Hot-Melt Extrusion as an Effective Technique for Obtaining an Amorphous System of Curcumin and Piperine with Improved Properties Essential for Their Better Biological Activities
Abstract
1. Introduction
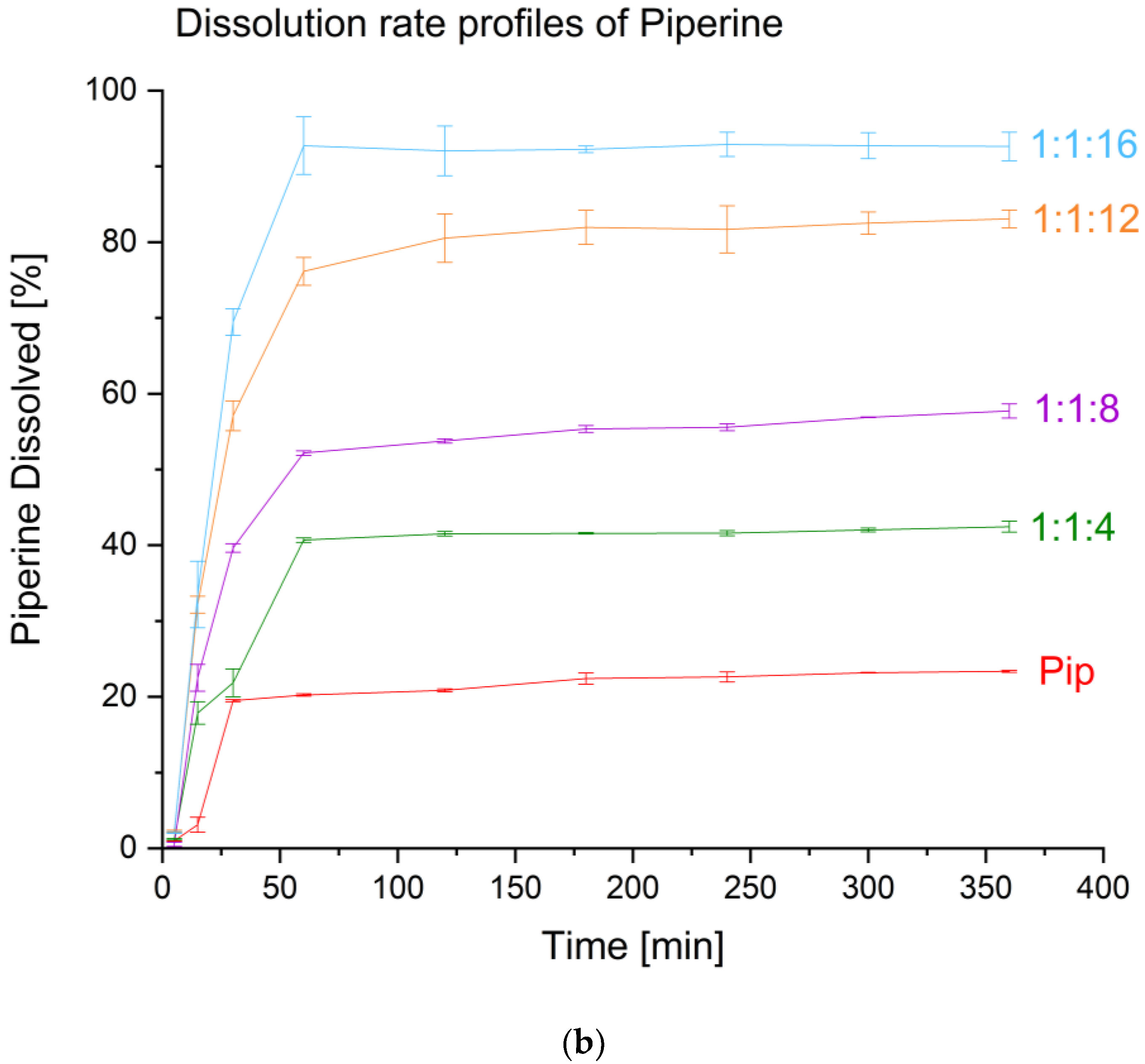
2. Results and Discussion
3. Materials and Methods
3.1. Materials
3.2. Preparation of the Systems
3.3. Solid-State IDENTIFICATION
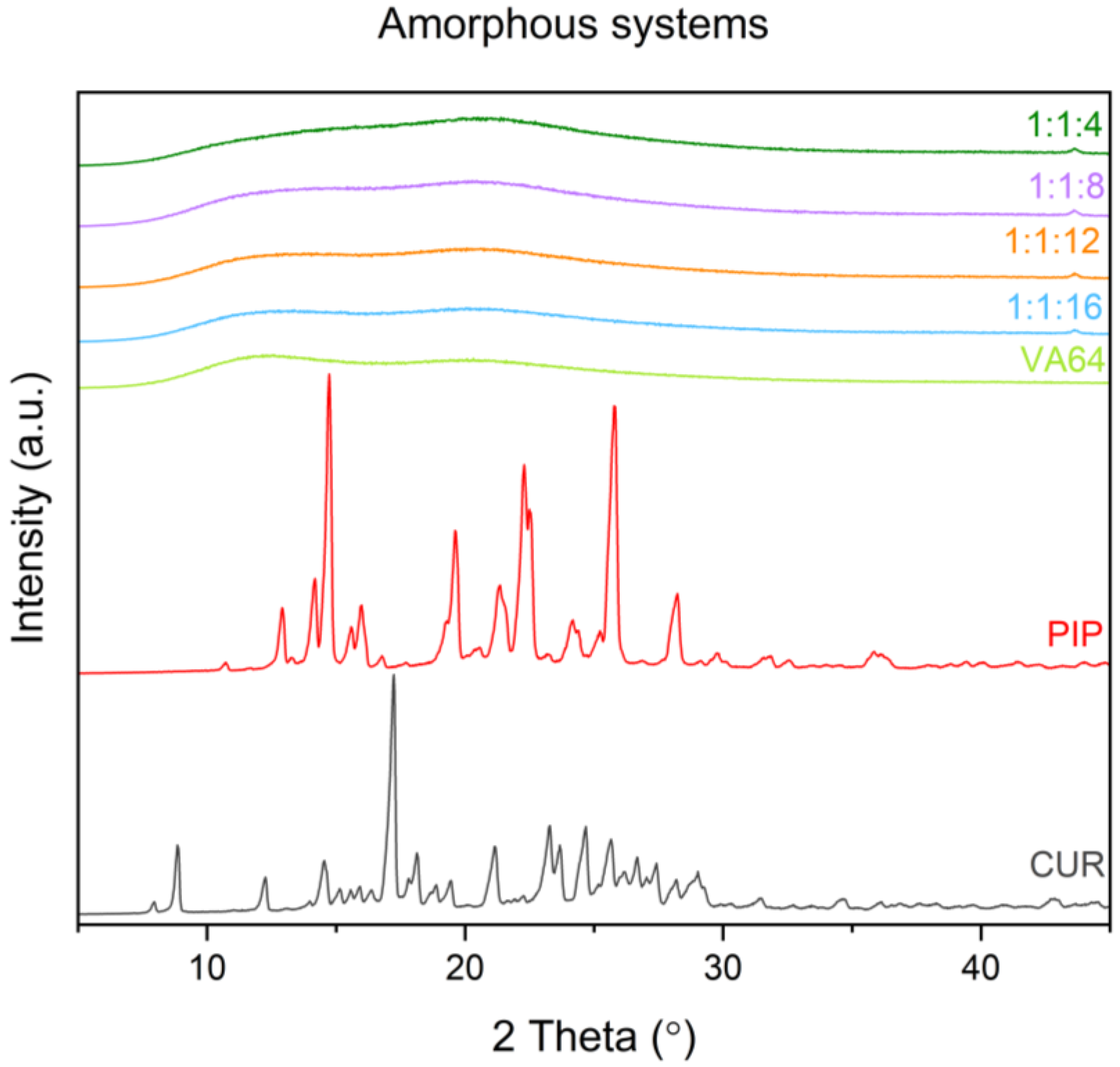
3.3.1. X-ray Powder Diffraction (XRPD)
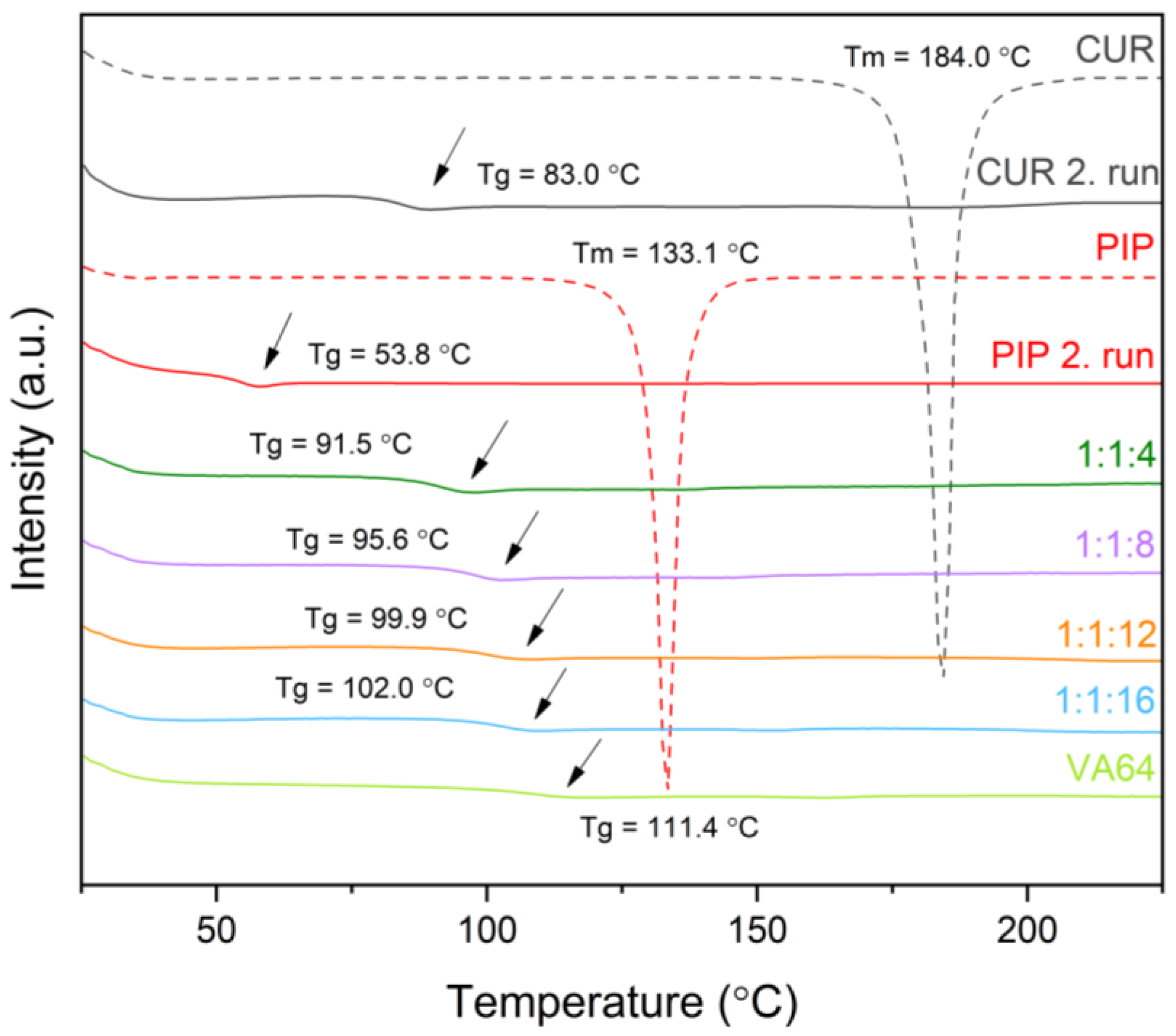
3.3.2. Differential Scanning Calorimetry (DSC)
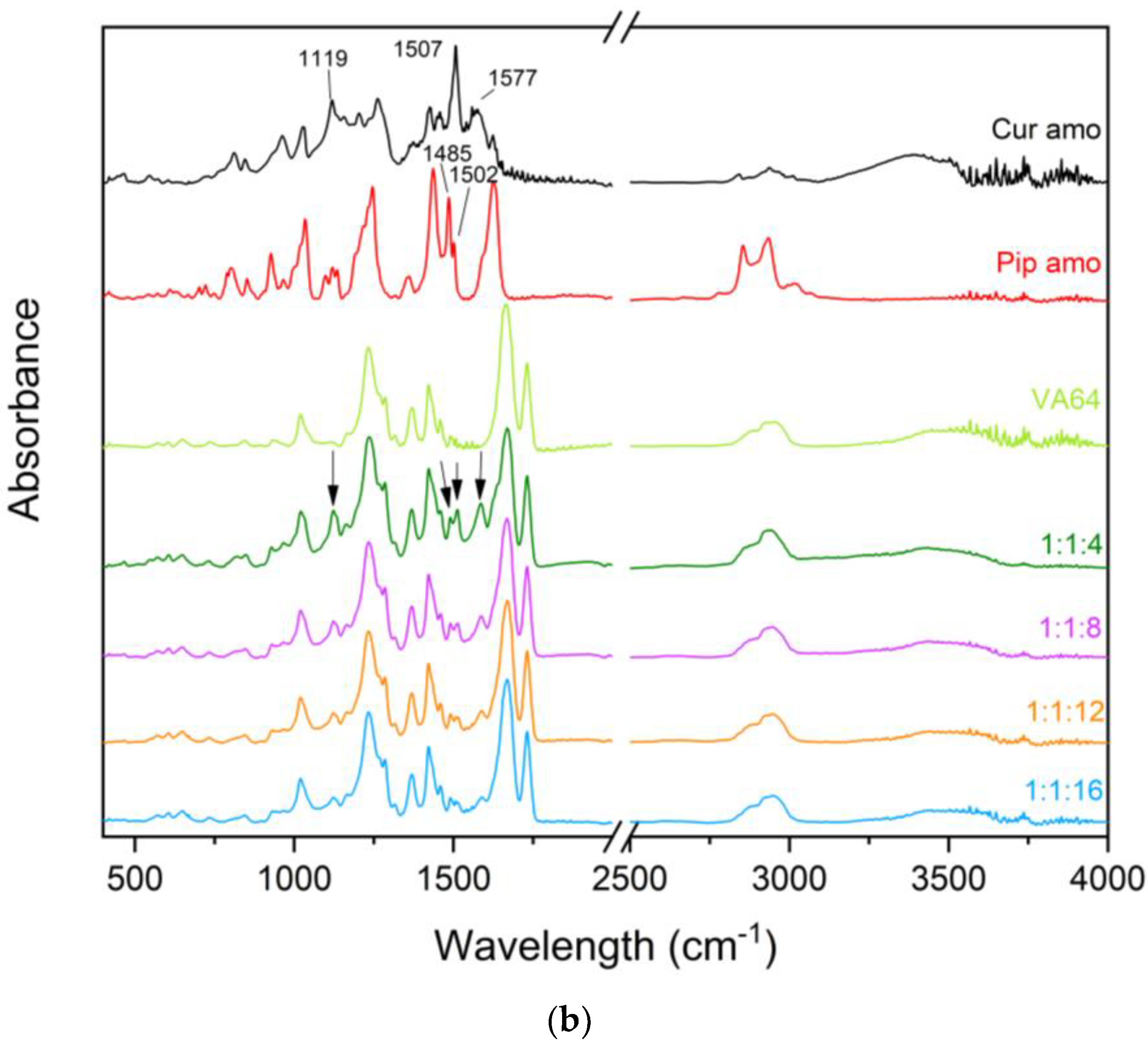
3.3.3. Fourier Transform Infrared Spectroscopy with Attenuated Total Reflectance (FTIR-ATR)
3.3.4. Physical Stability
3.4. Physicochemical Characterization
3.4.1. HPLC Conditions
3.4.2. Media for Dissolution and Solubility Studies
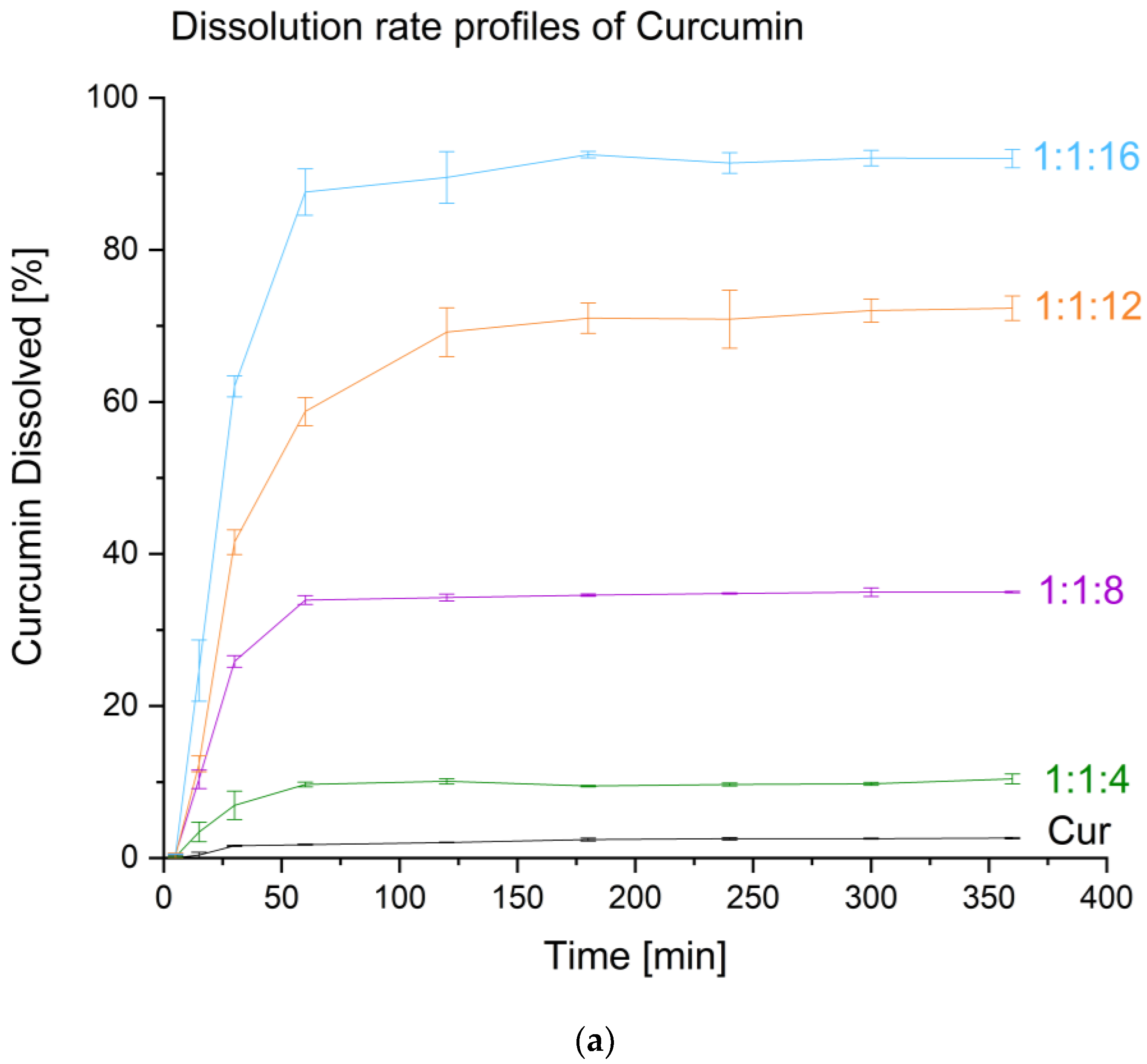
3.4.3. Dissolution Studies
3.4.4. Apparent Solubility Studies
3.4.5. Permeability Studies
3.5. Biological Activities Studies
3.5.1. Antioxidant Activity Assay
3.5.2. Determination of Butyrylcholinesterase (BuChE) Inhibition
3.6. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Siviero, A.; Gallo, E.; Maggini, V.; Gori, L.; Mugelli, A.; Firenzuoli, F.; Vannacci, A. Curcumin, a golden spice with a low bioavailability. J. Herb. Med. 2015, 5, 57–70. [Google Scholar] [CrossRef]
- Pulido-Moran, M.; Moreno-Fernandez, J.; Ramirez-Tortosa, C.; Ramirez-Tortosa, M. Curcumin and health. Molecules 2016, 21, 264. [Google Scholar] [CrossRef] [PubMed]
- Stanić, Z. Curcumin, a compound from natural sources, a true scientific challenge—A review. Plant Foods Hum. Nutr. 2017, 72, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Liczbiński, P.; Michałowicz, J.; Bukowska, B. Molecular mechanism of curcumin action in signaling pathways: Review of the latest research. Phyther. Res. 2020, 34, 1992–2005. [Google Scholar] [CrossRef] [PubMed]
- Haq, I.; Imran, M.; Nadeem, M.; Tufail, T.; Gondal, T.A.; Mubarak, M.S. Piperine: A review of its biological effects. Phytother. Res. 2021, 35, 680–700. [Google Scholar] [CrossRef] [PubMed]
- Chopra, B.; Dhingra, A.K.; Kapoor, R.P.; Prasad, D.N. Piperine and its various physicochemical and biological aspects: A review. Open Chem. J. 2016, 3, 75–96. [Google Scholar] [CrossRef]
