The Research Progress of Extraction, Purification and Analysis Methods of Phenolic Compounds from Blueberry: A Comprehensive Review
Abstract
1. Introduction
2. Research Progress of Extraction, Purification, and Detection Methods of Some Phenolic Compounds in Blueberries
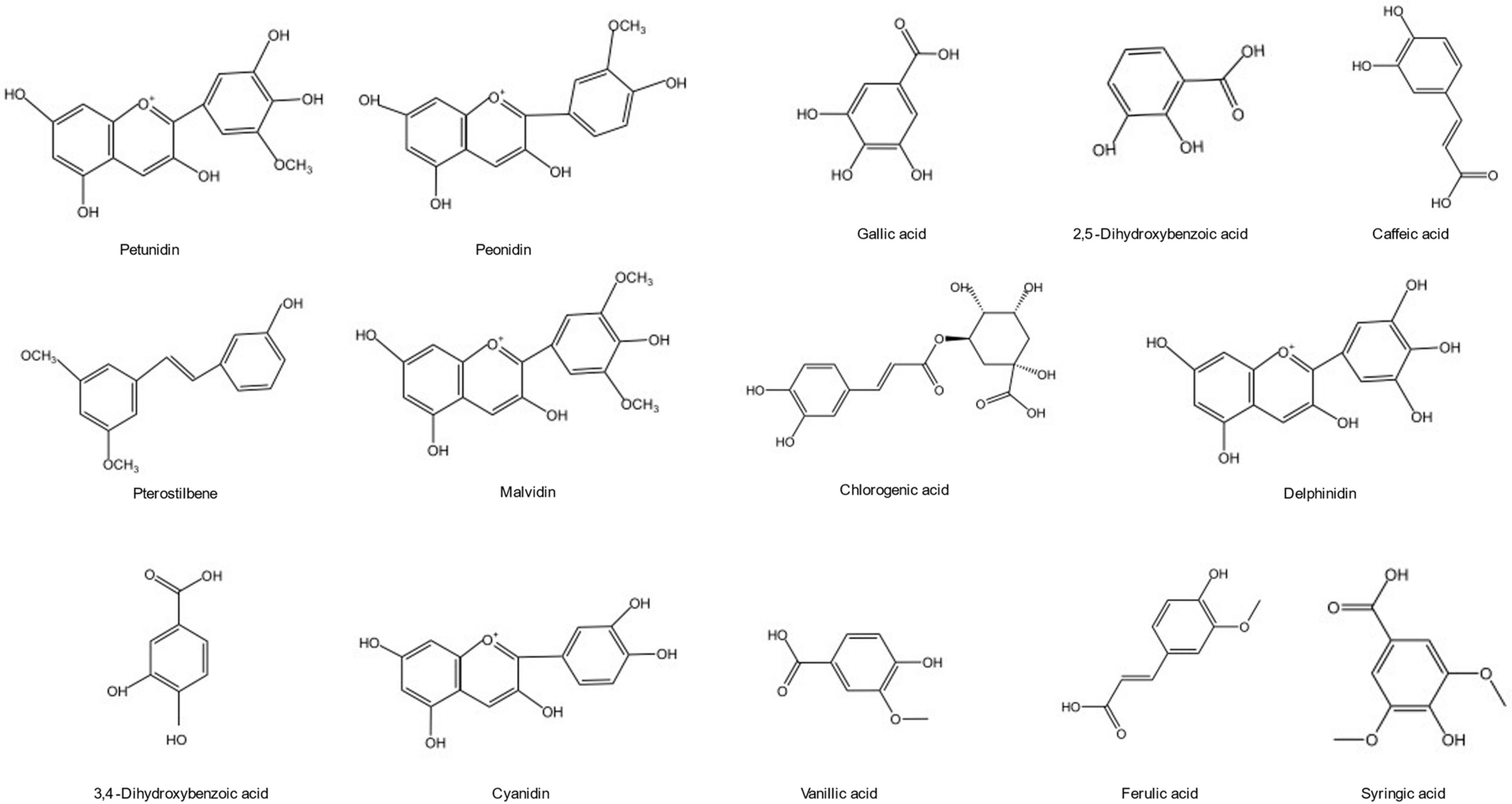
2.1. Anthocyanines
2.1.1. Extraction Methods
Solvent Extraction
Ultrasound-Assisted Extraction
Microwave-Assisted Extraction (MAE)
Extraction Using Deep Eutectic Solvent
Enzyme-Assisted Extraction
Extraction Using Supercritical Fluid Carbon Dioxide
Combined Extraction Method
2.1.2. Separation and Purification
High Performance Liquid Chromatography (HPLC)
Column Chromatography
Membrane Separation
Progress in Coupled Separation Techniques
2.1.3. Detection and Analysis Methods
Ultraviolet-Visible (UV-VIS) Spectrophotometry
Chromatographic Method
Mass Spectrometry
Coupled Detection Methods
2.2. Pterostilbene
2.2.1. Extraction Methods
2.2.2. Separation and Purification
2.2.3. Detection and Analysis Method Mass Spectrometry (MS)
Chromatographic Method
Coupled Detection Methods
2.3. Phenolic Acids
2.3.1. Extraction Methods
PEF Law
Ultrasound-Assisted Solvent Extraction (UASE)
Coupled Extraction Techniques
2.3.2. Separation and Purification
Resin Adsorption Method
Chromatographic Method
2.3.3. Detection and Analysis Methods
Chromatographic Method
Capillary Electrophoresis (CE)
Coupled Detection Techniques
2.4. Tannins
2.4.1. Extraction Methods
Solid–Liquid Extraction (SLE)
Supercritical Fluid Extraction (SFE)
Microwave-Assisted Extraction (MAE)
Pressurized Water Extraction (PWE)
Ultrasound-Assisted Extraction (UAE)
2.4.2. Separation and Purification
2.4.3. Detection and Analysis
3. Outlook
3.1. Food Value and Prospects of Blueberries
3.2. Health Value and Prospects of Blueberries
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Reque, P.M.; Steffens, R.S.; Silva, A.M.D.; Jablonski, A.; Flôres, S.H.; Rios, A.D.O.; Jong, E.V.D. Characterization of blueberry fruits (Vaccinium spp.) and derived products. Food Sci. Technol. 2014, 34, 773–779. [Google Scholar] [CrossRef]
- Ferlemi, A.-V.; Lamari, F.N. Berry Leaves: An Alternative Source of Bioactive Natural Products of Nutritional and Medicinal Value. Antioxidants 2016, 5, 17. [Google Scholar] [CrossRef] [PubMed]
- Duan, Y.; Tarafdar, A.; Chaurasia, D.; Singh, A.; Bhargava, P.C.; Yang, J.; Li, Z.; Ni, X.; Tian, Y.; Li, H.; et al. Blueberry fruit valorization and valuable constituents: A review. Int. J. Food Microbiol. 2022, 381, 109890. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Yong, H.; Yao, X.; Hu, H.; Yun, D.; Xiao, L. Recent advances in phenolic–protein conjugates: Synthesis, characterization, biological activities and potential applications. RSC Adv. 2019, 9, 35825–35840. [Google Scholar] [CrossRef] [PubMed]
- Kostka, T.; Ostberg-Potthoff, J.J.; Stärke, J.; Guigas, C.; Matsugo, S.; Mirčeski, V.; Stojanov, L.; Veličkovska, S.K.; Winterhalter, P.; Esatbeyoglu, T. Bioactive Phenolic Compounds from Lingonberry (Vaccinium vitis-idaea L.): Extraction, Chemical Characterization, Fractionation and Cellular Antioxidant Activity. Antioxidants 2022, 11, 467. [Google Scholar] [CrossRef] [PubMed]
- Martini, D.; Marino, M.; Venturi, S.; Tucci, M.; Klimis-Zacas, D.; Riso, P.; Porrini, M.; Del Bo’, C. Blueberries and their bioactives in the modulation of oxidative stress, inflammation and cardio/vascular function markers: A systematic review of human intervention studies. J. Nutr. Biochem. 2023, 111, 109154. [Google Scholar] [CrossRef]
- Ferreira, L.F.; Minuzzi, N.M.; Rodrigues, R.F.; Pauletto, R.; Rodrigues, E.; Emanuelli, T.; Bochi, V.C. Citric acid water-based solution for blueberry bagasse anthocyanins recovery: Optimization and comparisons with microwave-assisted extraction (MAE). Lwt 2020, 133, 110064. [Google Scholar] [CrossRef]
- Hu, A.-J.; Hao, S.-T.; Zheng, J.; Chen, L.; Sun, P.-P. Multi-Frequency Ultrasonic Extraction of Anthocyanins from Blueberry Pomace and Evaluation of Its Antioxidant Activity. J. AOAC Int. 2020, 104, 811–817. [Google Scholar] [CrossRef]
- Aliaño-González, M.J.; Jarillo, J.A.; Carrera, C.; Ferreiro-González, M.; Álvarez, J.; Palma, M.; Ayuso, J.; Barbero, G.F.; Espada-Bellido, E. Optimization of a Novel Method Based on Ultrasound-Assisted Extraction for the Quantification of Anthocyanins and Total Phenolic Compounds in Blueberry Samples (Vaccinium corymbosum L.). Foods 2020, 9, 1763. [Google Scholar] [CrossRef]
- Xue, H.; Xu, H.; Wang, X.; Shen, L.; Liu, H.; Liu, C.; Qin, Q.; Zheng, X.; Li, Q. Effects of Microwave Power on Extraction Kinetic of Anthocyanin from Blueberry Powder considering Absorption of Microwave Energy. J. Food Qual. 2018, 2018, 9680184. [Google Scholar] [CrossRef]
- da Silva, D.T.; Smaniotto, F.A.; Costa, I.F.; Baranzelli, J.; Muller, A.; Somacal, S.; Monteiro, C.S.; Vizzotto, M.; Rodrigues, E.; Barcia, M.T.; et al. Natural deep eutectic solvent (NADES): A strategy to improve the bioavailability of blueberry phenolic compounds in a ready-to-use extract. Food Chem. 2021, 364, 130370. [Google Scholar] [CrossRef] [PubMed]
