Biological Activities of Sargassum Algae Mediated ZnO and Co Doped ZnO Nanoparticles as Enhanced Antioxidant and Anti-Diabetic Agents
Abstract
1. Introduction
2. Results and Discussion
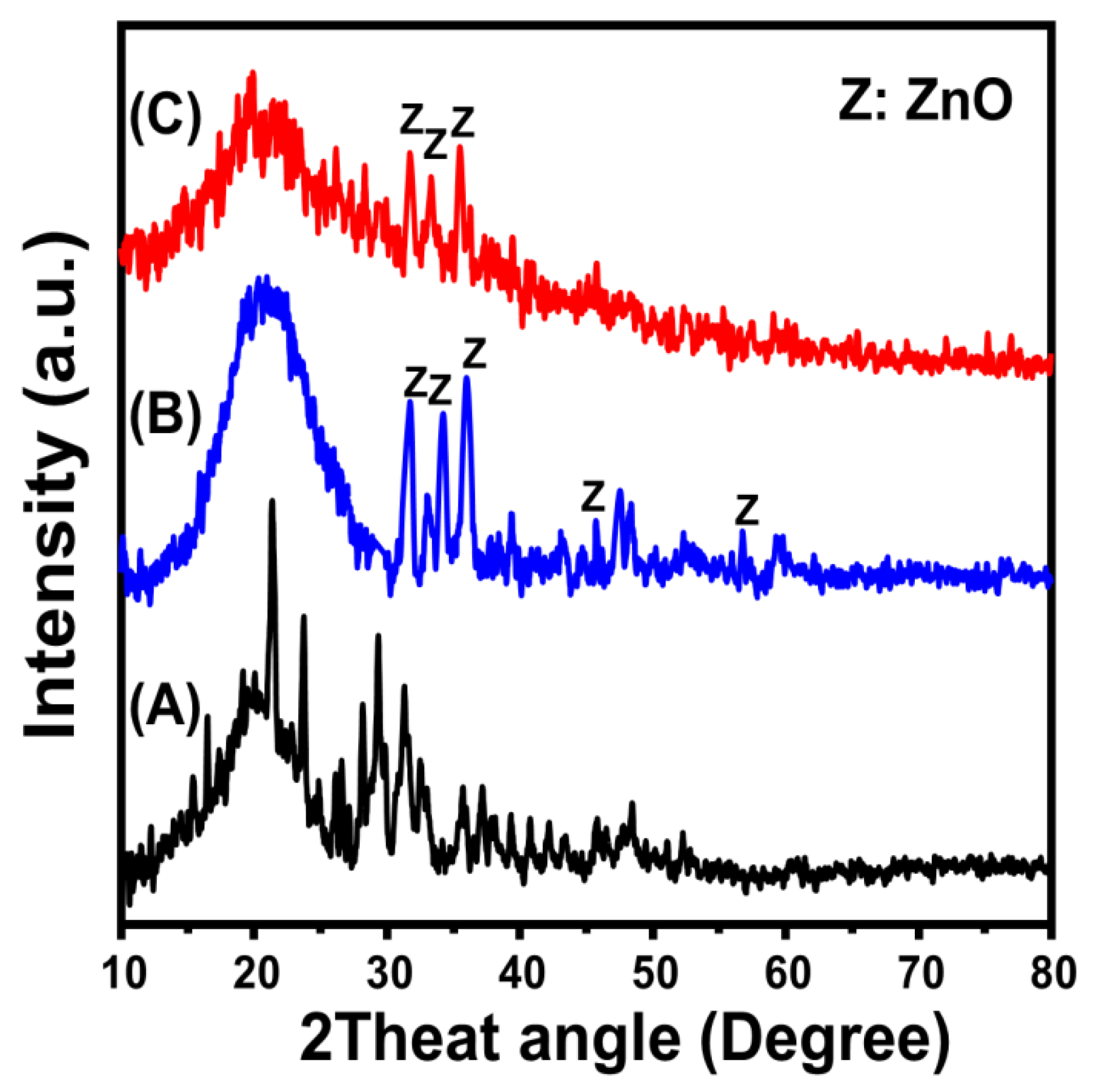
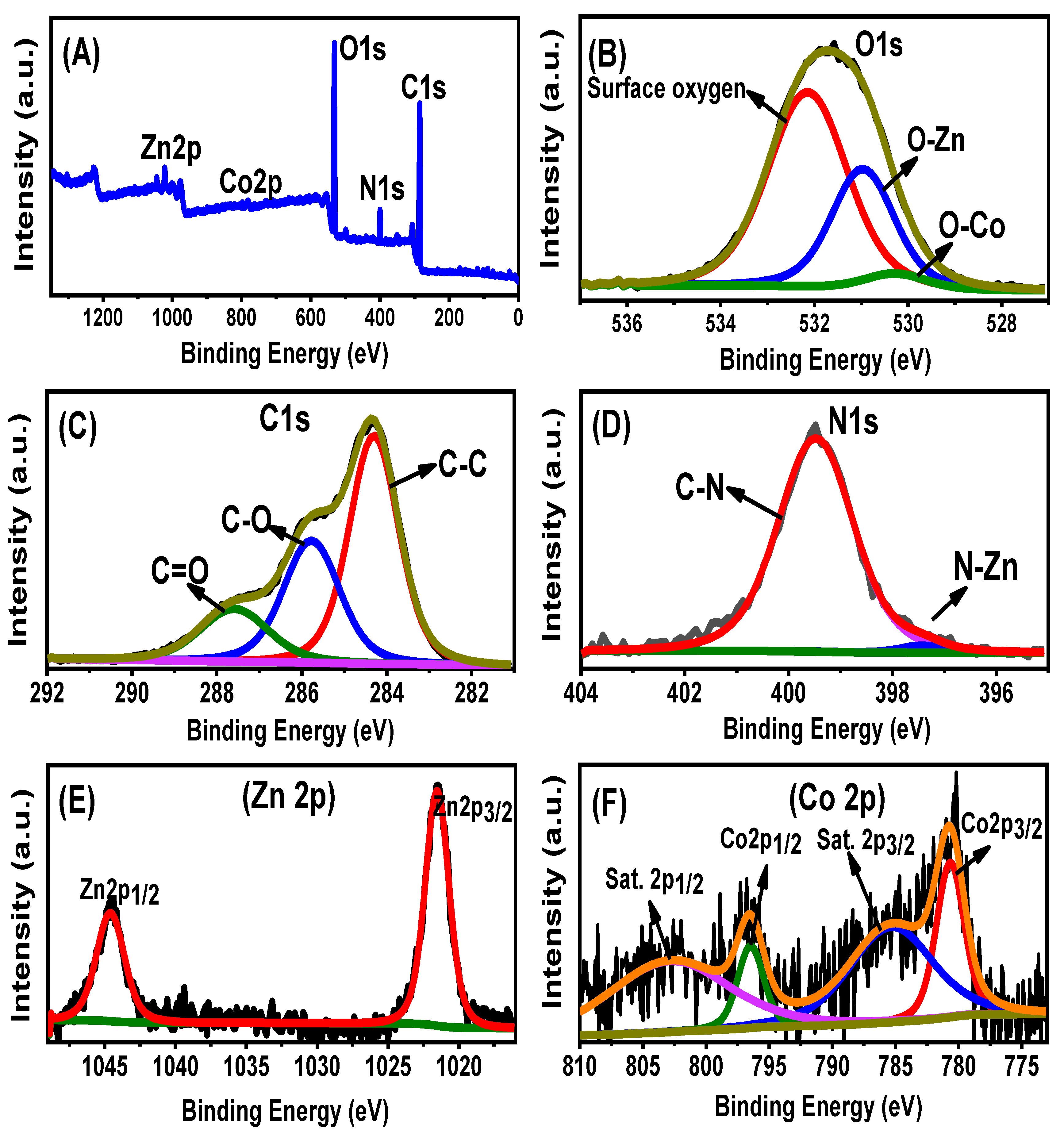
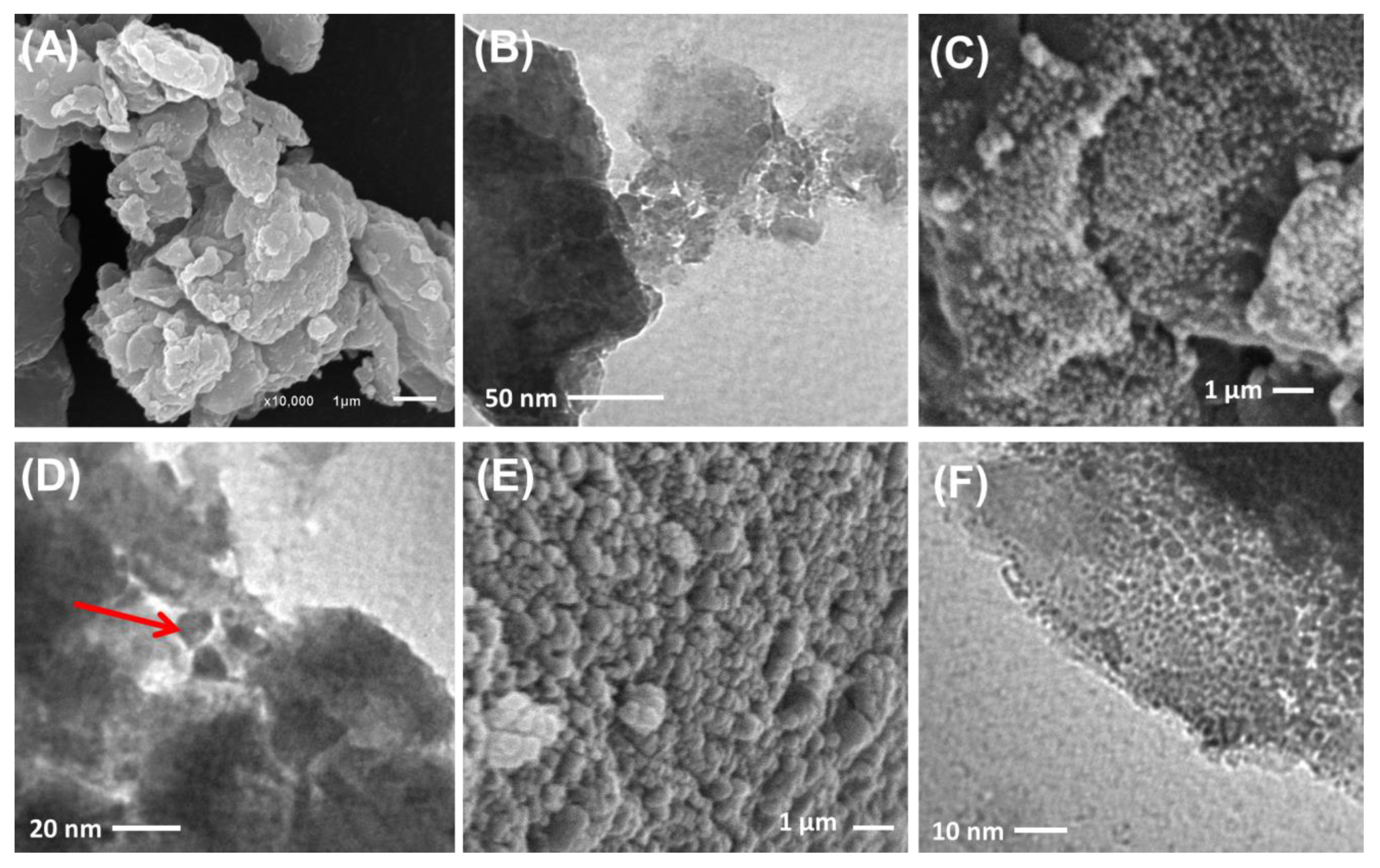
2.1. Characterization of the Synthetic Structures
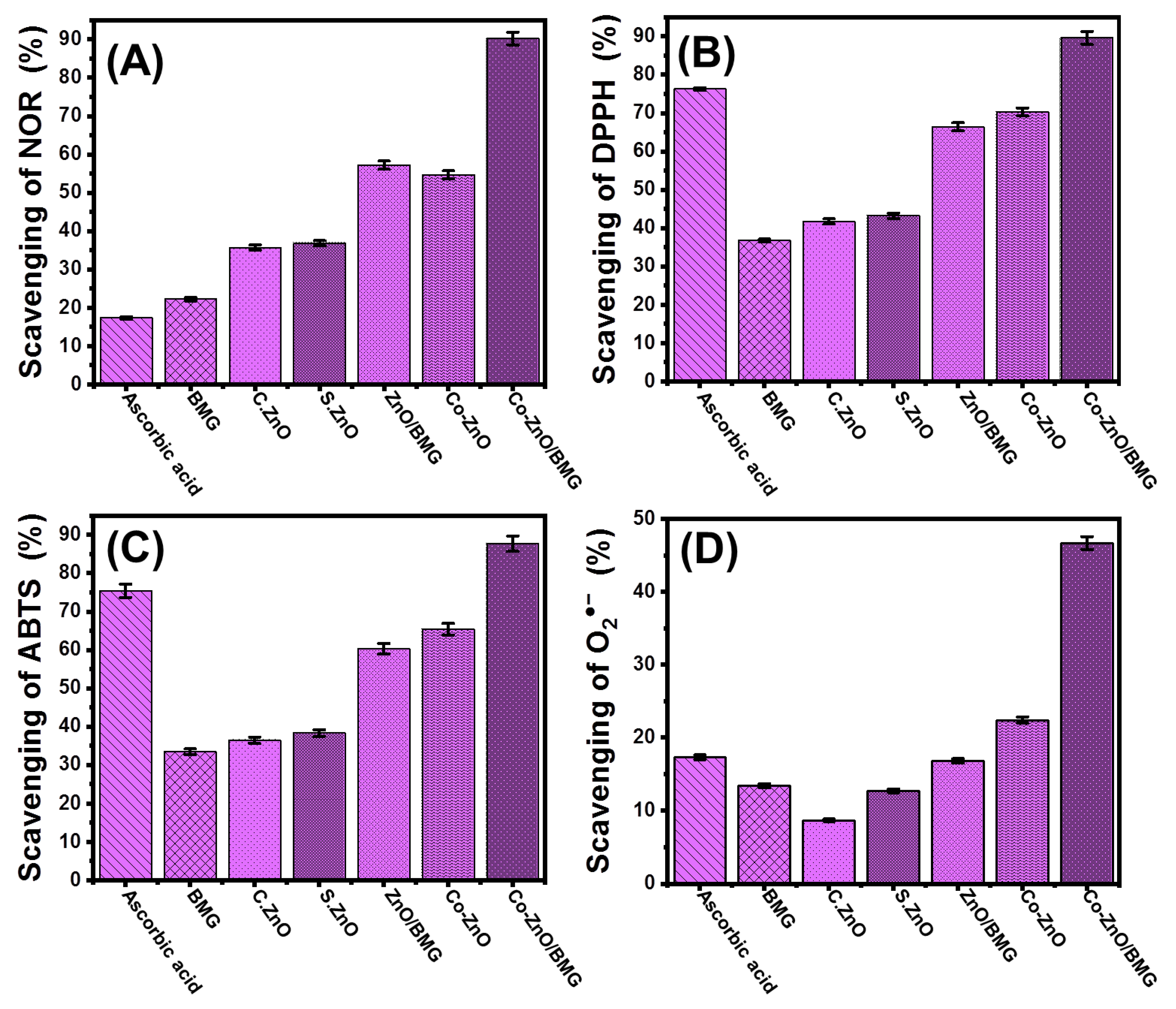
2.2. Antioxidant Properties
2.2.1. Nitric Oxide Scavenging
2.2.2. DPPH Radical Scavenging
2.2.3. ABTS Radical Scavenging
2.2.4. Superoxide Radical Scavenging
2.3. Anti-Diabetic Properties
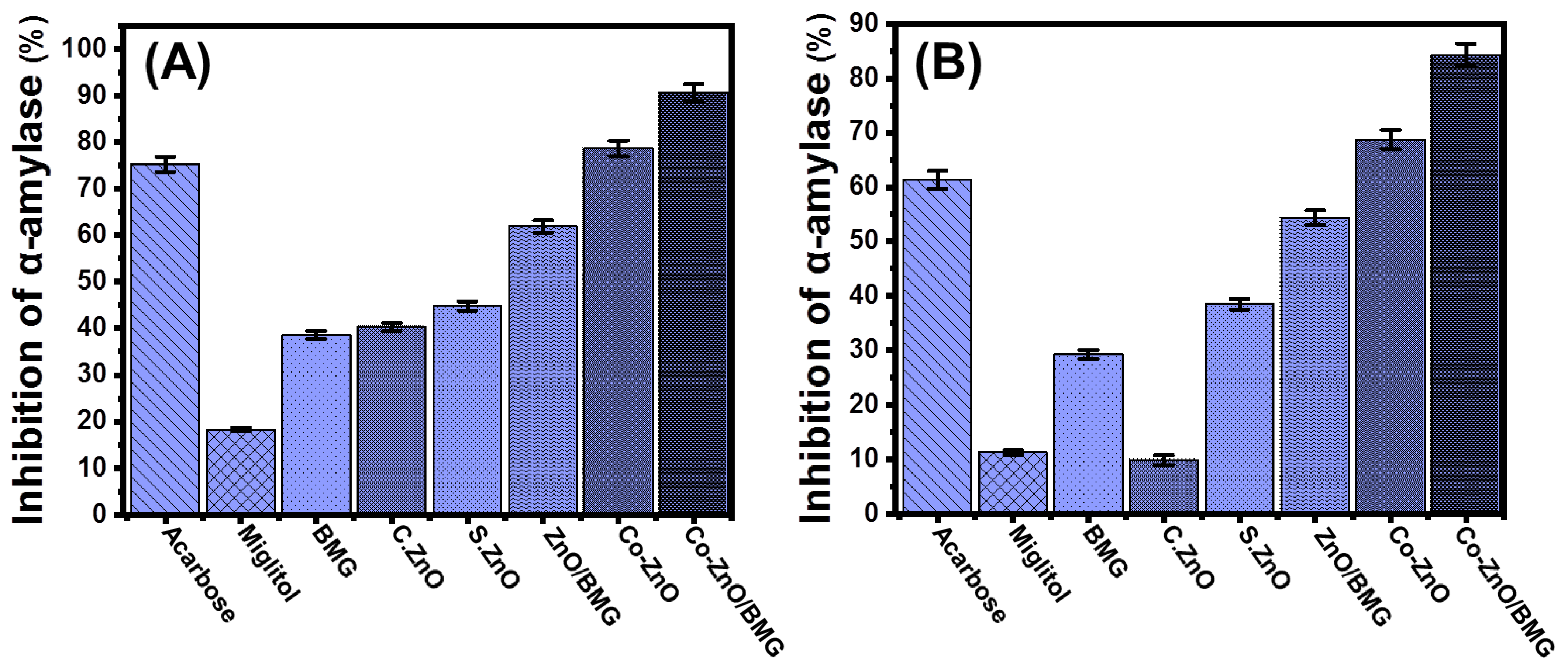
2.3.1. Porcine Pancreatic α-Amylase Inhibition Assay
2.3.2. Murine Pancreatic α-Amylase Inhibition
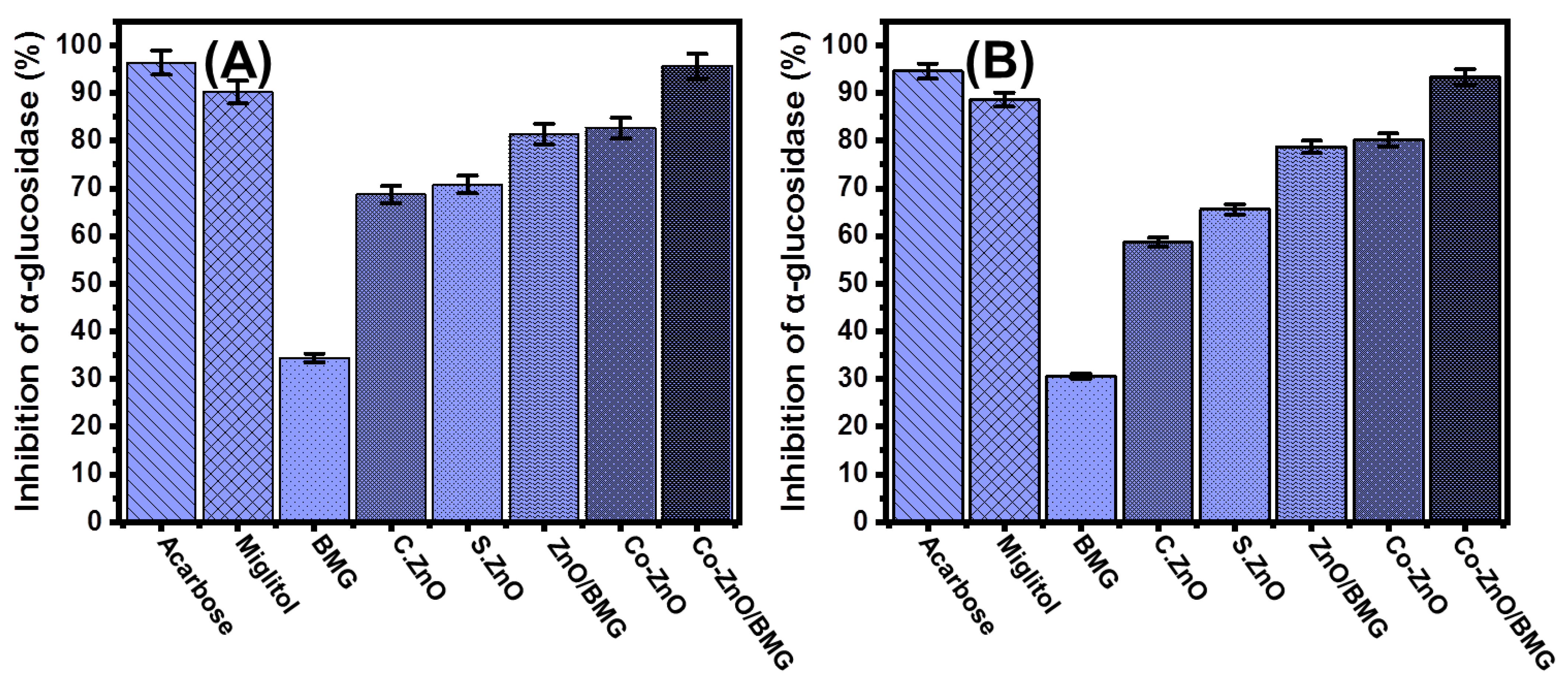
2.3.3. Pancreatic α-Glucosidase Inhibition
2.3.4. Murine Intestinal α-Glucosidase Inhibition
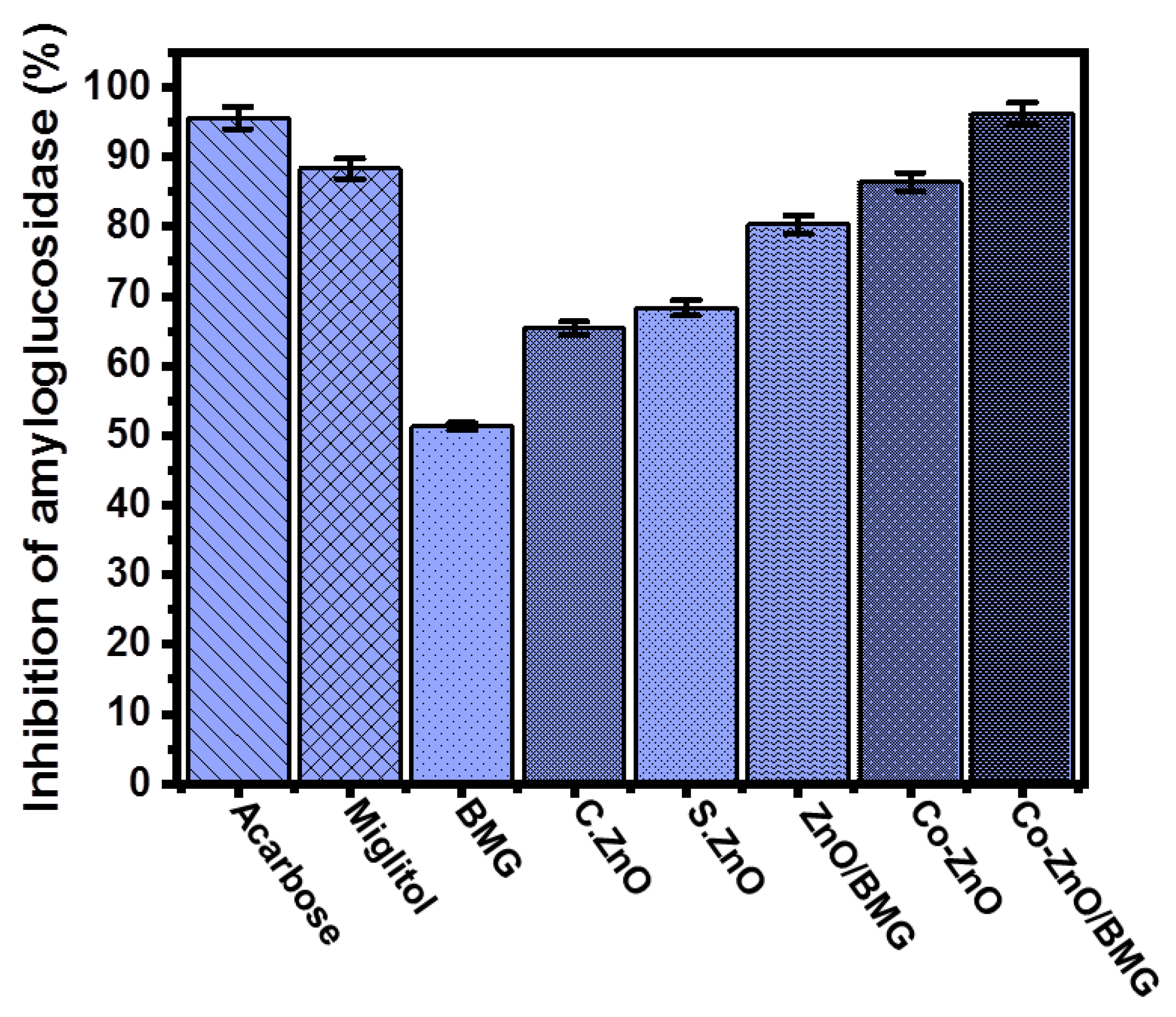
2.3.5. Amyloglucosidase Inhibition
3. Materials and Methods
3.1. Materials and Chemicals
3.2. Synthesis of the Tested Structures
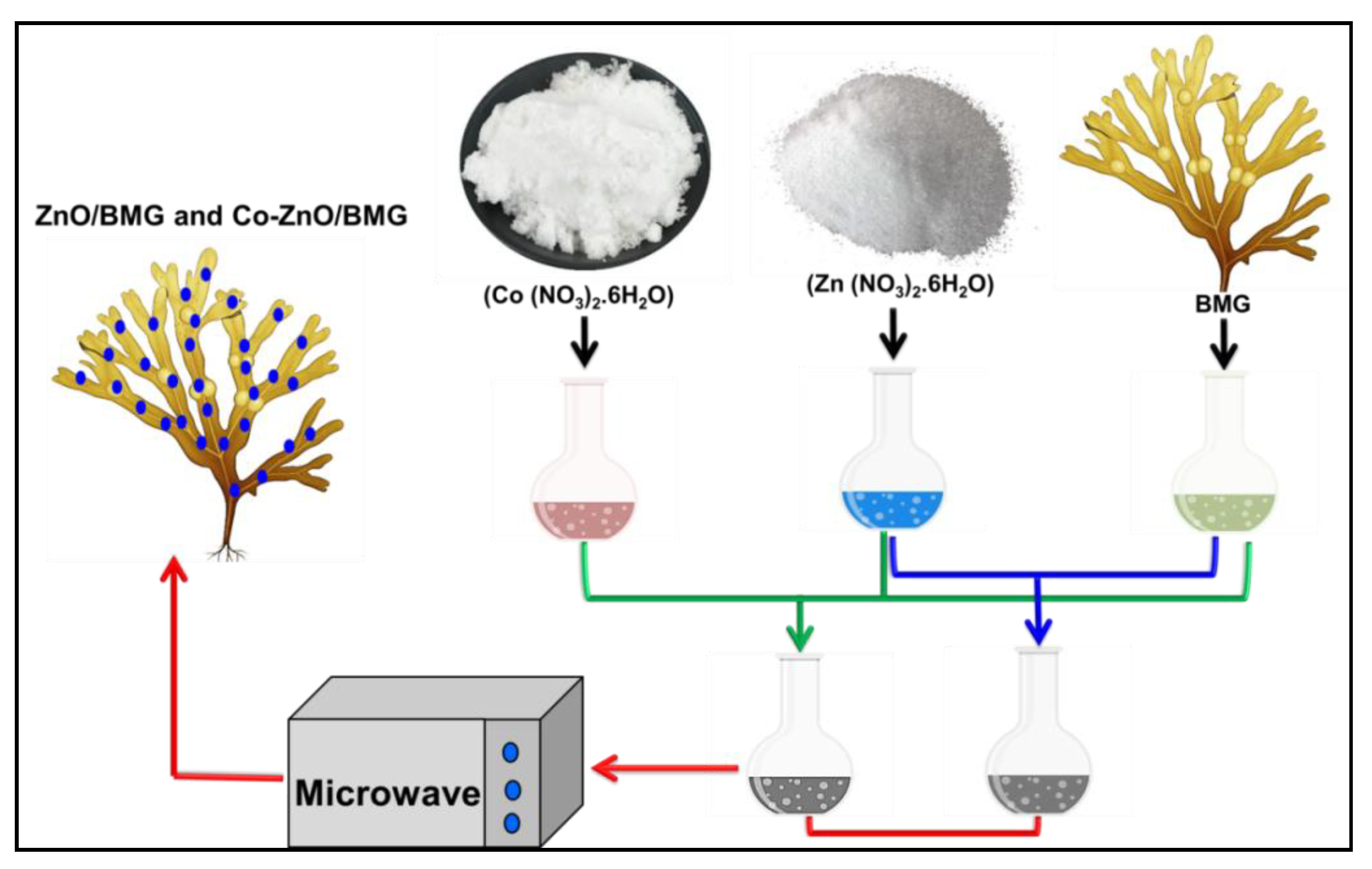
3.2.1. Preparation of Incorporated Brown Macroalgae (BMG)
3.2.2. Synthesis of ZnO and Co-ZnO Composites with BMG (ZnO/BMG and Co-ZnO/BMG)
3.3. Characterization Techniques
3.4. Antioxidant Studies
3.4.1. Scavenging of Nitric Oxide Radical (NOR)
3.4.2. Scavenging of the DPPH Radical
3.4.3. Scavenging of ABTS Radical
3.4.4. Scavenging of Superoxide Radical
3.5. Anti-Diabetic Studies
3.5.1. Inhibition Assay of Porcine Pancreatic α-Amylase
3.5.2. Inhibition Assay of Crude Murine Pancreatic α-Amylase
3.5.3. Inhibition Assay of α-Glucosidase
3.5.4. Inhibition Assay of Crude Murine Intestinal α-Glucosidase
3.5.5. Amyloglucosidase Inhibition Assay
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
Recommendation
References
- Arvanag, F.M.; Bayrami, A.; Habibi-Yangjeh, A.; Pouran, S.R. A comprehensive study on antidiabetic and antibacterial activities of ZnO nanoparticles biosynthesized using Silybum marianum L. seed extract. Mater. Sci. Eng. C 2019, 97, 397–405. [Google Scholar] [CrossRef] [PubMed]
- Behl, T.; Kaur, I.; Sehgal, A.; Sharma, E.; Kumar, A.; Grover, M.; Bungau, S. Unfolding Nrf2 in diabetes mellitus. Mol. Biol. Rep. 2021, 48, 927–939. [Google Scholar] [CrossRef] [PubMed]
- Billacura, M.P.; Lavilla, C.J.; Cripps, M.J.; Hanna, K.; Sale, C.; Turner, M.D. β-alanine scavenging of free radicals protects mitochondrial function and enhances both insulin secretion and glucose uptake in cells under metabolic stress. Adv. Redox Res. 2022, 6, 100050. [Google Scholar] [CrossRef]
