Abstract
Recently, single-atom catalysts (SACs) have attracted wide attention in the field of environmental engineering. Compared with their nanoparticle counterparts, SACs possess high atomic efficiency, unique catalytic activity, and selectivity. This review summarizes recent studies on the environmental remediation applications of SACs in (1) gaseous: volatile organic compounds (VOCs) treatment, NOx reduction, CO2 reduction, and CO oxidation; (2) aqueous: Fenton-like advanced oxidation processes (AOPs), hydrodehalogenation, and nitrate/nitrite reduction. We present the treatment activities and reaction mechanisms of various SACs and propose challenges and future opportunities. We believe that this review will provide constructive inspiration and direction for future SAC research in environmental engineering.
1. Introduction
Large amounts of pollutants are discharged into the environment as a result of economic growth, leaving serious pollution problems that require urgent treatment. Compared to traditional physical adsorption or biological treatments, chemical catalysis is considered an effective approach to quickly degrade pollutants [1], with less generation of secondary solid waste or sludge. Developing appropriate catalysts that can not only efficiently eliminate pollutants but also operate stably and sustainably is of great importance [2].
Conventional heterogeneous catalysts are typically designed on a nanometer scale. However, the atomic utilization of nanoparticles (NPs) is limited because only the outmost layer of atoms participates in the surface catalytic reaction [3], which hinders the further improvement of catalytic activity. Moreover, noble metal catalysts containing costly Pd, Pt, Au, Ru, etc. are required to achieve higher atomic efficiency to obtain economic benefits. To solve these issues, researchers have devoted themselves to decreasing the size of nanocatalysts to maximize the exposure of active surface sites, meanwhile achieving additional benefits such as quantum size effects [4,5] and unsaturated coordination [6].
The idea of single-atom catalysts (SACs) was first proposed by Zhang and coworkers [7] in 2011, which describes a type of catalyst reaching the theoretical size limit of “single-atom”. Compared to bulk nanocatalysts, single-atom catalysts possess several advantages. From the perspective of catalyst structure, the sufficient interactions generated by the chemical bond between the metal and the support provide higher numbers of interfaces and active sites for the catalytic reaction [8,9,10,11]. The unsaturated coordination facilitates the adsorption of pollutants on the SAC site and dynamic electron transport, contributing to a better redox reaction [7,10,12]. The strong metal-support bonding also prevents the aggregation of atoms [13,14] and the environmental risk of metal leaching [15]. With close to 100% atomic efficiency, the metal loading greatly decreases to achieve a similar degradation capacity as nanocatalysts, further reducing the cost of the catalyst.
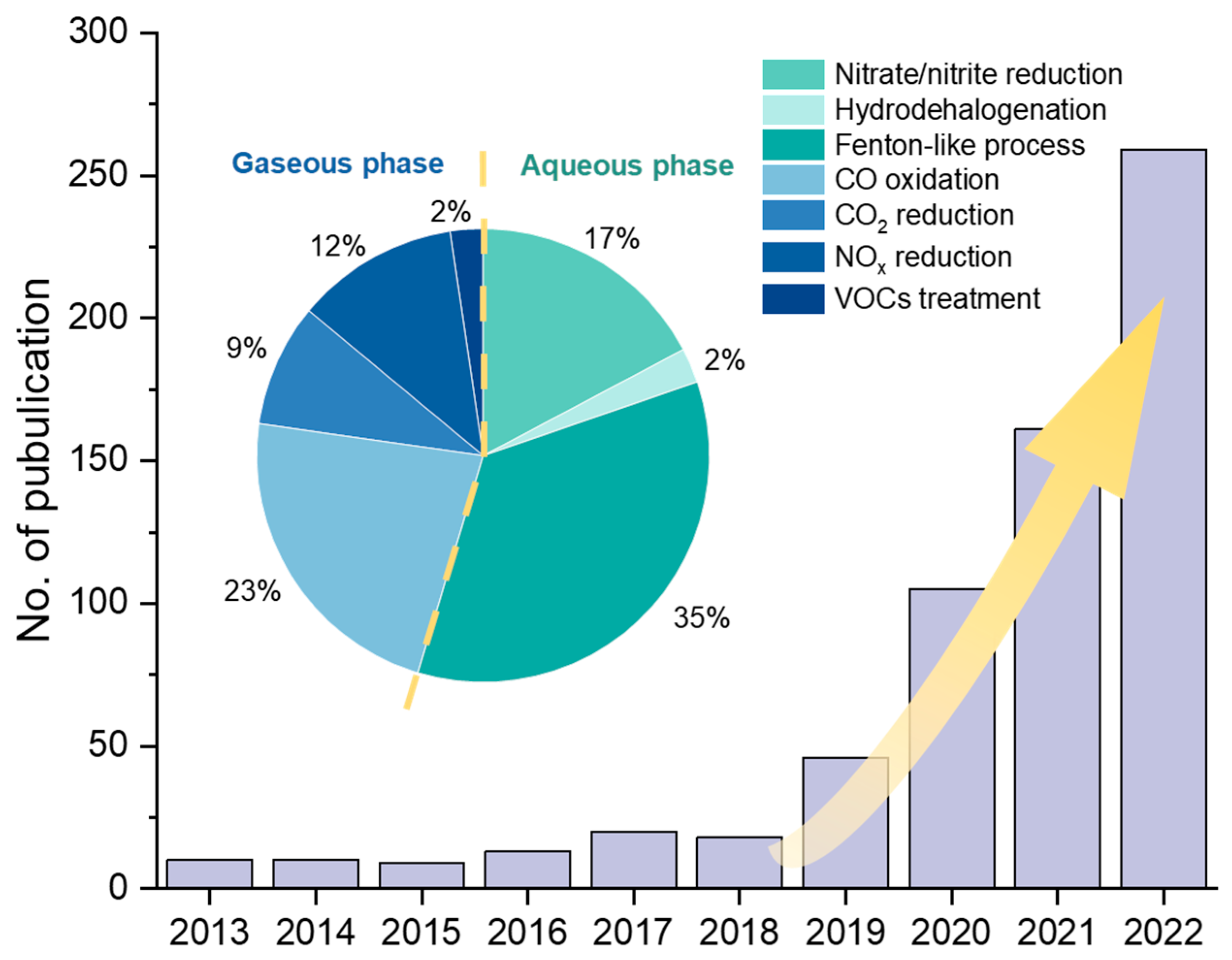
In the field of environmental engineering, remarkable progress has been made in SAC research (Figure 1), particularly involving CO oxidation [7,16,17], CO2 reduction [18,19,20,21], NOx degradation [22,23,24], volatile organic compounds (VOCs) degradation [23,25], aqueous advanced oxidation processes (AOPs) [26,27,28], hydrodehalogenation [29], nitrate reduction [30,31], etc. To date, there are few systematic summaries and reviews of SACs’ applications in environmental engineering. Therefore, in this review, we summarize recent studies on SAC applications in gaseous and aqueous pollution control, respectively, focusing on treatment efficiencies and reaction mechanisms. We further propose suggestions on the synthesis strategies and discuss the challenges and directions for future SAC research in the environmental engineering field.
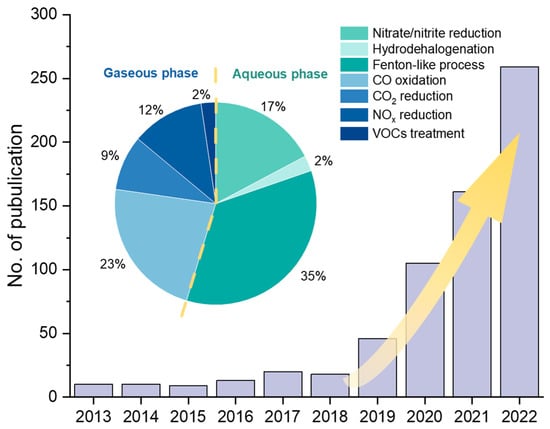
Figure 1.
Number of publications of SAC research in environmental remediation applications and the proportion in various fields in the last decade. The publication data from 2013 to 2022 was collected from the Web of Science in April 2023.
2. Progress of SACs in Gaseous Pollution Control
2.1. VOC Treatments
Volatile organic compounds (VOCs) are ubiquitous air pollutants that are mainly emitted from fossil fuel combustion, transportation, and industrial and household activities [32,33]. There are a wide variety of VOCs, including non-methane hydrocarbons (e.g., alkanes, aromatics), oxygen-containing organic compounds (e.g., aldehydes, ketones, alcohols, ethers), halogenated hydrocarbons, nitrogen- and sulfur-containing compounds, etc. The outdoor VOCs are important precursors of photochemical smog [34], and the indoor VOCs are detrimental to human health, with the probability of causing cancer [35]. The Chinese Fourteenth Five-Year Plan (2021–2025) [36] proposes to further advance the comprehensive management of VOC emissions and requires a more than 10% reduction of the total VOC emissions compared to 2020. Given the adverse impacts of VOCs on the environment and the new legislation in place, it is critical to develop efficient and applicable technologies to reduce VOC emissions. Catalytic oxidation is one of the most promising approaches due to its desirable features, such as high efficiency and energy savings [37], among traditional VOC abatement technologies including adsorption, condensation, thermal incineration, and biological degradation [33].
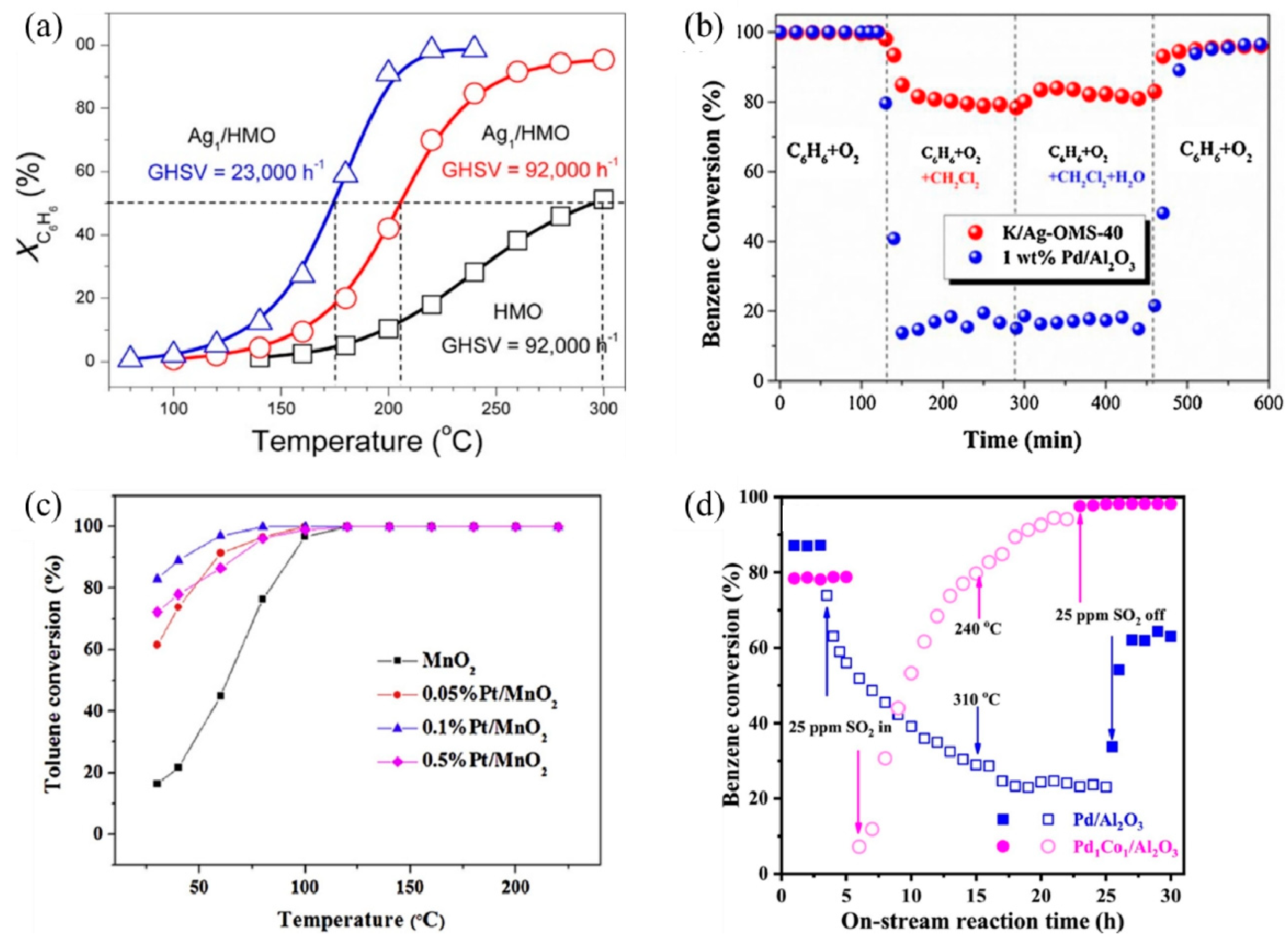
SACs can maximize atomic efficiency, minimize the usage of noble metals, and achieve high activity and selectivity [9,38,39], thus attracting much attention in VOC treatments. In recent years, several noble metal SACs have been developed for VOC catalytic oxidation and showed superior performance compared to their nanoparticle counterparts, including Ag [40,41], Au [23,42], Pt [43,44,45], and Pd [46]. The single-atom Ag based on nanostructured hollandite manganese oxide (Ag1/HMO) [40] prepared by a thermal diffusion method achieved 100% conversion of benzene oxidation at 220 °C at a GHSV of 23,000 h−1 (Figure 2a). The isolated Ag adatoms possessed an excellent ability to activate lattice oxygen and gaseous O2 owing to their upshifted 4d orbitals. Comparably, the Ag atoms incorporated into cryptomelane-type manganese oxide (K/Ag–OMS-40) [41] showed higher benzene conversion, excellent stability, and enhanced tolerance to chlorine poisoning and moisture than 1 wt% Pd/Al2O3 (Figure 2b) [40,41]. The increased number of Mn octahedral defects and newly formed Ag–O–Mn interaction entities accelerated charge transfer [41], facilitating the benzene conversion.
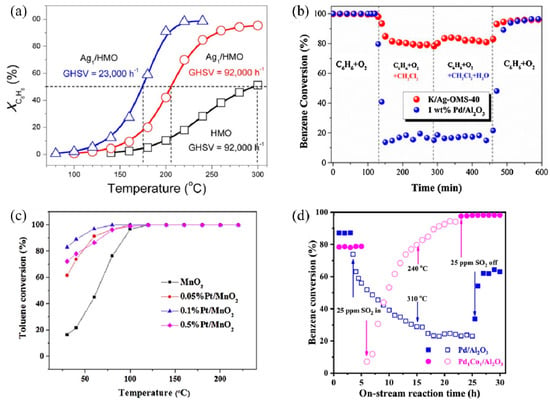
Figure 2.
Catalytic performance of different SACs for several VOC treatments. (a) Conversion of benzene (XC6H6) as a function of temperature over Ag1/HMO and HMO at different GHSVs Reaction conditions: benzene, 200 ppm; O2, 20% and balanced by N2; flow rate, 100 mL min−1. Copyright 2017, American Chemical Society [40]. (b) Comparison of C6H6 conversion between K/Ag–OMS–40 (GHSV = 45,000 h−1) and 1 wt% Pd/Al2O3 (GHSV = 40,000 h−1) and stability test in terms of chlorine and moisture tolerance at a temperature of 300 °C. Copyright 2018, Elsevier B.V. [41]. (c) Temperature-dependent toluene conversion by MnO2 and Pt-deposited MnO2 catalysts (toluene inlet concentration: 10 ppm, 21% O2, N2 as balance gas, GHSV: 60 L g−1 h−1). Copyright 2019, Elsevier B.V. [43]. (d) Benzene conversion as a function of on-stream reaction time in the presence or absence of SO2 over the as-obtained samples. Copyright 2021, Elsevier B.V. [47].
Au SACs also play an important role in low-temperature HCHO oxidation. Au1/α-MnO2 [42] and Au1/CeO2 [23] both exhibited remarkable activity and stability as the doped Au facilitates the formation of oxygen vacancies, active oxygen species, and charged Au species as active sites [23,42]. Au1/α-MnO2 completely degraded the 500 ppm HCHO pollutant stream at 75 °C, with a WHSV of 6 L g−1 h−1. As for Au1/CeO2, among different CeO2 morphologies, CeO2 rod-supported Au (Au/r–CeO2) as an optimal catalyst successfully achieved complete mineralization of HCHO at 85 °C. Additionally, Pt SACs exhibit good VOC catalytic performance as well. For example, the Pt1/MnO2 [43] synthesized via hydrothermal process achieved 100% conversion of indoor-level toluene at ambient temperature due to the formation of surface active oxygen species, including hydroxyl radicals (•OH) (Figure 2c). Chen et al. [45] screened out 0.47 wt% Pt1/Mn–TiO2 as the optimal catalyst with extraordinary activity and acceptable cost, which completely eliminated HCHO (100 ppm) at room temperature.
