Role of Silica on Clay-Catalyzed Ozonation for Total Mineralization of Bisphenol-A
Abstract
:1. Introduction
2. Results and Discussion
2.1. UV-Vis Spectrum Evolution during Non-Catalytic Ozonation
2.2. Effect of Clay Catalyst Addition
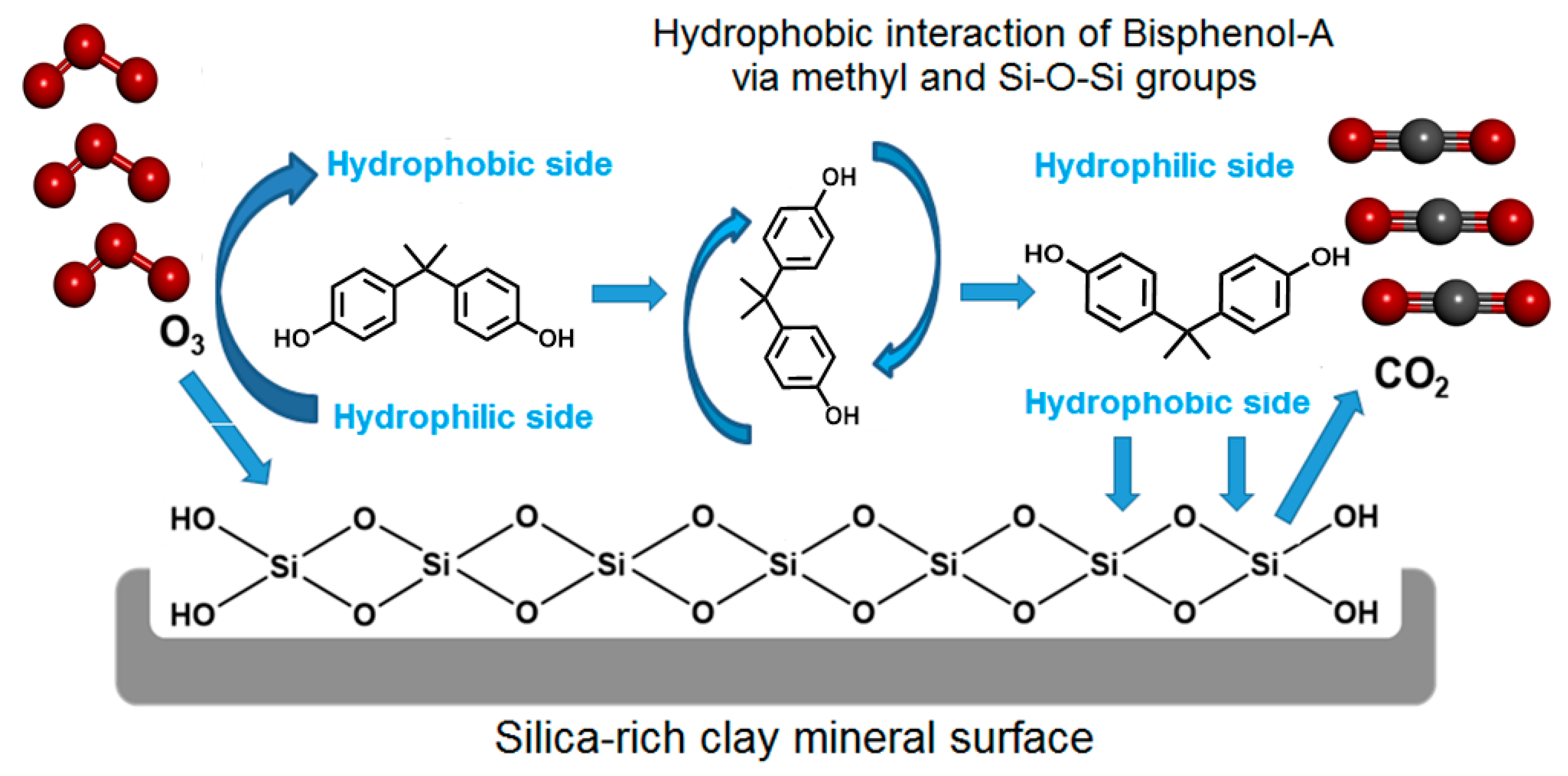
2.3. Adsorption on HMt and Hydrophobicity
2.4. Effect of BPA on Clay Dispersion
2.5. Role of Silica Content and pH
2.6. Role of Iron
3. Materials and Methods
3.1. Catalyst Preparation
3.2. Material Characterization
3.3. Ozonation Tests and Product Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Barriuso, E.; Calvet, R.; Schiavon, M.; Soulas, G. Les pesticides et les polluants organiques des sols: Transformations et dissipation. Etude Gest. Sols. 1996, 3, 279–295. [Google Scholar]
- Shahidi, D.; Roy, R.; Azzouz, A. Advances in catalytic oxidation of organic pollutants-Prospects for thorough mineralization by natural clay catalysts. Appl. Catal. B Environ. 2015, 174–175, 277–292. [Google Scholar] [CrossRef]
- Azzouz, A.; Mirila, D.-C.; Nistor, D.-I.; Boudissa, F.; Roy, R. Advances in the oxidative degradation of organic pollutants: Prospects for catalyzed oxidation processes targeting total mineralization. In Advances in Chemestry Research; Taylor, J.C., Ed.; Nova Science Publishers: Hauppauge, NY, USA, 2019; Volume 49, p. 55467. [Google Scholar]
- Weber, R.; Watson, A.; Forter, M.; Oliaei, F. Persistent organic pollutants and landfills-a review of past experiences and future challenges. Waste Manag. Res. 2011, 29, 107–121. [Google Scholar] [CrossRef] [PubMed]
- Yong, R.N.; Rao, S.M. Mechanistic evaluation of mitigation of petroleum hydrocarbon contamination by soil medium. Can. Geotech. J. 1991, 28, 84–91. [Google Scholar] [CrossRef]
- Meite, L.; Szabo, R.; Mazellier, P.; de Laat, J. Kinetics of phototransformation of emerging contaminants in aqueous solution. Rev. Des Sci. L’eau 2010, 23, 31–39. [Google Scholar]
- Trabelsi, H. Etude de la Dégradabilité et de la Toxicité des Colorants par Ozonation et Photocatalyse. Master’s Thesis, Yahia Fares University of Medea, Medea, Algeria, 2014. [Google Scholar]
- Azzouz, A.; Hausler, R.; El-Akhrass, M. Pesticides and removal approaches. In Sorbents Materials for Controlling Environmental Pollution; Núñez-Delgado, A., Ed.; Elsevier: Amsterdam, The Netherlands, 2021; Chapter 17. [Google Scholar]
- Mougin, C.; Chaplain, V.; Rama-Mercier, R.; Sohier, L.; Sigoillot, J.-C.; Asther, M. Utilisation de champignons filamenteux pour la dépollution de sols pollués par des polluants organiques. Déchets Sci. Tech. 1996, 4, 20–22. [Google Scholar] [CrossRef]
- Meeker, J.D.; Ehrlich, S.; Toth, T.L.; Wright, D.L.; Calafat, A.M.; Trisini, A.T.; Ye, X.; Hauser, R. Semen quality and sperm DNA damage in relation to urinary bisphenol A among men from an infertility clinic. Reprod. Toxicol. 2010, 30, 532–539. [Google Scholar] [CrossRef]
- Lassen, T.H.; Frederiksen, H.; Jensen, T.K.; Petersen, J.H.; Joensen, U.N.; Main, K.M.; Skakkebaek, N.E.; Juul, A.; Jørgensen, N.; Andersson, A.-M. Urinary Bisphenol A Levels in Young Men: Association with Reproductive Hormones and Semen Quality. Environ. Health Perspect. 2014, 122, 478–484. [Google Scholar] [CrossRef]