- Hsu, W.H.; Chang, H.M.; Lee, Y.L.; Prasannan, A.; Hu, C.C.; Wang, J.S.; Lai, J.Y.; Yang, J.M.; Jebaranjitham, N.; Tsai, H.C. Biode-gradable polymer-nanoclay composites as intestinal sleeve implants installed in digestive tract for obesity and type 2 diabetes treatment. Mater. Sci. Eng. C 2020, 110, 110676. [Google Scholar] [CrossRef]
- Sagandira, C.R.; Khasipo, A.Z.; Sagandira, M.B.; Watts, P. An overview of the synthetic routes to essential oral anti-diabetes drugs. Tetrahedron 2021, 96, 132378. [Google Scholar] [CrossRef]
- Robkhob, P.; Ghosh, S.; Bellare, J.; Jamdade, D.; Tang, I.-M.; Thongmee, S. Effect of silver doping on antidiabetic and antioxidant potential of ZnO nanorods. J. Trace Elem. Med. Biol. 2020, 58, 126448. [Google Scholar] [CrossRef]
- Paul, S.; Hajra, D. Study of glucose uptake enhancing potential of fenugreek (Trigonella foenum graecum) leaves extract on 3T3 L1 cells line and evaluation of its antioxidant potential. Pharmacogn. Res. 2018, 10, 347. [Google Scholar] [CrossRef]
- Dedvisitsakul, P.; Watla-Iad, K. Antioxidant activity and antidiabetic activities of Northern Thai indigenous edible plant extracts and their phytochemical constituents. Heliyon 2022, 8, 10740. [Google Scholar] [CrossRef]
- Feldman, E.L.; Callaghan, B.C.; Pop-Busui, R.; Zochodne, D.W.; Wright, D.E.; Bennett, D.L.; Bril, V.; Russell, J.W.; Viswanathan, V. Diabetic neuropathy. Nat. Rev. Dis. Primer 2019, 5, 1–18. [Google Scholar] [CrossRef]
- Kim, M.-S.; Jung, Y.S.; Jang, D.; Cho, C.H.; Lee, S.-H.; Han, N.S.; Kim, D.-O. Antioxidant capacity of 12 major soybean isoflavones and their bioavailability under simulated digestion and in human intestinal Caco-2 cells. Food Chem. 2022, 374, 131493. [Google Scholar] [CrossRef] [PubMed]
- Asmat, U.; Abad, K.; Ismail, K. Diabetes mellitus and oxidative stress—A concise review. Saudi Pharm. J. 2016, 24, 547–553. [Google Scholar] [CrossRef] [PubMed]
- Ben Ahmed, Z.; Hefied, F.; Yousfi, M.; Demeyer, K.; Heyden, Y.V. Study of the antioxidant activity of Pistacia atlantica Desf. Gall extracts and evaluation of the responsible compounds. Biochem. Syst. Ecol. 2022, 100, 104358. [Google Scholar] [CrossRef]
- Yilmazer-Musa, M.; Griffith, A.M.; Michels, A.J.; Schneider, E.; Frei, B. Grape Seed and Tea Extracts and Catechin 3-Gallates Are Potent Inhibitors of α-Amylase and α-Glucosidase Activity. J. Agric. Food Chem. 2012, 60, 8924–8929. [Google Scholar] [CrossRef]