Moreover, non-noble metal SACs are also applied in VOC catalytic oxidation. An Al SAC-doped graphene was proposed through density functional theory (DFT) calculations for the catalytic oxidation of HCHO at room temperature [48]. Through a pathway of HCHO→HCOOH→CO→CO2, the energy barriers for breaking the C–H bond in HCHO and the C–O bond in HCOOH were both 0.82 eV, serving as the kinetic limiting steps. A bimetal single-atom Pd1Co1/Al2O3 catalyst with double active sites showed enhanced catalytic performance and sulfur resistance for benzene oxidation, over which a 90% benzene conversion was realized at 256 °C, and a gradual recovery of activity after the introduction of 25 ppm SO2 was observed (Figure 2d) [47]. In situ temperature-programmed experiments, in situ diffuse reflectance infrared Fourier transform spectroscopy (DRIFTS), and X-ray absorption fine structure (XAFS) characterizations demonstrated the synergistic behaviors between Co1 and Pd1 sites. The O = Co = O species formed rapidly on the Co1 site to activate oxygen, while benzene selectively tended to adsorb on the Pd1 site. According to previous studies, due to the π-bond in the benzene molecule, a parallel or flat configuration is formed on the close-packed transition metal surfaces [49]. The Pd1 and Co1 double active sites inhibited the competitive adsorption between benzene and oxygen, thus enhancing the reactivity. Meanwhile, the PdO–SO3 complex formed after the addition of SO2 was decomposed into PdO, reactive oxygen species (ROS), and aluminum sulfite at low temperatures, while ROS and PdO sites continued to participate in the reaction, leading to high sulfur resistance.
2.2. CO Oxidation
Carbon monoxide (CO), an odorless and toxic gas due to its high affinity with hemoglobin in the blood [50], widely exists in the exhaust of the automobile and multiple industrial processes [51]. Over the past few decades, CO oxidation methods have been investigated to deal with CO emissions [52]. To overcome the low activity, poor stability, and high cost of current catalysts [53,54], numerous SACs have attracted considerable attention in CO oxidation both experimentally and theoretically, including noble metal catalysts (Pt (Figure 3) [7], Au [55,56,57,58], Pd [59]), non-noble metal catalysts (Fe [60], Co [61], Ni [62]), and metal-free catalysts (Si [63], B and S [64]).
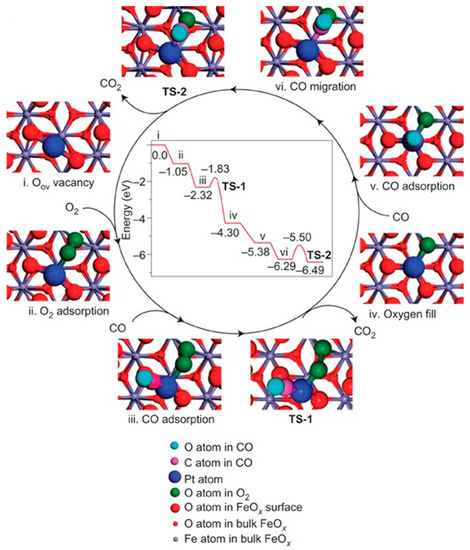
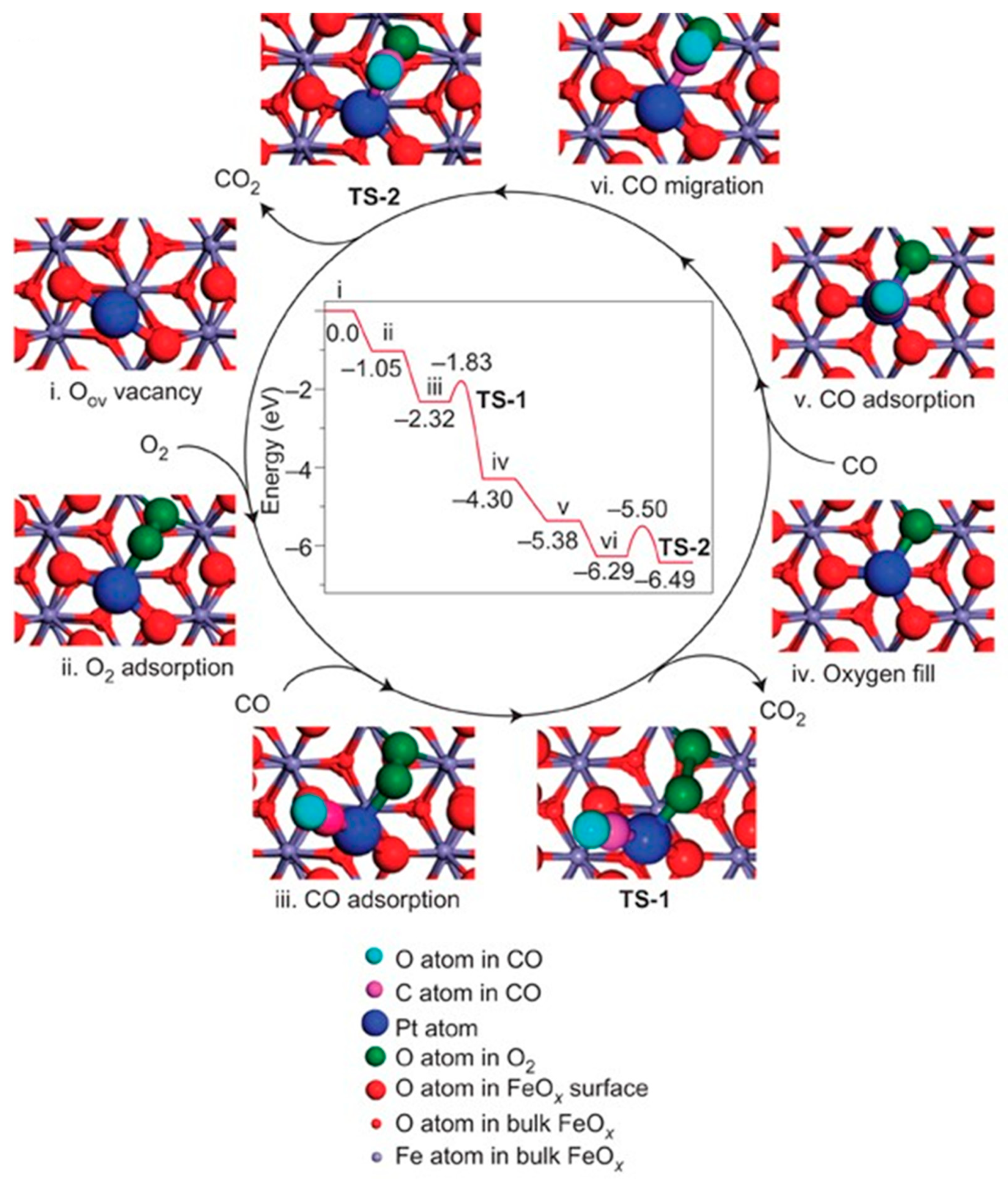
Figure 3.
The proposed reaction pathways and energy profile (in eV) for CO oxidation on the single-atom catalyst Pt1/FeOx. Copyright 2011, Nature Publishing Group [7].
Gold nanocatalysts have shown outstanding performance in low-temperature CO oxidation [55,56,57,58]. As large Au particles are inert for O2 activation, it is important to reduce the particle size. The Au1/FeOx SAC [65] with an extremely low loading of 0.015 wt% achieved a high turnover frequency (TOF) of 0.49 s−1 at 24 °C, which was almost 10 times higher than that of the Au/Fe2O3 catalyst with a loading of 4.4 wt% at 27 °C [66]. It also achieved higher sintering resistance than Au nanocatalysts. By means of extensive first-principles calculations [67], undergoing a local reconstruction, single-atom Au in Ni- and Cu-doped Au@TiO2 were atomically deposited at oxygen vacancies on the TiO2 and formed stable “O–Au–O” species. The oxidation states of the Au cation SAC can be tuned via substrate doping with a transition metal to further improve the O2 activation. The highly oxidized Au single atom showed magnetism and promoted activity and stability for O2 activation and CO oxidation.
The high cost of noble metals can be an obstacle to their practical application, so it is necessary to exploit non-noble and non-mental catalysts. It was elucidated theoretically that the Fe1/C2N monolayer can catalyze CO oxidation via a two-step mechanism due to the localized metal 3d orbitals near the Fermi level [60]. The mechanism of CO oxidation mediated by single Cu atom-doped clusters CuAl4O7–9− was experimentally identified, and CO was found to be crucial to stabilizing Cu in CuAl4O9− around the +1 oxidation state [68]. Moreover, the single-atom Si can be stably embedded into the center of N4 in graphene (Si–GN4) and effectively regulate the electronic structure of the GN4 system, enhancing O2 adsorption [63]. According to the first-principles method, Si–GN4 had excellent stability and catalytic activity at high temperatures. The steps of the complete CO oxidation on Si SAC were as follows: CO + O2→OOCO→CO2 + Oads, 0.57 eV, followed by a second reaction: CO + Oads→CO2, 0.72 eV. Lee and Yan et al. [64] reported the CO oxidation mechanism on a sulfur-doped hexagonal boron nitride (h–BN) non-mental catalyst. The sulfur-doped h–BN accelerated the oxidation of CO by reducing the energy barrier of O2 chemisorption.
2.3. NO and N2O Reduction
High volumes of NOx exist in gaseous wastes from industrial activities and automobile exhaust gas. Selective catalytic reduction (SCR) is the key industrial technology for NOx removal by converting it to N2 with reducing gases (e.g., H2 and NH3) at high temperatures. Conventionally, metal oxides and molecular sieves ((M)2/nO·Al2O3·xSiO2·pH2O) are commonly used as supports to load active metals for NOx SCR. However, the additional secondary metals (usually in the oxide form) tend to aggregate into large nanoparticles, decreasing the distribution of active sites and inhibiting metal–metal interactions for good NOx reduction performance.
Therefore, in recent years, researchers have started to design bimetallic catalysts in the single-atom alloy (SAA) structure. In SAA, a small amount of an active metal is well distributed on the surface of another less active or less expensive metal to improve activity via enhancing metal–metal and metal–NOx interactions. For example, Wen et al. investigated the reduction of NO with H2 on pure Ni and single-atom-Ir-doped Ni (Ir/Ni) surfaces by DFT calculations and microdynamics models [69]. The results showed that the doping of Ir greatly reduced the energy barrier of N2 generation and increased the energy barrier of N2O production (Figure 4a,b). In another study, a Cu–Pd dual-atom alloy (DAA) using Al2O3 as the support completely converted NO to N2 at 175 °C [70], with the N–O bond breaking of the (NO)2 dimer determined as the rate-limiting step. Single-atom Pd isolated by a large amount of Cu (Cu/Pd = 5) significantly improved the catalytic activity and N2 selectivity. After N–O bond breaking, N2O is decomposed into N2 smoothly on the Cu surface, which makes Cu and Cu-rich catalysts have high N2 selectivity. Both single-atom Pd and Cu active sites contribute to this highly efficient deNOx system.
Tang’s group has systematically designed several SAAs for NOx–SCR. A single-atom Mo1/Fe2O3 catalyst was synthesized for NO SCR [71], in which atomic Mo was anchored on reducible α-Fe2O3(001), thus a single-atom Mo ion and an adjacent Fe ion were constructed as a dinuclear site. In Mo1/Fe2O3, Mo ions provided Brønsted acid sites that converted to Lewis acid sites during SCR. This dinuclear structure showed high SCR TOFs comparable to V2O5/TiO2. Further, this group assembled single-atom V1 and W1 loaded on TiO2 (V1–W1/TiO2) [72], which realized tunable electronic interactions, thus performing significantly higher SCR rates (Figure 4c). Experimental and theoretical results indicated that the synergistic electron effect between V1 and W1 enriches high-energy spin charge around the Fermi level, enhancing the adsorption of reactant (NH3 or O2) and accelerating the surface reactions compared to individual V or W atoms. Besides, a dinuclear Ce1–W1/TiO2 catalyst was also developed to explore the synergistic effect between Ce and W in SCR [73]. The synergy of Ce1–W1 reduces the lowest unoccupied states of Ce1 near the Fermi level, boosting adsorption and oxidization of NH3, and renders the frontier orbital electrons of W1, speeding up O2 activation. Due to the strong electronic interaction within Ce1-W1 atom pairs, the TOF of Ce1–W1/TiO2 at 250 °C was four times higher than the sum of Ce1/TiO2 and W1/TiO2 (Figure 4d).
With CO as the reducing agent, CO–SCR is regarded as a promising NO–SCR route because of its capacity to control two pollutant gases at the same time. However, the narrow reaction temperature window and the weak resistance to SO2 and O2 limit the application of CO–SCR. Ji et al. [74] developed a novel Ir SAC (IrW–WO3/KIT-6), with 1% Ir loaded on mesoporous SiO2 (KIT-6), and formed Ir–W intermetallic nanoparticles. At 250 °C and in the presence of 1% O2, NO was completely converted to N2 with 100% selectivity. At a wide temperature window (250–400 °C), the NO conversion rate of 80% and the N2 selectivity of 95% were achieved, better than those of Ir isolate-single-atomic-sites (Ir1–WO3/KIT-6) and Ir nanoparticles (Irn–WO3/KIT-6); IrW–WO3/KIT-6 also showed excellent SO2 resistance. Furthermore, the team also developed a Pt SAC with negatively charged single-atom Pt (0.02 wt%) embedded on CuO squares and supported by CoAlO nanosheets (Pt−CuO/CoAlO) [75], showing 91% NO conversion and 80% N2 selectivity in 3% O2 at 200 °C. The interfacial electron transfer from CoAlO to CuO improved the electron density near Pt, thus enhancing NO adsorption, while Cu served as the adsorption site for CO. The Pt−CuO/CoAlO also showed no activity loss after 200 ppm SO2 heating for 15 h due to weakened SO2 adsorption on active sites.
The reaction between CO and NO also has implications for automobile exhaust treatment. SACs were also found to be efficient in emission control, typically in three-way catalysts (TWCs), achieving synergistic treatment of NO, CO, and hydrocarbons (HCs). Wang et al. [76] reported a dual-site catalyst composed of strongly coupled atomic Pt and Pd on CeO2, which was fabricated via a multi-step heating strategy. Compared with Pt SAC and Pd SAC, Pt–Pd SAC showed a lower T90 of NO and C3H6 conversion, while the T90 of CO oxidation was Pt–Pd SAC ≈ Pt SAC > Pd SAC.
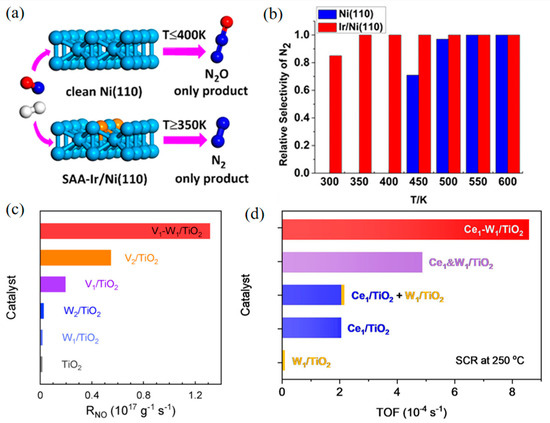
Figure 4.
The single-atom alloy (SAA) Ir/Ni (110) promotes the reduction of NO into N2. (a) Mechanism diagram. (b) Relative selectivity of N2. Copyright 2019, American Chemical Society [69]. (c) Catalytic activities in terms of the reaction rates over the samples in SCR. Copyright 2022, Wiley-VCH [72]. (d) TOFs in SCR over Ce1&W1/TiO2, Ce1&W1/TiO2, Ce1/TiO2 + W1/TiO2, Ce1/TiO2, W1/TiO2, and TiO2 at 250 °C. Copyright 2022, American Chemical Society [73].
Figure 4.
The single-atom alloy (SAA) Ir/Ni (110) promotes the reduction of NO into N2. (a) Mechanism diagram. (b) Relative selectivity of N2. Copyright 2019, American Chemical Society [69]. (c) Catalytic activities in terms of the reaction rates over the samples in SCR. Copyright 2022, Wiley-VCH [72]. (d) TOFs in SCR over Ce1&W1/TiO2, Ce1&W1/TiO2, Ce1/TiO2 + W1/TiO2, Ce1/TiO2, W1/TiO2, and TiO2 at 250 °C. Copyright 2022, American Chemical Society [73].
