Dipsacus and Scabiosa Species—The Source of Specialized Metabolites with High Biological Relevance: A Review
Abstract
:1. Introduction
2. Traditional Medicinal Uses and Pharmacopoeial Monographs
2.1. Dipsacus spp.
2.2. Scabiosa spp.
3. Chemical Constituents of Dipsacus and Scabiosa Species
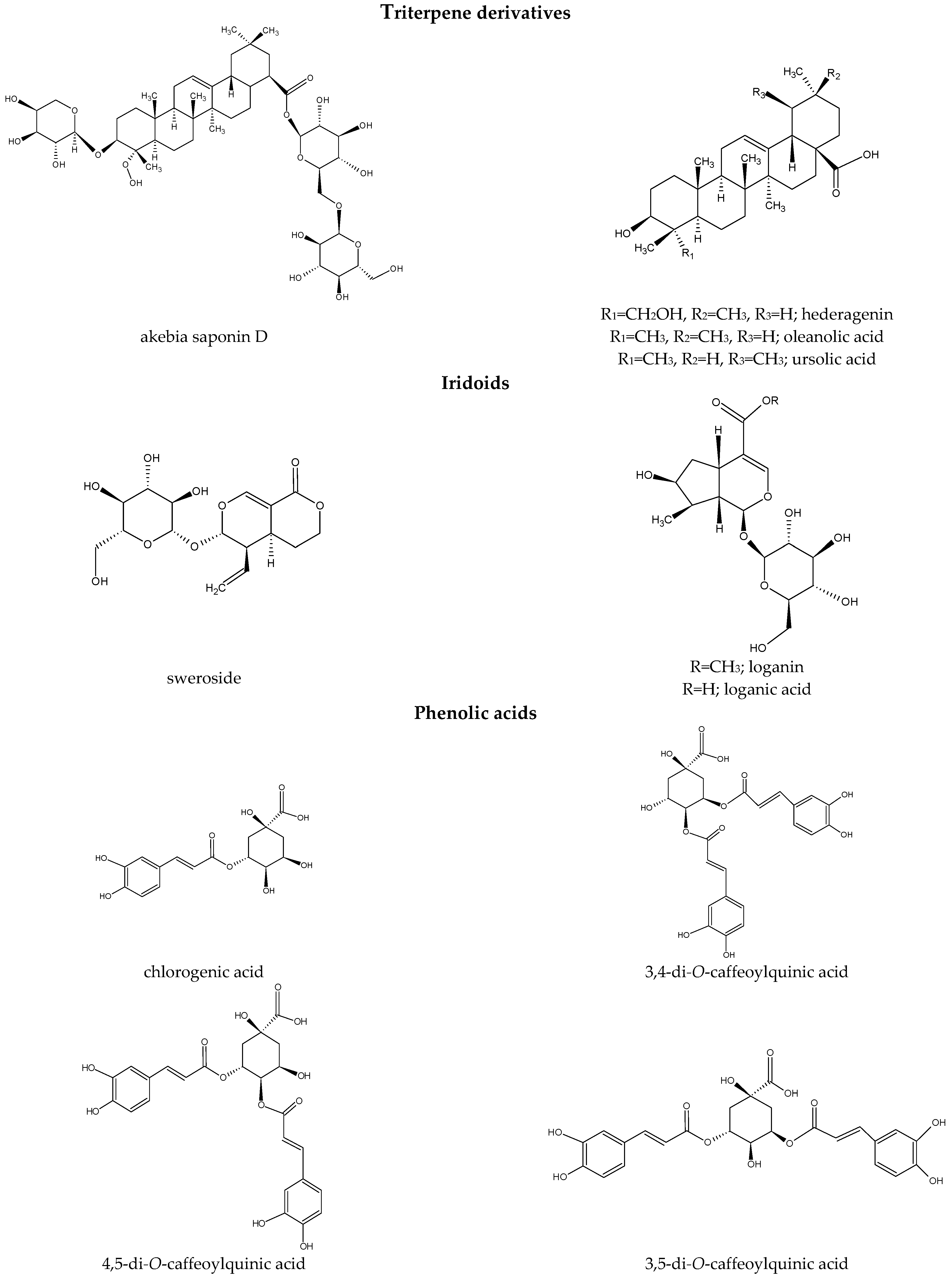
3.1. Terpenoid Derivatives
| No. | Compound Name | Identification | Species (Part of Plant) |
|---|---|---|---|
| 1. | (6α,11α)-6-[(2′-O-acetyl- α-l-arabinopyranosyl)oxy]-3-oxotaraxast-20-ene-11,28-diyl diacetate | NMR | D. asper (roots) [12] |
| 2. | 2′-O-acetyl-akebia saponin D; 2′-O-acetyl- 2′-O-acetyl-3-O-α-l-arabinopyranosyl-23-hydroxyolea- 12-en-28-oic acid; 3-O-(2-O-acetyl)-α-l-arabinopyranosyl-hederagenin 28-O-β-d-glucopyranosyl-(1-6)-β-d-glucopyranoside | HPLC-ESI-QTOF-MS/MS [93], R-ESI-MS, 1H NMR, 13C NMR [12,22,96] | D. asper (roots) [12,22,93,96] |
| 3. | 2α-hydroxyursolic acid | NMR | D. asper (roots) [12] |
| 4. | 2α-hydroxy- 3β-O-trans-feruloyloxy-olean-12-en-28-oic acid | NMR | D. asper (roots) [12] |
| 5. | 2α,3β-dihydroxy- 23-norolea-4(24),12-dien-28-oic acid | NMR | D. asper (roots) [12] |
| 6. | 2α,3β-dihydroxy-23-norurs-4(24),11,13(18)-trien-28-oic acid | HR-ESI-MS, 1H NMR, 13C NMR, 1H-1H COSY, ROESY | D. asper (roots) [12] |
| 7. | 2α,3β-dihydroxy- 24-norurs-4(23),12-dien-28-oic acid | NMR | D. asper (roots) [12] |
| 8. | 2α,3β,24-trihydroxy-23-norurs-12-en-28-oic acid | HR-ESI-MS, 1H NMR, 13C NMR, DEPT, 1H-1H COSY, HMBC, NOESY | D. asper (roots) [12] |
| 9. | 2α,23α-dihydroxy-3β-O-trans-feruloyloxy- olean-12-en-28-oic acid | NMR | D. asper (roots) [12] |
| 10. | 2′,3′-O-diacetyl-3-O-α-l-arabinopyranosyl-23-hydroxyolea-12-en-28-oic acid | HR-ESI-MS, 1H NMR, 13C NMR, HMBC | D. asper (roots) [12] |
| 11. | 2′,4′-O-diacetyl-3-O-α-l-arabinopyranosyl-23-hydroxyolea-12-en-28-oic acid | HR-ESI-MS, 1H NMR, 13C NMR, HMBC | D. asper (roots) [12] |
| 12. | 3β-O-trans-feruloyl-2α-hydroxy-urs-12-en-28-oic acid | NMR | D. asper (roots) [12] |
| 13. | 3β- O-trans-feruloyl-2α,23α-dihydroxy-urs-12-en-28-oic acid | NMR | D. asper (roots) [12] |
| 14. | 3β-hydroxy-24-norurs- 4(23),12-dien-28-oic acid | NMR | D. asper (roots) [12] |
| 15. | 3-O-α-l-arabinopyranosylhederagenin 28-O-β-d-glucopyranoside | NMR | D. asper (roots) [96] |
| 16. | 3-O-α-l-arabinopyranosyl-28-O-β-d-glucopyranosyl-(1→6)-β-d-glucopyranosyloleanolic acid | NMR [96], HPLC-ESI-QTOF-MS/MS [93] | D. asper (roots) [92,93,96] |
| 17. | 3-O-β-d-Glucopyranosyl-(1→3)-α-l-rhamnopyranosyl-(1→2)-α-l-arabinopyranosyl- 23-hydroxyolean-18-en-28-oic acid 28-O-β-Dglucopyranosyl-(1→6)-β-d-glucopyranosyl ester | HR-ESI-MS, 1D-TOCSY, 2D-HSQC, TOCSY-HSQC, COSY, HMBC | D. asper (roots) [96] |
| 18. | 3-O-[β-d-xylopyranosyl- (1→4)-β-d-glucopyranosyl-(1→4)][α-l-rhamnopyranosyl-(1→3)]-β- d-glucopyranosyl-(1→3)-α-l-rhamnopyranosyl-(1→2)-α-l-arabinopyranosylhederagenin | NMR | D. asper (roots) [96] |
| 19. | 3′-O-acetyl-akebia saponin D; 3′-O-acetyl-3-O-α-l-arabinopyranosylhederagenin 28-O-β-d-glucopyranosyl-(1→6)-β-d-glucopyranoside; 3′-O-acetyl-3- O-α-l-arabinopyranosyl-23-hydroxyolea-12-en-28-oic acid; 3-O-(3-O-acetyl)-α-l-arabinopyranosyl-hederagenin 28-O-β-d-glucopyranosyl-(1-6)-β-d-glucopyranoside | HR-ESI-MS, 1H NMR, 13C NMR [12,22,96], HPLC-ESI-QTOF-MS/MS [93] | D. asper (roots) [12,22,93,96] |
| 20. | 4′-O-acetyl-akebia saponin D (asperosaponin Ⅳ) a; 4′-O-acetyl-3-O-α-l-arabinopyranosylhederagenin 28-O- β-d-glucopyranosyl-(1→6)-β-d-glucopyranoside; 4′-O-acetyl-3-O-α-l-arabinopyranosyl-23-hydroxyolea- 12-en-28-oic acid; 3-O-(4-O-acetyl)- α-l-arabinopyranosyl-hederagenin 28-O-β-d-glucopyranosyl-(1-6)-β-d-glucopyranoside | HPLC-ESI-QTOF-MS/MS [93], HR-ESI-MS, 1H NMR, 13C NMR [12,22,96] | D. asper (roots) [12,22,92,93,96] |
| 21. | 11α,12α-epoxy-3,6β-dihydroxy-24-norurs-3-en-2-on- (28→13)-olide | NMR | D. asper roots) [12] |
| 22. | 23α-hydroxy-olean-12-en-3- one | NMR | D. asper (roots) [12] |
| 23. | 25-acetoxy-28-dehydroxyrubiarbonone E | HR-ESI-MS, 1D NMR, 2D NMR, 1H-1H COSY, HMBC, NOESY | D. asper (roots) [12] |
| 24. | Akebia saponin PA (cauloside A; leontoside A; 3-O-α-l-arabinopyranosyl hederagenin) a | HPLC-ESI-QTOF-MS/MS [93], FAB-MS, 1D NMR, 2D NMR [21,22], HR-ESI-MS [22] | D. asper/D. asperoides (roots) [27,93] D. asper (roots) [6,21,22] D. asper (roots) [12] |
| 25. | Akebia saponins X-Y | HPLC-ESI-QTOF-MS/MS [93] | D. asper (roots) [93] |
| 26. | Asperosaponin B | D. asper (roots) [93] | |
| 27. | Asperosaponin E (3-O-β-d-glucopyranosyl-(1→4)-[a-l-rhamnopyranosyl-(1→6)]-β-d-glucopyranosyl-(1→3)-a-l-rhamnopyranosyl-(1→2)-a-l-arabinopyranosyl oleanolic acid) | HPLC-ESI-QTOF-MS/MS [93] | D. asper (roots) [93] |
| 28. | Asperosaponin F (3-O-α-l-rhamnopyranosyl-(→›6)-β-d-glucopyranosyl-(1→3)-α-l-rhamnopyranosyl-(1→2)-α-l-arabinopyranosyl oleanolic acid) | HPLC-ESI-QTOF-MS/MS [93] | D. asper (roots) [93] |
| 29. | Asperosaponin G (3-O-β-glucopyranosyl-(1→3)-α-l-rhamnopyranosyl-(1→2)-α-l-arabinopyranosyl oleanolic acid) | HPLC-ESI-QTOF-MS/MS [93] | D. asper (roots) [93] |
| 30. | Asperosaponin V | UPLC-Q-TOF-MS | D. asper (roots) [66] |
| 31. | Asperosaponin VI (akebia saponin D) a (3-O-α-l-arabinopyranosyl hederagenin-28-β-d-glucopyranoside-(1→6)- β-d-glucopyranoside) | UHPLC-MS/MS [65], UHPLC-Q-TOF-MS [66], LC-ESI-MS [90], HPLC-ESI-QTOF-MS/MS [93], HPLC-DAD [95] | D. asper/D. asperoides (roots) [65,66,89,90,92,93,95] |
| 32. | Colchiside (3-O-β-d-xylopyranosyl-23-O-β-d-glucopyranosyl-28-O-β-d-(6-O-acetyl)- glucopyranosyl hederagenin) | 1D NMR, 2D NMR, DEPT, TOCSY, HMQC, HMBC | D. asper (roots) [21] |
| 33. | Dipsacoside A | UPLC-Q-TOF-MS | D. asper Wall. (roots) [66] |
| 34. | Dipsacoside B | UHPLC-MS/MS [65] | D. asperoides (roots) [27,65] |
| 35. | Dipsacus saponin A | HR-ESI-MS, 1H NMR, 13C NMR [22] | D. asper (roots) [6,22,92] |
| 36. | Dipsacus saponin B | UPLC-Q-TOF-MS [66], HPLC-ESI-QTOF-MS/MS [93] | D. asper/D. asperoides (roots) [6,27,66,93] |
| 37. | Dipsacus saponin C | ESI-QTOF-MS/MS [93] | D. asper/D. asperoides (roots) [6,27,91,93] |
| 38. | Dipsacus saponins J-K | ESI-QTOF-MS/MS [93] | D. asper (roots) [6,93] |
| 39. | Dipsacus saponin L (3-O-α-l-rhamnopyranosyl-(1→2)-α-l-arabinopyranosyl hederagenin) | ESI-QTOF-MS/MS | D. asper (roots) [93] |
| 40. | Dipsacus saponin M (3-O-α-l-rhamnopyranosyl-(1→2)-α-l-arabinopyranosyl hed-eragenin 28-O-β-d-glucopyranosyl-(1→6)-β-d-glucopyranosyl) | ESI-QTOF-MS/MS | D. asper (roots) [93] |
| 41. | Dipsacus saponin N (3-O-β-d-xylopyranosyl-(1→3)-α-l-rhamnopyranosyl-(1→2)-α-l-arabinopyranosyl hederagenin) | ESI-QTOF-MS/MS | D. asper (roots) [93] |
| 42. | Dipsacus saponin O | ESI-QTOF-MS/MS | D. asper (roots) [93] |
| 43. | Dipsacus saponin P (3-O-β-d-xylopyranosyl-(1→3)-α-l-rhamnopyranosyl-(1→2)-α-l-arabinopyranosyl hederagenin 28-O-β-d-glucopyranosyl-(1→6)-β-d-glucopyranosyl) | ESI-QTOF-MS/MS | D. asper (roots) [93] |
| 44. | Dipsacus saponin R (3-O-α-l-rhamnopyranosyl(1→3)-β-d-glucopyranosyl (1→3)-α-l-rhamnopyranosyl-(1→2)-α-l-arabinopyranosyl-hederagenin) | ESI-QTOF-MS/MS | D. asper (roots) [93] |
| 45. | Dipsacus saponin V | - | D. asper [6] |
| 46. | Dipsacus saponin VI | HR-ESI-MS, 1H NMR, 13C NMR [22] | D. asper (roots) [6,22] |
| 47. | Dipsacus saponin VII | - | D. asper [6] |
| 48. | Dipsacus saponins IX-XI | - | D. asper [6] |
| 49. | Dipsacus saponin XII | ESI-QTOF-MS/MS [93] | D. asper (roots) [6,93] |
| 50. | Dipsacus saponin XIII | - | D. asper [6] |
| 51. | Elmalienoside B | NMR | D. asper (roots) [96] |
| 52. | α-hederin | - | D. asper [6] |
| 53. | Hederagenin | - | D. asper [6] |
| 54. | Hederagenin 28- O-β-d-glucopyranosyl-(1→6)-β-d-glucopyranoside | NMR | D. asper (roots) [96] |
| 55. | Hederagonic acid | NMR | D. asper (roots) [12] |
| 56. | HN saponin F | ESI-QTOF-MS/MS [93] | D. asper (roots) [6,93] |
| 57. | Hookeroside A-B | NMR | S. tschilliensis (whole plants) [7,51] |
| 58. | Japondipsaponin E1 | - | D. japonicus (roots) [5,88] |
| 59. | Kalopanax saponin A | - | D. asper (roots) [5] |
| 60. | Oleanolic acid | 1H NMR, 13C NMR [82], GC-MS [49] | D. asper (roots) [6,89] S. arenaria (aerial parts) [82] S. stellata (aerial parts) [49] S. tschilliensis (flowers) [94] |
| 61. | Macranthoidin A | ESI-QTOF-MS/MS [93], NMR [12,96] | D. asper (roots) [6,12,93,96] |
| 62. | Macranthoside B | - | D. asper [6] |
| 63. | Maslinic acid | NMR | D. asper (roots) [12] |
| 64. | Maslinic acid-pentosyl-rhamnosyl-glucoside | LC-MS/MS | S. atropurpurea subsp. maritima (leaves) [81] |
| 65. | Oleanolic acid-pentosyl-rhamnosyl-glucosyl-glucosyl-di-glucoside | LC-MS/MS | S. atropurpurea subsp. maritima (leaves) [81] |
| 66. | Oleanolic acid-pentosyl-rhamnosyl-pentosyl-glucosyl-di-glucoside | LC-MS/MS | S. atropurpurea subsp. maritima (leaves) [81] |
| 67. | Palustroside III (3-O-[β-d-glucopyranosyl-(1→2)-β-d-glucuronopyranosyl]-28-O-[β-d-glucopyranosyl]-hederagenin) | - | S. stellata (whole plants) [7,48] |
| 68. | Saponin XII (3-O-[β-d-glucopyranosyl(1→4)][α-l-rhamnopyranosyl(1→3)]- emph-β-d-glucopyranosyl(1→3)-α-l-rhamnopyranosyl(1→2)-α-arabinopyranosyl hederagenin 28-O-β-d-glucopyranosyl(1→6)-β-d-glucopyranoside) | 1D NMR, 2D NMR, DEPT, HR-ESI-MS | D. japonicus (roots) [40] |
| 69. | Scabiosaponin A (3-O-β-d-glucopyranosyl-(1→4)-β-d-xylopyranosyl-(1→3)-α-l-rhamnopyranosyl-(1→2)-α-l-arabinopyranosyloleanolic acid 28-O-β-d-glucopyranosyl-(1→6)-β-d-glucopyranoside) | 2D NMR, DEPT, DQF-COSY, TOCSY, HMQC, HMQC-TOCSY, HMBC, NOESY [51] | S. tschilliensis (whole plants) [7,51] |
| 70. | Scabiosaponin B (3-O-β-d-xylopyranosyl-(1→4)-β-d-glucopyranosyl-(1→4)-β-d-xylopyranosyl-(1→3)-α-l-rhamnopyranosyl-(1→2)-α-l-arabinopyranosyloleanolic acid 28-O-β-d-glucopyranosyl-(1→6)-β-d-glucopyranoside) | 2D NMR, DEPT, DQF-COSY, TOCSY, HMQC, HMQC-TOCSY, HMBC, NOESY [51] | S. tschilliensis (whole plants) [7,51] |
| 71. | Scabiosaponin C (3-O-[β-d-glucopyranosyl-(1→4)-β-d-xylopyranosyl-(1→3)-α-l-rhamnopyranosy-(1→2)][β-d-glucopyranosyl-(1→4)]-α-l-arabinopyranosyloleanolic acid 28-O-β-d-glucopyranosyl-(1→6)-β-d-glucopyranoside) | 2D NMR, DEPT, DQF-COSY, TOCSY, HMQC, HMQC-TOCSY, HMBC, NOESY [51] | S. tschilliensis (whole plants) [7,51] |
| 72. | Scabiosaponin D (3-O-[α-l-rhamnopyranosyl-(1→2)][β-d-glucopyranosyl-(1→4)]-α-l-arabinopyranosyloleanolic acid 28-O-β-d-glucopyranosyl-(1→6)-β-d-glucopyranoside) | 2D NMR, DEPT, DQF-COSY, TOCSY, HMQC, HMQC-TOCSY, HMBC, NOESY [51] | S. tschilliensis (whole plants) [7,51] |
