Comparison of Ten Metal-Doped LaFeO3 Samples on Photocatalytic Degradation of Antibiotics in Water under Visible Light: Role of Surface Area and Aqueous Phosphate Ions
Abstract
:1. Introduction
2. Results and Discussion
2.1. Microstructural Characterization
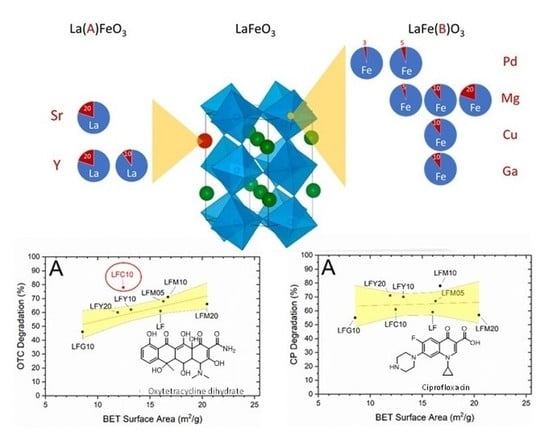
2.2. Brunauer–Emmett–Teller (BET) Specific Surface Area (SSA)
2.3. Scanning Electron Microscopy (SEM) and Energy Dispersive X-ray Analysis (EDX)
2.4. Photocatalytic Degradation of CP and OTC
2.5. Photocatalytic Degradation of CP and OTC
2.6. BET Specific Surface Area (SSA) and CP Degradation
3. Materials and Methods
3.1. Chemicals
3.2. Microstructural Characterization
3.3. Scanning Electron Microscopy (SEM) and Energy Dispersive X-ray Analysis (EDX)
3.4. Photocatalytic Studies
3.5. UV–Vis Spectroscopy
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Patel, M.; Kumar, R.; Kishor, K.; Mlsna, T.; Pittman, C.U.; Mohan, D. Pharmaceuticals of Emerging Concern in Aquatic Systems: Chemistry, Occurrence, Effects, and Removal Methods. Chem. Rev. 2019, 119, 3510–3673. [Google Scholar] [CrossRef] [PubMed]
- Cantarella, M.; Impellizzeri, G.; Di Mauro, A.; Privitera, V.; Carroccio, S.C. Innovative Polymeric Hybrid Nanocomposites for Application in Photocatalysis. Polymers 2021, 13, 1184. [Google Scholar] [CrossRef]
- Hoffmann, M.R.; Martin, S.T.; Choi, W.; Bahnemann, D.W. Environmental Applications of Semiconductor Photocatalysis. Chem. Rev. 1995, 95, 69–96. [Google Scholar] [CrossRef]
- Li, S.; Jing, L.; Fu, W.; Yang, L.; Xin, B.; Fu, H. Photoinduced Charge Property of Nanosized Perovskite-Type LaFeO3 and Its Relationships with Photocatalytic Activity under Visible Irradiation. Mater. Res. Bull. 2007, 42, 203–212. [Google Scholar] [CrossRef]
- Parida, K.M.; Reddy, K.H.; Martha, S.; Das, D.P.; Biswal, N. Fabrication of Nanocrystalline LaFeO3: An Efficient Sol-Gel Auto-Combustion Assisted Visible Light Responsive Photocatalyst for Water Decomposition. Int. J. Hydrogen Energy 2010, 35, 12161–12168. [Google Scholar] [CrossRef]
- Natali Sora, I.; Fumagalli, D. Fast Photocatalytic Degradation of Pharmaceutical Micropollutants and Ecotoxicological Effects. Environ. Sci. Pollut. Res. 2017, 24, 12556–12561. [Google Scholar] [CrossRef] [PubMed]
- Birben, N.C.; Lale, E.; Pelosato, R.; Demirel, C.S.U.; Sora, I.N.; Bekbolet, M. Photocatalytic Bactericidal Performance of LaFeo3 under Solar Light: Kinetics, Spectroscopic and Mechanistic Evaluation. Water 2021, 13, 2785. [Google Scholar] [CrossRef]
- Koba-Ucun, O.; Arslan-Alaton, I.; Sora, I.N.; Bekbölet, M. Persulfate-Enhanced Lanthanum Iron Oxide-Mediated Photocatalysis Can Effectively Degrade an Aqueous Industrial Dye and Mineralize Water and Wastewater. Desalination Water Treat. 2022, 267, 215–230. [Google Scholar] [CrossRef]
- Arslan-Alaton, I.; Koba-Ucun, O.; Turkten, N.; Sora, I.N.; Bekbolet, M. More about Persulfate-Assisted Ferrilanthanide-Mediated Photocatalysis of Textile Industry Dye Reactive Black 5: Surface Properties and Structural Assessment. Water 2023, 15, 906. [Google Scholar] [CrossRef]