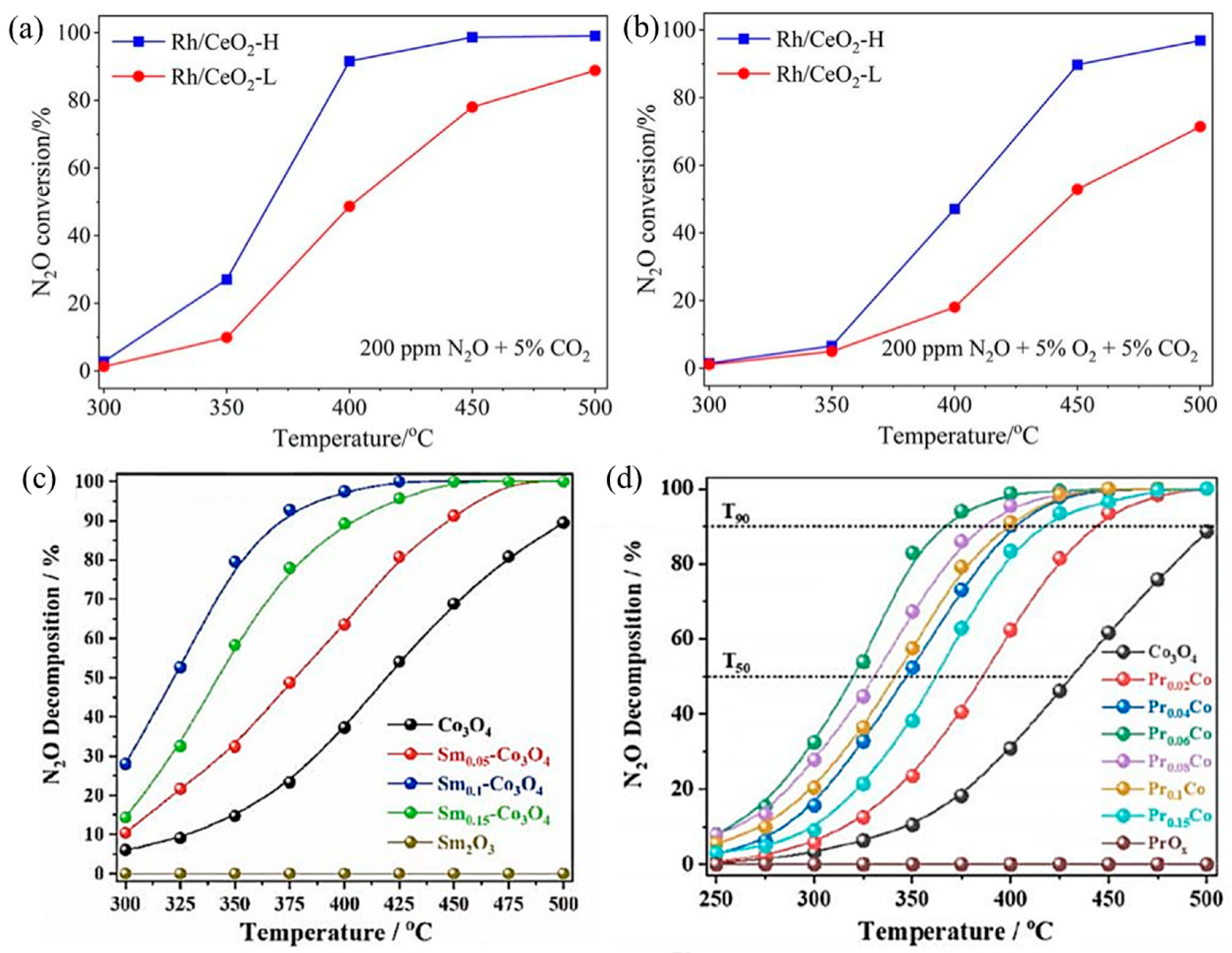
N2O largely exists in the gas exhausts of nitric acid, adipate, and caprolactam industrial production and is also a byproduct during NH3–SCR to treat NOx. With an extremely high global warming potential (GWP) that is 298 times CO2-equivalent and 25 times CH4-equivalent, N2O is an important greenhouse gas [77]. For N2O direct decomposition (deN2O), it is generally decomposed at high temperatures (500–600 °C) by metal-loaded oxides or molecular sieves. In order to reduce the amount of noble metals, they are usually loaded on carriers with large specific surface areas, such as NiO, Co3O4, Al2O3, CeO2, and SiO2, to make them dispersed and improve deN2O activity. The catalytic performance of SACs depends largely on the coordination environment of metal sites. For example, Xie et al. obtained two different Rh1/CeO2 SACs with high and low coordination numbers (CN) by adjusting synthesis procedures [78]. The Rh1/CeO2 with higher Rh CN (Rh/CeO2-H) was more active in deN2O, which resulted from faster O2 desorption, more surface oxygen vacancies, and higher reducibility (Figure 5a,b). Li’s group loaded rare earth elements Sm [79] and Pr [80] onto Co3O4, respectively. By introducing Sm into Co3O4, the presence of Sm promoted the regeneration of the active site and improved the reducibility and oxygen desorption capacity of Co3O4. The catalytic performance of Sm0.1–Co3O4 showed ~52% N2O decomposition at 325 °C and over 90% N2O decomposition at 375 °C (Figure 5c). In addition, in Pr–Co3O4, the “Pr 4f–O 2p–Co 3d” network generated by Pr single-atom doping in Co3O4 redistributed electrons in the Co3O4 lattice, which greatly improved the N2O decomposition performance (Figure 5d). The T50 decreased from ∼430 °C of Co3O4 to ∼320 °C of Pr0.06Co, and the T90 decreased from ∼500 °C of Co3O4 to ∼367 °C of Pr0.06Co.
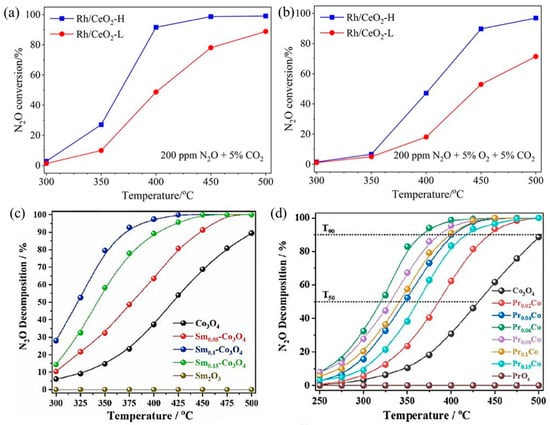
Figure 5.
Catalytic performance for N2O decomposition over Rh/CeO2-L and Rh/CeO2-H catalysts under conditions of (a) 0.02% N2O + 5% CO2 and (b) 0.02% N2O + 5% O2 + 5% CO2; Ar balanced. Weight hour space velocity (WHSV) was fixed at 100,000 mL g−1·h−1. Copyright 2023, Chinese Society of Rare Earths [78]. (c) N2O decomposition activity normalized by specific surface area (SBET) on Co3O4 and Sm-doped Co3O4 samples. Copyright 2021, Elsevier B.V. [79]. (d) N2O decomposition activity normalized by SBET on Co3O4 and Pr-doped Co3O4 samples. Copyright 2022, American Chemical Society [80].
2.4. CO2 Reduction
Electrochemical reduction of CO2 into various chemical feedstocks and fuels not only reduces the negative environmental impact of CO2 but also alleviates the problem of fossil fuel shortage [81,82,83]. In recent years, researchers have developed SACs as efficient catalysts for the electrochemical reduction of CO2 (CO2RR). Numerous heterogeneous catalysts, for example, metals [84,85,86], metal oxides [87,88], metal sulfides [89], metal organic frameworks (MOFs) [90], and their composites [91], have been used. In general, Ni and Fe SACs exhibited superior catalytic performance for CO evolution, while Co, Mn, and Zn SACs were relatively inert to CO2RR [92].
In a study by Zhang et al. [93], an isolated nickel monatomic electrode was prepared with high-density Ni(I) sites anchored to a nitrogen-doped carbon nanotube array and further encapsulated in a nickel–copper alloy on carbon fiber paper (NiI–NCNT@Ni9Cu). The nickel–copper alloy was encased in the carbon-fiber paper. The combination of the single-atomic Ni(I) site and the self-supported array structure resulted in excellent CO2RR performance. The electron configuration of the d band of Ni was modified by introducing Cu, which enhanced the adsorption of hydrogen, thus hindering the hydrogen evolution reaction (HER). The specific current density of a single Ni atom electrode was 32.87 mA cm−2, with a TOF of 1962 h−1 at an overpotential of 620 mV and a Faradaic efficiency of 97% at around −0.73 V vs. RHE. Tang’s group [94] developed a Fe–N–C catalyst for CO2RR via a novel one-step calcination method, which achieved high selectivity of CO2RR to CO. Compared with pristine N–C material, Fe–N–C achieved a higher maximum Faradaic efficiency of 73% and a Tafel slope of 68 mV dec−1, respectively. The excellent CO2RR performance of the catalyst was ascribed to the active Fe–Nx sites, rich functional groups, and abundant microporous structure.
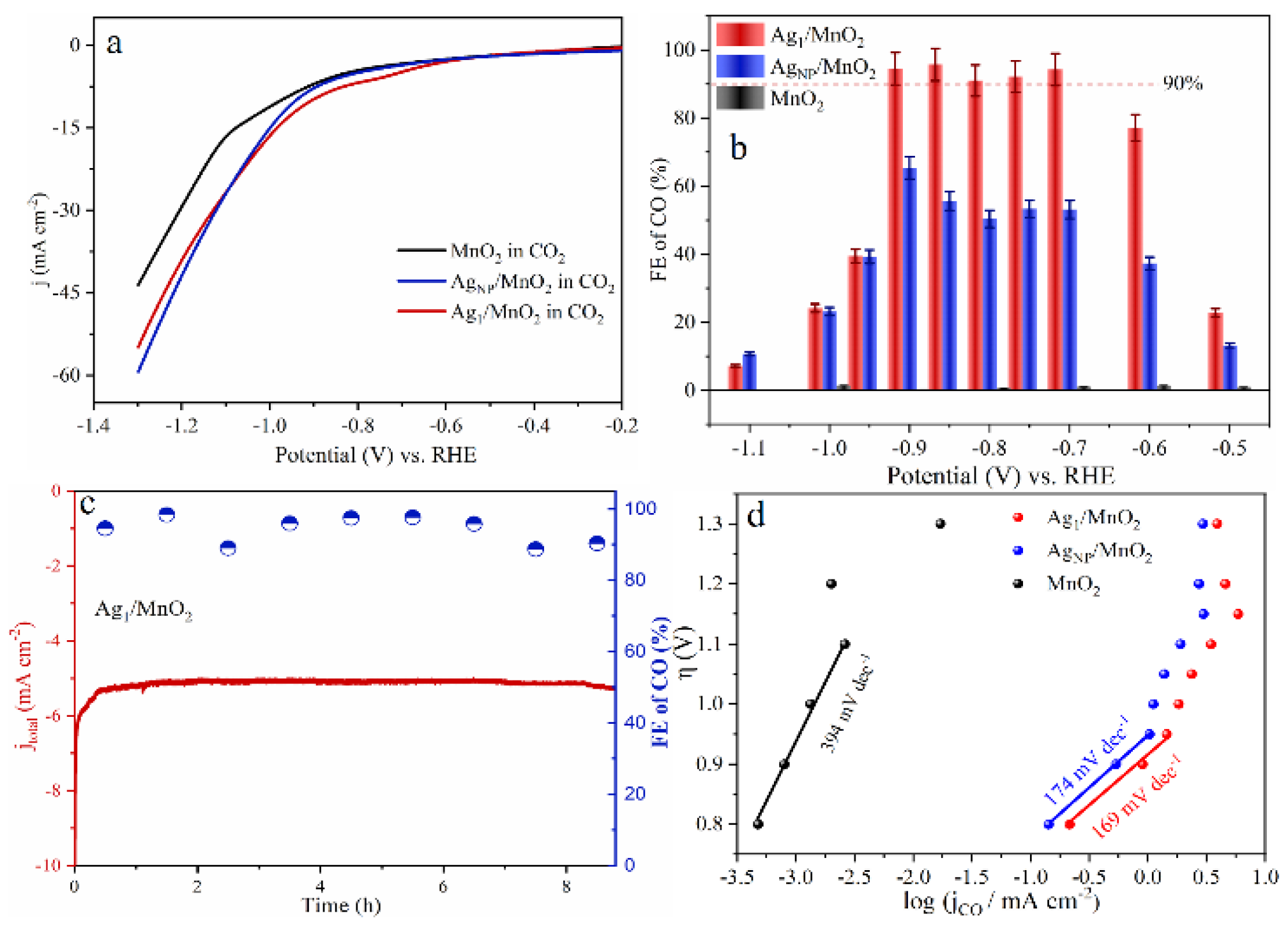
As for noble metal catalysts, Au and Ag show relatively high CO2RR catalytic activity. It was shown that when comparing Ag and Au, when the NP size decreases, Au NPs will lead to the enhancement of the competitive HER, resulting in an increase in by-products, while Ag NPs can selectively enhance CO2RR [95]. Zhang et al. synthesized Ag1 monatomic catalyst (Ag1/MnO2) by thermal conversion of Ag NPs and surface reconstruction of MnO2 [96]. Ag1/MnO2 exhibited a Faradaic efficiency of 95.7% at −0.85 V vs. RHE (Figure 6b), with excellent stability in the reaction (Figure 6c). The Ag1/MnO2 showed improved CO2RR performance than conventional Ag nanocatalysts (AgNP/MnO2) and other reported Ag-based catalysts (Figure 6a,b,d). For current SACs in CO2RR, the low density of active sites, poor conductivity, and mass transfer resistance towards single atomic electrodes still limit their catalytic performance. In order to prevent metal aggregation on the cathode during reductive reactions and maintain the atomic dispersion, most current studies have only achieved a relatively low SAC metal loading below 5 wt%. Therefore, further research is still needed to improve the metal loading capacity of SACs.
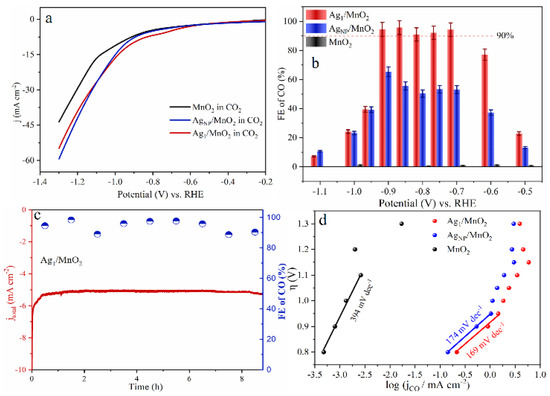
Figure 6.
(a) Linear Sweep Voltammetry (LSV) curves of MnO2, AgNP/MnO2, and Ag1/MnO2 in a CO2-saturated 0.5 M KHCO3 electrolyte. (b) Faradaic efficiency of CO. (c) Long-term electrolysis experiments on Ag1/MnO2 at electrolysis potentials of −0.9 V vs. RHE. (d) Tafel plots of three samples. Copyright 2021, Angew Chem Int Ed Engl. [96].
3. Progress of SACs in Aqueous Pollution Control
3.1. H2O2-Based Fenton-like Processes
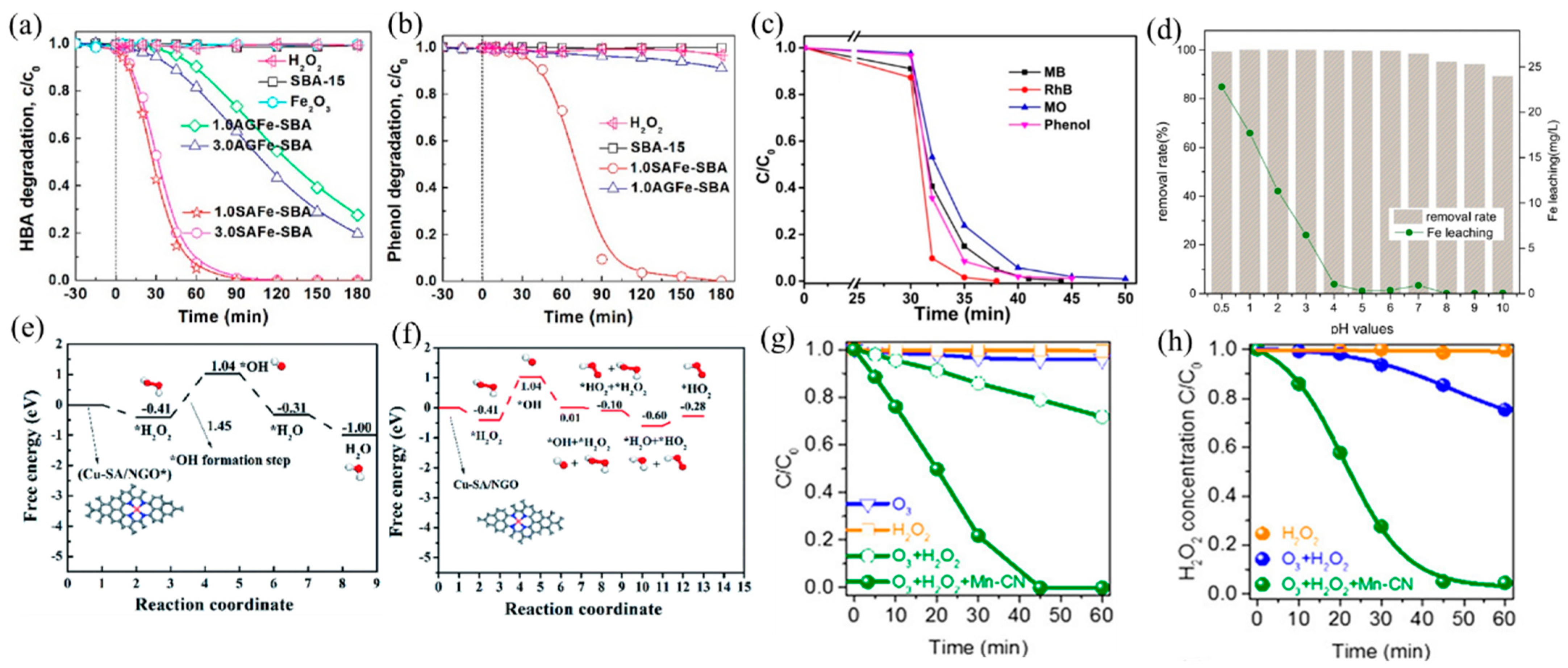
Fenton process with H2O2 generates strongly oxidizing •OH for aqueous organic pollutant decomposition. The traditional Fe-based catalysts have been reported the most, among which Fe SACs have a better catalytic ability than nanoparticle catalysts. Yin et al. [97] reported a SAFe–SBA catalyst with single-atom Fe dispersed into the nanopores of SBA-15. The well-dispersed Fe atoms promoted the decomposition of H2O2 into •OH, leading to a better catalytic performance of SAFe–SBA than aggregated iron sites (AGFe–SBA). The degradation efficiency of both HBA and phenol reached 100% after 180 min (Figure 7a,b). In addition, the Fe SACs formed by Fe sites embedded in g–C3N4 effectively degraded a variety of dyes and organic pollutants (methylene blue (MB), methyl orange (MO), rhodamine B (RhB), and phenol) (Figure 7c), owing to the improved production of •OH from H2O2 activated by Fe(II)–Nx active sites [28].
