Alginate–Chitosan Membranes for the Encapsulation of Lavender Essential Oil and Development of Biomedical Applications Related to Wound Healing
Abstract
1. Introduction
2. Results and Discussion
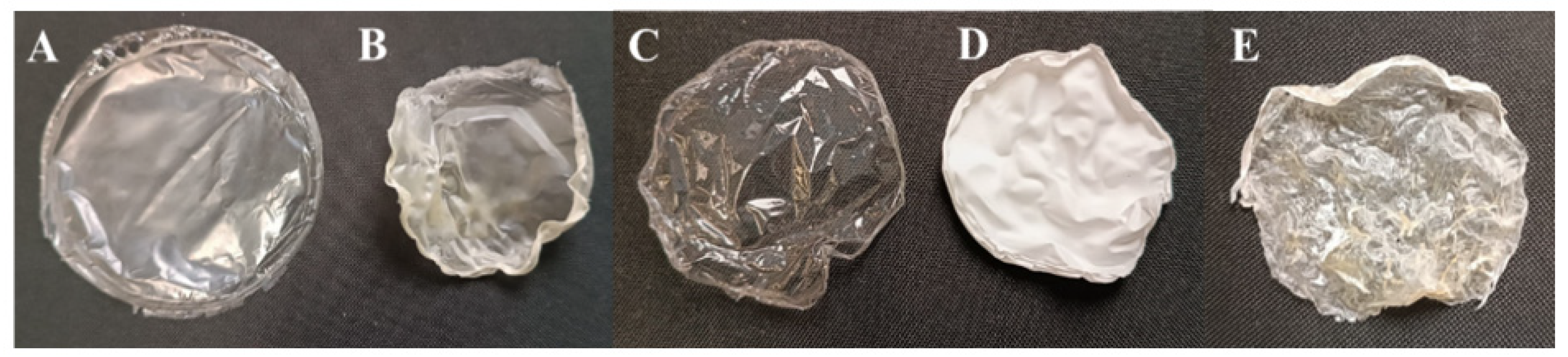
2.1. Preparation of Membranes
2.2. Chemical Characterization: Fourier Transform Infrared (FTIR)-Attenuated Total Reflectance (ATR) Spectroscopy
2.3. Study of Water Uptake and Degradation
2.4. LEO Release Profile
2.5. Biological Analysis of the Most Promising Membranes
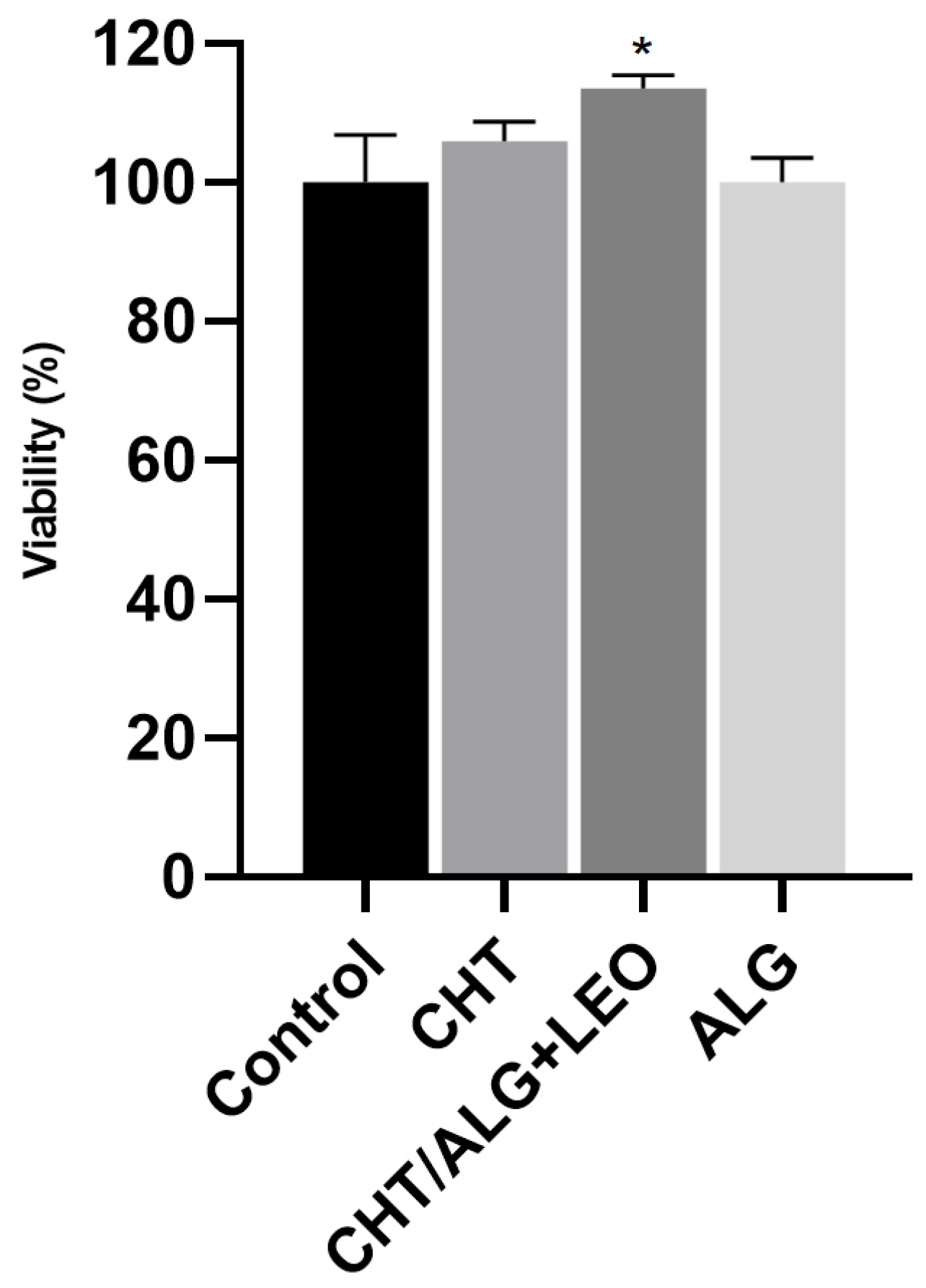
2.5.1. Cell Viability Assessment
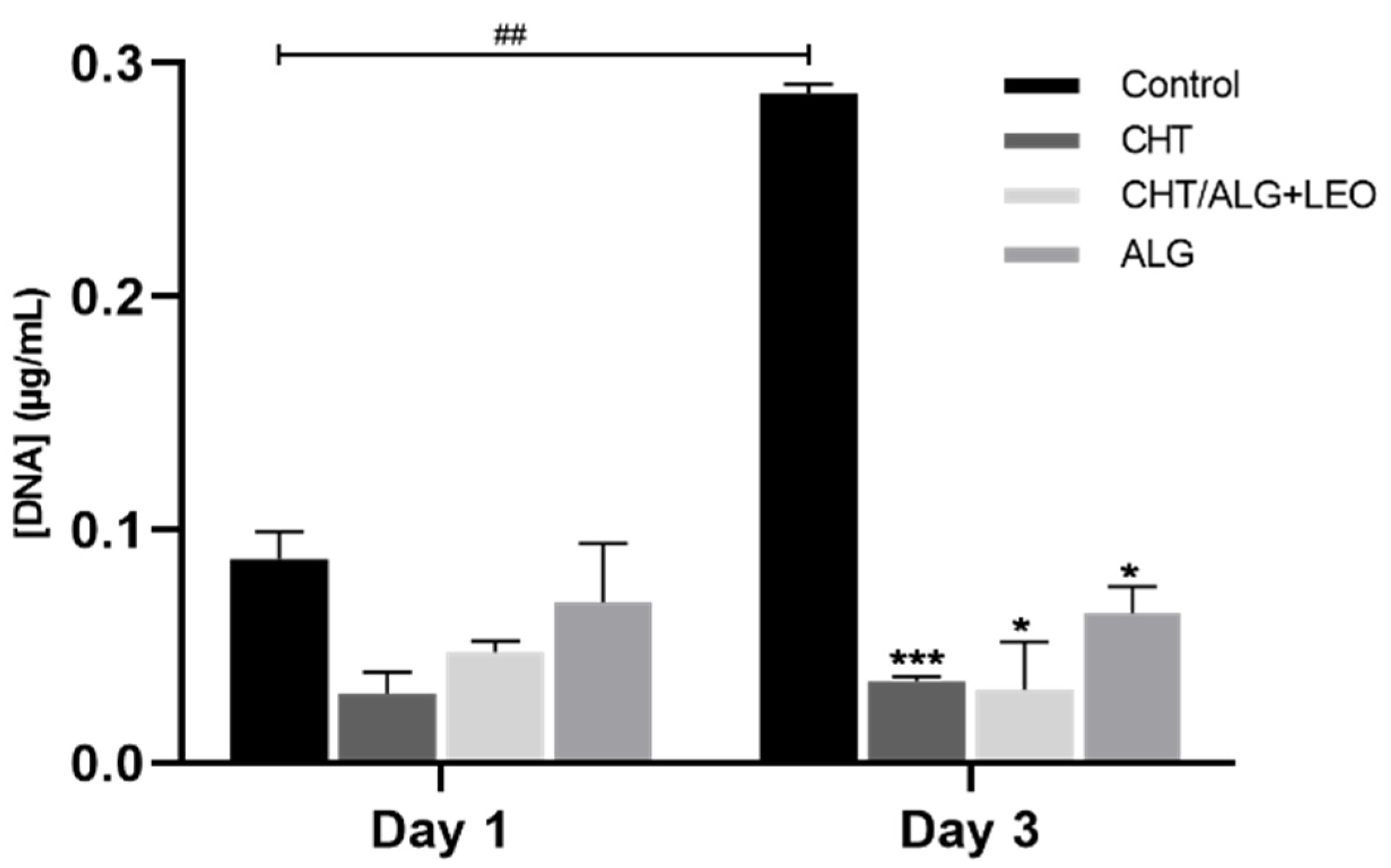
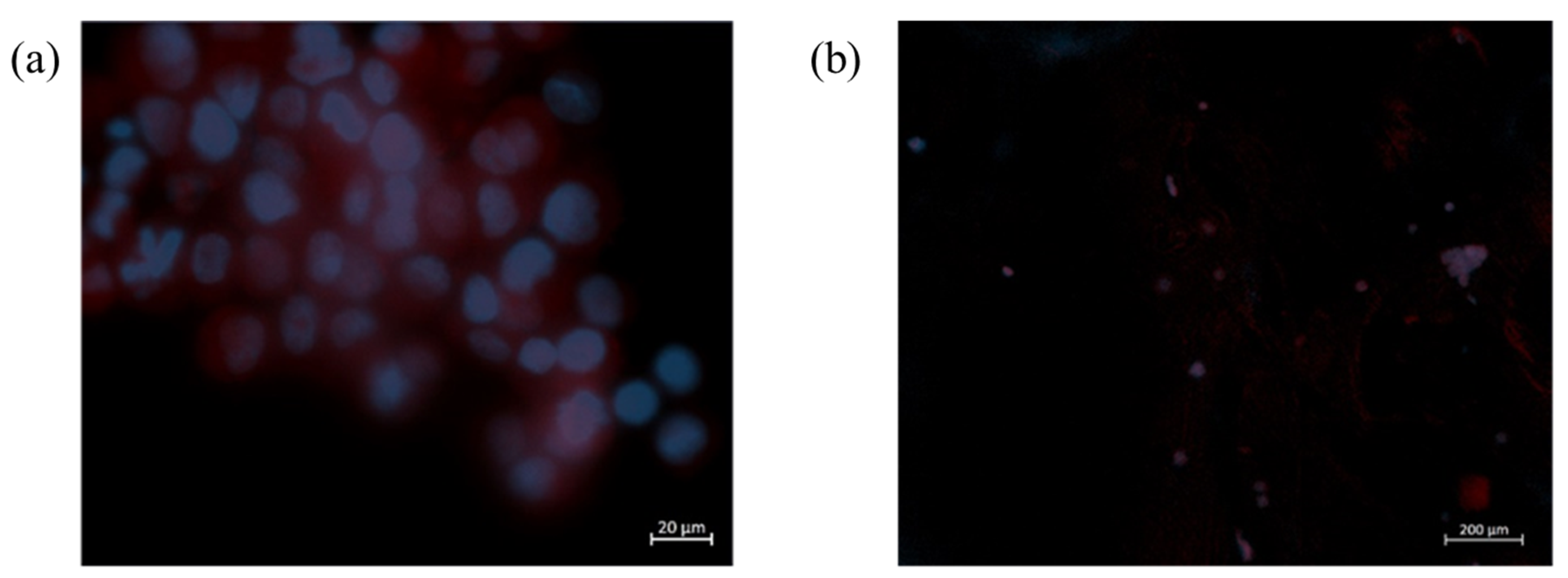
2.5.2. Biocompatibility Assays
3. Materials and Methods
3.1. Preparation of Membranes
3.1.1. Materials
3.1.2. Preparation of Membranes
3.2. Determination of Water Uptake and Degradation
3.3. Controlled Release of LEO
3.4. Morphological Characterization: FTIR-ATR Spectroscopy
3.5. Cell Culture
3.6. Cell Viability Assessment
3.7. Biocompatibility Assays
3.8. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Fiorentini, F.; Suarato, G.; Grisoli, P.; Zych, A.; Bertorelli, R.; Athanassiou, A. Plant-Based Biocomposite Films as Potential Antibacterial Patches for Skin Wound Healing. Eur. Polym. J. 2021, 150, 110414. [Google Scholar] [CrossRef]
- Li, M.; Liang, Y.; Liang, Y.; Pan, G.; Guo, B. Injectable Stretchable Self-Healing Dual Dynamic Network Hydrogel as Adhesive Anti-Oxidant Wound Dressing for Photothermal Clearance of Bacteria and Promoting Wound Healing of MRSA Infected Motion Wounds. Chem. Eng. J. 2022, 427, 132039. [Google Scholar] [CrossRef]
- Samuelson, R.; Lobl, M.; Higgins, S.; Clarey, D.; Wysong, A. The Effects of Lavender Essential Oil on Wound Healing: A Review of the Current Evidence. J. Altern. Complement. Med. 2020, 26, 680–690. [Google Scholar] [CrossRef] [PubMed]