- García, F.E.; Litter, M.I.; Sora, I.N. Assessment of the Arsenic Removal From Water Using Lanthanum Ferrite. ChemistryOpen 2021, 10, 790–797. [Google Scholar] [CrossRef]
- Wheeler, G.P.; Baltazar, V.U.; Smart, T.J.; Radmilovic, A.; Ping, Y.; Choi, K.-S. Combined Theoretical and Experimental Investigations of Atomic Doping to Enhance Photon Absorption and Carrier Transport of LaFeO3 Photocathodes. Chem. Mater. 2019, 31, 5890–5899. [Google Scholar] [CrossRef]
- Humayun, M.; Ullah, H.; Usman, M.; Habibi-Yangjeh, A.; Tahir, A.A.; Wang, C.; Luo, W. Perovskite-Type Lanthanum Ferrite Based Photocatalysts: Preparation, Properties, and Applications. J. Energy Chem. 2022, 66, 314–338. [Google Scholar] [CrossRef]
- Phan, T.T.N.; Nikoloski, A.N.; Bahri, P.A.; Li, D. Heterogeneous Photo-Fenton Degradation of Organics Using Highly Efficient Cu-Doped LaFeO3 under Visible Light. J. Ind. Eng. Chem. 2018, 61, 53–64. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, L.; Chen, Z.; Hu, J.; Li, S.; Wang, Z.; Liu, J.; Wang, X. Semiconductor Heterojunction Photocatalysts: Design, Construction, and Photocatalytic Performances. Chem. Soc. Rev. 2014, 43, 5234–5244. [Google Scholar] [CrossRef] [PubMed]
- Dong, S.; Wang, Y.; Zhao, Y.; Zhou, X.; Zheng, H. La3+/La(OH)3 Loaded Magnetic Cationic Hydrogel Composites for Phosphate Removal: Effect of Lanthanum Species and Mechanistic Study. Water Res. 2017, 126, 433–441. [Google Scholar] [CrossRef] [PubMed]
- Blair, B.; Nikolaus, A.; Hedman, C.; Klaper, R.; Grundl, T. Evaluating the Degradation, Sorption, and Negative Mass Balances of Pharmaceuticals and Personal Care Products during Wastewater Treatment. Chemosphere 2015, 134, 395–401. [Google Scholar] [CrossRef]
- Sarmah, A.K.; Meyer, M.T.; Boxall, A.B.A. A Global Perspective on the Use, Sales, Exposure Pathways, Occurrence, Fate and Effects of Veterinary Antibiotics (VAs) in the Environment. Chemosphere 2006, 65, 725–759. [Google Scholar] [CrossRef]
- Bolon, M.K. The Newer Fluoroquinolones. Infect. Dis. Clin. N. Am. 2009, 23, 1027–1051. [Google Scholar] [CrossRef]
- Pelosato, R.; Bolognino, I.; Fontana, F.; Sora, I.N. Applications of Heterogeneous Photocatalysis to the Degradation of Oxytetracycline in Water: A Review. Molecules 2022, 27, 2743. [Google Scholar] [CrossRef]
- Bahnemann, W.; Muneer, M.; Haque, M.M. Titanium Dioxide-Mediated Photocatalysed Degradation of Few Selected Organic Pollutants in Aqueous Suspensions. Catal. Today 2007, 124, 133–148. [Google Scholar] [CrossRef]
- Ilham, F.; Triyono, D.; Abdillah, M.N. Room Temperature Electrical Impedance Analysis of LaFe1-XMgxO3(x = 0.01 and 0.05) Ceramics. J. Phys. Conf. Ser. 2021, 1816, 012059. [Google Scholar] [CrossRef]
- Caronna, T.; Fontana, F.; Sora, I.N.; Pelosato, R. Chemical Synthesis and Structural Characterization of the Substitution Compound LaFe1-XCuxO3 (x = 0–0.40). Mater. Chem. Phys. 2009, 116, 645–648. [Google Scholar] [CrossRef]