| 73. | Scabiosaponin E (3-O-β-d-xylopyranosyl-(1→3)-α-l-rhamnopyranosyl-(1→2)-β-d-xylopyranosyloleanolic acid 28-O-β-d-glucopyranosyl-(1→6)- β-d-glucopyranoside) | 2D NMR, DEPT, DQF-COSY, TOCSY, HMQC, HMQC-TOCSY, HMBC, NOESY [51] | S. tschilliensis (whole plants) [7,51] |
| 74. | Scabiosaponin F (3-O-β-d-glucopyranosyl- (1→3)-α-l-rhamnopyranosyl-(1→2)-β-d-xylopyranosyloleanolic acid 28-O-β-d-glucopyranosyl-(1→6)-β-d-glucopyranoside) | 2D NMR, DEPT, DQF-COSY, TOCSY, HMQC, HMQC-TOCSY, HMBC, NOESY [51] | S. tschilliensis (whole plants) [7,51] |
| 75. | Scabiosaponin G (3-O-β-d-glucopyranosyl-(1→4)-β-d-glucopyranosyl-(1→3)-α-l-rhamnopyranosyl-(1→2)-β-d-xylopyranosyloleanolic acid 28-O-β-d-glucopyranosyl-(1→6)-β-d-glucopyranoside) | 2D NMR, DEPT, DQF-COSY, TOCSY, HMQC, HMQC-TOCSY, HMBC, NOESY [51] | S. tschilliensis (whole plants) [7,51] |
| 76. | Scabiosaponin H (3-O-β-d-glucopyranosyl-(1→4)-β-d-glucopyranosyl-(1→3)-α-l-rhamnopyranosyl-(1→2)-α-L-arabinopyranosylpomolic acid 28-O-β-d-glucopyranosyl-(1→6)-β-d-glucopyranoside) | 2D NMR, DEPT, DQF-COSY, TOCSY, HMQC, HMQC-TOCSY, HMBC, NOESY [51] | S. tschilliensis (whole plants) [7,51] |
| 77. | Scabiosaponin I (3-O-β-d-glucopyranosyl-(1→3)-α-l-rhamnopyranosyl-(1→2)-α-l-arabinopyranosylpomolic acid 28-O-β-d-glucopyranosyl-(1→6)-β-d-glucopyranoside) | 2D NMR, DEPT, DQF-COSY, TOCSY, HMQC, HMQC-TOCSY, HMBC, NOESY [51] | S. tschilliensis (whole plants) [7,51] |
| 78. | Scabiosaponin J (3-O-β-d-glucopyranosyl-(1→3)-α-l-rhamnopyranosyl-(1→2)-α-l-arabinopyranosylsiaresinolic acid 28-O-β-d-glucopyranosyl-(1→6)-β-d-glucopyranoside) | 2D NMR, DEPT, DQF-COSY, TOCSY, HMQC, HMQC-TOCSY, HMBC, NOESY [51] | S. tschilliensis (whole plants) [7,51] |
| 79. | Scabiosaponin K (3-O-β-d-glucopyranosyl-(1→4)-β-d-xylopyranosyl-(1→3)-α-l-rhamnopyranosyl-(1→2)-α-l-arabinopyranosylsiaresinolic acid 28-O-β-d-glucopyranosyl-(1→6)-β-d-glucopyranoside) | 2D NMR, DEPT, DQF-COSY, TOCSY, HMQC, HMQC-TOCSY, HMBC, NOESY [51] | S. tschilliensis (whole plants) [7,51] |
| 80. | Scabiostellatoside A (3-O-[β-d-glucopyranosyl-(1→4)-β-d-xylopyranosyl-(1→3)-α-l-rhamnopyranosyl-(1→2)-α-l-arabinopyranonosyl]-28-O-[β-d-glucopyranosyl-(1→6)-β-d-glucopyranosyl]-oleanolic acid) | HR-ESI-MS, 1D NMR, 2D NMR, HMBC, COSY, TOCSY, HSQC, ROESY [48] | S. stellata (whole plants) [7,48] |
| 81. | Scabiostellatoside B (3-O-α-l-rhamnopyranosyl-(1→3)-β-d-xylopyranosyl-(1→3)-α-l-rhamnopyranosyl-(1→2)-β-d-xylopyranosyl]-28-O-[β-d-glucopyranosyl-(1→6)-β-d-glucopyranosyl]-oleanolic acid) | HR-ESI-MS, 1D NMR, 2D NMR, HMBC, COSY, TOCSY, HSQC, HSQC-TOCSY, ROESY [48] | S. stellata (whole plants) [7,48] |
| 82. | Scabiostellatoside C (3-O-[β-d-glucopyranosyl-(1→4)-α-l-rhamnopyranosyl-(1→3)-β-d-xylopyranosyl-(1→3)-α-l-rhamnopyranosyl-(1→2)-a-l-arabinopyranosyl]-28-O-[β-d-glucopyranosyl-(1→6)-β-d-glucopyranosyl]-oleanolic acid) | HR-ESI-MS, 1D NMR, 2D NMR, HMBC, COSY, TOCSY, HSQC, HSQC-TOCSY, ROESY [48] | S. stellata (whole plants) [7,48] |
| 83. | Scabiostellatoside D (3-O-[β-d-glucopyranosyl-(1→4)-α-l-rhamnopyranosyl-(1→3)-β-d-xylopyranosyl-(1→3)-α-l-rhamnopyranosyl-(1→2)-β-d-xylopyranonosyl]-28-O-[β-d-glucopyranosyl-(1→6)-β-d-glucopyranosyl]-oleanolic acid) | HR-ESI-MS, 1D NMR, 2D NMR, HMBC, COSY, TOCSY, HSQC, HSQC-TOCSY, ROESY [48] | S. stellata (whole plants) [7,48] |
| 84. | Scabiostellatoside E (3-O-[α-l-rhamnopyranosyl- (1→3)-β-d-xylopyranosyl-(1→3)-α-l-rhamnopyranosyl-(1→2)-{β-d-glucopyranosyl-(1→4)-}α-l-arabinopyranosyl]-28-O-[β-Dglucopyranosyl-(1→6)-β-d-glucopyranosyl]-oleanolic acid) | HR-ESI-MS, 1D NMR, 2D NMR, HMBC, COSY, TOCSY, HSQC, HSQC-TOCSY, ROESY [48] | S. stellata (whole plants) [7,48] |
| 85. | Scabiostellatoside F (3-O-[β-d-glucopyranosyl-(1→4)-β-d-glucopyranosyl-(1→4)-β-d-glucopyranosyl-(1→4)-α-l-rhamnopyranosyl-(1→3)-β-d-xylopyranosyl-(1→3)-α-l-rhamnopyranosyl-(1→2)-β-d-xylopyranosyl]- oleanolic acid) | HR-ESI-MS, 1D NMR, 2D NMR, HMBC, COSY, TOCSY, HSQC, HSQC-TOCSY, ROESY [48] | S. stellata (whole plants) [7,48] |
| 86. | Scabiostellatoside G (3-O-[β-d-glucopyranosyl-(1→2)-β-d-glucuronopyranosyl]-28-O-[β-d-glucopyranosyl-(1→6)-β-d-glucopyranosyl]-hederagenin) | HR-ESI-MS, 1D NMR, 2D NMR, HMBC, COSY, TOCSY, HSQC, HSQC-TOCSY, ROESY [48] | S. stellata (whole plants) [7,48] |
| 87. | Scabiostellatoside H (3-O-[α-l-rhamnopyranosyl-(1→2)-β-d-glucuronopyranosyl]-28-O-[β-d-glucopyranosyl]-asiatic acid) | HR-ESI-MS, 1D NMR, 2D NMR, HMBC, COSY, TOCSY, HSQC, HSQC-TOCSY, ROESY [48] | S. stellata (whole plants) [7,48] |
| 88. | Urceolide | - | S. tschilliensis (flowers) [94] |
| 89. | Ursolic acid | NMR [12], GC-MS [49] | D. asper (roots) [12] S. stellata (whole plants) [7,48,49] |
Iridoids
| No. | Compound Name | Species (Part of Plant) |
|---|---|---|
| 1. | 3′-O-β-d-glucopyranosyl sweroside | D. asper (roots) [89] |
| 2. | 6′-O-β-d-apiofuranosyl sweroside | D. asper (roots) [89,99] |
| 3. | 7-O-caffeoyl-sylvestroside I | S. stellata (whole plants) [7,75] |
| 4. | 7-O-(p-coumaroyl)-sylvestroside I | S. stellata (whole plants) [7,75] |
| 5. | Atropurpurin A | S. atropurpurea subsp. maritima (whole plants) [78] |
| 6. | Atropurpurin B | S. atropurpurea subsp. maritima (whole plants) [78] |
| 7. | Cantleyoside | D. asper (roots) [6,89,92,93,97,99] D. fullonum (leaves and roots) [33] S. atropurpurea subsp. maritima (roots) [7,84] |
| 8. | Cocculoside | D. asper (roots) [96] |
| 9. | Dipsanoside A and dipsanoside B | D. asper (roots) [6,22,92,93,99] |
| 10. | Dipsanosides C-G | D. asper (roots) [6,89] |
| 11. | Dipsanoside H | D. asper [6] |
| 12. | Dipsanosides M-N | D. asper (roots) [6,13] |
| 13. | Dipasperoside A | D. asper (roots) [100] |
| 14. | Dipasperoside B | D. asper (roots) [98] |
| 15. | Eustomoruside | S. stellata (whole plants) [7,75] |
| 16. | Eustomoside | S. stellata (whole plants) [7,75] |
| 17. | Rhamnopyranosyl-cantleyoside | D. asper (roots) [99] |
| 18. | Lisianthioside | D. asper (roots) [6,89] |
| 19. | Loganic acid | D. asper (roots) [6,22,65,66,92,93,95,99] D. fullonum (leaves, roots) [33,34] S. atropurpurea subsp. maritima (flowers) [7,84] |
| 20. | Loganic acid ethyl ester | D. asper (roots) [97] |
| 21. | Loganin | D. asper/D. asperoides (roots) [6,22,27,65,66,89,92,93,95,96,97,99] D. fullonum (leaves, roots) [33,34] S. atropurpurea subsp. maritima (flowers) [7,84] |
| 22. | Secologanin-methyl-hemiacetal | S. atropurpurea subsp. maritima (whole plants) [78] |
| 23. | Septemfidoside | S. stellata (whole plants) [7,75] |
| 24. | Sweroside | D. asper/D. asperoides (roots) [6,22,27,65,66,92,93,96,99] D. fullonum leaves, roots) [33] S. stellata (whole plants) [7,75] S. atropurpurea subsp. maritima (flowers) [7,84] S. tschilliensis (flowers) [94] |
| 25. | Sylvestroside I | D. asper (roots) [22,92,99] S. stellata (whole plants) [7,75] S. tschilliensis (flowers) [94] |
| 26. | Sylvestroside II | S. tschilliensis (flowers) [94] |
| 27. | Sylvestroside III | D. asper [6] D. fullonum (leaves, roots) [33,34] |
| 28. | Sylvestroside IV | D. asper (roots) [6] D. fullonum (leaves) [34] |
| 29. | Triplostoside A | D. asper (roots) [6,89,92,93,99] |
3.2. Phenolic Acids
| No. | Compound Name | Species (Part of Plant) |
|---|---|---|
| 1. | 1-O-caffeoylquinic acid | D. asper (roots) [6] S. stellata (whole plants) [76] |
| 2. | 2′-O-caffeoyl-d-glucopyranoside ester | D. asper (roots) [89] |
| 3. | 2,6-dihydroxycinnamic acid | D. asper (roots) [6,89] |
| 4. | 3-O-caffeoylquinic acid methyl ester | S. atropurpurea subsp. maritima (leaves) [81] S. atropurpurea L. (aerial parts) [43] |
| 5. | 3,4-di-O-caffeoylquinic acid (isochlorogenic acid B) a | D. asper (roots) [6,24,66,92,93,95,99] S. atropurpurea sbsp. maritima (leaves) [81] S. comosa (inflorescences) [74] S. stellata (whole plants) [76] S. tschilliensis (inflorescences) [74] |
| 6. | 3,4-dihydroxybenzoic acid | D. asper (roots) [92] |
| 7. | 3,5-di-O-caffeoylquinic acid (isochlorogenic A) a | D. asper/D. asperoides (roots) [6,24,27,65,66,92,93,95,99] D. fullonum (leaves, roots) [33] S. atropurpurea subsp. maritima (leaves) [6] S. comosa (inflorescences) [74] S. stellata (whole plants) [75,76] S. tschilliensis (inflorescences) [74] |
| 8. | 4-O-caffeoylquinic acid (cryptochlorogenic acid) a | D. asper (roots) [6,65,66] D. fullonum (leaves, roots) [33] S. atropurpurea subsp. maritima (leaves) [81] S. stellata (whole plants) [76] |
| 9. | 4,5-di-O-caffeoylquinic acid (isochlorogenic acid C) a | D. asper (roots) [6,24,92,93,95,99] S. comosa (inflorescences) [74] S. stellata (whole plants) [75,76] S. tschilliensis (inflorescences) [74] |
| 10. | 5-O-feruloylquinic acid | S. stellata (whole plants) [76] |
| 11. | 5-O-p-coumaroylquinic acid | S. stellata (whole plants) [76] |
| 12. | Caffeic acid | D. asper/D. asperoides (roots) [6,29,89,92,93,95,97,99] S. comosa (inflorescences) [74] S. tschilliensis (inflorescences) [53,74] S. atropurpurea (stems) [46] |
| 13. | Caffeic acid methyl ester | S. tschilliensis (flowers) [94] |
| 14. | Chlorogenic acid | D. asper/D. asperoides (roots) [6,27,29,65,93,95,97,99] D. fullonum (leaves, roots) [33,34,101] S. atropurpurea subsp. maritima (leaves) [81] S. atropurpurea (stems) [46] S. comosa (inflorescences) [74] S. tschilliensis (inflorescences) [53,74] |
| 15. | Methyl 3,4-di-O-caffeoylquinate | D. asper (roots) [6,24] |
| 16. | Methyl 3,5-di-O-caffeoylquinate | D. asper (roots) [6,24] |
| 17. | Methyl 4,5-di-O-caffeoylquinate | D. asper (roots) [6,24] |
| 18. | Neochlorogenic acid | D. fullonum (leaves, roots) [33] S. atropurpurea subsp. maritima (leaves) [81] |
| 19. | p-coumaric acid | S. comosa (inflorescences) [74] S. tschilliensis (inflorescences) [74] S. atropurpurea (stems) [46] S. arenaria (roots) [73] |
| 20. | p-coumaric acid 3-glucoside | S. atropurpurea. subsp. maritima (leaves) [81] |
| 21. | p-coumaroylquinic acid | S. atropurpurea subsp. maritima (leaves) [81] |
| 22. | p-hydroxycinnamic acid | S. atropurpurea (stems) [46] |
| 23. | Protocatechuic acid | S. comosa (inflorescences) [74] S. tschilliensis (inflorescences) [74] |
| 24. | Protocatechuic acid 3-glucoside | S. atropurpurea subsp. maritima (leaves) [81] |
| 25. | Quinic acid | S. comosa inflorescences) [74] S. tschilliensis inflorescences [74] S. atropurpurea subsp. maritima (leaves) [81] |
| 26. | Sinapic acid | S. stellata (whole plants) [76] |
| 27. | Vanillic acid | D. asper (roots) [6,89,92] |
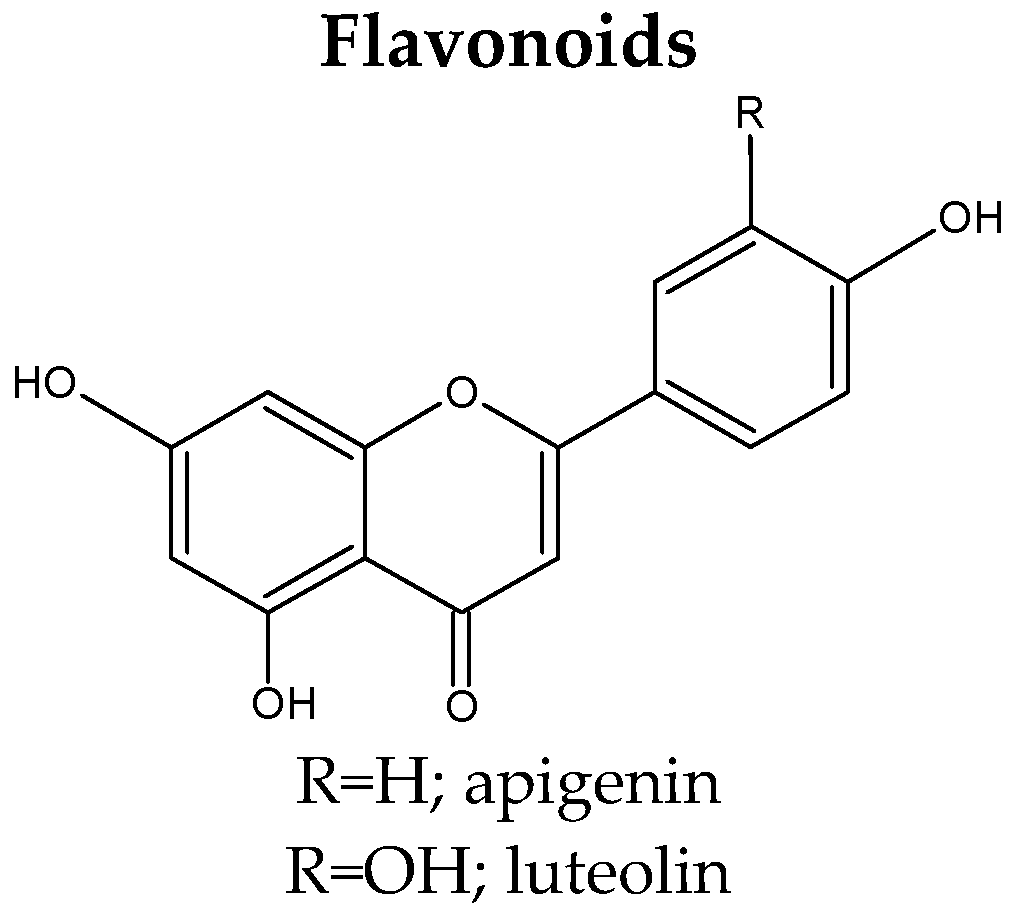