- Syed, M.H.; Zahari, M.A.K.M.; Khan, M.M.R.; Beg, M.D.H.; Abdullah, N. An Overview on Recent Biomedical Applications of Biopolymers: Their Role in Drug Delivery Systems and Comparison of Major Systems. J. Drug Deliv. Sci. Technol. 2023, 80, 104121. [Google Scholar] [CrossRef]
- Duceac, I.A.; Coseri, S. Biopolymers and Their Derivatives: Key Components of Advanced Biomedical Technologies. Biotechnol. Adv. 2022, 61, 108056. [Google Scholar] [CrossRef]
- Skaugrud, O.; Hagen, A.; Borgersen, B.; Dornish, M. Biomedical and Pharmaceutical Applications of Alginate and Chitosan. Biotechnol. Genet. Eng. Rev. 1999, 16, 23–40. [Google Scholar] [CrossRef]
- Zhang, W.; Hou, X.; Wang, H.; Kong, D.; Zhou, Y. Preparation of Chitosan-Sodium Alginate/Bioactive Glass Composite Cartilage Scaffolds with High Cell Activity and Bioactivity. Ceram. Int. 2023, 49, 1987–1996. [Google Scholar] [CrossRef]
- Matica, M.A.; Aachmann, F.L.; Tøndervik, A.; Sletta, H.; Ostafe, V. Chitosan as a Wound Dressing Starting Material: Antimicrobial Properties and Mode of Action. Int. J. Mol. Sci. 2019, 20, 5889. [Google Scholar] [CrossRef]
- Ahmady, A.R.; Razmjooee, K.; Saber-Samandari, S.; Toghraie, D. Fabrication of Chitosan-Gelatin Films Incorporated with Thymol-Loaded Alginate Microparticles for Controlled Drug Delivery, Antibacterial Activity and Wound Healing: In-Vitro and in-Vivo Studies. Int. J. Biol. Macromol. 2022, 223, 567–582. [Google Scholar] [CrossRef]
- Lu, J.; Chen, Y.; Ding, M.; Fan, X.; Hu, J.; Chen, Y.; Li, J.; Li, Z.; Liu, W. A 4arm-PEG Macromolecule Crosslinked Chitosan Hydrogels as Antibacterial Wound Dressing. Carbohydr. Polym. 2022, 277, 118871. [Google Scholar] [CrossRef]
- Ren, W.; Cheng, W.; Wang, G.; Liu, Y. Developments in Antimicrobial Polymers. J. Polym. Sci. Part A Polym. Chem. 2017, 55, 632–639. [Google Scholar] [CrossRef]
- López-Mata, M.A.; Ruiz-Cruz, S.; de Jesús Ornelas-Paz, J.; Del Toro-Sánchez, C.L.; Márquez-Ríos, E.; Silva-Beltrán, N.P.; Cira-Chávez, L.A.; Burruel-Ibarra, S.E. Mechanical, Barrier and Antioxidant Properties of Chitosan Films Incorporating Cinnamaldehyde. J. Polym. Environ. 2018, 26, 452–461. [Google Scholar] [CrossRef]
