Enhancement of Polyphenols and Antioxidant Activity in Germinated Black Highland Barley by Ultrasonication
Abstract
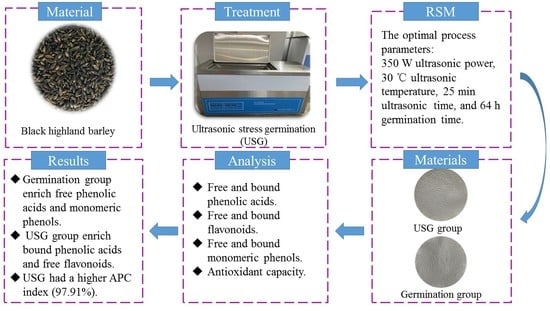
:1. Introduction
2. Results and Discussion
2.1. Response Surface Test
2.2. Effects of USG on the Phenolic Compounds in BHB
2.3. Effects of USG on the In Vitro Antioxidant Activity of BHB
2.4. Correlations between Antioxidant Capacity and Phenolic Compounds
3. Materials and Methods
3.1. Materials and Reagents
3.2. Sample Preparation
3.3. Research Procedures
3.3.1. Extraction of Free and Bound Phenols
3.3.2. Determination of Total Phenolic Contents (TPC) and Total Flavonoid Contents (TFC)
3.3.3. Determination of In Vitro Antioxidant Activity of Black Barley Polyphenol Extracts
3.3.4. Response Surface Test
3.3.5. Composition of Phenolic Substances
3.3.6. Antioxidant Potency Composite Index (APC index)
3.3.7. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Obadi, M.; Sun, J.; Xu, B. Highland barley: Chemical composition, bioactive compounds, health effects, and applications. Food Res. Int. 2021, 140, 110065. [Google Scholar] [CrossRef]
- Liu, Z.F.; Yao, Z.J.; Yu, C.Q.; Zhong, Z.M. Assessing crop water demand and deficit for the growth of spring highland barley in Tibet China. J. Integr. Agric. 2013, 12, 541–551. [Google Scholar] [CrossRef]
- Guo, T.L.; Horvath, C.; Chen, L.; Chen, J.; Zheng, B. Understanding the nutrient composition and nutritional functions of highland barley (qingke): A review. Trends Food Sci. Technol. 2020, 103, 109–117. [Google Scholar] [CrossRef]
- Liu, K.; Zhang, B.J.; Chen, L.; Li, X.; Zheng, B. Hierarchical structure and physicochemical properties of highland barley starch following heat moisture treatment. Food Chem. 2019, 271, 102–108. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.; Zhang, B.J.; Chen, L.; Li, X.X.; Zheng, B. In vitro and in vivo antioxidant activity of polyphenols extracted from black highland barley. Food Chem. 2016, 194, 1003–1012. [Google Scholar]
- Zhang, X.P.; Shao, J.J.; Cui, Q.H.; Ni, W.T.; Yang, Y.; Yan, B.B. Bioactivities of dietary polyphenols and their effects on intestinal microbiota. Mini-Rev. Med. Chem. 2023, 23, 361–377. [Google Scholar]
- Lin, S.; Guo, H.; Gong, J.D.B.; Lu, M.; Lu, M.Y.; Wang, L.; Zhang, Q.; Qin, W.; Wu, D.T. Phenolic profiles, β-glucan contents, and antioxidant capacities of colored Qingke (Tibetan hulless barley) cultivars. J. Cereal Sci. 2018, 81, 69–75. [Google Scholar] [CrossRef]
- Li, J.X.; Zhang, H.; Yang, X.J.; Zhu, L.; Wu, G.C.; Qi, X.G.; Zhang, H. Trapping of reactive carbonyl species by fiber-bound polyphenols from whole grains under simulated physiological conditions. Food Res. Int. 2022, 156, 111142. [Google Scholar] [CrossRef]
- Xu, L.; Wang, P.; Ali, B.; Yang, N.; Chen, Y.S.; Wu, F.F.; Xu, X.M. Changes of the phenolic compounds and antioxidant activities in germinated adlay seeds. J. Sci. Food Agric. 2017, 97, 4227–4234. [Google Scholar] [CrossRef]