3.3. Flavonoids
| No. | Compound Name | Species (Part of Plant) |
|---|---|---|
| 1. | 3-O-[3-O-acetyl-6-O-(p-coumaroyl)-β-d-glucopyranosyl]-kaempferol | S. stellata (whole plants) [48] |
| 2. | Apigenin | S. tschilliensis (flowers) [94] S. comosa (inflorescences) [74] S. tschilliensis (inflorescences) [74] |
| 3. | Apigenin-2″-O-pentosyl-8-C-glucoside | S. stellata (whole plants) [76] |
| 4. | Apigenin-4′-glucoside (apigenin-4′-O-β-d-glucopyranoside) a | S. comosa (inflorescences) [74] S. tschilliensis (inflorescences/flowers) [74,94] |
| 5. | Apigenin-7-arabino(1~6)-glucoside | S. comosa (inflorescences) [74] S. tschilliensis (inflorescences) [74] |
| 6. | Apigenin-7-glucoside (apigenin-7-O-β-d-glucopyranoside) a | S. atropurpurea subsp. maritima (leaves) [81] S. comosa (inflorescences) [74] S. tschilliensis (inflorescences/flowers) [74,94] |
| 7. | Apigenin-7-O-rutinoside | S. tschilliensis (flowers) [94] |
| 8. | Diosmetin-6(or 8)-C-glucoside | S. stellata (whole plants) [76] |
| 9. | Diosmetin-7-O-glucoside | S. atropurpurea subsp. maritima (leaves) [81] |
| 10. | Hyperin (hyperoside; quercetin 3-O-galactoside) a | S. atropurpurea (stems) [46] S. stellata (whole plants) [7,75,76] |
| 11. | Icariin | S. tschiliensis (inflorescences) [53] |
| 12. | Isoorientin (luteolin-6-C-glucoside) a | D. fullonum (leaves) [34] D. sativus L. (leaves) [42] S. comosa (inflorescences) [74] S. stellata (whole plants) [7,75,76] S. tschilliensis (inflorescences) [74] |
| 13. | Isoquercitrin (quercetin 3-glucoside) a | S. atropurpurea (stems) [46] S. comosa (inflorescences) [74] S. tschilliensis (inflorescences) [74] |
| 14. | Isovitexin (saponaretin; apigenin-6-C-glucoside) a | D. fullonum (leaves) [33,34] D. sativus (leaves) [42] |
| 15. | Kaempferol-3-O-β-d-(3,6-di-p-(hydroxycinnamoyl)-glucopyranoside | S. tschilliensis (flowers) [94] |
| 16. | Kaempferol-3-O-β-d-6-O-(p-hydroxycinnamoyl)-glucopyranoside | S. tschilliensis (flowers) [94] |
| 17. | Kaempferol-3-O-rutinoside derivative | S. stellata (whole plants) [76] |
| 18. | Lucenin-2 (luteolin-6,8-di-C-glucoside) a | S. stellata (whole plants) [76] |
| 19. | Luteolin | S. atropurpurea subsp. maritima (leaves) [81] S. atropurpurea (aerial parts, stems) [7,43,46] S. comosa (inflorescences) [74] S. tschilliensis (inflorescences/flowers) [74,94] |
| 20. | Luteolin-2″-O-pentosyl-6-C-hexoside | S. stellata (whole plants) [76] |
| 21. | Luteolin-4′-O-β-d-glucopyranoside (luteolin-4′-glucoside) a | S. comosa (inflorescences) [74] S. tschilliensis (flowers) [74,94] |
| 22. | Luteolin 3′-glucoside | S. atropurpurea subsp. maritima (leaves) [81] |
| 23. | Luteolin hexoside | S. atropurpurea (stems) [46] |
| 24. | Luteolin-6-C-glucoside-7-O-glucoside | S. stellata (whole plants) [76] |
| 25. | Luteolin 7-rutinoside (luteolin-7-O-β-d-rutinoside) a | S. atropurpurea (aerial parts) [43] S. atropurpurea subsp. maritima (leaves) [81] |
| 26. | Luteolin-7,3′-diglucoside | S. atropurpurea subsp. maritima (leaves) [81] |
| 27. | Luteolin 7-O-β-d-glucoside (cynaroside; luteolin-7-O-β-d-glucopyranoside) a | S. arenaria (aerial parts) [82] S. atropurpurea (stems) [46] S. atropurpurea subsp. maritima (leaves/aerial parts) [43,81] S. tschilliensis (flowers) [81] |
| 28. | Myricetin | S. arenaria (roots) [73] |
| 29. | Orientin (luteolin 8-C-β-d-glucopyranoside, luteolin 8-C-glucoside) a | D. fullonum (leaves) [33] |
| 30. | Quercetin-3-rutinoside | S. comosa (inflorescences) [74] S. tschilliensis (inflorescences) [74] |
| 31. | Quercetin 3,4′-diglucoside | S. atropurpurea subsp. maritima (leaves) [81] |
| 32. | Quercimeritrin (quercetin 7-glucoside) a | S. atropurpurea (stems) [46] |
| 33. | Quercitrin (quercitin-3-O-rhamnoside) a | S. tschiliensis (inflorescences) [53] |
| 34. | Rutin (quercetin 3-rutinoside) a | S. tschiliensis (inflorescences) [53] |
| 35. | Saponarin (apigenin-6-C-glucoside-7-O-glucoside) a | D. fullonum (leaves, roots) [33,34] D. sativus (leaves) [42] |
| 36. | Swertiajaponin (isoorientin 7-methyl ether) a | S. stellata (whole plants) [7,75] |
| 37. | Tamarixetin derivative (3-β-l-rhamnosyl-(1→2)[β-l-rhamnosyl-(1→6)]β-d-glucoside | S. stellata (whole plants) [7,76] |
| 38. | Tiliroside | S. stellata (whole plants) [7,76] |
3.4. Lignans
| No. | Compound Name | Species (Part of Plant) |
|---|---|---|
| 1. | (7R, 8S, 7′R, 8′S)-fraxiresinol-4′-O-β-d-glucopyranoside | D. asper (roots) [99] |
| 2. | (7R, 8S, 7′R, 8′S)-prinsepiol- 4-O-β-d-glucopyranoside | D. asper (roots) [99] |
| 3. | (7R, 8S, 7′R, 8′S)-8-hydroxypinoresinol- 4′-O-β-d-glucopyranoside 8′-hydroxypinoresinol-4′-O-β-d-glucopyranoside | D. asper (roots) [96,99] |
| 4. | (+)-1-hydroxy-2,6-bis-epi-pinoresinol | D. asper (roots) [6] |
| 5. | (+)-8-hydroxy-7,7′-bis-epi-pinoresinol | D. asper (roots) [13] |
| 6. | Dipsalignan A ((+)-8-hydroxy-7,7′-bis-epi-fraxiresinol) | D. asper (roots) [6,13] |
| 7. | Dipsalignan B ((+)-(7S, 8S, 7′R, 8′S)-prinsepiol) | D. asper (roots) [6,13] |
| 8. | Dipsalignan C ((+)-(7S, 8S, 7′R, 8′S)-5-methoxyprinsepiol) | D. asper (roots) [6,13] |
| 9. | Dipsalignan D ((+)-(7S, 8R, 7′S, 8′R)-5-methoxyprinsepiol) | D. asper (roots) [6,13] |
| 10. | Syringaresinol-4′,4″-O-bis-β-d-glucoside | D. asper (roots) [97] |
| 11. | Syringaresinol hexoside | S. atropurpurea (stems) [46] |
3.5. Polysaccharides
3.6. Essential Oils
| Compounds (Content) | Species (Part of Plant) |
|---|---|
| n-hexadecanoic acid (2.92–16.00%); 11,14,17-eicosatrienoic acid, methyl ester (15.86% only in dried material); 9,12,15-octadecatrienoic acid, methyl ester (0.65–3.2%) | D. fullonum (roots; fresh or dried) [35] |
| Phytol (61.08–72.31%); 9,12,15-octadecatrienoic acid, methyl ester (6.06% only in fresh material); cyclohexane, cyclopropylidene (2.90–5.20%); n-hexadecanoic acid (2.01–2.34%); 3-buten-2-one, 4-(2,6,6-trimethyl-1-cyclohexen-1-yl) (1.5–3.2%) | D. fullonum (leaves; fresh or dried) [35] |
| Linalool (11.78%); trans-geraniol (8.58%); 1,8-cineole (7.91%); β-caryophyllene (5.58%); α-terpineol (5.32%); β-selinene (5.15%); and spathulenol (5.04%); geranyl acetone (3.88%); α-pinene (3.57%) | D. japonicus (flowering aerial parts) [102] |
| 1,8 cineole (8.1–33.8%); tetradecene (5.7–24.1%); (E)-β-ionone (5.9–20.7%); dihydroactinidiolide (26.1% in chloroform fraction, not detected in hexane fraction); (Z)-jasmone (5.6% in hexane fraction, not detected in chloroform fraction); eugenol (3.6% in hexane fraction, not detected in chloroform fraction); (E)-β-damascenone (3.0–6.4%); (E)-geranylacetone (3.0–9.2%); linalool (3.3–4.9%); 2-hydroxy-5-methylacetophenone (2.9–4.4%); cis-linalool oxide (1.6–3.0%) | S. atropurpurea (stems; volatile fraction extracted by hexane or chloroform) [46] |
| Chrysanthenone (23.43%), camphor (12.98%) and α-thujone (10.7%); α-fenchol (4.08%); sabinene (3.11%); trans-alloocimene (3.03%); | S. arenaria (vegetative parts; stems and leaves) [83] |
| Chrysanthenone (38.52%), camphor (11.7%) and α-thujone (9.5%); α-fenchol (5.86%); filifolone (3.72%); longifolene (3.96%) | S. arenaria (flowers) [83] |
| α-thujone (34.39%), camphor (17.48%), and β-thujone (15.29%); camphene (3.62%); 1,8-cineole (3.48%); sabinene (3.46%) | S. arenaria (fruits) [83] |
3.7. Fatty Acids
| No. | Compound Name | Species (Part of Plant) |
|---|---|---|
| 1. | Behenic acid | S. stellata (aerial parts) [49] |
| 2. | Dodecanoic acid | S. stellata (aerial parts) [49] |
| 3. | Dotriacontanic acid | D. asper [6] |
| 4. | Eicosanoic acid | S. stellata (aerial parts) [49] |
| 5. | Hexadecatrienoic acid | S. stellata (aerial parts) [49] |
| 6. | Lignoceric acid | S. stellata (aerial parts) [49] |
| 7. | Linoleic acid | S. stellata (aerial parts) [49] |
| 8. | Linolenic acid | S. stellata (aerial parts) [49] |
| 9. | Myristic acid | S. stellata (aerial parts) [49] |
| 10. | Palmitic acid | S. stellata (aerial parts) [49] |
| 11. | Pentacosanoic acid | D. asper (roots) [6] |
| 12. | Stearic acid | S. stellata (aerial parts) [49] S. tschilliensis (flowers) [94] |
| 13. | Triacontanoic acid | S. stellata (aerial parts) [49] |
4. Safety of Use
5. Pharmacokinetic and Bioavailability of Some Specialized Metabolites of Dipsaci radix
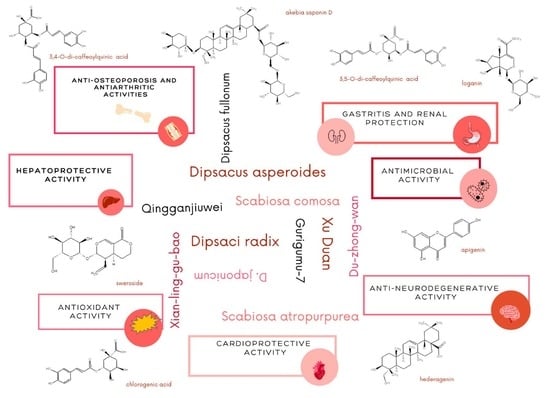
6. Biological Activities of Dipsacus and Scabiosa Species
6.1. Strengthening the Bone Tissue and Antiarthritic Activity
6.2. Anti-Neurodegenerative Activity
6.3. Hepatoprotective Activity
6.4. Cardioprotective Activity
6.5. Renal and Gastritis Protection
6.6. Anti-Asthmatic Effect
6.7. Anti-Diabetic Activity
6.8. Anti-Inflammatory Activity
6.9. Antioxidant Activity
6.10. Anticancer Activity
6.11. Antimicrobial and Anti-Insecticidal Activity
6.12. Others
7. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Pizzino, G.; Irrera, N.; Cucinotta, M.; Pallio, G.; Mannino, F.; Arcoraci, V.; Squadrito, F.; Altavilla, D.; Bitto, A. Oxidative Stress: Harms and Benefits for Human Health. Oxid. Med. Cell. Longev. 2017, 2017, 8416763. [Google Scholar] [CrossRef] [PubMed]
- Forman, H.J.; Zhang, H. Targeting oxidative stress in disease: Promise and limitations of antioxidant therapy. Nat. Rev. Drug Discov. 2021, 20, 689–709. [Google Scholar] [CrossRef] [PubMed]
- Vona, R.; Pallotta, L.; Cappelletti, M.; Severi, C.; Matarrese, P. The Impact of Oxidative Stress in Human Pathology: Focus on Gastrointestinal Disorders. Antioxidants 2021, 10, 201. [Google Scholar] [CrossRef] [PubMed]
- The Global Biodiversity Information Facility. Available online: https://www.gbif.org/ (accessed on 21 January 2023).
- Zhao, Y.-M.; Shi, Y.-P. Phytochemicals and biological activities of Dipsacus species. Chem. Biodivers. 2011, 8, 414–430. [Google Scholar] [CrossRef]
- Tao, Y.; Lin, C.; Yan, J. Traditional uses, processing methods, phytochemistry, pharmacology and quality control of Dipsacus asper Wall. ex C.B. Clarke: A review. J. Ethnopharmacol. 2020, 258, 112912. [Google Scholar] [CrossRef] [PubMed]
- Pinto, D.C.G.A.; Rahmouni, N.; Beghidja, N.; Silva, A.M.S. Scabiosa genus: A rich source of bioactive metabolites. Medicines 2018, 5, 110. [Google Scholar] [CrossRef] [PubMed]
- The World Flora Online Database. Available online: http://www.worldfloraonline.org/ (accessed on 4 February 2023).
- Plants of the World Online Database. Available online: https://powo.science.kew.org/ (accessed on 27 October 2022).
- Flora of China Database. Available online: http://www.efloras.org/ (accessed on 4 February 2023).
