Point-of-Care and Dual-Response Detection of Hydrazine/Hypochlorite-Based on a Smart Hydrogel Sensor and Applications in Information Security and Bioimaging
Abstract
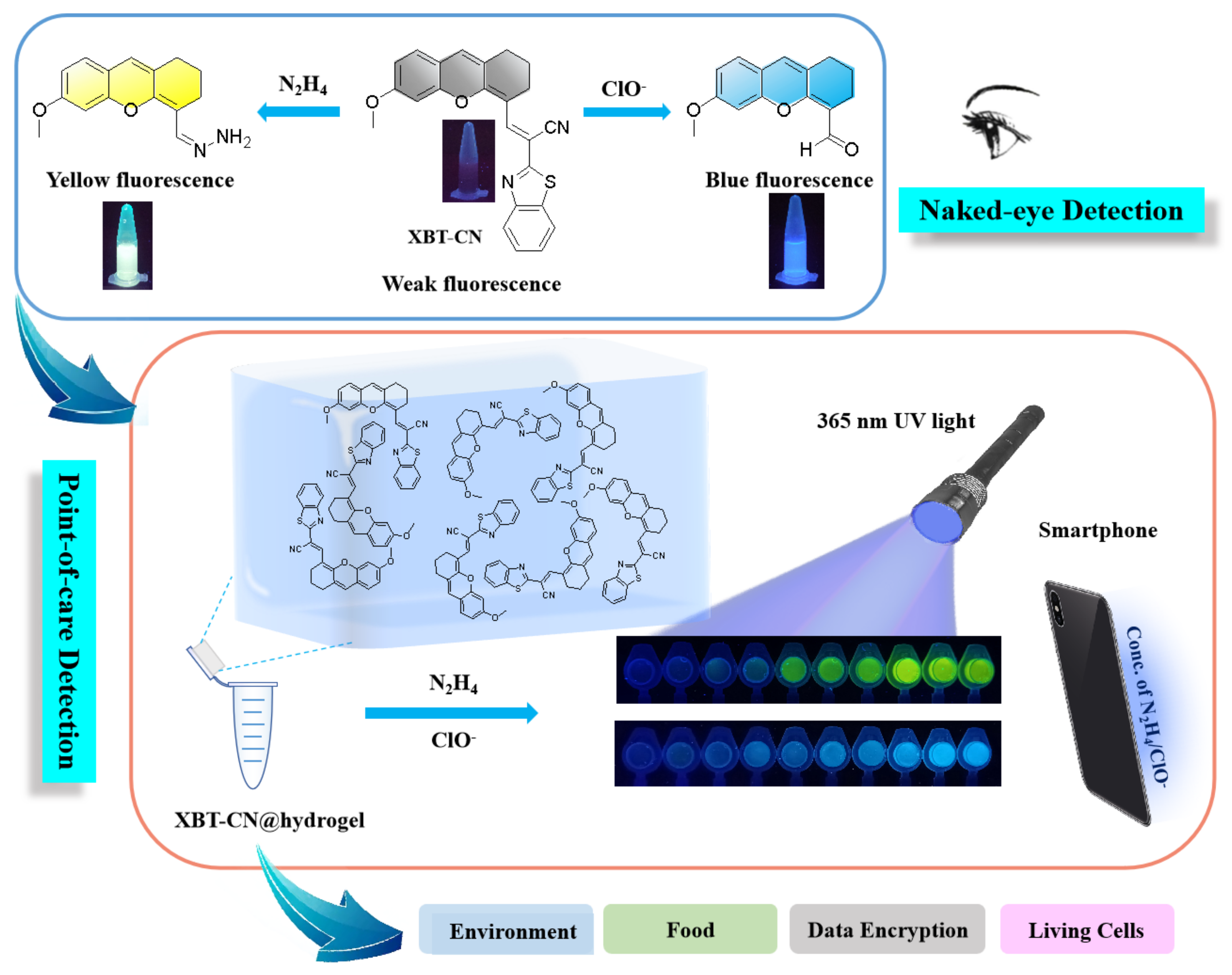
:1. Introduction
2. Results and Discussion
2.1. Design and Synthesis of the Probe XBT-CN
2.2. UV Response of XBT-CN to N2H4 and ClO−
2.3. Fluorescence Response of XBT-CN to N2H4 and ClO−
2.4. Selectivity and Anti-Interference Performance of XBT-CN
2.5. Effect of pH and Response Time
2.6. Sensing Mechanism Study of XBT-CN to N2H4 and ClO−
2.7. Point-of-Care Detection of N2H4/ClO− by Hydrogel Test Kit with Smartphone
2.8. Application in Real Samples
2.9. Analysis of ClO− in MPO/H2O2/Cl− System
2.10. Role as Encryption Ink in Data Security
2.11. Fluorescence Imaging in Living Cells
3. Materials and Methods
3.1. Materials and Instruments
3.2. Synthesis
3.2.1. Synthesis of Compound 1
3.2.2. Synthesis of Compound 2
3.2.3. Synthesis of Compound 3
3.2.4. Synthesis of Compound XBT-CN
3.3. Spectroscopic Measurements
3.4. Determination of the Fluorescence Quantum Yield
3.5. Selectivity Study
3.6. HPLC Analysis
3.7. Density Functional Theory
3.8. Preparation of the XBT-CN@Hydrogel Portable Test Kit
3.9. Detection of N2H4 and ClO− in Real Samples by Hydrogel Test Kit
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Serov, A.; Kwak, C. Direct hydrazine fuel cells: A review. Appl. Catal. B Environ. 2010, 98, 1–9. [Google Scholar] [CrossRef]
- Ramachandraih, C.T.; Subramanyam, N.; Bar, K.J.; Baker, G.; Yeragani, V.K. Antidepressants: From MAOIs to SSRIs and more. Indian J. Psychiatr. 2011, 53, 180–182. [Google Scholar] [CrossRef]
- Nguyen, H.N.; Chenoweth, J.A.; Bebarta, V.S.; Albertson, T.E.; Nowadly, C.D. The toxicity, pathophysiology, and treatment of acute hydrazine propellant exposure: A systematic review. Mil. Med. 2021, 186, 319–326. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.Y.; Albertson, T.E.; Olson, K.R. Treatment of drug-induced seizures. Br. J. Clin. Pharmacol. 2016, 81, 412–419. [Google Scholar] [CrossRef] [PubMed]
- Sinha, B.K.; Mason, R.P. Biotransformation of hydrazine dervatives in the mechanism of toxicity. J. Drug Metabol. Toxicol. 2014, 5, 1000168. [Google Scholar] [CrossRef]
- Finkelstein, D.A.; Imbeault, R.; Garbarino, S.; Rou, L.; Guay, D. Trends in catalysis and catalyst cost effectiveness for N2H4 fuel cells and sensors: A rotating disk electrode (RDE) study. J. Phys. Chem. C 2016, 12, 4717–4738. [Google Scholar] [CrossRef]
- Qin, H.Y.; Liu, Z.X.; Yin, W.X.; Zhu, J.K.; Li, Z.P. Effects of hydrazine addition on gas evolution and performance of the direct borohydride fuel cell. J. Power Sources 2008, 185, 895–898. [Google Scholar] [CrossRef]