- Metcalfe, C.D.; Metcalfe, T.L.; Kiparissis, Y.; Koenig, B.G.; Khan, C.; Hughes, R.J.; Croley, T.R.; March, R.E.; Potter, T. Estrogenic potency of chemicals detected in sewage treatment plant effluents as determined by in vivo assays with Japanese medaka (Oryzias latipes). Environ. Toxicol. Chem. 2001, 20, 297–308. [Google Scholar] [CrossRef]
- Schulte-Oehlmann, U.; Tillmann, M.; Casey, D.; Duft, M.; Markert, B.; Oehlmann, J. Östrogenartige wirkungen von bisphenol a auf vorderkiemenschnecken (Mollusca: Gastropoda: Prosobranchia). Umweltwiss. Schadst.-Forsch. 2001, 13, 319. [Google Scholar] [CrossRef]
- Chen, J.; Saili, K.S.; Liu, Y.; Li, L.; Zhao, Y.; Jia, Y.; Bai, C.; Tanguay, R.L.; Dong, Q.; Huang, C. Developmental bisphenol A exposure impairs sperm function and reproduction in zebrafish. Chemosphere 2017, 169, 262–270. [Google Scholar] [CrossRef] [PubMed]
- Lang, I.A.; Galloway, T.S.; Scarlett, A.; Henley, W.E.; Depledge, M.; Wallace, R.B.; Melzer, D. Association of Urinary Bisphenol A concentration with medical disorders and laboratory abnormalities in adults. JAMA J. Am. Med. Assoc. 2008, 300, 1303–1310. [Google Scholar] [CrossRef]
- Schecter, A.; Malik, N.; Haffner, D.; Smith, S.; Harris, T.R.; Paepke, O.; Birnbaum, L. Bisphenol A (BPA) in U.S. Food. Environ. Sci. Technol. 2010, 44, 9425–9430. [Google Scholar] [CrossRef] [PubMed]
- Campbell, C.G.; Borglin, S.E.; Green, F.B.; Grayson, A.; Wozei, E.; Stringfellow, W.T. Biologically directed environmental monitoring, fate, and transport of estrogenic endocrine disrupting compounds in water: A review. Chemosphere 2006, 65, 1265–1280. [Google Scholar] [CrossRef] [PubMed]
- Jackson, J.; Sutton, R. Sources of endocrine-disrupting chemicals in urban wastewater, Oakland, CA. Sci. Total Environ. 2008, 405, 153–160. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Avila, J.; Bonet, J.; Velasco, G.; Lacorte, S. Determination and occurrence of phthalates, alkylphenols, bisphenol A, PBDEs, PCBs and PAHs in an industrial sewage grid discharging to a Municipal Wastewater Treatment Plant. Sci. Total Environ. 2009, 407, 4157–4167. [Google Scholar] [CrossRef] [PubMed]
- Gómez, M.J.; Martínez Bueno, M.J.; Lacorte, S.; Fernández-Alba, A.R.; Agüera, A. Pilot survey monitoring pharmaceuticals and related compounds in a sewage treatment plant located on the Mediterranean coast. Chemosphere 2007, 66, 993–1002. [Google Scholar] [CrossRef]
- Kuch, H.M.; Ballschmiter, K. Determination of Endocrine-Disrupting Phenolic Compounds and Estrogens in Surface and Drinking Water by HRGC−(NCI)−MS in the Picogram per Liter Range. Environ. Sci. Technol. 2001, 35, 3201–3206. [Google Scholar] [CrossRef]
- Braunrath, R.; Podlipna, D.; Padlesak, S.; Cichna-Markl, M. Determination of bisphenol A in canned foods by immunoaffinity chromatography, HPLC, and fluorescence detection. J. Agric. Food Chem. 2005, 53, 8911–8917. [Google Scholar] [CrossRef]
- Oppeneer, S.J.; Robien, K. Bisphenol A exposure and associations with obesity among adults: A critical review. Public Health Nutr. 2014, 18, 1847–1863. [Google Scholar] [CrossRef]