- Stojanović-Radić, Z.; Pejčić, M.; Dimitrijević, M.; Aleksić, A.; V. Anil Kumar, N.; Salehi, B.; Cho, C.W.; Sharifi-Rad, J. Piperine—A major principle of black pepper: A review of its bioactivity and studies. Appl. Sci. 2019, 9, 4270. [Google Scholar] [CrossRef]
- Bi, X.; Yuan, Z.; Qu, B.; Zhou, H.; Liu, Z.; Xie, Y. Piperine enhances the bioavailability of silybin via inhibition of efflux transporters BCRP and MRP2. Phytomedicine 2019, 54, 98–108. [Google Scholar] [CrossRef]
- Aswar, M.; Bhalekar, M.; Trimukhe, A.; Aswar, U. Self-microemulsifying drug delivery system (SMEDDS) of curcumin attenuates depression in olfactory bulbectomized rats. Heliyon 2020, 6, e04482. [Google Scholar] [CrossRef]
- Thenmozhi, K.; Yoo, Y.J. Enhanced solubility of piperine using hydrophilic carrier-based potent solid dispersion systems. Drug Dev. Ind. Pharm. 2017, 43, 1501–1509. [Google Scholar] [CrossRef]
- Grill, A.E.; Koniar, B.; Panyam, J. Co-delivery of natural metabolic inhibitors in a self-microemulsifying drug delivery system for improved oral bioavailability of curcumin. Drug Deliv. Transl. Res. 2014, 4, 344–352. [Google Scholar] [CrossRef] [PubMed]
- Ipar, V.S.; Dsouza, A.; Devarajan, P.V. Enhancing curcumin oral bioavailability through nanoformulations. Eur. J. Drug Metab. Pharmacokinet. 2019, 44, 459–480. [Google Scholar] [CrossRef] [PubMed]
- Kazi, M.; Shahba, A.A.; Alrashoud, S.; Alwadei, M.; Sherif, A.Y.; Alanazi, F.K. Bioactive self-nanoemulsifying drug delivery systems (Bio-SNEDDS) for combined oral delivery of curcumin and piperine. Molecules 2020, 25, 1703. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Li, Q.; McClements, D.J.; Han, Y.; Dai, L.; Mao, L.; Gao, Y. Co-delivery of curcumin and piperine in zein-carrageenan core-shell nanoparticles: Formation, structure, stability and in vitro gastrointestinal digestion. Food Hydrocoll. 2020, 99, 105334. [Google Scholar] [CrossRef]
- Tang, J.; Ji, H.; Ren, J.; Li, M.; Zheng, N.; Wu, L. Solid lipid nanoparticles with TPGS and Brij 78: A co-delivery vehicle of curcumin and piperine for reversing P-glycoprotein-mediated multidrug resistance in vitro. Oncol. Lett. 2017, 13, 389–395. [Google Scholar] [CrossRef] [PubMed]
- Abolhassani, H.; Safavi, M.S.; Handali, S.; Nosrati, M.; Shojaosadati, S.A. Synergistic effect of self-assembled curcumin and piperine co-loaded human serum albumin nanoparticles on suppressing cancer cells. Drug Dev. Ind. Pharm. 2020, 46, 1647–1655. [Google Scholar] [CrossRef] [PubMed]
