Microbial Synthesis of Heme b: Biosynthetic Pathways, Current Strategies, Detection, and Future Prospects
Abstract
1. Introduction
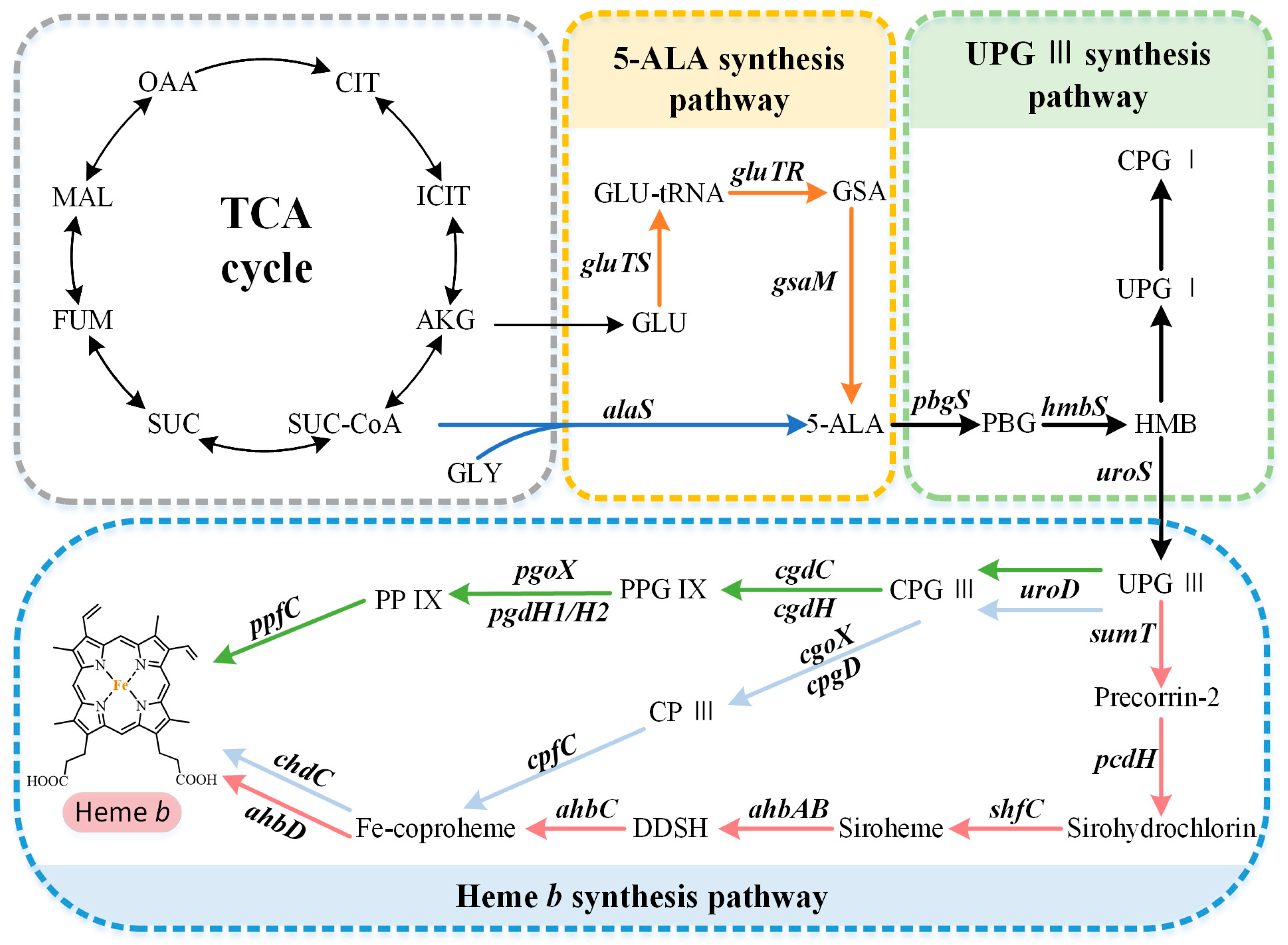
2. Biosynthetic Pathway of Heme b
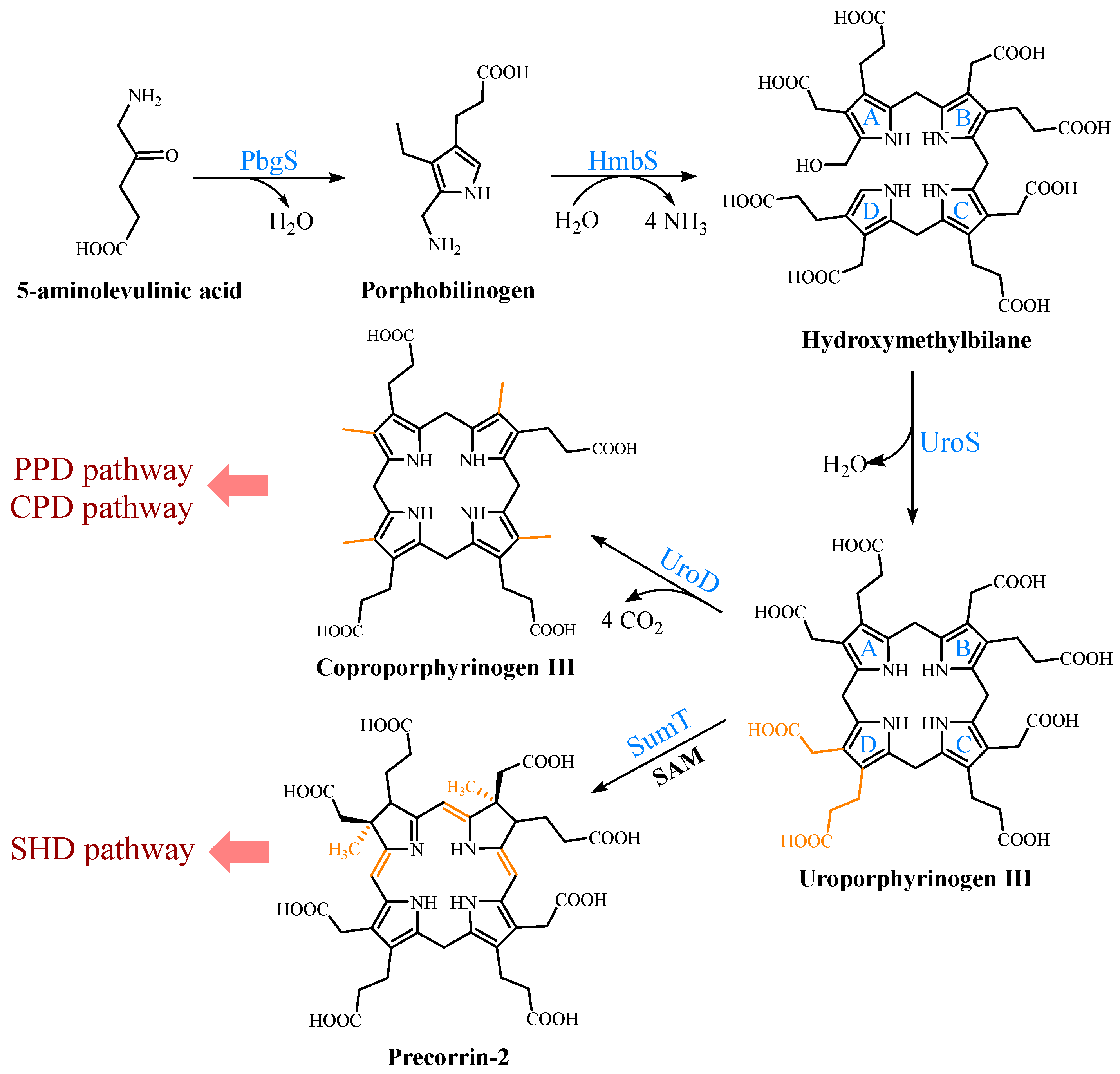
2.1. Biosynthetic Pathways of the Precursor 5-ALA
2.2. Formation of the Common Tetrapyrrole Core UPG III
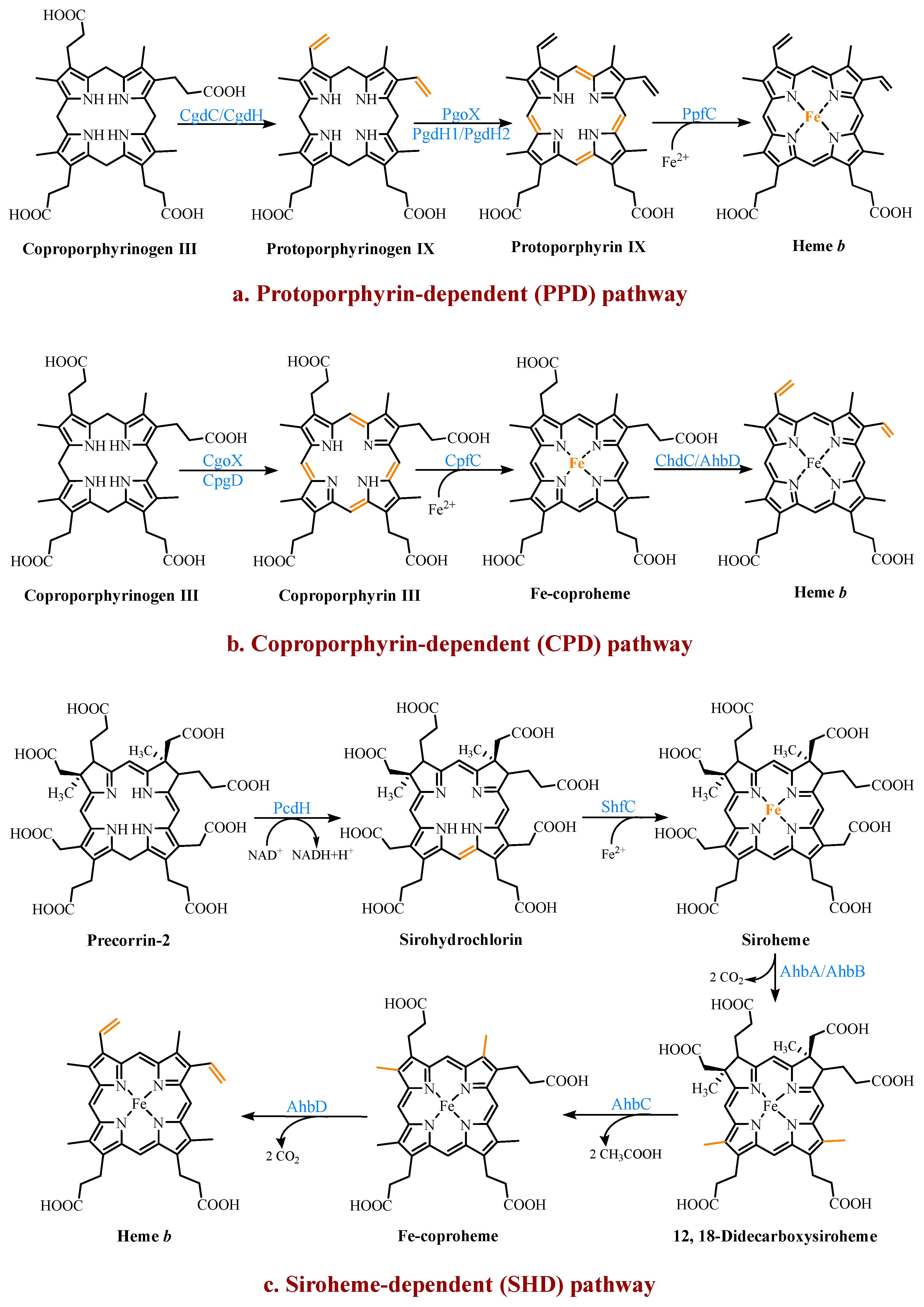
2.3. Multiple Pathways for Synthesizing Heme b
2.3.1. The Protoporphyrin-Dependent (PPD) Branch
2.3.2. The Coproporphyrin-Dependent (CPD) Branch
2.3.3. The Siroheme-Dependent (SHD) Pathway
3. Metabolic Engineering Strategies for Heme b Biosynthesis
3.1. Screening and Comparing the Heme b Biosynthetic Pathways
3.2. Increasing the Supply of the Key Precursor 5-ALA
3.2.1. Engineering the Metabolic Flow in the TCA Cycle
3.2.2. Optimizing the 5-ALA Biosynthetic Pathway
3.3. Balancing the Expression Levels of the Gene Encoding Enzymes in the Pathway from 5-ALA to Heme b
3.4. Blocking Downstream Pathways
3.5. Improving the Efficiency of the Cellular Export
3.6. Optimizing the Iron Concentration by Engineering the Intracellular Iron Ion Metabolism
| Chassis | 5-ALA Synthetic Pathway | Heme b Synthesis Pathway | Detection | Description | Culture Mode | Titer (mg/L) | Heme Secretion Ratio (%) | Ref. |
|---|---|---|---|---|---|---|---|---|
| E. coli K-12 JM109 | C4 | PPD | LC-MS | Overexpression of alaSRC, pbgS, hmbS, uroS, uroDSP, cgdC, and ppfCBS | Flask | 2.03 ± 0.18 | ND 1 | [10] |
| E. coli W3110 | C4 | - | Spectrophotometer | Overexpression of alaSRS, macB, and dctA | Fed-batch | 6.4 | ND 1 | [60] |
| E. coli W3110 | C4 | - | LC-MS | Overexpression of alaSRS, macB, and dctA and optimization of the fermentation conditions | Fed-batch | 0.12 | ND 1 | [90] |
| E. coli W3110 (DE3) | C4 | - | HPLC | Overexpression of alaSRS, macB, and coaA and the addition of glycine, succinate | Flask | 0.49 2 | ND 1 | [65] |
| E. coli W3110 | C4 | - | LC-MS | Overexpression of alaSRS, coaA and the addition of glycine, succinate, and FeCl3·6H2O | Flask | 9.1 2 | ND 1 | [64] |
| E. coli BL21 (DE3) | C5 | PPD | HPLC | Overexpression of gluTRfbr, gsaM, pbgS, hmbS, uroS, uroD, cgdC, pgoX, and ppfC, and deletion of yfeX, ldhA, and pta | Flask | 7.88 | 16.0 | [9] |
| E. coli BL21 (DE3) | C5 | PPD | HPLC | Overexpression of gluTRfbr, gsaM, pbgS, hmbS, uroS, uroD, cgdC, pgoX, ppfC, and ccmABC, deletion of yfeX, ldhA, and pta and optimization of the fermentation conditions | Fed-batch | 239.2 | 63.3 | [9] |
| E. coli BL21 (DE3) | C5 | PPD | HPLC | Overexpression of gluTRfbr, gsaM, pbgS, hmbS, uroS, uroD, cgdC, pgoX, ppfC, and ccmABC, deletion of yfeX, ldhA, and pta and optimization of the fermentation conditions | Fed-batch | 1034.3 | 45.5 | [12] |
| C. glutamicum ATCC 13032 | C5 | - | Fluorescence | Overexpression of gluTSEC, gluTREC, and gsaMSA | Flask | 4.22 ± 0.62 | ND 1 | [13] |
| C. glutamicum ATCC 13826 | C5 | CPD | HPLC | Overexpression of gluTRST, gsaMEC, and chdCATG | Flask | 27.22 ± 0.65 | ND 1 | [55] |
| C. glutamicum ATCC 14067 | C4+C5 | CPD | HPLC | Overexpression of gluTRMST, gsaMEC, alaSRC, uroDAUG, cgoX, cpfC, chdC, dtxR, hrtA, and hrtB, and deletion of hrrS, htaA, and hmuT | Flask | 38.16 ± 0.52 | 21.7 | [54] |
| C. glutamicum ATCC 14067 | C4+C5 | CPD | HPLC | Overexpression of gluTRMST, gsaMEC, alaSRC, uroDAUG, cgoX, cpfC, chdC, dtxR, hrtA, and hrtB, deletion of hrrS, htaA, and hmuT, optimization of the fermentation conditions, and addition of the cell wall inhibitor ethambutol | Fed-batch | 309.18 ± 16.43 | 78.58 | [54] |
| C. glutamicum ATCC 14067 | C4+C5 | CPD | HPLC | Overexpression of gluTRMST, gsaMEC, alaSRC, uroDAUG, cgoX, cpfC, chdC, dtxR, hrtA, and hrtB, deletion of hrrS, htaA, and hmuT, and optimization of the fermentation conditions | Fed-batch | 111.87 ± 6.48 | 91.25 | [54] |
