Different In Silico Approaches Using Heterocyclic Derivatives against the Binding between Different Lineages of SARS-CoV-2 and ACE2
Abstract
:1. Introduction
2. Results
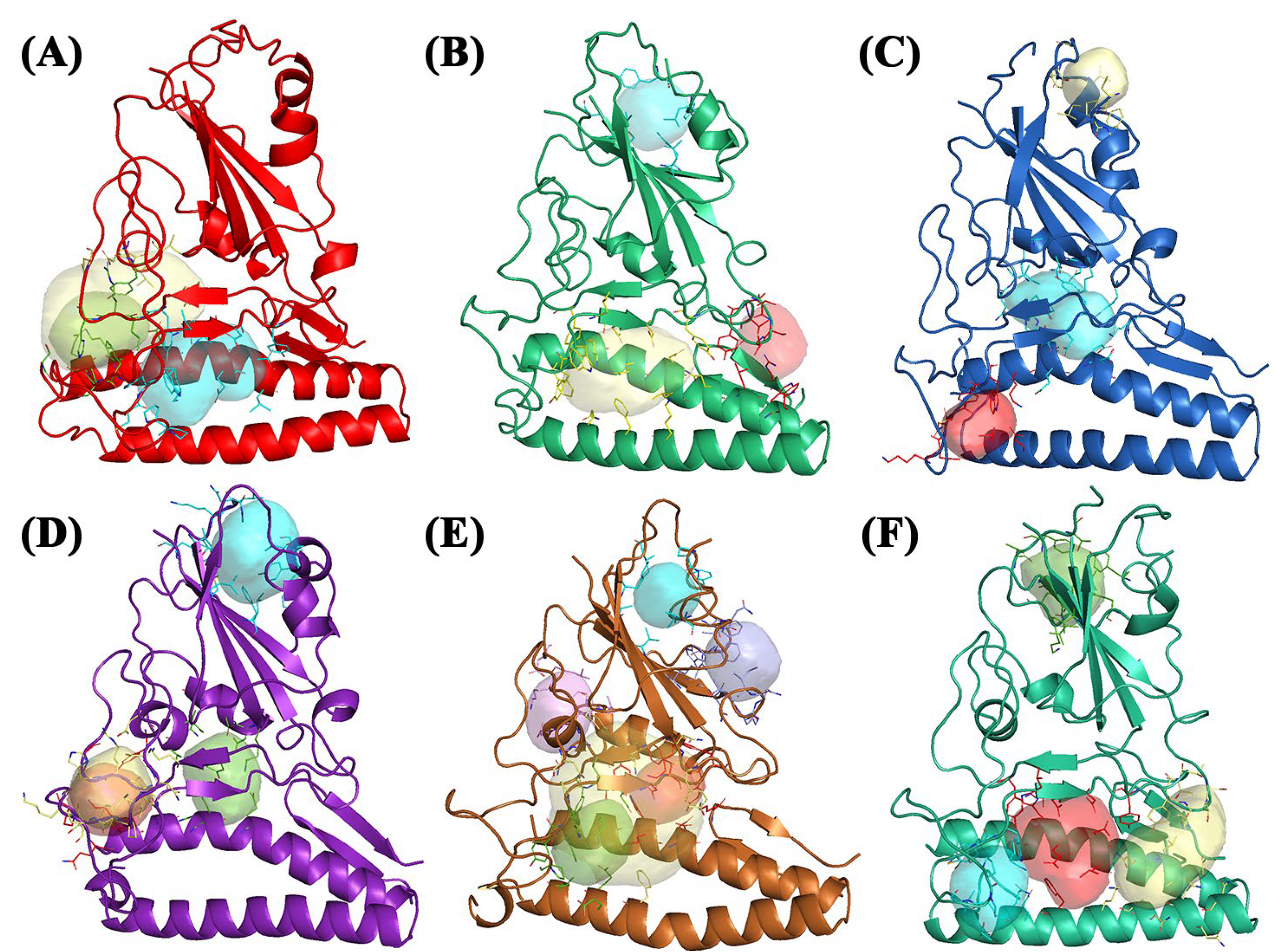
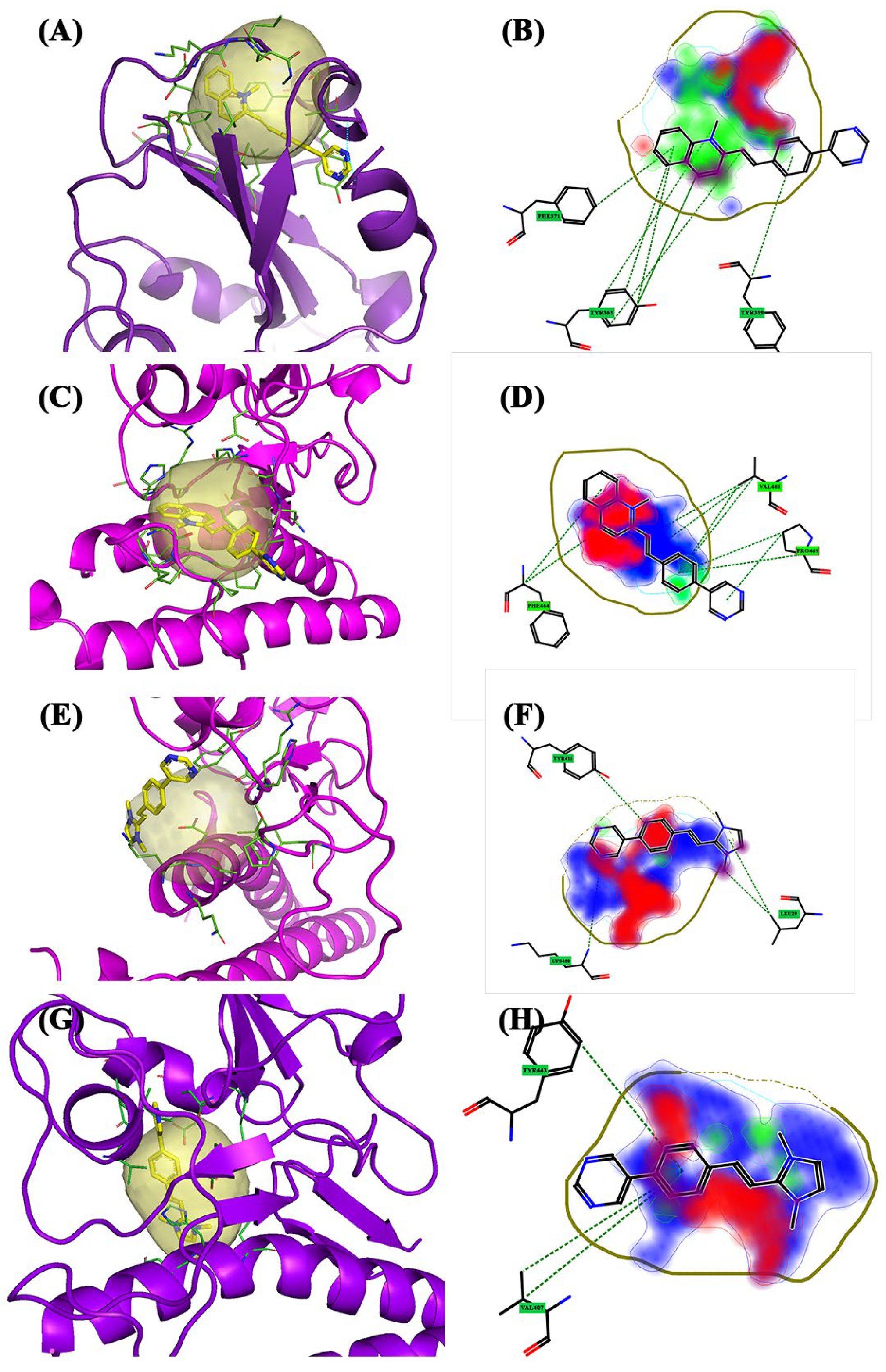
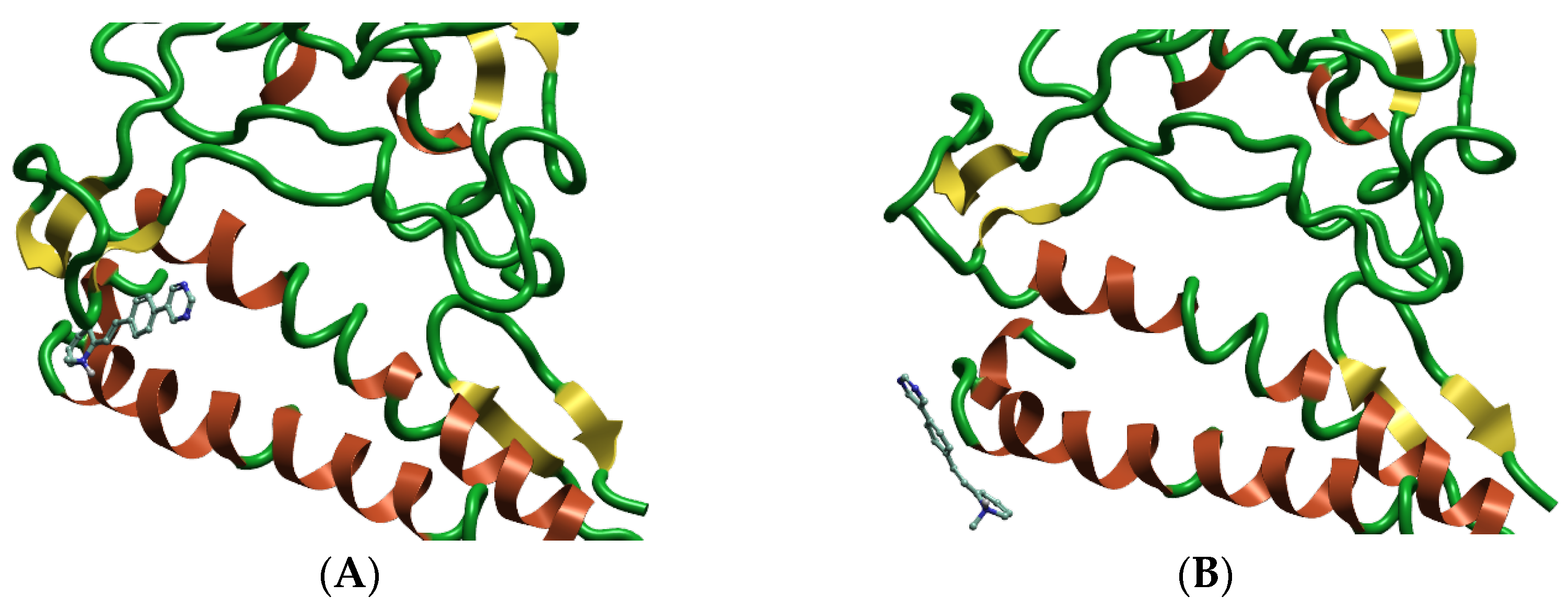
2.1. Binding Site Identification and Molecular Docking Studies

| Variant | Amino Acid ACE2 | Amino Acid Spike Protein | |
|---|---|---|---|
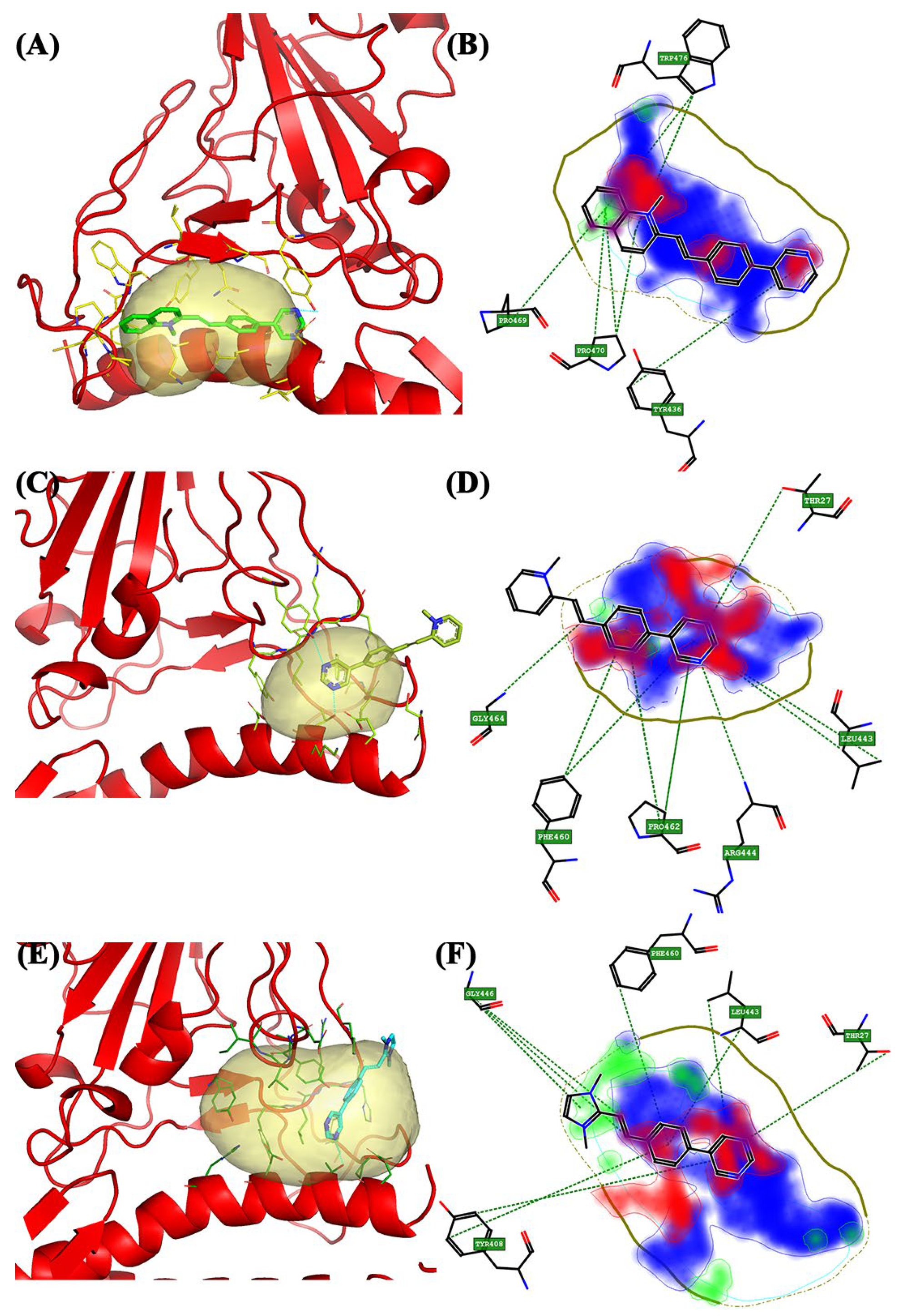
| Wild-Type | Pocket 1 | Lys31, His34, Glu35, Ala36, Asp38, and Leu39 | Tyr436, Tyr442, Pro469, Pro470, Cys474, Tyr475, Trp476, Leu478, Asn479, and Asp480 |
| Pocket 2 | Ser19, Glu23, Lys26, Thr27, and Asp30 | Tyr408, Tyr442, Leu443, Arg444, His445, Phe460, Ser461, and Pro462 | |
| Pocket 2.5 | Glu23, Thr27, Asp30, and His34 | Gly403, Val404, Ile405, Asp407, Tyr408, Tyr440, Tyr442. Leu443, Arg444, His445, Lys447, Phe460, and Pro462 | |
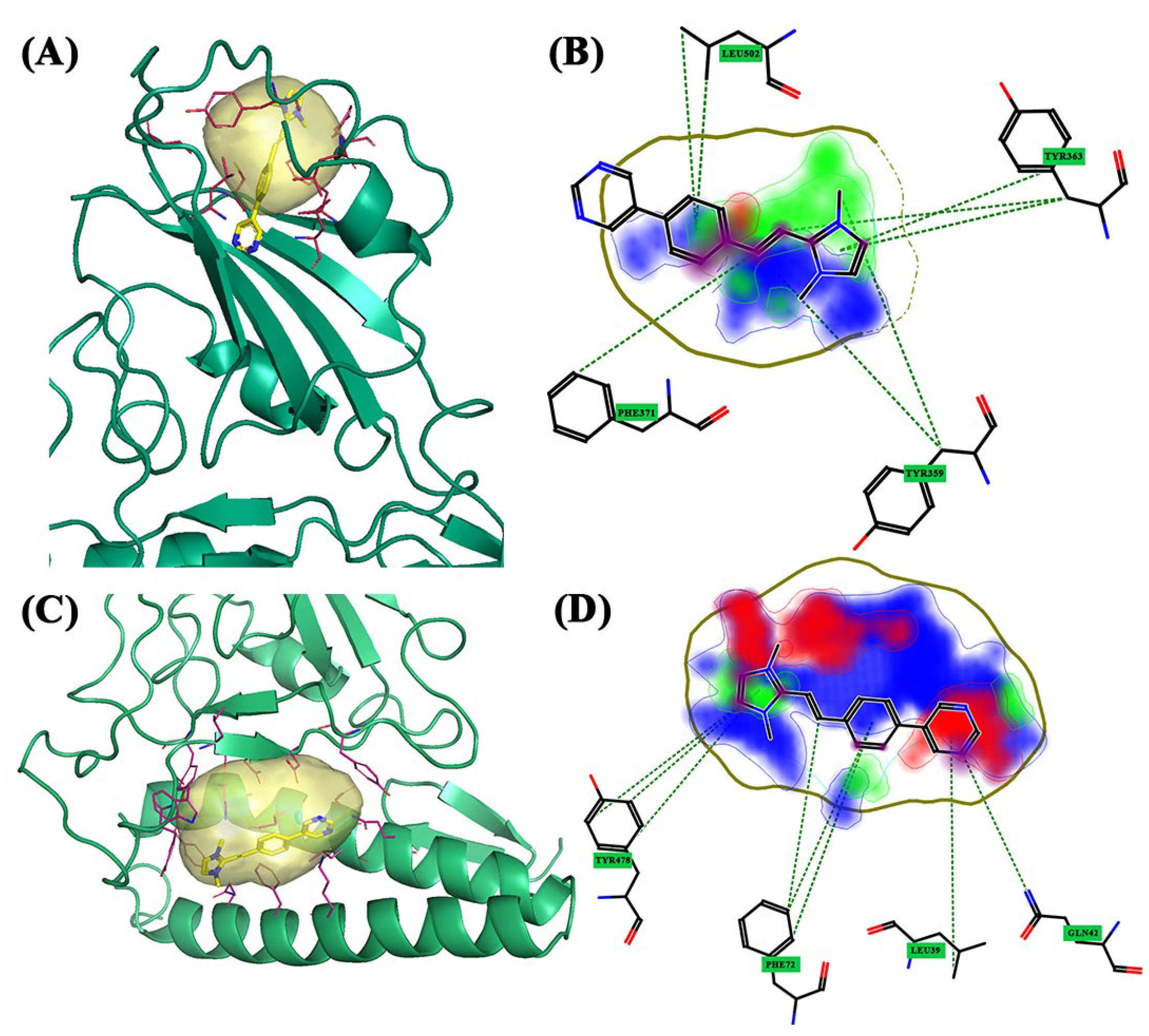
| Alpha | Pocket 1 | Tyr359, Leu362, Tyr363, Phe371, Ser377, Cys422, Leu424, and Leu502 | |
| Pocket 2 | Phe28, Lys31, Glu35, Asp38, Leu39, Gln42, Lys68, Phe72, and Gln76 | Tyr439, Tyr445, Tyr478, Trp479, Leu481, Asn482, and Asp483 | |
| Pocket 3 | Leu45, Trp48, Asn49, and Arg103 | Thr434, Ser435, Thr436, Tyr492, Thr493, and Thr494 | |
| Beta | Pocket 1 | Asn33, His34, Glu35, Glu37, Asp38, and Lys99 | Lys393, Asp395, Asp396, Arg398, Gln399, Asn407, Ile408, Tyr443, Asn482, Asp483, Tyr484, and Tyr494 |
| Pocket 2 | Cys330, Pro331, Phe332, Ala357, Tyr359, Val361, and Leu362 | ||
| Pocket 3 | Lys36, Glu75, and Leu79 | Lys474, Leu475, Asn476, Cys477, Tyr478, and Trp479 | |
| Delta | Pocket 1 | Ala357, Tyr359, Ser360, Tyr363, Phe371, Cys373, Thr379, Lys380, Leu381, Asn382, Val385, Cys422, Leu424, Leu502, and Phe504 | |
| Pocket 2 | Arg444, Leu446, Arg447, His448, Asp457, Ser459, Asn460, Val461, Pro462, Phe463, Ser464, Gly467, Lys468, Pro469, and Pro480 | ||
| Pocket 3 | Gln24, Lys26, Thr27, and Asp30 | Tyr411, Leu446, Arg447, His448, Gly449, Phe463, and Pro465 | |
| Pocket 4 | Asn33, His34, and Glu37 | Lys393, Asp395, Asp396, Gln399, Val407, Ile408, and Tyr443 | |
| Gamma | Pocket 1 | Pro331, Phe332, Val335, Lys350, Ile352, Leu362, Ala387, and Val500 | |