- Javed, B.; Zhao, X.; Cui, D.; Curtin, J.; Tian, F. Enhanced Anticancer Response of Curcumin-and Piperine-Loaded Lignin-gp (NIPAM-co-DMAEMA) Gold Nanogels against U-251 MG Glioblastoma Multiforme. Biomedicines 2021, 9, 1516. [Google Scholar] [CrossRef]
- Kim, D.; Kim, Y.; Tin, Y.-Y.; Soe, M.-T.-P.; Ko, B.; Park, S.; Lee, J. Recent technologies for amorphization of poorly water-soluble drugs. Pharmaceutics 2021, 13, 1318. [Google Scholar] [CrossRef]
- Qiang, W.; Löbmann, K.; McCoy, C.P.; Andrews, G.P.; Zhao, M. Microwave-induced in situ amorphization: A new strategy for tackling the stability issue of amorphous solid dispersions. Pharmaceutics 2020, 12, 655. [Google Scholar] [CrossRef]
- Censi, R.; Gigliobianco, M.R.; Casadidio, C.; Di Martino, P. Hot melt extrusion: Highlighting physicochemical factors to be investigated while designing and optimizing a hot melt extrusion process. Pharmaceutics 2018, 10, 89. [Google Scholar] [CrossRef]
- Tiwari, R.V.; Patil, H.; Repka, M.A. Contribution of hot-melt extrusion technology to advance drug delivery in the 21st century. Expert Opin. Drug Deliv. 2016, 13, 451–464. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Han, J.; Jiang, A.; Huang, R.; Fu, T.; Wang, L.; Zheng, Q.; Li, W.; Li, J. Involvement of metabolism-permeability in enhancing the oral bioavailability of curcumin in excipient-free solid dispersions co-formed with piperine. Int. J. Pharm. 2019, 561, 9–18. [Google Scholar] [CrossRef] [PubMed]
- Althobaiti, A.A.; Ashour, E.A.; Almutairi, M.; Almotairy, A.; Al Yahya, M.; Repka, M.A. Formulation Development of Curcumin-piperine solid dispersion via hot-melt extrusion. J. Drug Deliv. Sci. Technol. 2022, 76, 103753. [Google Scholar] [CrossRef]
- Knapik-Kowalczuk, J.; Chmiel, K.; Pacułt, J.; Bialek, K.; Tajber, L.; Paluch, M. Enhancement of the physical stability of amorphous sildenafil in a binary mixture, with either a plasticizing or antiplasticizing compound. Pharmaceutics 2020, 12, 460. [Google Scholar] [CrossRef] [PubMed]
- Theil, F.; Anantharaman, S.; Kyeremateng, S.O.; van Lishaut, H.; Dreis-Kühne, S.H.; Rosenberg, J.; Magerlein, M.; Woehrle, G.H. Frozen in time: Kinetically stabilized amorphous solid dispersions of nifedipine stable after a quarter century of storage. Mol. Pharm. 2017, 14, 183–192. [Google Scholar] [CrossRef] [PubMed]