- Granato, D.; Fidelis, M.; Haapakoski, M.; Lima, A.D.S.; Viil, J.; Hellström, J.; Rätsep, R.; Kaldmäe, H.; Bleive, U.; Azevedo, L.; et al. Enzyme-assisted extraction of anthocyanins and other phenolic compounds from blackcurrant (Ribes nigrum L.) press cake: From processing to bioactivities. Food Chem. 2022, 391, 133240. [Google Scholar] [CrossRef] [PubMed]
- Qin, G.; Han, H.; Ding, Y.; Wang, L. Optimization of extracting technology of anthocyanins from blueberry pomace by supercritical carbon dioxide. Appl. Chem. Ind. 2019, 48, 109–112. [Google Scholar]
- Machado, A.P.D.F.; Pereira, A.L.D.; Barbero, G.F.; Martínez, J. Recovery of anthocyanins from residues of Rubus fruticosus, Vaccinium myrtillus and Eugenia brasiliensis by ultrasound assisted extraction, pressurized liquid extraction and their combination. Food Chem. 2017, 231, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Jovanović, M.S.; Krgović, N.; Živković, J.; Stević, T.; Zdunić, G.; Bigović, D.; Šavikin, K. Ultrasound-Assisted Natural Deep Eutectic Solvents Extraction of Bilberry Anthocyanins: Optimization, Bioactivities, and Storage Stability. Plants 2022, 11, 2680. [Google Scholar] [CrossRef] [PubMed]
- Nagarajan, S.; Mohandas, S.; Ganesan, K.; Xu, B.; Ramkumar, K.M. New Insights into Dietary Pterostilbene: Sources, Metabolism, and Health Promotion Effects. Molecules 2022, 27, 6316. [Google Scholar] [CrossRef] [PubMed]
- Cheung, L.K.; Sanders, A.D.; Pratap-Singh, A.; Dee, D.R.; Dupuis, J.H.; Baldelli, A.; Yada, R.Y. Effects of high pressure on protein stability, structure, and function—Theory and applications. In Effect of High-Pressure Technologies on Enzymes; Academic Press: Cambridge, MA, USA, 2023. [Google Scholar] [CrossRef]
- Lončarić, A.; Celeiro, M.; Jozinović, A.; Jelinić, J.; Kovač, T.; Jokić, S.; Babić, J.; Moslavac, T.; Zavadlav, S.; Lores, M. Green Extraction Methods for Extraction of Polyphenolic Compounds from Blueberry Pomace. Foods 2020, 9, 1521. [Google Scholar] [CrossRef]
- Frosi, I.; Montagna, I.; Colombo, R.; Milanese, C.; Papetti, A. Recovery of chlorogenic acids from agri-food wastes: Updates on green extraction techniques. Molecules 2021, 26, 4515. [Google Scholar] [CrossRef]
- Shi, M.; Loftus, H.; McAinch, A.J.; Su, X.Q. Blueberry as a source of bioactive compounds for the treatment of obesity, type 2 diabetes and chronic inflammation. J. Funct. Foods 2017, 30, 16–29. [Google Scholar] [CrossRef]
- Ngamsamer, C.; Sirivarasai, J.; Sutjarit, N. The Benefits of Anthocyanins against Obesity-Induced Inflammation. Biomolecules 2022, 12, 852. [Google Scholar] [CrossRef]
- Trushina, E.N.; Mustaphina, O.K.; Aksenov, I.V.; Krasutsky, A.G.; Nikityuk, D.B. Protective effect of anthocyanins on apoptosis of gastrocnemius muscle myocytes of rats after intense exercise. Probl. Nutr. 2022, 91, 47–53. [Google Scholar] [CrossRef] [PubMed]
- Surien, O.; Masre, S.F.; Basri, D.F.; Ghazali, A.R. Chemopreventive Effects of Oral Pterostilbene in Multistage Carcinogenesis of Skin Squamous Cell Carcinoma Mouse Model Induced by DMBA/TPA. Biomedicines 2022, 10, 2743. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Zorita, S.; Milton-Laskibar, I.; Macarulla, M.T.; Biasutto, L.; Fernández-Quintela, A.; Miranda, J.; Lasa, A.; Segues, N.; Bujanda, L.; Portillo, M.P. Pterostilbene modifies triglyceride metabolism in hepatic steatosis induced by high-fat high-fructose feeding: A comparison with its analog resveratrol. Food Funct. 2021, 12, 3266–3279. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.; Guo, F.; Mai, H.; Liu, J.; Xia, Z.; Zhu, G.; Zhang, J.; Ma, L.; Fu, P. Pterostilbene, a Bioactive Component of Blueberries, Alleviates Renal Interstitial Fibrosis by Inhibiting Macrophage-Myofibroblast Transition. Am. J. Chin. Med. 2020, 48, 1715–1729. [Google Scholar] [CrossRef]
- Li, Y.; Sun, C.; Zhang, Y.; Chen, X.; Huang, H.; Han, L.; Xing, H.; Zhao, D.; Chen, X.; Zhang, Y. Phase I Metabolism of Pterostilbene, a Dietary Resveratrol Derivative: Metabolite Identification, Species Differences, Isozyme Contribution, and Further Bioactivation. J. Agric. Food Chem. 2022, 71, 331–346. [Google Scholar] [CrossRef]
- Pan, J.; Shi, M.; Li, L.; Liu, J.; Guo, F.; Feng, Y.; Ma, L.; Fu, P. Pterostilbene, a bioactive component of blueberries, alleviates renal fibrosis in a severe mouse model of hyperuricemic nephropathy. Biomed. Pharmacother. 2018, 109, 1802–1808. [Google Scholar] [CrossRef]
- Fraga-Corral, M.; García-Oliveira, P.; Pereira, A.G.; Lourenço-Lopes, C.; Jimenez-Lopez, C.; Prieto, M.A.; Simal-Gandara, J. Technological Application of Tannin-Based Extracts. Molecules 2020, 25, 614. [Google Scholar] [CrossRef]