- Khang, D.T.; Dung, T.N.; Elzaawely, A.A.; Xuan, T.D. Phenolic profiles and antioxidant activity of germinated legumes. Foods 2016, 5, 27. [Google Scholar] [CrossRef]
- Domínguez-Arispuro, D.M.; Cuevas-Rodríguez, E.O.; Milán-Carrillo, J.; León-López, L.; Gutiérrez-Dorado, R.; Reyes-Moreno, C. Optimal germination condition impacts on the antioxidant activity and phenolic acids profile in pigmented desi chickpea (Cicer arietinum L.) seeds. J. Food Sci. Technol. 2018, 55, 638–647. [Google Scholar] [CrossRef]
- Živković, A.; Polak, T.; Cigić, B.; Požrl, T. Germinated buckwheat: Effects of dehulling on phenolics profile and antioxidant activity of buckwheat seeds. Foods 2021, 10, 740. [Google Scholar] [CrossRef] [PubMed]
- Tang, S.Y.; Mao, G.; Yuan, Y.; Weng, Y.D.; Zhu, R.Y.; Cai, C.G.; Mao, J.W. Optimization of oat seed steeping and germination temperatures to maximize nutrient content and antioxidant activity. J. Food Process. Preserv. 2020, 44, e14683. [Google Scholar] [CrossRef]
- Gong, L.X.; Wen, T.T.; Wang, J. The role of the microbiome in mediating health effects of dietary components. J. Agric. Food Chem. 2020, 68, 1280–12835. [Google Scholar] [CrossRef]
- Yao, F.; Zhang, J.Y.; Xiao, X.; Dong, Y.; Zhou, X.H. Antitumor activities and apoptosis-regulated mechanisms of fermented barley extract in the transplantation tumor model of human HT-29 cells in nude mice. Biomed. Environ. Sci. 2017, 30, 10–21. [Google Scholar] [PubMed]
- Li, R.; Li, Z.J.; Wu, N.N.; Tan, B. Effect of pre-treatment on the functional properties of germinated whole grains: A review. Cereal Chem. 2022, 99, 253–269. [Google Scholar] [CrossRef]
- Sharma, N.; Goyal, S.K.; Alam, T.; Fatma, S.; Chaoruangrit, A.; Niranjan, K. Effect of high pressure soaking on water absorption, gelatinization, and biochemical properties of germinated and non-germinated foxtail millet grains. J. Cereal Sci. 2018, 83, 162–170. [Google Scholar] [CrossRef]
- Xia, Q.; Li, Y.F. Mild high hydrostatic pressure pretreatments applied before soaking process to modulate wholegrain brown rice germination: An examination on embryo growth and physicochemical properties. Food Res. Int. 2018, 106, 817–824. [Google Scholar] [CrossRef] [PubMed]
- Du, Y.; Chen, Z.X.; Liang, F.; Zhou, W.J.; Li, T.Y.; Tu, Z.X.; Tan, Y.J.; Li, J. Effects of hypoxia stress germination on nutrients, physicochemical properties and cooking characteristics of highland barley. J. Cereal Sci. 2022, 103, 103411. [Google Scholar] [CrossRef]
- Alfalahi, A.O.; Alobaidy, B.S.; Almarie, A.A.; Dhanoon, O.M.; Qasem, J.R.; Almehemdi, A.F.; Najda, A. Ultrasonic treatment enhances germination and affects antioxidant gene expression in soybean (Glycine max L. Merr). Agronomy 2022, 12, 2446. [Google Scholar] [CrossRef]