| Pocket 2 | Ile400, Ala401, Pro402, Gly403, Asn407, Ala409, Asp410, Lys414, Leu415, and Asp417 | ||
| Pocket 3 | His34, Glu35, Glu37, Asp38, and Lys99 | Lys393, Tyr443, Asp483, Phe486, and Tyr494 | |
| Pocket 3.5 | Phe28, Lys31, His34, Glu35, Asp38, Phe72, Gln76, and Lys99 | Tyr345, Lys393, Asp396, Tyr439, Asn440, Lys442, Tyr443, Tyr445, Asn460, Tyr478, Trp479, Pro480, Leu481, Asn482, Asp483, and Tyr484 | |
| Pocket 4 | Phe28, Lys31, His34, Glu35, Asp38, Phe72, Gln76, and Leu79 | Tyr445, Pro473, Tyr478, Trp479, Leu481, and Asn482 | |
| Pocket 5 | Phe336, Asn337, Ala338, Phe348, Trp426, Asn427, Thr428, Arg429, Asn430, Ile431, and Arg498 | ||
| Omicron | Pocket 1 | Lys31, Glu75, Gln76, Thr78, and Leu79 | Pro472, Pro473, Ala474, and Trp479 |
| Pocket 2 | Gln42, Leu45, Ala46, Asn49, Asn58, Gln60, Asn61, Asn64, Ala65, and Lys68 | Arg103, Ser435, Thr496, Gly497, Tyr487, Thr488, and Thr489 | |
| Pocket 3 | Lys31, His34, Glu35, Asp38, Leu39, Gln42, Lys68, Phe72, and Glu75 | Tyr439, Tyr445, Trp479, Leu481, Asn482, and Asp483 | |
| Pocket 4 | Tyr363, Phe369, Asn371, Lys372, Cys373, Val376, Thr379, Gly421, Cys422, Val423, Leu502, and Phe503 |
| Variant | Ligand | GLOB-SUM | GLOB-PROD | Distance | DRY | H | O | |
|---|---|---|---|---|---|---|---|---|
| Wild-type | Pocket 1 | BCC2 | 1.583 | 0.504 | 12.719 | 0.735 | 0.825 | 0.102 |
| Pocket 2 | BCC1 | 1.076 | 0.477 | 13.753 | 0.334 | 0.561 | 0.258 | |
| Pocket 2.5 | BCC3 | 1.515 | 0.591 | 12.079 | 0.766 | 0.945 | 0.182 | |
| Alpha | Pocket 1 | BCC3 | 1.801 | 0.556 | 11.731 | 1.090 | 0.758 | 0.204 |
| Pocket 2 | BCC3 | 1.601 | 0.613 | 11.754 | 0.794 | 0.978 | 0.159 | |
| Beta | Pocket 1 | BCC1 | 1.393 | 0.529 | 13.057 | 0.488 | 0.868 | 0.074 |
| Pocket 2 | BCC1 | 1.843 | 0.511 | 12.249 | 1.270 | 0.595 | 0.121 | |
| Pocket 3 | BCC2 | 1.594 | 0.465 | 12.545 | 0.895 | 0.711 | 0.192 | |
| Delta | Pocket 1 | BCC2 | 2.448 | 0.618 | 10.406 | 1.782 | 0.790 | 0.211 |
| Pocket 2 | BCC2 | 1.458 | 0.254 | 13.385 | 0.838 | 0.621 | 0.032 | |
| Pocket 3 | BCC3 | 1.018 | 0.434 | 14.206 | 0.248 | 0.686 | 0.083 | |
| Pocket 4 | BCC3 | 1.362 | 0.371 | 13.562 | 0.590 | 0.799 | 0.042 | |
| Gamma | Pocket 1 | BCC2 | 1.772 | 0.460 | 12.756 | 1.218 | 0.563 | 0.068 |