- Shamilov, A.A.; Olennikov, D.N.; Pozdnyakov, D.I.; Bubenchikova, V.N.; Garsiya, E.R.; Larskii, M.V. Caucasian Blueberry: Comparative Study of Phenolic Compounds and Neuroprotective and Antioxidant Potential of Vaccinium myrtillus and Vaccinium arctostaphylos Leaves. Life 2022, 12, 2079. [Google Scholar] [CrossRef]
- Yang, L.; Wang, Z.-M.; Wang, Y.; Li, R.-S.; Wang, F.; Wang, K. Phenolic constituents with neuroprotective activities from Hypericum wightianum. Phytochemistry 2019, 165, 112049. [Google Scholar] [CrossRef]
- Xie, Y.; Yang, W.; Tang, F.; Chen, X.; Ren, L. Antibacterial Activities of Flavonoids: Structure-Activity Relationship and Mechanism. Curr. Med. Chem. 2014, 22, 132–149. [Google Scholar] [CrossRef]
- Winiarska-Mieczan, A.; Kwiecień, M.; Jachimowicz-Rogowska, K.; Donaldson, J.; Tomaszewska, E.; Baranowska-Wójcik, E. Anti-Inflammatory, Antioxidant, and Neuroprotective Effects of Polyphenols—Polyphenols as an Element of Diet Therapy in Depressive Disorders. Int. J. Mol. Sci. 2023, 24, 2258. [Google Scholar] [CrossRef] [PubMed]
- Tobar-Bolaños, G.; Casas-Forero, N.; Orellana-Palma, P.; Petzold, G. Blueberry juice: Bioactive compounds, health impact, and concentration technologies—A review. J. Food Sci. 2021, 86, 5062–5077. [Google Scholar] [CrossRef] [PubMed]
- Ayvaz, H.; Cabaroglu, T.; Akyildiz, A.; Pala, C.U.; Temizkan, R.; Ağçam, E.; Ayvaz, Z.; Durazzo, A.; Lucarini, M.; Direito, R.; et al. Anthocyanins: Metabolic Digestion, Bioavailability, Therapeutic Effects, Current Pharmaceutical/Industrial Use, and Innovation Potential. Antioxidants 2022, 12, 48. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Wang, S.; Wu, Q.; Battino, M.; Giampieri, F.; Bai, W.; Tian, L. Recovering high value-added anthocyanins from blueberry pomace with ultrasound-assisted extraction. Food Chem. X 2022, 16, 100476. [Google Scholar] [CrossRef]
- Ehlenfeldt, M.K.; Prior, R.L. Oxygen Radical Absorbance Capacity (ORAC) and Phenolic and Anthocyanin Concentrations in Fruit and Leaf Tissues of Highbush Blueberry. J. Agric. Food Chem. 2001, 49, 2222–2227. [Google Scholar] [CrossRef]
- Tan, J.; Han, Y.; Han, B.; Qi, X.; Cai, X.; Ge, S.; Xue, H. Extraction and purification of anthocyanins: A review. J. Agric. Food Res. 2022, 8, 100306. [Google Scholar] [CrossRef]
- Yao, G.-L.; Ma, X.-H.; Cao, X.-Y.; Chen, J. Effects of Power Ultrasound on Stability of Cyanidin-3-glucoside Obtained from Blueberry. Molecules 2016, 21, 1564. [Google Scholar] [CrossRef]
- Nunes, A.N.; Borges, A.; Matias, A.A.; Bronze, M.R.; Oliveira, J. Alternative Extraction and Downstream Purification Processes for Anthocyanins. Molecules 2022, 27, 368. [Google Scholar] [CrossRef]
- Grillo, G.; Gunjević, V.; Radošević, K.; Redovniković, I.R.; Cravotto, G. Deep Eutectic Solvents and Nonconventional Technologies for Blueberry-Peel Extraction: Kinetics, Anthocyanin Stability, and Antiproliferative Activity. Antioxidants 2020, 9, 1069. [Google Scholar] [CrossRef]
- Yuan, J.; Li, H.; Tao, W.; Han, Q.; Dong, H.; Zhang, J.; Jing, Y.; Wang, Y.; Xiong, Q.; Xu, T. An effective method for extracting anthocyanins from blueberry based on freeze-ultrasonic thawing technology. Ultrason. Sonochem. 2020, 68, 105192. [Google Scholar] [CrossRef]
- Arruda, H.S.; Silva, E.K.; Araujo, N.M.P.; Pereira, G.A.; Pastore, G.M.; Junior, M.R.M. Anthocyanins Recovered from Agri-Food By-Products Using Innovative Processes: Trends, Challenges, and Perspectives for Their Application in Food Systems. Molecules 2021, 26, 2632. [Google Scholar] [CrossRef]
- Diaconeasa, Z.; Iuhas, C.I.; Ayvaz, H.; Mortas, M.; Farcaş, A.; Mihai, M.; Danciu, C.; Stanilă, A. Anthocyanins from Agro-Industrial Food Waste: Geographical Approach and Methods of Recovery—A Review. Plants 2022, 12, 74. [Google Scholar] [CrossRef] [PubMed]
- Gligor, O.; Mocan, A.; Moldovan, C.; Locatelli, M.; Crișan, G.; Ferreira, I.C.F.R. Enzyme-assisted extractions of polyphenols—A comprehensive review. Trends Food Sci. Technol. 2019, 88, 302–315. [Google Scholar] [CrossRef]
- Jin, Y.; Zhang, Y.; Liu, D.; Liu, D.; Zhang, C.; Qi, H.; Gu, H.; Yang, L.; Zhou, Z. Efficient Homogenization-Ultrasound-Assisted Extraction of Anthocyanins and Flavonols from Bog Bilberry (Vaccinium uliginosum L.) Marc with Carnosic Acid as an Antioxidant Additive. Molecules 2019, 24, 2537. [Google Scholar] [CrossRef]
- Boateng, I.D. Evaluating the status quo of deep eutectic solvent in food chemistry. Potentials and limitations. Food Chem. 2023, 406, 135079. [Google Scholar] [CrossRef]
- Zhou, Y.; Long, S.; Xu, Q.; Yan, C.; Yang, J.; Zhou, Y. Optimization and application of HPLC for simultaneous separation of six well-known major anthocyanins in blueberry. Prep. Biochem. Biotechnol. 2021, 51, 961–970. [Google Scholar] [CrossRef]
- Wang, E.; Yin, Y.; Xu, C.; Liu, J. Isolation of high-purity anthocyanin mixtures and monomers from blueberries using combined chromatographic techniques. J. Chromatogr. A 2014, 1327, 39–48. [Google Scholar] [CrossRef] [PubMed]