- Chen, J.; Yao, D.; Yuan, H.; Zhang, S.; Tian, J.; Guo, W.; Liang, W.; Li, H.; Zhang, Y. Dipsacus asperoides polysaccharide induces apoptosis in osteosarcoma cells by modulating the PI3K/Akt pathway. Carbohydr. Polym. 2013, 95, 780–784. [Google Scholar] [CrossRef]
- Yu, J.-H.; Yu, Z.-P.; Wang, Y.-Y.; Bao, J.; Zhu, K.-K.; Yuan, T.; Zhang, H. Triterpenoids and triterpenoid saponins from Dipsacus asper and their cytotoxic and antibacterial activities. Phytochemistry 2019, 162, 241–249. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Ma, G.; Zhang, D.; Huang, W.; Ding, G.; Hu, H.; Tu, G.; Guo, B. New Lignans and Iridoid Glycosides from Dipsacus asper Wall. Molecules 2015, 20, 2165–2175. [Google Scholar] [CrossRef] [PubMed]
- Tan, L.-H.; Zhang, D.; Yu, B.; Zhao, S.-P.; Wang, J.-W.; Yao, L.; Cao, W.-G. Antioxidant activity and optimization of extraction of polysaccharide from the roots of Dipsacus asperoides. Int. J. Biol. Macromol. 2015, 81, 332–339. [Google Scholar] [CrossRef]
- Tao, Y.; Ren, Y.; Li, W.; Cai, B.; Di, L.; Shi, L.; Hu, L. Comparative pharmacokinetic analysis of extracts of crude and wine-processed Dipsacus asper in rats by a sensitive ultra-performance liquid chromatography-tandem mass spectrometry approach. J. Chromatogr. B. 2016, 1036, 33–41. [Google Scholar] [CrossRef]
- Li, T.; Hua, Q.; Li, N.; Cui, Y.; Zhao, M. Protective effect of a polysaccharide from Dipsacus asper Wall on streptozotocin (STZ)-induced diabetic nephropathy in rat. Int. J. Biol. Macromol. 2019, 133, 1194–1200. [Google Scholar] [CrossRef]
- Liu, K.; Liu, Y.; Xu, Y.; Nandakumar, K.S.; Tan, H.; He, C.; Dang, W.; Lin, J.; Zhou, C. Asperosaponin VI protects against bone destructions in collagen induced arthritis by inhibiting osteoclastogenesis. Phytomedicine 2019, 63, 153006. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.-G.; Zhang, R.; Li, C.; Ma, X.; Liu, L.; Wang, J.-P.; Mei, Q.-B. The osteoprotective effect of Radix Dipsaci extract in ovariectomized rats. J. Ethnopharmacol. 2009, 123, 74–81. [Google Scholar] [CrossRef]
- Chen, D.X.; Li, L.Y.; Zhang, X.; Wang, Y.; Zhang, Z. Genetic diversity and population structure of wild Dipsacus asperoides in China as indicated by ISSR markers. Genet. Mol. Res. 2014, 13, 6340–6349. [Google Scholar] [CrossRef]
- Han, J.-S.; Lee, B.-S.; Han, S.-R.; Han, H.-Y.; Chung, M.-K.; Min, B.-S.; Seok, J.H.; Kim, Y.-B. A subchronic toxicity study of Radix Dipsaci water extract by oral administration in F344 rats. Regul. Toxicol. Pharmacol. 2016, 81, 136–145. [Google Scholar] [CrossRef] [PubMed]
- Choi, N.H.; Jang, J.Y.; Choi, G.J.; Choi, Y.H.; Jang, K.S.; Nguyen, V.T.; Min, B.-S.; Dang, Q.L.; Kim, J.-C. Antifungal activity of sterols and dipsacus saponins isolated from Dipsacus asper roots against phytopathogenic fungi. Pestic. Biochem. Phys. 2017, 141, 103–108. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Zhang, J.; Sun, X.; Xiao, C.; Wang, X.; Zhou, T. Comprehensive separation of iridoid glycosides and triterpenoid saponins from Dipsacus asper with salt-containing solvent by high-speed countercurrent chromatography coupled with recycling mode. J. Sep. Sci. 2020, 43, 1265–1274. [Google Scholar] [CrossRef]
- Li, F.; Yang, X.; Bi, J.; Yang, Z.; Zhang, C. Antiosteoporotic activity of Du-Zhong-Wan water extract in ovariectomized rats. Pharm. Biol. 2016, 54, 1857–1864. [Google Scholar] [CrossRef]
- Hung, T.M.; Na, M.K.; Thuong, P.T.; Su, N.D.; Sok, D.E.; Song, K.S.; Seong, Y.H.; Bae, K.H. Antioxidant activity of caffeoyl quinic acid derivatives from the roots of Dipsacus asper Wall. J. Ethnopharmacol. 2006, 108, 188–192. [Google Scholar] [CrossRef]
- Gu, M.; Jin, J.; Ren, C.; Chen, X.; Gao, W.; Wang, X.; Wu, Y.; Tian, N.; Pan, Z.; Wu, A.; et al. Akebia Saponin D suppresses inflammation in chondrocytes via NRF2/HO-1/NF-κB axis and ameliorates osteoarthritis in Mice. Food Funct. 2020, 11, 10852–10863. [Google Scholar] [CrossRef]
- Park, J.-Y.; Park, S.-D.; Koh, Y.J.; Kim, D.-I.; Lee, J.-H. Aqueous extract of Dipsacus asperoides suppresses lipopolysaccharide-stimulated inflammatory responses by inhibiting the ERK1/2 signaling pathway in RAW 264.7 macrophages. J. Ethnopharmacol. 2019, 231, 253–261. [Google Scholar] [CrossRef]
- Shin, N.-R.; Lee, A.Y.; Park, G.; Ko, J.-W.; Kim, J.-C.; Shin, I.-S.; Kim, J.-S. Therapeutic Effect of Dipsacus asperoides C. Y. Cheng et T. M. Ai in Ovalbumin-Induced Murine Model of Asthma. Int. J. Mol. Sci. 2019, 20, 1855. [Google Scholar] [CrossRef] [PubMed]
- Wagner, H.; Bauer, R.; Melchart, D.; Xiao, P.-G.; Staudinger, A. (Eds.) Chromatographic Fingerprint Analysis of Herbal Medicines, Thin-layer and High Performance Liquid Chromatography of Chinese Drugs, Second, Revised and Enlarged Edition; Springer: Wien, Austria, 2011; Volume 1, pp. 677–689. [Google Scholar]
- Pearson, J.L.; Lee, S.; Suresh, H.; Low, M.; Nang, M.; Singh, S.; Lamin, F.; Kazzem, M.; Sullivan, S.; Khoo, C.S. The Liquid Chromatographic Determination of Chlorogenic and Caffeic Acids in Xu Duan (Dipsacus asperoides) Raw Herb. ISRN Anal. Chem. 2014, 2014, 968314. [Google Scholar] [CrossRef]
- Chun, J.M.; Lee, A.Y.; Nam, J.Y.; Lim, K.S.; Choe, M.S.; Lee, M.Y.; Kim, C.; Kim, J.-S. Effects of Dipsacus asperoides Extract on Monosodium Iodoacetate-Induced Osteoarthritis in Rats Based on Gene Expression Profiling. Front. Pharmacol. 2021, 12, 615157. [Google Scholar] [CrossRef]
- Zhang, Z.-J.; Qian, Y.-H.; Hu, H.-T.; Yang, J.; Yang, G.-D. The herbal medicine Dipsacus asper Wall extract reduces the cognitive deficits and overexpression of β-amyloid protein induced by aluminum exposure. Life Sci. 2003, 73, 2443–2454. [Google Scholar] [CrossRef]
- Gong, L.-L.; Yang, S.; Liu, H.; Zhang, W.; Ren, L.-L.; Han, F.-F.; Lv, Y.-L.; Wan, Z.-R.; Liu, L.-H. Anti-nociceptive and anti-inflammatory potentials of Akebia saponin D. Eur. J. Pharmacol. 2018, 845, 85–90. [Google Scholar] [CrossRef] [PubMed]
- Oszmiański, J.; Wojdyło, A.; Juszczyk, P.; Nowicka, P. Roots and leaf extracts of Dipsacus fullonum L. and their biological activities. Plants 2020, 9, 78. [Google Scholar] [CrossRef] [PubMed]
- Saar-Reismaa, P.; Bragina, O.; Kuhtinskaja, M.; Reile, I.; Laanet, P.-R.; Kulp, M.; Vaher, M. Extraction and fractionation of bioactives from Dipsacus fullonum L. leaves and evaluation of their anti-Borrelia activity. Pharmaceuticals 2022, 15, 87. [Google Scholar] [CrossRef]
- Witkowska-Banaszczak, E. Dipsacus fullonum L. leaves and roots-identification of the components of the essential oils and alpha-amylase inhibitory activities of methanolic extracts. Acta Pol. Pharm. 2018, 75, 951–957. [Google Scholar] [CrossRef]
- Bullitta, S.; Piluzza, G.; Viegi, L. Plant resources used for traditional ethnoveterinary phytotherapy in Sardinia (Italy). Genet. Resour. Crop Evol. 2007, 54, 1447–1464. [Google Scholar] [CrossRef]
- Jeelani, S.M.; Rather, G.A.; Sharma, A.; Lattoo, S.K. In perspective: Potential medicinal plant resources of Kashmir Himalayas, their domestication and cultivation for commercial exploitation. J. Appl. Res. Med. Aromat. Plants. 2018, 8, 10–25. [Google Scholar] [CrossRef]
- Kanta, C.; Sharma, I.P.; Shiekh, M.A. Ethnobotanical studies on medicinal plants of Langate area, Kupwara, Jammu and Kashmir, India. J. Med. Plants Stud. 2018, 6, 94–97. [Google Scholar]
- Hassan, S.; Sajjad, N.; Khan, S.U.; Gupta, S.; Bhat, M.A.; Ali, R.; Ahmad, Z.; Ganie, S.A.; Hamid, R. Dipsacus inermis Wall. modulates inflammation by inhibiting NF-κB pathway: An in vitro and in vivo study. J. Ethnopharmacol. 2020, 254, 112710. [Google Scholar] [CrossRef]
- Cham, B.T.; Linh, N.T.T.; Thao, D.T.; Anh, N.T.H.; Tam, N.T.; Anh, B.K.; Muscari, I.; Adorisio, S.; Sung, T.V.; Thuy, T.T.; et al. Cell growth inhibition of saponin XII from Dipsacus japonicus Miq. on acute myeloid leukemia cells. Molecules 2020, 25, 3325. [Google Scholar] [CrossRef]
- World Health Organization; Regional Office for the Western Pacific. Medicinal Plants in Viet Nam; Series No. 3; Dan, N.V., Ed.; WHO Regional Office for the Western Pacific: Manila, Philippines, 1990; pp. 151–152. ISBN 9290611014. Available online: https://apps.who.int/iris/handle/10665/207579 (accessed on 21 January 2023).
- Yang, B.; Feng, X.; Xu, J.; Lei, H.; Zhang, L. Multi-Component HPLC Analysis and Antioxidant Activity Characterization of Extracts from Dipsacus sativus (Linn.) Honck. Int. J. Food Prop. 2016, 19, 1000–1006. [Google Scholar] [CrossRef]
- Elhawary, S.S.; Eltantawy, M.E.; Sleem, A.A.; Abdallah, H.M.; Mohamed, N.M. Investigation of Phenolic Content and Biological Activities of Scabiosa atropurpurea L. World Appl. Sci. J. 2011, 15, 311–317. [Google Scholar]
- Bonet, M.À.; Parada, M.; Selga, A.; Vallés, J. Studies on pharmaceutical ethnobotany in the regions of L’Alt Empordà and Les Guilleries (Catalonia, Iberian Peninsula). J. Ethnopharmacol. 1999, 68, 145–168. [Google Scholar] [CrossRef]
- Erarslan, Z.B.; Yeşil, Y. The anatomical properties of Scabiosa atropurpurea L. (Caprifoliaceae). Istanbul J. Pharm. 2018, 48, 1–5. [Google Scholar] [CrossRef]
- Hrichi, S.; Chaabane-Banaoues, R.; Bayar, S.; Flamini, G.; Majdoub, Y.O.E.; Mangraviti, D.; Mondello, L.; Mzoughi, R.E.; Babba, H.; Mighri, Z.; et al. Botanical and Genetic Identification Followed by Investigation of Chemical Composition and Biological Activities on the Scabiosa atropurpurea L. Stem from Tunisian Flora. Molecules 2020, 25, 5032. [Google Scholar] [CrossRef]
- Ma, Y.; Yuan, H.; Jin, R.; Bao, X.; Wang, H.; Su, X.; Mu, M.G.S.L.; Liang, J.; Zhang, J.; Wu, X. Flavonoid-rich Scabiosa comosa inflorescence extract attenuates CCl4-induced hepatic fibrosis by modulating TGF-β-induced Smad3 phosphorylation. Biomed. Pharmacother. 2018, 106, 426–433. [Google Scholar] [CrossRef]
- Lehbili, M.; Magid, A.A.; Kabouche, A.; Voutquenne-Nazabadioko, L.; Morjani, H.; Harakat, D.; Kabouche, Z. Triterpenoid saponins from Scabiosa stellata collected in North-eastern Algeria. Phytochemistry 2018, 150, 40–49. [Google Scholar] [CrossRef]
- Rahmouni, N.; Pinto, D.C.G.A.; Santos, S.A.O.; Beghidja, N.; Silva, A.M.S. Lipophilic composition of Scabiosa stellata L.: An underexploited plant from Batna (Algeria). Chem. Pap. 2018, 72, 753–762. [Google Scholar] [CrossRef]
- Mouffouk, C.; Hambaba, L.; Haba, H.; Mouffouk, S.; Bensouici, C.; Mouffouk, S.; Hachemi, M.; Khadraoui, H. Acute toxicity and in vivo anti-inflammatory effects and in vitro antioxidant and anti-arthritic potential of Scabiosa stellata. Orient. Pharm. Exp. Med. 2018, 18, 335–348. [Google Scholar] [CrossRef]
- Zheng, Q.; Koike, K.; Han, L.-K.; Okuda, H.; Nikaido, T. New Biologically Active Triterpenoid Saponins from Scabiosa tschiliensis. J. Nat. Prod. 2004, 67, 604–613. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Liu, K.; Xu, D.; Wang, Q.; Bi, K.; Song, Y.; Li, J.; Zhang, L. Rapid micropropagation system in vitro and antioxidant activity of Scabiosa tschiliensis Grunning. Plant Growth Regul. 2013, 69, 305–310. [Google Scholar] [CrossRef]
- Wang, J.; Liu, K.; Li, X.; Bi, K.; Zhang, Y.; Huang, J.; Zhang, R. Variation of active constituents and antioxidant activity in Scabiosa tschiliensis Grunning from different stages. J. Food Sci. Technol. 2017, 54, 2288–2295. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Jun, M.; Park, S.; Park, S. Lineage-Specific Variation in IR Boundary Shift Events, Inversions, and Substitution Rates among Caprifoliaceae s.l. (Dipsacales) Plastomes. Int. J. Mol. Sci. 2021, 22, 10485. [Google Scholar] [CrossRef] [PubMed]
- Carlson, S.E.; Linder, H.P.; Donoghue, M.J. The historical biogeography of Scabiosa (Dipsacaceae): Implications for Old World plant disjunctions. J. Biogeogr. 2012, 39, 1086–1100. [Google Scholar] [CrossRef]
- Essghaier, B.; Toukabri, N.; Dridi, R.; Hannachi, H.; Limam, I.; Mottola, F.; Mokni, M.; Zid, M.F.; Rocco, L.; Abdelkarim, M. First Report of the Biosynthesis and Characterization of Silver Nanoparticles Using Scabiosa atropurpurea subsp. maritima Fruit Extracts and Their Antioxidant, Antimicrobial and Cytotoxic Properties. Nanomaterials 2022, 12, 1585. [Google Scholar] [CrossRef]
- Ryder, M.L. Teasel Growing for Cloth Raising. Folk Life 1969, 7, 117–119. [Google Scholar] [CrossRef]
- Rector, B.G.; Harizanova, V.; Sforza, V.; Widmer, T.; Wiedenmann, R.N. Prospects for biological control of teasels, Dipsacus spp., a new target in the United States. Biol. Control 2006, 36, 1–14. [Google Scholar] [CrossRef]
- Akar, Z. Chemical compositions by using LC-MS/MS and GC-MS and antioxidant activities of methanolic extracts from leaf and flower parts of Scabiosa columbaria subsp. columbaria var. columbaria L. Saudi. J. Biol. Sci. 2021, 28, 6639–6644. [Google Scholar] [CrossRef]