| S. cerevisiae BY4741 | C4 | PPD | Fluorescence | Overexpression of alaS, pbgS, hmbS, cgdC, pgoX, ppfC, and fet4 and deletion of shm1, hmx1, gcv2, and gcv1 | Flask | 53.5 | ND 1 | [14] |
| S. cerevisiae CEN.PK2-1C | C4 | PPD | Fluorescence | Overexpression of alaS (copy number is 2), cgdCP, pgoXP40-539, and ppfCP31-393 and deletion of hmx1 | Flask | 0.3 | ND 1 | [67] |
| Pichia pastoris L10A1T | C4 | PPD | Heme detection kit | AOX1p-HmbS-AOX1p-AlaS-AOX1p-PbgS-AOX1p-UroD-AOX1p-PgoX-AOX1p-CgdC-AOX1p-UroS-AOX1p-PpfC integrated into the his4 locus and complementation of ku70 | Fed-batch | 132 | ND 1 | [91] |
4. Detection of Heme b
4.1. Spectrophotometric Heme b Assay of Pyridine Hemochrome
4.2. Heme b Assay Based on Protoporphyrin Fluorescence
4.3. Heme b Analysis via High-Performance Liquid Chromatography (HPLC)
4.4. Heme b Biosensors
| Method Name | Sample | Description of the Method Conditions | Detection Time (min) | Linearity Range, Limits of Detection, and Quantification (LOQ) | Ref. |
|---|---|---|---|---|---|
| Spectrophotometric heme b assay of pyridine hemochrome | Tissue homogenate, homogenate or sonicated lysate of the tissue culture cells |
| 30 | <7 μg/mL | [92] |
| Heme b assay based on protoporphyrin fluorescence | Tissue culture cells |
| 30 | 1 nM~1 μM | [93] |
| Heme b analysis via high-performance liquid chromatography (HPLC) | Tissue culture cells or tissue (sonicated lysate or homogenate) |
| 40 | ND 1 | [54] |
| Heme b biosensors | Tissue culture cells |
| ND 1 | ND 1 | [14] |
5. Concluding Remarks and Future Perspectives
5.1. Exploration of Natural High-Producers as Novel Chassis Strains
5.2. Elucidation of Heme-Regulating Mechanisms
5.3. Target Gene Mining and Genome-Scale Design
5.4. Construction of Artificial Microbial Consortia for the Heme b Synthesis
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Ponka, P. Cell biology of heme. Am. J. Med. Sci. 1999, 318, 241–256. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhang, L. The chemical and structural bases of heme recognition: Binding interactions of heme with proteins and peptides. World Sci. 2011, 7484, 161–196. [Google Scholar]
- Lee, J.; Yesilkanal, A.E.; Wynne, J.P.; Frankenberger, C.; Liu, J.; Yan, J.; Elbaz, M.; Rabe, D.C.; Rustandy, F.D.; Tiwari, P.; et al. Effective breast cancer combination therapy targeting BACH1 and mitochondrial metabolism. Nature 2019, 568, 254–258. [Google Scholar] [CrossRef] [PubMed]
- Waltz, E. Appetite grows for biotech foods with health benefits. Nat. Biotechnol. 2019, 37, 573–575. [Google Scholar] [CrossRef]
- Zhang, J.; Cui, Z.; Zhu, Y.; Zhu, Z.; Qi, Q.; Wang, Q. Recent advances in microbial production of high-value compounds in the tetrapyrrole biosynthesis pathway. Biotechnol. Adv. 2022, 55, 107904. [Google Scholar] [CrossRef]
- Schmitz, L.M.; Rosenthal, K.; Lutz, S. Recent advances in heme biocatalysis engineering. Biotechnol. Bioeng. 2019, 116, 3469–3475. [Google Scholar] [CrossRef]
- Fischer, H.; Zeile, K. Synthese des Hämatoporphyrins, Protoporphyrins und Hämins. Eur. J. Org. Chem. 2010, 468, 98–116. [Google Scholar] [CrossRef]
- Espinas, N.A.; Kobayashi, K.; Takahashi, S.; Mochizuki, N.; Masuda, T. Evaluation of unbound free heme in plant cells by differential acetone extraction. Plant Cell Physiol. 2012, 53, 1344–1354. [Google Scholar] [CrossRef]
- Kwon, S.J.; Boer, A.L.; Petri, R.; Schmidt-Dannert, C. High-level production of porphyrins in metabolically engineered Escherichia coli: Systematic extension of a pathway assembled from overexpressed genes involved in heme biosynthesis. Appl. Environ. Microbiol. 2003, 69, 4875–4883. [Google Scholar] [CrossRef]
- Dailey, H.A.; Dailey, T.A.; Gerdes, S.; Jahn, D.; Jahn, M.; O’Brian, M.R.; Warren, M.J. Prokaryotic heme biosynthesis: Multiple pathways to a common essential Product. Microbiol. Mol. Biol. Rev. 2017, 81, e00048-16. [Google Scholar] [CrossRef]