- Xue, J.; Kannan, P.; Kumosani, T.A.; Al-Malki, A.L.; Kannan, K. Resin-based dental sealants as a source of human exposure to bisphenol analogues, bisphenol A diglycidyl ether, and its derivatives. Environ. Res. 2018, 162, 35–40. [Google Scholar] [CrossRef] [PubMed]
- Numata, M.; Yarita, T.; Aoyagi, Y.; Yamazaki, M.; Takatsu, A. Investigation of saponification for determination of polychlorinated biphenyls in marine sediments. Chemosphere 2005, 58, 865–875. [Google Scholar] [CrossRef] [PubMed]
- Northcott, G.L.; Jones, K.C. Validation of procedures to quantify nonextractable polycyclic aromatic hydrocarbon residues in soil. J. Environ. Qual. 2003, 32, 571–582. [Google Scholar] [CrossRef] [PubMed]
- Larouk, S.; Ouargli, R.; Shahidi, D.; Olhund, L.; Shiao, T.C.; Chergui, N.; Sehili, T.; Roy, R.; Azzouz, A. Catalytic ozonation of Orange-G through highly interactive contributions of hematite and SBA-16–To better understand azo-dye oxidation in nature. Chemosphere 2017, 168, 1648–1657. [Google Scholar] [CrossRef] [PubMed]
- Arslan, İ.; Akmehmet Balcioǧlu, I.; Tuhkanen, T. Oxidative treatment of simulated dyehouse effluent by uv and near-UV light assisted Fenton’s reagent. Chemosphere 1999, 39, 2767–2783. [Google Scholar] [CrossRef]
- Tang, S.; Yuan, D.; Zhang, Q.; Liu, Y.; Zhang, Q.; Liu, Z.; Huang, H. Fe-Mn bi-metallic oxides loaded on granular activated carbon to enhance dye removal by catalytic ozonation. Environ. Sci. Pollut. Res. 2016, 23, 18800–18808. [Google Scholar] [CrossRef]
- Faria, P.C.C.; Orfão, J.J.M.; Pereira, M.F.R. Mineralisation of coloured aqueous solutions by ozonation in the presence of activated carbon. Water Res. 2005, 39, 1461–1470. [Google Scholar] [CrossRef]
- Faria, P.C.C.; Órfão, J.J.M.; Pereira, M.F.R. Ozone Decomposition in Water Catalyzed by Activated Carbon: Influence of Chemical and Textural Properties. Ind. Eng. Chem. Res. 2006, 45, 2715–2721. [Google Scholar] [CrossRef]
- Pirgalıoğlu, S.; Özbelge, T.A. Comparison of non-catalytic and catalytic ozonation processes of three different aqueous single dye solutions with respect to powder copper sulfide catalyst. Appl. Catal. A Gen. 2009, 363, 157–163. [Google Scholar] [CrossRef]
- Keykavoos, R.; Mankidy, R.; Ma, H.; Jones, P.; Soltan, J. Mineralization of bisphenol A by catalytic ozonation over alumina. Sep. Purif. Technol. 2013, 107, 310–317. [Google Scholar] [CrossRef]
- Azzouz, A.; Kotbi, A.; Niquette, P.; Sajin, T.; Ursu, A.V.; Rami, A.; Monette, F.; Hausler, R. Ozonation of oxalic acid catalyzed by ion-exchanged montmorillonite in moderately acidic media. React. Kinet. Mech. Catal. 2010, 99, 289–302. [Google Scholar] [CrossRef]
- Ma, H.; Wang, B.; Luo, X. Studies on degradation of Methyl Orange wastewater by combined electrochemical process. J. Hazard. Mater. 2007, 149, 492–498. [Google Scholar] [CrossRef] [PubMed]
- Sasidharan Pillai, I.M.; Gupta, A.K. Effect of inorganic anions and oxidizing agents on electrochemical oxidation of methyl orange, malachite green and 2,4-dinitrophenol. J. Electroanal. Chem. 2016, 762, 66–72. [Google Scholar] [CrossRef]
- Xu, L.; Wang, J. Fenton-like degradation of 2,4-dichlorophenol using Fe3O4 magnetic nanoparticles. Appl. Catal. B Environ. 2012, 123–124, 117–126. [Google Scholar] [CrossRef]