| Pocket 3 | BCC2 | 1.238 | 0 | 14.837 | 0.722 | 0.516 | 0 | |
| Pocket 3.5 | BCC2 | 2.213 | 0.665 | 10.196 | 1.340 | 0.964 | 0.240 | |
| Pocket 4 | BCC1 | 1.363 | 0.433 | 13.159 | 0.723 | 0.685 | 0.136 | |
| Pocket 5 | BCC2 | 1.640 | 0.486 | 12.615 | 1.034 | 0.710 | 0.086 | |
| Omicron | Pocket 1 | BCC1 | 1.132 | 0.479 | 13.589 | 0.512 | 0.634 | 0.129 |
| Pocket 2 | BCC1 | 1.796 | 0.633 | 10.882 | 1.017 | 0.965 | 0.346 | |
| Pocket 3 | BCC2 | 1.697 | 0.600 | 12.015 | 0.875 | 0.814 | 0.190 | |
| Pocket 4 | BCC3 | 1.535 | 0.413 | 12.913 | 0.794 | 0.775 | 0.114 |
2.2. Molecular Dynamics Studies
2.3. Quantum Mechanical Studies
3. Discussion
4. Materials and Methods
4.1. In Silico Mutagnesis
4.2. Binding Site Identification and Molecular Docking Studies
4.3. Molecular Dynamics Studies
4.4. Quantum Mechanical Studies
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
Abbreviations
References
- Wang, M.; Zhao, R.; Gao, L.; Gao, X.; Wang, D.; Cao, J. SARS-CoV-2: Structure, Biology, and Structure Based Therapeutics Development. Front. Cell. Infect. Microbiol. 2020, 10, 587269. [Google Scholar] [CrossRef] [PubMed]
- He, F.; Deng, Y.; Li, W. Coronavirus disease 2019: What we know? J. Med. Virol. 2020, 92, 719–725. [Google Scholar] [CrossRef] [PubMed]
- Cui, J.; Li, F.; Shi, Z.L. Origin and evolution of pathogenic coronaviruses. Nat. Rev. Microbiol. 2019, 17, 181–192. [Google Scholar] [CrossRef]
- Yoshimoto, F.K. A Biochemical Perspective of the Nonstructural Proteins (NSPs) and the Spike Protein of SARS CoV-2. Protein J. 2021, 40, 260–295. [Google Scholar] [CrossRef] [PubMed]
- Carota, G.; Ronsisvalle, S.; Panarello, F.; Tibullo, D.; Nicolosi, A.; Li Volti, G. Role of Iron Chelation and Protease Inhibition of Natural Products on COVID-19 Infection. J. Clin. Med. 2021, 10, 2306. [Google Scholar] [CrossRef] [PubMed]
- Shang, J.; Wan, Y.; Luo, C.; Ye, G.; Geng, Q.; Auerbach, A.; Li, F. Cell entry mechanisms of SARS-CoV-2. Proc. Natl. Acad. Sci. USA 2020, 117, 11727–11734. [Google Scholar] [CrossRef] [PubMed]
- Li, F. Structure, Function, and Evolution of Coronavirus Spike Proteins. Annu. Rev. Virol. 2016, 3, 237–261. [Google Scholar] [CrossRef]