- Bano, I.; Arshad, M.; Yasin, T.; Ghauri, M.A.; Younus, M. Chitosan: A Potential Biopolymer for Wound Management. Int. J. Biol. Macromol. 2017, 102, 380–383. [Google Scholar] [CrossRef] [PubMed]
- Del Prado-Audelo, M.L.; Caballero-Florán, I.H.; Sharifi-Rad, J.; Mendoza-Muñoz, N.; González-Torres, M.; Urbán-Morlán, Z.; Florán, B.; Cortes, H.; Leyva-Gómez, G. Chitosan-Decorated Nanoparticles for Drug Delivery. J. Drug Deliv. Sci. Technol. 2020, 59, 101896. [Google Scholar] [CrossRef]
- Aderibigbe, B.A.; Buyana, B. Alginate in Wound Dressings. Pharmaceutics 2018, 10, 42. [Google Scholar] [CrossRef]
- Mrudulakumari Vasudevan, U.; Lee, O.K.; Lee, E.Y. Alginate Derived Functional Oligosaccharides: Recent Developments, Barriers, and Future Outlooks. Carbohydr. Polym. 2021, 267, 118158. [Google Scholar] [CrossRef]
- Baysal, K.; Aroguz, A.Z.; Adiguzel, Z.; Baysal, B.M. Chitosan/Alginate Crosslinked Hydrogels: Preparation, Characterization and Application for Cell Growth Purposes. Int. J. Biol. Macromol. 2013, 59, 342–348. [Google Scholar] [CrossRef] [PubMed]
- Tønnesen, H.H.; Karlsen, J. Alginate in Drug Delivery Systems. Drug Dev. Ind. Pharm. 2002, 28, 621–630. [Google Scholar] [CrossRef]
- Zhou, H.; Yang, N.; Hou, J.; Yu, C.; Jin, Z.; Zeng, P.; Yang, L.; Fu, Y.; Shen, Y.; Guo, S. Effects of CaCl2, HCl, Acetic Acid or Citric Acid on Dynamic Mechanical Performances and Physicochemical Properties of Sodium Alginate Edible Films. Food Packag. Shelf Life 2022, 34, 100935. [Google Scholar] [CrossRef]
- Manuja, A.; Kumar, B.; Athira, S.; Sarkar, P.; Riyesh, T.; Kumar, N.; Tripathi, B.N.; Mann, B. Zinc Oxide Nanoparticles Encapsulated in Polysaccharides Alginate/Gum Acacia and Iron Oxide Nanomatrices Show Enhanced Biocompatibility and Permeability to Intestinal Barrier. Food Hydrocoll. Heal. 2022, 2, 100050. [Google Scholar] [CrossRef]