- Malik, A.R.; Sharif, S.; Shaheen, F.; Khalid, M.; Iqbal, Y.; Faisal, A.; Aziz, M.H.; Atif, M.; Ahmad, S.; Fakhar-e-Alam, M.; et al. Green synthesis of RGO-ZnO mediated Ocimum basilicum leaves extract nanocomposite for antioxidant, antibacterial, anti-diabetic and photocatalytic activity. J. Saudi Chem. Soc. 2022, 26, 101438. [Google Scholar] [CrossRef]
- Singh, T.A.; Sharma, A.; Tejwan, N.; Ghosh, N.; Das, J.; Sil, P.C. A state of the art review on the synthesis, antibacterial, antioxidant, antidiabetic and tissue regeneration activities of zinc oxide nanoparticles. Adv. Colloid Interface Sci. 2021, 295, 102495. [Google Scholar] [CrossRef] [PubMed]
- Sarani, M.; Tosan, F.; Hasani, S.A.; Barani, M.; Adeli-Sardou, M.; Khosravani, M.; Niknam, S.; Kouhbanani, M.A.J.; Beheshtkhoo, N. Study of in vitro cytotoxic performance of biosynthesized α-Bi2O3 NPs, Mn-doped and Zn-doped Bi2O3 NPs against MCF-7 and HUVEC cell lines. J. Mater. Res. Technol. 2022, 19, 140–150. [Google Scholar] [CrossRef]
- Hamidian, K.; Sarani, M.; Barani, M.; Khakbaz, F. Cytotoxic performance of green synthesized Ag and Mg dual doped ZnO NPs using Salvadora persica extract against MDA-MB-231 and MCF-10 cells. Arab. J. Chem. 2022, 15, 103792. [Google Scholar] [CrossRef]
- Madan, H.; Sharma, S.; Udayabhanu; Suresh, D.; Vidya, Y.; Nagabhushana, H.; Rajanaik, H.; Anantharaju, K.; Prashantha, S.; Maiya, P.S. Facile green fabrication of nanostructure ZnO plates, bullets, flower, prismatic tip, closed pine cone: Their antibacterial, antioxidant, photoluminescent and photocatalytic properties. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2016, 152, 404–416. [Google Scholar] [CrossRef]
- Ansari, A.; Ali, A.; Khan, N.; Umar, M.S.; Owais, M.; Shamsuzzaman. Synthesis of steroidal dihydropyrazole derivatives using green ZnO NPs and evaluation of their anticancer and antioxidant activity. Steroids 2022, 188, 109113. [Google Scholar] [CrossRef]
- Sharma, A.; Nagraik, R.; Sharma, S.; Sharma, G.; Pandey, S.; Azizov, S.; Chauhan, P.K.; Kumar, D. Green synthesis of ZnO na-noparticles using Ficus palmata: Antioxidant, antibacterial and antidiabetic studies. Results Chem. 2022, 4, 100509. [Google Scholar] [CrossRef]
- Saad, A.M.; Abukhadra, M.R.; Ahmed, S.A.-K.; Elzanaty, A.M.; Mady, A.H.; Betiha, M.A.; Shim, J.-J.; Rabie, A.M. Photocatalytic degradation of malachite green dye using chitosan supported ZnO and Ce–ZnO nano-flowers under visible light. J. Environ. Manag. 2020, 258, 110043. [Google Scholar] [CrossRef] [PubMed]