- Jin, H.; Yu, H.; Wang, H.; Zhang, J. Comparative Proteomic Analysis of Dipsacus asperoides Roots from Different Habitats in China. Molecules 2020, 25, 3605. [Google Scholar] [CrossRef]
- Wong, R.W.K.; Rabie, A.B.M.; Hägg, E.U.O. The Effect of Crude Extract from Radix Dipsaci on Bone in Mice. Phytother. Res. 2007, 21, 596–598. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Zhou, C.; Li, Y.; Gao, F.; Wu, H.; Yang, L.; Qiu, W.; Zhu, L.; Du, X.; Lin, W.; et al. Asperosaponin VI promotes progesterone receptor expression in decidual cells via the notch signaling pathway. Fitoterapia 2016, 113, 58–63. [Google Scholar] [CrossRef]
- Cong, G.; Cui, L.; Zang, M.; Hao, L. Attenuation of renal ischemia/reperfusion injury by a polysaccharide from the roots of Dipsacus asperoides. Int. J. Biol. Macromol. 2013, 56, 14–19. [Google Scholar] [CrossRef] [PubMed]
- Ke, K.; Li, Q.; Yang, X.; Xie, Z.; Wang, Y.; Shi, J.; Chi, L.; Xu, W.; Hu, L.; Shi, H. Asperosaponin VI promotes bone marrow stromal cell osteogenic differentiation through the PI3K/AKT signaling pathway in an osteoporosis model. Sci. Rep. 2016, 6, 35233. [Google Scholar] [CrossRef] [PubMed]
- Tao, Y.; Du, Y.; Su, D.; Li, W.; Cai, B. UHPLC–MS/MS quantification combined with chemometrics for the comparative analysis of different batches of raw and wine-processed Dipsacus asper. J. Sep. Sci. 2017, 40, 1686–1693. [Google Scholar] [CrossRef]
- Tao, Y.; Huang, S.; Li, W.; Cai, B. Simultaneous Determination of Ten Bioactive Components in Raw and Processed Radix Dipsaci by UPLC-Q-TOF-MS. J. Chromatogr. Sci. 2019, 57, 122–129. [Google Scholar] [CrossRef]
- Tao, Y.; Chen, X.; Ren, Y.C.; Cai, B.C. Study on regulation of wine-processed dipsacus asper on OPG/RANK/RANKL axis system in osteoporosis rats. J. Mod. Med. Health 2016, 32, 1127–1129. [Google Scholar]
- Tao, Y.; Chen, X.; Li, W.; Cai, B.; Di, L.; Shi, L.; Hu, L. Global and Untargeted Metabolomics Evidence of the Protective Effect of Different Extracts of Dipsacus asper Wall. ex C.B. Clarke on Estrogen Deficiency after Ovariectomia in Rats. J. Ethnopharmacol. 2017, 199, 20–29. [Google Scholar] [CrossRef] [PubMed]
- Zhao, B.T.; Jeong, S.Y.; Moon, D.C.; Son, K.H.; Son, J.K.; Woo, M.H. Quantitative determination and pattern recognition analyses of bioactive marker compounds from Dipsaci Radix by HPLC. Arch. Pharm. Res. 2013, 36, 1345–1353. [Google Scholar] [CrossRef]
- Chun, J.M.; Lee, A.Y.; Moon, B.C.; Choi, G.; Kim, J.-S. Effects of Dipsacus asperoides and Phlomis umbrosa Extracts in a Rat Model of Osteoarthritis. Plants 2021, 10, 2030. [Google Scholar] [CrossRef] [PubMed]
- Kaul, M.K. High altitude botanicals in integrative medicine-Case studies from Northwest Himalaya. Indian J. Trad. Knowl. 2010, 9, 18–25. [Google Scholar]
- Besbes Hlila, M.; Omri, A.; Ben Jannet, H.; Lamari, A.; Aouni, M.; Selmi, B. Phenolic composition, antioxidant and anti-acetylcholinesterase activities of the Tunisian Scabiosa arenaria. Pharm. Biol. 2013, 51, 525–532. [Google Scholar] [CrossRef] [PubMed]
- Besbes Hlila, M.; Mosbah, H.; Mssada, K.; Benn Jannet, H.; Aouni, M.; Selmi, B. Acetylcholinesterase inhibitory and antioxidant properties of roots extracts from the Tunisian Scabiosa arenaria Forssk. Ind. Crops Prod. 2015, 67, 62–69. [Google Scholar] [CrossRef]
- Ma, J.N.; Bolraa, S.; Ji, M.; He, Q.Q.; Ma, C.M. Quantification and antioxidant and anti-HCV activities of the constituents from the inflorescences of Scabiosa comosa and S. tschilliensis. Nat. Prod. Res. 2016, 30, 590–594. [Google Scholar] [CrossRef]
- Lehbili, M.; Magid, A.A.; Hubert, J.; Kabouche, A.; Voutquenne-Nazabadioko, L.; Renault, J.-H.; Nuzillard, J.-M.; Morjani, H.; Abedini, A.; Gangloff, S.C.; et al. Two new bis-iridoids isolated from Scabiosa stellata and their antibacterial, antioxidant, anti-tyrosinase and cytotoxic activities. Fitoterapia 2018, 125, 41–48. [Google Scholar] [CrossRef]
- Rahmouni, N.; Pinto, D.C.G.A.; Beghidja, N.; Benayache, S.; Silva, A.M.S. Scabiosa stellata L. phenolic content clarifies its antioxidant activity. Molecules 2018, 23, 1285. [Google Scholar] [CrossRef]
- Mouffouk, C.; Mouffouk, S.; Hambaba, L.; Haba, H.; Mouffouk, S. Evaluation of cytotoxic effect, anticholinesterase, antioxidant, antiarthritic and antibacterial activities of the Algerian species Scabiosa stellata L. In Proceedings of the International Conference on Veterinary, Agriculture and Life Science, The Eurasia Proceedings of Science, Technology, Engineering & Mathematics, Antalya, ICVALS 2019, Antalya, Turkey, 26–29 October 2019; Volume 8, pp. 1–11, ISSN 2602-3199. [Google Scholar]
- Kılınc, H.; Masullo, M.; Lauro, G.; D’Urso, G.; Alankus, O.; Bifulco, G.; Piacente, S. Scabiosa atropurpurea: A rich source of iridoids with α-glucosidase inhibitory activity evaluated by in vitro and in silico studies. Phytochemistry 2023, 205, 113471. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Wang, Y.; Ma, F.; Han, M.; Wang, Z.; Xue, P.; Lu, J. Systematic profiling of the effective ingredients and mechanism of Scabiosa comosa and S. tschilliensis against hepatic fibrosis combined with network pharmacology. Sci. Rep. 2021, 11, 2600. [Google Scholar] [CrossRef]
- Menggensilimu, Y.H.; Zhao, C.; Bao, X.; Wang, H.; Liang, J.; Yan, Y.; Zhang, C.; Jin, R.; Ma, L.; Zhang, J.; et al. Anti-liver fibrosis effect of total flavonoids from Scabiosa comosa Fisch. ex Roem. et Schult. on liver fibrosis in rat models and its proteomics analysis. Ann. Palliat. Med. 2020, 9, 272–285. [Google Scholar] [CrossRef]
- Toumia, I.B.; Sobeh, M.; Ponassi, M.; Banelli, B.; Dameriha, A.; Wink, M.; Ghedira, L.C.; Rosano, C. A Methanol Extract of Scabiosa atropurpurea Enhances Doxorubicin Cytotoxicity against Resistant Colorectal Cancer Cells In Vitro. Molecules 2020, 25, 5265. [Google Scholar] [CrossRef]
- Besbes Hlila, M.; Mosbah, H.; Majouli, K.; Ben Nejma, A.; Ben Jannet, H.; Mastouri, M.; Aouni, M.; Selmi, B. Antimicrobial Activity of Scabiosa arenaria Forssk. Extracts and Pure Compounds Using Bioguided Fractionation. Chem. Biodivers. 2016, 13, 1262–1272. [Google Scholar] [CrossRef]
- Besbes, M.; Amel Omri, A.; Cheraif, I.; Daami, M.; Ben Jannet, H.; Mastouri, M.; Aouni, M.; Selmi, B. Chemical Composition and Antimicrobial Activity of Essential Oils from Scabiosa arenaria Forssk. Growing Wild in Tunisia. Chem. Biodivers. 2012, 9, 829–839. [Google Scholar] [CrossRef] [PubMed]
- Polat, E.; Alankus-Caliskan, Ö.; Karayildirim, T.; Bedir, E. Iridoids from Scabiosa atropurpurea L. subsp. maritima Arc. (L.). Biochem. Syst. Ecol. 2010, 38, 253–255. [Google Scholar] [CrossRef]
- Ge, H.Y.; Zhang, S.H.; Zhao, B.S.; Yu, C.K. Clinical effect of Mongolian medicine Qinggan Jiuwei powder in treatment of alcoholic liver fibrosis. J. Clin. Hepatol. 2017, 33, 2316–2320. [Google Scholar] [CrossRef]
- Ge, H.; Wang, A.; Su, Y.; Yu, C.L.; Gao, L.; Li, Y. Ameliorative effects of Qingganjiuwei powder, a traditional Mongolian medicine, against CCl4-induced liver fibrosis in rats. J. Ethnopharmacol. 2021, 264, 113226. [Google Scholar] [CrossRef]
- Xu, H.; Ma, Q.; Ma, J.; Wu, Z.; Wang, Y.; Ma, C. Hepato-protective effects and chemical constituents of a bioactive fraction of the traditional compound medicine-Gurigumu-7. BMC Complement. Altern. Med. 2016, 16, 179. [Google Scholar] [CrossRef] [PubMed]
- Wei, F.; Lou, Z.C.; Gao, M.; Miao, Z.C. Application of new techniques of NMR in the structure elucidation of Japondipsaponin E-1 isolated from Dipsacus japonicus Miq. Yaoxue Xuebao 1995, 30, 831–837. [Google Scholar]
- Tian, X.-Y.; Wang, Y.-H.; Liu, H.-Y.; Yu, S.-S.; Fang, W.-S. On the Chemical Constituents of Dipsacus asper. Chem. Pharm. Bull. 2007, 55, 1677–1681. [Google Scholar] [CrossRef]
- Jeong, S.-I.; Zhou, B.; Bae, J.-B.; Kim, N.-S.; Kim, S.-G.; Kwon, J.; Kim, D.-K.; Shin, T.-Y.; Jeon, H.; Lim, J.-P.; et al. Apoptosis-inducing Effect of Akebia Saponin D from the Roots of Dipsacus asper Wall in U937 Cells. Arch. Pharm. Res. 2008, 31, 1399–1404. [Google Scholar] [CrossRef]
- Hwang, S.A.; Hwang, I.Y.; Jung, J.; Kang, H.K.; Son, K.H.; Jeong, C.S. Dipsacus saponin C from Dipsacus asper Reduces the Risk of Gastritis and Gastric Ulcer in Rats. Food Nutr. Sci. 2012, 3, 931–941. [Google Scholar] [CrossRef]
- Li, F.; Tanaka, K.; Watanabe, S.; Tezuka, Y.; Saiki, I. Dipasperoside A, a Novel Pyridine Alkaloid-Coupled Iridoid Glucoside from the Roots of Dipsacus asper. Chem. Pharm. Bull. 2013, 61, 1318–1322. [Google Scholar] [CrossRef]
- Ling, Y.; Liu, K.; Zhang, Q.; Liao, L.; Lu, Y. High performance liquid chromatography coupled to electrospray ionization and quadrupole time-of-flight–mass spectrometry as a powerful analytical strategy for systematic analysis and improved characterization of the major bioactive constituents from Radix Dipsaci. J. Pharm. Biomed. Anal. 2014, 98, 120–129. [Google Scholar] [CrossRef]
- Wang, G.-V.; Zhao, Z.-L.; Xue, P.-F.; Ma, F.-X.; Zhang, D.-Y.; Wang, N.-N.; Li, M.-H. Chemical constituents from flowers of Scabiosa tschilliensis. Zhongguo Zhong Yao Za Zhi 2015, 40, 807–813. [Google Scholar]
- Du, W.; Li, X.; Yang, Y.; Yue, X.; Jiang, D.; Ge, W.; Cai, B. Quantitative determination, principal component analysis and discriminant analysis of eight marker compounds in crude and sweated Dipsaci Radix by HPLC-DAD. Pharm. Biol. 2017, 55, 2129–2135. [Google Scholar] [CrossRef]
- Yu, Z.-P.; Wang, Y.-Y.; Yu, S.-J.; Bao, J.; Yu, J.-H.; Zhang, H. Absolute structure assignment of an iridoid-monoterpenoid indole alkaloid hybrid from Dipsacus asper. Fitoterapia 2019, 135, 99–106. [Google Scholar] [CrossRef]
- Ji, D.; Zhang, C.; Li, J.; Yang, H.; Shen, J.; Yang, Z. A New Iridoid Glycoside from the Roots of Dipsacus asper. Molecules 2012, 17, 1419–1424. [Google Scholar] [CrossRef]
- Li, F.; Tanaka, K.; Watanabe, S.; Tezuka, Y.; Dipasperoside, B. A New Trisiridoid Glucoside from Dipsacus asper. Nat. Prod. Commun. 2016, 11, 891–894. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Zhang, Y.; Yang, Y.; Liu, J.; Zheng, W.; Ma, B.; Guo, B. Qualitative and quantitative analysis of furofuran lignans, iridoid glycosides, and phenolic acids in Radix Dipsaci by UHPLC-Q-TOF/MS and UHPLC-PDA. J. Pharm. Biomed. Anal. 2018, 154, 40–47. [Google Scholar] [CrossRef]
- Li, F.; Nishidono, Y.; Tanaka, K.; Watanabe, S.; Tezuka, Y. A New Monoterpenoid Glucoindole Alkaloid from Dipsacus asper. Nat. Prod. Commun. 2020, 15, 1934578X20917292. [Google Scholar] [CrossRef]
- Kuhtinskaja, M.; Vaher, M. Extraction and analysis of bioactive compounds from Dipsacus Fullonum and Galium Verum for Lyme borreliosis treatment. Biomed. J. Sci. Tech. Res. 2018, 11, 8614–8616. [Google Scholar] [CrossRef]
- Liu, Z.L.; Jiang, G.H.; Zhou, L.; Liu, Q.Z. Analysis of the essential oil of Dipsacus japonicus flowering aerial parts and its insecticidal activity against Sitophilus zeamais and Tribolium castaneum. Z. Naturforsch. C J. Biosci. 2013, 68, 13–18. [Google Scholar] [CrossRef]
- Zeng, J.; Huang, T.; Xue, M.; Chen, J.; Feng, L.; Du, R.; Feng, Y. Current knowledge and development of hederagenin as a promising medicinal agent: A comprehensive review. RSC Adv. 2018, 8, 24188. [Google Scholar] [CrossRef]
- Xu, J.; Hu, Z.; He, H.; Ou, X.; Yang, Y.; Xiao, C.; Yang, C.; Li, L.; Jiang, W.; Zhou, T. Transcriptome analysis reveals that jasmonic acid biosynthesis and signaling is associated with the biosynthesis of asperosaponin VI in Dipsacus asperoides. Front. Plant Sci. 2022, 13, 1022075. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Wang, X.; Zhao, S.; Sun, W.; Tong, S. Preparative separation of structural isomeric pentacyclic triterpene oleanolic acid and ursolic acid from natural products by pH-zone-refining countercurrent chromatography. RSC Adv. 2019, 9, 38860. [Google Scholar] [CrossRef]
- Deng, S.; West, B.J.; Jensen, C.J. UPLC-TOF-MS Characterization and Identification of Bioactive Iridoids in Cornus mas Fruit. J. Anal. Methods Chem. 2013, 2013, 710972. [Google Scholar] [CrossRef]
- Chiu, C.-H.; Lin, K.-H.; Lin, H.-H.; Chu, W.-X.; Lai, Y.-C.; Chao, P.-Y. Analysis of Chlorogenic Acid in Sweet Potato Leaf Extracts. Plants 2022, 11, 2063. [Google Scholar] [CrossRef]
- Bligh, S.W.A.; Ogegbo, O.; Wang, Z.T. Flavonoids by HPLC. In Natural Products; Ramawat, K., Mérillon, J.M., Eds.; Springer: Berlin/Heidelberg, Germany, 2013; pp. 2107–2144. [Google Scholar] [CrossRef]
- PubChem Database. Available online: https://pubchem.ncbi.nlm.nih.gov/ (accessed on 21 January 2023).
