APA StyleLiang, Y., Deng, L., Feng, Z., Ouyang, Q., Wu, X., Quan, W., Zhu, Y., Ye, H., Wu, K., & Luo, H. (2023). A Chitosan-Based Flocculation Method for Efficient Recovery of High-Purity B-Phycoerythrin from a Low Concentration of Phycobilin in Wastewater. Molecules, 28(8), 3600. https://doi.org/10.3390/molecules28083600