- Zhao, X.R.; Choi, K.R.; Lee, S.Y. Metabolic engineering of Escherichia coli for secretory production of free haem. Nat. Catal. 2018, 1, 720–728. [Google Scholar] [CrossRef]
- Choi, K.R.; Yu, H.E.; Lee, H.; Lee, S.Y. Improved production of heme using metabolically engineered Escherichia coli. Biotechnol. Bioeng. 2022, 119, 3178–3193. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Jin, H.; Liu, W.; Wang, Q.; Qi, Q. Engineering Corynebacterium glutamicum to produce 5-aminolevulinic acid from glucose. Microb. Cell Fact. 2015, 14, 183. [Google Scholar] [CrossRef] [PubMed]
- Ishchuk, O.P.; Domenzain, I.; Sanchez, B.J.; Muniz-Paredes, F.; Martinez, J.L.; Nielsen, J.; Petranovic, D. Genome-scale modeling drives 70-fold improvement of intracellular heme production in Saccharomyces cerevisiae. Proc. Natl. Acad. Sci. USA 2022, 119, e2108245119. [Google Scholar] [CrossRef]
- Beale, S.I.; Castelfranco, P.A. 14C incorporation from exogenous compounds into δ-aminolevulinic acid by greening cucumber cotyledons. BBRC 1973, 52, 143–149. [Google Scholar] [CrossRef]
- Kaufholz, A.L.; Hunter, G.A.; Ferreira, G.C.; Lendrihas, T.; Hering, V.; Layer, G.; Jahn, M.; Jahn, D. Aminolaevulinic acid synthase of Rhodobacter capsulatus: High-resolution kinetic investigation of the structural basis for substrate binding and catalysis. Biochem. J. 2013, 451, 205–216. [Google Scholar] [CrossRef]
- Luer, C.; Schauer, S.; Virus, S.; Schubert, W.D.; Heinz, D.W.; Moser, J.; Jahn, D. Glutamate recognition and hydride transfer by Escherichia coli glutamyl-tRNA reductase. FEBS J. 2007, 274, 4609–4614. [Google Scholar] [CrossRef]
- Ilag, L.L.; Jahn, D. Activity and spectroscopic properties of the Escherichia coli glutamate 1-semialdehyde aminotransferase and the putative active site mutant K265R. Biochemistry 1992, 31, 7143–7151. [Google Scholar] [CrossRef]
- Nogaj, L.A.; Beale, S.I. Physical and kinetic interactions between glutamyl-tRNA reductase and glutamate-1-semialdehyde aminotransferase of Chlamydomonas reinhardtii. J. Biol. Chem. 2005, 280, 24301–24307. [Google Scholar] [CrossRef]
- Breinig, S.; Kervinen, J.; Stith, L.; Wasson, A.S.; Fairman, R.; Wlodawer, A.; Zdanov, A.; Jaffe, E.K. Control of tetrapyrrole biosynthesis by alternate quaternary forms of porphobilinogen synthase. Nat. Struct. Biol. 2003, 10, 757–763. [Google Scholar] [CrossRef]
- Coates, L.; Beaven, G.; Erskine, P.T.; Beale, S.I.; Wood, S.P.; Shoolingin-Jordan, P.M.; Cooper, J.B. Structure of Chlorobium vibrioforme 5-aminolaevulinic acid dehydratase complexed with a diacid inhibitor. Acta Crystallogr. D Biol. Crystallogr. 2005, 61, 1594–1598. [Google Scholar] [CrossRef] [PubMed]
- Jaffe, E.K. The porphobilinogen synthase family of metalloenzymes. Acta Crystallogr. D Biol. Crystallogr. 2000, 56, 115–128. [Google Scholar] [CrossRef] [PubMed]
- Jordan, P.M. The biosynthesis of 5-aminolaevulinic acid and its transformation into uroporphyrinogen III. New Compr. Biochem. 1991, 19, 1–66. [Google Scholar]
- Shoolingin-Jordan, P.M.; Warren, M.; Fau-Awan, S.J.; Awan, S.J. Discovery that the assembly of the dipyrromethane cofactor of porphobilinogen deaminase holoenzyme proceeds initially by the reaction of preuroporphyrinogen with the apoenzyme. Biochem. J. 1996, 316, 373–61996. [Google Scholar] [CrossRef]
- Silva, P.J.; Ramos, M.J. Comparative density functional study of models for the reaction mechanism of uroporphyrinogen III synthase. J. Phys. Chem. B 2008, 112, 3144–3148. [Google Scholar] [CrossRef]
- Lindsey, J.S.; Ptaszek, M.; Taniguchi, M. Simple formation of an abiotic porphyrinogen in aqueous solution. Orig. Life Evol. Biosph. 2009, 39, 495–515. [Google Scholar] [CrossRef]