- Zhuang, H.; Shao, J.; Wu, P.; Yu, G.; Fu, K.; Sun, Z.; Cao, M.; Liu, Y.; Zhou, Y. Nitric Oxide Releasing Alginate Microspheres for Antimicrobial Application. Int. J. Biol. Macromol. 2023, 224, 1244–1251. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, A.P.; Sanchez, E.M.S.; Da Costa, A.C.; Moraes, A.M. The Influence of Preparation Conditions on the Characteristics of Chitosan-Alginate Dressings for Skin Lesions. J. Appl. Polym. Sci. 2008, 109, 2703–2710. [Google Scholar] [CrossRef]
- Caetano, G.F.; Frade, M.A.C.; Andrade, T.A.M.; Leite, M.N.; Bueno, C.Z.; Moraes, Â.M.; Ribeiro-Paes, J.T. Chitosan-Alginate Membranes Accelerate Wound Healing. J. Biomed. Mater. Res. Part B Appl. Biomater. 2015, 103, 1013–1022. [Google Scholar] [CrossRef]
- Istiqomah, A.; Utami, M.R.; Firdaus, M.; Suryanti, V.; Kusumaningsih, T. Antibacterial Chitosan-Dioscorea Alata Starch Film Enriched with Essential Oils Optimally Prepared by Following Response Surface Methodology. Food Biosci. 2022, 46, 101603. [Google Scholar] [CrossRef]
- Jovanović, J.; Ćirković, J.; Radojković, A.; Mutavdžić, D.; Tanasijević, G.; Joksimović, K.; Bakić, G.; Branković, G.; Branković, Z. Chitosan and Pectin-Based Films and Coatings with Active Components for Application in Antimicrobial Food Packaging. Prog. Org. Coat. 2021, 158, 106349. [Google Scholar] [CrossRef]
- Turasan, H.; Sahin, S.; Sumnu, G. Encapsulation of Rosemary Essential Oil. LWT—Food Sci. Technol. 2015, 64, 112–119. [Google Scholar] [CrossRef]
- Benavides, S.; Villalobos-Carvajal, R.; Reyes, J.E. Physical, Mechanical and Antibacterial Properties of Alginate Film: Effect of the Crosslinking Degree and Oregano Essential Oil Concentration. J. Food Eng. 2012, 110, 232–239. [Google Scholar] [CrossRef]
