Review of Characteristics and Analytical Methods for Determination of Thiabendazole
Abstract
:1. Introduction
2. Physicochemical Properties
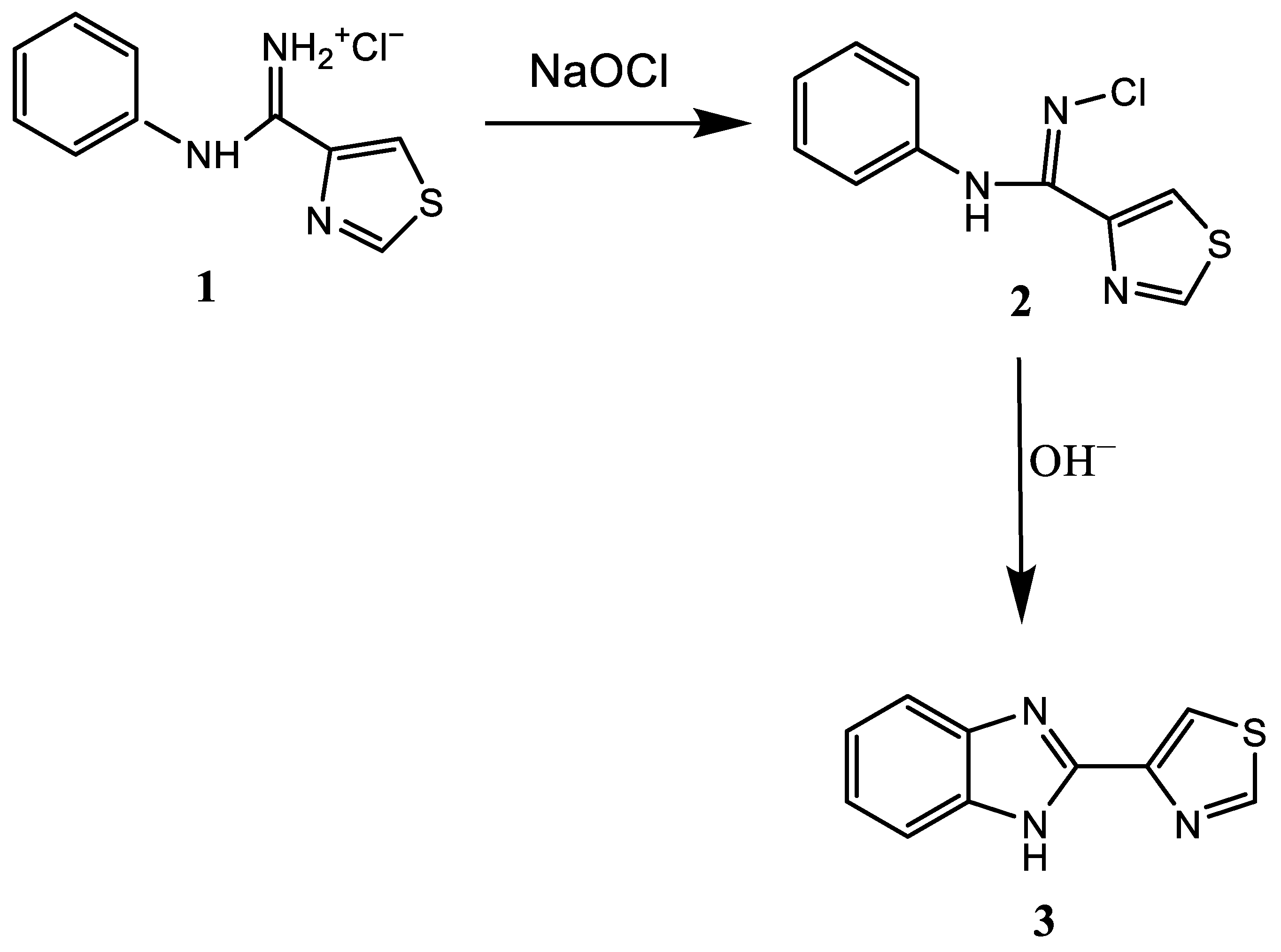
3. Synthesis
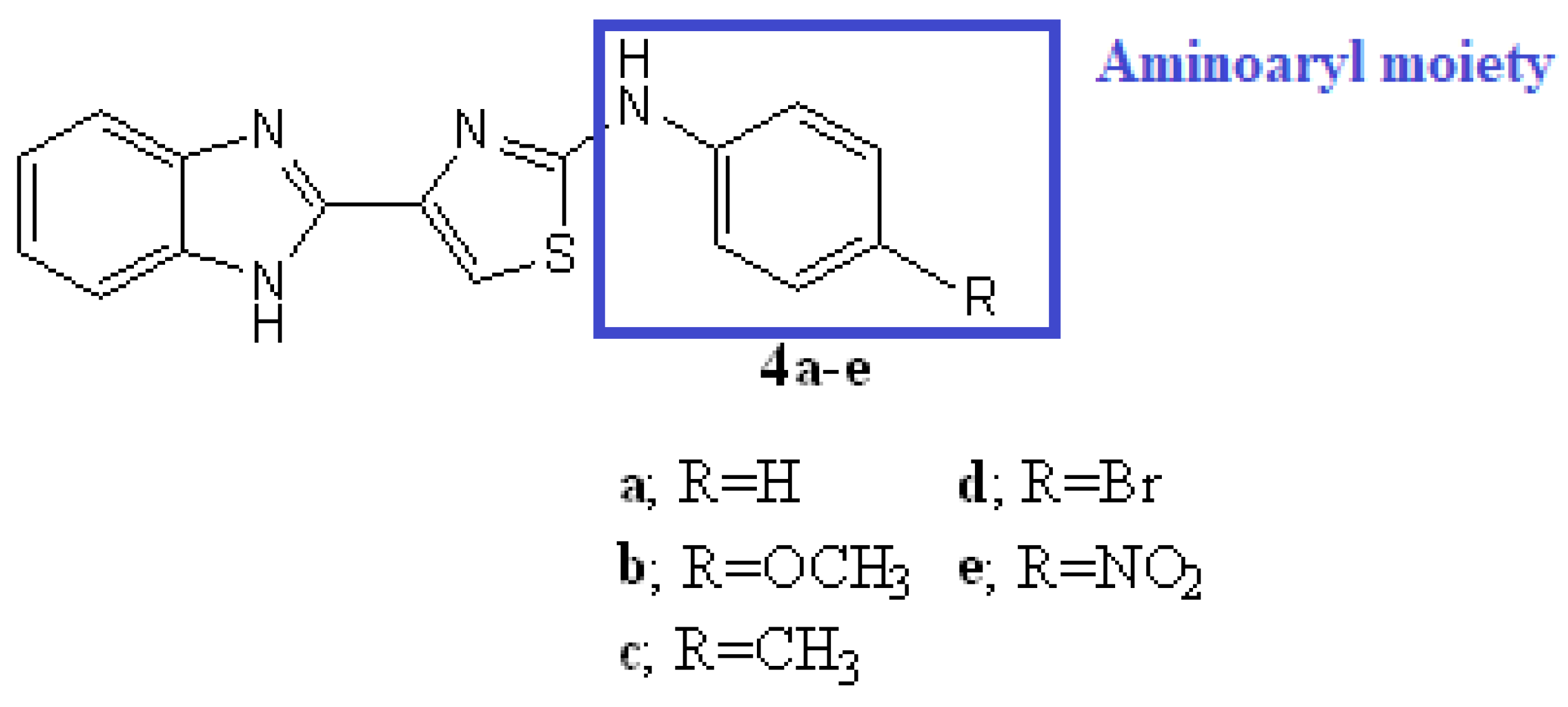
4. Structural Modifications
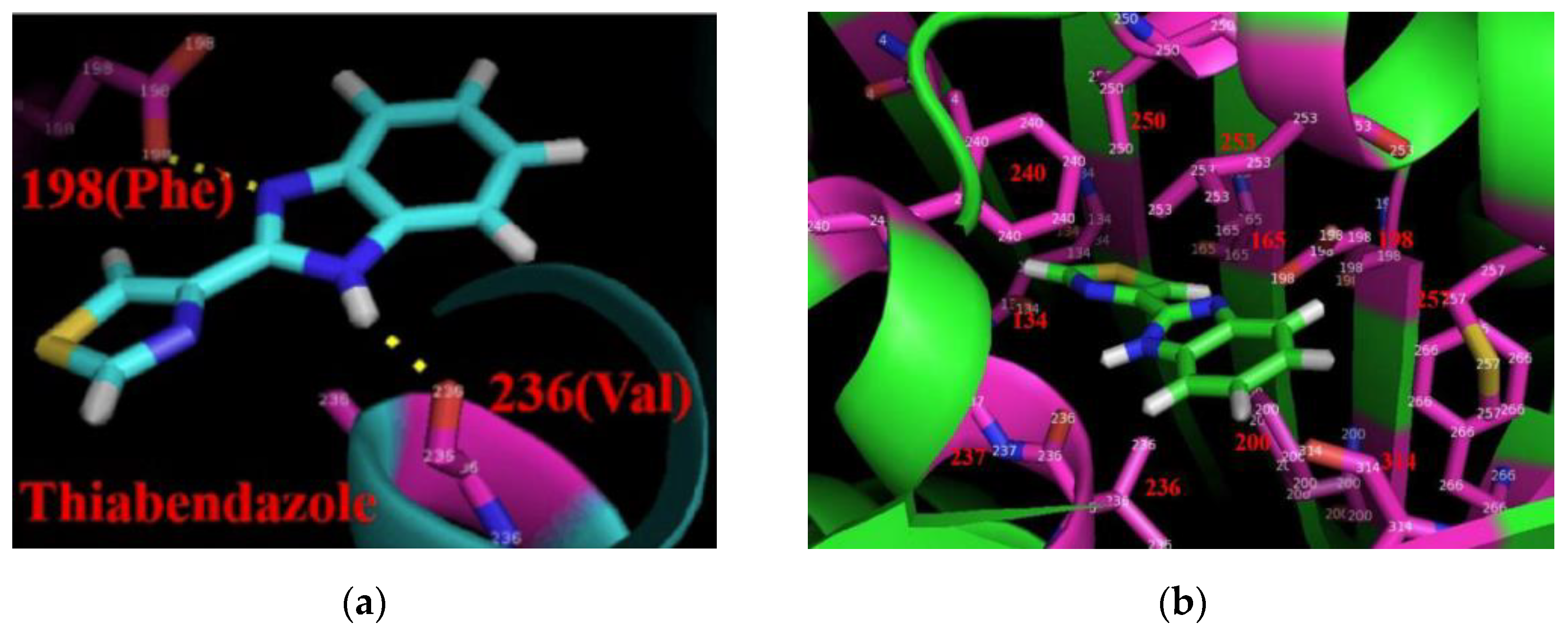
5. Mechanism of Action
6. Pharmacokinetics
7. Adverse Effects and Drug Interactions
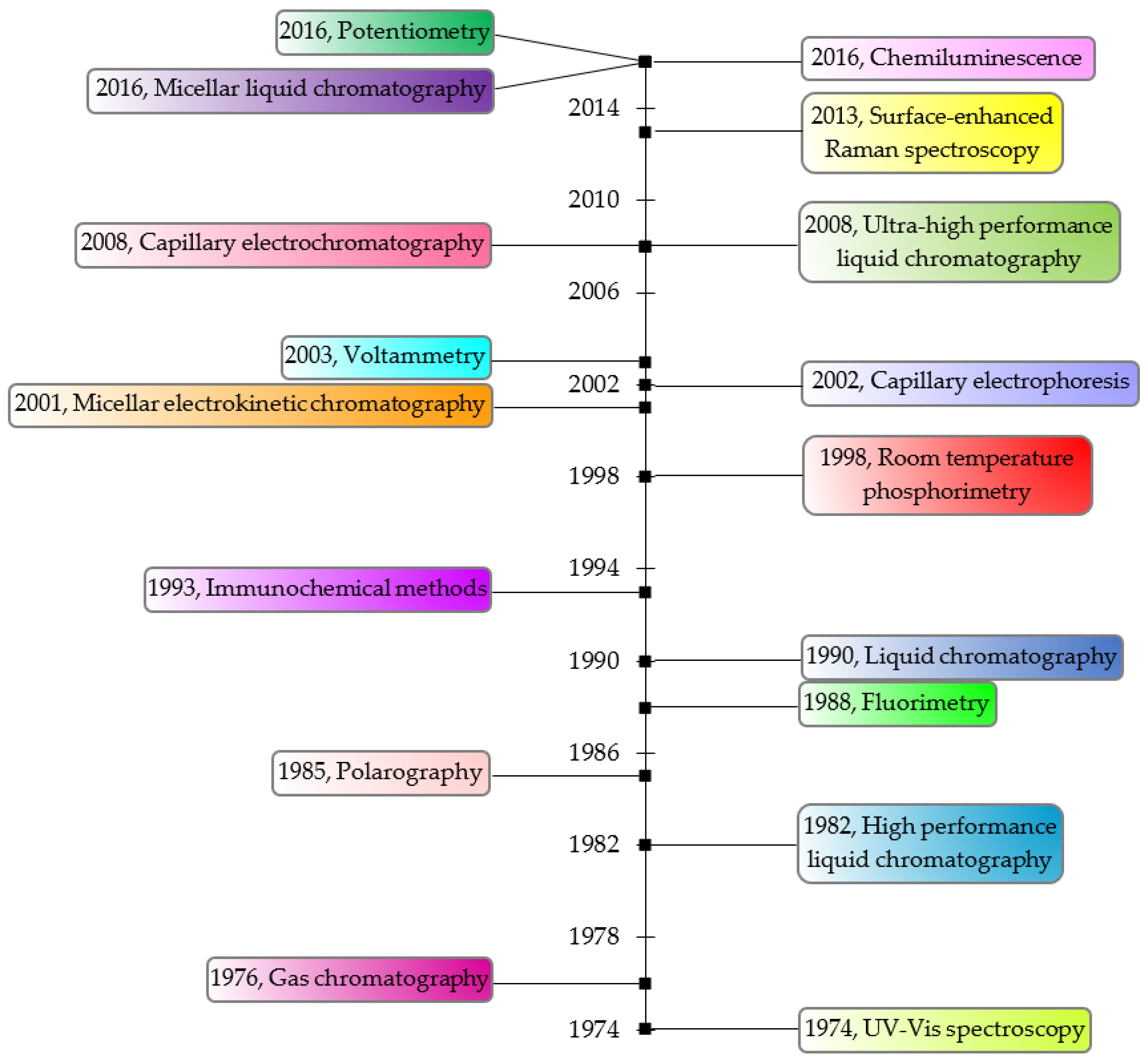
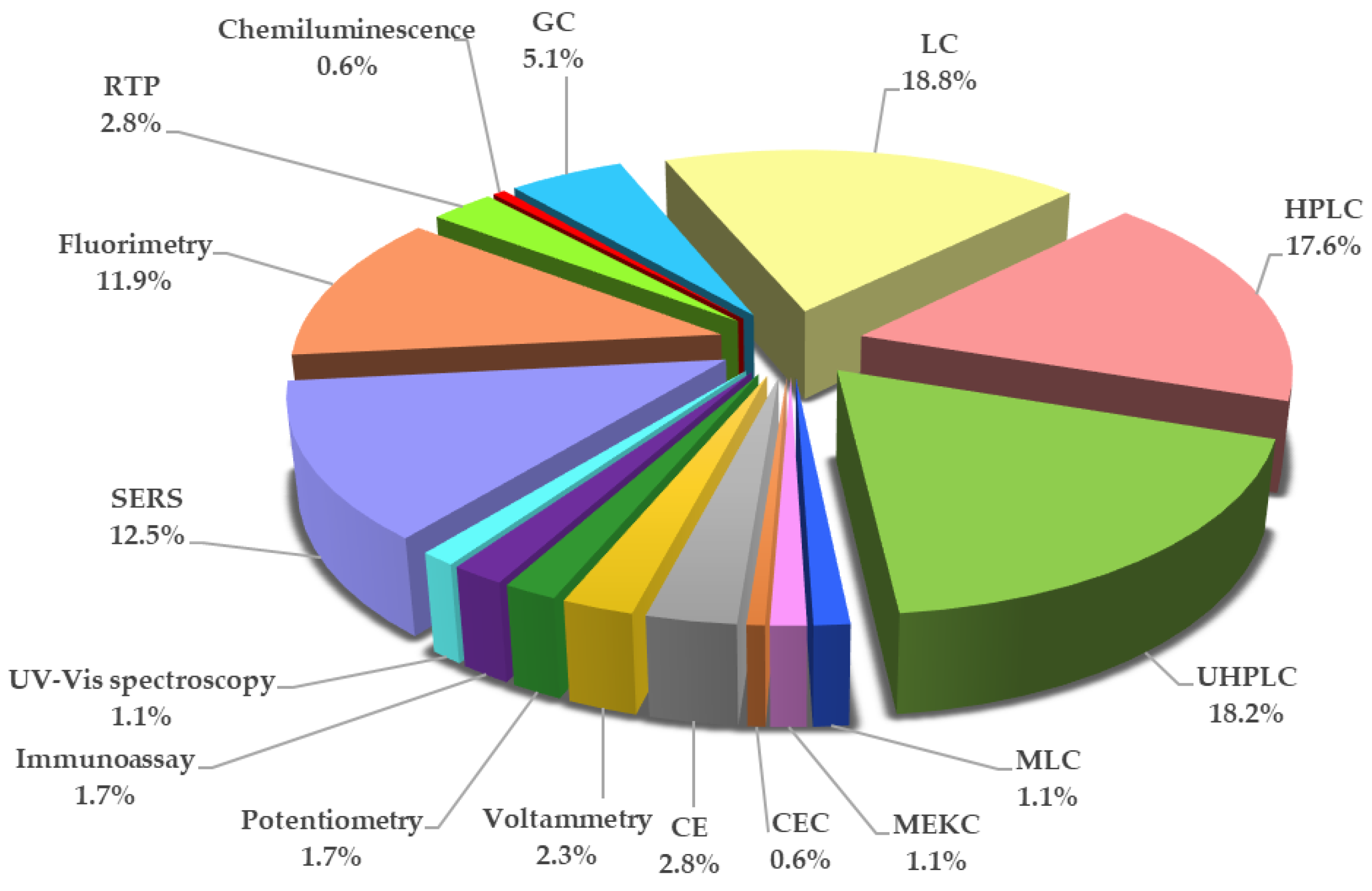