- Hashimoto, K.; Nakamura, K.; Kuroda, T.; Yabe, I.; Nakamatsu, T.; Kawasaki, H. The protein encoded by NCgl1221 in Corynebacterium glutamicum functions as a mechanosensitive channel. Biosci. Biotechnol. Biochem. 2010, 74, 2546–2549. [Google Scholar] [CrossRef]
- Akhtar, M. Mechanism and stereochemistry of the enzymes involved in the conversion of uroporphyrinogen III into haem. New Compr. Biochem. 1991, 19, 67–99. [Google Scholar]
- Ishida, T.; Yu, L.; Akutsu, H.; Ozawa, K.; Kawanishi, S.; Seto, A.; Inubushi, T.; Sano, S. A primitive pathway of porphyrin biosynthesis and enzymology in Desulfovibrio vulgaris. Proc. Natl. Acad. Sci USA 1998, 95, 4853–4858. [Google Scholar] [CrossRef]
- Bali, S.; Lawrence, A.D.; Lobo, S.A.; Saraiva, L.M.; Golding, B.T.; Palmer, D.J.; Howard, M.J.; Ferguson, S.J.; Warren, M.J. Molecular hijacking of siroheme for the synthesis of heme and d1 heme. Proc. Natl. Acad. Sci. USA 2011, 108, 18260–18265. [Google Scholar] [CrossRef]
- Vévodová, J.; Graham, R.M.; Raux, E.; Schubert, H.L.; Roper, D.I.; Brindley, A.A.; Ian Scott, A.; Roessner, C.A.; Stamford, N.P.; Elizabeth Stroupe, M.; et al. Structure/function studies on a S-adenosyl-L-methionine-dependent uroporphyrinogen III methyltransferase (SUMT), a key regulatory enzyme of tetrapyrrole biosynthesis. J. Mol. Biol. 2004, 344, 419–433. [Google Scholar] [CrossRef]
- Batlle, A.M.; Benson, A.; Rimington, C. Purification and properties of coproporphyrinogenase. Biochem. J. 1965, 97, 731–740. [Google Scholar] [CrossRef]
- Cavallaro, G.; Decaria, L.; Rosato, A. Genome-based analysis of heme biosynthesis and uptake in prokaryotic systems. J. Proteome Res. 2008, 7, 4946–4954. [Google Scholar] [CrossRef]
- Layer, G.; Reichelt, J.; Jahn, D.; Heinz, D.W. Structure and function of enzymes in heme biosynthesis. Protein Sci. 2010, 19, 1137–1161. [Google Scholar] [CrossRef]
- Rand, K.; Noll, C.; Schiebel, H.M.; Kemken, D.; Dülcks, T.; Kalesse, M.; Heinz, D.W.; Layer, G. The oxygen-independent coproporphyrinogen III oxidase HemN utilizes harderoporphyrinogen as a reaction intermediate during conversion of coproporphyrinogen III to protoporphyrinogen IX. Biol. Chem. 2010, 391, 55–63. [Google Scholar] [CrossRef]
- Qin, X.; Sun, L.; Wen, X.; Yang, X.; Tan, Y.; Jin, H.; Cao, Q.; Zhou, W.; Xi, Z.; Shen, Y. Structural insight into unique properties of protoporphyrinogen oxidase from Bacillus subtilis. J. Struct. Biol. 2010, 170, 76–82. [Google Scholar] [CrossRef]
- Möbius, K.; Arias-Cartin, R.; Breckau, D.; Hännig, A.L.; Riedmann, K.; Biedendieck, R.; Schröder, S.; Becher, D.; Magalon, A.; Moser, J.; et al. Heme biosynthesis is coupled to electron transport chains for energy generation. Proc. Natl. Acad. Sci. USA 2010, 107, 10436–10441. [Google Scholar] [CrossRef]
- Kobayashi, K.; Masuda, T.; Tajima, N.; Wada, H.; Sato, N. Molecular phylogeny and intricate evolutionary history of the three isofunctional enzymes involved in the oxidation of protoporphyrinogen IX. Genome Biol. Evol. 2014, 6, 2141–2155. [Google Scholar] [CrossRef]
- Kato, K.; Tanaka, R.; Sano, S.; Tanaka, A.; Hosaka, H. Identification of a gene essential for protoporphyrinogen IX oxidase activity in the cyanobacterium Synechocystis sp. PCC6803. Proc. Natl. Acad. Sci. USA 2010, 107, 16649–16654. [Google Scholar] [CrossRef]
- Shepherd, M.; Dailey, T.A.; Dailey, H.A. A new class of [2Fe-2S]-cluster-containing protoporphyrin (IX) ferrochelatases. Biochem. J. 2006, 397, 47–52. [Google Scholar] [CrossRef]
- Moody, M.D.; Dailey, H.A. Ferric iron reductase of Rhodopseudomonas sphaeroides. J. Bacteriol. 1985, 163, 1120–1125. [Google Scholar] [CrossRef]
- Medlock, A.E.; Shiferaw, M.T.; Marcero, J.R.; Vashisht, A.A.; Wohlschlegel, J.A.; Phillips, J.D.; Dailey, H.A. Identification of the mitochondrial heme metabolism complex. PLoS ONE 2015, 10, e0135896. [Google Scholar] [CrossRef]
