Nb2O5 Coating to Improve the Cyclic Stability and Voltage Decay of Li-Rich Cathode Material for Lithium-Ion Battery
Abstract
:1. Introduction
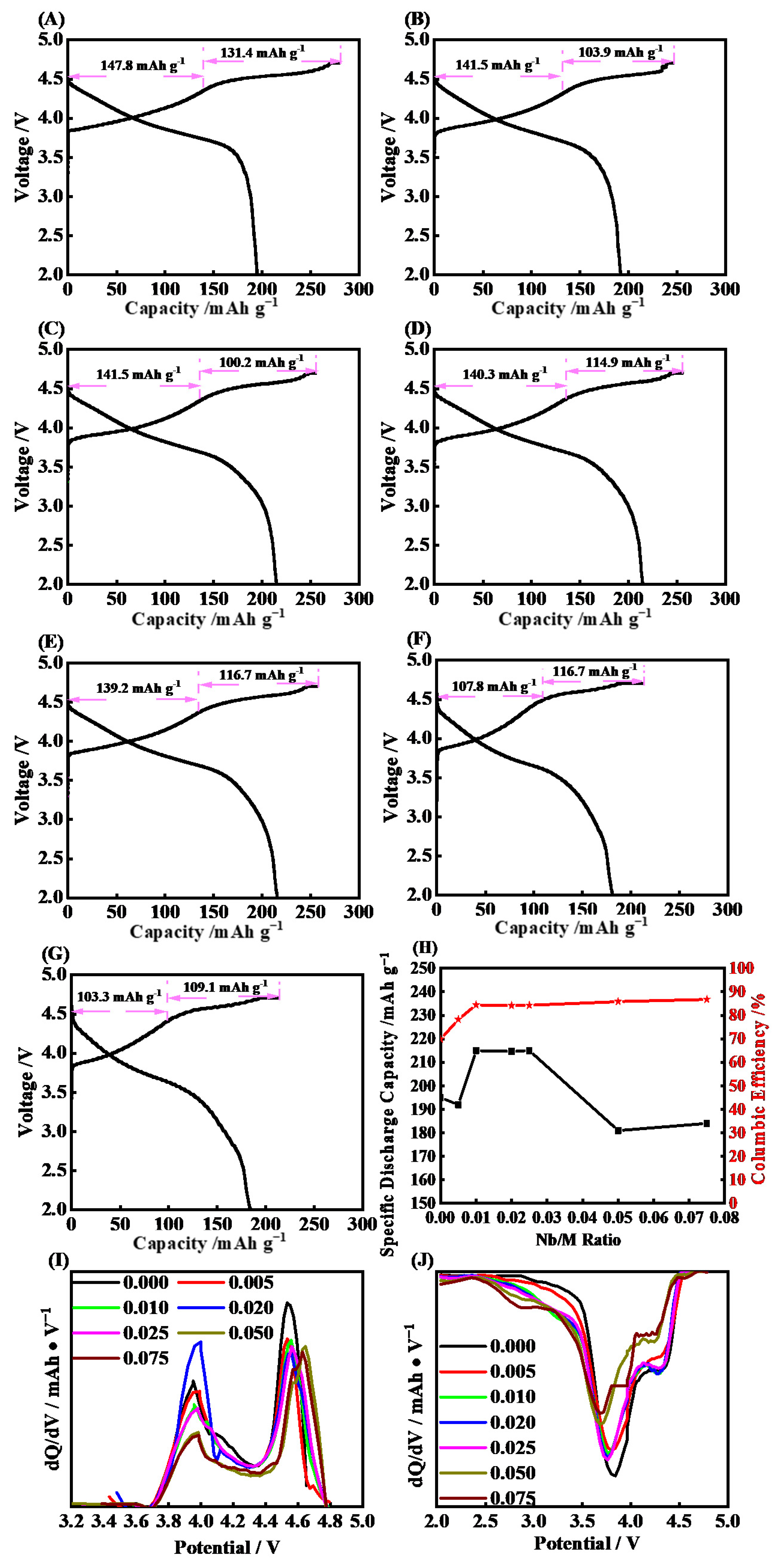
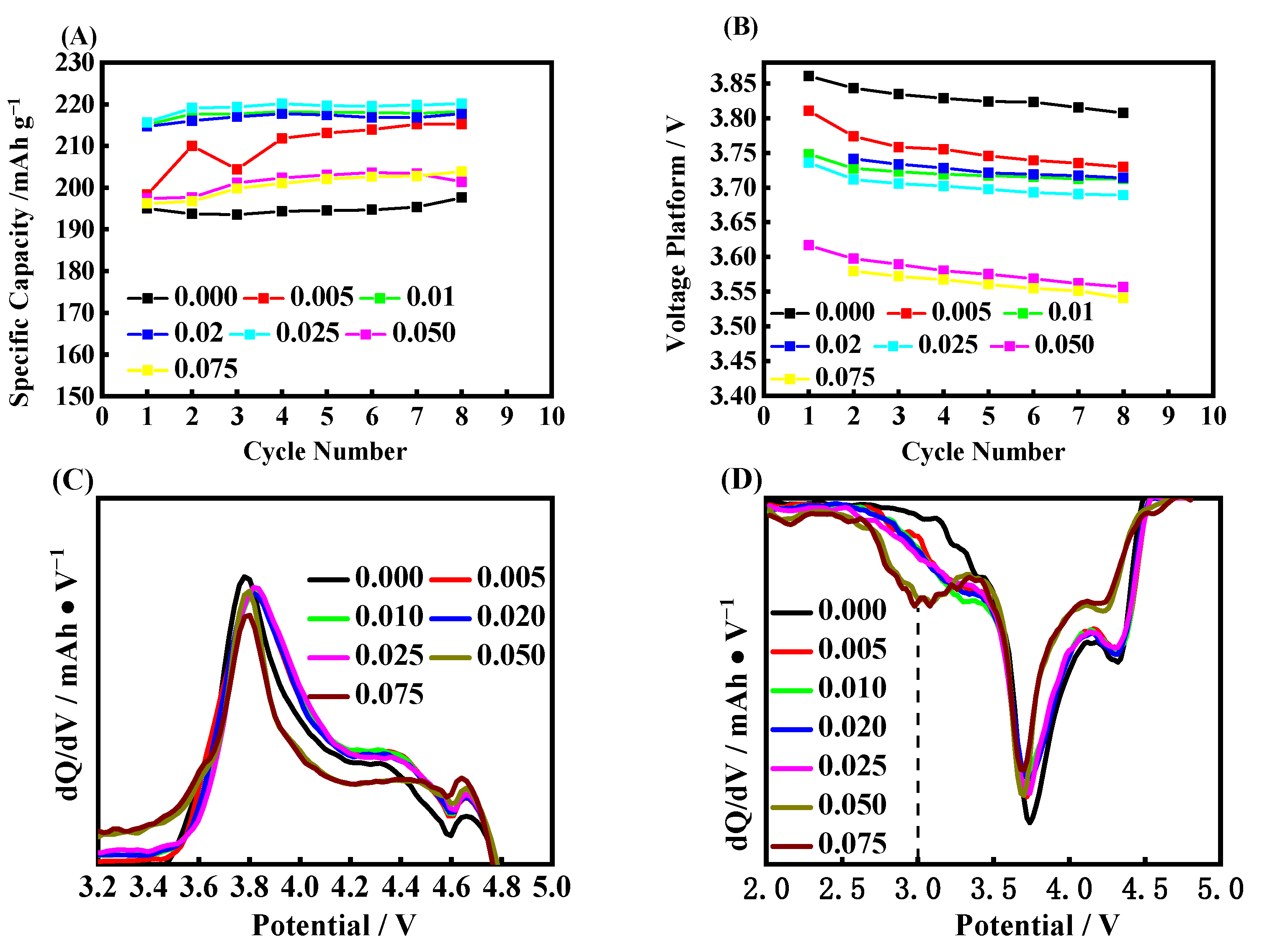
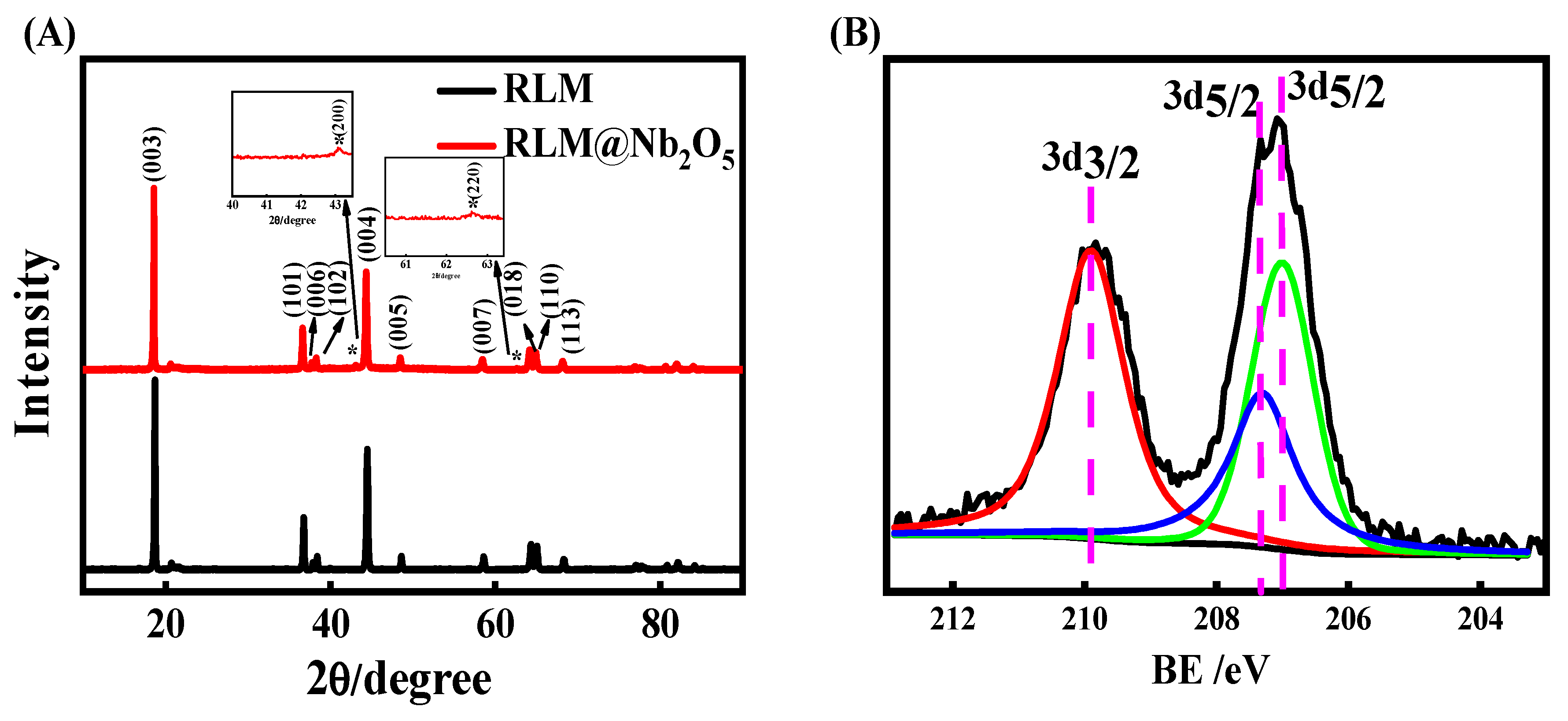
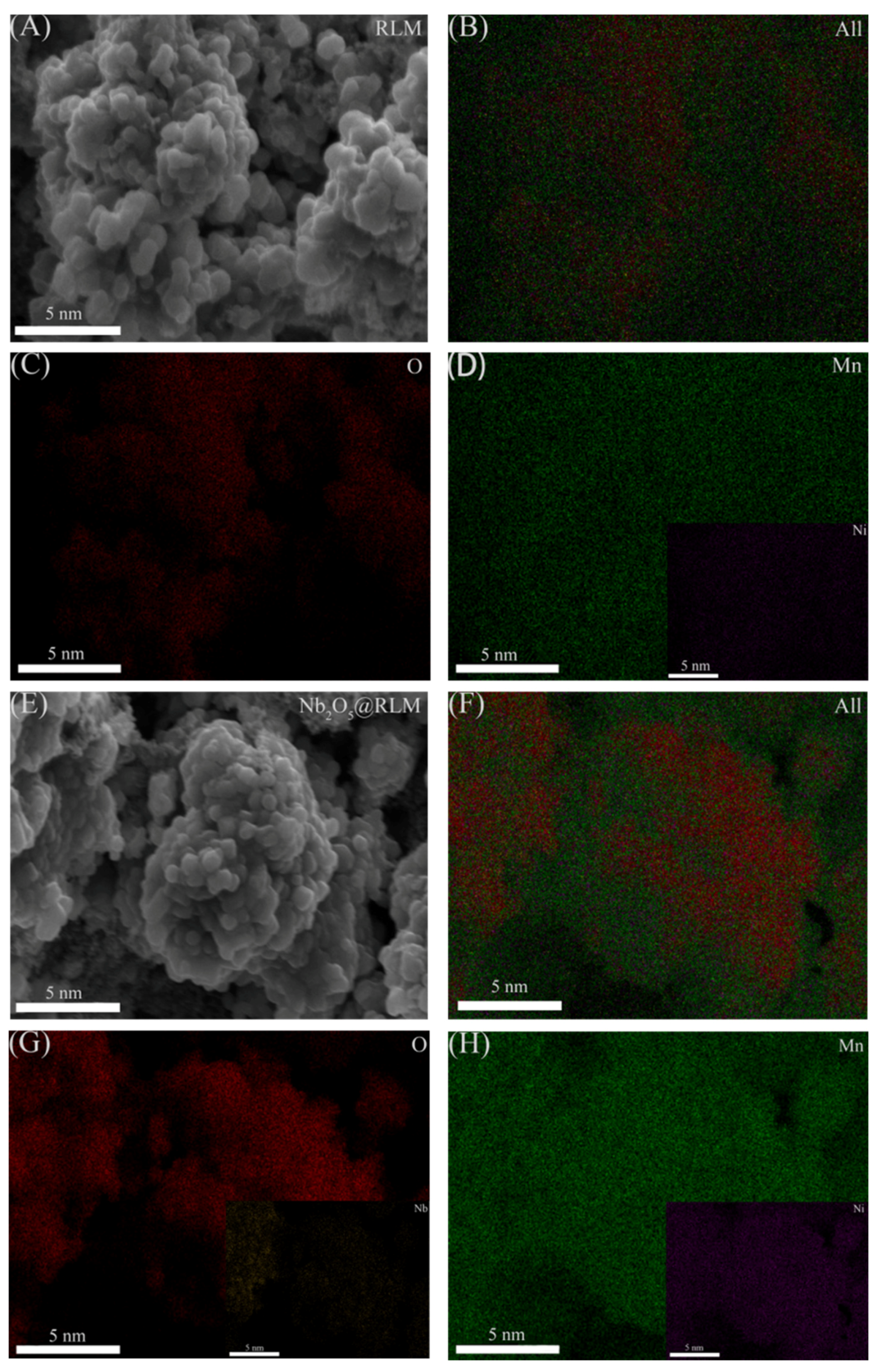
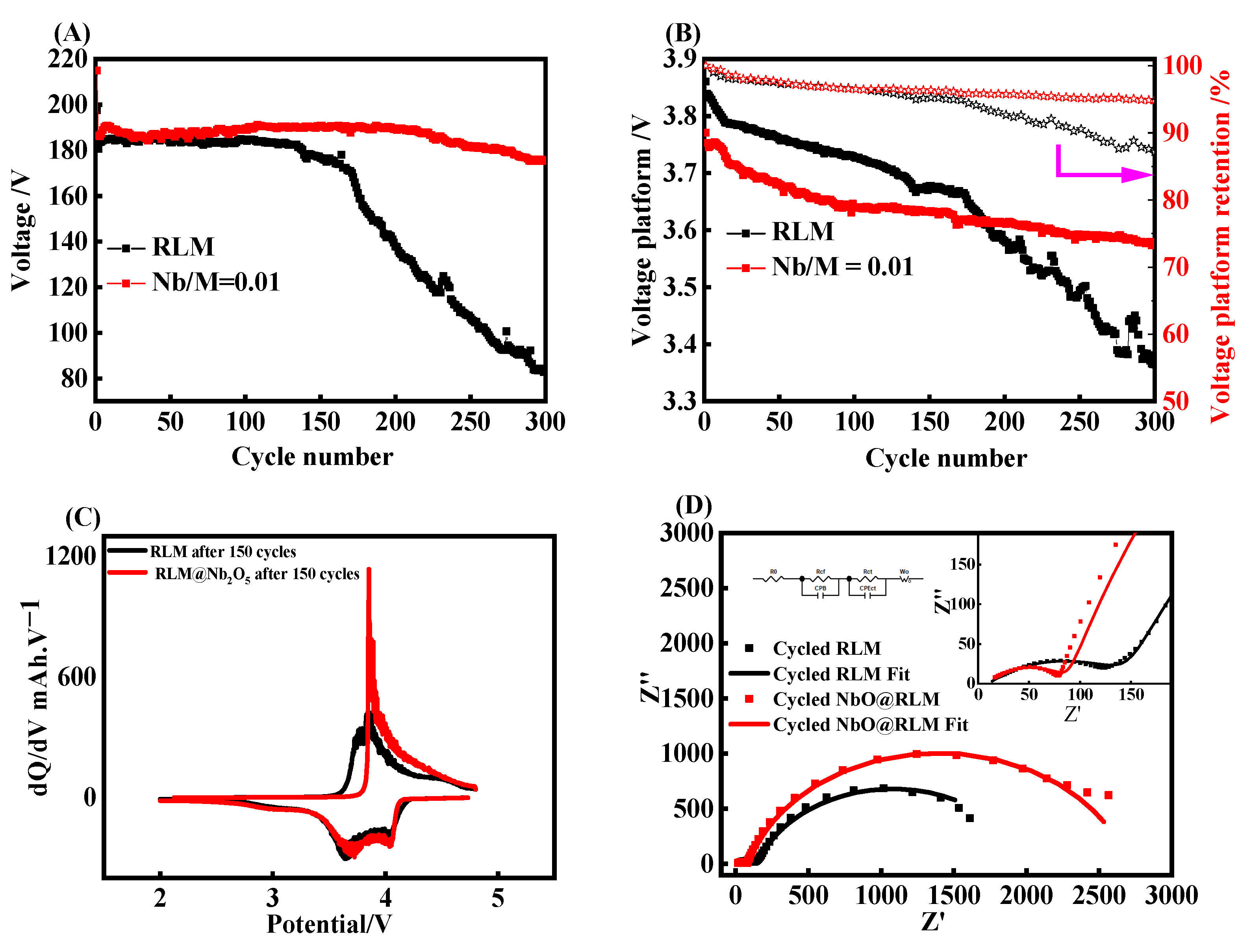
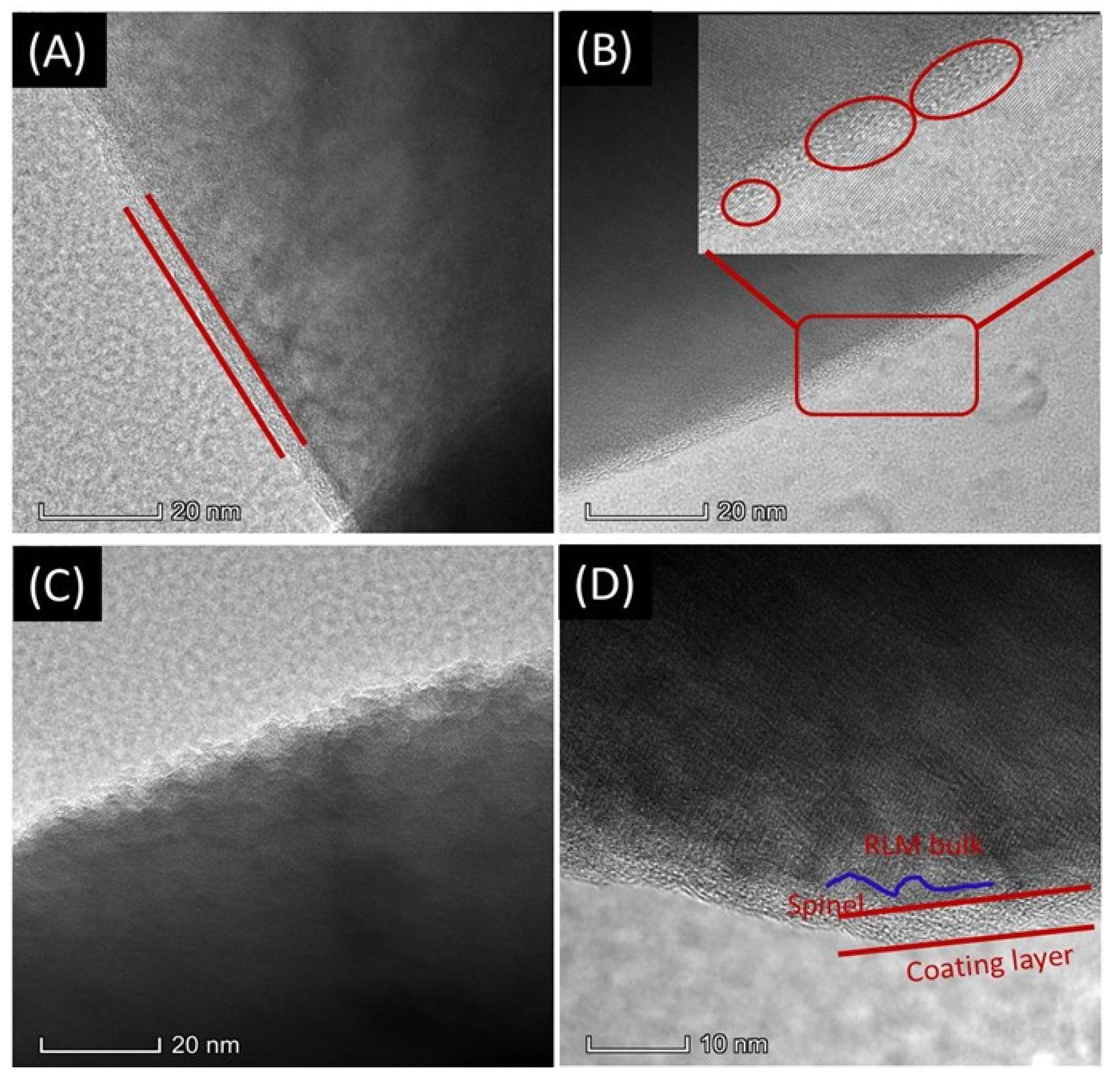
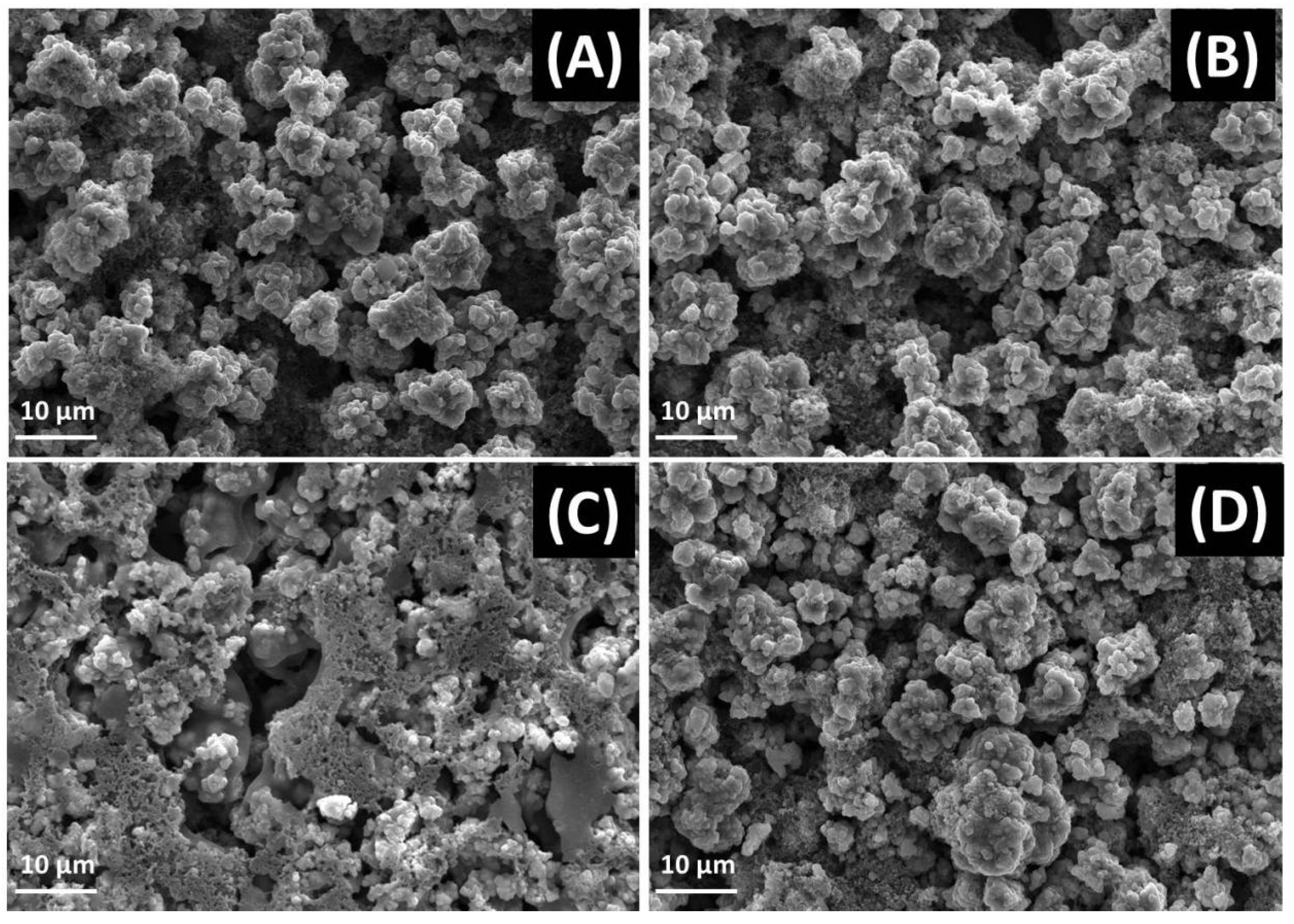
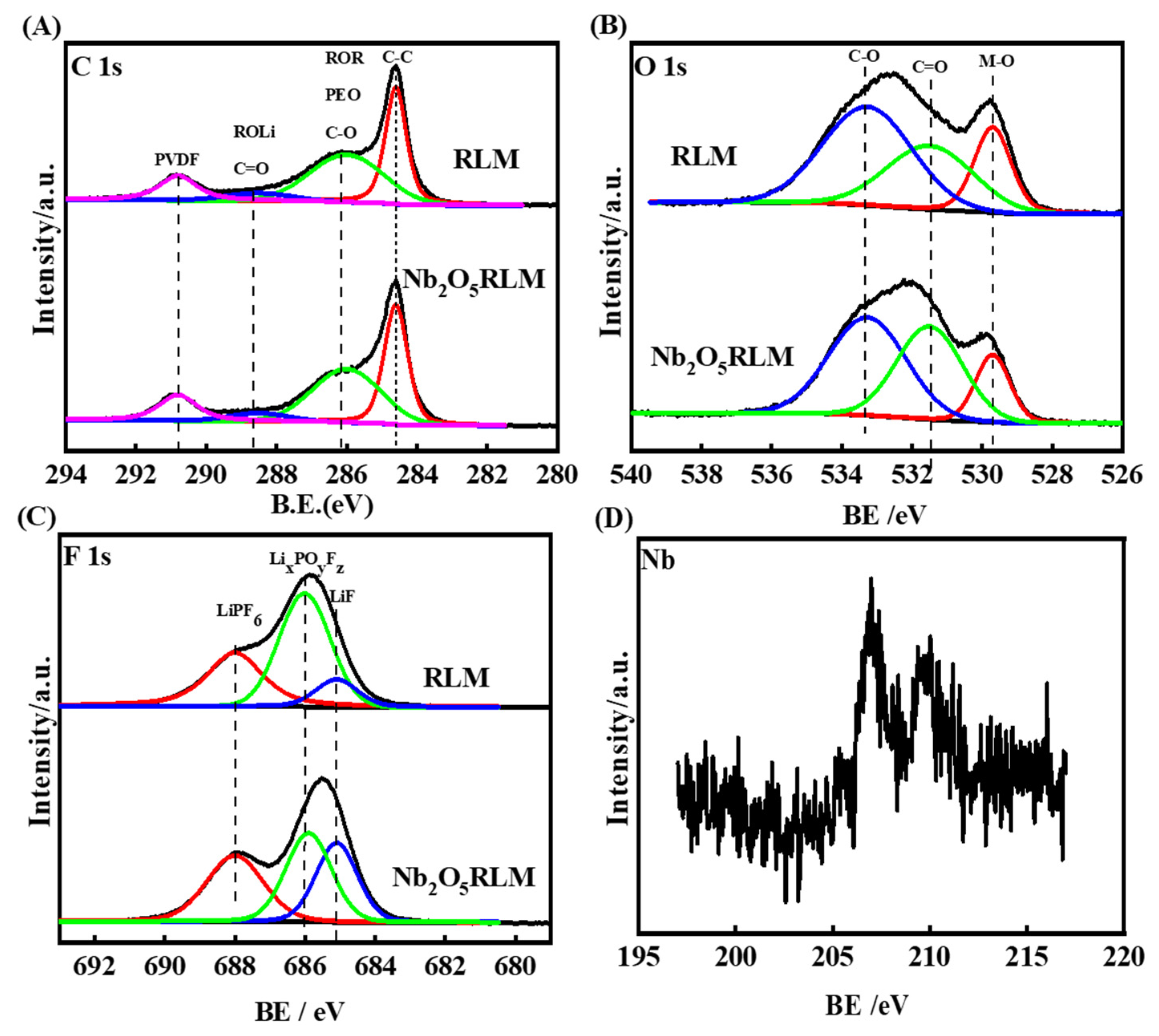
2. Results and Discussion
3. Experiment Section
3.1. Preparation of Nb2O5 Coated Samples
3.2. Physical Characterizations
3.3. Electrochemical Measurements
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Hu, X.S.; Zhang, K.; Liu, K.L.; Lin, X.K.; Dey, S.; Onori, S. Advanced Fault Diagnosis for Lithium-Ion Battery Systems: A Review of Fault Mechanisms, Fault Features, and Diagnosis Procedures. IEEE Ind. Electron. Mag. 2020, 14, 65–91. [Google Scholar] [CrossRef]
- Hu, S.J.; Pillai, A.S.; Liang, G.M.; Pang, W.K.; Wang, H.Q.; Li, Q.Y.; Guo, Z.P. Li-Rich Layered Oxides and Their Practical Challenges: Recent Progress and Perspectives. Electrochem. Energy Rev. 2019, 2, 277–311. [Google Scholar] [CrossRef]
- Zhao, S.; Yan, K.; Zhang, J.; Sun, B.; Wang, G. Reaction Mechanisms of Layered Lithium-Rich Cathode Materials for High-Energy Lithium-Ion Batteries. Angew. Chem. Int. Ed. 2020, 60, 2208–2220. [Google Scholar] [CrossRef] [PubMed]
- Eum, D.; Kim, B.; Kim, S.J.; Park, H.; Kang, K. Voltage decay and redox asymmetry mitigation by reversible cation migration in lithium-rich layered oxide electrodes. Nat. Mater. 2020, 19, 1–9. [Google Scholar] [CrossRef]