- Degenhardt, A.; Knapp, H.; Winterhalter, P. Separation and Purification of Anthocyanins by High-Speed Countercurrent Chromatography and Screening for Antioxidant Activity. J. Agric. Food Chem. 2000, 48, 338–343. [Google Scholar] [CrossRef]
- Xiao, H.U.; Sun, A.D.; Zhang, D.Q. Separation of anthocyanins from Perilla frutescens by high speed counter-current chromatography. J. Chin. Med. Mater. 2010, 33, 1586. [Google Scholar]
- Xue, H.; Shen, L.; Wang, X.; Liu, C.; Liu, C.; Liu, H.; Zheng, X. Isolation and Purification of Anthocyanin from Blueberry Using Macroporous Resin Combined Sephadex LH-20 Techniques. Food Sci. Technol. Res. 2019, 25, 29–38. [Google Scholar] [CrossRef]
- Yao, L.; Zhang, N.; Wang, C.; Wang, C. Highly Selective Separation and Purification of Anthocyanins from Bilberry Based on a Macroporous Polymeric Adsorbent. J. Agric. Food Chem. 2015, 63, 3543–3550. [Google Scholar] [CrossRef] [PubMed]
- Liao, Z.; Zhang, X.; Chen, X.; Battino, M.; Giampieri, F.; Bai, W.; Tian, L. Recovery of value-added anthocyanins from mulberry by a cation exchange chromatography. Curr. Res. Food Sci. 2022, 5, 1445–1451. [Google Scholar] [CrossRef] [PubMed]
- Avram, A.M.; Morin, P.; Brownmiller, C.; Howard, L.R.; Sengupta, A.; Wickramasinghe, S.R. Concentrations of polyphenols from blueberry pomace extract using nanofiltration. Food Bioprod. Process. 2017, 106, 91–101. [Google Scholar] [CrossRef]
- Chorfa, N.; Savard, S.; Belkacemi, K. An efficient method for high-purity anthocyanin isomers isolation from wild blueberries and their radical scavenging activity. Food Chem. 2016, 197 Pt B, 1226–1234. [Google Scholar] [CrossRef]
- Sedbare, R.; Raudone, L.; Zvikas, V.; Viskelis, J.; Liaudanskas, M.; Janulis, V. Development and Validation of the UPLC-DAD Methodology for the Detection of Triterpenoids and Phytosterols in Fruit Samples of Vaccinium macrocarpon Aiton and Vaccinium oxycoccos L. Molecules 2022, 27, 4403. [Google Scholar] [CrossRef] [PubMed]
- AOAC. Official Methods of Analysis; AOAC Press: Philadelphia, PA, USA, 2005. [Google Scholar]
- Delgado-Povedano, M.D.M.; de Villiers, A.; Hann, S.; Causon, T. Identity confirmation of anthocyanins in berries by LC–DAD–IM-QTOFMS. Electrophoresis 2020, 42, 473–481. [Google Scholar] [CrossRef] [PubMed]
- Myjavcová, R.; Marhol, P.; Křen, V.; Šimánek, V.; Ulrichová, J.; Palíková, I.; Papoušková, B.; Lemr, K.; Bednář, P. Analysis of anthocyanin pigments in Lonicera (Caerulea) extracts using chromatographic fractionation followed by microcolumn liquid chromatography-mass spectrometry. J. Chromatogr. A 2010, 1217, 7932–7941. [Google Scholar] [CrossRef]
- Longo, L.; Vasapollo, G. Determination of Anthocyanins in Ruscus aculeatus L. Berries. J. Agric. Food Chem. 2004, 53, 475–479. [Google Scholar] [CrossRef]
- Kalogiouri, N.P.; Karadimou, C.; Avgidou, M.S.; Petsa, E.; Papadakis, E.-N.; Theocharis, S.; Mourtzinos, I.; Menkissoglu-Spiroudi, U.; Koundouras, S. An Optimized HPLC-DAD Methodology for the Determination of Anthocyanins in Grape Skins of Red Greek Winegrape Cultivars (Vitis vinifera L.). Molecules 2022, 27, 7107. [Google Scholar] [CrossRef]
- Saha, S.; Singh, J.; Paul, A.; Sarkar, R.; Khan, Z.; Banerjee, K. Anthocyanin Profiling Using UV-Vis Spectroscopy and Liquid Chromatography Mass Spectrometry. J. AOAC Int. 2020, 103, 23–39. [Google Scholar] [CrossRef]
- Thuy, N.M.; Minh, V.Q.; Ben, T.C.; Nguyen, M.T.T.; Ha, H.T.N.; Van Tai, N. Identification of Anthocyanin Compounds in Butterfly Pea Flowers (Clitoria ternatea L.) by Ultra Performance Liquid Chromatography/Ultraviolet Coupled to Mass Spectrometry. Molecules 2021, 26, 4539. [Google Scholar] [CrossRef]
- Gavrilova, V.; Kajdžanoska, M.; Gjamovski, V.; Stefova, M. Separation, Characterization and Quantification of Phenolic Compounds in Blueberries and Red and Black Currants by HPLC−DAD−ESI-MSn. J. Agric. Food Chem. 2011, 59, 4009–4018. [Google Scholar] [CrossRef]
- Prior, R.L.; Lazarus, S.A.; Cao, G.; Muccitelli, H.; Hammerstone, J.F. Identification of Procyanidins and Anthocyanins in Blueberries and Cranberries (Vaccinium spp.) Using High-Performance Liquid Chromatography/Mass Spectrometry. J. Agric. Food Chem. 2001, 49, 1270–1276. [Google Scholar] [CrossRef] [PubMed]
- Lin, W.-S.; Leland, J.V.; Ho, C.-T.; Pan, M.-H. Occurrence, Bioavailability, Anti-inflammatory, and Anticancer Effects of Pterostilbene. J. Agric. Food Chem. 2020, 68, 12788–12799. [Google Scholar] [CrossRef] [PubMed]
- Devgun, M.; Nanda, A.; Ansari, S.H. Comparison of conventional and non conventional methods of extraction of heartwood of Pterocarpus marsupium Roxb. Acta Pol. Pharm. 2012, 69, 475–485. [Google Scholar]
- Catenacci, L.; Vicatos, A.I.; Sorrenti, M.; Bonferoni, M.C.; Caira, M.R. Native Cyclodextrins as Complexation Agents for Pterostilbene: Complex Preparation and Characterization in Solution and in the Solid State. Pharmaceutics 2021, 14, 8. [Google Scholar] [CrossRef]