- Xia, Q.; Tao, H.; Li, Y.F.; Pan, D.D.; Cao, J.X.; Liu, L.L.; Zhou, X.W.; Barba, F.J. Characterizing physicochemical, nutritional and quality attributes of wholegrain Oryza sativa L. subjected to high intensity ultrasound-stimulated pre-germination. Food Control 2020, 108, 106827. [Google Scholar] [CrossRef]
- Naumenko, N.; Potoroko, I.; Kalinina, I. Stimulation of antioxidant activity and γ-aminobutyric acid synthesis in germinated wheat grain Triticum aestivum L. by ultrasound: Increasing the nutritional value of the product. Ultrason. Sonochem. 2022, 86, 106000. [Google Scholar] [CrossRef] [PubMed]
- Ding, J.Z.; Ulanov, A.V.; Dong, M.Y.; Yang, T.W.; Nemzer, B.V.; Xiong, S.B.; Zhao, S.M.; Feng, H. Enhancement of gama-aminobutyric acid (GABA) and other health-related metabolites in germinated red rice (Oryza sativa L.) by ultrasonication. Ultrason. Sonochem. 2018, 40, 791–797. [Google Scholar] [CrossRef] [PubMed]
- Ding, J.Z.; Johnson, J.; Chu, Y.F.; Feng, H. Enhancement of γ- aminobutyric acid, avenanthramides, and other health-promoting metabolites in germinating oats (Avena sativa L.) treated with and without power ultrasound. Food Chem. 2019, 283, 239–247. [Google Scholar] [CrossRef] [PubMed]
- Hassan, S.; Imran, M.; Ahmad, M.H.; Khan, M.I.; Xu, C.M.; Khan, M.K.; Muhammad, N. Phytochemical characterization of ultrasound-processed sorghum sprouts for the use in functional foods. Int. J. Food Prop. 2020, 23, 853–863. [Google Scholar] [CrossRef]
- Tang, Y.; Xiao, L.; Wu, X.Y.; Li, W.L.; Wu, T.; Zhang, P. Impact of germination pretreatment on the polyphenol profile, antioxidant activities, and physicochemical properties of three color cultivars of highland barley. J. Cereal Sci. 2021, 97, 103152. [Google Scholar] [CrossRef]
- Yang, R.Q.; Guo, Y.X.; Wang, S.F.; Gu, Z.X. Ca2+ and aminoguanidine on γ-aminobutyric acid accumulation in germinating soybean under hypoxia–NaCl stress. J. Food Drug Anal. 2015, 23, 287–293. [Google Scholar] [CrossRef]
- López-Ribera, I.; Vicient, C.M. Use of ultrasonication to increase germination rates of Arabidopsis seeds. Plant Methods 2017, 13, 31. [Google Scholar] [CrossRef]
- Oreopoulou, A.; Tsimogiannis, D.; Oreopoulou, V. Extraction of polyphenols from aromatic and medicinal plants: An overview of the methods and the effect of extraction parameters. In Polyphenols in Plants; Academic Press: London, UK, 2019; pp. 243–259. [Google Scholar]
- Xiang, J.L.; Yuan, Y.; Du, L.; Zhang, Y.Y.; Li, C.Q.; Beta, T. Modification on phenolic profiles and enhancement of antioxidant activity of proso millets during germination. Food Chem. X 2023, 18, 100628. [Google Scholar] [CrossRef]
- Ti, H.H.; Zhang, R.F.; Zhang, M.W.; Li, Q.; Wei, Z.C.; Zhang, Y.; Tang, X.J.; Deng, Y.Y.; Liu, L.; Ma, Y.X. Dynamic changes in the free and bound phenolic compounds and antioxidant activity of brown rice at different germination stages. Food Chem. 2014, 161, 337–344. [Google Scholar] [CrossRef]