- Mercurio, I.; Tragni, V.; Busto, F.; De Grassi, A.; Pierri, C.L. Protein structure analysis of the interactions between SARS-CoV-2 spike protein and the human ACE2 receptor: From conformational changes to novel neutralizing antibodies. Cell Mol. Life Sci. 2021, 78, 1501–1522. [Google Scholar] [CrossRef]
- Kadam, S.B.; Sukhramani, G.S.; Bishnoi, P.; Pable, A.A.; Barvkar, V.T. SARS-CoV-2, the pandemic coronavirus: Molecular and structural insights. J. Basic Microbiol. 2021, 61, 180–202. [Google Scholar] [CrossRef]
- Senapati, S.; Banerjee, P.; Bhagavatula, S.; Kushwaha, P.P.; Kumar, S. Contributions of human ACE2 and TMPRSS2 in determining host–pathogen interaction of COVID-19. J. Genet. 2021, 100, 12. [Google Scholar] [CrossRef]
- LaTourrette, K.; Garcia-Ruiz, H. Determinants of Virus Variation, Evolution, and Host Adaptation. Pathogens 2022, 11, 1039. [Google Scholar] [CrossRef]
- Ruan, Y.J.; Wei, C.L.; Ee, A.L.; Vega, V.B.; Thoreau, H.; Su, S.T.; Chia, J.M.; Ng, P.; Chiu, K.P.; Lim, L.; et al. Comparative full-length genome sequence analysis of 14 SARS coronavirus isolates and common mutations associated with putative origins of infection. Lancet 2003, 36, 1779–1785. [Google Scholar] [CrossRef] [PubMed]
- Harvey, W.T.; Carabelli, A.M.; Jackson, B.; Gupta, R.K.; Thomson, E.C.; Harrison, E.M.; Ludden, C.; Reeve, R.; Rambaut, A.; COVID-19 Genomics UK (COG-UK); et al. SARS-CoV-2 variants, spike mutations and immune escape. Nat. Rev. Microbiol. 2021, 19, 409–424. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Zhang, L.; Chen, S.; Ji, W.; Li, C.; Ren, L. Recent progress on the mutations of SARS-CoV-2 spike protein and suggestions for prevention and controlling of the pandemic. Infect. Genet. Evol. 2021, 93, 104971. [Google Scholar] [CrossRef]
- Thomson, E.C.; Rosen, L.E.; Shepherd, J.G.; Spreafico, R.; da Silva Filipe, A.; Wojcechowskyj, J.A.; Davis, C.; Piccoli, L.; Pascall, D.J.; Dillen, J.; et al. Circulating SARS-CoV-2 spike N439K variants maintain fitness while evading antibody-mediated immunity. Cell 2021, 184, 1171–1187.e20. [Google Scholar] [CrossRef]
- Carabelli, A.M.; Peacock, T.P.; Thorne, L.G.; Harvey, W.T.; Hughes, J.; De Silva, T.I.; Peacock, S.J.; Barclay, W.S.; De Silva, T.I.; Towers, G.J.; et al. SARS-CoV-2 variant biology: Immune escape, transmission and fitness. Nat. Rev. Microbiol. 2023, 21, 162. [Google Scholar] [CrossRef] [PubMed]