- Masoumi, A.; Heinemann, I.U.; Rohde, M.; Koch, M.; Jahn, M.; Jahn, D. Complex formation between protoporphyrinogen IX oxidase and ferrochelatase during haem biosynthesis in Thermosynechococcus elongatus. Microbiology 2008, 154, 3707–3714. [Google Scholar] [CrossRef]
- Dailey, H.A.; Gerdes, S.; Dailey, T.A.; Burch, J.S.; Phillips, J.D. Noncanonical coproporphyrin-dependent bacterial heme biosynthesis pathway that does not use protoporphyrin. Proc. Natl. Acad. Sci. USA 2015, 112, 2210–2215. [Google Scholar] [CrossRef]
- Al-Karadaghi, S.; Hansson, M.; Nikonov, S.; Jonsson, B.; Hederstedt, L. Crystal structure of ferrochelatase: The terminal enzyme in heme biosynthesis. Structure 1997, 5, 1501–1510. [Google Scholar] [CrossRef]
- Medlock, A.; Swartz, L.; Dailey, T.A.; Dailey, H.A.; Lanzilotta, W.N. Substrate interactions with human ferrochelatase. Proc. Natl. Acad. Sci. USA 2007, 104, 1789–1793. [Google Scholar] [CrossRef]
- Medlock, A.E.; Carter, M.; Dailey, T.A.; Dailey, H.A.; Lanzilotta, W.N. Product release rather than chelation determines metal specificity for ferrochelatase. J. Mol. Biol. 2009, 393, 308–319. [Google Scholar] [CrossRef]
- Corrigall, A.V.; Siziba, K.B.; Maneli, M.H.; Shephard, E.G.; Ziman, M.; Dailey, T.A.; Kirsch, R.E.; Meissner, P.N. Purification of and kinetic studies on a cloned protoporphyrinogen oxidase from the aerobic bacterium Bacillus subtilis. Arch. Biochem. Biophys. 1998, 358, 251–256. [Google Scholar] [CrossRef]
- Zappa, S.; Li, K.; Bauer, C.E. The tetrapyrrole biosynthetic pathway and its regulation in Rhodobacter capsulatus. Adv. Exp. Med. Biol. 2010, 675, 229–250. [Google Scholar]
- Bali, S.; Palmer, D.J.; Schroeder, S.; Ferguson, S.J.; Warren, M.J. Recent advances in the biosynthesis of modified tetrapyrroles: The discovery of an alternative pathway for the formation of heme and heme d 1. Cell Mol. Life Sci. 2014, 71, 2837–2863. [Google Scholar] [CrossRef]
- Stroupe, M.E.; Leech, H.K.; Daniels, D.S.; Warren, M.J.; Getzoff, E.D. CysG structure reveals tetrapyrrole-binding features and novel regulation of siroheme biosynthesis. Nat. Struct. Biol. 2003, 10, 1064–1073. [Google Scholar] [CrossRef]
- Smith, M.A.; King, P.J.; Grimm, B. Transient-State kinetic analysis of synechococcus glutamate 1-semialdehyde aminotransferase. Biochemistry 1998, 37, 319–329. [Google Scholar] [CrossRef]
- Ge, B.; Chen, Y.; Yu, Q.; Lin, X.; Li, J.; Qin, S. Regulation of the heme biosynthetic pathway for combinational biosynthesis of phycocyanobilin in Escherichia coli. Process Biochem. 2018, 71, 23–30. [Google Scholar] [CrossRef]
- Ko, Y.J.; Kim, M.; You, S.K.; Shin, S.K.; Chang, J.; Choi, H.J.; Jeong, W.Y.; Lee, M.E.; Hwang, D.H.; Han, S.O. Animal-free heme production for artificial meat in Corynebacterium glutamicum via systems metabolic and membrane engineering. Metab. Eng. 2021, 66, 217–228. [Google Scholar] [CrossRef]
- Seok, J.; Ko, Y.J.; Lee, M.E.; Hyeon, J.E.; Han, S.O. Systems metabolic engineering of Corynebacterium glutamicum for the bioproduction of biliverdin via protoporphyrin independent pathway. J. Biol. Eng. 2019, 13, 28. [Google Scholar] [CrossRef]
- Kallscheuer, N.; Vogt, M.; Marienhagen, J. A novel synthetic pathway enables microbial production of polyphenols independent from the endogenous aromatic amino acid metabolism. ACS Synth. Biol. 2017, 6, 410–415. [Google Scholar] [CrossRef]
- Jiang, M.R.; Hong, K.Q.; Mao, Y.F.; Ma, H.W.; Chen, T.; Wang, Z.Z. Natural 5-aminolevulinic acid: Sources, biosynthesis, detection and applications. Front. Bioeng. Biotechnol. 2022, 10, 841443. [Google Scholar] [CrossRef]
- Yi, Y.C.; Shih, I.T.; Yu, T.H.; Lee, Y.J.; Ng, I.S. Challenges and opportunities of bioprocessing 5-aminolevulinic acid using genetic and metabolic engineering: A critical review. BIBO 2021, 8, 100. [Google Scholar] [CrossRef]