- Singh, P.; Choudhuri, I.; Rai, H.M.; Mishra, V.; Kumar, R.; Pathak, B.; Sagdeo, A.; Sagdeo, P.R. Fe Doped LaGaO3: Good White Light Emitters. RSC Adv. 2016, 6, 100230–100238. [Google Scholar] [CrossRef]
- Saha, J.; Jana, Y.M.; Mukherjee, G.D.; Mondal, R.; Kumar, S.; Gupta, H.C. Structure, Mössbauer Spectroscopy and Vibration Phonon Spectra in Valence-Bond Force-Field Model Approach for Distorted Perovskites AFeO3 (A = La, Y). Mater. Chem. Phys. 2020, 240, 122286. [Google Scholar] [CrossRef]
- Zhang, R.; Villanueva, A.; Alamdari, H.; Kaliaguine, S. Cu- and Pd-Substituted Nanoscale Fe-Based Perovskites for Selective Catalytic Reduction of NO by Propene. J. Catal. 2006, 237, 368–380. [Google Scholar] [CrossRef]
- Verlicchi, P.; Al Aukidy, M.; Zambello, E. Occurrence of Pharmaceutical Compounds in Urban Wastewater: Removal, Mass Load and Environmental Risk after a Secondary Treatment-A Review. Sci. Total Environ. 2012, 429, 123–155. [Google Scholar] [CrossRef] [PubMed]
- Albini, A.; Monti, S. Photophysics and Photochemistry of Fluoroquinolones. Chem. Soc. Rev. 2003, 32, 238–250. [Google Scholar] [CrossRef] [PubMed]
- Grätzel, C.K.; Jirousek, M.; Grätzel, M. Decomposition of Organophosphorus Compounds on Photoactivated TiO2 Surfaces. J. Mol. Catal. 1990, 60, 375–387. [Google Scholar] [CrossRef]
- Fox, M.A.; Dulay, M.T. Heterogeneous Photocatalysis. Chem. Rev. 1993, 93, 341–357. [Google Scholar] [CrossRef]
- Wang, G.; Cheng, C.; Zhu, J.; Wang, L.; Gao, S.; Xia, X. Enhanced Degradation of Atrazine by Nanoscale LaFe1-xCuxO3-δ Perovskite Activated Peroxymonosulfate: Performance and Mechanism. Sci. Total Environ. 2019, 673, 565–575. [Google Scholar] [CrossRef]
- Zhang, H.; Zhang, R.; Wu, Z.; Yang, F.; Luo, M.; Yao, G.; Ao, Z.; Lai, B. Cobalt-Doped Boosted the Peroxymonosulfate Activation Performance of LaFeO3 Perovskite for Atrazine Degradation. Chem. Eng. J. 2023, 452, 139427. [Google Scholar] [CrossRef]
- Zhao, Y.; Huang, B.; An, H.; Dong, G.; Feng, J.; Wei, T.; Ren, Y.; Ma, J. Enhanced Activation of Peroxymonosulfate by Sr-Doped LaFeO3 Perovskite for Orange I Degradation in the Water. Sep. Purif. Technol. 2021, 256, 117838. [Google Scholar] [CrossRef]
- Harja, M.; Ciobanu, G. Studies on Adsorption of Oxytetracycline from Aqueous Solutions onto Hydroxyapatite. Sci. Total Environ. 2018, 628–629, 36–43. [Google Scholar] [CrossRef] [PubMed]
- Turkten, N.; Sora, I.N.; Tomruk, A.; Bekbolet, M. Photocatalytic Degradation of Humic Acids Using LaFeO3. Catalysts 2018, 8, 630. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, L.; Hu, C.; Wang, X.; Lyu, L.; Sheng, G. Enhanced Degradation of Organic Pollutants over Cu-Doped LaAlO3 Perovskite through Heterogeneous Fenton-like Reactions. Chem. Eng. J. 2018, 332, 572–581. [Google Scholar] [CrossRef]