- Nobari, N.; Behboudnia, M.; Maleki, R. Palladium-free electroless deposition of pure copper film on glass substrate using hydrazine as reducing agent. Appl. Surf. Sci. 2016, 385, 9–17. [Google Scholar] [CrossRef]
- Niemeier, J.K.; Kjell, D.P. Hydrazine and aqueous hydrazine solutions: Evaluating safety in chemical processes. Org. Process Res. Dev. 2013, 17, 1580–1590. [Google Scholar] [CrossRef]
- Ioannidou, H.A.; Koutentis, P.A. The conversion of Isothiazoles into pyrazoles using hydrazine. Tetrahedron 2009, 65, 7023–7037. [Google Scholar] [CrossRef]
- Aigner, B.A.; Darsow, U.; Grosber, M.; Ring, J.; Plőtz, S.G. Multiple basal cell carcinomas after long-term exposure to hydrazine: Case report and review of the literature. Dermatology 2010, 221, 300–302. [Google Scholar] [CrossRef]
- Vivekanandan, P.; Gobianand, K.; Priya, S.; Vijayalakshmi, P.; Karthikeyan, S. Protective effect of picroliv against hydrazine-induced hyperlipidemia and hepatic steatosis in rats. Drug Chem. Toxicol. 2007, 30, 241–252. [Google Scholar] [CrossRef]
- Liu, K.; Huang, S.J.; Li, T.R.; Sun, J.; Fan, L.; Wang, X.F.; Li, H.X.; Li, Y.J.; Zhang, W.; Yang, Z.Y. A benzocoumarin-based fluorescent probe for highly specific ultra-sensitive fast detecting endogenous/exogenous hypochlorous acid and its applications. J. Photoch. Photobiol. A Chem. 2022, 427, 113843. [Google Scholar] [CrossRef]
- Nguyen, K.H.; Hao, Y.Q.; Zeng, K.; Fan, S.N.; Li, F.; Yuan, S.K.; Ding, X.J.; Xu, M.T.; Liu, Y.N. A benzothiazole-based fluorescent probe for hypochlorous acid detection and imaging in living cells. Spectrochim. Acta 2018, 199, 189–193. [Google Scholar] [CrossRef]
- Zhu, B.C.; Wu, L.; Zhang, M.; Wang, Y.W.; Liu, C.Y.; Wang, Z.K.; Duan, Q.X.; Jia, P. A highly specific and ultrasensitive near-infrared fluorescent probe for imaging basal hypochlorite in the mitochondria of living cells. Biosens. Bioelectron. 2018, 107, 218–223. [Google Scholar] [CrossRef] [PubMed]
- Swierczyńska, M.; Słowinski, D.; Grzelakowska, A.; Szala, M.; Romanski, J.; Pierzchała, K.; Siarkiewicz, P.; Michalski, R.; Podsiadły, R. Selective, stoichiometric and fast-response fluorescent probe based on 7-nitrobenz-2-oxa-1,3-diazole fluorophore for hypochlorous acid detection. Dye. Pigment. 2021, 193, 109563. [Google Scholar] [CrossRef]
- Wu, W.L.; Zhao, Z.M.; Dai, X.; Su, L.; Zhao, B.X. A fast-response colorimetric and fluorescent probe for hypochlorite and its real applications in biological imaging. Sens. Actuator B Chem. 2016, 232, 390–395. [Google Scholar] [CrossRef]
- Zhao, X.J.; Jiang, Y.R.; Chen, Y.X.; Yang, B.Q.; Li, Y.T.; Liu, Z.H.; Liu, C. A new “off-on” NIR fluorescence probe for determination and bio-imaging of mitochondrial hypochlorite in living cells and zebrafish. Spectrochim. Acta 2019, 219, 509–516. [Google Scholar] [CrossRef]