- Kwon, Y.D.; Kwon, O.H.; Lee, H.S.; Kim, P. The effect of NADP-dependent malic enzyme expression and anaerobic C4 metabolism in Escherichia coli compared with other anaplerotic enzymes. J. Appl. Microbiol. 2007, 103, 2340–2345. [Google Scholar] [CrossRef]
- Kwon, O.H.; Kim, S.; Hahm, D.H.; Lee, S.Y.; Kim, P. Potential application of the recombinant Escherichia coli synthesized Heme as a bioavailable iron source. J. Microbiol. Biotechnol. 2009, 19, 604–609. [Google Scholar]
- Rock, C.O.; Calder, R.B.; Karim, M.A.; Jackowski, S. Pantothenate kinase regulation of the intracellular concentration of coenzyme A. J. Biol. Chem. 2000, 275, 1377–1383. [Google Scholar] [CrossRef] [PubMed]
- Rock, C.O.; Park, H.W.; Jackowski, S. Role of feedback regulation of pantothenate kinase (CoaA) in control of coenzyme A levels in Escherichia coli. J. Bacteriol. 2003, 185, 3410–3415. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.J.; Kwon, Y.D.; Lee, S.Y.; Kim, P. An engineered Escherichia coli having a high intracellular level of ATP and enhanced recombinant protein production. Appl. Microbiol. Biotechnol. 2012, 94, 1079–1086. [Google Scholar] [CrossRef] [PubMed]
- Pranawidjaja, S.; Choi, S.I.; Lay, B.W.; Kim, P. Analysis of heme biosynthetic pathways in a recombinant Escherichia coli. J. Microbiol. Biotechnol. 2015, 25, 880–886. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.J.; Kim, H.J.; Lee, J.Y.; Kwon, A.S.; Jun, S.Y.; Kang, S.H.; Kim, P. Effect of gene amplifications in porphyrin pathway on heme biosynthesis in a recombinant Escherichia coli. J. Microbiol. Biotechnol. 2013, 23, 668–673. [Google Scholar]
- Wang, L.; Wilson, S.; Elliott, T. A mutant hemA protein with positive charge close to the N terminus is stabilized against heme-regulated proteolysis in Salmonella typhimurium. J. Bacteriol. 1999, 181, 6033–6041. [Google Scholar] [CrossRef]
- Xue, J.; Zhou, J.; Li, J.; Du, G.; Chen, J.; Wang, M.; Zhao, X. Systematic engineering of Saccharomyces cerevisiae for efficient synthesis of hemoglobins and myoglobins. Bioresour. Technol. 2022, 370, 128556. [Google Scholar] [CrossRef]
- Kang, Z.; Wang, Y.; Gu, P.; Wang, Q.; Qi, Q. Engineering Escherichia coli for efficient production of 5-aminolevulinic acid from glucose. Metab. Eng. 2011, 13, 492–498. [Google Scholar] [CrossRef]
- Lo, T.M.; Teo, W.S.; Ling, H.; Chen, B.; Kang, A.; Chang, M.W. Microbial engineering strategies to improve cell viability for biochemical production. Biotechnol. Adv. 2013, 31, 903–914. [Google Scholar] [CrossRef]
- Biggs, B.W.; De Paepe, B.; Santos, C.N.; De Mey, M.; Kumaran Ajikumar, P. Multivariate modular metabolic engineering for pathway and strain optimization. Curr. Opin. Biotechnol. 2014, 29, 156–162. [Google Scholar] [CrossRef]
- Zhang, J.; Kang, Z.; Chen, J.; Du, G. Optimization of the heme biosynthesis pathway for the production of 5-aminolevulinic acid in Escherichia coli. Sci. Rep. 2015, 5, 8584. [Google Scholar] [CrossRef] [PubMed]
- Hu, B.; Yu, H.; Zhou, J.; Li, J.; Chen, J.; Du, G.; Lee, S.Y.; Zhao, X. Whole-cell P450 biocatalysis using engineered Escherichia coli with fine-tuned heme Biosynthesis. Adv. Sci. 2022, 10, e2205580. [Google Scholar] [CrossRef] [PubMed]
- Letoffe, S.; Heuck, G.; Delepelaire, P.; Lange, N.; Wandersman, C. Bacteria capture iron from heme by keeping tetrapyrrol skeleton intact. Proc. Natl. Acad. Sci. USA 2009, 106, 11719–11724. [Google Scholar] [CrossRef] [PubMed]
- Dailey, H.A.; Septer, A.N.; Daugherty, L.; Thames, D.; Gerdes, S.; Stabb, E.V.; Dunn, A.K.; Dailey, T.A.; Phillips, J.D. The Escherichia coli protein YfeX functions as a porphyrinogen oxidase, not a heme dechelatase. mBio 2011, 2, e00248-11. [Google Scholar] [CrossRef] [PubMed]
- Turlin, E.; Heuck, G.; Simões Brandão, M.I.; Szili, N.; Mellin, J.R.; Lange, N.; Wandersman, C. Protoporphyrin (PPIX) efflux by the MacAB-TolC pump in Escherichia coli. Microbiologyopen 2014, 3, 849–859. [Google Scholar] [CrossRef]