- Yusof, N.A.A.; Zain, N.M.; Pauzi, N. Synthesis of ZnO nanoparticles with chitosan as stabilizing agent and their antibacterial properties against Gram-positive and Gram-negative bacteria. Int. J. Biol. Macromol. 2018, 124, 1132–1136. [Google Scholar] [CrossRef] [PubMed]
- Shaaban, S.; Adam, M.S.S.; El-Metwaly, N.M. Novel organoselenium-based N-mealanilic acid and its zinc (II) chelate: Catalytic, anticancer, antimicrobial, antioxidant, and computational assessments. J. Mol. Liq. 2022, 363, 119907. [Google Scholar] [CrossRef]
- Meer, B.; Andleeb, A.; Iqbal, J.; Ashraf, H.; Meer, K.; Ali, J.S.; Drouet, S.; Anjum, S.; Mehmood, A.; Khan, T.; et al. Bio-Assisted Synthesis and Characterization of Zinc Oxide Nanoparticles from Lepidium sativum and Their Potent Antioxidant, Antibacterial and Anticancer Activities. Biomolecules 2022, 12, 855. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, T.; Azhar, S.; Zafar, M.; Kiran, H.; Kebaili, I.; Alrobei, H. Synthesis and characterization of Ag-ZnO nano-composites for investigation of variations in the germination of peanut and kidney beans. Appl. Nanosci. 2021, 11, 2767–2777. [Google Scholar] [CrossRef]
- Rabie, A.M.; Abukhadra, M.R.; Rady, A.M.; Ahmed, S.A.; Labena, A.; Mohamed, H.S.H.; Betiha, M.A.; Shim, J.-J. Instantaneous photocatalytic degradation of malachite green dye under visible light using novel green Co–ZnO/algae composites. Res. Chem. Intermed. 2020, 46, 1955–1973. [Google Scholar] [CrossRef]
- Atugoda, T.; Gunawardane, C.; Ahmad, M.; Vithanage, M. Mechanistic interaction of ciprofloxacin on zeolite modified seaweed (Sargassum crassifolium) derived biochar: Kinetics, isotherm and thermodynamics. Chemosphere 2021, 281, 130676. [Google Scholar] [CrossRef]
- Alreshidi, M.; Badraoui, R.; Adnan, M.; Patel, M.; Alotaibi, A.; Saeed, M.; Ghandourah, M.; Al-Motair, K.A.; Arif, I.A.; Albulaihed, Y.; et al. Phytochemical profiling, antibacterial, and antibiofilm activities of Sargassum sp. (brown algae) from the Red Sea: ADMET prediction and molecular docking analysis. Algal Res. 2023, 69, 102912. [Google Scholar] [CrossRef]
- El Shafay, S.M.; Ali, S.S.; El-Sheekh, M. Antimicrobial activity of some seaweeds species from Red sea, against multidrug resistant bacteria. Egypt. J. Aquat. Res. 2016, 42, 65–74. [Google Scholar] [CrossRef]
- Khairy, H.M.; El-Sheikh, M.A. Antioxidant activity and mineral composition of three Mediterranean common seaweeds from Abu-Qir Bay, Egypt. Saudi J. Biol. Sci. 2015, 22, 623–630. [Google Scholar] [CrossRef]