- Xu, D.; Liu, J.; Zheng, W.; Gao, Q.; Gao, Y.; Leng, X. Identification of Polysaccharides from Dipsacus asperoides and Their Effects on Osteoblast Proliferation and Differentiation in a High-Glucose Environment. Front. Pharmacol. 2022, 13, 851956. [Google Scholar] [CrossRef]
- Sun, X.; Wei, B.; Peng, Z.; Fu, Q.; Wang, C.; Zhen, J.; Sun, J. Protective effects of Dipsacus asper polysaccharide on osteoporosis in vivo by regulating RANKL/RANK/OPG/VEGF and PI3K/Akt/eNOS pathway. Int. J. Biol. Macromol. 2019, 129, 579–587. [Google Scholar] [CrossRef] [PubMed]
- Huang, T.-Y.; Shahrousvand, M.; Hsu, Y.-T.; Su, W.-T. Polycaprolactone/Polyethylene Glycol Blended with Dipsacus asper Wall Extract Nanofibers Promote Osteogenic Differentiation of Periodontal Ligament Stem Cells. Polymers 2021, 13, 2245. [Google Scholar] [CrossRef]
- Niu, Y.B.; Li, Y.H.; Kong, X.H.; Zhang, R.; Sun, Y.; Li, Q.; Li, C.; Liu, L.; Wang, J.; Mei, Q.B. The beneficial effect of Radix Dipsaci total saponins on bone metabolism in vitro and in vivo and the possible mechanisms of action. Osteoporos. Int. 2012, 23, 2649–2660. [Google Scholar] [CrossRef] [PubMed]
- Niu, Y.-T.; Xie, L.; Deng, R.-R.; Zhang, X.-Y. In the presence of TGF-β1, Asperosaponin VI promotes human mesenchymal stem cell differentiation into nucleus pulposus like-cells. BMC Complement. Med. Ther. 2021, 21, 32. [Google Scholar] [CrossRef] [PubMed]
- Zhou, D.; Zhang, H.; Xue, X.; Tao, Y.; Wang, S.; Ren, X.; Su, J. Safety Evaluation of Natural Drugs in Chronic Skeletal Disorders: A Literature Review of Clinical Trials in the Past 20 years. Front. Pharmacol. 2022, 12, 801287. [Google Scholar] [CrossRef]
- Zhan, H.S.; Shi, Y.Y.; Zhao, Y.F.; Hu, X.Y. Phase III Clinical Investigation of Xu Duan Zhuang Gu Capsules to the Primary Osteoporotic Patient. Chin. J. Osteoporo. 2009, 15, 197–200. [Google Scholar]
- Xiao, T.T.; Xu, M.; Yang, X.H.; Kok, L.; Chow, Y.L.; Zhao, Z.Z.; Leung, K.S.-Y.; Yang, Z.J.; Tian, X.Y.; Li, Z.; et al. The evaluation on embryo toxicity of Dipsaci Radix with mice and embryonic stem cells. J. Ethnopharmacol. 2014, 151, 114–122. [Google Scholar] [CrossRef]
- Huang, Y.-X.; Liu, E.-W.; Wang, L.; Huo, Y.; Wang, Q.; Olaleye, O.; Wang, T.; Gao, X.-M. LC/MS/MS determination and pharmacokinetic studies of six compounds in rat plasma following oral administration of the single and combined extracts of Eucommia ulmoides and Dipsacus asperoides. Chin. J. Nat. Med. 2014, 12, 469–476. [Google Scholar] [CrossRef]
- Wang, Q.-H.; Yang, X.-L.; Xiao, W.; Wang, Z.-Z.; Ding, G.; Huang, W.-Z.; Yang, Z.-L.; Zhang, C.-F. Microcrystalline Preparation of Akebia Saponin D for its Bioavailability Enhancement in Rats. Am. J. Chin. Med. 2015, 43, 513–528. [Google Scholar] [CrossRef]
- Tao, Y.; Du, Y.; Li, W.; Cai, B.; Di, L.; Shi, L.; Hu, L. Integrating UHPLC–MS/MS quantification and DAS analysis to investigate the effects of wine-processing on the tissue distributions of bioactive constituents of herbs in rats: Exemplarily shown for Dipsacus asper. J. Chromatogr. B. Anal. Technol. Biomed. Life Sci. 2017, 1055–1056, 135–143. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.-S.; Kim, Y.-C.; Zadeh, H.; Park, Y.-J.; Pi, S.-H.; Shin, H.-S.; You, H.-K. Effects of the dichloromethane fraction of Dipsaci Radix on the osteoblastic differentiation of human alveolar bone marrow-derived mesenchymal stem cells. Biosci. Biotechnol. Biochem. 2011, 75, 13–19. [Google Scholar] [CrossRef]
- Liu, M.; Xiao, G.G.; Rong, P.; Zhang, Z.; Dong, J.; Zhao, H.; Li, H.; Li, Y.; Pan, J.; Liu, H.; et al. Therapeutic effects of radix dipsaci, pyrola herb, and cynomorium songaricum on bone metabolism of ovariectomized rat. BMC Complement. Altern. Med. 2012, 12, 67. Available online: http://www.biomedcentral.com/1472-6882/12/67 (accessed on 21 January 2023). [CrossRef]
- Niu, Y.; Li, C.; Pan, Y.; Li, Y.; Kong, X.; Wang, S.; Zhai, Y.K.; Wu, X.; Fan, W.; Mei, Q. Treatment of Radix Dipsaci extract prevents long bone loss induced by modeled microgravity in hindlimb unloading rats. Pharm. Biol. 2015, 53, 110–116. [Google Scholar] [CrossRef]
- Niu, Y.-B.; Kong, X.-H.; Li, Y.-H.; Fan, L.; Pan, Y.-L.; Li, C.-R.; Wu, X.-L.; Lu, T.-L.; Mei, Q.-B. Radix Dipsaci total saponins stimulate MC3T3-E1 cell differentiation via the bone morphogenetic protein-2/MAPK/Smad-dependent Runx2 pathway. Mol. Med. Rep. 2015, 11, 4468–4472. [Google Scholar]
- Wang, X.; He, Y.; Guo, B.; Tsang, M.-C.; Tu, F.; Dai, Y.; Yao, Z.; Zheng, L.; Xie, X.; Wang, N.; et al. In Vivo Screening for Anti-osteoporotic Fraction from Extract of Herbal Formula Xianlinggubao in Ovariectomized Mice. PLoS ONE 2015, 10, e0118184. [Google Scholar] [CrossRef]
- He, J.; Li, X.; Wang, Z.; Bennett, S.; Chen, K.; Xiao, Z.; Zhan, J.; Chen, S.; Hou, Y.; Chen, J.; et al. Therapeutic Anabolic and Anticatabolic Benefits of Natural Chinese Medicines for the Treatment of Osteoporosis. Front. Pharmacol. 2019, 10, 1344. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Xue, K.; Gao, Y.; Huai, Y.; Wang, W.; Miao, Z.; Dang, K.; Jiang, S.; Qian, A. Systems pharmacology dissection of action mechanisms of Dipsaci Radix for osteoporosis. Life Sci. 2019, 235, 116820. [Google Scholar] [CrossRef]
- Wu, Q.-C.; Tang, X.-Y.; Dai, Z.-Q.; Dai, Y.; Xiao, H.-H.; Yao, X.-S. Sweroside promotes osteoblastic differentiation and mineralization via interaction of membrane estrogen receptor-α and GPR30 mediated p38 signalling pathway on MC3T3-E1 cells. Phytomedicine 2020, 68, 153146. [Google Scholar] [CrossRef] [PubMed]
- Aibar-Almazán, A.; Voltes-Martínez, A.; Castellote-Caballero, Y.; Afanador-Restrepo, D.F.; Carcelén-Fraile, M.d.C.; López-Ruiz, E. Current Status of the Diagnosis and Management of Osteoporosis. Int. J. Mol. Sci. 2022, 23, 9465. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, H.M.M.; Yeh, J.-Y.; Tang, Y.-C.; Cheng, W.T.-K.; Ou, B.-R. Molecular screening of Chinese medicinal plants for progestogenic and antiprogestogenic activity. J. Biosci. 2014, 39, 453–461. [Google Scholar] [CrossRef] [PubMed]
- Niu, J.; Wang, Y.; Meng, Y.; Qi, W.; Wen, J. Asperosaponin VI induces osteogenic differentiation of human umbilical cord mesenchymal stem cells via the estrogen signaling pathway. Medicine 2022, 101, e32344. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Zhong, Q.; Wang, J.; Wang, M.; Fang, F.; Xia, Z.; Zhong, R.; Huang, H.; Ke, Z.; Wei, Y.; et al. Beneficial Effects and Toxicity Studies of Xian-ling-gu-bao on Bone Metabolism in Ovariectomized Rats. Front. Pharmacol. 2017, 8, 273. [Google Scholar] [CrossRef]
- Tang, X.-Y.; Dai, Z.-Q.; Wu, Q.-C.; Zeng, J.-X.; Gao, M.-X.; Xiao, H.-H.; Yao, Z.-H.; Dai, Y.; Yao, X.-S. Simultaneous determination of multiple components in rat plasma and pharmacokinetic studies at a pharmacodynamic dose of Xian-Ling-Gu-Bao capsule by UPLC-MS/MS. J. Pharm. Biomed. Anal. 2020, 177, 112836. [Google Scholar] [CrossRef]
- Tian, S.; Zou, Y.; Wang, J.; Li, Y.; An, B.-Z.; Liu, Y.-Q. Protective effect of Du-Zhong-Wan against osteoporotic fracture by targeting the osteoblastogenesis and angiogenesis couple factor SLIT3. J. Ethnopharmacol. 2022, 295, 115399. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.; Liang, W.; Mao, L.; Yin, Y.; Zhang, L.; Li, C.; Liu, C. Synergy effects of Asperosaponin VI and bioactive factor BMP-2 on osteogenesis and anti-osteoclastogenesis. Bioact. Mater. 2022, 10, 335–344. [Google Scholar] [CrossRef] [PubMed]
- Park, E.; Lee, C.G.; Lim, E.; Hwang, S.; Yun, S.H.; Kim, J.; Jeong, H.; Yong, Y.; Yun, S.-H.; Choi, C.W.; et al. Osteoprotective Effects of Loganic Acid on Osteoblastic and Osteoclastic Cells and Osteoporosis-Induced Mice. Int. J. Mol. Sci. 2021, 22, 233. [Google Scholar] [CrossRef]
- Shen, Y.; Teng, L.; Qu, Y.; Huang, Y.; Peng, Y.; Tang, M.; Fu, Q. Hederagenin Suppresses Inflammation and Cartilage Degradation to Ameliorate the Progression of Osteoarthritis: An In vivo and In vitro Study. Inflammation 2023, 46, 655–678. [Google Scholar] [CrossRef]
- Jung, H.W.; Jung, J.K.; Son, K.H.; Lee, D.H.; Kang, T.M.; Kim, Y.S.; Park, Y.-K. Inhibitory effects of the root extract of Dipsacus asperoides C.Y. Cheng et al. T.M.Ai on collagen-induced arthritis in mice. J. Ethnopharmacol. 2012, 139, 98–103. [Google Scholar] [CrossRef]
- Li, X.-R.; Li, J.; Ren, Q.; Sun, S. The molecular mechanism of treating osteoarthritis with dipsacus saponins by inhibiting chondrocyte apoptosis. Exp. Ther. Med. 2017, 14, 4527–4532. [Google Scholar] [CrossRef]
- Bai, J.; Xie, N.; Hou, Y.; Chen, X.; Hu, Y.; Zhang, Y.; Meng, X.; Wang, X.; Tang, C. The enhanced mitochondrial dysfunction by cantleyoside confines inflammatory response and promotes apoptosis of human HFLS-RA cell line via AMPK/Sirt 1/NF-κB pathway activation. Biomed Pharmacother. 2022, 149, 112847. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Wang, C.M.; Jiang, H.J.; Tian, X.G.; Li, W.; Liang, W.; Yang, J.; Zhong, C.; Chen, Y.; Li, T. Protective Effects of Sweroside on IL-1β-Induced Inflammation in Rat Articular Chondrocytes Through Suppression of NF-κB and mTORC1 Signaling Pathway. Inflammation 2019, 42, 496–505. [Google Scholar] [CrossRef] [PubMed]
- Available online: https://www.who.int/news-room/fact-sheets/detail/dementia (accessed on 21 January 2023).
- Yu, X.; Wang, L.-N.; Du, Q.-M.; Ma, L.; Chen, L.; You, R.; Liu, L.; Ling, J.-J.; Yang, Z.-L.; Ji, H. Akebia Saponin D attenuates amyloid β-induced cognitive deficits and inflammatory response in rats: Involvement of Akt/NF-κB pathway. Behav. Brain Res. 2012, 235, 200–209. [Google Scholar] [CrossRef]
- Qian, Y.-H.; Liu, Y.; Hu, H.-T.; Ren, H.-M.; Chen, X.-L.; Xu, J.-H. The effects of the total saponin of Dipsacus asperoides on the damage of cultured neurons induced by β-amyloid protein 25-35. Anat. Sci. Int. 2002, 77, 196–200. [Google Scholar] [CrossRef] [PubMed]
- Xie, Z.-S.; Zhao, J.-P.; Wu, L.-M.; Chu, S.; Cui, Z.-H.; Sun, Y.-R.; Wang, H.; Ma, H.-F.; Ma, D.-R.; Wang, P.; et al. Hederagenin improves Alzheimer’s disease through PPARα/TFEB-mediated autophagy. Phytomedicine 2023, 112, 154711. [Google Scholar] [CrossRef]
- Chen, C.; Ai, Q.; Shi, A.; Wang, N.; Wang, L.; Wei, Y. Oleanolic acid and ursolic acid: Therapeutic potential in neurodegenerative diseases, neuropsychiatric diseases and other brain disorders. Nutr. Neurosci. 2023, 26, 414–428. [Google Scholar] [CrossRef]
- Brinza, I.; Raey, M.A.E.; El-Kashak, W.; Eldahshan, O.A.; Hritcu, L. Sweroside Ameliorated Memory Deficits in ScopolamineInduced Zebrafish (Danio rerio) Model: Involvement of Cholinergic System and Brain Oxidative Stress. Molecules 2022, 27, 5901. [Google Scholar] [CrossRef]
- Ahmedy, O.A.; Abdelghany, T.M.; El-Shamarka, M.E.A.; Khattab, M.A.; El-Tanbouly, D.M. Apigenin attenuates LPS-induced neurotoxicity and cognitive impairment in mice via promoting mitochondrial fusion/mitophagy: Role of SIRT3/PINK1/Parkin pathway. Psychopharmacology 2022, 239, 3903–3917. [Google Scholar] [CrossRef]
- Wang, Z.Y.; Zhang, X.D.; Whang, W.K. The Effect of Terpenoids of Dipsacus Asperoides Against Alzheimer’s Disease and Development of Simultaneous Analysis by High Performance Liquid Chromatography. Nat. Prod. Commun. 2021, 16, 1934578X211044603. [Google Scholar] [CrossRef]
- Tan, Z.; Sun, H.; Xue, T.; Gan, C.; Liu, H.; Xie, Y.; Yao, Y.; Ye, T. Liver Fibrosis: Therapeutic Targets and Advances in Drug Therapy. Front. Cell Dev. Biol. 2021, 9, 730176. [Google Scholar] [CrossRef]
- Gong, J.; Yang, F.; Yang, Q.; Tang, X.; Shu, F.; Xu, L.; Wang, Z.; Yang, L. Sweroside ameliorated carbon tetrachloride (CCl4)-induced liver fibrosis through FXR-miR-29a signaling pathway. J. Nat. Med. 2020, 74, 17–25. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.; Jang, J.H.; Kim, S.W.; Han, S.H.; Ma, K.H.; Jang, J.K.; Kang, H.C.; Cho, Y.Y.; Lee, H.S.; Lee, J.Y. Sweroside Prevents Non-Alcoholic Steatohepatitis by Suppressing Activation of the NLRP3 Inflammasome. Int. J. Mol. Sci. 2020, 21, 2790. [Google Scholar] [CrossRef]
- Meng, Z.; Zhu, B.; Gao, M.; Wang, G.; Zhou, H.; Lu, J.; Guan, S. Apigenin alleviated PA-induced pyroptosis by activating autophagy in hepatocytes. Food Funct. 2022, 13, 5559–5570. [Google Scholar] [CrossRef]
- Gong, L.-L.; Wang, Z.-H.; Li, G.-R.; Liu, L.-H. Protective Effects of Akebia Saponin D Against Rotenone-Induced Hepatic Mitochondria Dysfunction. J. Pharmacol. Sci. 2014, 126, 243–252. [Google Scholar] [CrossRef]
- Gong, L.-L.; Yang, S.; Zhang, W.; Han, F.-F.; Lv, Y.-L.; Wan, Z.-R.; Liu, H.; Jia, Y.-J.; Ling-ling, X.; Liu, L.-H. Akebia saponin D alleviates hepatic steatosis through BNip3 induced mitophagy. J. Pharmacol. Sci. 2018, 136, 189–195. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Available online: https://www.who.int/health-topics/cardiovascular-diseases#tab=tab_1 (accessed on 1 April 2023).
- Yang, S.; Zhang, W.; Xuan, L.-L.; Han, F.-F.; Lv, Y.-L.; Wan, Z.-R.; Liu, H.; Ren, L.-L.; Gong, L.-L.; Liu, L.-H. Akebia Saponin D inhibits the formation of atherosclerosis in ApoE−/− mice by attenuating oxidative stress-induced apoptosis in endothelial cells. Atherosclerosis 2019, 285, 23–30. [Google Scholar] [CrossRef]
- Li, J.; Zhao, C.; Zhu, Q.; Wang, Y.; Li, G.; Li, X.; Li, Y.; Wu, N.; Ma, C. Sweroside Protects Against Myocardial Ischemia-Reperfusion Injury by Inhibiting Oxidative Stress and Pyroptosis Partially via Modulation of the Keap1/Nrf2 Axis. Front. Cardiovasc. Med. 2021, 8, 650368. [Google Scholar] [CrossRef]
- Ma, L.Q.; Yu, Y.; Chen, H.; Li, M.; Ihsan, A.; Tong, H.Y.; Huang, X.J.; Gao, Y. Sweroside Alleviated Aconitine-Induced Cardiac Toxicity in H9c2 Cardiomyoblast Cell Line. Front. Pharmacol. 2018, 9, 1138. [Google Scholar] [CrossRef]
- Li, C.; Gao, Y.; Tian, J.; Xing, Y.; Zhu, H.; Shen, J. Long-term oral Asperosaponin VI attenuates cardiac dysfunction, myocardial fibrosis in a rat model of chronic myocardial infarction. Food Chem. Toxicol. 2012, 50, 1432–1438. [Google Scholar] [CrossRef]
- Muruganathan, N.; Dhanapal, A.R.; Baskar, V.; Muthuramalingam, P.; Selvaraj, D.; Aara, H.; Shiek Abdullah, M.Z.; Sivanesan, I. Recent Updates on Source, Biosynthesis, and Therapeutic Potential of Natural Flavonoid Luteolin: A Review. Metabolites 2022, 12, 1145. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Kim, M.-Y.; Cho, J.Y. Immunopharmacological Activities of Luteolin in Chronic Diseases. Int. J. Mol. Sci. 2023, 24, 2136. [Google Scholar] [CrossRef] [PubMed]
- Thomas, S.D.; Jha, N.K.; Jha, S.K.; Sadek, B.; Ojha, S. Pharmacological and Molecular Insight on the Cardioprotective Role of Apigenin. Nutrients 2023, 15, 385. [Google Scholar] [CrossRef]