- Keppel, M.; Piepenbreier, H.; Gatgens, C.; Fritz, G.; Frunzke, J. Toxic but tasty-temporal dynamics and network architecture of heme-responsive two-component signaling in Corynebacterium glutamicum. Mol. Microbiol. 2019, 111, 1367–1381. [Google Scholar] [CrossRef]
- Anzaldi, L.L.; Skaar, E.P. Overcoming the heme paradox: Heme toxicity and tolerance in bacterial pathogens. Infect. Immun. 2010, 78, 4977–4989. [Google Scholar] [CrossRef]
- Feissner, R.E.; Richard-Fogal, C.L.; Frawley, E.R.; Kranz, R.G. ABC transporter-mediated release of a haem chaperone allows cytochrome c biogenesis. Mol. Microbiol. 2006, 61, 219–231. [Google Scholar] [CrossRef]
- Allen, C.E.; Schmitt, M.P. HtaA is an iron-regulated hemin binding protein involved in the utilization of heme iron in Corynebacterium diphtheriae. J. Bacteriol. 2009, 191, 2638–2648. [Google Scholar] [CrossRef]
- Keppel, M.; Davoudi, E.; Gatgens, C.; Frunzke, J. Membrane topology and heme binding of the histidine kinases HrrS and ChrS in Corynebacterium glutamicum. Front. Microbiol. 2018, 9, 183. [Google Scholar] [CrossRef]
- D’Angelo, P.; Lucarelli, D.; della Longa, S.; Benfatto, M.; Hazemann, J.L.; Feis, A.; Smulevich, G.; Ilari, A.; Bonamore, A.; Boffi, A. Unusual heme iron-lipid acyl chain coordination in Escherichia coli flavohemoglobin. Biophys. J. 2004, 86, 3882–3892. [Google Scholar] [CrossRef]
- Radmacher, E.; Stansen, K.C.; Besra, G.S.; Alderwick, L.J.; Maughan, W.N.; Hollweg, G.; Sahm, H.; Wendisch, V.F.; Eggeling, L. Ethambutol, a cell wall inhibitor of Mycobacterium tuberculosis, elicits L-glutamate efflux of Corynebacterium glutamicum. Microbiology 2005, 151, 1359–1368. [Google Scholar] [CrossRef] [PubMed]
- Jang, S.; Imlay, J.A. Micromolar intracellular hydrogen peroxide disrupts metabolism by damaging iron-sulfur enzymes. J. Biol. Chem. 2007, 282, 929–937. [Google Scholar] [CrossRef] [PubMed]
- Li, M.M.; Wang, Y.X.; Tang, L. Effect of Fur overexpression on heme synthesis in Escherichia coli. Food Ferment. Ind. 2019, 45, 18–25. [Google Scholar]
- Burnham, B.F.; Pierce, W.S.; Williams, K.R.; Boyer, M.H.; Kirby, C.K. Control of porphyrin biosynthesis through a negative-feedback mechanism. Studies with preparations of δ-aminolaevulate synthetase and δ-aminolaevulate dehydratase from Rhodopseudomonas spheroides. Biochem. J. 1963, 87, 462–472. [Google Scholar] [CrossRef] [PubMed]
- O’Brian, M.R. Perception and homeostatic control of iron in the rhizobia and related bacteria. Annu. Rev. Microbiol. 2015, 69, 229–245. [Google Scholar] [CrossRef]
- Li, F.; Wang, Y.; Gong, K.; Wang, Q.; Liang, Q.; Qi, Q. Constitutive expression of RyhB regulates the heme biosynthesis pathway and increases the 5-aminolevulinic acid accumulation in Escherichia coli. FEMS Microbiol. Lett. 2014, 350, 209–215. [Google Scholar] [CrossRef]
- Brune, I.; Werner, H.; Huser, A.T.; Kalinowski, J.; Puhler, A.; Tauch, A. The DtxR protein acting as dual transcriptional regulator directs a global regulatory network involved in iron metabolism of Corynebacterium glutamicum. BMC Genom. 2006, 7, 21. [Google Scholar] [CrossRef]
- Cornelis, P.; Wei, Q.; Andrews, S.C.; Vinckx, T. Iron homeostasis and management of oxidative stress response in bacteria. Metallomics. 2011, 3, 540–549. [Google Scholar] [CrossRef]
- Lee, M.J.; Chun, S.J.; Kim, H.J.; Kwon, A.S.; Jun, S.Y.; Kang, S.H.; Kim, P. Porphyrin derivatives from a recombinant Escherichia coli grown on chemically defined medium. J. Microbiol. Biotechnol. 2012, 22, 1653–1658. [Google Scholar] [CrossRef]