- Xu, J.G.; Tian, C.R.; Hu, Q.P.; Luo, J.Y.; Wang, X.D.; Tian, X.D. Dynamic changes in phenolic compounds and antioxidant activity in oats (Avena nuda L.) during steeping and germination. J. Agric. Food Chem. 2009, 57, 10392–10398. [Google Scholar] [CrossRef]
- Chen, Z.J.; Yu, L.L.; Wang, X.K.; Gu, Z.X.; Beta, T. Changes of phenolic profiles and antioxidant activity in canaryseed (Phalaris canariensis L.) during germination. Food Chem. 2016, 194, 608–618. [Google Scholar] [CrossRef]
- Estivi, L.; Brandolini, A.; Condezo-Hoyos, L.; Hidalgo, A. Impact of low-frequency ultrasound technology on physical, chemical and technological properties of cereals and pseudocereals. Ultrason. Sonochem. 2022, 86, 106044. [Google Scholar] [CrossRef]
- Kalita, D.; Jain, S.; Srivastava, B.; Goud, V.V. Sono-hydro priming process (ultrasound modulated hydration): Modelling hydration kinetic during paddy germination. Ultrason. Sonochem. 2021, 70, 105321. [Google Scholar] [CrossRef]
- Miano, A.C.; Pereira, J.D.; Costa, C.N.; Augusto, P.E.D. Enhancing mung bean hydration using the ultrasound technology: Description of mechanisms and impact on its germination and main components. Sci. Rep. 2016, 6, 38996. [Google Scholar] [CrossRef]
- Kentish, S.; Feng, H. Applications of power ultrasound in food processing. Annu. Rev. Food Sci. Technol. 2014, 5, 263–284. [Google Scholar] [CrossRef]
- Wang, L.D.; Li, X.Q.; Gao, F.; Liu, Y.; Lang, S.J.; Wang, C.Y.; Zhang, D.J. Effect of ultrasound combined with exogenous GABA treatment on polyphenolic metabolites and antioxidant activity of mung bean during germination. Ultrason. Sono-Chem. 2023, 94, 106311. [Google Scholar] [CrossRef]
- Yang, X.J.; Dang, B.; Fan, M.T. Free and bound phenolic compound content and antioxidant activity of different cultivated blue highland barley varieties from the Qinghai-Tibet Plateau. Molecules 2018, 23, 879. [Google Scholar] [CrossRef]
- Keriene, I.; Mankeviciene, A.; Bliznikas, S.; Jablonskyte-Rasce, D.; Maiksteniene, S.; Cesnuleviciene, R. Biologically active phenolic compounds in buckwheat, oats and winter spelt wheat. Zemdirbyste 2015, 102, 289–296. [Google Scholar] [CrossRef]
- Ge, X.Z.; Saleh, A.S.M.; Jing, L.Z.; Zhao, K.; Su, C.Y.; Zhang, B.; Zhang, Q.; Li, W.H. Germination and drying induced changes in the composition and content of phenolic compounds in naked barley. J. Food Compos. Anal. 2021, 95, 103594. [Google Scholar] [CrossRef]
- Boubakri, H.; Jdey, A.; Taamalli, A.; Taamalli, W.; Jebara, M.; Brini, F.; Riciputi, Y.; Pasini, F.; Christian, M.; Verardo, V. Phenolic composition as measured by liquid chromatography/mass spectrometry and biological properties of Tunisian barley. Int. J. Food Prop. 2017, 20, 1783–1797. [Google Scholar] [CrossRef]