- Cao, X.; Li, H.; Qiao, Y.; Jia, M.; Zhou, H. Structure design enables stable anionic and cationic redox chemistry in a T2-type Li-excess layered oxide cathode. Sci. Bull. 2021, 67, 381–388. [Google Scholar] [CrossRef]
- Li, M.; Liu, T.; Bi, X.; Chen, Z.; Amine, K.; Zhong, C.; Lu, J. Cationic and anionic redox in lithium-ion based batteries. Chem. Soc. Rev. 2020, 49, 1688–1705. [Google Scholar] [CrossRef]
- Mccalla, E.; Abakumov, A.M.; Saubanere, M.; Foix, D.; Berg, E.J.; Rousse, G.; Doublet, M.L.; Gonbeau, D.; Novak, P.; Van Tendeloo, G. Visualization of O-O peroxo-like dimers in high-capacity layered oxides for Li-ion batteries. Science 2015, 350, 1516. [Google Scholar] [CrossRef]
- House, R.A.; Rees, G.J.; Pérez-Osorio, M.; Marie, J.J.; Boivin, E.; Robertson, A.W.; Nag, A.; Garcia-Fernandez, M.; Zhou, K.J.; Bruce, P.G. First-cycle voltage hysteresis in Li-rich 3d cathodes associated with molecular O2 trapped in the bulk. Nat. Energy 2020, 5, 777–785. [Google Scholar] [CrossRef]
- Mt, A.; Rk, A.; Ky, B. High-capacity Li-excess lithium nickel manganese oxide as a Co-free positive electrode material. Mater. Res. Bull. 2020, 137, 111178. [Google Scholar]
- Si, M.; Wang, D.; Zhao, R.; Pan, D.; Zhang, C.; Yu, C.; Lu, X.; Zhao, H.; Bai, Y. Local Electric-Field-Driven Fast Li Diffusion Kinetics at the Piezoelectric LiTaO3 Modified Li-Rich Cathode–Electrolyte Interphase. Adv. Sci. 2020, 7, 1902538. [Google Scholar] [CrossRef]