- Shao, Y.; Xue, C.; Liu, W.; Zuo, S.; Wei, P.; Huang, L.; Lian, J.; Xu, Z. High-level secretory production of leghemoglobin in Pichia pastoris through enhanced globin expression and heme biosynthesis. Bioresour. Technol. 2022, 363, 127884. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.Y.; Liu, J.B.; Zhang, T.; Cui, Z.Z.; Zhuang, H. Study on testing methods of ferroheme. Sci. Technol. Food Ind. 2010, 1, 378–380. [Google Scholar]
- Sinclair, P.R.; Gorman, N.; Jacobs, J.M. Measurement of heme concentration. Curr. Protoc. Toxicol. 2001, 8.3.1–8.3.7. [Google Scholar] [CrossRef] [PubMed]
- Paul, K.G. The molar light absorption of pyridine ferroprotoporphrin (pyridine haemochromogen). Acta Chim. Sin. 1953, 7, 1284–1287. [Google Scholar] [CrossRef]
- Berry, E.A.; Trumpower, B.L. Simultaneous determination of hemes a, b, and c from pyridine hemochrome spectra. Anal. Biochem. 1987, 161, 1–15. [Google Scholar] [CrossRef]
- Sassa, S. Sequential induction of heme pathway enzymes during erythroid differentiation of mouse friend leukemia virus-infected cells. J. Exp. Med. 1976, 143, 305–315. [Google Scholar] [CrossRef]
- Bonkovsky, H.L.; Wood, S.G.; Howell, S.K.; Sinclair, P.R.; Lincoln, B.; Healey, J.F.; Sinclair, J.F. High-performance liquid chromatographic separation and quantitation of tetrapyrroles from biological materials. Anal. Biochem. 1986, 155, 56–64. [Google Scholar] [CrossRef]
- Hanna, D.A.; Harvey, R.M.; Martinez-Guzman, O.; Yuan, X.; Chandrasekharan, B.; Raju, G.; Outten, F.W.; Hamza, I.; Reddi, A.R. Heme dynamics and trafficking factors revealed by genetically encoded fluorescent heme sensors. Proc. Natl. Acad. Sci. USA 2016, 113, 7539–7544. [Google Scholar] [CrossRef]
- Czajka, J.J.; Oyetunde, T.; Tang, Y.J. Integrated knowledge mining, genome-scale modeling, and machine learning for predicting Yarrowia lipolytica bioproduction. Metab. Eng. 2021, 67, 227–236. [Google Scholar] [CrossRef]
- Callaway, E. What’s next for AlphaFold and the AI protein-folding revolution. Nature 2022, 604, 234–238. [Google Scholar] [CrossRef]
- Champion, M.; Brennan, K.; Croonenborghs, T.; Gentles, A.J.; Pochet, N.; Gevaert, O. Module analysis captures pancancer epigenetically deregulated cancer driver genes for smoking and antiviral response. EBioMedicine 2018, 27, 156–166. [Google Scholar] [CrossRef]
- Yang, X.; Mao, Z.; Zhao, X.; Wang, R.; Zhang, P.; Cai, J.; Xue, C.; Ma, H. Integrating thermodynamic and enzymatic constraints into genome-scale metabolic models. Metab. Eng. 2021, 67, 133–144. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Li, Y.; Jin, K.; Zhang, L.; Li, J.; Liu, Y.; Du, G.; Lv, X.; Chen, J.; Ledesma-Amaro, R.; et al. CRISPR-dCas12a-mediated genetic circuit cascades for multiplexed pathway optimization. Nat. Chem. Biol. 2023, 19, 367–377. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Wang, Z.; Su, T.; Sun, H.; Zhu, Y.; Qi, Q.; Wang, Q. Tuning the binding affinity of heme-responsive biosensor for precise and dynamic pathway regulation. iScience 2020, 23, 101067. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, Q.; Zhao, J.; Zheng, Y.; Chen, T.; Wang, Z. Microbial Synthesis of Heme b: Biosynthetic Pathways, Current Strategies, Detection, and Future Prospects. Molecules 2023, 28, 3633. https://doi.org/10.3390/molecules28083633
Yang Q, Zhao J, Zheng Y, Chen T, Wang Z. Microbial Synthesis of Heme b: Biosynthetic Pathways, Current Strategies, Detection, and Future Prospects. Molecules. 2023; 28(8):3633. https://doi.org/10.3390/molecules28083633
Chicago/Turabian StyleYang, Qiuyu, Juntao Zhao, Yangyang Zheng, Tao Chen, and Zhiwen Wang. 2023. "Microbial Synthesis of Heme b: Biosynthetic Pathways, Current Strategies, Detection, and Future Prospects" Molecules 28, no. 8: 3633. https://doi.org/10.3390/molecules28083633
APA StyleYang, Q., Zhao, J., Zheng, Y., Chen, T., & Wang, Z. (2023). Microbial Synthesis of Heme b: Biosynthetic Pathways, Current Strategies, Detection, and Future Prospects. Molecules, 28(8), 3633. https://doi.org/10.3390/molecules28083633