- Zhao, H.F.; Dong, J.J.; Lu, J.; Chen, J.; Li, Y.; Shan, L.; Lin, Y.; Fen, W.; Gu, G.X. Effects of Extraction solvent mixtures on antioxidant activity evaluation and their extraction capacity and selectivity for free phenolic compounds in barley (Hordeum vulgare L.). J. Agric. Food Chem. 2006, 54, 7277–7286. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Aal, E.S.M.; Choo, T.M.; Dhillon, S.; Rabalski, I. Free and bound phenolic acids and total phenolics in black, blue, and yellow barley and their contribution to free radical scavenging capacity. Cereal Chem. 2012, 89, 198–204. [Google Scholar] [CrossRef]
- Zhang, W.G.; Lan, Y.L.; Dang, B.; Zhang, J.; Zheng, W.C.; Du, Y.; Yang, X.J.; Li, Z.H. Polyphenol profile and in vitro antioxi-dant and enzyme inhibitory activities of different solvent extracts of highland barley bran. Molecules 2023, 28, 1665. [Google Scholar] [CrossRef] [PubMed]
- Jin, H.M.; Dang, B.; Zhang, W.G.; Zheng, W.C.; Yang, X.J. Polyphenol and anthocyanin composition and activity of highland barley with different colors. Molecules 2022, 27, 3411. [Google Scholar] [CrossRef] [PubMed]
- Guo, W.W.; Beta, T. Phenolic acid composition and antioxidant potential of insoluble and soluble dietary fibre extracts derived from select whole-grain cereals. Food Res. Int. 2013, 51, 518–525. [Google Scholar] [CrossRef]
- Seeram, N.P.; Aviram, M.; Zhang, Y.J.; Henning, S.M.; Feng, L.; Dreher, M.; Heber, D. Comparison of antioxidant potency of commonly consumed polyphenol-rich beverages in the United States. J. Agric. Food Chem. 2008, 56, 1415–1422. [Google Scholar] [CrossRef]




| Number | X1: Germination Time (h) | X2: Ultrasound Power (W) | X3: Ultrasound Time (min) | TPC (mg/100 g DW) | TFC (mg/100 g DW) |
|---|---|---|---|---|---|
| 1 | −1 | −1 | 0 | 581.66 ± 3.69 | 46.06 ± 0.45 |
| 2 | 1 | −1 | 0 | 600.74 ± 3.30 | 56.78 ± 0.31 |
| 3 | −1 | 1 | 0 | 581.23 ± 1.17 | 47.69 ± 0.15 |
| 4 | 1 | 1 | 0 | 629.25 ± 3.36 | 56.70 ± 1.33 |
| 5 | −1 | 0 | −1 | 598.94 ± 0.97 | 49.28 ± 1.48 |
| 6 | 1 | 0 | −1 | 649.46 ± 4.20 | 58.32 ± 0.56 |
| 7 | −1 | 0 | 1 | 574.25 ± 4.87 | 45.34 ± 0.53 |
| 8 | 1 | 0 | 1 | 645.70 ± 4.02 | 57.60 ± 0.39 |
| 9 | 0 | −1 | −1 | 593.19 ± 2.33 | 55.17 ± 1.84 |
| 10 | 0 | 1 | −1 | 630.20 ± 3.52 | 56.26 ± 0.81 |
| 11 | 0 | −1 | 1 | 591.37 ± 7.78 | 53.75 ± 1.13 |
| 12 | 0 | 1 | 1 | 601.80 ± 4.56 | 53.27 ± 0.29 |
| 13 | 0 | 0 | 0 | 684.17 ± 6.11 | 58.47 ± 1.72 |
| 14 | 0 | 0 | 0 | 687.60 ± 2.09 | 59.12 ± 1.47 |
| 15 | 0 | 0 | 0 | 668.43 ± 8.78 | 59.17 ± 0.64 |
| 16 | 0 | 0 | 0 | 679.51 ± 3.37 | 58.63 ± 1.34 |
| 17 | 0 | 0 | 0 | 686.39 ± 9.54 | 58.88 ± 1.19 |
| Source of Variation | TPC | TFC | ||||
|---|---|---|---|---|---|---|
| F-Value | p-Value | Significance | F-Value | p-Value | Significance | |
| Model | 31.01 | <0.0001 | ** | 354.08 | <0.0001 | ** |
| X1 | 46.95 | 0.0002 | ** | 1852.18 | <0.0001 | ** |
| X2 | 7.49 | 0.0290 | * | 5.13 | 0.0578 | |
| X3 | 4.52 | 0.0711 | 90.51 | <0.0001 | ** | |
| X1 X2 | 2.20 | 0.1816 | 6.43 | 0.0389 | * | |
| X1 X3 | 1.15 | 0.3190 | 22.82 | 0.0020 | ** | |
| X2 X3 | 1.86 | 0.2153 | 5.42 | 0.0527 | ||
| X12 | 54.28 | 0.0002 | ** | 754.47 | <0.0001 | ** |