- Mistry, P.; Suryanarayanan, R. Strength of drug-polymer interactions: Implications for crystallization in dispersions. Cryst. Growth Des. 2016, 16, 5141–5149. [Google Scholar] [CrossRef]
- Knapik, J.; Wojnarowska, Z.; Grzybowska, K.; Tajber, L.; Mesallati, H.; Paluch, K.J.; Paluch, M. Molecular dynamics and physical stability of amorphous nimesulide drug and its binary drug-polymer systems. Mol. Pharm. 2016, 13, 1937–1946. [Google Scholar] [CrossRef]
- Kwon, J.; Giri, B.R.; Song, E.S.; Bae, J.; Lee, J.; Kim, D.W. Spray-dried amorphous solid dispersions of atorvastatin calcium for improved supersaturation and oral bioavailability. Pharmaceutics 2019, 11, 461. [Google Scholar] [CrossRef]
- Qin, Y.; Xiao, C.; Li, X.; Huang, J.; Si, L.; Sun, M. Enteric Polymer–Based Amorphous Solid Dispersions Enhance Oral Absorption of the Weakly Basic Drug Nintedanib via Stabilization of Supersaturation. Pharmaceutics 2022, 14, 1830. [Google Scholar] [CrossRef]
- Hua, S. Advances in oral drug delivery for regional targeting in the gastrointestinal tract-influence of physiological, pathophysiological and pharmaceutical factors. Front. Pharmacol. 2020, 11, 524. [Google Scholar] [CrossRef]
- Butreddy, A.; Sarabu, S.; Almutairi, M.; Ajjarapu, S.; Kolimi, P.; Bandari, S.; Repka, M.A. Hot-melt extruded hydroxypropyl methylcellulose acetate succinate based amorphous solid dispersions: Impact of polymeric combinations on supersaturation kinetics and dissolution performance. Int. J. Pharm. 2022, 615, 121471. [Google Scholar] [CrossRef] [PubMed]
- DiNunzio, J.C.; Miller, D.A.; Yang, W.; McGinity, J.W.; Williams III, R.O. Amorphous compositions using concentration enhancing polymers for improved bioavailability of itraconazole. Mol. Pharm. 2008, 5, 968–980. [Google Scholar] [CrossRef] [PubMed]
- Childs, S.L.; Kandi, P.; Lingireddy, S.R. Formulation of a danazol cocrystal with controlled supersaturation plays an essential role in improving bioavailability. Mol. Pharm. 2013, 10, 3112–3127. [Google Scholar] [CrossRef] [PubMed]
- Yeap, Y.Y.; Trevaskis, N.L.; Quach, T.; Tso, P.; Charman, W.N.; Porter, C.J.H. Intestinal bile secretion promotes drug absorption from lipid colloidal phases via induction of supersaturation. Mol. Pharm. 2013, 10, 1874–1889. [Google Scholar] [CrossRef] [PubMed]