- Laha, S.; Chakraborty, J.; Das, S.; Manna, S.K.; Biswas, S.; Chatterjee, R. Characterizations of SARS-CoV-2 mutational profile, spike protein stability and viral transmission. Infect. Genet. Evol. 2020, 85, 104445. [Google Scholar] [CrossRef] [PubMed]
- Yan, R.; Zhang, Y.; Li, Y.; Xia, L.; Guo, Y.; Zhou, Q. Structural basis for the recognition of SARS-CoV-2 by full-length human ACE2. Science 2020, 367, 1444. [Google Scholar] [CrossRef]
- Pedebos, C.; Khalid, S. Simulations of the spike: Molecular dynamics and SARS-CoV-2. Nat. Rev. Microbiol. 2022, 20, 192. [Google Scholar] [CrossRef]
- Lan, J.; Ge, J.; Yu, J.; Shan, S.; Zhou, H.; Fan, S.; Zhang, Q.; Shi, X.; Wang, Q.; Zhang, L.; et al. Structure of the SARS-CoV-2 spike receptor-binding domain bound to the ACE2 receptor. Nature 2020, 581, 215–220. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Meng, F.; Xie, Y.; Wang, W.; Meng, Y.; Li, L.; Liu, T.; Qi, J.; Ni, X.; Zheng, S.; et al. In Silico Discovery of Small Molecule Modulators Targeting the Achilles’ Heel of SARS-CoV-2 Spike Protein. ACS Cent. Sci. 2023, 9, 252–265. [Google Scholar] [CrossRef]
- Wang, L.; Wu, Y.; Yao, S.; Ge, H.; Zhu, Y.; Chen, K.; Chen, W.Z.; Zhang, Y.; Zhu, W.; Wang, H.Y.; et al. Discovery of potential small molecular SARS-CoV-2 entry blockers targeting the spike protein. Acta Pharm. Sin. 2022, 43, 788–796. [Google Scholar] [CrossRef] [PubMed]
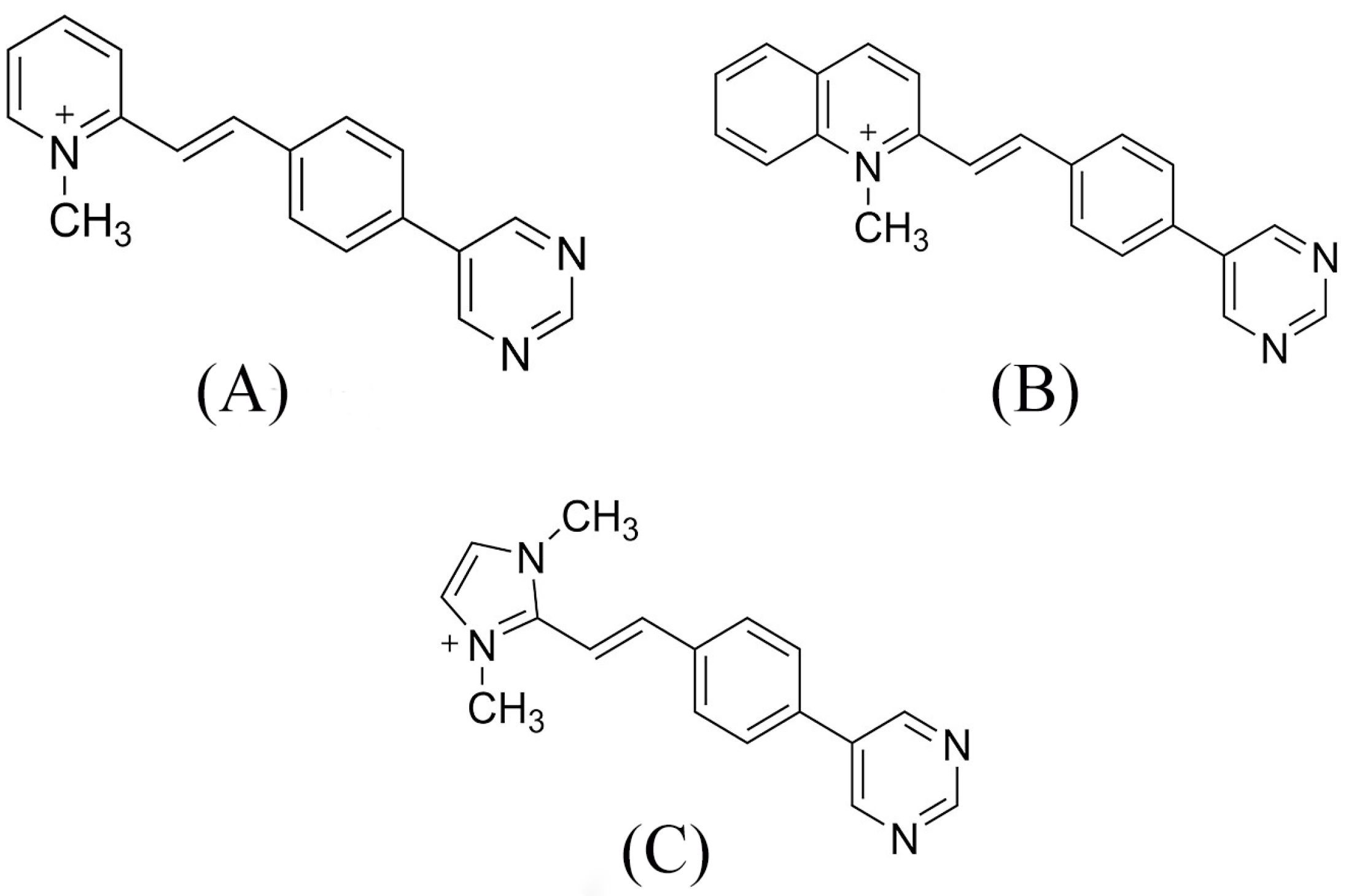
- Barresi, V.; Bonaccorso, C.; Consiglio, G.; Goracci, L.; Musso, N.; Musumarra, G.; Satriano, C.; Fortuna, C.G. Modeling, design and synthesis of new heteroaryl ethylenes active against the MCF-7 breast cancer cell-line. Mol. Biosyst. 2013, 9, 2426–2429. [Google Scholar] [CrossRef] [PubMed]
- Mazzoli, A.; Carlotti, B.; Bonaccorso, C.; Fortuna, C.G.; Mazzucato, U.; Miolo, G.; Spalletti, A. Photochemistry and DNA-affinity of some pyrimidine-substituted styryl-azinium iodides. Photochem. Photobiol. Sci. 2011, 10, 1830–1836. [Google Scholar] [CrossRef]