| X22 | 101.82 | <0.0001 | ** | 238.06 | <0.0001 | ** |
| X32 | 37.48 | 0.0005 | ** | 107.99 | <0.0001 | ** |
| Lack of fit | 2.33 | 0.2161 | 1.54 | 0.3343 | ||
| R2 | 0.9755 | 0.9978 | ||||
| Adjusted R2 (R2adj) | 0.9441 | 0.9950 | ||||
| Untreated Group | Germination Group | USG Group | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Free | Bound | Total | Free | Bound | Total | Free | Bound | Total | |
| Flavonoids | |||||||||
| Kaempferol | 0.36 ± 0.05 b | 0.87 ± 0.14 b | 1.22 ± 0.19 b | 0.47 ± 0.02 b | ND | 0.47 ± 0.02 c | 24.81 ± 0.80 a | 2.40 ± 0.08 a | 27.22 ± 0.81 a |
| Maltol | 3.35 ± 1.31 a | 1.74 ± 0.11 b | 5.09 ± 1.38 a | 3.22 ± 0.94 a | 2.24 ± 0.24 b | 5.47 ± 0.61 a | 2.09 ± 0.33 b | 3.10 ± 0.54 a | 5.19 ± 0.28 a |
| Taxifolin | 0.29 ± 0.01 a | 0.58 ± 0.05 a | 0.87 ± 0.06 a | 0.21 ± 0.05 b | 0.50 ± 0.08 a | 0.70 ± 0.06 b | 0.15 ± 0.01 b | 0.30 ± 0.04 b | 0.45 ± 0.05 c |
| Rutin | 0.51 ± 0.03 a | 0.57 ± 0.02 a | 1.09 ± 0.03 a | 0.48 ± 0.05 a | ND | 0.48 ± 0.05 b | 0.47 ± 0.01 a | ND | 0.47 ± 0.01 b |
| Diosmin | 36.94 ± 0.84 a | 1.30 ± 0.27 a | 38.24 ± 1.11 a | 16.74 ± 0.48 c | 0.45 ± 0.24 b | 17.19 ± 0.36 c | 19.87 ± 1.27 b | 0.47 ± 0.01 b | 20.34 ± 1.26 b |
| Kaempferol-3-O-rutinoside | 0.42 ± 0.05 a | ND | 0.51 ± 0.06 b | 0.28 ± 0.03 b | 0.20 ± 0.10 a | 0.48 ± 0.09 b | 0.37 ± 0.03 a | 0.32 ± 0.05 a | 0.69 ± 0.03 a |
| Myricetin | 0.12 ± 0.01 b | 0.21 ± 0.04 a | 0.33 ± 0.02 a | 0.22 ± 0.01 a | ND | 0.22 ± 0.01 b | 0.13 ± 0.00 b | ND | 0.13 ± 0.00 c |
| Quercetin | 0.25 ± 0.05 a | 0.69 ± 0.05 a | 0.95 ± 0.04 a | ND | 0.19 ± 0.05 b | 0.19 ± 0.05 c | 0.13 ± 0.00 b | 0.26 ± 0.06 b | 0.38 ± 0.06 b |
| Luteolin | 5.70 ± 0.00 b | ND | 5.74 ± 0.01 b | ND | ND | ND | 13.11 ± 0.58 a | ND | 13.11 ± 0.58 a |
| Diosmetin | 60.07 ± 0.69 b | 5.52 ± 0.26 a | 65.58 ± 0.95 b | 26.53 ± 0.47 c | 4.89 ± 0.07 b | 31.42 ± 0.47 c | 125.98 ± 2.66 a | ND | 125.98 ± 2.66 a |
| Catechin | 132.59 ± 2.40 c | ND | 132.59 ± 2.40 c | 239.58 ± 4.05 b | ND | 239.58 ± 4.05 b | 276.06 ± 2.30 a | ND | 276.06 ± 2.30 a |
| Epicatechin | 4.58 ± 0.19 b | ND | 4.58 ± 0.24 b | 18.28 ± 0.50 a | 0.13 ± 0.09 a | 18.41 ± 0.44 a | 3.22 ± 0.03 c | ND | 3.22 ± 0.03 c |
| Puerarin | ND | ND | ND | 0.24 ± 0.11 a | ND | 0.24 ± 0.11 a | ND | ND | ND |
| Homoorientin | 3.72 ± 0.06 b | 0.59 ± 0.06 b | 4.30 ± 0.08 b | 1.40 ± 0.02 c | 0.54 ± 0.02 b | 1.94 ± 0.02 c | 4.12 ± 0.10 a | 0.82 ± 0.03 a | 4.95 ± 0.10 a |
| Vitexin | 0.46 ± 0.01 b | ND | 0.50 ± 0.02 a | 0.35 ± 0.01 c | ND | 0.35 ± 0.01 b | 0.48 ± 0.01 a | ND | 0.48 ± 0.01 a |
| Naringin | ND | 0.16 ± 0.10 a | 0.25 ± 0.10 a | ND | ND | ND | ND | 0.14 ± 0.07 a | 0.14 ± 0.07 a |
| Isovitexin | 4.12 ± 0.06 c | ND | 4.22 ± 0.06 c | 9.80 ± 0.03 a | ND | 9.80 ± 0.03 a | 7.49 ± 0.10 b | 0.14 ± 0.02 a | 7.62 ± 0.12 b |
| Procyanidin A2 | ND | ND | ND | 0.32 ± 0.02 a | ND | 0.32 ± 0.02 a | ND | ND | ND |