- Yan, L.; Hu, C.; Li, J. A fluorescence turn-on probe for rapid monitoring of hypochlorite based on coumarin Schiff base. Anal. Bioanal. Chem. 2018, 410, 7457–7464. [Google Scholar] [CrossRef] [PubMed]
- Huo, F.J.; Zhang, J.J.; Yang, Y.T.; Chao, J.B.; Yin, C.X.; Zhang, Y.B.; Chen, T.G. A fluorescein based highly specific colorimetric and fluorescent probe for hypochlorites in aqueous solution and its application in tap water. Sens. Actuator B Chem. 2012, 166, 44–49. [Google Scholar] [CrossRef]
- Chan, C.M.; Zhang, W.N.; Xue, Z.L.; Fang, Y.Y.; Qiu, F.X.; Pan, J.M.; Tian, J.W. Near-infrared photoacoustic probe for reversible imaging of the ClO−/GSH redox cycle in vivo. Anal. Chem. 2022, 94, 5918–5926. [Google Scholar] [CrossRef]
- Zhang, Y.R.; Liu, Y.; Feng, X.; Zhao, B.X. Recent progress in the development of fluorescent probes for the detection of hypochlorous acid. Sens. Actuator B Chem. 2017, 240, 18–36. [Google Scholar] [CrossRef]
- McAdam, K.; Kimpton, H.; Essen, S.; Davis, P.; Vas, C.; Wright, C.; Porter, A.; Rodu, B. Analysis of hydrazine in smokeless tobacco products by gas chromatography-mass spectrometry. Chem. Cent. J. 2015, 9, 13. [Google Scholar] [CrossRef]
- Karimi-Maleh, H.; Moazampour, M.; Ensafi, A.A.; Mallakpour, S.; Hatami, M. An electrochemical nanocomposite modified carbon paste electrode as a sensor for simultaneous determination of hydrazine and phenol in water and wastewater samples. Environ. Sci. Pollut. Res. Int. 2014, 21, 5879–5888. [Google Scholar] [CrossRef]
- Oh, J.A.; Shin, H.S. Simple determination of hydrazine in waste water by headspace solid-phase micro extraction and gas chromatography-tandem mass spectrometry after derivatization with trifluoro pentanedione. Anal. Chim. Acta 2017, 950, 57–63. [Google Scholar] [CrossRef]
- Sharma, S.; Ganeshan, S.K.; Pattnaik, P.K.; Kanungo, S.; Chappanda, K.N. Laser induced flexible graphene electrodes for electrochemical sensing of hydrazine. Mater. Lett. 2020, 262, 127150. [Google Scholar] [CrossRef]
- Tang, X.; Zhu, Z.; Wang, Y.; Han, J.; Ni, L.; Zhang, H.Q.; Li, J.; Mao, Y.L. A cyanobiphenyl based fluorescent probe for rapid and specific detection of hypochlorite and its bio-imaging applications. Sens. Actuator B Chem. 2018, 262, 57–63. [Google Scholar] [CrossRef]
- Jiang, Q.; Wang, Z.L.; Li, M.X.; Song, J.; Yang, Y.Q.; Xu, X.; Xu, H.J.; Wang, S.F. A novel nopinone-based fluorescent probe for colorimetric and ratiometric detection of hypochlorite and its applications in water samples and living cells. Analyst 2020, 145, 1033–1040. [Google Scholar] [CrossRef]