- Shahidi, D.; Roy, R.; Azzouz, A. Total removal of oxalic acid via synergistic parameter interaction in montmorillonite catalyzed ozonation. J. Environ. Chem. Eng. 2014, 2, 20–30. [Google Scholar] [CrossRef]
- Karthikeyan, S.; Titus, A.; Gnanamani, A.; Mandal, A.B.; Sekaran, G. Treatment of textile wastewater by homogeneous and heterogeneous Fenton oxidation processes. Desalination 2011, 281, 438–445. [Google Scholar] [CrossRef]
- Azzouz, A. Advances in oxidative decomposition of oxalic acid in aqueous media. In Advances in Reserach Chemistry; Taylor, J.C., Ed.; Nova-Science Publishers: NewYork, NY, USA, 2012; Volume 14. [Google Scholar]
- Long, J.; Guo, Y.; Yu, G.; Komarneni, S.; Wang, Y. Evaluation of the Effect of Catalysts on Ozone Mass Transfer and Pollutant Abatement during Laboratory Catalytic Ozonation Experiments: Implications for Practical Water and Wastewater Treatment. ACS EST Eng. 2023, 3, 387–397. [Google Scholar] [CrossRef]
- Tian, J.; Wei, J.; Liang, Y.; Guo, R.; Li, B.; Qu, R.; Zhou, D.; Wang, Z.; Sun, P. Catalytic ozonation of an imidazole ionic liquid via Fe3O4/ZnO nanocomposites: Performance, products and reaction mechanism. J. Environ. Chem. Eng. 2022, 10, 108726. [Google Scholar] [CrossRef]
- Tian, J.; Li, B.; Qu, R.; Zhou, D.; Sun, C.; Wang, Z.; Zhu, F. Influence of anions on ozonation of bisphenol AF: Kinetics, reaction pathways, and toxicity assessment. Chemosphere 2022, 286, 131864. [Google Scholar] [CrossRef]
- Nawrocki, J.; Fijołek, L. Effect of aluminium oxide contaminants on the process of ozone decomposition in water. Appl. Catal. B Environ. 2013, 142–143, 533–537. [Google Scholar] [CrossRef]
- Benghaffour, A.; Foka-Wembe, E.-N.; Dami, M.; Dewez, D.; Azzouz, A. Insight into natural medium remediation through ecotoxicity correlation with clay catalyst selectivity in organic molecule ozonation. Dalton Trans. 2022, 11, 4366–4376. [Google Scholar] [CrossRef] [PubMed]
- Boudissa, F.; Mirilà, D.; Arus, V.-A.; Terkmani, T.; Semaan, S.; Proulx, M.; Nistor, I.-D.; Roy, R.; Azzouz, A. Acid-treated clay catalysts for organic dye ozonation–Thorough mineralization through optimum catalyst basicity and hydrophilic character. J. Hazard. Mater. 2019, 364, 356–366. [Google Scholar] [CrossRef] [PubMed]
- Boudissa, F.; Zekkari, M.; Arus, V.-A.; Ouargli-Saker, R.; Nabil, B.; Roy, R.; Azzouz, A. Clay-catalyzed ozonation of endocrine-disrupting compounds in solvent-free media–to better understand soil catalytic capacity. Dalton Trans. 2020, 49, 16693–16706. [Google Scholar] [CrossRef]
- Foka Wembe, E.N.; Benghafour, A.; Dewez, D.; Azzouz, A. Clay-Catalyzed Ozonation of Organic Pollutants in Water and Toxicity on Lemna minor: Effects of Molecular Structure and Interactions. Molecules 2023, 28, 222. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Ma, J.; Sun, Z.; Liu, H. Mechanism of heterogeneous catalytic ozonation of nitrobenzene in aqueous solution with modified ceramic honeycomb. Appl. Catal. B Environ. 2009, 89, 326–334. [Google Scholar] [CrossRef]
- Yong, K.; Wu, J.; Andrews, S. Heterogeneous Catalytic Ozonation of Aqueous Reactive Dye. Ozone Sci. Eng. 2005, 27, 257–263. [Google Scholar] [CrossRef]