- Leanza, D.; Mirolo, M.; Vaz, C.; Novák, P.; Elkazzi, M. Surface Degradation and Chemical Electrolyte Oxidation Induced by the Oxygen Released from Layered Oxide Cathodes in LiIon Batteries. Batter. Supercaps 2019, 2, 482–492. [Google Scholar] [CrossRef]
- Yao, L.; Liang, F.; Jin, J.; Chowdari, B.; Wen, Z. Improved electrochemical property of Ni-rich LiNi0.6Co0.2Mn0.2O2 cathode via in-situ ZrO2 coating for high energy density lithium ion batteries. Chem. Eng. J. 2020, 389, 124403. [Google Scholar]
- Chen, Z.; Liu, Y.; Lu, Z.; Hu, R.; Cui, J.; Xu, H.; Ouyang, Y.; Zhang, Y.; Zhu, M. Plasma-assisted coating of nanosized SnO2 on LiNi0.5Co0.2Mn0.3O2 cathodes for enhanced cyclic stability of lithium-ion batteries. J. Alloys Compd. 2019, 803, 71–79. [Google Scholar] [CrossRef]
- Du, Z.L.; Peng, W.J.; Wang, Z.X.; Guo, H.J.; Hu, Q.Y.; Li, X.H. Improving the electrochemical performance of Li-rich Li1.2Ni0.13Co0.13Mn0.54O2 cathode material by LiF coating. Ionics 2018, 24, 3717–3724. [Google Scholar] [CrossRef]
- Liu, Y.J.; Fan, X.J.; Zhang, Z.Q.; Wu, H.H.; Liu, D.M.; Dou, A.C.; Su, M.R.; Zhang, Q.B.; Chu, D.W. Enhanced Electrochemical Performance of Li-Rich Layered Cathode Materials by Combined Cr Doping and LiAlO2 Coating. Acs Sustain. Chem. Eng. 2019, 7, 2225–2235. [Google Scholar]
- Fu, C.C.; Wang, J.Y.; Wang, J.F.; Meng, L.L.; Zhang, W.M.; Li, X.T.; Li, L.P. A LiPF6-electrolyte-solvothermal route for the synthesis of LiF/LixPFyOz-coated Li-rich cathode materials with enhanced cycling stability. J. Mater. Chem. A 2019, 7, 23149–23161. [Google Scholar]