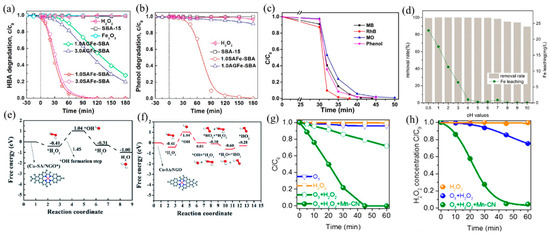
Figure 7.
(a) HBA and (b) phenol adsorption and oxidation by H2O2 activation on catalysts. Copyright 2019, American Chemical Society [97]. (c) Removal efficiency of different organic pollutants using an I–FeNx/g–C3N4-5 catalyst [28]. Reaction conditions: 200 mg L−1 organics (MB, MO, RhB, and phenol), 77 mM H2O2, 0.5 g L−1 catalyst, 308 K, and visible light. Copyright 2018, American Chemical Society. (d) Effect of pH values on AR 73 removal and Fe leaching. Copyright 2016, Elsevier B.V. [98]. Free energy diagrams for Cu–SA/NGO in •OH generation under acidic (e) and neutral (f) conditions. Copyright 2020, The Royal Society of Chemistry [26]. (g) Degradation curves of OA in ozonation, the H2O2 process, and the peroxone process with or without Mn–CN. (h) Corresponding H2O2 decay curves in the H2O2 process, peroxone process, and peroxone process with Mn–CN. Copyright 2019, American Chemical Society [99].
The narrow pH range (2–4) hinders the real application of the Fenton processes [100], which can be improved by adjusting the support and coordination environment of the catalyst. Ma et al. [98] found that dispersing SA Fe–g–C3N4 onto graphitized mesoporous carbon composite (GMC) broadened the working pH window. The obtained catalyst exhibited high catalytic activity in the range of pH = 4–10, attributable to the well-dispersed Fe–Nx and π–π stacking of GMC that promoted the adsorption and decomposition of H2O2 (Figure 7d). Besides, Wu and coworkers [26] prepared a high density of Cu SACs on N-doped graphene (Cu–SA/NGO), which also achieved efficient H2O2 decomposition at neutral pH facilitated by Cu–N4 active sites and low energy barriers of reaction (Figure 7e,f). At acidic conditions, H2O2 can easily be adsorbed on Cu–N4 sites and generate OH* and •OH, while at neutral conditions, OH* s formed when adsorbed H2O2 reacted with another H2O2 molecule to form oxidative HO2*. Gong and coworkers [99] developed Mn–N4-doped g–C3N4 (Mn–CN), which catalyzed the formation of •OH with H2O2 and additional oxidant O3 and degraded oxalic acid (OA). Because of the dispersion of isolated Mn atoms, Mn–CN showed excellent catalytic performance, and oxalic acid was completely degraded within 45 min (Figure 7g,h). Different from the traditional H2O2 reaction, this work proposed a new pathway: H2O2 adsorbed on Mn–N4 sites formed HOO–Mn–N4 species, which reacted with O3 to generate HO2• and O3•−, finally producing •OH.
3.2. Persulfate-Based Fenton-like Processes
In recent years, persulfate-based AOPs have been widely applied in water purification, which is mainly based on the chain reactions initiated by persulfate (PMS, PDS) molecules, generating strongly oxidizing ROS including SO4•−, •OH, O2•−, and 1O2 [101,102]. The process has a strong oxidation capacity and a wide range of solutions for environmental adaptation. In persulfate-AOPs, Co-based, Fe-based, Cu-based, and Mn-based catalysts are widely studied.
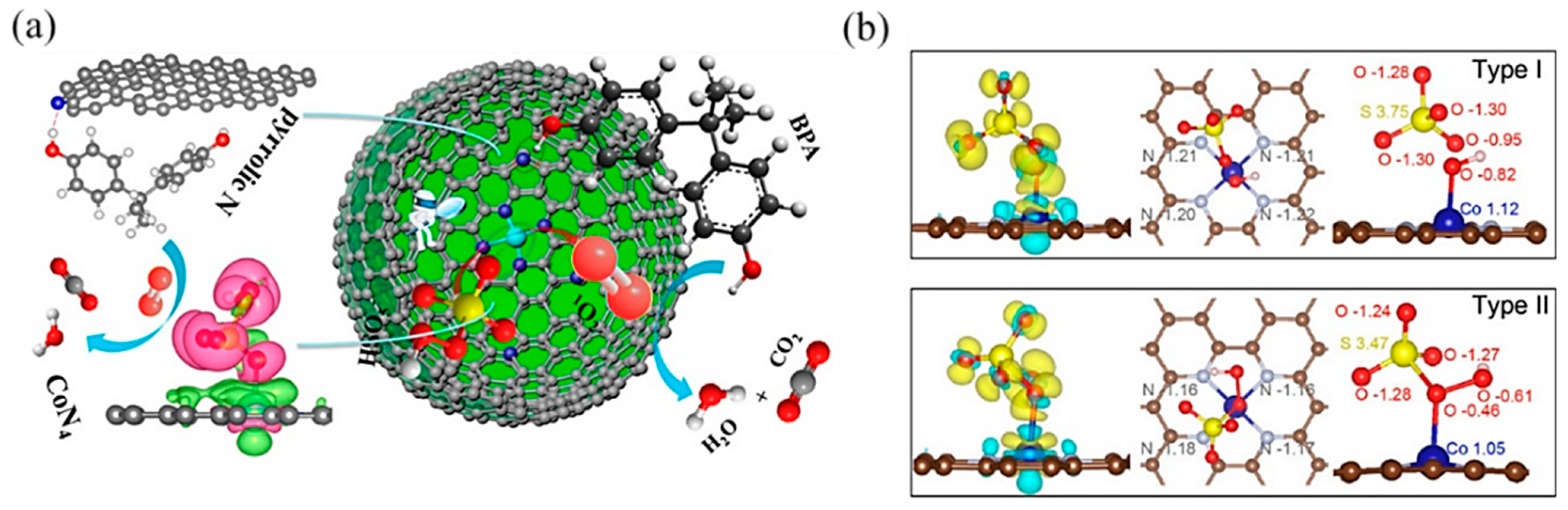
In PMS-based AOPs, Co-based SACs have been extensively studied [101]. Single Co atoms anchored onto porous N-doped graphene showed dual reaction sites [103]: the Co atom was the reaction site, while the adjacent pyrrolic N was the adsorption site (Figure 8a). It activated PMS to degrade BPA with high efficiency because the dual reactive sites reduced the transport distance of ROS and improved the mass transfer efficiency. Likewise, Kim’s group [104] reported pyridine N-coordinated single-atom Co loaded on a polyromantic macrostructure (Co–TPML) (Figure 8b), which also showed outstanding PMS activation and achieved high pollutant removal efficiency, resulting from a high-density and ultrafine dispersion of Co single atoms. With beneficial π-conjugation of TPML and strong metal–support interactions, peroxide adsorption and activation were enhanced. Furthermore, this group developed a single-atom Co-loaded 2D Graphene Oxide (GO)-based membrane [105], in which vitamin C was applied as a mild reducing agent to improve the atomically Co dispersion and maintain the structure of GO layers. This study observed that the Co1–GO membrane showed excellent ability for 1,4-dioxane degradation with the addition of PMS. The kinetics of 1,4-dioxane degradation were over 640 times greater than those in suspension, which was the highest among reported studies in persulfate-based 1,4-dioxane degradation. This catalyst–membrane combination was able to repel macromolecular organic matter, reducing its scavenging effect on free radicals. In addition, studies have found that the porous carbon material support can promote electron transfer [106], which is conducive to improving the efficiency of PMS-based AOPs.
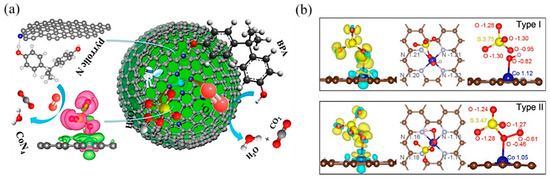
Figure 8.
(a) The proposed overall Fenton-like reaction mechanism on a single-Co-atom catalyst. Copyright 2018, American Chemical Society [103]. (b) Adsorption configuration and charge density of PMS on Co–TPML through coordination with H-adjacent (Type I) and S-adjacent (Type II) O atoms in the peroxide bond, respectively. Yellow and cyan denote the electron accumulation and electron depletion, respectively. Copyright 2020, American Chemical Society [104].
Compared with PMS, PDS is more difficult to activate with a short peroxide O–O bond (1.322 Å) in the structure of –O3S–O–O–SO3– [107]. Because of its cheaper cost, lower toxicity, and lower pH limits, PDS is expected to be more widely applied in actual water treatment. Generally, in PDS/SAC systems, synergistic effects between the atomic metal and the support play an important role [15]. Li and coworkers [108] developed Cu single sites dispersed on carbon nitride (SAS–Cu1.0), showing remarkable performance in tetracycline degradation due to the enhanced PDS adsorption and activation. Under UV light and 0.1 mM sodium persulfate, the tetracycline (TC) degradation rate of SAS-Cu1.0 reached 82.5% in 30 min, while the degradation rates of carbon nitride (CN) and CN–NanoCu were 53.5% and 78.1%, respectively. The result revealed that the degradation mechanism on single-atom Cu involved both radical and nonradical pathways, leading to the promotion of charge separation and transfer.
3.3. Electrocatalytic Hydrodehalogenation
Organic halides that contain C–X bonds (X = Cl, Br, I, and F), such as chlorobenzene, 4-chlorophenol, and bromophenol, are commonly found in water bodies contaminated by pharmaceuticals, pesticides, surfactants, and after disinfection by chlorine [109]. Due to their strong carbon–halogen bonds and the ability to destruct biological enzymes, organohalogens are difficult to destroy by biological methods, leading to their persistent existence in water and posing a serious threat to human health, the ecological environment, and agricultural production. To solve this problem, hydrodehalogenation is proposed as an effective dehalogenation scheme in which two distinct partially charged Hδ− and Hδ+ atoms formed from H atoms are utilized to attack carbon–halogen bonds [110,111]. Direct catalytic hydrodehalogenation is the most studied dehalogenation method at present. Numerous mono-metal and bimetallic catalysts have been developed. However, high catalyst costs, strict reaction conditions, and unsatisfactory catalytic efficiency make this technology a dilemma. In comparison, electrocatalytic and photocatalytic hydrodehalogenation, which are environmentally friendly and energy saving, have become a hot research field in recent years. In 1975, Geer et al.’s experiment on hydrodehalogenation of hexachlorobezene (HCB) by electrocatalysis proved that the complete degradation of chlorinated organic compounds could be achieved by controlling the potential [112].
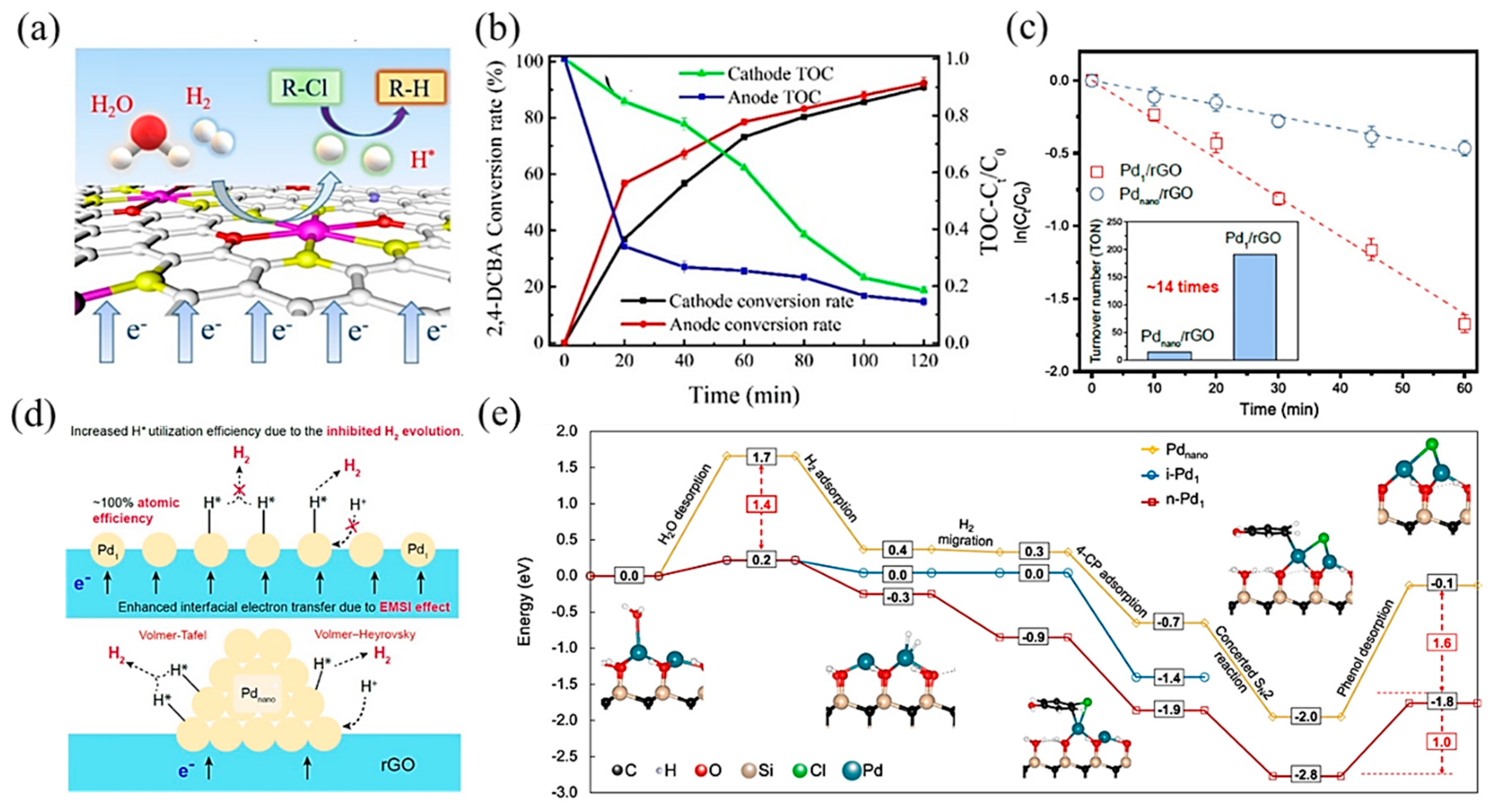
Transition metal-based SACs have been used due to their excellent electrocatalytic hydrodehalogenation performance. Wang et al. [113] synthesized single-atom Co on sulfide graphene (Co–SG), achieving high atomic H* production by electrochemical reduction of H2O and electrolysis of hydrogen. With the synergistic effects among Co active sites, S-doped graphene, and the interfacial structure, the conversion rate of 2,4-DCBA reached 91.1% and the TOC concentration was reduced by 80% (Figure 9a,b). Zhao and coworkers [114] developed a Fe/Cu bimetallic single-atom catalyst dispersed on N-dope porous carbon (FeCuSA–NPC), leading to a stronger chlorinated pollutant degradation effect. In this process, dichlorination on the Cu single atom and hydroxyl radical oxidation on the Fe single atom formed a synergistic effect, which led to a high removal activity for 3-chlorophenol (3-CP), 2,4-dichlorophenol (2,4-DCP), and 2,4,6-trichlorophenol (2,4,6-TCP), with kinetics between 545.1 and 1374 min−1 gmetal−1.