- Kasprzyk-Hordern, B.; Ziółek, M.; Nawrocki, J. Catalytic ozonation and methods of enhancing molecular ozone reactions in water treatment. Appl. Catal. B Environ. 2003, 46, 639–669. [Google Scholar] [CrossRef]
- Chaliha, S.; Bhattacharyya, K.G. Using Mn(II)−MCM41 as an Environment-Friendly Catalyst to Oxidize Phenol, 2-Chlorophenol, and 2-Nitrophenol in Aqueous Solution. Ind. Eng. Chem. Res. 2008, 47, 1370–1379. [Google Scholar] [CrossRef]
- Beltrán, F.J.; Rivas, F.J.; Fernández, L.A.; Álvarez, P.M.; Montero-de-Espinosa, R. Kinetics of Catalytic Ozonation of Oxalic Acid in Water with Activated Carbon. Ind. Eng. Chem. Res. 2002, 41, 6510–6517. [Google Scholar] [CrossRef]
- Kaptijn, J.P. The Ecoclear® Process. Results from Full-Scale Installations. Ozone Sci. Eng. 1997, 19, 297–305. [Google Scholar] [CrossRef]
- Jans, U.; Hoigné, J. Activated Carbon and Carbon Black Catalyzed Transformation of Aqueous Ozone into OH-Radicals. Ozone Sci. Eng. 1998, 20, 67–90. [Google Scholar] [CrossRef]
- Shahidi, D.; Moheb, A.; Abbas, R.; Larouk, S.; Roy, R.; Azzouz, A. Total mineralization of sulfamethoxazole and aromatic pollutants through Fe2+-montmorillonite catalyzed ozonation. J. Hazard. Mater. 2015, 298, 338–350. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Park, H.; Yoon, J. Ozonation characteristics of bisphenol a in water. Environ. Technol. 2003, 24, 241–248. [Google Scholar] [CrossRef] [PubMed]
- Irmak, S.; Erbatur, O.; Akgerman, A. Degradation of 17β-estradiol and bisphenol A in aqueous medium by using ozone and ozone/UV techniques. J. Hazard. Mater. 2005, 126, 54–62. [Google Scholar] [CrossRef]
- Deborde, M.; Rabouan, S.; Duguet, J.P.; Legube, B. Kinetics of aqueous ozone-induced oxidation of some endocrine disrupters. Environ. Sci. Technol. 2005, 39, 6086–6092. [Google Scholar] [CrossRef]
- Choi, K.J.; Kim, S.G.; Kim, C.W.; Park, J.K. Removal efficiencies of endocrine disrupting chemicals by coagulation/flocculation, ozonation, powdered/granular activated carbon adsorption, and chlorination. Korean J. Chem. Eng. 2006, 23, 399–408. [Google Scholar] [CrossRef]
- Xu, B.; Gao, N.; Rui, M.; Wang, H.; Wu, H. Degradation of endocrine disruptor bisphenol A in drinking water by ozone oxidation. Front. Environ. Sci. Eng. China 2007, 1, 350–356. [Google Scholar] [CrossRef]
- Kim, S.E.; Park, N.S.; Yamada, H.; Tsuno, H. Modeling of Decomposition Characterisitics of Estrogenic Chemicals during Ozonation. Environ. Technol. 2008, 29, 287–296. [Google Scholar] [CrossRef]
- Baig, S.; Hansmann, G.; Paolini, B. Ozone oxidation of oestrogenic active substances in wastewater and drinking water. Water Sci. Technol. 2008, 58, 451–458. [Google Scholar] [CrossRef]
- Deborde, M.; Rabouan, S.; Mazellier, P.; Duguet, J.-P.; Legube, B. Oxidation of bisphenol A by ozone in aqueous solution. Water Res. 2008, 42, 4299–4308. [Google Scholar] [CrossRef]
- Rivas, F.J.; Encinas, Á.; Acedo, B.; Beltrán, F.J. Mineralization of bisphenol A by advanced oxidation processes. J. Chem. Technol. Biotechnol. 2009, 84, 589–594. [Google Scholar] [CrossRef]