| Procyanidin B2 | 52.32 ± 0.74 a | ND | 52.32 ± 0.74 a | 41.05 ± 0.17 c | ND | 41.05 ± 0.17 c | 43.71 ± 1.06 b | ND | 43.71 ± 1.06 b |
| Total flavonoids | 305.79 ± 3.73 c | 12.23 ± 0.38 a | 318.09 ± 3.81 c | 357.62 ± 2.86 b | 9.13 ± 0.16 b | 366.32 ± 3.44 b | 522.14 ± 3.14 a | 7.95 ± 0.46 c | 530.09 ± 2.73 a |
| Phenolic acids | |||||||||
| Ferulic acid | 20.46 ± 0.34 a | 179.21 ± 5.56 b | 199.67 ± 5.25 a | 12.56 ± 0.06 b | 141.58 ± 1.97 c | 154.13 ± 1.97 b | 10.17 ± 0.78 b | 202.69 ± 1.85 a | 212.86 ± 2.05 a |
| Protocatechuic acid | 2.40 ± 0.06 b | 5.52 ± 0.09 b | 7.92 ± 0.10 b | 34.83 ± 1.49 a | 8.49 ± 0.48 a | 43.31 ± 1.01 a | 1.46 ± 0.21 b | 5.86 ± 0.07 b | 7.32 ± 0.18 b |
| p-Hydroxybenzoic acid | 2.87 ± 0.14 c | 1.29 ± 0.55 a | 4.17 ± 0.64 c | 13.61 ± 0.15 a | 1.28 ± 0.21 a | 14.88 ± 0.30 a | 8.35 ± 0.19 b | 0.94 ± 0.23 a | 9.39 ± 0.19 b |
| Homogentisic acid | 3.16 ± 0.10 c | 0.39 ± 0.20 b | 3.55 ± 0.29 c | 4.11 ± 0.18 b | 0.58 ± 0.49 b | 4.68 ± 0.65 b | 4.42 ± 0.15 a | 15.26 ± 0.49 a | 19.68 ± 0.60 a |
| p-Coumaric acid | 5.76 ± 0.39 c | 8.55 ± 0.22 c | 14.31 ± 0.25 c | 66.16 ± 0.36 a | 12.64 ± 0.41 b | 78.80 ± 0.58 a | 40.56 ± 0.70 b | 13.71 ± 0.37 a | 54.28 ± 0.71 b |
| Vanillic acid | 12.51 ± 0.41 c | 4.65 ± 0.45 a | 17.15 ± 0.26 b | 20.87 ± 1. 78 a | 3.53 ± 0.37 b | 24.39 ± 1.83 a | 15.30 ± 0.39 b | 3.60 ± 0.45 b | 18.90 ± 0.31 b |
| Total phenolic acids | 47.16 ± 1.05 c | 199.62 ± 11.42 b | 246.78.11 ± 5.14 b | 155.12 ± 3.17 a | 168.08 ± 1.04 c | 320.20 ± 3.29 a | 80.25 ± 0.95 b | 242.06 ± 2.44 a | 322.32 ± 2.53 a |
| Monomeric phenols | |||||||||
| Phlorogucinol | 51.34 ± 1.07 c | 9.78 ± 0.70 b | 61.12 ± 1.72 c | 82.73 ± 0.58 a | 13.41 ± 0.27 a | 96.14 ± 0.67 a | 57.55 ± 1.10 b | 9.29 ± 0.33 b | 66.85 ± 0.89 b |
| Pyrogallol | 0.16 ± 0.02 a | 2.60 ± 0.36 b | 2.76 ± 0.33 b | ND | 3.63 ± 0.29 a | 3.63 ± 0.29 a | 0.41 ± 0.18 a | 1.58 ± 0.15 c | 1.99 ± 0.24 c |
| 4-Hydroxybenzaldehyde | 2.01 ± 0.11 c | 2.45 ± 0.17 c | 4.47 ± 0.14 c | 5.45 ± 0.04 a | 5.59 ± 0.08 a | 11.04 ± 0.06 a | 3.65 ± 0.46 b | 2.80 ± 0.15 b | 6.45 ± 0.31 b |
| Sesamol | 0.15 ± 0.02 b | 0.17 ± 0.09 a | 0.32 ± 0.11 a | 0.35 ± 0.12 a | ND | 0.35 ± 0.12 a | ND | 0.20 ± 0.04 a | 0.20 ± 0.04 b |
| 6-Gingerol | ND | ND | ND | ND | 0.36 ± 0.00 a | 0.36 ± 0.00 b | 0.51 ± 0.06 a | ND | 0.51 ± 0.06 a |
| Vanillin | 4.53 ± 0.17 a | 16.34 ± 1.38 b | 20.87 ± 1.22 b | 1.90 ± 0.12 c | 16.08 ± 0.66 b | 17.99 ± 0.56 c | 2.85 ± 0.39 b | 20.05 ± 0.51 a | 22.90 ± 0.85 a |
| Total monomeric phenols | 58.20 ± 1.30 c | 31.33 ± 1.97 b | 89.53 ± 2.73 c | 90.43 ± 0.74 a | 39.08 ± 1.07 a | 129.51 ± 1.12 a | 64.97 ± 1.67 b | 33.92 ± 0.87 b | 98.89 ± 1.55 b |
| Total phenolic acids+ total flavonoids+ total monomeric phenols | 411.15 ± 4.14 c | 243.18 ± 4.75 b | 654.40 ± 2.08 c | 600.18 ± 1.93 b | 216.29 ± 2.18 c | 816.03 ± 3.38 b | 667.37 ± 4.08 a | 283.93 ± 2.34 a | 951.29 ± 2.62 a |