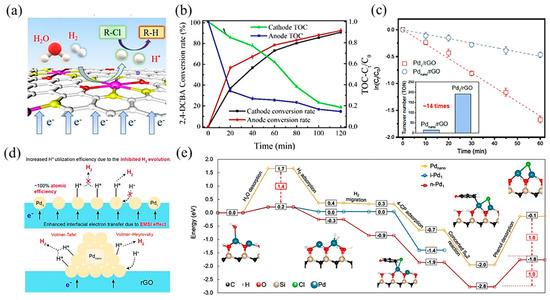
Figure 9.
(a) Schematic illustration of the electrocatalytic hydrodechlorination reaction mechanism. (b) Total Organic Carbon (TOC) concentration ratio and conversion rate of 2,4-DCBA over time. Copyright 2020, American Chemical Society [113]. (c) Pseudo-first-order kinetic plots of 4-CP dechlorination with Pd1/rGO and Pdnano/rGO electrodes. The inset indicates the turnover number per Pd atom based on a reaction time of 30 min. (d) Proposed mechanism of enhanced cathodic hydrodechlorination with Pd1/rGO versus Pdnano/rGO. Copyright 2021, American Chemical Society [115]. (e) Hydrodehalogenation on i-Pd1, n-Pd1, and Pdnano. The blue, black, red, tan, and white spheres in geometrical models are Pd, C, O, Si, and H atoms, respectively. The solid and dashed lines represent the minimum energy path and other reaction pathways, respectively. Copyright 2021, Springer Nature [116].
Apart from transition metal catalysts, noble metal catalysts are also efficient in electrocatalytic hydrodehalogenation, among which, Pd-based catalysts have been studied in depth. Huang et al. [115] synthesized a single-atom Pd loaded on reduced graphene oxide (Pd1/rGO), which was more effective in chlorinated phenol dichlorination and showed higher atomic efficiency than Pd nanoparticle counterparts (Figure 9c). Mechanistic studies showed that this promotion effect was attributed to two aspects: (1) a strong interaction between the metal and support enhanced interfacial electron transfer through Pd–O bonds; (2) Pd1 restrained H2 evolution, contributing to atomic H (H*) utilization (Figure 9d). Further, Chu et al. [116] proposed that neighboring Pd single-atom catalysts, with shorter distances and more adjacent active sites between atoms, performed higher activity and selectivity in hydrogenating carbon–halogen bonds than isolated single-atom Pd. DFT calculations (Figure 9e) revealed that the cooperative effect between neighboring Pd atoms decreased the energies of water desorption and hydrogenated product desorption, which were the key meta-stable reaction steps. Besides, the neighboring structure was conducive to selectively hydrogenating the C–Cl bond without affecting the other bonds.
3.4. Photocatalytic Hydrodehalogenation
Photocatalytic hydrodehalogenation realizes the fracture of carbon–halogen bonds through photoexcitation and electron transfer. Numerous studies have proved that semiconductor catalysts doped with noble metals display superior photocatalytic hydrodehalogenation.
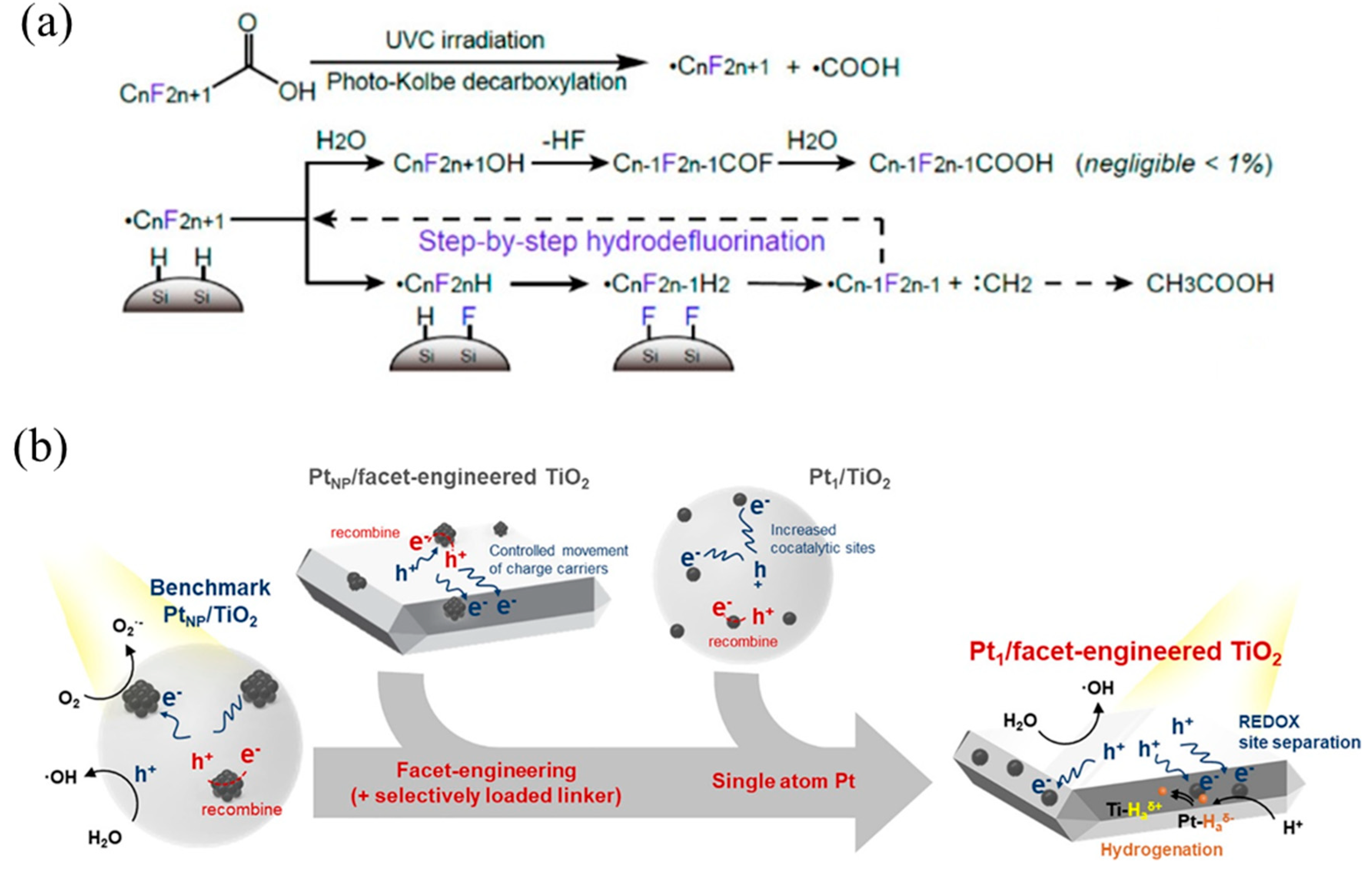
Kim’s team has reported single-atom Pt supported on SiC (Pt1/SiC) [29] and TiO2 (Pt1/TiO2) [117], respectively, achieving hydrodehalogenation of perfluorooctanoic acid (PFOA) by cleaving C–F bonds. As for Pt/SiC (Figure 10a), due to the high work function of Pt (~5.65 eV), it tended to attract photogenerated electrons from the SiC conduction band, and then H atoms were selectively reduced and formed Pt–H bonds through the Volmer reaction. Finally, H atoms spillover from Pt–H bonds were transferred to SiC to form Si–H, which was then redistributed with the C–F bond, thus achieving hydrodehalogenation. Likewise, Pt single atoms in Pt/TiO2 drove the photogenerated electrons on the conduction band to generate H atoms and spill over onto the TiO2 surface, further forming Ti–H bonds to break C–F (Figure 10b). On the contrary, Pt nanoparticles consumed photogenerated electrons to reduce O2, instead of hydrodehalogenation.
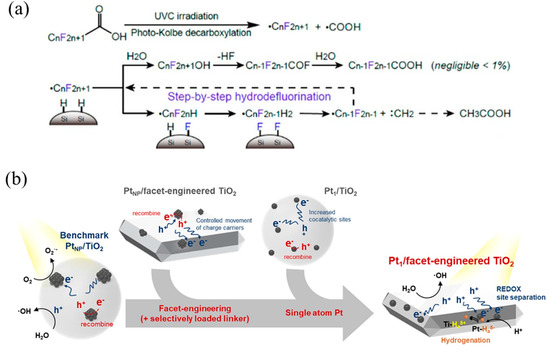
Figure 10.
(a) PFOA decomposition mechanism with Pt1/SiC showing photo-Kolbe decarboxylation and subsequent hydrodefluorination. Copyright 2018, American Chemical Society [29]. (b) Photocatalysis mechanisms of Pt nanoparticle-loaded TiO2, Pt single-atom-loaded TiO2, Pt nanoparticle-loaded facet-engineered TiO2, and Pt single-atom-loaded facet-engineered TiO2. Copyright 2021, American Chemical Society [117].
In addition, single-atom Ag was confirmed as an ideal catalyst to selectively dehalogenate under visible-light irradiation by Wang et al. [118]. Under mild visible light irradiation, AgF was successfully reduced to Ag(0) single atoms and Ag nanoparticles. Theoretical and experimental investigations suggested that such mixed species (MS-Ag) showed outstanding hydrodehalogenation and deiodination-arylation performance, resulting from the synergistic effects of the Ag single atoms and the light-harvesting unit of Ag nanoparticles. Notably, the yield of selective hydrodehalogenation of 4-iodoanisole was up to 99% when CsF was added.
3.5. Nitrate and Nitrite Reduction
Nitrate (NO3−) and nitrite (NO2−) are common inorganic nitrogen-containing pollutants in the aqueous phase and the main causes of eutrophication and algae blooms. Due to the excessive use of agricultural fertilizers and improper treatment of sewage, NO3− is prevalent in groundwater and surface water bodies, posing a great threat to human health and the environment. NO3− in sewage can be converted into NO2− by microorganisms, which will destroy the oxygen transport ability of hemoglobin when entering the human body, and even lead to poisoning or cancer. Nitrate reduction reaction (NO3RR) is a promising strategy to reduce the environmental pollution caused by NO3−, while producing N2 or NH3 as a valuable energy source.
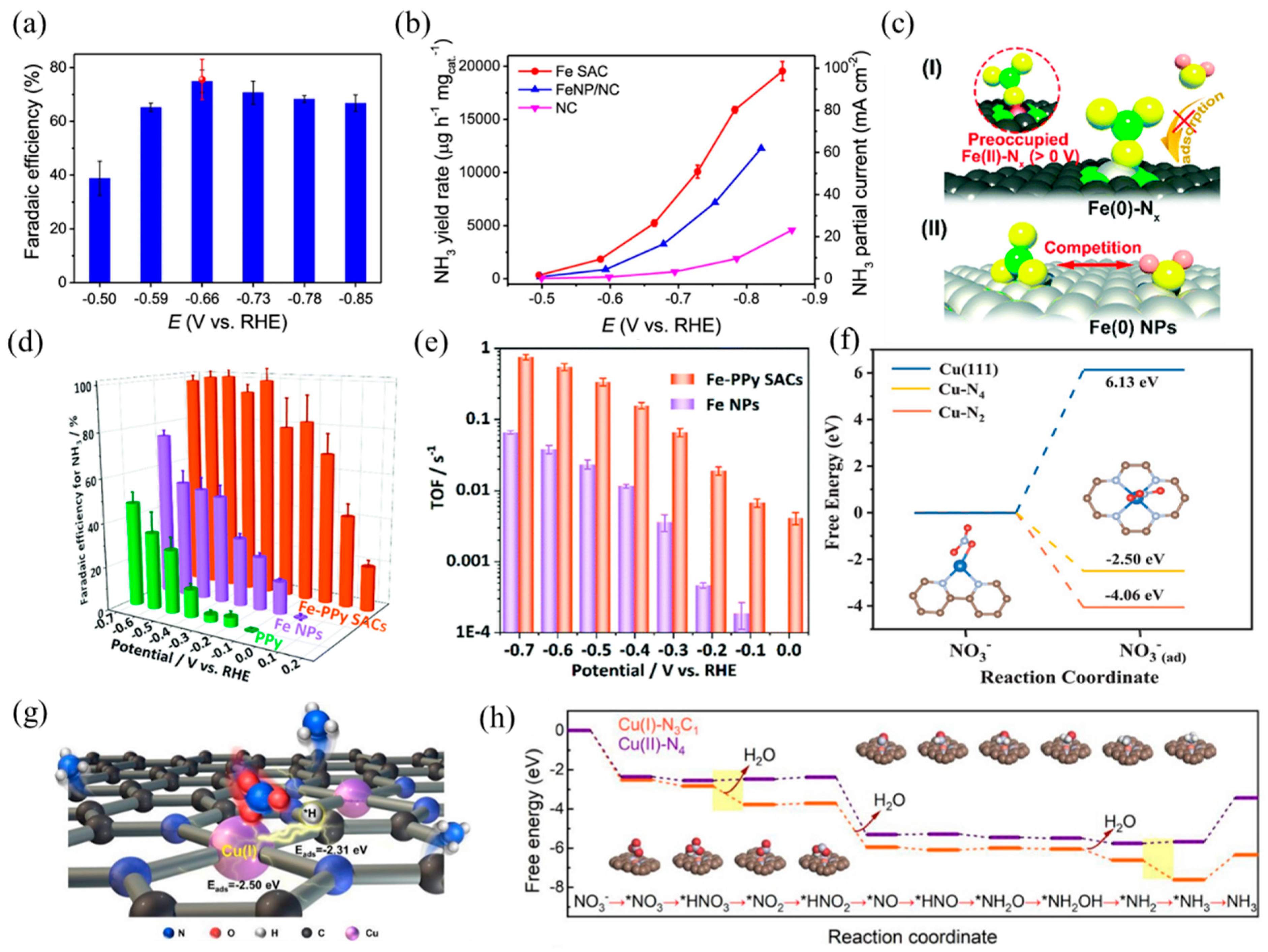
Single-atom electrocatalysts can realize efficient NO3RR and selectively obtain NH3, such as Fe- and Cu-based SACs. Primarily, the active center of the Fe-based catalyst is Fe–Nx. According to Wang et al. [30], isolated Fe single atoms in the form of Fe–N4 hindered N–N coupling, resulting in higher affinity towards N–H coupling and NH3 formation. Benefiting from these structure advantages, the nitrogen-coordinated Fe sites dispersed on carbon matrix exhibited remarkable capacities in NO3RR with a Faradaic efficiency of ~75% and a high NH3 yield of ~20,000 μg h−1 mgcat.−1 (Figure 11a,b). In addition, Liu et al. [31] prepared a highly active and selective Fe–CNS consisting of Fe single atoms loaded on S and N-doped carbon supports. S-doping created more defects on the support surface, which was beneficial to enhancing the stability of Fe single atoms. Along with Fe–N4, the presence of S sites adjusted the coordination environment and formed FeN4S2 as the dominant active site. The experimental results of NO3RR revealed that the prepared Fe–CNS catalyst performed excellent activity with a nitrate removal capacity of 7822 mg-N g−1 Fe and a high ammonia Faradaic efficiency of 78.4%. Yu et al. [119] found that nitrate preoccupied on Fe(II)–Nx and hindered the adsorption of H2O, thus inhibiting the competitive reaction of HER. In addition, the special thermodynamic and kinetic properties of the Fe SACs resulted in a more positive and narrower range of redox potentials than the Fe NPs (Figure 11c). As a result, Fe SACs achieved a higher NH3 yield and selectivity, with a maximum yield rate of 2.75 mgNH3 h−1 cm−2 and close to 100% Faradaic efficiency (Figure 11d). The TOFs of the Fe–PPy SACs reached 0.006–0.7 s−1 at 0–−0.7 V vs. RHE, while the TOFs of the Fe NPs were 0.00015–0.06 s−1 (Figure 11e).
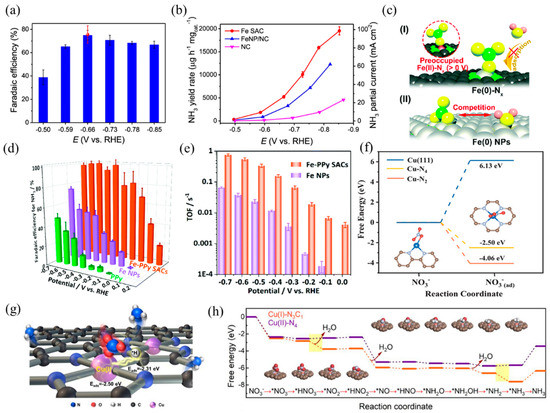
Figure 11.