- Garoma, T.; Matsumoto, S. Ozonation of aqueous solution containing bisphenol A: Effect of operational parameters. J. Hazard. Mater. 2009, 167, 1185–1191. [Google Scholar] [CrossRef] [PubMed]
- Gultekin, I.; Mavrov, V.; Ince, N.H. Degradation of Bisphenol-A by ozonation. J. Adv. Oxid. Technol. 2009, 12, 242–248. [Google Scholar] [CrossRef]
- Leal, L.; Temmink, H.; Zeeman, G.; Buisman, C. Removal of micropollutants from aerobically treated grey water via ozone and activated carbon. Water Res. 2011, 45, 2887–2896. [Google Scholar] [CrossRef]
- Park, J.-N.; Wang, J.; Choi, K.Y.; Dong, W.-Y.; Hong, S.-I.; Lee, C.W. Hydroxylation of phenol with H2O2 over Fe2+ and/or Co2+ ion-exchanged NaY catalyst in the fixed-bed flow reactor. J. Mol. Catal. A Chem. 2006, 247, 73–79. [Google Scholar] [CrossRef]
- Zekkari, M.; Ouargli-Saker, R.; Boudissa, F.; Lachachi, A.K.; El Houda Sekkal, K.N.; Tayeb, R.; Boukoussa, B.; Azzouz, A. Silica-catalyzed ozonation of 17α -ethinyl-estradiol in aqueous media-to better understand the role of silica in soils. Chemosphere 2022, 298, 134312. [Google Scholar] [CrossRef]
- Zhao, D.; Feng, J.; Huo, Q.; Melosh, N.; Fredrickson, G.H.; Chmelka, B.F.; Stucky, G.D. Triblock Copolymer Syntheses of Mesoporous Silica with Periodic 50 to 300 Angstrom Pores. Science 1998, 279, 548. [Google Scholar] [CrossRef]
- . Ouargli, R.; Larouk, S.; Terrab, I.; Hamacha, R.; Benharrats, N.; Bengheddach, A.; Azzouz, A. Intrinsic Activity of SBA-like Silica in the Catalytic Ozonation of Organic Pollutants. Ozone Sci. Eng. 2016, 38, 48–61. [Google Scholar] [CrossRef]
- Kruk, M.; Jaroniec, M.; Ko, C.H.; Ryoo, R. Characterization of the Porous Structure of SBA-15. Chem. Mater. 2000, 12, 1961–1968. [Google Scholar] [CrossRef]
- Zhao, D.; Huo, Q.; Feng, J.; Chmelka, B.F.; Stucky, G.D. Nonionic Triblock and Star Diblock Copolymer and Oligomeric Surfactant Syntheses of Highly Ordered, Hydrothermally Stable, Mesoporous Silica Structures. J. Am. Chem. Soc. 1998, 120, 6024–6036. [Google Scholar] [CrossRef]
- Leland, J.K.; Bard, A.J. Photochemistry of colloidal semiconducting iron oxide polymorphs. J. Phys. Chem. 1987, 91, 5076–5083. [Google Scholar] [CrossRef]
- Garoma, T.; Matsumoto, S.A.; Wu, Y.; Klinger, R. Removal of Bisphenol A and its Reaction-Intermediates from Aqueous Solution by Ozonation. Ozone Sci. Eng. 2010, 32, 338–343. [Google Scholar] [CrossRef]
- Mazellier, P.; Leverd, J. Transformation of 4-tert-octylphenol by UV irradiation and by an H2O2/UV process in aqueous solution. Photochem. Photobiol. Sci. 2003, 2, 946–953. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zhao, J.; Li, Z.; Li, G.; Li, W.; Gao, X. Catalytic ozonation of bisphenol A in aqueous solution using Mn-Ce/HZSM-5 as catalyst. Water Sci. Technol. 2015, 72, 696. [Google Scholar] [CrossRef]
- Huber, M.M.; Ternes, T.A.; von Gunten, U. Removal of estrogenic activity and formation of oxidation products during ozonation of 17alpha-ethinylestradiol. Environ. Sci. Technol. 2004, 38, 5177–5186. [Google Scholar] [CrossRef]
- Ma, J.; Graham, N.J.D. Degradation of atrazine by manganese-catalysed ozonation—Influence of radical scavengers. Water Res. 2000, 34, 3822–3828. [Google Scholar] [CrossRef]