- Berginc, K.; Trontelj, J.; Basnet, N.S.; Kristl, A. Physiological barriers to the oral delivery of curcumin. Die Pharm. Int. J. Pharm. Sci. 2012, 67, 518–524. [Google Scholar]
- Kusuhara, H.; Furuie, H.; Inano, A.; Sunagawa, A.; Yamada, S.; Wu, C.; Fukizawa, S.; Morimoto, N.; Ieiri, I.; Morishita, M. Pharmacokinetic interaction study of sulphasalazine in healthy subjects and the impact of curcumin as an in vivo inhibitor of BCRP. Br. J. Pharmacol. 2012, 166, 1793–1803. [Google Scholar] [CrossRef] [PubMed]
- Ge, S.; Yin, T.; Xu, B.; Gao, S.; Hu, M. Curcumin affects phase II disposition of resveratrol through inhibiting efflux transporters MRP2 and BCRP. Pharm. Res. 2016, 33, 590–602. [Google Scholar] [CrossRef]
- Dei Cas, M.; Ghidoni, R. Dietary curcumin: Correlation between bioavailability and health potential. Nutrients 2019, 11, 2147. [Google Scholar] [CrossRef]
- Singh, S.; Jamwal, S.; Kumar, P. Piperine enhances the protective effect of curcumin against 3-NP induced neurotoxicity: Possible neurotransmitters modulation mechanism. Neurochem. Res. 2015, 40, 1758–1766. [Google Scholar] [CrossRef]
- Bishnoi, M.; Chopra, K.; Rongzhu, L.; Kulkarni, S.K. Protective effect of curcumin and its combination with piperine (bioavailability enhancer) against haloperidol-associated neurotoxicity: Cellular and neurochemical evidence. Neurotox. Res. 2011, 20, 215–225. [Google Scholar] [CrossRef]
- Erfen, Ş.; Akbay Çetin, E. Therapeutic and Preventive Effects of Piperine and its Combination with Curcumin as a Bioenhancer Against Aluminum-Induced Damage in the Astrocyte Cells. Neurotox. Res. 2022, 40, 2027–2045. [Google Scholar] [CrossRef] [PubMed]
- Abdul Manap, A.S.; Wei Tan, A.C.; Leong, W.H.; Yin Chia, A.Y.; Vijayabalan, S.; Arya, A.; Wong, E.H.; Rizwan, F.; Bindal, U.; Koshy, S. Synergistic effects of curcumin and piperine as potent acetylcholine and amyloidogenic inhibitors with significant neuroprotective activity in SH-SY5Y cells via computational molecular modeling and in vitro assay. Front. Aging Neurosci. 2019, 11, 206. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Kukreti, R.; Saso, L.; Kukreti, S. Oxidative stress: A key modulator in neurodegenerative diseases. Molecules 2019, 24, 1583. [Google Scholar] [CrossRef] [PubMed]