(a) NH3 Faradaic efficiency of Fe SAC at each given potential. Red dot is Faradaic efficiency estimated by three independent NMR tests. (b) NH3 yield rate and partial current density of Fe SAC, FeNP/NC, and NC. Copyright 2021, Springer Nature [30]. (c) The proposed preoccupied NO3RR mechanism for the single-site center (I) and the classical competitive mechanism for the bulk surface (II). (d) Faradaic efficiency for ammonia and (e) TOFs of the Fe–PPy SACs and Fe NPs based on the result of SI-SECM for ammonia production. Copyright 2021, The Royal Society of Chemistry [119]. (f) Calculated free energies for NO3− adsorption on Cu (111), Cu–N4, and Cu–N2 surfaces, respectively. The brown, gray, blue, and red balls represent C, N, Cu, and O atoms, respectively. Copyright 2020 Wiley-VCH [120]. (g) TOC and (h) calculated activation energy for NO3RR using Cu(I)–N3C1 and Cu(II)–N4 as models. Copyright 2022, American Chemical Society [121].
Besides, Cu-based SACs with the Cu–Nx coordination structure are also suitable for NO3RR, according to previous studies. Feng et al. [120] reported a Cu SAC anchored on nitrogen-doped carbon nanosheets (Cu–N–C) with high activity, selectivity, and stability in NO3RR. XAFS analysis and DFT calculations revealed that the mixed coordination structures of Cu–N2 and Cu–N4 dispersed on carbon caused the adsorption of NO3− and NO2− (Figure 11f), inhibiting the release of NO2−. At −1.3 V vs. SCE with an initial 50 mg L−1 NO3-N, the selectivity of the NO2-N product was only 5%. Fan and coworkers [121] studied the NO3RR properties of atomic Cu supported on micro/mesoporous nitrogen-doped carbon (Cu MNC). The Cu(I) sites (Cu(I)–N3C1) concentrated the charge around the center Cu atoms, causing the adsorption of *NO3 and *H to adjacent Cu and C sites by balanced adsorption energy. Compared with Cu(II)–N4, Cu(I)–N3C1 decreased the activation energy of rate-limiting steps, thus promoting the formation of NH3 (Figure 11g,h). When applied to nitrate reduction (100 mg-N L−1), Cu MNC achieved a promising NH3 yield rate per active site of 5466 mmol gCu−1 h−1 and a conversion rate of 94.8% within 6 h.
As for noble metal SACs, isolated Ru sites dispersed on nitrogen-doped carbon (Ru SA–NC) were demonstrated to be an effective catalyst for both nitrate and nitrite electroreduction to NH3 [122]. Ru SA–NC achieved Faradaic efficiencies of 97.8% at −0.6 V vs. RHE (NO2− reduction) and 72.8% at −0.4 V vs. RHE (NO3− reduction), respectively. A bimetallic catalyst with a single-atom Ru-modified Cu nanowire array loaded on Cu foam (Ru–Cu NW/CF) was proposed by Lee’s group [123], which showed efficient electrocatalytic nitrite reduction. Due to the inhibition of N–N coupling by the active site of single-atom Ru, at the overpotential of −0.6 V vs. RHE, the Faradaic efficiency reached 94.1% and the NH3 yield was up to 211.73 mg h−1 cm−2. Kamiya et al. [124] prepared an atomically dispersed Pt-modified covalent triazine framework hybridized with carbon nanoparticles (Pt–CTF/CP), which showed a NO2− reduction activity comparable to that of bulk Pt surfaces. It is worth noting that nitrate reduction reactions were almost not detectable. Since nitrate adsorbed on the single Pt atom is in an unstable monodentate form, the nitrate may not have enough adsorption energy to be activated.
4. Conclusions and Outlook
With close to 100% atomic efficiency and high catalytic activity, SACs are considered to bring new opportunities for environmental pollution remediation and are becoming a prevalent research frontier. The well-controlled atomically dispersed structure of SACs fills the gap between heterogeneous and homogeneous catalytic reactions and provides a new direction for understanding the catalytic mechanism at the atomic level. Compared with bulk NPs, SACs’ unique electronic characteristics and atomic sites help them achieve reactions that cannot be catalyzed by NPs. Additionally, with higher atomic utilization and less metal loading, SACs have a lower cost of raw materials, showing economic advantages in practical engineered applications. In the past decade, researchers have developed a variety of SACs that have been successfully applied to solve practical environmental problems, such as the purification of industrial gaseous pollutants and the treatment of organic pollutants in wastewater. In general, SACs show excellent catalytic activity, selectivity, and stability in various catalytic reactions.
However, current SACs still have non-negligible shortcomings that should be overcome. Due to ultra-low metal loading, the catalytic efficiency of SACs is unsatisfactory. To improve the reaction efficiency, it is a tough challenge to avoid single-atom aggregation when increasing the metal content. From the above discussions, we found that the coordination structure and interactions between the atomic metal and the support have an important impact on the physicochemical properties and catalytic performances of SACs. Nevertheless, there is still a lack of clarity on the structure–catalytic correlation. Besides, unlike laboratory experiments, complex compositions in the actual gas or water bodies might interfere with the catalytic reaction via surface contamination and deactivation of the catalyst. A long-term reaction may also lead to the loss of metal atoms or aggregation. In engineering applications, the integration of SACs into existing devices or systems is also an important issue. To promote further development of SACs in environmental engineering, the following research directions are proposed:
- (1)
- Development of new synthetic strategies: Increasing the number and density of coordination sites can effectively improve the loading of metal single atoms. More loading sites can be created by fabricating defects and unsaturated coordination centers. The methods for synthesizing stable SACs with relatively high metal loadings should be further developed. Studies revealed that when the SAC content increases from ~1% to ~5%, monatomic metals will form neighboring SACs or SAC ensembles without metal−metal bonding. However, it still maintains high atomic utilization and a unique coordination environment [101]. Recently, atom-trapping methods have been applied to load 1–3 wt% of SACs onto reducible supports (e.g., CeO2, FeOx), preventing metal aggregation at high temperatures [17]. It was demonstrated that a single-atom Cu catalyst prepared by atom-trapping on CeO2 effectively prevented sintering and deactivation via the regulated charge state of the Cu through facile charge transfer between the active site and the support [125]. Moreover, using graphene quantum dots as the carbon carrier, the transition metal SAC content was further increased to nearly 40% [126]. Appropriate supports, such as porous carbon and MOF, can strengthen metal–substrate interactions. In addition, it is important to develop a synthetic strategy that can precisely regulate the atomic active center and create more selective metal active centers for a specific catalytic reaction. Through doping heteroatoms and designing bimetallic sites, creating synergistic interactions between various elements may greatly contribute to the enhancement of SAC performance.
- (2)
- Study on catalytic mechanisms: At present, most of the characterization techniques are ex situ, such as high-angle annular dark-field–scanning transmission electron microscopy (HAADF–STEM) and X-ray absorption spectroscopy (XAS), which make it difficult to provide in situ characterization of the alterations of the physicochemical properties and electronic structures of SACs during the reactions. Hence, it is necessary to develop advanced in situ characterization technology to further study the complex pathways of catalytic reactions at the atomic level. Nowadays, some cutting-edge in situ characterization techniques have been reported to detect the evolution of catalyst sites and the interactions between active sites and reactants during the reaction process. For example, Hensen et al. [59] used an in situ near ambient pressure X-ray photoelectron spectrometer (NAP–XPS) to follow the surface electronic structure of Pd–CeO2 SAC during CO oxidation and in situ infrared spectroscopy to probe the interaction between surface sites and reactants. Thereby, the structure–function relationships of Pd/CeO2 catalysts were established. In addition, in situ and operando infrared and XAS were used to detect CO oxidation mechanisms on an Ir single atom, detailing reaction steps [127]. Datye et al. [128] also used CO as a probe molecule during in situ DRIFTS to effectively detect the property changes of Pt1/CeO2 under reaction conditions. The model establishment and theoretical calculations by DFT are beneficial to understanding the formation of the intermediate products and energy barriers (i.e., the rate-determining step) during the reaction, which can guide the design of future catalysts. However, when faced with complicated environmental media and operating parameters, DFT is not suitable due to the high cost of time. As a more handy and advanced technology, machine learning (ML) and quantitative structure–activity relationship (QASR) can efficiently establish the relationship between catalyst performance and certain specific descriptors, such as operational parameters.
- (3)
- Optimization for practical applications: To stabilize the interactions between metal atoms and support, the synthesis methods of a certain metal–support combination are specific, which may hinder the large-scale synthesis of SACs. Developing a simple and general synthesis strategy is beneficial to reducing the cost of large-scale SAC production. The integration of SACs into reactors or systems to achieve pilot-scale and large-scale is another troublesome challenge to overcome. Besides, it is of great importance to improve the adaptability to different complex environments and the stability of the reaction system.
Author Contributions
Conceptualization, Z.W., H.W. and X.W.; methodology, Z.W., H.W. and X.W.; software, Z.L.; validation, Z.L. and X.W.; formal analysis, R.H.; investigation, Z.Z.; resources, Z.W.; data curation, R.H. and Z.Z.; writing—original draft preparation, Z.L., R.H. and Z.Z.; writing—review and editing, Z.W. and X.W.; visualization, Z.L.; supervision, X.W.; project administration, Z.W.; funding acquisition, X.W. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.
Acknowledgments
We thank our group members for their suggestions and help in the manuscript preparation.
Conflicts of Interest
The authors declare no conflict of interest.
Sample Availability
Not applicable.
References
- Thomas, N.; Dionysiou, D.D.; Pillai, S.C. Heterogeneous Fenton catalysts: A review of recent advances. J. Hazard. Mater. 2021, 404, 124082. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Yang, Z.; Li, Y.; Zhang, G. Advances in single-atom catalysts: Design, synthesis and environmental applications. J. Hazard. Mater. 2022, 429, 128285. [Google Scholar] [CrossRef] [PubMed]
- Schauermann, S.; Hoffmann, J.; Johánek, V.; Hartmann, J.; Libuda, J.; Freund, H.J. Catalytic activity and poisoning of specific sites on supported metal nanoparticles. Angew. Chem. Int. Ed. 2002, 41, 2532–2535. [Google Scholar] [CrossRef]
- Li, J.; Li, X.; Zhai, H.; Wang, L. Au20: A tetrahedral cluster. Science 2003, 299, 864–867. [Google Scholar] [CrossRef]
- Valden, M.; Lai, X.; Goodman, D. Onset of catalytic activity of gold clusters on titania with the appearance of nonmetallic properties. Science 1998, 281, 1647–1650. [Google Scholar] [CrossRef]
- Gao, C.; Low, J.; Long, R.; Kong, T.; Zhu, J.; Xiong, Y. Heterogeneous single-atom photocatalysts: Fundamentals and applications. Chem. Rev. 2020, 120, 12175–12216. [Google Scholar] [CrossRef]
- Qiao, B.; Wang, A.; Yang, X.; Allard, L.F.; Jiang, Z.; Cui, Y.; Liu, J.; Li, J.; Zhang, T. Single-atom catalysis of CO oxidation using Pt1/FeOx. Nat. Chem. 2011, 3, 634–641. [Google Scholar] [CrossRef]
- Liu, L.; Corma, A. Metal catalysts for heterogeneous catalysis: From single atoms to nanoclusters and nanoparticles. Chem. Rev. 2018, 118, 4981–5079. [Google Scholar] [CrossRef]
- Yang, X.; Wang, A.; Qiao, B.; Li, J.; Liu, J.; Zhang, T. Single-atom catalysts: A new frontier in heterogeneous catalysis. Acc. Chem. Res. 2013, 46, 1740–1748. [Google Scholar] [CrossRef]
- Li, Z.; Ji, S.; Liu, Y.; Cao, X.; Tian, S.; Chen, Y.; Niu, Z.; Li, Y. Well-defined materials for heterogeneous catalysis: From nanoparticles to isolated single-atom sites. Chem. Rev. 2019, 120, 623–682. [Google Scholar] [CrossRef]
- Zhang, N.; Li, L.; Chu, Y.; Zheng, L.; Sun, S.; Zhang, G.; He, H.; Zhao, J. High Pt utilization efficiency of electrocatalysts for oxygen reduction reaction in alkaline media. Catal. Today 2019, 332, 101–108. [Google Scholar] [CrossRef]
- Abdelghafar, F.; Xu, X.; Jiang, S.P.; Shao, Z. Designing single-atom catalysts toward improved alkaline hydrogen evolution reaction. Mater. Rep. Energy 2022, 2, 100144. [Google Scholar] [CrossRef]
- Mitchell, S.; Vorobyeva, E.; Pérez-Ramírez, J. The Multifaceted Reactivity of Single-Atom Heterogeneous Catalysts. Angew. Chem. Int. Ed. 2018, 57, 15316–15329. [Google Scholar] [CrossRef]
- Hu, P.; Huang, Z.; Amghouz, Z.; Makkee, M.; Xu, F.; Kapteijn, F.; Dikhtiarenko, A.; Chen, Y.; Gu, X.; Tang, X. Electronic Metal–Support Interactions in Single-Atom Catalysts. Angew. Chem. Int. Ed. 2014, 53, 3418–3421. [Google Scholar] [CrossRef]
- Cai, T.; Teng, Z.; Wen, Y.; Zhang, H.; Wang, S.; Fu, X.; Song, L.; Li, M.; Lv, J.; Zeng, Q. Single-atom site catalysts for environmental remediation: Recent advances. J. Hazard. Mater. 2022, 440, 129772. [Google Scholar] [CrossRef]
- Peterson, E.J.; DeLaRiva, A.T.; Lin, S.; Johnson, R.S.; Guo, H.; Miller, J.T.; Hun Kwak, J.; Peden, C.H.; Kiefer, B.; Allard, L.F. Low-temperature carbon monoxide oxidation catalysed by regenerable atomically dispersed palladium on alumina. Nat. Commun. 2014, 5, 4885. [Google Scholar] [CrossRef]
- Jones, J.; Xiong, H.; DeLaRiva, A.T.; Peterson, E.J.; Pham, H.; Challa, S.R.; Qi, G.; Oh, S.; Wiebenga, M.H.; Pereira Hernández, X.I. Thermally stable single-atom platinum-on-ceria catalysts via atom trapping. Science 2016, 353, 150–154. [Google Scholar] [CrossRef]
- Yang, J.; Li, W.; Wang, D.; Li, Y. Electronic metal-support interaction of single-atom catalysts and applications in electrocatalysis. Adv. Mater. 2020, 32, 2003300. [Google Scholar] [CrossRef]
- Cai, S.; Zhang, M.; Li, J.; Chen, J.; Jia, H. Anchoring Single-Atom Ru on CdS with Enhanced CO2 Capture and Charge Accumulation for High Selectivity of Photothermocatalytic CO2 Reduction to Solar Fuels. Sol. RRL 2021, 5, 2000313. [Google Scholar] [CrossRef]
- Zhang, Y.; Jiao, L.; Yang, W.; Xie, C.; Jiang, H. Rational Fabrication of Low-Coordinate Single-Atom Ni Electrocatalysts by MOFs for Highly Selective CO2 Reduction. Angew. Chem. Int. Ed. 2021, 60, 7607–7611. [Google Scholar] [CrossRef]
- Ni, W.; Liu, Z.; Zhang, Y.; Ma, C.; Deng, H.; Zhang, S.; Wang, S. Electroreduction of Carbon Dioxide Driven by the Intrinsic Defects in the Carbon Plane of a Single Fe–N4 Site. Adv. Mater. 2021, 33, 2003238. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Zhang, S.; Zhu, Y.; Patlolla, A.; Shan, J.; Yoshida, H.; Takeda, S.; Frenkel, A.I.; Tao, F. Catalysis and in situ studies of Rh1/Co3O4 nanorods in reduction of NO with H2. ACS Catal. 2013, 3, 1011–1019. [Google Scholar] [CrossRef]
- Chen, J.; Jiang, M.; Chen, J.; Xu, W.; Jia, H. Selective immobilization of single-atom Au on cerium dioxide for low-temperature removal of C1 gaseous contaminants. J. Hazard. Mater. 2020, 392, 122511. [Google Scholar] [CrossRef] [PubMed]
- Narula, C.K.; Allard, L.F.; Moses-DeBusk, M.; Stocks, G.M.; Wu, Z. Single Pd atoms on θ-Al2O3 (010) surface do not catalyze NO oxidation. Sci. Rep. 2017, 7, 560. [Google Scholar] [CrossRef]
- Wang, X.; Jin, B.; Jin, Y.; Wu, T.; Ma, L.; Liang, X. Supported Single Fe Atoms Prepared via Atomic Layer Deposition for Catalytic Reactions. ACS Appl. Nano Mater. 2020, 3, 2867–2874. [Google Scholar] [CrossRef]
- Wu, Q.; Wang, J.; Wang, Z.; Xu, Y.; Xing, Z.; Zhang, X.; Guan, Y.; Liao, G.; Li, X. High-loaded single Cu atoms decorated on N-doped graphene for boosting Fenton-like catalysis under neutral pH. J. Mater. Chem. A 2020, 8, 13685–13693. [Google Scholar] [CrossRef]
- Song, H.; Wei, L.; Chen, C.; Wen, C.; Han, F. Photocatalytic production of H2O2 and its in situ utilization over atomic-scale Au modified MoS2 nanosheets. J. Catal. 2019, 376, 198–208. [Google Scholar] [CrossRef]
- An, S.; Zhang, G.; Wang, T.; Zhang, W.; Li, K.; Song, C.; Miller, J.T.; Miao, S.; Wang, J.; Guo, X. High-Density Ultra-small Clusters and Single-Atom Fe Sites Embedded in Graphitic Carbon Nitride (g-C3N4) for Highly Efficient Catalytic Advanced Oxidation Processes. ACS Nano 2018, 12, 9441–9450. [Google Scholar] [CrossRef]
- Huang, D.; de Vera, G.A.; Chu, C.; Zhu, Q.; Stavitski, E.; Mao, J.; Xin, H.; Spies, J.A.; Schmuttenmaer, C.A.; Niu, J.; et al. Single-Atom Pt Catalyst for Effective C–F Bond Activation via Hydrodefluorination. ACS Catal. 2018, 8, 9353–9358. [Google Scholar] [CrossRef]
- Wu, Z.; Karamad, M.; Yong, X.; Huang, Q.; Cullen, D.A.; Zhu, P.; Xia, C.; Xiao, Q.; Shakouri, M.; Chen, F.; et al. Electrochemical ammonia synthesis via nitrate reduction on Fe single atom catalyst. Nat. Commun. 2021, 12, 2870. [Google Scholar] [CrossRef]
- Li, J.; Li, M.; An, N.; Zhang, S.; Song, Q.; Yang, Y.; Liu, X. Atomically dispersed Fe atoms anchored on S and N–codoped carbon for efficient electrochemical denitrification. Proc. Natl. Acad. Sci. USA 2021, 118, e2105628118. [Google Scholar] [CrossRef]
- McDonald, B.C.; de Gouw, J.A.; Gilman, J.B.; Jathar, S.H.; Akherati, A.; Cappa, C.D.; Jimenez, J.L.; Lee-Taylor, J.; Hayes, P.L.; McKeen, S.A.; et al. Volatile chemical products emerging as largest petrochemical source of urban organic emissions. Science 2018, 359, 760–764. [Google Scholar] [CrossRef]
- He, C.; Cheng, J.; Zhang, X.; Douthwaite, M.; Pattisson, S.; Hao, Z.P. Recent Advances in the Catalytic Oxidation of Volatile Organic Compounds: A Review Based on Pollutant Sorts and Sources. Chem. Rev. 2019, 119, 4471–4568. [Google Scholar] [CrossRef]
- He, Z.R.; Wang, X.M.; Ling, Z.H.; Zhao, J.; Guo, H.; Shao, M.; Wang, Z. Contributions of different anthropogenic volatile organic compound sources to ozone formation at a receptor site in the Pearl River Delta region and its policy implications. Atmos. Chem. Phys. 2019, 19, 8801–8816. [Google Scholar] [CrossRef]
- Yue, X.C.; Ma, N.L.; Sonne, C.; Guan, R.R.; Lam, S.S.; Le, Q.V.; Chen, X.M.; Yang, Y.F.; Gu, H.P.; Rinklebe, J.; et al. Mitigation of indoor air pollution: A review of recent advances in adsorption materials and catalytic oxidation. J. Hazard. Mater. 2021, 405, 13. [Google Scholar] [CrossRef]
- Xinhua News Agency. The 14th Five-Year Plan for National Economic and Social Development of the People’s Republic of China and the Outline of the Long-Range Goals for 2035. Available online: http://www.gov.cn/xinwen/2021-03/13/content_5592681.htm (accessed on 1 April 2023).