- Arus, V.A.; Nousir, S.; Sennour, R.; Shiao, T.C.; Nistor, I.D.; Roy, R.; Azzouz, A. Intrinsic affinity of acid-activated bentonite towards hydrogen and carbon dioxide. Int. J. Hydrogen Energy 2018, 43, 7964–7972. [Google Scholar] [CrossRef]
- Zburavlev, L. The surface chemistry of amorphous silica. Colloids Surf. A Physicochem. Eng. Aspecets 2000, 173, 1–38. [Google Scholar] [CrossRef]
- Duval, Y.; Mielczarski, J.; Pokrovsky, O.; Mielczarski, E.; Ehrhardt, J. Evidence of the existence of three types of species at the quartz−aqueous solution interface at pH 0−10: XPS surface group quantification and surface complexation modeling. J. Phys. Chem. B 2002, 106, 2937–2945. [Google Scholar] [CrossRef]
- Pokrovsky, O.S.; Golubev, S.; Mielczarski, J. Kinetic evidences of the existence of positively charged species at the quartz-aqueous solution interface. J. Colloid Interface Sci. 2006, 296, 189–194. [Google Scholar] [CrossRef]
- Sulpizi, M.; Gaigeot, M.-P.; Sprik, M. The Silica–Water Interface: How the Silanols Determine the Surface Acidity and Modulate the Water Properties. J. Chem. Theory Comput. 2012, 8, 1037–1047. [Google Scholar] [CrossRef] [PubMed]
- Brown, M.A.; Huthwelker, T.; Beloqui Redondo, A.; Janousch, M.; Faubel, M.; Arrell, C.A.; Scarongella, M.; Chergui, M.; van Bokhoven, J.A. Changes in the Silanol Protonation State Measured In Situ at the Silica–Aqueous Interface. J. Phys. Chem. Lett. 2012, 3, 231–235. [Google Scholar] [CrossRef]
- Sturza, R.; Melenciuc, M.; Nistor, I.-D.; Boudissa, F.; Terkmani, T.; Hadj-Abdelkader, N.e.H.; Abdelkrim, A. Recyclable Porous Materials For The Uptake Of Bis-Phenol-A; Institutional Repository of the Technical University of Moldova: Chișinău, Moldova, 2017; p. 16. [Google Scholar]
- Sreethawong, T.; Chavadej, S. Color removal of distillery wastewater by ozonation in the absence and presence of immobilized iron oxide catalyst. J. Hazard. Mater. 2008, 155, 486–493. [Google Scholar] [CrossRef]
- Sennour, R.; Shiao, T.C.; Arus, V.A.; Tahir, M.N.; Bouazizi, N.; Roy, R.; Azzouz, A. Cu0-Loaded organo-montmorillonite with improved affinity towards hydrogen: An insight into matrix-metal and non-contact hydrogen-metal interactions. Phys. Chem. Chem. Phys. 2017, 19, 29333–29343. [Google Scholar] [CrossRef] [PubMed]
- Didi, M.A.; Makhoukhi, B.; Azzouz, A.; Villemin, D. Colza oil bleaching through optimized acid activation of bentonite. A comparative study. Appl. Clay Sci. 2009, 42, 336–344. [Google Scholar] [CrossRef]
- Makhoukhi, B.; Didi, M.A.; Villemin, D.; Azzouz, A. Acid activation of Bentonite for use as a vegetable oil bleaching agent. Grasas Aceites 2009, 60, 343–349. [Google Scholar] [CrossRef]
- Ouargli, R.; Bouazizi, N.; Khelil, M.; Nousir, S.; Benslama, R.; Ammar, S.; Azzouz, A. SBA-15-supported iron nanoparticles with improved optical properties, conductance and capacitance. Chem. Phys. Lett. 2017, 673, 30–37. [Google Scholar] [CrossRef]
- Boukoussa, B.; Hakiki, A.; Nunes-Beltrao, A.P.; Hamacha, R.; Azzouz, A. Assessment of the intrinsic interactions of nanocomposite polyaniline/SBA-15 with carbon dioxide: Correlation between the hydrophilic character and surface basicity. J. CO2 Util. 2018, 26, 171–178. [Google Scholar] [CrossRef]