- Zhang, P.S.; Wang, H.; Hong, Y.X.; Yu, M.L.; Zeng, R.J.; Long, Y.F.; Chen, J. Selective visualization of endogenous hypochlorous acid in zebrafish during lipopolysaccharide-induced acute liver injury using a polymer micelles-based ratiometric fluorescent probe. Biosens. Bioelectron. 2018, 99, 318–324. [Google Scholar] [CrossRef]
- Ren, M.G.; Li, Z.H.; Deng, B.B.; Wang, L.; Lin, W.Y. Single fluorescent probe separately and continuously visualize H2S and HClO in lysosomes with different fluorescence signals. Anal. Chem. 2019, 91, 2932–2938. [Google Scholar] [CrossRef]
- Zhu, M.Q.; Zhao, Z.Y.; Huang, Y.; Fan, F.G.; Wang, F.; Li, W.L.; Wu, X.W.; Hua, R.M.; Wang, Y. Hydrazine exposure: A near-infrared ICT-based fluorescent probe and its application in bioimaging and sewage analysis. Sci. Total Environ. 2021, 759, 143102. [Google Scholar] [CrossRef]
- Tang, C.; Tong, H.J.; Liu, B.; Wang, X.N.; Jin, Y.S.; Tian, E.L.; Wang, F. Robust ERα-targeted near-infrared fluorescence probe for selective hydrazine imaging in breast cancer. Anal. Chem. 2022, 94, 14012–14020. [Google Scholar] [CrossRef]
- Wang, L.Y.; Xin, S.Q.; Xie, F.R.; Ran, X.G.; Tang, H.; Cao, D.R. A novel windmill-shaped AIE-active pyrrolopyrrole cyanine: Design, synthesis and efficient hydrazine detection. J. Mater. Chem. C 2022, 10, 14605–14615. [Google Scholar] [CrossRef]
- Li, D.H.; Liu, L.; Yang, H.G.; Ma, J.; Wang, H.L.; Pan, J.M. A novel dual-response triphenylamine-based fluorescence sensor for special detection of hydrazine in water. Mat. Sci. Eng. B 2022, 276, 115556. [Google Scholar] [CrossRef]
- Zhang, K.; Huang, Y.; Shen, Y.J.; Ma, S.; Chen, T.T. Combination of imine bond and samarium emitter enables turn-off fluorescence detection of hydrazine in vapor and water samples. Talanta 2021, 225, 122065. [Google Scholar] [CrossRef]
- Huang, X.H.; Luo, T.C.; Zhang, C.; Li, J.R.; Jia, Z.J.; Chen, X.L.; Hu, Y.J.; Huang, H. Dual-ratiometric fluorescence probe for viscosity and hypochlorite based on AIEgen with mitochondria-targeting ability. Talanta 2022, 241, 123235. [Google Scholar] [CrossRef]
- Xu, X.H.; Ding, H.C.; Zhang, Q.; Liu, G.; Pu, S.Z. A ratiometric fluorescent probe with an extremely large emission shift for detecting ClO− and its application in test strips and cell imaging. Dye. Pigment. 2022, 207, 110776. [Google Scholar] [CrossRef]
- Zeng, C.H.; Xu, Z.Y.; Song, C.; Qin, T.Y.; Jia, T.H.; Zhao, C.; Wang, L.; Liu, B.; Peng, X.J. Naphthalene-based fluorescent probe for on-site detection of hydrazine in the environment. J. Hazard. Mater. 2023, 445, 130416. [Google Scholar] [CrossRef]
- Li, N.N.; Gao, Y.E.; Xu, X.Y.; Qiu, P.; Gao, Y.; Yan, M.; Zhang, Q.; Lin, W.Y.; Xing, Z.Y.; Zong, Z.A. Fluorescence probe with aggregation induced emission effect and application for colormetric detection of endogenous and exogenous hypochlorite. Dye. Pigment. 2023, 210, 110965. [Google Scholar] [CrossRef]