- Wang, W.B.; Liang, Y.; Jin, Y.Q.; Zhang, J.; Su, J.G.; Li, Q.M. E484K mutation in SARS-CoV-2 RBD enhances binding affinity with hACE2 but reduces interactions with neutralizing antibodies and nanobodies: Binding free energy calculation studies. J. Mol. Graph. Model. 2021, 109, 108035. [Google Scholar] [CrossRef] [PubMed]
- Mahmood, T.B.; Hossan, M.I.; Mahmud, S.; Shimu, M.S.S.; Alam, M.J.; Bhuyan, M.M.R.; Emran, T.B. Missense mutations in spike protein of SARS-CoV-2 delta variant contribute to the alteration in viral structure and interaction with hACE2 receptor. Immun. Inflam Dis. 2022, 10, e683. [Google Scholar] [CrossRef] [PubMed]
- Cherian, S.; Potdar, V.; Jadhav, S.; Yadav, P.; Gupta, N.; Das, M.; Rakshit, P.; Singh, S.; Abraham, P.; Panda, S.; et al. SARS-CoV-2 Spike Mutations, L452R, T478K, E484Q and P681R, in the Second Wave of COVID-19 in Maharashtra, India. Microorganisms 2021, 9, 1542. [Google Scholar] [CrossRef]
- Rath, S.L.; Padhi, A.K.; Mandal, N. Scanning the RBD-ACE2 molecular interactions in Omicron variant. Biochem. Biophys. Res. Commun. 2022, 592, 18–23. [Google Scholar] [CrossRef] [PubMed]
- Yang, T.; Yu, P.; Chang, Y.; Liang, K.; Tso, H.; Ho, M.; Chen, W.; Lin, H.; Wu, H.; Hsu, S.D. Effect of SARS-CoV-2 B.1.1.7 mutations on spike protein structure and function. Nat. Struct. Mol. Biol. 2021, 28, 731–739. [Google Scholar] [CrossRef] [PubMed]
- Mohammad, A.; Abubaker, J.; Al-Mulla, F. Structural modelling of SARS-CoV-2 alpha variant (B.1.1.7) suggests enhanced furin binding and infectivity. Virus Res. 2021, 303, 198522. [Google Scholar] [CrossRef] [PubMed]
- Yépez, Y.; Marcano-Ruiz, M.; Bezerra, R.S.; Fam, B.; Ximenez, J.P.; Silva Jr, W.A.; Bortolini, M.C. Evolutionary history of the SARS-CoV-2 Gamma variant of concern (P.1): A perfect storm. Genet. Mol. Biol. 2022, 45, e20210309. [Google Scholar] [CrossRef]
- Salleh, M.Z.; Derrick, J.P.; Deris, Z.Z. Structural evaluation of the spike glycoprotein variants on SARS-CoV-2 transmission and immune evasion. Int. J. Mol. Sci. 2021, 22, 7425. [Google Scholar] [CrossRef] [PubMed]
- Bureš, F. Fundamental aspects of property tuning in push–pull molecules. RSC Adv. 2014, 4, 58826–58851. [Google Scholar] [CrossRef]