- Bouazizi, N.; Ouargli, R.; Nousir, S.; Slama, R.B.; Azzouz, A. Properties of SBA-15 modified by iron nanoparticles as potential hydrogen adsorbents and sensors. J. Phys. Chem. Solids 2015, 77, 172–177. [Google Scholar] [CrossRef]
- Azzouz, A.; Ursu, A.-V.; Nistor, D.; Sajin, T.; Assaad, E.; Roy, R. TPD study of the reversible retention of carbon dioxide over montmorillonite intercalated with polyol dendrimers. Thermochim. Acta 2009, 496, 45–49. [Google Scholar] [CrossRef]
- Azzouz, A.; Nousir, S.; Platon, N.; Ghomari, K.; Shiao, T.C.; Hersant, G.; Bergeron, J.-Y.; Roy, R. Truly reversible capture of CO2 by montmorillonite intercalated with soya oil-derived polyglycerols. Int. J. Greenh. Gas Control. 2013, 17, 140–147. [Google Scholar] [CrossRef]
- Huber, M.M.; Canonica, S.; Park, G.-Y.; von Gunten, U. Oxidation of Pharmaceuticals during Ozonation and Advanced Oxidation Processes. Environ. Sci. Technol. 2003, 37, 1016–1024. [Google Scholar] [CrossRef] [PubMed]








| Catalyst | Specific Surface Area (m2·g−1) * | WRC a nmol.g−1 | Si/Al Mole Ratio | Clay Suspension | Particle Size (μm) | ||||
|---|---|---|---|---|---|---|---|---|---|
| pH | Zeta Potential | Alone f | with BPA g | ||||||
| Alone b | with BPA c | Alone d | with BPA e | ||||||
| Bentonite | 51 | 126 | 2.50 | 9.69 | 7.92 | −31.25 | −27.44 | 1.24 | 1.404 |
| NaMt | 59 | 96 | 2.50 | 8.77 | 6.55 | −42.19 | −38.79 | 0.649 | 0.534 |
| Fe(II)Mt | 54 | 195 | 2.50 | 2.33 | 2.38 | +16.20 | −48.71 | 2.45 | 3.897 |
| HMt-1 | 86 | 76 | 2.69 | 4.18 | 4.27 | −43.02 | −35.24 | 1.99 | 1.830 |
| HMt-4 | 118 | 68.2 | 3.00 | 4.15 | 4.10 | −38.36 | −41.20 | 2.04 | 1.287 |
| HMt-8 | 131 | 62.3 | 3.47 | 4.04 | 3.96 | −43.12 | −34.88 | 2.14 | 1.297 |
| HMt-15 | 139 | 50.1 | 4.03 | 3.92 | 3.86 | −42.18 | −39.60 | 2.26 | 1.341 |
| HMt-24 | 140 | 52.8 | 4.36 | 3.96 | 3.86 | −37.62 | −36.72 | 2.35 | 1.290 |
| SBA-15 | 850 | - | - | 5.81 | 5.27 | −31.49 | −22.22 | 1.92 | 1.58 |
| Hematite Concentration (g·L−1) | t (min) | Removal Yield (%) a | Initial pH of the Reaction Mixture | Removal Yield (%) b |
|---|---|---|---|---|
| 1 | 15 | 54 | 1.8 | 40 |
| 25 | 94 | 33 | ||
| 2 | 15 | 23 | 2.8 | 31 |
| 25 | 25 | 31 | ||
| 3 | 15 | 89 | 3.8 | 54 |
| 25 | 87 | 94 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Boudissa, F.; Arus, V.-A.; Foka-Wembe, E.-N.; Zekkari, M.; Ouargli-Saker, R.; Dewez, D.; Roy, R.; Azzouz, A. Role of Silica on Clay-Catalyzed Ozonation for Total Mineralization of Bisphenol-A. Molecules 2023, 28, 3825. https://doi.org/10.3390/molecules28093825
Boudissa F, Arus V-A, Foka-Wembe E-N, Zekkari M, Ouargli-Saker R, Dewez D, Roy R, Azzouz A. Role of Silica on Clay-Catalyzed Ozonation for Total Mineralization of Bisphenol-A. Molecules. 2023; 28(9):3825. https://doi.org/10.3390/molecules28093825
Chicago/Turabian StyleBoudissa, Farida, Vasilica-Alisa Arus, Eric-Noel Foka-Wembe, Meriem Zekkari, Rachida Ouargli-Saker, David Dewez, René Roy, and Abdelkrim Azzouz. 2023. "Role of Silica on Clay-Catalyzed Ozonation for Total Mineralization of Bisphenol-A" Molecules 28, no. 9: 3825. https://doi.org/10.3390/molecules28093825