- Bagher, Z.; Ehterami, A.; Safdel, M.H.; Khastar, H.; Semiari, H.; Asefnejad, A.; Davachi, S.M.; Mirzaii, M.; Salehi, M. Wound Healing with Alginate/Chitosan Hydrogel Containing Hesperidin in Rat Model. J. Drug Deliv. Sci. Technol. 2020, 55, 101379. [Google Scholar] [CrossRef]
- Edris, A. Pharmaceutical and Therapeutic Potentials of Essential Oils and Their Individual Volatile Constituents: A Review. Phytother. Res. 2007, 21, 308–323. [Google Scholar] [CrossRef]
- Peana, A.T.; D’Aquila, P.S.; Panin, F.; Serra, G.; Pippia, P.; Moretti, M.D.L. Anti-Inflammatory Activity of Linalool and Linalyl Acetate Constituents of Essential Oils. Phytomedicine 2002, 9, 721–726. [Google Scholar] [CrossRef] [PubMed]
- Cruz Sánchez, E.; García-Vargas, J.M.; Gracia, I.; Rodríguez, J.F.; García, M.T. Pilot-Plant-Scale Extraction of Antioxidant Compounds from Lavender: Experimental Data and Methodology for an Economic Assessment. Processes 2022, 10, 2708. [Google Scholar] [CrossRef]
- Mori, H.-M.; Kawanami, H.; Kawahata, H.; Aoki, M. Wound Healing Potential of Lavender Oil by Acceleration of Granulation and Wound Contraction through Induction of TGF-β in a Rat Model. BMC Complement. Altern. Med. 2016, 16, 144. [Google Scholar] [CrossRef]
- Cavanagh, H.M.A.; Wilkinson, J.M. Lavender Essential Oil: A Review. Aust. Infect. Control 2005, 10, 35–37. [Google Scholar] [CrossRef]
- Ma, S.; Zheng, Y.; Zhou, R.; Ma, M. Characterization of Chitosan Films Incorporated with Different Substances of Konjac Glucomannan, Cassava Starch, Maltodextrin and Gelatin, and Application in Mongolian Cheese Packaging. Coatings 2021, 11, 84. [Google Scholar] [CrossRef]
- Mutlu, B.; Erci, F.; Çakir Koç, R. Production of Alginate Films Containing Hypericum Perforatum Extract as an Antibacterial and Antioxidant Wound Dressing Material. J. Bioact. Compat. Polym. 2022, 37, 134–148. [Google Scholar] [CrossRef]