- Imchen, T.; Sing, K.S. Marine algae colorants: Antioxidant, anti-diabetic properties and applications in food industry. Algal Res. 2023, 69, 102898. [Google Scholar] [CrossRef]
- Johnson, M.; Kanimozhi, S.A.; Malar, T.R.J.J.; Shibila, T.; Freitas, P.; Tintino, S.; Menezes, I.; da Costa, J.; Coutinho, H. The antioxidative effects of bioactive products from Sargassum polycystum C. Agardh and Sargassum duplicatum J. Agardh against inflammation and other pathological issues. Complement. Ther. Med. 2019, 46, 19–23. [Google Scholar] [CrossRef]
- Labhane, P.K.; Sonawane, G.H.; Sonawane, S.H. Influence of rare-earth metal on the zinc oxide nanostructures: Application in the photocatalytic degradation of methylene blue and p-nitro phenol. Green Process. Synth. 2017, 7, 360–371. [Google Scholar] [CrossRef]
- Chang, C.-J.; Lin, C.-Y.; Chen, J.-K.; Hsu, M.-H. Ce-doped ZnO nanorods based low operation temperature NO2 gas sensors. Ceram. Int. 2014, 40, 10867–10875. [Google Scholar] [CrossRef]
- Moubayed, N.M.; Al Houri, H.J.; Al Khulaif, M.M.; Al Farraj, D.A. Antimicrobial, antioxidant properties and chemical composition of seaweeds collected from Saudi Arabia (Red Sea and Arabian Gulf). Saudi J. Biol. Sci. 2017, 24, 162–169. [Google Scholar] [CrossRef]
- Do Nascimento, W.J., Jr.; da Silva, M.G.C.; Vieira, M.G.A. Competitive biosorption of Cu2+ and Ag+ ions on brown macro-algae waste: Kinetic and ion-exchange studies. Environ. Sci. Pollut. Res. 2019, 26, 23416–23428. [Google Scholar] [CrossRef] [PubMed]
- Biswas, B.; Arun, A.; Bisht, Y.; Singg, R.; Kumar, J.; Bhaskar, T. Bioresource technology effects of temperature and solvent on hydrothermal liquefaction of Sargassum tenerrimum algae. Bioresour. Technol. 2017, 242, 344–350. [Google Scholar] [CrossRef] [PubMed]
- Cechinel, M.A.; Mayer, D.A.; Mazur, L.P.; Silva, L.G.; Girardi, A.; Vilar, V.J.P.; Souza, A.A.U.; Souza, S.U. Application of eco-friendly cation exchangers (Gracilaria caudata and Gracilaria cervicornis) for metal ions separation and recovery from a syn-thetic petrochemical wastewater: Batch and fi xed bed studies. J. Clean. Prod. 2018, 172, 1928–1945. [Google Scholar] [CrossRef]
- Yang, X.; Wang, J.; El-Sherbeeny, A.M.; AlHammadi, A.A.; Park, W.H.; Abukhadra, M.R. Insight into the adsorption and oxi-dation activity of a ZnO/piezoelectric quartz core-shell for enhanced decontamination of ibuprofen: Steric, energetic, and oxidation studies. Chem. Eng. J. 2022, 431, 134312. [Google Scholar] [CrossRef]