- Bojadzic, D.; Alcazar, O.; Chen, J.; Chuang, S.T.; Condor Capcha, J.M.; Shehadeh, L.A.; Buchwald, P. Small-molecule inhibitors of the coronavirus spike: ACE2 protein–protein interaction as blockers of viral attachment and entry for SARS-CoV-2. ACS Infect. Dis. 2021, 7, 1519–1534. [Google Scholar] [CrossRef] [PubMed]
- Berman, H.M.; Westbrook, J.; Feng, Z.; Gilliland, G.; Bhat, T.N.; Weissig, H.; Shindyalov, I.N.; Bourne, P.E. The protein data bank. Nucleic Acids Res. 2000, 28, 235–242. [Google Scholar] [CrossRef]
- Kuhn, M.; Firth-Clark, S.; Tosco, P.; Mey, A.S.J.S.; Mackey, M.; Michel, J. Assessment of Binding Affinity via Alchemical Free-Energy Calculations. J. Chem. Inf. Model. 2020, 60, 3120. [Google Scholar] [CrossRef]
- Baroni, M.; Cruciani, G.; Sciabola, S.; Perruccio, F.; Mason, J.S. A Common Reference Framework for Analyzing/Comparing Proteins and Ligands. Fingerprints for Ligands and Proteins (FLAP): Theory and Application. J. Chem. Inf. Model. 2007, 47, 279–294. [Google Scholar] [CrossRef] [PubMed]
- Carosati, E.; Sciabola, S.; Cruciani, G. Hydrogen Bonding Interactions of Covalently Bonded Fluorine Atoms: From Crystallographic Data to a New Angular Function in the GRID Force Field. J. Med. Chem. 2004, 47, 5114–5125. [Google Scholar] [CrossRef] [PubMed]
- Goodford, P.J. A Computational Procedure for Determining Energetically Favorable Binding Sites on Biologically Important Macromolecules. J. Med. Chem. 1985, 28, 849–857. [Google Scholar]
- Dapprich, S.; Komáromi, I.; Byun, K.S.; Morokuma, K.; Frisch, M.J. A New ONIOM Implementation in Gaussian 98. 1. The Calculation of Energies, Gradients and Vibrational Frequencies and Electric Field Derivatives. J. Mol. Struct. (Theochem) 1999, 461–462, 1–21. [Google Scholar] [CrossRef]
- Chung, L.W.; Sameera, W.M.C.; Ramozzi, R.; Page, A.J.; Hatanaka, M.; Petrova, G.P.; Harris, T.V.; Li, X.; Ke, Z.; Li, H.B.; et al. The ONIOM Method and its applications. Chem. Rev. 2015, 115, 5678–5796. [Google Scholar] [CrossRef] [PubMed]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A.; et al. Gaussian 09, Revision B.01; Gaussian Inc.: Wallingford, UK, 2010. [Google Scholar]



| Variant | Ligand | Bennet (kcal/mol) | FEP (kcal/mol) | TI (kcal/mol) | Consensus (kcal/mol) | |
|---|---|---|---|---|---|---|