- Xiao, W.; Nie, Y.; Miao, C.; Wang, J.L.; Tan, Y.; Wen, M.Y. Structural design of high-performance Ni-rich LiNi0.83Co0.11Mn0.06O2 cathode materials enhanced by Mg2+ doping and Li3PO4 coating for lithium ion battery. J. Colloid Interface Sci. 2022, 607, 1071–1082. [Google Scholar] [CrossRef]
- Deng, Z.; Wei, Q.; Wan, G.; Zhao, G.; Shi, S.; Mou, P.; Teng, S.; Du, C.; Wang, G. Synergistic effect of nanosheet structure and carbon coating engineering to enhance lithium storage performance of molybdenum niobium oxides. Mater. Today Sustain. 2022, 19, 100176. [Google Scholar] [CrossRef]
- Zhang, X.D.; Shi, J.L.; Liang, J.Y.; Yin, Y.X.; Zhang, J.N.; Yu, X.Q.; Guo, Y.G. Suppressing Surface Lattice Oxygen Release of Li-Rich Cathode Materials via Heterostructured Spinel Li4Mn5O12 Coating. Adv. Mater. 2018, 30, 1801751. [Google Scholar]
- Zhang, W.; Sun, Y.G.; Deng, H.Q.; Ma, J.M.; Zeng, Y.; Zhu, Z.Q.; Lv, Z.S.; Xia, H.R.; Ge, X.; Cao, S.K.; et al. Dielectric Polarization in Inverse Spinel-Structured Mg2TiO4 Coating to Suppress Oxygen Evolution of Li-Rich Cathode Materials. Adv. Mater. 2020, 32, 2000496. [Google Scholar] [CrossRef]
- Xie, H.X.; Cui, J.X.; Yao, Z.; Ding, X.K.; Zhang, Z.H.; Luo, D.; Lin, Z. Revealing the role of spinel phase on Li-rich layered oxides: A review. Chem. Eng. J. 2022, 427, 131978. [Google Scholar] [CrossRef]
- Li, J.L.; Jia, T.K.; Tang, C.J.; Yu, D.S.; Sun, J.; Zhang, W.Z.; Wang, Y.J.; Lee, J.H.; Kim, N.H. Stabilizing voltage and prolonged cycling life of Li-rich Mn-based oxides through spinel “lithium ion pump” heteroepitaxial coating strategy. Scr. Mater. 2021, 204, 114133. [Google Scholar] [CrossRef]