- Meshram, S.P.; Adhyapak, P.V.; Pardeshi, S.K.; Mulla, I.S.; Amalnerkar, D.P. Sonochemically generated cerium doped ZnO nanorods for highly efficient photocatalytic dye degradation. Powder Technol. 2017, 318, 120–127. [Google Scholar] [CrossRef]
- Li, P.C.; Liao, G.M.; Kumar, S.R.; Shih, C.-M.; Yang, C.-C.; Wang, D.-M.; Lue, S.J. Fabrication and characterization of chitosan nanoparticle-incorporated quaternized poly (vinyl alcohol) composite membranes as solid electrolytes for direct methanol al-kaline fuel cells. Electrochim. Acta 2016, 187, 616–628. [Google Scholar] [CrossRef]
- Alam, M.; Asiri, A.M.; Uddin, M.; Islam, M.; Rahman, M.M. Wet-chemically prepared low-dimensional ZnO/Al2O3/Cr2O3 na-noparticles for xanthine sensor development using an electrochemical method. RSC Adv. 2018, 8, 12562–12572. [Google Scholar] [CrossRef] [PubMed]
- Woo, H.; Kim, E.; Kim, J.-H.; Yun, S.-W.; Park, J.C.; Kim, Y.-T.; Park, K.H. Shape and composition control of monodisperse hybrid Pt-CoO nanocrystals by controlling the reaction kinetics with additives. Sci. Rep. 2017, 7, 3851. [Google Scholar] [CrossRef] [PubMed]
- Dhall, A.; Self, W. Cerium Oxide Nanoparticles: A Brief Review of Their Synthesis Methods and Biomedical Applications. Antioxidants 2018, 7, 97. [Google Scholar] [CrossRef]
- Sharpe, E.; Andreescu, D.; Andreescu, S. Artificial nanoparticle antioxidants. In Oxidative Stress: Diagnostics, Prevention, and Therapy; American Chemical Society: Washington, DC, USA, 2011; pp. 235–253. [Google Scholar]
- Parul, R.; Kundu, S.K.; Saha, P. In vitro nitric oxide scavenging activity of methanol extracts of three Bangladeshi medicinal plants. Pharma Innov. 2013, 1, 83. [Google Scholar]
- Song, Y.; Yang, F.; Ma, M.; Kang, Y.; Hui, A.; Quan, Z.; Wang, A. Green synthesized Se–ZnO/attapulgite nanocomposites using Aloe vera leaf extract: Characterization, antibacterial and antioxidant activities. LWT 2022, 165, 113762. [Google Scholar] [CrossRef]
- Wang, Z.-J.; Xu, J.-J.; Ji, F.-Y.; Luo, S.-Z.; Li, X.-J.; Mu, D.-D.; Jiang, S.-T.; Zheng, Z. Fabrication and characterization of soy β-conglycinin-dextran-polyphenol nanocomplexes: Improvement on the antioxidant activity and sustained-release property of curcumin. Food Chem. 2022, 395, 133562. [Google Scholar] [CrossRef]
- Dappula, S.S.; Kandrakonda, Y.R.; Shaik, J.B.; Mothukuru, S.L.; Lebaka, V.R.; Mannarapu, M.; Amooru, G.D. Biosynthesis of zinc oxide nanoparticles using aqueous extract of Andrographis alata: Characterization, optimization and assessment of their antibacterial, antioxidant, antidiabetic and anti-Alzheimer’s properties. J. Mol. Struct. 2023, 1273, 134264. [Google Scholar] [CrossRef]