- Li, J.Q.; Liu, H.; Deng, Y.Z.; Liu, G.; Chen, Y.F.; Yang, J. Emerging nanostructured materials for the catalytic removal of volatile organic compounds. Nanotechnol. Rev. 2016, 5, 147–181. [Google Scholar] [CrossRef]
- Fei, H.L.; Dong, J.C.; Chen, D.L.; Hu, T.D.; Duan, X.D.; Shakir, I.R.; Huang, Y.; Duan, X.F. Single atom electrocatalysts supported on graphene or graphene-like carbons. Chem. Soc. Rev. 2019, 48, 5207–5241. [Google Scholar] [CrossRef]
- Shang, Y.N.; Xu, X.; Gao, B.Y.; Wang, S.B.; Duan, X.G. Single-atom catalysis in advanced oxidation processes for environmental remediation. Chem. Soc. Rev. 2021, 50, 5281–5322. [Google Scholar] [CrossRef]
- Chen, Y.X.; Huang, Z.W.; Zhou, M.J.; Ma, Z.; Chen, J.M.; Tang, X.F. Single Silver Adatoms on Nanostructured Manganese Oxide Surfaces: Boosting Oxygen Activation for Benzene Abatement. Environ. Sci. Technol. 2017, 51, 2304–2311. [Google Scholar] [CrossRef]
- Deng, H.; Kang, S.Y.; Ma, J.Z.; Zhang, C.B.; He, H. Silver incorporated into cryptomelane-type Manganese oxide boosts the catalytic oxidation of benzene. Appl. Catal. B-Environ. 2018, 239, 214–222. [Google Scholar] [CrossRef]
- Chen, J.; Yan, D.X.; Xu, Z.; Chen, X.; Xu, W.J.; Jia, H.P.; Chen, J. A Novel Redox Precipitation to Synthesize Au-Doped alpha-MnO2 with High Dispersion toward Low-Temperature Oxidation of Formaldehyde. Environ. Sci. Technol. 2018, 52, 4728–4737. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.Y.; Sui, S.H.; Zheng, X.M.; Cao, R.R.; Zhang, P.Y. One-pot synthesis of atomically dispersed Pt on MnO2 for efficient catalytic decomposition of toluene at low temperatures. Appl. Catal. B-Environ. 2019, 257, 12. [Google Scholar] [CrossRef]
- Hoang, S.; Guo, Y.B.; Binder, A.J.; Tang, W.X.; Wang, S.B.; Liu, J.Y.; Huan, T.D.; Lu, X.X.; Wang, Y.; Ding, Y.; et al. Activating low-temperature diesel oxidation by single-atom Pt on TiO2 nanowire array. Nat. Commun. 2020, 11, 10. [Google Scholar]
- Chen, J.; Jiang, M.Z.; Xu, W.J.; Chen, J.; Hong, Z.X.; Jia, H.P. Incorporating Mn cation as anchor to atomically disperse Pt on TiO2 for low-temperature removal of formaldehyde. Appl. Catal. B-Environ. 2019, 259, 11. [Google Scholar] [CrossRef]
- Liu, P.X.; Zhao, Y.; Qin, R.X.; Gu, L.; Zhang, P.; Fu, G.; Zheng, N.F. A vicinal effect for promoting catalysis of Pd1/TiO2: Supports of atomically dispersed catalysts play more roles than simply serving as ligands. Sci. Bull. 2018, 63, 675–682. [Google Scholar] [CrossRef]
- Hou, Z.Q.; Dai, L.Y.; Liu, Y.X.; Deng, J.G.; Jing, L.; Pei, W.B.; Gao, R.Y.; Feng, Y.; Dai, H.X. Highly efficient and enhanced sulfur resistance supported bimetallic single-atom palladium-cobalt catalysts for benzene oxidation. Appl. Catal. B-Environ. 2021, 285, 12. [Google Scholar] [CrossRef]
- Liu, G.L.; Zhou, J.H.; Zhao, W.N.; Ao, Z.M.; An, T.C. Single atom catalytic oxidation mechanism of formaldehyde on Al doped graphene at room temperature. Chin. Chem. Lett. 2020, 31, 1966–1969. [Google Scholar] [CrossRef]
- Morin, C.; Simon, D.; Sautet, P. Chemisorption of Benzene on Pt(111), Pd(111), and Rh(111) Metal Surfaces: A Structural and Vibrational Comparison from First Principles. J. Phys. Chem. B 2004, 108, 5653–5665. [Google Scholar] [CrossRef]
- Rose, J.J.; Wang, L.; Xu, Q.Z.; McTiernan, C.F.; Shiva, S.; Tejero, J.; Gladwin, M.T. Carbon Monoxide Poisoning: Pathogenesis, Management, and Future Directions of Therapy. Am. J. Respir. Crit. Care Med. 2017, 195, 596–606. [Google Scholar] [CrossRef]
- Sinthika, S.; Vala, S.T.; Kawazoe, Y.; Thapa, R. CO Oxidation Prefers the Eley-Rideal or Langmuir-Hinshelwood Pathway: Monolayer vs Thin Film of SiC. ACS Appl. Mater. Interfaces 2016, 8, 5290–5299. [Google Scholar] [CrossRef]
- Qiao, B.; Liang, J.; Wang, A.; Liu, J.; Zhang, T. Single atom gold catalysts for low-temperature CO oxidation. Chin. J. Catal. 2016, 37, 1580–1586. [Google Scholar] [CrossRef]
- Wang, W.W.; Du, P.P.; Zou, S.H.; He, H.Y.; Wang, R.X.; Jin, Z.; Shi, S.; Huang, Y.Y.; Si, R.; Song, Q.S.; et al. Highly Dispersed Copper Oxide Clusters as Active Species in Copper-Ceria Catalyst for Preferential Oxidation of Carbon Monoxide. ACS Catal. 2015, 5, 2088–2099. [Google Scholar] [CrossRef]
- Esrafili, M.D.; Mousavian, P. A DFT study on the possibility of using a single Cu atom incorporated nitrogen-doped graphene as a promising and highly active catalyst for oxidation of CO. Int. J. Quantum Chem. 2019, 119, 10. [Google Scholar] [CrossRef]
- Haruta, M.; Kobayashi, T.; Sano, H.; Yamada, N. Novel Gold Catalysts for the Oxidation of Carbon Monoxide at a Temperature far Below 0 °C. Chem. Lett. 1987, 16, 405–408. [Google Scholar] [CrossRef]
- Widmann, D.; Behm, R.J. Activation of Molecular Oxygen and the Nature of the Active Oxygen Species for CO Oxidation on Oxide Supported Au Catalysts. Acc. Chem. Res. 2014, 47, 740–749. [Google Scholar] [CrossRef]
- Fujitani, T.; Nakamura, I. Mechanism and Active Sites of the Oxidation of CO over Au/TiO2. Angew. Chem. Int. Ed. 2011, 50, 10144–10147. [Google Scholar] [CrossRef]
- Schubert, M.M.; Hackenberg, S.; van Veen, A.C.; Muhler, M.; Plzak, V.; Behm, R.J. CO oxidation over supported gold catalysts-”inert” and “active” support materials and their role for the oxygen supply during reaction. J. Catal. 2001, 197, 113–122. [Google Scholar] [CrossRef]
- Muravev, V.; Spezzati, G.; Su, Y.Q.; Parastaev, A.; Chiang, F.K.; Longo, A.; Escudero, C.; Kosinov, N.; Hensen, E.J.M. Interface dynamics of Pd-CeO2 single-atom catalysts during CO oxidation. Nat. Catal. 2021, 4, 469–478. [Google Scholar] [CrossRef]
- He, B.L.; Shen, J.S.; Tian, Z.X. Iron-embedded C2N monolayer: A promising low-cost and high-activity single-atom catalyst for CO oxidation. Phys. Chem. Chem. Phys. 2016, 18, 24261–24269. [Google Scholar] [CrossRef]
- Liu, S.G.; Huang, S.P. Atomically dispersed Co atoms on MoS2 monolayer: A promising high-activity catalyst for CO oxidation. Appl. Surf. Sci. 2017, 425, 478–483. [Google Scholar] [CrossRef]
- Liang, J.X.; Yang, X.F.; Wang, A.Q.; Zhang, T.; Li, J. Theoretical investigations of non-noble metal single-atom catalysis: Ni1/FeOx for CO oxidation. Catal. Sci. Technol. 2016, 6, 6886–6892. [Google Scholar] [CrossRef]
- Tang, Y.N.; Chen, W.G.; Shen, Z.G.; Chang, S.S.; Zhao, M.Y.; Dai, X.Q. Nitrogen coordinated silicon-doped graphene as a potential alternative metal-free catalyst for CO oxidation. Carbon 2017, 111, 448–458. [Google Scholar] [CrossRef]
- Liu, B.P.; Lee, J.Y.; Yan, S.H. Enhanced Catalytic Oxidation of CO on Sulfur-Doped Boron Nitride. ChemNanoMat 2020, 6, 9. [Google Scholar] [CrossRef]
- Qiao, B.T.; Liang, J.X.; Wang, A.Q.; Xu, C.Q.; Li, J.; Zhang, T.; Liu, J.Y. Ultrastable single-atom gold catalysts with strong covalent metal-support interaction (CMSI). Nano Res. 2015, 8, 2913–2924. [Google Scholar] [CrossRef]
- Haruta, M.; Yamada, N.; Kobayashi, T.; Iijima, S. Gold catalysts prepared by coprecipitation for low-temperature oxidation of hydrogen and of carbon monoxide. J. Catal. 1989, 115, 301–309. [Google Scholar] [CrossRef]
- Shi, J.L.; Zhao, X.J.; Zhang, L.Y.; Xue, X.L.; Guo, Z.X.; Gao, Y.F.; Li, S.F. An oxidized magnetic Au single atom on doped TiO2 (110) becomes a high performance CO oxidation catalyst due to the charge effect. J. Mater. Chem. A 2017, 5, 19316–19322. [Google Scholar] [CrossRef]
- Zou, X.P.; Wang, L.N.; Li, X.N.; Liu, Q.Y.; Zhao, Y.X.; Ma, T.M.; He, S.G. Noble-Metal-Free Single-Atom Catalysts CuAl4O7-9- for CO Oxidation by O2. Angew. Chem. Int. Ed. 2018, 57, 10989–10993. [Google Scholar] [CrossRef]
- Wen, H.; Huai, L.; Jin, X.; Liu, J. Mechanism of nitric oxide reduction by hydrogen on Ni (110) and Ir/Ni (110): First principles and microkinetic modeling. J. Phys. Chem. C 2019, 123, 4825–4836. [Google Scholar] [CrossRef]
- Xing, F.; Jeon, J.; Toyao, T.; Shimizu, K.-I.; Furukawa, S. A Cu–Pd single-atom alloy catalyst for highly efficient NO reduction. Chem. Sci. 2019, 10, 8292–8298. [Google Scholar] [CrossRef]
- Qu, W.; Liu, X.; Chen, J.; Dong, Y.; Tang, X.; Chen, Y. Single-atom catalysts reveal the dinuclear characteristic of active sites in NO selective reduction with NH3. Nat. Commun. 2020, 11, 1532. [Google Scholar] [CrossRef]
- Qu, W.; Yuan, H.; Ren, Z.; Qi, J.; Xu, D.; Chen, J.; Chen, L.; Yang, H.; Ma, Z.; Liu, X.; et al. An Atom-Pair Design Strategy for Optimizing the Synergistic Electron Effects of Catalytic Sites in NO Selective Reduction. Angew. Chem. Int. Ed. 2022, 61, e202212703. [Google Scholar] [CrossRef]
- Fang, X.; Qu, W.; Qin, T.; Hu, X.; Chen, L.; Ma, Z.; Liu, X.; Tang, X. Abatement of Nitrogen Oxides via Selective Catalytic Reduction over Ce1–W1 Atom-Pair Sites. Environ. Sci. Technol. 2022, 56, 6631–6638. [Google Scholar] [CrossRef]
- Ji, Y.; Liu, S.; Zhu, H.; Xu, W.; Jiang, R.; Zhang, Y.; Yu, J.; Chen, W.; Jia, L.; Jiang, J.; et al. Isolating Contiguous Ir Atoms and Forming Ir–W Intermetallics with Negatively Charged Ir for Efficient NO Reduction by CO. Adv. Mater. 2022, 34, 2205703. [Google Scholar] [CrossRef]
- Ji, Y.; Liu, S.; Song, S.; Xu, W.; Li, L.; Zhang, Y.; Chen, W.; Li, H.; Jiang, J.; Zhu, T.; et al. Negatively Charged Single-Atom Pt Catalyst Shows Superior SO2 Tolerance in NOx Reduction by CO. ACS Catal. 2023, 13, 224–236. [Google Scholar] [CrossRef]
- Zhou, X.; Han, K.; Li, K.; Pan, J.; Wang, X.; Shi, W.; Song, S.; Zhang, H. Dual-Site Single-Atom Catalysts with High Performance for Three-Way Catalysis. Adv. Mater. 2022, 34, 2201859. [Google Scholar] [CrossRef]
- Liu, X.; Zhou, T.; Liu, Y.; Zhang, X.; Li, L.; Pan, G. Effect of mid-season drainage on CH4 and N2O emission and grain yield in rice ecosystem: A meta-analysis. Agric. Water Manag. 2019, 213, 1028–1035. [Google Scholar] [CrossRef]
- Xie, S.; Kim, D.; Ye, K.; Tetard, L.; Liu, F. Regulating local coordination environment of rhodium single atoms in Rh/CeO2 catalysts for N2O decomposition. J. Rare Earths 2023. [Google Scholar] [CrossRef]
- Liu, H.; Chen, J.; Wang, Y.; Xiong, S.; Su, Z.; Wang, Y.; Yang, W.; Chu, X.; Yang, W.; Peng, Y. Boosting nitrous oxide direct decomposition performance based on samarium doping effects. Chem. Eng. J. 2021, 414, 128643. [Google Scholar] [CrossRef]
- Liu, H.; Yang, S.; Wang, G.; Liu, H.; Peng, Y.; Sun, C.; Li, J.; Chen, J. Strong Electronic Orbit Coupling between Cobalt and Single-Atom Praseodymium for Boosted Nitrous Oxide Decomposition on Co3O4 Catalyst. Environ. Sci. Technol. 2022, 56, 16325–16335. [Google Scholar] [CrossRef]
- Li, F.; Tang, Q. The critical role of hydride (H−) ligands in electrocatalytic CO2 reduction to HCOOH by [Cu25H22(PH3)12]Cl nanocluster. J. Catal. 2020, 387, 95–101. [Google Scholar] [CrossRef]
- Li, F.; Thevenon, A.; Rosas-Hernández, A.; Wang, Z.; Li, Y.; Gabardo, C.M.; Ozden, A.; Dinh, C.T.; Li, J.; Wang, Y. Molecular tuning of CO2-to-ethylene conversion. Nature 2020, 577, 509–513. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Reddu, V.; Fisher, A.C.; Wang, X. Electrocatalytic reduction of carbon dioxide: Opportunities with heterogeneous molecular catalysts. Energy Environ. Sci. 2020, 13, 374–403. [Google Scholar] [CrossRef]
- Dong, W.J.; Yoo, C.J.; Lee, J.L. Monolithic nanoporous In–Sn alloy for electrochemical reduction of carbon dioxide. ACS Appl. Mater. Interfaces 2017, 9, 43575–43582. [Google Scholar] [CrossRef] [PubMed]