- Jasiecki, J.; Targońska, M.; Wasąg, B. The role of butyrylcholinesterase and iron in the regulation of cholinergic network and cognitive dysfunction in Alzheimer’s disease pathogenesis. Int. J. Mol. Sci. 2021, 22, 2033. [Google Scholar] [CrossRef] [PubMed]
- Bisset, S.; Sobhi, W.; Bensouici, C. Antioxidant Activity and Inhibitory Effect of Curcumin on Some Enzymes Involved in Several Diseases: Acetylcholinesterase, Butyrylcholinesterase, α-Glucosidase and Tyrosinase. Curr. Enzym. Inhib. 2022, 18, 172–179. [Google Scholar]
- Jaipea, S.; Saehlim, N.; Sutcharitruk, W.; Athipornchai, A.; Ingkaninan, K.; Saeeng, R. Synthesis of piperine analogues as AChE and BChE inhibitors for the treatment of Alzheimer’s disease. Phytochem. Lett. 2023, 53, 216–221. [Google Scholar] [CrossRef]
- Yen, F.-L.; Wu, T.-H.; Tzeng, C.-W.; Lin, L.-T.; Lin, C.-C. Curcumin nanoparticles improve the physicochemical properties of curcumin and effectively enhance its antioxidant and antihepatoma activities. J. Agric. Food Chem. 2010, 58, 7376–7382. [Google Scholar] [CrossRef]
- Wdowiak, K.; Rosiak, N.; Tykarska, E.; Żarowski, M.; Płazińska, A.; Płaziński, W.; Cielecka-Piontek, J. Amorphous Inclusion Complexes: Molecular Interactions of Hesperidin and Hesperetin with HP-Β-CD and Their Biological Effects. Int. J. Mol. Sci. 2022, 23, 4000. [Google Scholar] [CrossRef]
- Jiang, L.; Xia, N.; Wang, F.; Xie, C.; Ye, R.; Tang, H.; Zhang, H.; Liu, Y. Preparation and characterization of curcumin/β-cyclodextrin nanoparticles by nanoprecipitation to improve the stability and bioavailability of curcumin. LWT 2022, 171, 114149. [Google Scholar] [CrossRef]
- Mangolim, C.S.; Moriwaki, C.; Nogueira, A.C.; Sato, F.; Baesso, M.L.; Neto, A.M.; Matioli, G. Curcumin–β-cyclodextrin inclusion complex: Stability, solubility, characterisation by FT-IR, FT-Raman, X-ray diffraction and photoacoustic spectroscopy, and food application. Food Chem. 2014, 153, 361–370. [Google Scholar] [CrossRef]
- Hu, L.; Shi, Y.; Li, J.H.; Gao, N.; Ji, J.; Niu, F.; Chen, Q.; Yang, X.; Wang, S. Enhancement of oral bioavailability of curcumin by a novel solid dispersion system. Aaps Pharmscitech 2015, 16, 1327–1334. [Google Scholar] [CrossRef] [PubMed]