- Othman, F. Preparation and Characterization of Sodium Alginate-Based Edible Film with Antibacterial Additive Using Lemongrass Oil (Penyediaan Dan Pencirian Filem Boleh Dimakan Berasaskan Natrium Alginat Dengan Bahan Tambah Antibakteria Menggunakan Minyak Serai). Sains Malays. 2022, 51, 485–494. [Google Scholar] [CrossRef]
- Sofi, H.S.; Akram, T.; Tamboli, A.H.; Majeed, A.; Shabir, N.; Sheikh, F.A. Novel Lavender Oil and Silver Nanoparticles Simultaneously Loaded onto Polyurethane Nanofibers for Wound-Healing Applications. Int. J. Pharm. 2019, 569, 118590. [Google Scholar] [CrossRef]
- Yan, H.; Feng, Y.; Hu, W.; Cheng, C.; Liu, R.; Wang, C.; Li, J.; Lin, Q. Preparation and Evaluation of Alginate-Chitosan-Bentonite Based Beads for the Delivery of Pesticides in Controlled-Release Formulation. Asian J. Chem. 2013, 25, 9936–9940. [Google Scholar] [CrossRef]
- Silva, J.M.; Duarte, A.R.C.; Caridade, S.G.; Picart, C.; Reis, R.L.; Mano, J.F. Tailored Freestanding Multilayered Membranes Based on Chitosan and Alginate. Biomacromolecules 2014, 15, 3817–3826. [Google Scholar] [CrossRef]
- Silva, J.M.; Rodrigues, L.C.; Silva, S.S.; Reis, R.L.; Duarte, A.R.C. Engineered Tubular Structures Based on Chitosan for Tissue Engineering Applications. J. Biomater. Appl. 2018, 32, 841–852. [Google Scholar] [CrossRef]
- EĞRİ, Ö. Production of Lavender Oil Loaded Antibacterial Polymeric Membranes. Cumhur. Sci. J. 2020, 41, 160–168. [Google Scholar] [CrossRef]
- Danila, A.; Muresan, E.I.; Ibanescu, S.-A.; Popescu, A.; Danu, M.; Zaharia, C.; Türkoğlu, G.C.; Erkan, G.; Staras, A.-I. Preparation, Characterization, and Application of Polysaccharide-Based Emulsions Incorporated with Lavender Essential Oil for Skin-Friendly Cellulosic Support. Int. J. Biol. Macromol. 2021, 191, 405–413. [Google Scholar] [CrossRef] [PubMed]