- Young, M.J.; Letourneau, S.; Warburton, R.E.; Dose, W.M.; Johnson, C.; Greeley, J.; Elam, J.W. High-Rate Spinel LiMn2O4 (LMO) Following Carbonate Removal and Formation of Li-Rich Interface by ALD Treatment. J. Phys. Chem. C 2019, 123, 23783–23790. [Google Scholar] [CrossRef]
- Zhu, W.; Tai, Z.G.; Shu, C.Y.; Chong, S.K.; Guo, S.W.; Ji, L.J.; Chen, Y.Z.; Liu, Y.N. The superior electrochemical performance of a Li-rich layered cathode material with Li-rich spinel Li4Mn5O12 and MgF2 double surface modifications. J. Mater. Chem. A 2020, 8, 7991–8001. [Google Scholar] [CrossRef]
- Abood, M.; Salim, E.T.; Saimon, J.A. Optical investingations of Nb2O5 at different temperature for optoelectronic devices. J. Ovonic Res. 2019, 15, 109–115. [Google Scholar]
- Arico, C.; Ouendi, S.; Taberna, P.L.; Roussel, P.; Simon, P.; Lethien, C. Fast Electrochemical Storage Process in Sputtered Nb2O5 Porous Thin Films. Acs Nano 2019, 13, 5826–5832. [Google Scholar] [CrossRef]
- Meng, J.S.; He, Q.; Xu, L.H.; Zhang, X.C.; Liu, F.; Wang, X.P.; Li, Q.; Xu, X.M.; Zhang, G.B.; Niu, C.J.; et al. Identification of Phase Control of Carbon-Confined Nb2O5 Nanoparticles toward High-Performance Lithium Storage. Adv. Energy Mater. 2019, 9, 1802695. [Google Scholar] [CrossRef]
- Yuan, H.; Song, W.B.; Wang, M.; Gu, Y.J.; Chen, Y.B. Lithium-ion conductive coating layer on nickel rich layered oxide cathode material with improved electrochemical properties for Li-ion battery. J. Alloys Compd. 2019, 784, 1311–1322. [Google Scholar] [CrossRef]
- Pan, W.; Peng, W.; Yan, G.; Guo, H.; Wang, Z.; Li, X.; Gui, W.; Wang, J.; Chen, N. Suppressing the Voltage Decay and Enhancing the Electrochemical Performance of Li1.2Mn0.54Co0.13Ni0.13O2 by Multifunctional Nb2O5 Coating. Energy Technol. 2018, 6, 2139–2145. [Google Scholar] [CrossRef]