- Liu, J.; Pu, H.; Liu, S.; Kan, J.; Jin, C. Synthesis, characterization, bioactivity and potential application of phenolic acid grafted chitosan: A review. Carbohydr. Polym. 2017, 174, 999–1017. [Google Scholar] [CrossRef] [PubMed]
- Waszczuk, M.; Bianchi, S.E.; Pittol, V.; Martiny, S.; Delagustin, M.G.; Meirelles, G.D.C.; Raabe, V.B.; Barbosa, F.D.S.; Lacerda, D.D.S.; Araújo, A.S.; et al. The challenge of improving pterostilbene (PTS) solubility for solid and semi-solid dosage forms: The obtention of binary and ternary systems. Int. J. Pharm. 2023, 635, 122736. [Google Scholar] [CrossRef]
- Becker, L.; Carré, V.; Poutaraud, A.; Merdinoglu, D.; Chaimbault, P. MALDI Mass Spectrometry Imaging for the Simultaneous Location of Resveratrol, Pterostilbene and Viniferins on Grapevine Leaves. Molecules 2014, 19, 10587–10600. [Google Scholar] [CrossRef]
- Kambiranda, D.M.; Basha, S.M.; Stringer, S.J.; Obuya, J.O.; Snowden, J.J. Multi-year Quantitative Evaluation of Stilbenoids Levels Among Selected Muscadine Grape Cultivars. Molecules 2019, 24, 981. [Google Scholar] [CrossRef]
- Waszczuk, M.; Bianchi, S.E.; Martiny, S.; Pittol, V.; Lacerda, D.S.; Araújo, A.S.d.R.; Bassani, V.L. Development and validation of a specific-stability indicating liquid chromatography method for quantitative analysis of pterostilbene: Application in food and pharmaceutical products. Anal. Methods 2020, 12, 4310–4318. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Bonilla, P.; López-Nicolás, J.M.; Méndez-Cazorla, L.; García-Carmona, F. Development of a reversed phase high performance liquid chromatography method based on the use of cyclodextrins as mobile phase additives to determine pterostilbene in blueberries. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2011, 879, 1091–1097. [Google Scholar] [CrossRef]
- Rodríguez-Cabo, T.; Rodríguez, I.; Cela, R. Determination of hydroxylated stilbenes in wine by dispersive liquid–liquid microextraction followed by gas chromatography mass spectrometry. J. Chromatogr. A 2012, 1258, 21–29. [Google Scholar] [CrossRef]
- Mazzotti, F.; Di Donna, L.; Benabdelkamel, H.; Gabriele, B.; Napoli, A.; Sindona, G. The assay of pterostilbene in spiked matrices by liquid chromatography tandem mass spectrometry and isotope dilution method. J. Mass Spectrom. 2010, 45, 358–363. [Google Scholar] [CrossRef] [PubMed]
- Xie, L.; Bolling, B.W. Characterisation of stilbenes in California almonds (Prunus dulcis) by UHPLC–MS. Food Chem. 2014, 148, 300–306. [Google Scholar] [CrossRef]
- Zhang, L.; Tian, G.; Huang, L.; Zhou, M.; Zhu, J.; Yi, L.; Mi, M. Pterostilbene attenuates intestinal epithelial barrier loss induced by high loading intensity of exercise. Front. Nutr. 2022, 9, 965180. [Google Scholar] [CrossRef] [PubMed]
- Mattila, P.; Hellström, J.; Törrönen, R. Phenolic Acids in Berries, Fruits, and Beverages. J. Agric. Food Chem. 2006, 54, 7193–7199. [Google Scholar] [CrossRef]
- Wang, L.; Pan, X.; Jiang, L.; Chu, Y.; Gao, S.; Jiang, X.; Zhang, Y.; Chen, Y.; Luo, S.; Peng, C. The Biological Activity Mechanism of Chlorogenic Acid and Its Applications in Food Industry: A Review. Front. Nutr. 2022, 9, 943911. [Google Scholar] [CrossRef]
- Wianowska, D.; Gil, M. Recent advances in extraction and analysis procedures of natural chlorogenic acids. Phytochem. Rev. 2018, 18, 273–302. [Google Scholar] [CrossRef]
- Wang, T.; Xu, W.-J.; Wang, S.-X.; Kou, P.; Wang, P.; Wang, X.-Q.; Fu, Y.-J. Integrated and sustainable separation of chlorogenic acid from blueberry leaves by deep eutectic solvents coupled with aqueous two-phase system. Food Bioprod. Process. 2017, 105, 205–214. [Google Scholar] [CrossRef]
- Xie, L.; Chong, K.Y.; Stefanova, R.; Hui, J.P.M.; Zhang, J.; Brooks, M.S.-L. Recovery of chlorogenic acid from haskap leaves (Lonicera caerulea) using aqueous two-phase extraction. Biomass Convers. Biorefinery 2021, 13, 3741–3750. [Google Scholar] [CrossRef]
- Jiang, H.; Yang, L.; Xing, X.; Yan, M.; Guo, X.; Yang, B.; Wang, Q.-H.; Kuang, H.-X. Development of an analytical method for separation of phenolic acids by ultra-performance convergence chromatography (UPC 2) using a column packed with a sub-2-μm particle. J. Pharm. Biomed. Anal. 2018, 153, 117–125. [Google Scholar] [CrossRef] [PubMed]
- Dong, G.; Xu, J.; Gu, Y.; Wei, Y. A general separation method of phenolic acids using pH-zone-refining counter-current chromatography and its application to oat bran. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2015, 992, 36–42. [Google Scholar] [CrossRef]
- Ma, T.; Dong, H.; Geng, Y.; Guo, L.; Wang, X. Preparative separation of eight phenolic acids from Echinacea purpurea L. Moench using pH-zone-refining counter-current chromatography and evaluation of their immunomodulatory effects and synergistic potential. Anal. Methods 2023, 15, 778–787. [Google Scholar] [CrossRef]