8. Analytical Methods for TBZ Determination
8.1. UV-Vis Spectroscopy
8.2. Surface-Enhanced Raman Spectroscopy
8.3. Fluorimetry
8.4. Room Temperature Phosphorimetry
8.5. Chemiluminescence
8.6. Gas Chromatography
8.7. Liquid Chromatography
8.7.1. High Performance Liquid Chromatography
8.7.2. Ultra-High-Performance Liquid Chromatography
8.8. Micellar Liquid Chromatography
8.9. Micellar Electrokinetic Chromatography
8.10. Capillary Electrochromatography
8.11. Capillary Electrophoresis
8.12. Voltammetry
8.13. Potentiometry
8.14. Immunoassay
9. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
Sample Availability
References
- World Health Organization. Class Names and the International Numbering System for Food Additives; World Health Organization: Geneva, Switzerland, 2021.
- Zhang, C.; Zhong, B.; Yang, S.; Pan, L.; Yu, S.; Li, Z.; Li, S.; Su, B.; Meng, X. Synthesis and Biological Evaluation of Thiabendazole Derivatives as Anti-Angiogenesis and Vascular Disrupting Agents. Bioorg. Med. Chem. 2015, 23, 3774–3780. [Google Scholar] [CrossRef] [PubMed]
- Alsammarraie, F.K.; Lin, M.; Mustapha, A.; Lin, H.; Chen, X.; Chen, Y.; Wang, H.; Huang, M. Rapid Determination of Thiabendazole in Juice by SERS Coupled with Novel Gold Nanosubstrates. Food Chem. 2018, 259, 219–225. [Google Scholar] [CrossRef] [PubMed]
- Estevez, M.C.; Belenguer, J.; Gomez-Montes, S.; Miralles, J.; Escuela, A.M.; Montoya, A.; Lechuga, L.M. Indirect Competitive Immunoassay for the Detection of Fungicide Thiabendazole in Whole Orange Samples by Surface Plasmon Resonance. Analyst 2012, 137, 5659–5665. [Google Scholar] [CrossRef]
- U.S. Food and Drug Administration. Pesticide Residue Monitoring Program Fiscal Year 2020 Pesticide Report; U.S. Food and Drug Administration: Silver Spring, MD, USA, 2020.