- Phillips, P.J.; Bareno, J.; Li, Y.; Abraham, D.P.; Klie, R.F. On the Localized Nature of the Structural Transformations of Li2MnO3 Following Electrochemical Cycling. Adv. Energy Mater. 2015, 5, 1501252. [Google Scholar] [CrossRef]
- Nayak, P.K.; Erickson, E.M.; Schipper, F.; Penki, T.R.; Munichandraiah, N.; Adelhelm, P.; Sclar, H.; Amalraj, F.; Markovsky, B.; Aurbach, D. Review on Challenges and Recent Advances in the Electrochemical Performance of High Capacity Li- and Mn-Rich Cathode Materials for Li-Ion Batteries. Adv. Energy Mater. 2018, 8, 1702397. [Google Scholar] [CrossRef]
- Chen, H.; Liu, B.; Wang, Y.; Guan, H.; Zhou, H.M. Insight into wide temperature electrolyte based on lithiumdifluoro (oxalate)borate for high voltage lithium-ion batteries. J. Alloys Compd. 2021, 876, 159966. [Google Scholar] [CrossRef]
- Yang, F.; Li, W.Y.; Rui, Y.C.; Tang, B.H.J. Improved Specific Capacity of Nb2O5 by Coating on Carbon Materials for Lithium-Ion Batteries. Chemelectrochem 2018, 5, 3468–3477. [Google Scholar] [CrossRef]
- Zou, Y.; Cheng, Y.; Lin, J.D.; Xiao, Y.K.; Ren, F.C.; Zhou, K.; Wang, M.S.; Wu, D.Y.; Yang, Y.; Zheng, J.M. Boosting high voltage cycling of LiCoO2 cathode via triisopropanolamine cyclic borate electrolyte additive. J. Pow. Sources 2022, 532, 231372. [Google Scholar] [CrossRef]
- Li, S.W.; Wang, C.; Meng, C.X.; Ning, Y.X.; Zhang, G.H.; Fu, Q. Electrolyte-dependent formation of solid electrolyte interphase and ion intercalation revealed by in situ surface characterizations. J. Energy Chem. 2022, 67, 718–726. [Google Scholar] [CrossRef]