- Gu, C.; Karthikeyan, K.G. Sorption of the Antimicrobial Ciprofloxacin to Aluminum and Iron Hydrous Oxides. Environ. Sci. Technol. 2005, 39, 9166–9173. [Google Scholar] [CrossRef]
- Yahya, M.S.; Oturan, N.; El Kacemi, K.; El Karbane, M.; Aravindakumar, C.T.; Oturan, M.A. Oxidative Degradation Study on Antimicrobial Agent Ciprofloxacin by Electro-Fenton Process: Kinetics and Oxidation Products. Chemosphere 2014, 117, 447–454. [Google Scholar] [CrossRef]
- Zhao, C.; Pelaez, M.; Duan, X.; Deng, H.; O’Shea, K.; Fatta-Kassinos, D.; Dionysiou, D.D. Role of PH on Photolytic and Photocatalytic Degradation of Antibiotic Oxytetracycline in Aqueous Solution under Visible/Solar Light: Kinetics and Mechanism Studies. Appl. Catal. B Environ. 2013, 134–135, 83–92. [Google Scholar] [CrossRef]
- Kulshrestha, P.; Giese, R.F., Jr.; Aga, D.S. Investigating the Molecular Interactions of Oxytetracycline in Clay and Organic Matter: Insights on Factors Affecting Its Mobility in Soil. Environ. Sci. Technol. 2004, 38, 4097–4105. [Google Scholar] [CrossRef] [PubMed]
- Jiao, S.; Zheng, S.; Yin, D.; Wang, L.; Chen, L. Aqueous Oxytetracycline Degradation and the Toxicity Change of Degradation Compounds in Photoirradiation Process. J. Environ. Sci. 2008, 20, 806–813. [Google Scholar] [CrossRef]
- Chen, H.; Peng, Y.-P.; Chen, K.-F.; Lai, C.-H.; Lin, Y.-C. Rapid Synthesis of Ti-MCM-41 by Microwave-Assisted Hydrothermal Method towards Photocatalytic Degradation of Oxytetracycline. J. Environ. Sci. 2016, 44, 76–87. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.-Y.; Zhou, K.; Chen, B.-Y.; Chang, C.-T. Graphene/TiO2/ZSM-5 Composites Synthesized by Mixture Design Were Used for Photocatalytic Degradation of Oxytetracycline under Visible Light: Mechanism and Biotoxicity. Appl. Surf. Sci. 2016, 362, 329–334. [Google Scholar] [CrossRef]
- Jo, W.-K.; Kumar, S.; Isaacs, M.A.; Lee, A.F.; Karthikeyan, S. Cobalt Promoted TiO2/GO for the Photocatalytic Degradation of Oxytetracycline and Congo Red. Appl. Catal. B Environ. 2017, 201, 159–168. [Google Scholar] [CrossRef]
- Pereira, J.H.O.S.; Vilar, V.J.P.; Borges, M.T.; González, O.; Esplugas, S.; Boaventura, R.A.R. Photocatalytic Degradation of Oxytetracycline Using TiO2 under Natural and Simulated Solar Radiation. Sol. Energy 2011, 85, 2732–2740. [Google Scholar] [CrossRef]
- Sudhaik, A.; Raizada, P.; Shandilya, P.; Singh, P. Magnetically Recoverable Graphitic Carbon Nitride and NiFe2O4 Based Magnetic Photocatalyst for Degradation of Oxytetracycline Antibiotic in Simulated Wastewater under Solar Light. J. Environ. Chem. Eng. 2018, 6, 3874–3883. [Google Scholar] [CrossRef]
- Hassandoost, R.; Pouran, S.R.; Khataee, A.; Orooji, Y.; Joo, S.W. Hierarchically Structured Ternary Heterojunctions Based on Ce3+/Ce4+ Modified Fe3O4 Nanoparticles Anchored onto Graphene Oxide Sheets as Magnetic Visible-Light-Active Photocatalysts for Decontamination of Oxytetracycline. J. Hazard. Mater. 2019, 376, 200–211. [Google Scholar] [CrossRef] [PubMed]