| Group | FRAP Reducing Power (umol/100 g DW) | ABTS+· Scavenging Ability (umol/100 g DW) | DPPH·Scavenging Ability (umol/100 g DW) | APC Index (%) |
|---|---|---|---|---|
| Untreated group | 3217.53 ± 30.82 c | 3663.47 ± 43.15 b | 5739.32 ± 28.23 c | 76.52 (3) |
| Germination group | 4883.24 ± 12.56 a | 4501.00 ± 24.44 a | 6408.69 ± 73.11 b | 97.29 (2) |
| USG group | 4632.18 ± 19.86 b | 4449.89 ± 42.47 a | 6976.23 ± 47.19 a | 97.91 (1) |
| Free Phenolic Exaction | Bound Phenolic Exaction | Total Phenols | |||||||
|---|---|---|---|---|---|---|---|---|---|
| DPPH | FRAP | ABTS | DPPH | FRAP | ABTS | DPPH | FRAP | ABTS | |
| Phenolic content | 0.759 * | 0.974 ** | 0.979 ** | 0.906 ** | 0.963 ** | −0.278 | 0.935 ** | 0.965 ** | 0.981 ** |
| Flavonoid content | 0.137 | 0.863 ** | 0.845 ** | 0.114 | 0.642 | 0.305 | 0.304 | 0.794 * | 0.743 * |
| Kaempferol | 0.959 ** | 0.428 | 0.463 | 0.503 | −0.068 | −0.445 | 0.825 ** | 0.351 | 0.43 |
| Diosmin | −0.548 | −0.996 ** | −0.987 ** | −0.828 ** | −0.912 ** | −0.011 | −0.809 ** | −0.998 ** | −0.989 ** |
| Catechin | 0.817 ** | 0.945 ** | 0.952 ** | 0.969 ** | 0.925 ** | 0.949 ** | |||
| Isovitexin | 0.321 | 0.943 ** | 0.930 ** | 0.646 | 0.968 ** | 0.942 ** | |||
| Procyanidin B2 | −0.485 | −0.983 ** | −0.978 ** | −0.754 * | −0.989 ** | −0.979 ** | |||
| Homogentisic acid | 0.827 ** | 0.919 ** | 0.941 ** | 0.783 * | 0.302 | -0.449 | 0.868 ** | 0.431 | 0.506 |
| p-Hydroxybenzoic acid | 0.228 | 0.908 ** | 0.890 ** | −0.405 | −0.189 | 0.078 | 0.512 | 0.914 ** | 0.873 ** |
| p-Coumaric acid | 0.299 | 0.938 ** | 0.922 ** | 0.977 ** | 0.904 ** | −0.337 | 0.649 | 0.970 ** | 0.942 ** |
| 4-Hydroxybenzaldehyde | 0.212 | 0.877 ** | 0.869 ** | 0.249 | 0.746* | 0.154 | 0.34 | 0.818 ** | 0.766 * |
| Level | X1: Germination Time (h) | X2: Ultrasound Power (W) | X3: Ultrasound Time (min) |
|---|---|---|---|
| −1 | 48 | 300 | 20 |
| 0 | 60 | 350 | 25 |
| 1 | 72 | 400 | 30 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, J.; Guo, J.; Dang, B.; Zhang, W.; Zheng, W.; Yang, X. Enhancement of Polyphenols and Antioxidant Activity in Germinated Black Highland Barley by Ultrasonication. Molecules 2023, 28, 3679. https://doi.org/10.3390/molecules28093679
Zhang J, Guo J, Dang B, Zhang W, Zheng W, Yang X. Enhancement of Polyphenols and Antioxidant Activity in Germinated Black Highland Barley by Ultrasonication. Molecules. 2023; 28(9):3679. https://doi.org/10.3390/molecules28093679
Chicago/Turabian StyleZhang, Jie, Junling Guo, Bin Dang, Wengang Zhang, Wancai Zheng, and Xijuan Yang. 2023. "Enhancement of Polyphenols and Antioxidant Activity in Germinated Black Highland Barley by Ultrasonication" Molecules 28, no. 9: 3679. https://doi.org/10.3390/molecules28093679
APA StyleZhang, J., Guo, J., Dang, B., Zhang, W., Zheng, W., & Yang, X. (2023). Enhancement of Polyphenols and Antioxidant Activity in Germinated Black Highland Barley by Ultrasonication. Molecules, 28(9), 3679. https://doi.org/10.3390/molecules28093679