- Zhong, X.L.; Yang, Q.; Chen, Y.S.; Jiang, Y.L.; Dai, Z.H. Aggregation-induced fluorescence probe for hypochlorite imaging in mitochondria of living cells and zebrafish. J. Mater. Chem. B 2020, 8, 7375–7381. [Google Scholar] [CrossRef] [PubMed]
- Zhu, B.T.; Wu, X.L.; Rodrigues, J.; Hu, X.C.; Sheng, R.L.; Bao, G.M. A dual-analytes responsive fluorescent probe for discriminative detection of ClO− and N2H4 in living cells. Spectrochim. Acta 2021, 246, 118953. [Google Scholar] [CrossRef]
- Wang, L.; Pan, Q.; Chen, Y.; Ou, Y.F.; Li, H.Y.; Li, B.W. A dual-response ratiometric fluorescent probe for hypochlorite and hydrazine detection and its imaging in living cells. Spectrochim. Acta 2020, 241, 118672. [Google Scholar] [CrossRef]
- Das, S.; Patra, L.; Das, P.P.; Ghoshal, K.; Gharami, S.; Walton, J.W.; Bhattacharyyae, M.; Mondal, T.K. A new ratiometric switch “two-way” detects hydrazine and hypochlorite via a “dye-release” mechanism with a PBMC bioimaging study. Phys. Chem. Chem. Phys. 2022, 24, 20941–20952. [Google Scholar] [CrossRef]
- He, X.J.; Deng, Z.A.; Xu, W.; Li, Y.H.; Xu, C.C.; Chen, H.; Shen, J.L. A novel dual-response chemosensor for bioimaging of exogenous/endogenous hypochlorite and hydrazine in living cells, pseudomonas aeruginosa and zebrafish. Sens. Actuator B Chem. 2020, 321, 128450. [Google Scholar] [CrossRef]
- Qiu, L.M.; Wan, J.Y.; Lu, Y.H.; Zhang, P.T.; Qin, D.S.; Yan, J.; Xiao, H.B. A dual-site colorimetric fluorescent probe for rapid detection of hydrazine/hypochlorite and its application in two-photon fluorescent bioimaging. Results Chem. 2022, 4, 100311. [Google Scholar] [CrossRef]
- Stephens, P.J.; Devlin, F.J.; Chabalowski, C.F.; Frisch, M.J. Ab initio calculation of vibrational absorption and circular dichroism spectra using density functional force fields. J. Phys. Chem. 1994, 98, 11623–11627. [Google Scholar] [CrossRef]
- Rassolov, V.A.; Ratner, M.A.; Pople, J.A.; Redfern, P.C.; Curtiss, L.A. 6-31G* basis set for third-row atoms. J. Comput. Chem. 2001, 22, 976–984. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, M.A.; Robb, J.R.; Cheesman, G.; Scalmani, V.; Petersson, G.A.; Nakatsuji, H.; Li, M.; et al. Gaussian 09, Revision C. 01; Gaussian, Inc.: Wallingford, CT, USA, 2009. [Google Scholar]
- Zhao, Y.; Truhlar, D.G. The M06 suite of density functionals for main group thermochemistry, thermochemical kinetics, noncovalent interactions, excited states, and transition elements: Two new functionals and systematic testing of four M06-class functionals and 12 other functionals. Theor. Chem. Acc. 2008, 120, 215–241. [Google Scholar] [CrossRef]