- Hekmatfar, M.; Kazzazi, A.; Eshetu, G.G.; Hasa, I.; Passerini, S. Understanding the Electrode/Electrolyte Interface Layer on the Li-Rich Nickel Manganese Cobalt Layered Oxide Cathode by XPS. Acs Appl. Mater. Interfaces 2019, 11, 43166–43179. [Google Scholar] [CrossRef]
- Ding, H.B.; Wang, X.L.; Wang, J.J.; Zhang, H.B.; Liu, G.X.; Yu, W.S.; Dong, X.T.; Wang, J.X. Morphology-controllable synthesis and excellent electrochemical performance of Ni-rich layered NCM622 as cathode materials for lithium-ion batteries via glycerin-assisted solvothermal method. J. Pow. Sources 2023, 553, 232307. [Google Scholar] [CrossRef]
- Hy, S.; Felix, F.; Rick, J.; Su, W.N.; Hwang, B.J. Direct In situ Observation of Li2O Evolution on Li-Rich High-Capacity Cathode Material, LiNixLi(1−2x)/3Mn(2−x)/3O2 (0 <= x <= 0.5). J. Am. Chem. Soc. 2014, 136, 999–1007. [Google Scholar]
- Zhao, Y.F.; Yao, K.X.; Teng, B.Y.; Zhang, T.; Han, Y. A perfluorinated covalent triazine-based framework for highly selective and water-tolerant CO2 capture. Energy Environ. Sci. 2013, 6, 3684–3692. [Google Scholar] [CrossRef]
- Yu, X.Q.; Lyu, Y.C.; Gu, L.; Wu, H.M.; Bak, S.M.; Zhou, Y.N.; Amine, K.; Ehrlich, S.N.; Li, H.; Nam, K.W.; et al. Understanding the Rate Capability of High-Energy-Density Li-Rich Layered Li1.2Ni0.15Co0.1Mn0.55O2 Cathode Materials. Adv. Energy Mater. 2014, 4, 1300950. [Google Scholar] [CrossRef]