- Garrido, B.; González, S.; Hermosilla, J.; Millao, S.; Quilaqueo, M.; Guineo, J.; Acevedo, F.; Pesenti, H.; Rolleri, A.; Shene, C. Carbonate-β-cyclodextrin-based nanosponge as a nanoencapsulation system for piperine: Physicochemical characterization. J. Soil Sci. Plant Nutr. 2019, 19, 620–630. [Google Scholar] [CrossRef]
- Gorgani, L.; Mohammadi, M.; Najafpour, G.D.; Nikzad, M. Piperine—The bioactive compound of black pepper: From isolation to medicinal formulations. Compr. Rev. Food Sci. Food Saf. 2017, 16, 124–140. [Google Scholar] [CrossRef] [PubMed]
- Quilaqueo, M.; Millao, S.; Luzardo-Ocampo, I.; Campos-Vega, R.; Acevedo, F.; Shene, C.; Rubilar, M. Inclusion of piperine in β-cyclodextrin complexes improves their bioaccessibility and in vitro antioxidant capacity. Food Hydrocoll. 2019, 91, 143–152. [Google Scholar] [CrossRef]
- Liu, K.; Liu, H.; Li, Z.; Li, W.; Li, L. In vitro dissolution study on inclusion complex of piperine with ethylenediamine-β-cyclodextrin. J. Incl. Phenom. Macrocycl. Chem. 2020, 96, 233–243. [Google Scholar] [CrossRef]
- Dong, L.; Mai, Y.; Liu, Q.; Zhang, W.; Yang, J. Mechanism and Improved Dissolution of Glycyrrhetinic Acid Solid Dispersion by Alkalizers. Pharmaceutics 2020, 12, 82. [Google Scholar] [CrossRef]
- Akram, A.; Irfan, M.; Abualsunun, W.A.; Bukhary, D.M.; Alissa, M. How to Improve Solubility and Dissolution of Irbesartan by Fabricating Ternary Solid Dispersions: Optimization and In-Vitro Characterization. Pharmaceutics 2022, 14, 2264. [Google Scholar] [CrossRef]

| System (Cur:Pip:VA 64) | Compound | |||
|---|---|---|---|---|
| CUR | PIP | |||
| Conc. (mg/mL) | Improv. (-fold) | Conc. (mg/mL) | Improv. (-fold) | |
| Raw | 0.0001 ± 0.0000 e | - | 0.006 ± 0.0001 e | - |
| 1:1:4 | 0.689 ± 0.002 d | 5514 | 0.153 ± 0.001 d | 23 |
| 1:1:8 | 0.910 ± 0.003 c | 7283 | 0.669 ± 0.028 c | 103 |
| 1:1:12 | 1.049 ± 0.009 b | 8396 | 0.826 ± 0.010 b | 138 |
| 1:1:16 | 1.187 ± 0.010 a | 9496 | 1.050 ± 0.016 a | 161 |
| System (Cur:Pip:VA 64) | Compound | |||||
|---|---|---|---|---|---|---|
| CUR | PIP | |||||
| Conc. (mg/mL) | Improv. (-fold) | Conc. (mg/mL) | Improv. (-fold) | |||
| Model | GIT | Raw | 3.34 × 10−6 ± 1.94 × 10−6, e | - | 5.86 × 10−4 ± 1.35 × 10−4, e | - |
| 1:1:4 | 1.09 × 10−3 ± 4.09 × 10−4, d | 326 | 5.33 × 10−3 ± 1.76 × 10−3, d | 9 | ||
| 1:1:8 | 2.60 × 10−2 ± 4.57 × 10−3, c | 7776 | 1.21 × 10−1 ± 8.88 × 10−3, c | 207 | ||