- Adersh, A.R.; Kulkarni, S.; Ghosh, P.; More, B.A.; Gandhi, M.N.C. Surface defect rich ZnO quantum dots as antioxidant inhibitingα-amylase and α-glucosidase: A potential anti-diabetic nanomedicine. J. Mater. Chem. B 2015, 3, 4597–4606. [Google Scholar]
- Liu, Y.; Ying, D.; Cai, Y.; Le, X. Improved antioxidant activity and physicochemical properties of curcumin by adding ovalbumin and its structural characterization. Food Hydrocoll. 2017, 72, 304–311. [Google Scholar] [CrossRef]
- Hamasaki, T.; Kashiwagi, T.; Imada, T.; Nakamichi, N.; Aramaki, S.; Toh, K.; Morisawa, S.; Shimakoshi, H.; Hisaeda, Y.; Shirahata, S. Kinetic analysis of superoxide anion radical-scavenging and hydroxyl radical-scavenging activities of platinum nanoparticles. Langmuir 2008, 24, 7354–7364. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.; Wang, N.; Dong, X.; Wang, C.; Du, Z.; Mei, L.; Yong, Y.; Huang, C.; Li, Y.; Gu, Z.; et al. Graphdiyne Nanoparticles with High Free Radical Scavenging Activity for Radiation Protection. ACS Appl. Mater. Interfaces 2018, 11, 2579–2590. [Google Scholar] [CrossRef] [PubMed]
- Shu, G.; Xu, D.; Xie, S.; Chang, L.-J.; Liu, X.; Yang, J.; Li, Y.; Wang, X. The antioxidant, antibacterial, and infected wound healing effects of ZnO quantum dots-chitosan biocomposite. Appl. Surf. Sci. 2023, 611, 155727. [Google Scholar] [CrossRef]
- Deng, J.; Wang, J.; Hu, H.; Hong, J.; Yang, L.; Zhou, H.; Xu, D. Application of mesoporous calcium silicate nanoparticles as a po-tential SD carrier to improve the solubility of curcumin. J. Dispers. Sci. Technol. 2022, 1–9. [Google Scholar] [CrossRef]
- Vinotha, V.; Iswarya, A.; Thaya, R.; Govindarajan, M.; Alharbi, N.S.; Kadaikunnan, S.; Khaled, J.M.; Al-Anbr, M.N.; Vaseeharan, B. Synthesis of ZnO nanoparticles using insulin-rich leaf extract: Anti-diabetic, antibiofilm and anti-oxidant properties. J. Photochem. Photobiol. B Biol. 2019, 197, 111541. [Google Scholar] [CrossRef]
- Rehana, D.; Mahendiran, D.; Kumar, R.S.; Rahiman, A.K. In vitro antioxidant and antidiabetic activities of zinc oxide nano-particles synthesized using different plant extracts. Bioprocess Biosyst. Eng. 2017, 40, 943–957. [Google Scholar] [CrossRef]
- Alkaladi, A.; Abdelazim, A.; Afifi, M. Antidiabetic Activity of Zinc Oxide and Silver Nanoparticles on Streptozotocin-Induced Diabetic Rats. Int. J. Mol. Sci. 2014, 15, 2015–2023. [Google Scholar] [CrossRef]