- Song, J.-S.; Lim, K.-M.; Kang, S.; Noh, J.-Y.; Kim, K.; Bae, O.-N.; Chung, J.-H. Procoagulant and prothrombotic effects of herbal medicine, Dipsacus asper and its active ingredient, dipsacus saponin C, on human platelets. J. Thromb. Haemost. 2012, 10, 895–906. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; He, L. The protective effect of hederagenin on renal fibrosis by targeting muscarinic acetylcholine receptor. Bioengineered 2022, 13, 8689–8698. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Hu, T.; Liu, H.; Lv, Y.-L.; Zhang, W.; Li, H.; Xuan, L.; Gong, L.-L.; Liu, L.-H. Akebia saponin D ameliorates metabolic syndrome (MetS) via remodeling gut microbiota and attenuating intestinal barrier injury. Biomed. Pharmacother. 2021, 138, 111441. [Google Scholar] [CrossRef]
- Li, F.; Wei, R.; Huang, M.; Chen, J.; Li, P.; Ma, Y.; Chen, X. Luteolin can ameliorate renal interstitial fibrosis-induced renal anaemia through the SIRT1/FOXO3 pathway. Food Funct. 2022, 13, 11896–11914. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Wang, J.; Wang, Q. Hederagenin Exerts Potential Antilipemic Effect via p38MAPK Pathway in Oleic Acid-induced HepG2 cells and in Hyperlipidemic Rats. Ann. Acad. Bras. Cienc. 2022, 94, e20201909. [Google Scholar] [CrossRef]
- Luo, J.-F.; Zhou, H.; Lio, C.-K. Akebia Saponin D Inhibits the Inflammatory Reaction by Inhibiting the IL-6-STAT3-DNMT3b Axis and Activating the Nrf2 Pathway. Molecules 2022, 27, 6236. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Dong, Z.; Lan, X.; Liao, Z.; Chen, M. Sweroside Alleviated LPS-Induced Inflammation via SIRT1 Mediating NF-κB and FOXO1 Signaling Pathways in RAW264.7 Cells. Molecules 2019, 24, 872. [Google Scholar] [CrossRef]
- Piluzza, G.; Bullitta, S. Correlations between phenolic content and antioxidant properties in twenty-four plant species of traditional ethnoveterinary use in the Mediterranean area. Pharm. Biol. 2011, 49, 240–247. [Google Scholar] [CrossRef]
- Roman, B.; Muzykiewicz-Szymańska, A.; Ossowicz-Rupniewska, P.; Klimowicz, A.; Janus, E. The application of amino acid ionic liquids as additives in the ultrasound-assisted extraction of plant material. RSC Adv. 2021, 11, 25983. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.-Y.; Lee, D.H.; Joo, E.J.; Son, K.H.; Kim, Y.S. Akebia saponin PA induces autophagic and apoptotic cell death in AGS human gastric cancer cells. Food Chem. Toxicol. 2013, 59, 703–708. [Google Scholar] [CrossRef] [PubMed]
- Kuhtinskaja, M.; Bragina, O.; Kulp, M.; Vaher, M. Anticancer effect of the iridoid glycoside fraction from Dipsacus fullonum L. leaves. Nat. Prod. Commun. 2020, 15, 1934578X20951417. [Google Scholar] [CrossRef]
- Telang, N.; Nair, H.B.; Wong, G.Y.C. Anti-Proliferative and Pro-Apoptotic Effects of Dipsacus Asperoides in a Cellular Model for Triple-Negative Breast Cancer. Arch. Breast Cancer 2022, 9, 66–75. [Google Scholar] [CrossRef]
- Carraz, M.; Lavergne, C.; Jullian, V.; Wright, M.; Gairin, J.E.; de la Cruz, M.G.; Bourdy, G. Antiproliferative activity and phenotypic modification induced by selected Peruvian medicinal plants on human hepatocellular carcinoma Hep3B cells. J. Ethnopharmacol. 2015, 166, 185–199. [Google Scholar] [CrossRef] [PubMed]
- Chouhan, S.; Sharma, K.; Guleria, S. Antimicrobial Activity of Some Essential Oils-Present Status and Future Perspectives. Medicines 2017, 4, 58. [Google Scholar] [CrossRef]
- Liebold, T.; Straubinger, R.K.; Rauwald, H.W. Growth inhibiting activity of lipophilic extracts from Dipsacus sylvestris Huds. roots against Borrelia burgdorferi s. s. in vitro. Pharmazie 2011, 66, 628–630. [Google Scholar] [PubMed]
- Feng, J.; Leone, J.; Schweig, S.; Zhang, Y. Evaluation of natural and botanical medicines for activity against Growing and non-growing forms of B. burgdorferi. Front. Med. 2020, 7, 6. [Google Scholar] [CrossRef]
- Zhang, J.; Yi, S.; Li, Y.; Xiao, C.; Liu, C.; Jiang, W.; Yang, C.; Zhou, T. The antidepressant effects of asperosaponin VI are mediated by the suppression of microglial activation and reduction of TLR4/NF-κB-induced IDO expression. Psychopharmacology 2020, 237, 2531–2545. [Google Scholar] [CrossRef]
- Jiang, X.; Yi, S.; Liu, Q.; Su, D.; Li, L.; Xiao, C.; Zhang, J. Asperosaponin VI ameliorates the CMS-induced depressive-like behaviors by inducing a neuroprotective microglial phenotype in hippocampus via PPAR-γ pathway. J. Neuroinflamm. 2022, 19, 115. [Google Scholar] [CrossRef] [PubMed]
- Alghamdi, A.; Almuqbil, M.; Alrofaidi, M.A.; Burzangi, A.S.; Alshamrani, A.A.; Alzahrani, A.R.; Kamal, M.; Imran, M.; Alshehri, S.; Mannasaheb, B.A.; et al. Potential Antioxidant Activity of Apigenin in the Obviating Stress-Mediated Depressive Symptoms of Experimental Mice. Molecules 2022, 27, 9055. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Yuan, Z.; Jian, L.; Jiang, Q.; Zhang, S.; Tan, J. The Modified Bushen Antai Recipe Upregulates Estrogen and Progesterone Receptors at the Maternal-Fetal Interface in Pregnant Rats with Mifepristone-Induced Pregnancy Loss. Evid. Based Complement. Altern. Med. 2019, 2019, 8312020. [Google Scholar] [CrossRef] [PubMed]
| Accepted Species Name (Synonym Name a) | Common Name | Geographical Occurrence | Habitat | Traditional Usage (Part of Plant) |
|---|---|---|---|---|
| D. asperoides C.Y.Cheng & T.M.Ai [8] D. asper Wall. ex DC. [9] D. asper Wall. [4] (D. asper Wall. ex C.B.Clarke (doubtful) [4]) | Roots are called Xu duan, chuan xu duan, (in Chinese); Sichuan teasel, Himalayan Teasel Root (in English) [4,10,11,12,13,14,15,16,17] | China (Hubei, Hunan, Yunnan, Shanxi, Jiangxi, Sichuan, Gansu, Xizang, Guangxi, and Guizhou Provinces), Korea, Japan, Myanmar [9,12,18,19,20,21,22,23] | Moist fields, margins of forests, thickets, among herbs, by streams, roadsides, and mountains; 1500–3700 m [10,24,25] | Roots are used in traditional Chinese and Korean medicine to treat low back pain, knee pain, osteoporosis or bone diseases, bone fractures, rheumatic arthritis, lumbago, traumatic hematoma, uterine bleeding, and threatened abortion [6,11,14,15,22,26,27,28,29,30,31,32] |
| D. fullonum L. [4,8,9] (D. sylvestris Huds. [8,9]) | Cardo, Fuller’s teasel, teasel, wild teasel (in English) [4,9,33,34] | Europe to the Caucasus, North-Western Africa [9] | - | Herb is used for treatment of Lyme disease [35] and for eye infection in cattle (in Sardinia) [36] |
| D. inermis Wall. [4,8,9] (D. asper Wall. ex. DC. [8]) | zang xu duan (in Chinese); Wopal haakh/Wopal Hak (in Kashmiri) [10,37,38] | Afghanistan, Bangladesh, India, People’s Republic of China (Yunnan Province), Myanmar, Nepal, Pakistan, Thailand, Vietnam, Kashmir Himalaya [9,10,37] | Forests, grassy slopes, by streams; 2100–3900 m [10] | Himalayan herb is used in traditional Ayurvedic medicine and Kashmiri traditional medicine against various inflammation-related disorders, body weakness, cough, and sore throat. It has stomachic and carminative properties [37,38,39] |
| D. japonicus Miq. [4,8,9] | ri ben xu duan, (in Chinese); Japanese teasel (in English) [4,10] | People’s Republic of China, Korea, central and south Japan, and Vietnam [9,40] | Grassy slopes and roadsides, savannas; below 1000–2600 m [10,41] | Roots are used in the traditional medicine of China and Vietnam as a remedy for relieving joint pain and inflammation [40] |
| D. sativus (L.) Honc. [4,8,9] | Fuller’s Teasel (in English) [4] | Europa (France, Italy), Caucasus [9] | - | Tea of leaves is used for the treatment of cardiovascular diseases [42] |
| S. arenaria Forssk. [8,9] | - | Algeria, Egypt, Libya, Morocco, Palestine, Sinai, and Tunisia [9] | - | - |
| S. atropurpurea L. [8,9] | Mourningbride (in English); Mor uyuzotu or Şeytanotu (in Turkey); Ambarina (in Northern Peru); Escabiosa (in north-east Catalonia, Iberian Peninsula) [4,43,44,45] | Algeria, Tunisia, Turkey, Europe (Albania, Azores, Baleares, Bulgaria, Canary Is., Corse, East Aegean Is., France, Greece, Italy, Madeira, Morocco, Portugal, Sicilia, Spain) [9] | Around roadsides, dry fields, and dunes [45] | Flowers/aerial parts are used traditionally in Catalonia for bronchitis, acne, cold and cough, and for measles and furuncleas, in Northern Peru for menstrual regulation, and in the Iberian Peninsula as a veterinary diuretic [43,44,45,46] |
| S. comosa Fisch. ex Roem & Schult. [8,9] | lan pen hua, (in Chinese) [10] | People’s Republic of China (Gansu, Hebei, Henan, Heilongjiang, Jilin, Liaoning, Shaanxi, Shanxi Provinces, Nei, and Ningxia), Korea, Inner Mongolia, Russia (Far East, Siberia) [9,10] | Sandy dunes, dry mountain slopes, steppes; 300–1600(–3000) m [10] | In traditional Mongolian and Tibetan medicine, inflorescences are used in the treatment of liver diseases [7,47] |
| Lomelosia stellata (L.) Raf. [4,8,9] (S. stellata L. [8,9]) | Starflower pincushions (in English) [4,48] | Endemic to North Africa (Algeria, Libya, Morocco, Tunisia), Europe (France, Portugal, Spain) [9,48] | Dry sunny grassland and rocky hillsides [49] | Leaves and flowers are used in the traditional medicine of Morocco to treat heel cracks and for the treatment of various respiratory diseases including bronchitis, bronchial pneumonia, influenza, and asthma [48,50] |
| S. tschiliensis Grüning [8,9] (synonymous name of S. comosa Fisch. ex Roem & Schult. [8,9]) | Meng Gu Shan Luo Bo (in Chinese) [51] | Inner Mongolia autonomy district and China (the west of Hebei Province) [52] | Mountainous regions (300–1500 m) [52,53] | Inflorescences are used in inner Mongolia for the treatment of headache, fever, cough, and jaundice [51] |
| Species | Plant Material | Extract, Fraction, or Pure Compound | Antioxidant Assay | Antioxidant Activity | Positive Control | References |
|---|---|---|---|---|---|---|
| D. asper | Roots | Methanolic extract; 3,4-di-O-caffeoylquinic acid; methyl 3,4-di-O-caffeoyl quinate; 3,5-di-O-caffeoylquinic acid; methyl 3,5-di-O-caffeoyl quinate; 4,5-di-O-caffeoylquinic acid; methyl 4,5-di-O-caffeoyl quinate | DPPH; Cu2+ mediated low-density lipoprotein (LDL) | DPPH: methanolic extract IC50 = 90.2 µg/mL; 3,4-di-O-caffeoylquinic acid IC50 = 13.4 µM; methyl 3,4-di-O-caffeoyl quinate IC50 = 14.1 µM; 3,5-di-O-caffeoylquinic acid IC50 = 18.2 µM; methyl 3,5-di-O-caffeoyl quinate IC50 = 10.6 µM; 4,5-di-O-caffeoylquinic acid IC50 = 10.4 µM; methyl 4,5-di-O-caffeoyl quinate IC50 = 12.6 µM Cu2+ mediated LDL: methanolic extract IC50 = 134.4 µg/mL; 3,4-di-O-caffeoylquinic acid IC50 = 2.1 µM; methyl 3,4-di-O-caffeoyl quinate IC50 = 1.9 µM; 3,5-di-O-caffeoylquinic acid IC50 = 2.3 µM; methyl 3,5-di-O-caffeoyl quinate IC50 = 2.0 µM; 4,5-di-O-caffeoylquinic acid IC50 = 2.3 µM; methyl 4,5-di-O-caffeoyl quinate IC50 = 1.8 µM | DPPH: BHT IC50 = 145.8 µM; caffeic acid IC50 = 31.1 µM Cu2+ mediated LDL: BHT IC50 = 3 µM; caffeic acid IC50 = 5.2 µM; α-tocopherol IC50 = 23.4 µM | [24] |
| D. asperoides | Roots | Polysaccharide fraction | DPPH; ABTS | DPPH: EC50 = 0.355 mg/mL (at concentration 1 mg/mL 86.2% scavenging rate) ABTS: EC50 = 5.867 mg/mL (at concentration 10 mg/mL 92.6% scavenging rate) | - | [14] |
| D. fullonum | Whole plants | Acetone:water extract (7:3) | DPPH; ABTS | DPPH: 4.01 mmol TEAC/100 g d.w. ABTS: 3.58 mmol TEAC/100 g d.w. | - | [171] |
| Leaves | Aqueous extract; aqua [TEAH]+[Thr]−; aqua [TEAH]+[Met]− | DPPH; FRAP; CUPRAC | DPPH: aqua radical scavenging activity (RSA) 68.11%–73.81%; aqua [TEAH]+[Thr]− 57.55%–62.78%; aqua [TEAH]+[Met]− 58.73%–61.17% FRAP: aqua 13.14–14.85 mg FeSO4/g raw material; aqua [TEAH]+[Thr]− 1.45–17.08 mg FeSO4/g raw material; aqua [TEAH]+[Met]− 12.44–21.1 mg FeSO4/g raw material; CUPRAC: aqua 4.93–5.71 mg TEAC/g raw material; aqua [TEAH]+[Thr]− 5.08–5.74 mg TEAC/g raw material; aqua [TEAH]+[Met]− 4.39–6.09 mg TEAC/g raw material | - | [172] | |
| Leaves | 70% ethanol extract (v/v %); fraction NP7 (contained two chlorogenic acid derivatives, saponarin and isoorientin); fraction NP2 (contained bis-iridoids) | ORAC | 70% ethanol extract 10.8 mmol TEAC/100 mL; NP7 fraction 12.5 mmol TEAC/100 mL; NP2 fraction 0.78 mmol TEAC/100 mL | - | [34] | |
| Leaves | 50% methanol extract | ORAC | 14.78 mmol TEAC/100 g dry weight | - | [33] | |
| Roots | 50% methanol extract | ORAC | 10.87 mmol TEAC/100 g dry weight | - | [33] | |
| D. sativus | Leaves | Methanol extract | In vivo study with ICR mice pre-treated with D-galactose | SOD level in the peripheral blood plasma ↑; MDA level in the peripheral blood plasma ↓ | - | [42] |
| S. arenaria | Roots | 80% (v/v) hydro-methanolic extract; n-butanol, ethyl acetate, and water fractions | DPPH; ABTS; reducing power; β-carotene bleaching inhibition | DPPH: 80% methanolic extract IC50 = 260 µg/mL; n-butanol fraction IC50 = 26 µg/mL; ethyl acetate fraction IC50 = 19 µg/mL; water fraction IC50 = 105 µg/mL ABTS: 80% methanolic extract IC50 = 210 µg/mL; n-butanol fraction IC50 = 40 µg/mL; ethyl acetate fraction IC50 = 34 µg/mL; water fraction IC50 = 180 µg/mL reducing power: 80% methanolic extract EC50 = 190 µg/mL; n-butanol fraction EC50 = 28 µg/mL; ethyl acetate fraction EC50 = 66 µg/mL; water fraction IC50 = 510 µg/mL β-carotene bleaching inhibition: 80% methanolic extract IC50 = 720 µg/mL; n-butanol fraction IC50 = 18 µg/mL; ethyl acetate fraction IC50 = 26 µg/mL; water fraction IC50 = 450 µg/mL | DPPH: BHT IC50 = 18 µg/mL; ABTS: BHT IC50 = 50 µg/mL; reducing power: BHT EC50 = 20 µg/mL; β-carotene bleaching inhibition: BHT IC50 = 40 µg/mL | [73] |
| Stem and leaves; flowers; fruits | 80% (v/v) hydro-methanolic extract; n-butanolic, ethyl acetate, and water fractions | DPPH; ABTS; reducing power; β-carotene bleaching inhibition | DPPH: steam and leaves 80% methanolic extract IC50 = 21 µg/mL; n-butanol fraction IC50 = 19 µg/mL; ethyl acetate fraction IC50 = 19 µg/mL; water fraction IC50 = 48 µg/mL; DPPH: flowers 80% methanolic extract IC50 = 36 µg/mL; n-butanol fraction IC50 = 19 µg/mL; ethyl acetate fraction IC50 = 17 µg/mL; water fraction IC50 = 30 µg/mL; DPPH: fruits 80% methanolic extract IC50 = 32 µg/mL; n-butanol fraction IC50 = 20 µg/mL; ethyl acetate fraction IC50 = 18 µg/mL; water fraction IC50 = 25 µg/mL; ABTS: steam and leaves 80% methanolic extract IC50 = 810 µg/mL; ethyl acetate fraction IC50 = 110 µg/mL; ABTS: flowers n-butanol fraction IC50 = 140 µg/mL; ethyl acetate fraction IC50 = 54 µg/mL; ABTS: fruits ethyl acetate fraction IC50 = 170 µg/mL; β-carotene bleaching inhibition: steam and leaves 80% methanolic extract IC50 = 920 µg/mL; n-butanol fraction IC50 = 400 µg/mL; ethyl acetate fraction IC50 = 210 µg/mL; β-carotene bleaching inhibition: flowers 80% methanolic extract IC50 = 900 µg/mL; n-butanol fraction IC50 = 180 µg/mL; ethyl acetate fraction IC50 = 52 µg/mL; β-carotene bleaching inhibition: fruits 80% methanolic extract IC50 = 870 µg/mL; n-butanol fraction IC50 = 700 µg/mL; ethyl acetate fraction IC50 = 680 µg/mL; water fraction IC50 = 800 µg/mL; reducing power: steam and leaves 80% methanolic extract EC50 = 240 µg/mL; n-butanol fraction EC50 = 220 µg/mL; ethyl acetate fraction EC50 = 100 µg/mL; water fraction EC50 = 220 µg/mL; reducing power: flowers 80% methanolic extract EC50 = 40 µg/mL; n-butanol fraction EC50 = 26 µg/mL; ethyl acetate fraction EC50 = 20 µg/mL; water fraction EC50 = 77 µg/mL; reducing power: fruits n-butanol fraction EC50 = 800 µg/mL; ethyl acetate fraction EC50 = 52 µg/mL; water fraction EC50 = 64 µg/mL | DPPH: BHT IC50 = 18 µg/mL; ABTS: BHT EC50 = 50 µg/mL; β-carotene bleaching inhibition: BHT IC50 = 40 µg/mL; reducing power: BHT EC50 = 20 µg/mL | [72] | |