- Chen, H.; Song, X.; Huang, X. Development of magnetism-assisted in-tube solid phase microextraction of phenolic acids in fruit juices prior to high-performance liquid chromatography quantification. J. Sep. Sci. 2021, 44, 3418–3428. [Google Scholar] [CrossRef] [PubMed]
- Padilha, C.V.D.S.; Miskinis, G.A.; de Souza, M.E.A.O.; Pereira, G.E.; de Oliveira, D.; Bordignon-Luiz, M.T.; Lima, M.D.S. Rapid determination of flavonoids and phenolic acids in grape juices and wines by RP-HPLC/DAD: Method validation and characterization of commercial products of the new Brazilian varieties of grape. Food Chem. 2017, 228, 106–115. [Google Scholar] [CrossRef]
- de Souza Campos Junior, F.A.; Petrarca, M.H.; Meinhart, A.D.; de Jesus Filho, M.; Godoy, H.T. Multivariate optimization of extraction and validation of phenolic acids in edible mushrooms by capillary electrophoresis. Food Res. Int. 2019, 126, 108685. [Google Scholar] [CrossRef]
- Liu, S.; Marsol-Vall, A.; Laaksonen, O.; Kortesniemi, M.; Yang, B. Characterization and Quantification of Nonanthocyanin Phenolic Compounds in White and Blue Bilberry (Vaccinium myrtillus) Juices and Wines Using UHPLC-DAD−ESI-QTOF-MS and UHPLC-DAD. J. Agric. Food Chem. 2020, 68, 7734–7744. [Google Scholar] [CrossRef]
- Sun, J.; Gan, C.; Huang, J.; Wang, Z.; Wu, C.; Jiang, S.; Yang, X.; Peng, H.; Wei, F.; Yang, C. Determination of Triterpenoids and Phenolic Acids from Sanguisorba officinalis L. by HPLC-ELSD and Its Application. Molecules 2021, 26, 4505. [Google Scholar] [CrossRef]
- de Hoyos Martinez, P.L.; Merle, J.; Labidi, J.; Charrier-El Bouhtoury, F. Tannins extraction: A key point for their valorization and cleaner production. J. Clean. Prod. 2019, 206, 1138–1155. [Google Scholar] [CrossRef]
- Hagerman, A.E. Extraction of tannin from fresh and preserved leaves. J. Chem. Ecol. 1988, 14, 453–461. [Google Scholar] [CrossRef] [PubMed]
- Durgawale, T.P.; Durgawale, P.P.; Khanwelkar, C.C. Quantitative estimation of tannins by HPLC. Pharm. Lett. 2016, 8, 123–126. [Google Scholar]
- Das, A.K.; Islam, N.; Faruk, O.; Ashaduzzaman; Dungani, R. Review on tannins: Extraction processes, applications and possibilities. S. Afr. J. Bot. 2020, 135, 58–70. [Google Scholar] [CrossRef]
- Wang, Y.L.; Xi, G.S.; Zheng, Y.C.; Miao, F.S. Microwave-assisted extraction of flavonoids from Chinese herb Radix puerariae (Ge Gen). J. Med. Plants Res. 2010, 4, 304–308. [Google Scholar]
- Cong-Cong, X.U.; Bing, W.; Yi-Qiong, P.U.; Jian-Sheng, T.A.O.; Zhang, T. Advances in extraction and analysis of phenolic compounds from plant materials. Chin. J. Nat. Med. 2017, 15, 721–731. [Google Scholar]
- Silva, A.R.; Pinela, J.; García, P.A.; Ferreira, I.C.; Barros, L. Cytinus hypocistis (L.) L.: Optimised heat/ultrasound-assisted extraction of tannins by response surface methodology. Sep. Purif. Technol. 2021, 276, 119358. [Google Scholar] [CrossRef]
- Liu, D.; Ma, Y.; Wang, Y.; Su, Z.; Gu, M.; Janson, J.C. One-step separation and purification of hydrolysable tannins from Geranium wilfordii Maxim by adsorption chromatography on cross-linked 12% agarose gel. J. Sep. Sci. 2011, 34, 995–998. [Google Scholar] [CrossRef]
- Liu, D.; Su, Z.; Wang, C.; Gu, M.; Xing, S. Separation and purification of hydrolyzable tannin from Geranium wilfordii Maxim by reversed-phase and normal-phase high-speed counter-current chromatography. J. Sep. Sci. 2010, 33, 2266–2271. [Google Scholar] [CrossRef]
- Wang, C.; Zhang, M.; Wu, L.; Wang, F.; Li, L.; Zhang, S.; Sun, B. Qualitative and quantitative analysis of phenolic compounds in blueberries and protective effects on hydrogen peroxide-induced cell injury. J. Sep. Sci. 2021, 44, 2837–2855. [Google Scholar] [CrossRef]
- Romani, A.; Campo, M.; Pinelli, P. HPLC/DAD/ESI-MS analyses and anti-radical activity of hydrolyzable tannins from different vegetal species. Food Chem. 2012, 130, 214–221. [Google Scholar] [CrossRef]
- Varo, M.; Martín-Gómez, J.; Mérida, J.; Serratosa, M.P. Bioactive compounds and antioxidant activity of highbush blueberry (Vaccinium corymbosum) grown in southern Spain. Eur. Food Res. Technol. 2021, 247, 1199–1208. [Google Scholar] [CrossRef]
- Aguilera, J.M.; Toledo, T. Wild berries and related wild small fruits as traditional healthy foods. Crit. Rev. Food Sci. Nutr. 2022, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Zorenc, Z.; Veberic, R.; Mikulic-Petkovsek, M. Are processed bilberry products a good source of phenolics. J. Food Sci. 2018, 83, 1856–1861. [Google Scholar] [CrossRef] [PubMed]
- Irigoytia, M.B.; Irigoytia, K.; Sosa, N.; de Escalada Pla, M.; Genevois, C. Blueberry by-product as a novel food ingredient: Physicochemical characterization and study of its application in a bakery product. J. Sci. Food Agric. 2022, 102, 4551–4560. [Google Scholar] [CrossRef] [PubMed]
- Curutchet, A.; Cozzano, S.; Tárrega, A.; Arcia, P. Blueberry pomace as a source of antioxidant fibre in cookies: Consumer’s expectations and critical attributes for developing a new product. Food Sci. Technol. Int. 2019, 25, 642–648. [Google Scholar] [CrossRef]