- Dhobale, S.; Thite, T.; Laware, S.L.; Rode, C.V.; Koppikar, S.J.; Ghanekar, R.K.; Kale, S.N. Zinc oxide nanoparticles as novel al-pha-amylase inhibitors. J. Appl. Phys. 2008, 104, 094907. [Google Scholar] [CrossRef]
- Kitture, R.; Ghosh, S.; More, P.A.; Date, K.; Gaware, S.; Datar, S.; Chopade, B.A.; Kale, S.N. Curcumin-Loaded, Self-Assembled Aloevera Template for Superior Antioxidant Activity and Trans-Membrane Drug Release. J. Nanosci. Nanotechnol. 2015, 15, 4039–4045. [Google Scholar] [CrossRef]
- Sanap, S.P.; Ghosh, S.; Jabgunde, A.M.; Pinjari, R.V.; Gejji, S.P.; Singh, S.; Chopade, B.A.; Dhavale, D.D. Synthesis, computational study and glycosidase inhibitory activity of polyhydroxylated conidine alkaloids—A bicyclic iminosugar. Org. Biomol. Chem. 2010, 8, 3307–3315. [Google Scholar] [CrossRef]
- Lawande, P.P.; Sontakke, V.A.; Kumbhar, N.M.; Bhagwat, T.R.; Ghosh, S.; Shinde, V.S. Polyhydroxylated azetidine iminosugars: Synthesis, glycosidase inhibitory activity and molecular docking studies. Bioorg. Med. Chem. Lett. 2017, 27, 5291–5295. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rudayni, H.A.; Rabie, A.M.; Aladwani, M.; Alneghery, L.M.; Abu-Taweel, G.M.; Al Zoubi, W.; Allam, A.A.; Abukhadra, M.R.; Bellucci, S. Biological Activities of Sargassum Algae Mediated ZnO and Co Doped ZnO Nanoparticles as Enhanced Antioxidant and Anti-Diabetic Agents. Molecules 2023, 28, 3692. https://doi.org/10.3390/molecules28093692
Rudayni HA, Rabie AM, Aladwani M, Alneghery LM, Abu-Taweel GM, Al Zoubi W, Allam AA, Abukhadra MR, Bellucci S. Biological Activities of Sargassum Algae Mediated ZnO and Co Doped ZnO Nanoparticles as Enhanced Antioxidant and Anti-Diabetic Agents. Molecules. 2023; 28(9):3692. https://doi.org/10.3390/molecules28093692
Chicago/Turabian StyleRudayni, Hassan Ahmed, Abdelrahman M. Rabie, Malak Aladwani, Lina M. Alneghery, Gasem M. Abu-Taweel, Wail Al Zoubi, Ahmed A. Allam, Mostafa R. Abukhadra, and Stefano Bellucci. 2023. "Biological Activities of Sargassum Algae Mediated ZnO and Co Doped ZnO Nanoparticles as Enhanced Antioxidant and Anti-Diabetic Agents" Molecules 28, no. 9: 3692. https://doi.org/10.3390/molecules28093692
APA StyleRudayni, H. A., Rabie, A. M., Aladwani, M., Alneghery, L. M., Abu-Taweel, G. M., Al Zoubi, W., Allam, A. A., Abukhadra, M. R., & Bellucci, S. (2023). Biological Activities of Sargassum Algae Mediated ZnO and Co Doped ZnO Nanoparticles as Enhanced Antioxidant and Anti-Diabetic Agents. Molecules, 28(9), 3692. https://doi.org/10.3390/molecules28093692