- Cabana, J.; Kang, S.H.; Johnson, C.S.; Thackeray, M.M.; Grey, C.P. Structural and Electrochemical Characterization of Composite Layered-Spinel Electrodes Containing Ni and Mn for Li-Ion Batteries. J. Electrochem. Soc. 2009, 156, A730–A736. [Google Scholar] [CrossRef]
- Chernyavsky, V.; Kim, A.; Koshtyal, Y.; Rumyantsev, A.; Popovich, A.; Maximov, M.Y. Structural features of complete and partial activation of Li-rich cathodes studied by in-situ XRD. Electrochim. Acta 2022, 414, 140237. [Google Scholar] [CrossRef]

| RLM | RLM @ Nb2O5 | ||
|---|---|---|---|
| Rs/(Ω) | 10.46 | 9.675 | |
| Rf/(Ω) | 143.4 | 80.13 | |
| CPE1 | CPE1-T/(F) | 1.4348 × 10−4 | 4.4665 × 10−5 |
| CPE1-P/(F) | 0.46765 | 0.57493 | |
| Rct/(Ω) | 1856 | 2610 | |
| CPE2 | CPE2-T/(F) | 1.9901 × 10−2 | 6.5415 × 10−4 |
| CPE2-P/(F) | 0.80459 | 0.83649 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Y.; Yang, R.; Li, X.; Yang, W.; Lin, Y.; Zhang, G.; Wang, L. Nb2O5 Coating to Improve the Cyclic Stability and Voltage Decay of Li-Rich Cathode Material for Lithium-Ion Battery. Molecules 2023, 28, 3890. https://doi.org/10.3390/molecules28093890
Liu Y, Yang R, Li X, Yang W, Lin Y, Zhang G, Wang L. Nb2O5 Coating to Improve the Cyclic Stability and Voltage Decay of Li-Rich Cathode Material for Lithium-Ion Battery. Molecules. 2023; 28(9):3890. https://doi.org/10.3390/molecules28093890
Chicago/Turabian StyleLiu, Yanlin, Ruifeng Yang, Xinxi Li, Wensheng Yang, Yuanwei Lin, Guoqing Zhang, and Lijuan Wang. 2023. "Nb2O5 Coating to Improve the Cyclic Stability and Voltage Decay of Li-Rich Cathode Material for Lithium-Ion Battery" Molecules 28, no. 9: 3890. https://doi.org/10.3390/molecules28093890