- Chandra, P.; Rathore, A.S.; Kay, K.L.; Everhart, J.L.; Curtis, P.; Burton-Freeman, B.; Cassidy, A.; Kay, C.D. Contribution of Berry Polyphenols to the Human Metabolome. Molecules 2019, 24, 4220. [Google Scholar] [CrossRef] [PubMed]
- Palma, X.; Thomas-Valdés, S.; Cruz, G. Acute Consumption of Blueberries and Short-Term Blueberry Supplementation Improve Glucose Management and Insulin Levels in Sedentary Subjects. Nutrients 2021, 13, 1458. [Google Scholar] [CrossRef]
- Cobos, Á.; Díaz, O. ‘Superfoods’: Reliability of the Information for Consumers Available on the Web. Foods 2023, 12, 546. [Google Scholar] [CrossRef]
- Zhang, Q.; Cheng, Z.; Wang, Y.; Fu, L. Dietary protein-phenolic interactions: Characterization, biochemical-physiological consequences, and potential food applications. Crit. Rev. Food Sci. Nutr. 2020, 61, 3589–3615. [Google Scholar] [CrossRef]
- Castro-Castaneda, C.R.; Altamirano-Lamarque, F.; Ortega-Macías, A.G.; Cruz-Pavlovich, F.J.S.; la Rosa, A.G.-D.; Armendariz-Borunda, J.; Santos, A.; Navarro-Partida, J. Nutraceuticals: A Promising Therapeutic Approach in Ophthalmology. Nutrients 2022, 14, 5014. [Google Scholar] [CrossRef]
- Travica, N.; D’Cunha, N.M.; Naumovski, N.; Kent, K.; Mellor, D.; Firth, J.; Georgousopoulou, E.N.; Dean, O.M.; Loughman, A.; Jacka, F.; et al. The effect of blueberry interventions on cognitive performance and mood: A systematic review of randomized controlled trials. Brain Behav. Immun. 2019, 85, 96–105. [Google Scholar] [CrossRef] [PubMed]
- Stote, K.S.; Sweeney, M.I.; Kean, T.; Baer, D.J.; Novotny, J.A.; Shakerley, N.L.; Chandrasekaran, A.; Carrico, P.M.; Melendez, J.A.; Gottschall-Pass, K.T. The effects of 100% wild blueberry (Vaccinium angustifolium) juice consumption on cardiometablic biomarkers: A randomized, placebo-controlled, crossover trial in adults with increased risk for type 2 diabetes. BMC Nutr. 2017, 3, 45. [Google Scholar] [CrossRef] [PubMed]
- Stull, A.J.; Cash, K.C.; Champagne, C.M.; Gupta, A.K.; Boston, R.; Beyl, R.A.; Johnson, W.D.; Cefalu, W.T. Blueberries Improve Endothelial Function, but Not Blood Pressure, in Adults with Metabolic Syndrome: A Randomized, Double-Blind, Placebo-Controlled Clinical Trial. Nutrients 2015, 7, 4107–4123. [Google Scholar] [CrossRef] [PubMed]
- Gagnon, W.; Garneau, V.; Trottier, J.; Verreault, M.; Couillard, C.; Roy, D.; Marette, A.; Drouin-Chartier, J.-P.; Vohl, M.-C.; Barbier, O. Impact of Blueberry Consumption on the Human Fecal Bileacidome: A Pilot Study of Bile Acid Modulation by Freeze-Dried Blueberry. Nutrients 2022, 14, 3857. [Google Scholar] [CrossRef]
- Sidorova, Y.; Petrov, N.; Birulina, N.; Perova, I.; Zorin, S.; Kochetkova, A.; Mazo, V. Physiological and biochemical evaluation of the effectiveness of a new food ingredient—blueberry polyphenol concentrate. Vopr. Pitan. 2022, 91, 43–55. [Google Scholar] [CrossRef]
- Zhang, P.; Li, Y.; Wang, T.; Cai, Z.; Cao, H.; Zhang, H.; Cao, Y.; Chen, B.; Yang, D. Statistics on the bioactive anthocyanin/proanthocyanin products in China online sales. Food Sci. Nutr. 2021, 9, 5428–5434. [Google Scholar] [CrossRef]
- Singh, A.; Gu, Y.; Castellarin, S.D.; Kitts, D.D.; Pratap-Singh, A. Development and Characterization of the Edible Packaging Films Incorporated with Blueberry Pomace. Foods 2020, 9, 1599. [Google Scholar] [CrossRef]

| Phenolic Compound | Extraction Methods | Extraction Condition | Solvents | Ref. |
|---|---|---|---|---|
| Anthocyanin | SEM | 5 min (−1.41) and 100 °C | 1% citric acid | [7] |
| UAE | 1.400 W, 0 °C, PH6, 25 min; dual-frequency: 40 + 80 Hz, 350 W, 50 °C, 40 min | 1.34.2% MeOH | [8,9] | |
| MAE | 53–58 °C, 80 s, 100 W/g | 60% ethanol solvent | [10] | |
| DES/NADES | Blueberry powder (8 g; average particle size: 715.5 ± 12.3 μm) was mixed with 20 mL of a methanol:water:formic acid mixture (50:48.5:1.5; v/v/v). | Choline chloride:glycerol:cit-ric acid (0.5:2:0.5, molar ratio) NADES | [11] | |
| EAE | Cellulase auxiliary extraction | 4% acetic acid in 65% aqueous methanol | [12] | |
| SCDE | Extraction temperature 40 °C, pressure 34.7 MPa, CO2 flow rate 4.5 L/min, extraction time 1.86 h, | Carbon dioxide | [13] | |
| UAE + PLE | Ultrasonic bath for 8 min at 80 °C, PLE for 30 min | 50% and 70% ethanol v/v | [14] | |
| UAE + NADES | 37.63 min, 48.38 °C, 34.79% (w/w) water in NADES | choline chloride: sorbitol (1:1) | [15] | |
| pterostilbene | MAE | 1350 W at 100% power, 30 min | aqueous and ethanolic | [16] |
| Phenolic acid | PEF | 15 min (442.90 μg/gdw) at 50 H | Ethanol-based solvents | [17] |
| UASE | 80% methanol at room temperature for 15 min | 80% methanol | [18] | |
| MAE | 800 W, 50 °C, extraction time from 2 to 5 min | water | [18] | |
| NADES + ATPE | Liquid/solid ratio 17.01 mL/g, extraction temperature 59.03 °C, extraction time 24.12 min | 0.5% (v/v) formic acid aqueous water (A) and acetonitrile (B) | [19] |
| Phenolic Compound | Separation and Purification Methods | Separation and Purification Condition | Purity or Characteristic | Ref. |