- Low, Q.H.; Loo, N.W.X.; Calle-Vallejo, F.; Yeo, B.S. Enhanced electroreduction of carbon dioxide to methanol using zinc dendrites pulse-deposited on silver foam. Angew. Chem. Int. Ed. 2019, 58, 2256–2260. [Google Scholar] [CrossRef] [PubMed]
- Ting, L.R.L.; Pique, O.; Lim, S.Y.; Tanhaei, M.; Calle-Vallejo, F.; Yeo, B.S. Enhancing CO2 electroreduction to ethanol on copper–silver composites by opening an alternative catalytic pathway. ACS Catal. 2020, 10, 4059–4069. [Google Scholar] [CrossRef]
- Chen, C.S.; Wan, J.H.; Yeo, B.S. Electrochemical reduction of carbon dioxide to ethane using nanostructured Cu2O-derived copper catalyst and palladium (II) chloride. J. Phys. Chem. C 2015, 119, 26875–26882. [Google Scholar] [CrossRef]
- Li, T.; Wei, H.; Liu, T.; Zheng, G.; Liu, S.; Luo, J.-L. Achieving efficient CO2 electrochemical reduction on tunable in (OH)3-coupled Cu2O-derived hybrid catalysts. ACS Appl. Mater. Interfaces 2019, 11, 22346–22351. [Google Scholar] [CrossRef]
- Zakaria, S.N.A.; Hollingsworth, N.; Islam, H.U.; Roffey, A.; Santos-Carballal, D.; Roldan, A.; Bras, W.; Sankar, G.; Hogarth, G.; Holt, K.B. Insight into the nature of iron sulfide surfaces during the electrochemical hydrogen evolution and CO2 reduction reactions. ACS Appl. Mater. Interfaces 2018, 10, 32078–32085. [Google Scholar] [CrossRef]
- Cui, Q.; Qin, G.; Wang, W.; Geethalakshmi, K.R.; Du, A.; Sun, Q. Novel two-dimensional MOF as a promising single-atom electrocatalyst for CO2 reduction: A theoretical study. Appl. Surf. Sci. 2020, 500, 143993. [Google Scholar] [CrossRef]
- Wang, S.; Kou, T.; Varley, J.B.; Akhade, S.A.; Weitzner, S.E.; Baker, S.E.; Duoss, E.B.; Li, Y. Cu2O/CuS nanocomposites show excellent selectivity and stability for formate generation via electrochemical reduction of carbon dioxide. ACS Mater. Lett. 2020, 3, 100–109. [Google Scholar] [CrossRef]
- Xu, C.; Vasileff, A.; Zheng, Y.; Qiao, S. Recent progress of 3d transition metal single-atom catalysts for electrochemical CO2 reduction. Adv. Mater. Interfaces 2021, 8, 2001904. [Google Scholar] [CrossRef]
- Zhang, T.; Han, X.; Yang, H.; Han, A.; Hu, E.; Li, Y.; Yang, X.; Wang, L.; Liu, J.; Liu, B. Atomically Dispersed Nickel(I) on an Alloy-Encapsulated Nitrogen-Doped Carbon Nanotube Array for High-Performance Electrochemical CO2 Reduction Reaction. Angew. Chem. Int. Ed. 2020, 59, 12055–12061. [Google Scholar] [CrossRef]
- Lyu, H.; Ma, C.; Zhao, J.; Shen, B.; Tang, J. A novel one-step calcination tailored single-atom iron and nitrogen co-doped carbon material catalyst for the selective reduction of CO2 to CO. Sep. Purif. Technol. 2022, 303, 122221. [Google Scholar] [CrossRef]
- Sun, D.; Xu, X.; Qin, Y.; Jiang, S.P.; Shao, Z. Rational Design of Ag-Based Catalysts for the Electrochemical CO2 Reduction to CO: A Review. ChemSusChem 2020, 13, 39–58. [Google Scholar] [CrossRef]
- Zhang, N.; Zhang, X.; Tao, L.; Jiang, P.; Ye, C.; Lin, R.; Huang, Z.; Li, A.; Pang, D.; Yan, H. Silver single-atom catalyst for efficient electrochemical CO2 reduction synthesized from thermal transformation and surface reconstruction. Angew. Chem. Int. Ed. 2021, 60, 6170–6176. [Google Scholar] [CrossRef]
- Yin, Y.; Shi, L.; Li, W.; Li, X.; Wu, H.; Ao, Z.; Tian, W.; Liu, S.; Wang, S.; Sun, H. Boosting Fenton-Like Reactions via Single Atom Fe Catalysis. Environ. Sci. Technol. 2019, 53, 11391–11400. [Google Scholar] [CrossRef]
- Ma, J.; Yang, Q.; Wen, Y.; Liu, W. Fe-g-C3N4/graphitized mesoporous carbon composite as an effective Fenton-like catalyst in a wide pH range. Appl. Catal. B Environ. 2017, 201, 232–240. [Google Scholar] [CrossRef]
- Guo, Z.; Xie, Y.; Xiao, J.; Zhao, Z.; Wang, Y.; Xu, Z.; Zhang, Y.; Yin, L.; Cao, H.; Gong, J. Single-Atom Mn–N4 Site-Catalyzed Peroxone Reaction for the Efficient Production of Hydroxyl Radicals in an Acidic Solution. J. Am. Chem. Soc. 2019, 141, 12005–12010. [Google Scholar] [CrossRef]
- Yin, Y.; Li, W.; Xu, C.; Shi, L.; Zhang, L.; Ao, Z.; Liu, M.; Lu, M.; Duan, X.; Wang, S. Ultrafine copper nanoclusters and single sites for Fenton-like reactions with high atom utilities. Environ. Sci. Nano 2020, 7, 2595–2606. [Google Scholar] [CrossRef]
- Wu, X.; Kim, J.H. Outlook on Single Atom Catalysts for Persulfate-Based Advanced Oxidation. ACS EST Eng. 2022, 2, 1776–1796. [Google Scholar] [CrossRef]
- Yang, L.; Jiao, Y.; Xu, X.; Pan, Y.; Su, C.; Duan, X.; Sun, H.; Liu, S.; Wang, S.; Shao, Z. Superstructures with Atomic-Level Arranged Perovskite and Oxide Layers for Advanced Oxidation with an Enhanced Non-Free Radical Pathway. ACS Sustain. Chem. Eng. 2022, 10, 1899–1909. [Google Scholar] [CrossRef]
- Li, X.; Huang, X.; Xi, S.; Miao, S.; Ding, J.; Cai, W.; Liu, S.; Yang, X.; Yang, H.; Gao, J.; et al. Single Cobalt Atoms Anchored on Porous N-Doped Graphene with Dual Reaction Sites for Efficient Fenton-like Catalysis. J. Am. Chem. Soc. 2018, 140, 12469–12475. [Google Scholar] [CrossRef] [PubMed]
- Chu, C.; Yang, J.; Zhou, X.; Huang, D.; Qi, H.; Weon, S.; Li, J.; Elimelech, M.; Wang, A.; Kim, J.H. Cobalt single atoms on tetrapyridomacrocyclic support for efficient peroxymonosulfate activation. Environ. Sci. Technol. 2020, 55, 1242–1250. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Rigby, K.; Huang, D.; Hedtke, T.; Wang, X.; Chung, M.W.; Weon, S.; Stavitski, E.; Kim, J.H. Single-Atom Cobalt Incorporated in a 2D Graphene Oxide Membrane for Catalytic Pollutant Degradation. Environ. Sci. Technol. 2022, 56, 1341–1351. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.; Zhang, M.; Zou, R.; Xu, Q. Metal–Organic Framework-Based Catalysts with Single Metal Sites. Chem. Rev. 2020, 120, 12089–12174. [Google Scholar] [CrossRef]
- Wang, J.; Wang, S. Activation of persulfate (PS) and peroxymonosulfate (PMS) and application for the degradation of emerging contaminants. Chem. Eng. J. 2018, 334, 1502–1517. [Google Scholar] [CrossRef]
- Liu, J.; He, H.; Shen, Z.; Wang, H.H.; Li, W. Photoassisted highly efficient activation of persulfate over a single-atom Cu catalyst for tetracycline degradation: Process and mechanism. J. Hazard. Mater. 2022, 429, 128398. [Google Scholar] [CrossRef]
- Weon, S.; Huang, D.; Rigby, K.; Chu, C.; Wu, X.; Kim, J.H. Environmental Materials beyond and below the Nanoscale: Single-Atom Catalysts. ACS EST Eng. 2021, 1, 157–172. [Google Scholar] [CrossRef]
- Chaplin, B.P.; Reinhard, M.; Schneider, W.F.; Schüth, C.; Shapley, J.R.; Strathmann, T.J.; Werth, C.J. Critical Review of Pd-Based Catalytic Treatment of Priority Contaminants in Water. Environ. Sci. Technol. 2012, 46, 3655–3670. [Google Scholar] [CrossRef]
- Baumgartner, R.; Stieger, G.K.; McNeill, K. Complete Hydrodehalogenation of Polyfluorinated and Other Polyhalogenated Benzenes under Mild Catalytic Conditions. Environ. Sci. Technol. 2013, 47, 6545–6553. [Google Scholar] [CrossRef]
- Farwell, S.O.; Beland, F.A.; Geer, R.D. Reduction pathways of organohalogen compounds: Part I. Chlorinated benzenes. J. Electroanal. Chem. Interfacial Electrochem. 1975, 61, 303–313. [Google Scholar] [CrossRef]
- Li, N.; Song, X.; Wang, L.; Geng, X.; Wang, H.; Tang, H.; Bian, Z. Single-Atom Cobalt Catalysts for Electrocatalytic Hydrodechlorination and Oxygen Reduction Reaction for the Degradation of Chlorinated Organic Compounds. ACS Appl. Mater. Interfaces 2020, 12, 24019–24029. [Google Scholar] [CrossRef]
- Huang, D.; Kim, D.J.; Rigby, K.; Zhou, X.; Wu, X.; Meese, A.; Niu, J.; Stavitski, E.; Kim, J.H. Elucidating the Role of Single-Atom Pd for Electrocatalytic Hydrodechlorination. Environ. Sci. Technol. 2021, 55, 13306–13316. [Google Scholar] [CrossRef]
- Chu, C.; Huang, D.; Gupta, S.; Weon, S.; Niu, J.; Stavitski, E.; Muhich, C.; Kim, J.H. Neighboring Pd single atoms surpass isolated single atoms for selective hydrodehalogenation catalysis. Nat. Commun. 2021, 12, 5179. [Google Scholar] [CrossRef]
- Zhao, K.; Quan, X.; Su, Y.; Qin, X.; Chen, S.; Yu, H. Enhanced Chlorinated Pollutant Degradation by the Synergistic Effect between Dechlorination and Hydroxyl Radical Oxidation on a Bimetallic Single-Atom Catalyst. Environ. Sci. Technol. 2021, 55, 14194–14203. [Google Scholar] [CrossRef]
- Weon, S.; Suh, M.-J.; Chu, C.; Huang, D.; Stavitski, E.; Kim, J.H. Site-Selective Loading of Single-Atom Pt on TiO2 for Photocatalytic Oxidation and Reductive Hydrodefluorination. ACS EST Eng. 2021, 1, 512–522. [Google Scholar] [CrossRef]
- Wu, W.; Cui, E.; Zhang, Y.; Zhang, C.; Zhu, F.; Tung, C.; Wang, Y. Involving Single-Atom Silver(0) in Selective Dehalogenation by AgF under Visible-Light Irradiation. ACS Catal. 2019, 9, 6335–6341. [Google Scholar] [CrossRef]
- Li, P.; Jin, Z.; Fang, Z.; Yu, G. A single-site iron catalyst with preoccupied active centers that achieves selective ammonia electrosynthesis from nitrate. Energy Environ. Sci. 2021, 14, 3522–3531. [Google Scholar] [CrossRef]
- Zhu, T.; Chen, Q.; Liao, P.; Duan, W.; Liang, S.; Yan, Z.; Feng, C. Single-Atom Cu Catalysts for Enhanced Electrocatalytic Nitrate Reduction with Significant Alleviation of Nitrite Production. Small 2020, 16, 2004526. [Google Scholar] [CrossRef]
- Xue, Y.; Yu, Q.; Ma, Q.; Chen, Y.; Zhang, C.; Teng, W.; Fan, J.; Zhang, W. Electrocatalytic Hydrogenation Boosts Reduction of Nitrate to Ammonia over Single-Atom Cu with Cu(I)-N3C1 Sites. Environ. Sci. Technol. 2022, 56, 14797–14807. [Google Scholar] [CrossRef]
- Ke, Z.; He, D.; Yan, X.; Hu, W.; Williams, N.; Kang, H.; Pan, X.; Huang, J.; Gu, J.; Xiao, X. Selective NOX− Electroreduction to Ammonia on Isolated Ru Sites. ACS Nano 2023, 17, 3483–3491. [Google Scholar] [CrossRef] [PubMed]
- Tran, N.Q.; Duy, L.T.; Truong, D.C.; Nguyen Le, B.T.; Phan, B.T.; Cho, Y.; Liu, X.; Lee, H. Efficient ammonia synthesis via electroreduction of nitrite using single-atom Ru-doped Cu nanowire arrays. Chem. Commun. 2022, 58, 5257–5260. [Google Scholar] [CrossRef] [PubMed]
- Kamai, R.; Nakanishi, S.; Hashimoto, K.; Kamiya, K. Selective electrochemical reduction of nitrogen oxides by covalent triazine frameworks modified with single Pt atoms. J. Electroanal. Chem. 2017, 800, 54–59. [Google Scholar] [CrossRef]
- García-Vargas, C.E.; Collinge, G.; Yun, D.; Lee, M.-S.; Muravev, V.; Su, Y.; Pereira-Hernández, X.I.; Jiang, D.; Glezakou, V.-A.; Hensen, E.J.M.; et al. Activation of Lattice and Adatom Oxygen by Highly Stable Ceria-Supported Cu Single Atoms. ACS Catal. 2022, 12, 13649–13662. [Google Scholar] [CrossRef]
- Xia, C.; Qiu, Y.; Xia, Y.; Zhu, P.; King, G.; Zhang, X.; Wu, Z.; Kim, J.Y.; Cullen, D.A.; Zheng, D.; et al. General synthesis of single-atom catalysts with high metal loading using graphene quantum dots. Nat. Chem. 2021, 13, 887–894. [Google Scholar] [CrossRef]
- Lu, Y.; Wang, J.; Yu, L.; Kovarik, L.; Zhang, X.; Hoffman, A.S.; Gallo, A.; Bare, S.R.; Sokaras, D.; Kroll, T.; et al. Identification of the active complex for CO oxidation over single-atom Ir-on-MgAl2O4 catalysts. Nat. Catal. 2019, 2, 149–156. [Google Scholar] [CrossRef]
- Jiang, D.; Yao, Y.; Li, T.; Wan, G.; Pereira-Hernández, X.I.; Lu, Y.; Tian, J.; Khivantsev, K.; Engelhard, M.H.; Sun, C.; et al. Tailoring the Local Environment of Platinum in Single-Atom Pt1/CeO2 Catalysts for Robust Low-Temperature CO Oxidation. Angew. Chem. Int. Ed. 2021, 60, 26054–26062. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).