- Xu, K.; Yang, X.; Ruan, L.; Qi, S.; Liu, J.; Liu, K.; Pan, S.; Feng, G.; Dai, Z.; Yang, X.; et al. Superior Adsorption and Photocatalytic Degradation Capability of Mesoporous LaFeO3/g-C3N4 for Removal of Oxytetracycline. Catalysts 2020, 10, 301. [Google Scholar] [CrossRef]








| Sample | Nominal Composition | BET (m2 g−1) | Crystallite Size (nm) |
|---|---|---|---|
| LFC10 | LaFe0.90Cu0.10O3 | 12 | 27 |
| LFG10 | LaFe0.90Ga0.10O3 | 9 | 45 |
| LFM05 | LaFe0.95Mg0.05O3 | 16 | 25 |
| LFM10 | LaFe0.90Mg0.10O3 | 17 | 25 |
| LFM20 | LaFe0.80Mg0.20O3 | 20 | 18 |
| LFP03 | LaFe0.97Pd0.03O3 | n.a. | 39 |
| LFP05 | LaFe0.95Pd0.05O3 | n.a. | 31 |
| LFS20 | La0.80Sr0.20FeO3 | 17 | 26 |
| LFY10 | La0.90Y0.10FeO3 | 13 | 28 |
| LFY20 | La0.80Y0.20FeO3 | 12 | 24 |
| Sample | La | Fe | Cu | Mg | Ga | Pd | Y | Sr |
|---|---|---|---|---|---|---|---|---|
| LFC10 | 49 (50) | 46 (45) | 5.0 (5.0) | - | - | - | - | - |
| LFM05 | 50 (50) | 47 (47.5) | - | 3.0 (2.5) | - | - | - | - |
| LFM10 | 46 (50) | 49 (45) | - | 5.0 (5.0) | - | - | - | - |
| LFM20 | 45 (50) | 38 (40) | - | 17 (10) | - | - | - | - |
| LFG10 | 50 (50) | 44 (45) | - | - | 6.0 (5.0) | - | - | - |
| LFP03 | 46 (50) | 52 (48.5) | - | - | - | 2.0 (1.5) | - | - |
| LFP05 | 49 (50) | 48 (47.5) | - | - | - | 3.0 (2.5) | - | - |
| LFY10 | 45 (45) | 50 (50) | - | - | - | - | 5.0 (5.0) | - |
| LFY20 | 39 (40) | 49 (50) | - | - | - | - | 12 (10) | - |
| LFS20 | 40 (40) | 50 (50) | - | - | - | - | - | 10 (10) |
| Solution | LF | LFY10 | LFC10 | LFM10 |
|---|---|---|---|---|
| CP unbuffered | 41 | 30 | 39 | 22 |
| CP buffered | 62 | 55 | 72 | 46 |
| Catalyst | Catalyst Loading (mg L−1) | Pollutant | Pollutant (mol L−1) | Additive | k1 (min−1) | R2 |
|---|---|---|---|---|---|---|
| LFM10 | 130 | OTC | 5 × 10−6 M | H2O2 | 0.0051 | 0.999 |
| LFC10 | 130 | OTC | 5 × 10−6 M | H2O2 | 0.0068 | 0.980 |
| LFM10 | 130 | CP | 5 × 10−6 M | H2O2 | 0.0069 | 0.988 |
| LFC10 | 130 | CP | 5 × 10−6 M | H2O2 | 0.0042 | 0.951 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bolognino, I.; Pelosato, R.; Marcì, G.; Natali Sora, I. Comparison of Ten Metal-Doped LaFeO3 Samples on Photocatalytic Degradation of Antibiotics in Water under Visible Light: Role of Surface Area and Aqueous Phosphate Ions. Molecules 2023, 28, 3807. https://doi.org/10.3390/molecules28093807
Bolognino I, Pelosato R, Marcì G, Natali Sora I. Comparison of Ten Metal-Doped LaFeO3 Samples on Photocatalytic Degradation of Antibiotics in Water under Visible Light: Role of Surface Area and Aqueous Phosphate Ions. Molecules. 2023; 28(9):3807. https://doi.org/10.3390/molecules28093807
Chicago/Turabian StyleBolognino, Isabella, Renato Pelosato, Giuseppe Marcì, and Isabella Natali Sora. 2023. "Comparison of Ten Metal-Doped LaFeO3 Samples on Photocatalytic Degradation of Antibiotics in Water under Visible Light: Role of Surface Area and Aqueous Phosphate Ions" Molecules 28, no. 9: 3807. https://doi.org/10.3390/molecules28093807