| S. artropurpurea | Stems | Dichloromethane, chloroform, ethyl acetate, ethanol extracts; volatile fractions/hydro distillation V1 hexane, V2 chloroform | DPPH | Dichloromethane extract IC50 = 2.7085 mg/mL; chloroform extract IC50 = 2.0951 mg/mL; ethyl acetate extract IC50 = 0.4806 mg/mL; ethanol extract IC50 = 0.1383 mg/mL; V1 fraction IC50 = 0.4798 mg/mL; V2 fraction IC50 = 1.2944 mg/mL | Ascorbic acid IC50 = 0.084 mg/mL | [46] |
| S. artropurpurea subsp. maritima | Fruits | Water extract (silver nanoparticles) | DPPH; FRAP | DPPH: IC50 = 0.112 mg/mL FRAP; IC50 = 0.036 mg EAa/g d.w. | DPPH: ascorbic acid IC50 = 0.087 mg/mL; FRAP: ascorbic acid IC50 = 0.024 mg EAa/g d.w. | [56] |
| S. comosa | Inflorescences | 70% ethanol extract; 3,5-di-O-caffeoylquinic acid; chlorogenic acid; 4,5-di-O-caffeoylquinic acid; 3,4-di-O-caffeoylquinic acid; caffeic acid; luteolin-7-glucoside; luteolin-6-C-glucoside; quercetin-3-glucoside; quercetin-3-rutinoside | DPPH; ABTS | DPPH: 70% ethanol extract IC50 = 331.1 µg/mL; 3,5-di-O-caffeoylquinic acid IC50 = 3.63 µg/mL; chlorogenic acid IC50 = 4.67 µg/mL; 4,5-di-O-caffeoylquinic acid IC50 = 4.01 µg/mL; 3,4-di-O-caffeoylquinic acid IC50 = 4.78 µg/mL; caffeic acid IC50 = 3.96 µg/mL; protocatechuic acid IC50 = 4.15 µg/mL; luteolin IC50 = 6.03 µg/mL; luteolin-7-glucoside IC50 = 9.16 µg/mL; luteolin-6-C-glucoside IC50 = 5.74 µg/mL; quercetin-3-glucoside IC50 = 6.47 µg/mL; quercetin-3-rutinoside IC50 = 6.15 µg/mL ABTS: 70% ethanol extract IC50 = 223.5 µg/mL | - | [74] |
| S. stellata | Whole plants | 70% ethanol extract; fraction A 100% water; fraction B 25% methanol; fraction C 50% methanol; fraction D 75% methanol; fraction E 100% methanol; eustomoruside; eustomoside; hyperin | DPPH | 70% ethanol extract IC50 = 86 µg/mL; fraction A IC50 = 133 µg/mL; fraction B IC50 = 48.7 µg/mL; fraction C IC50 = 25 µg/mL; fraction D IC50 = 64.3 µg/mL; fraction E IC50 >200 µg/mL; eustomoruside IC50 = 7.1 µg/mL; eustomoside IC50 = 7.2 µg/mL; hyperin IC50 = 16 µg/mL | Ascorbic acid IC50 = 6.3 µg/mL | [75] |
| Whole plants | Petroleum ether; ethyl acetate; n-butanol extract | DPPH; ABTS; FRAP; CUPRAC; β-carotene; phosphomolybdate; ferrous and metal ions chelating | DPPH: petroleum ether IC50 = 171.61 µg/mL; ethyl acetate IC50 = 25.15 µg/mL; n-butanol extract IC50 = 21.22 µg/mL ABTS: petroleum ether IC50 = 64.1 µg/mL; ethyl acetate IC50 = 14.00 µg/mL; n-butanol extract IC50 = 24.99 µg/mL CUPRAC: petroleum ether IC50 = 100.95 µg/mL; ethyl acetate IC50 = 28.5 µg/mL; n-butanol extract IC50 = 42.16 µg/mL β-carotene: petroleum ether IC50 = 11.18 µg/mL; ethyl acetate IC50 = 10.34 µg/mL n; n-butanol extract IC50 = 50.01 µg/mL chelation in ferrous iron: ethyl acetate EC50 = 5.026 mg/mL n; n-butanol extract EC50 = 1.652 mg/mL chelation in metal iron: ethyl acetate EC50 > 200 mg/mL n; n-butanol extract EC50 = 145.35 mg/mL | DPPH: BHA IC50 = 6.82 µg/mL; BHT IC50 = 22.32 µg/mL; tannic acid IC50 = 7.74 µg/mL; ascorbic acid IC50 = 3.1 µg/mL; α-tocopherol IC50 = 13.02 µg/mL ABTS: BHA IC50 = 1.81 µg/mL; BHT IC50 = 1.29 µg/mL; tannic acid IC50 = 1.01 µg/mL; ascorbic acid IC50 = 1.74 µg/mL; α-tocopherol IC50 = 7.59 µg/mL CUPRAC: BHA IC50 = 3.64 µg/mL; BHT IC50 = 9.62 µg/mL; tannic acid IC50 = 3.76 µg/mL; ascorbic acid IC50 = 12.43 µg/mL; α-tocopherol IC50 = 19.92 µg/mL chelation in ferrous iron: EDTA EC50 = 0.0627 µg/mL chelation in metal iron: EDTA EC50 = 8.57 mg/mL | [50,77] | |
| Whole plants | n-butanol, ethyl acetate, dichloromethane fractions | DPPH; ABTS; FRAP | DPPH: n-butanol FRS50 = 64.46µg/mL; ethyl acetate FRS50 = 71.82 µg/mL; dichloromethane FRS50 > 250 µg/mL ABTS: n-butanol FRS50 = 27.87 µg/mL; ethyl acetate FRS50 = 40.41 µg/mL; dichloromethane FRS50 > 250 µg/mL FRAP: n-butanol EC50 = 161.11 µg/mL; ethyl acetate EC50 = 202.41 µg/mL; dichloromethane EC50 > 50 µg/mL | DPPH: ascorbic acid FRS50 = 8.21 µg/mL ABTS: Trolox FRS50 = 12.07 µg/mL FRAP: BHA EC50 = 18.03 µg/mL | [76] | |
| S. tschilliensis | Inflorescences | 70% ethanol extract | DPPH; ABTS | DPPH: IC50 = 272.8 µg/mL ABTS: IC50 = 199.7 µg/mL | - | [74] |
| Whole plants | 90% ethanol extract | DPPH | DPPH: IC50 = 26.502 µg/mL | Ascorbic acid IC50 = 5.41 µg/mL | [52] | |
| Whole plants in pre-flowering, flowering, and fruiting stages | 96% ethanol extract; water, n-butanol, ethyl acetate, petroleum ether fractions | DPPH; ABTS; inhibition of lipid peroxidation; OH scavenging activity | DPPH: 96% ethanol extract IC50 = 25.65–86.79 µg/mL; acetate fraction of pre-flowering stage of plants IC50 = 8.47 µg/mL ABTS: acetate fraction of pre-flowering stage of plants IC50 = 58.76 µg/mL OH scavenging activity: 96% ethanol extract IC50 = 206.47–772.45 µg/mL; acetate fraction of pre-flowering stage of plants IC50 = 67.64 µg/mL | DPPH: ascorbic acid IC50 = 7.6 µg/mL; ABTS: lipid peroxidation inhibition: OH scavenging activity: | [53] |
| Species | Plant Material | Extract or Specialized Metabolites | Cell Line | Assay | Result | Positive Control | References |
|---|---|---|---|---|---|---|---|
| D. asper | Roots | Phenolic acids (2,6-dihydroxycinnamic acid, vanillic acid, 2′-O-caffeoyl-d-glucopyranoside ester, caffeoylquinic acid) iridoids (dipsanosides C-G, 3′-O-β-d-glucopyranosyl sweroside, loganin, cantleyoside, triplostoside A, lisianthioside, and 6′-O-β-d-apiofuranosyl sweroside) | Lung carcinoma A549, hepatoma Bel7402, gastric carcinoma BGC-823, colon cancer HCT-8, and ovary cancer A2780 | MTT assay, cell viability (96 h) | 2,6-dihydroxycinnamic acid, A549 IC50 = 3.883 µg/mL, Bel7402 IC50 = 7.346 µg/mL, BGC-823 IC50 = 4.321 µg/mL; vanillic acid, Bel7402 IC50 = 6.437 µg/mL, HCT-8 IC50 = 5.218 µg/mL, A2780 IC50 = 7.395 µg/mL; 2′-O-caffeoyl-d-glucopyranoside ester, A549 IC50 = 5.663 µg/mL; Bel7402 IC50 = 5.545 µg/mL, BGC-823 IC50 = 6.432 µg/mL, HCT-8 IC50 = 5.7 µg/mL, A2780 IC50 = 6.380 µg/mL; caffeoylquinic acid, A549 IC50 = 5.713 µg/mL; Bel7402 IC50 = 5.586 µg/mL, BGC-823 IC50 = 6.204 µg/mL, HCT-8 IC50 = 5.37 µg/mL, A2780 IC50 = 6.679 µg/mL iridoids were not cytotoxic against A549, Bel7402, BGC-823, HCT-8, and A2780 cell lines | Fluorouracil, A549 IC50 = 0.177 µg/mL, Bel7402 IC50 = 0.542 µg/mL, BGC-823 IC50 = 0.695 µg/mL, HCT-8 IC50 = 0.67 µg/mL, A2780 IC50 = 0.569 µg/mL | [89] |
| Roots | Akebia saponin D | Human monocyte-like histiocytic U937 cells | MTT assay, cytotoxicity (48 h); flow cytometric analysis and DNA ladding (level of DNA fragmentation, sub-G1 peak); RT-PCR | Induction of cell cytotoxicity in a concentration-dependent manner (0.1–1000 µM); an increase in the percentage of Sub G1 cells and Bax and p53 gene expression | Doxorubicin, the cell viability 25% at a concentration of 0.1 µM | [90] | |
| Roots | 2′,3′-O-diacetyl-3-O-α-l-arabinopyranosyl-23-hydroxyolea-12-en- 28-oic acid; 2′,4′-O-diacetyl-3-O-α-l-arabinopyranosyl-23-hydroxyolea-12-en- 28-oic acid; 3β-O-trans-feruloyl-2α-hydroxy-urs-12-en-28-oic acid; leontoside A; 4′-O-acetyl-3-O-α-l-arabinopyranosyl-23-hydroxyolea-12-en-28-oic acid; 3′-O-acetyl-3-O-α-l-arabinopyranosyl-23-hydroxyolea-12-en-28-oic acid; 2′-O-acetyl-3-O-α-l-arabinopyranosyl-23-hydroxyolea-12-en-28-oic acid | Lung A549, liver H157 and HepG2, and breast MCF-7 cell lines | SRB method, cytotoxicity (48 h) | 2′,3′-O-diacetyl-3-O-α-L-arabinopyranosyl-23-hydroxyolea-12-en-28-oic acid, A549 IC50 = 6.67 µM; H157 IC50 = 9.7 µM; HepG2 IC50 = 15.89 µM; MCF-7 IC50 = 15.08 µM; 2′,4′-O-diacetyl-3-O-α-L-arabinopyranosyl-23-hydroxyolea-12-en-28-oic acid, A549 IC50 = 6.67 µM; H157 IC50 = 9.57 µM; HepG2 IC50 = 16.23 µM; MCF-7 IC50 = 15.23 µM; 3β-O-trans-feruloyl-2α-hydroxy-urs-12-en-28-oic acid, A549 IC50 = 12.8 µM; H157 IC50 = 5.66 µM; HepG2 IC50 = 9.5 µM; MCF-7 IC50 = 9.36 µM; leontoside A, A549 IC50 = 35.24 µM; H157 IC50 = 36.45 µM; HepG2 IC50 = 46.4 µM; MCF-7 IC50 = 52.06 µM; 4′-O-acetyl-3-O-α-l-arabinopyranosyl-23-hydroxyolea-12-en-28-oic acid, A549 IC50 = 22.94 µM; H157 IC50 = 21.21 µM; HepG2 IC50 = 27.13 µM; MCF-7 IC50 = 26.72 µM; 3′-O-acetyl-3-O-α-l-arabinopyranosyl-23-hydroxyolea-12-en-28-oic acid, A549 IC50 = 34.18 µM; H157 IC50 = 30.02 µM; HepG2 IC50 = 34.35 µM; MCF-7 IC50 = 37.32 µM; 2′-O-acetyl-3-O-α-l-arabinopyranosyl-23-hydroxyolea-12-en-28-oic acid, A549 IC50 = 33.14 µM; H157 IC50 = 27.54 µM; HepG2 IC50 = 34.5 µM; MCF-7 IC50 = 35.66 µM | Doxorubicin, A549 IC50 = 1.68 µM; H157 IC50 = 0.85 µM; HepG2 IC50 = 1.57 µM; MCF-7 IC50 = 0.9 µM | [12] | |
| Roots | 3-O-[β-d-xylopyranosyl-(1→4)-β-d-glucopyranosyl-(1→4)][α-l-rhamnopyranosyl-(1→3)]-β-d-glucopyranosyl-(1→3)-α-l-rhamnopyranosyl-(1→2)-α-l-arabinopyranosylhederagenin | Lung A549, liver H157 and HepG2, and breast MCF-7 cell lines | SRB method, cytotoxicity (48 h) | A549 IC50 = 6.94 µM; H157 IC50 = 9.06 µM | - | [96] | |
| D. asperoides | Roots | Water extract | Human mammary carcinoma derived triple negative MDA-MB-231 cells | Cell Titre Glo 2.0 assay, cell viability (7 d); flow cytometer (cell cycle); Western Blot; caspase 3/7 activity | Cell viability IC50 = 15 µg/mL; arrest cycle growth of cells in G2/M phase; induction of apoptosis by increasing pro-apoptotic caspase 3/7 activity and inhibiting the expression of BRAF, p-ERK, MEK, pPI3K, pAKT, and cyclin-dependent kinase 4/6 in a dose-dependent manner | - | [175] |
| Roots | Akebia saponin PA | Human gastric cancer AGS, MKN-45, SNU-638, and KATO III cell lines | MTT assay, cell viability (24 h); Annexing V/propidium (PI) staining; Western Blot | Cell viability AGS IC50 = 30.3 µM; cell viability MKN-45 IC50 = 24.1 µM; cell viability SNU-638 IC50 = 27.6 µM; cell viability KATO III IC50 = 36.5 µM; an increase in apoptotic cells (AGS) to 9.46%, 19.33%, and 48.20% at a concentration of 20 µM, 30 µM, and 40 µM, respectively; an increase in AGS cell number in the sub-G1 phase; activation of caspase-3, cleavage of PARP-1, mitogen-activated protein kinases (MAPKs), and p38/c-Jun N-terminal kinase (JNK); induction of autophagy through PI3K/AKT/mTOR and AMPK/mTOR pathways | - | [173] | |
| Roots | Water-soluble polysaccharide (ADAPW) with the molecular weight of 16 kDa | Human osteosarcoma cell line HOS cells | MTT assay, cell viability (24 h); Annexin V-FITC/PI staining; Western Blot | Inhibition of cell growth (at the concentration of 400 µg/mL, the cell survival rate ˂35%); induction of apoptosis in a concentration-dependent pattern (at a concentration of 100–400 µg/mL, the number of apoptotic cell increase to 23.7%-55.3%); down-regulation of PI3K and pAkt protein level; reduction of mitochondrial membrane potential; an increase in intracellular ROS level | [11] | ||
| D. fullonum | Leaves | Iridoid glycosides fraction of 80% methanol extract | Human breast cancer cell lines MCF7 and MDB-MB-231 and human cervical cancer cell line HeLa | WST-1 assay, cell viability (72 h) | Decrease in cell survival; the viability of cells 64%, 69.5%, and 78.9% for MCF7, MDB-MB-231, and HeLa, respectively | - | [174] |
| Leaves and flowers | 96% ethanol extract | Human hepatocellular carcinoma cell lines Hep3B, HepG2, PLC/PFR/5, and SNU-182 | ATPlite assay, cell viability (8 h) | Hep3B IC50 > 100 µg/mL; HepG2, PLC PFR5, SNU-182 antiproliferative effect not detected | - | [176] | |
| D. japonicus | Roots | Saponin XII | acute myeloid leukemia OCI-AML3 cells | Cell viability (24 h) and cell cycle progression were analyzed by flow cytometry to determine the DNA content of cell nuclei stained with propidium iodide (PI) | Apoptosis cell death at a concentration of 1–2 µg/mL (0.648–1.295 µM); an increase in the number of cells in the G0/G1 phase of the cell cycle and a decrease in the number of cells in the S and G2/M phases, and activation of caspase-3 | - | [40] |
| S. atropurpurea subsp. maritima | Leaves | Methanolic extract | Human epithelial colorectal adenocarcinoma Caco-2 | MTT assay, cell viability (48 h); Annexin-V/PI Double-Staining analysis of apoptotic cells, flow cytometer; Multicaspase assay; quantitative reverse transcription Real-Time PCR (RT-qPCR) | Plant extract at a concentration of IC10, IC20, or IC30 enhances the toxicity of doxorubicin; cell viability IC50 = 1.04 µg/mL; the combination of doxorubicin with plant extract at a concentration of IC50 and IC10 results in an increase in the percentage of apoptotic cells, the percentage of caspase-activated cells, mRNA levels of the apoptosis related-genes (Bax, caspase-3, p21), and a decrease in expression level of anti-apoptotic genes (Bcl-2) | doxorubicin cell viability IC50 = 2.41 µg/mL | [81] |
| Fruits | Water extract, silver nanoparticles | Human multiple myeloma U266 cell line and human breast cancer cell line MDA-MB-231 | MTT assay, cell viability (48 h) | U266 IC50 = 10 µg/mL; MDA-MB-231 IC50 = 12 µg/mL | - | [56] | |
| S. stellata | Whole plants | Scabiostellatosides A-H | Fibrosarcoma HT1080 cell line | WST1 assay, cell viability (72 h) | Scabiostellatoside B, IC50 = 49 µM; scabiostellatoside D, IC50 = 40 µM; scabiostellatoside E, IC50 = 38 µM; scabiostellatoside F, IC50 =12 µM; scabiostellatoside H, IC50 = 40 µM; scabiostellatoside A, scabiostellatoside C, scabiostellatoside G not cytotoxic at a concentration of 50 µM | Doxorubicin, IC50 = 0.59 µM | [48] |
| Whole plants | Iridoids (7-O-(E-caffeoyl)-sylvestroside I and 7-O-(E-p-coumaroyl)-sylvestroside I | Fibrosarcoma HT1080 cell line | WST1 assay, cell viability (72 h) | 7-O-(E-caffeoyl)-sylvestroside I, IC50 = 35.9 µg/mL; 7-O-(E-p-coumaroyl)-sylvestroside I, IC50 > 100 µg/mL | - | [75] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Skała, E.; Szopa, A. Dipsacus and Scabiosa Species—The Source of Specialized Metabolites with High Biological Relevance: A Review. Molecules 2023, 28, 3754. https://doi.org/10.3390/molecules28093754
Skała E, Szopa A. Dipsacus and Scabiosa Species—The Source of Specialized Metabolites with High Biological Relevance: A Review. Molecules. 2023; 28(9):3754. https://doi.org/10.3390/molecules28093754
Chicago/Turabian StyleSkała, Ewa, and Agnieszka Szopa. 2023. "Dipsacus and Scabiosa Species—The Source of Specialized Metabolites with High Biological Relevance: A Review" Molecules 28, no. 9: 3754. https://doi.org/10.3390/molecules28093754
APA StyleSkała, E., & Szopa, A. (2023). Dipsacus and Scabiosa Species—The Source of Specialized Metabolites with High Biological Relevance: A Review. Molecules, 28(9), 3754. https://doi.org/10.3390/molecules28093754