|---|---|---|---|---|
| Anthocyanin | HPLC method | Acetonitrile–water (containing 0.3% phosphoric acid) as the mobile phase gradient elution at 520 nm detection wavelength | 99% | [47] |
| Semi-preparative high performance liquid chromatography | Mobile phase A: methanol; mobile phase B: 3% formic acid. The initial gradient composition: 15% solvent A and 85% solvent B. The elution conditions: solvent B: 0 min, 85%; 3 min, 80%; 7 min, 75%; 10 min, 75%; 55 min, 30%; 60 min, 30%; 65 min. | The tree anthocyanin components’ purity are 97.7%, 99.3%, and 95.4% | [48] | |
| HSCCC | Duplexic mixture of tert-butyl methyl ether/n-butanol/acetonitrile/water (2:2:1:5) acidified with trifluoroacetic acid | Peak purity standard | [49] | |
| Macroporous resin method | Macroporous resin combined with the Sephadex LH-20 method | 90.96% | [51] | |
| Membrane separation method | Nanofiltration membranes (NF245 and NF270) used to separate and adsorb anthocyanins | Reduces anthocyanin waste by more than 60% | [54] | |
| DSC-C18 + DSC-SCX + HPLC | Hydrophobic silica gel (DSC-C18) and cation exchange resin (DSC-SCX) in two consecutive solid-phase extractions anthocyanins. | 100% | [55] | |
| Pterostilbene | New ways to increase solubility | PTS:BCD:HPMC ternary system using ethanol as a co-solvent | Low-bulk powders with a high content of PTS | [17,68] |
| Phenolic acid | UPC | Mobile phase: carbon dioxide methanol/acetonitrile (00:70, v/v) Flow rates of 30.1 mL/min; modifier: 17% TFA; the shortest time: 0.8 min. | A short separation time of 0.8 min | [69] |
| Phenolic Compound | Detection Method | Characteristic | Ref. |
|---|---|---|---|
| Anthocyanin | UV-VIS | Maximum absorption in the visible light range around 520 nm | [58] |
| HPLC | Chromatographic fractionation of methanol media achieves suitable chromatographic performance | [59] | |
| Mass spectrometry | Q-TOF and ESI are the most commonly used methods | [58] | |
| HPLC-DAD + MSn | Extracts pigments from berry peels with 0.1% HCl methanol, purified using C-18 solid phase columns | [60] | |
| UV-VIS + LC-MS | Provides additional information on the structural details of anthocyanins | [62] | |
| UPLC/UV/MS | The mobile phase A: water; mobile phase B: acetonitrile; each containing 0.1% formic acid. With a flow rate of 0.3 mL/min, this method provides a reliable determination of anthocyanins. | [62] | |
| HPLC + MSn | Distinguishes anthocyanins from pro-anthocyanidins | [64] | |
| Pterostilbene | MALDI mass spectrometry | Simultaneously localizes resveratrol, pterostilbene, and glucoside on grape leaves | [70] |
| RP-HPLC | The addition of 0 mM HP-β-CD to a 25:7 (v/v) methanol aqueous mobile phase at 12 °C and pH 50.50 significantly improved the main analytical parameters | [73] | |
| DLLME | A linear response: 5000 ng/mL (−1); accuracy: overall recovery of 1000% to 1% for samples spiked at different levels of 90 to 102 ng/mL (−50) with a standard deviation of less than 12% | [74] | |
| Phenolic acid | GC | Detects small phenolic acid compounds below 600 D | / |
| HPLC + Magnetically assisted tube solid phase microextraction | Increased the analyte extraction efficiency from 44.9–64.0% to 78.6–87.1% | [86] | |
| RP-HPLC | Has good linearity and precision and can be used for commercial characterization | [87] | |
| Capillary electrophoresis | Hydrochloric acid concentration, 2 mol·L−1; temperature, 80 °C; and time 30 min; is a green extraction detection method | [88] | |
| UHPLC-DAD-ESI-QTOF-MS + UHPLC-DAD | Quantifies and characterizes phenolic compounds other than anthocyanins in blueberry juice and fermented wine | [89] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bai, X.; Zhou, L.; Zhou, L.; Cang, S.; Liu, Y.; Liu, R.; Liu, J.; Feng, X.; Fan, R. The Research Progress of Extraction, Purification and Analysis Methods of Phenolic Compounds from Blueberry: A Comprehensive Review. Molecules 2023, 28, 3610. https://doi.org/10.3390/molecules28083610
Bai X, Zhou L, Zhou L, Cang S, Liu Y, Liu R, Liu J, Feng X, Fan R. The Research Progress of Extraction, Purification and Analysis Methods of Phenolic Compounds from Blueberry: A Comprehensive Review. Molecules. 2023; 28(8):3610. https://doi.org/10.3390/molecules28083610
Chicago/Turabian StyleBai, Xinyu, Lin Zhou, Li Zhou, Song Cang, Yuhan Liu, Rui Liu, Jie Liu, Xun Feng, and Ronghua Fan. 2023. "The Research Progress of Extraction, Purification and Analysis Methods of Phenolic Compounds from Blueberry: A Comprehensive Review" Molecules 28, no. 8: 3610. https://doi.org/10.3390/molecules28083610
APA StyleBai, X., Zhou, L., Zhou, L., Cang, S., Liu, Y., Liu, R., Liu, J., Feng, X., & Fan, R. (2023). The Research Progress of Extraction, Purification and Analysis Methods of Phenolic Compounds from Blueberry: A Comprehensive Review. Molecules, 28(8), 3610. https://doi.org/10.3390/molecules28083610