| Beta | Pocket 3 | BCC1 | −19.84 | −18.68 | −17.97 | −19.05 ± 1.87 |
| Pocket 3 | BCC2 | −19.45 | −20.85 | −20.45 | −20.03 ± 1.40 | |
| Delta | Pocket 2 | BCC1 | −24.58 | −22.22 | −21.93 | −23.31 ± 2.64 |
| Gamma | Pocket 4 | BCC1 | −23.91 | −19.63 | −20.20 | −21.94 ± 4.29 |
| Omicron | Pocket 1 | BCC1 | −23.99 | −20.19 | −20.27 | −22.11 ± 3.95 |
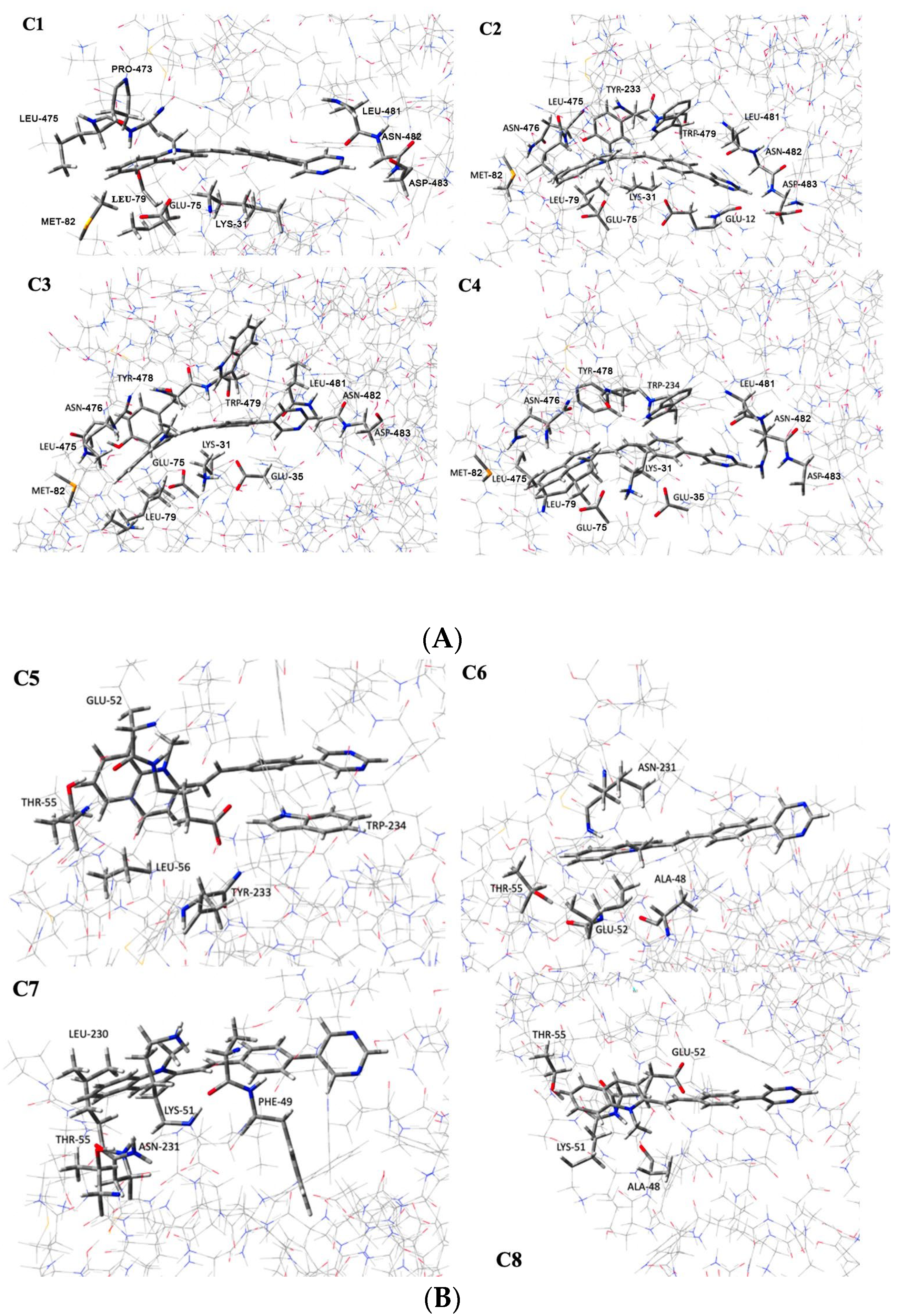
| Configuration | ΔE (kcal · mol−1) | Interacting Residues |
|---|---|---|
| C1 | −45.31 | Glu35, Glu75, Leu79, Met82, Pro473, Lys474, Leu475, Leu481, Asn482, and Asp483 |
| C2 | −55.75 | Glu35, Glu75, Leu79, Met82, Leu475, Asn476, Tyr478, Trp479, Leu481, and Asn482, Asp483 |
| C3 | −51.04 | Glu35, Glu75, Leu79, Met82, Leu475, Asn476, Tyr478, Trp479, Leu481, and Asn482, Asp483 |
| C4 | −53.72 | Glu35, Glu75, Leu79, Met82, Leu475, Asn476, Tyr478, Trp479, Leu481, Asn482, and Asp483 |
| C5 | −35.10 | Glu75, Thr78, Leu79, Tyr478, and Trp479 |
| C6 | −20.14 | Glu75, Ala71, Thr78, and Asn476 |
| C7 | −26.26 | Phe72, Lys74, Thr78, Leu475, and Asn476 |
| C8 | −20.29 | Ala71, Lys74, Glu75, and Thr78 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sipala, F.; Cavallaro, G.; Forte, G.; Satriano, C.; Giuffrida, A.; Fraix, A.; Spadaro, A.; Petralia, S.; Bonaccorso, C.; Fortuna, C.G.; et al. Different In Silico Approaches Using Heterocyclic Derivatives against the Binding between Different Lineages of SARS-CoV-2 and ACE2. Molecules 2023, 28, 3908. https://doi.org/10.3390/molecules28093908
Sipala F, Cavallaro G, Forte G, Satriano C, Giuffrida A, Fraix A, Spadaro A, Petralia S, Bonaccorso C, Fortuna CG, et al. Different In Silico Approaches Using Heterocyclic Derivatives against the Binding between Different Lineages of SARS-CoV-2 and ACE2. Molecules. 2023; 28(9):3908. https://doi.org/10.3390/molecules28093908
Chicago/Turabian StyleSipala, Federica, Gianfranco Cavallaro, Giuseppe Forte, Cristina Satriano, Alessandro Giuffrida, Aurore Fraix, Angelo Spadaro, Salvatore Petralia, Carmela Bonaccorso, Cosimo Gianluca Fortuna, and et al. 2023. "Different In Silico Approaches Using Heterocyclic Derivatives against the Binding between Different Lineages of SARS-CoV-2 and ACE2" Molecules 28, no. 9: 3908. https://doi.org/10.3390/molecules28093908