| 1:1:12 | 3.33 × 10−2 ± 2.87 × 10−3, b | 9976 | 1.66 × 10−1 ± 1.09 × 10−2, b | 284 | ||
| 1:1:16 | 4.20 × 10−2 ± 2.21 × 10−3, a | 12,578 | 2.01 × 10−1 ± 7.77 × 10−3, a | 343 | ||
| BBB | Raw | 1.86 × 10−5 ± 2.48 × 10−6, e | - | 2.10 × 10−3 ± 8.86 × 10−5, d | - | |
| 1:1:4 | 1.51 × 10−2 ± 1.86 × 10−3, d | 815 | 2.06 × 10−2 ± 3.73 × 10−3, c | 10 | ||
| 1:1:8 | 4.12 × 10−2 ± 1.40 × 10−3, c | 2218 | 2.99 × 10−1 ± 6.85 × 10−3, b | 142 | ||
| 1:1:12 | 4.45 × 10−2 ± 2.47 × 10−3, b | 2436 | 3.25 × 10−1 ± 2.97 × 10−2, a, b | 155 | ||
| 1:1:16 | 5.70 × 10−2 ± 1.40 × 10−3, a | 3069 | 3.45 × 10−1 ± 2.07 × 10−2, a | 164 | ||
| System (Cur:Pip:VA 64) | Assay | |
|---|---|---|
| DPPH | BChE | |
| % of Inhibition | % of Inhibition | |
| Raw | 10.69 ± 0.92 d | 1.24 ± 0.27 e |
| 1:1:4 | 48.16 ± 2.56 c | 42.87 ± 2.27 d |
| 1:1:8 | 87.87 ± 1.12 b | 77.46 ± 2.48 c |
| 1:1:12 | 96.85 ± 1.35 a | 92.35 ± 3.55 b |
| 1:1:16 | 96.97 ± 1.32 a | 98.52 ± 0.87 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wdowiak, K.; Pietrzak, R.; Tykarska, E.; Cielecka-Piontek, J. Hot-Melt Extrusion as an Effective Technique for Obtaining an Amorphous System of Curcumin and Piperine with Improved Properties Essential for Their Better Biological Activities. Molecules 2023, 28, 3848. https://doi.org/10.3390/molecules28093848
Wdowiak K, Pietrzak R, Tykarska E, Cielecka-Piontek J. Hot-Melt Extrusion as an Effective Technique for Obtaining an Amorphous System of Curcumin and Piperine with Improved Properties Essential for Their Better Biological Activities. Molecules. 2023; 28(9):3848. https://doi.org/10.3390/molecules28093848
Chicago/Turabian StyleWdowiak, Kamil, Robert Pietrzak, Ewa Tykarska, and Judyta Cielecka-Piontek. 2023. "Hot-Melt Extrusion as an Effective Technique for Obtaining an Amorphous System of Curcumin and Piperine with Improved Properties Essential for Their Better Biological Activities" Molecules 28, no. 9: 3848. https://doi.org/10.3390/molecules28093848
APA StyleWdowiak, K., Pietrzak, R., Tykarska, E., & Cielecka-Piontek, J. (2023). Hot-Melt Extrusion as an Effective Technique for Obtaining an Amorphous System of Curcumin and Piperine with Improved Properties Essential for Their Better Biological Activities. Molecules, 28(9), 3848. https://doi.org/10.3390/molecules28093848