- Qi, Y.L.; Chen, J.; Zhang, B.; Li, H.; Li, D.D.; Wang, B.Z.; Yang, Y.; Zhu, H.L. A turn-on fluorescent sensor for selective detection of hydrazine and its application in Arabidopsis thaliana. Spectrochim. Acta 2020, 227, 117707. [Google Scholar] [CrossRef]
- Guo, Z.R.; Niu, Q.F.; Yang, Q.X.; Li, T.D.; Wei, T.; Yang, L.; Chen, J.B.; Qin, X.Y. New “naked-eye” colori/fluorimetric “turn-on” chemosensor: Ultrafast and ultrasensitive detection of hydrazine in similar to 100% aqueous solution and its bio-imaging in living cells. Anal. Chim. Acta 2020, 1123, 64–72. [Google Scholar] [CrossRef]
- Wang, J.L.; Wang, H.; Yang, S.X.; Tian, H.Y.; Liu, Y.G.; Hao, Y.F.; Zhang, J.; Sun, B.G. A fluorescent probe for sensitive detection of hydrazine and its application in red wine and water. Anal. Sci. 2018, 34, 329–333. [Google Scholar] [CrossRef]
- Mu, S.; Gao, H.; Li, C.; Li, S.S.; Wang, Y.Y.; Zhang, Y.; Ma, C.M.; Zhang, H.X.; Liu, X.Y. A dual-response fluorescent probe for detection and bioimaging of hydrazine and cyanide with different fluorescence signals. Talanta 2021, 221, 121606. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.; Shi, G.J.; Wang, J.J.; Qin, H.F.; Zhang, Q.; Chen, S.J.; Wen, Y.H.; Guo, J.B.; Wang, K.P.; Hu, Z.Q. A highly-sensitive “turn on” probe based on coumarin β-diketone for hydrazine detection in PBS and living cells. Spectrochim. Acta 2021, 252, 119510. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.L.; Li, X.L.; Jiang, Y.H.; Zhang, Y.N.; Xie, Y.X.; Sun, Y.D.; Liu, C. A super large Stokes shift ratiometric fluorescent probe for highly selective sensing of ClO− in bio-imaging and real water samples. Spectrochim. Acta 2022, 283, 121736. [Google Scholar] [CrossRef]











Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Du, M.; Zhang, Y.; Xu, Z.; Dong, Z.; Zhao, S.; Du, H.; Zhao, H. Point-of-Care and Dual-Response Detection of Hydrazine/Hypochlorite-Based on a Smart Hydrogel Sensor and Applications in Information Security and Bioimaging. Molecules 2023, 28, 3896. https://doi.org/10.3390/molecules28093896
Du M, Zhang Y, Xu Z, Dong Z, Zhao S, Du H, Zhao H. Point-of-Care and Dual-Response Detection of Hydrazine/Hypochlorite-Based on a Smart Hydrogel Sensor and Applications in Information Security and Bioimaging. Molecules. 2023; 28(9):3896. https://doi.org/10.3390/molecules28093896
Chicago/Turabian StyleDu, Man, Yue Zhang, Zhice Xu, Zhipeng Dong, Shuchun Zhao, Hongxia Du, and Hua Zhao. 2023. "Point-of-Care and Dual-Response Detection of Hydrazine/Hypochlorite-Based on a Smart Hydrogel Sensor and Applications in Information Security and Bioimaging" Molecules 28, no. 9: 3896. https://doi.org/10.3390/molecules28093896