- Mahmud, M.; Rahman, A.F.M.M.; Salem, K.; Bari, L.; Qiu, H. Architecting Ultrathin Graphitic C3N4 Nanosheets Incorporated PVA/ Gelatin Bionanocomposite for Potential Biomedical Application: Effect on Drug Delivery, Release Kinetics, and Antibacterial Activity. ACS Appl. Bio Mater. 2022, 5, 5126–5139. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez-Jara, C.; Bilbao-Sainz, C.; McHugh, T.; Chiou, B.; Williams, T.; Villalobos-Carvajal, R. Physical, Mechanical and Transport Properties of Emulsified Films Based on Alginate with Soybean Oil: Effects of Soybean Oil Concentration, Number of Passes and Degree of Surface Crosslinking. Food Hydrocoll. 2020, 109, 106133. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cruz Sánchez, E.; García, M.T.; Pereira, J.; Oliveira, F.; Craveiro, R.; Paiva, A.; Gracia, I.; García-Vargas, J.M.; Duarte, A.R.C. Alginate–Chitosan Membranes for the Encapsulation of Lavender Essential Oil and Development of Biomedical Applications Related to Wound Healing. Molecules 2023, 28, 3689. https://doi.org/10.3390/molecules28093689
Cruz Sánchez E, García MT, Pereira J, Oliveira F, Craveiro R, Paiva A, Gracia I, García-Vargas JM, Duarte ARC. Alginate–Chitosan Membranes for the Encapsulation of Lavender Essential Oil and Development of Biomedical Applications Related to Wound Healing. Molecules. 2023; 28(9):3689. https://doi.org/10.3390/molecules28093689
Chicago/Turabian StyleCruz Sánchez, Encarnación, María Teresa García, Joana Pereira, Filipe Oliveira, Rita Craveiro, Alexandre Paiva, Ignacio Gracia, Jesús Manuel García-Vargas, and Ana Rita C. Duarte. 2023. "Alginate–Chitosan Membranes for the Encapsulation of Lavender Essential Oil and Development of Biomedical Applications Related to Wound Healing" Molecules 28, no. 9: 3689. https://doi.org/10.3390/molecules28093689
APA StyleCruz Sánchez, E., García, M. T., Pereira, J., Oliveira, F., Craveiro, R., Paiva, A., Gracia, I., García-Vargas, J. M., & Duarte, A. R. C. (2023). Alginate–Chitosan Membranes for the Encapsulation of Lavender Essential Oil and Development of Biomedical Applications Related to Wound Healing. Molecules, 28(9), 3689. https://doi.org/10.3390/molecules28093689