- Müller, C.; David, L.; Chiş, V.; Pînzaru, S.C. Detection of Thiabendazole Applied on Citrus Fruits and Bananas Using Surface Enhanced Raman Scattering. Food Chem. 2014, 145, 814–820. [Google Scholar] [CrossRef] [PubMed]
- Tsialla, Z.; Ucles-Moreno, A.; Petrou, P.; Fernandez-Alba, A.R.; Kakabakos, S.E. Development of an Indirect Enzyme Immunoassay for the Determination of Thiabendazole in White and Red Wines. Int. J. Environ. Anal. Chem. 2015, 95, 1299–1309. [Google Scholar] [CrossRef]
- EU Pesticides Database—MRLs—Pesticide Residue(s) and Maximum Residue Levels (Mg/Kg). Available online: https://ec.europa.eu/food/plant/pesticides/eu-pesticides-database/start/screen/mrls/details?lg_code=EN&pest_res_id_list=210&product_id_list= (accessed on 17 January 2023).
- United States Environmental Protection Agency R.E.D. FACTS Thiabendazole and Salts 2002. US EPA Archive Document. Available online: https://archive.epa.gov/pesticides/reregistration/web/pdf/thiabendazole_red.pdf (accessed on 17 January 2023).
- Dumancas, G.G.; Hikkaduwa Koralege, R.S.; Mojica, E.-R.E.; Murdianti, B.S.; Pham, P.J. Thiabendazole. In Encyclopedia of Toxicology; Elsevier: Amsterdam, The Netherlands, 2014; pp. 533–536. [Google Scholar]
- Zhang, J.; Zhao, C.; Gao, Y.; Jiang, Y.; Liang, H.; Zhao, G. Thiabendazole, a Well-Known Antifungal Drug, Exhibits Anti-Metastatic Melanoma B16F10 Activity via Inhibiting VEGF Expression and Inducing Apoptosis. Pharmazie 2013, 68, 962–968. [Google Scholar]
- Mothilal, K.K.; Karunakaran, C.; Rajendran, A.; Murugesan, R. Synthesis, X-ray Crystal Structure, Antimicrobial Activity and Photodynamic Effects of Some Thiabendazole Complexes. J. Inorg. Biochem. 2004, 98, 322–332. [Google Scholar] [CrossRef]
- Robinson, H.J.; Stoerk, H.C.; Graessle, O.E. Studies on the Toxicologic and Pharmacologic Properties of Thiabendazole. Toxicol. Appl. Pharmacol. 1965, 7, 53–63. [Google Scholar] [CrossRef]
- Thiabendazole|148-79-8. Available online: https://www.chemicalbook.com/ChemicalProductProperty_EN_CB1685813.htm (accessed on 12 January 2023).
- Brown, H.D.; Matzuk, A.R.; Ilves, I.R.; Peterson, L.H.; Harris, S.A.; Sarett, L.H.; Egerton, J.R.; Yakstis, J.J.; Campbell, W.C.; Cuckler, A.C. Antiparasitic Drugs. IV. 2-(4′-Thiazolyl)-Benzimidazole, a New Anthelmintic. J. Am. Chem. Soc. 1961, 83, 1764–1765. [Google Scholar] [CrossRef]
- Heterocyclic Compounds. Robert C. Elderfield. John Wiley & Sons, Inc., 440 Park Ave., South, New York 16, N.Y., 1961. Vii + 878cm. Price $37.50. J. Pharm. Sci. 1962, 51, 88. [CrossRef]
- Grenda, V.J.; Jones, R.E.; Gal, G.; Sletzinger, M. Novel Preparation of Benzimidazoles from N-Arylamidines. New Synthesis of Thiabendazole. J. Org. Chem. 1965, 30, 259–261. [Google Scholar] [CrossRef]
- Parziale, P.A.; Chang, T.-C.; Applegate, L.E. Process for Preparing Thiabendazole 1994. U.S. Patent 5,310,924, 10 May 1994. [Google Scholar]
- Sood, S.; Bala, R.; Kumar, V.; Singh, N.; Singh, K. Iodine Mediated Synthesis of Thiabendazole Derivatives and Their Antimicrobial Evaluation. Curr. Bioact. Compd. 2018, 14, 273–277. [Google Scholar] [CrossRef]
- Davidse, L.C.; Flach, W. Interaction of Thiabendazole with Fungal Tubulin. Biochim. Biophys. Acta 1978, 543, 82–90. [Google Scholar] [CrossRef] [PubMed]
- Sharp, M. Chapter 8 Benzimidazoles. Pharmacochem. Libr. 1997, 25, 195–238. [Google Scholar] [CrossRef]
- Vanden Bossche, H.; Thienpont, D.; Janssens, P.G. Capítulo 4: Pharmacology of Anthelmintics. In Chemotherapy of Gastrointestinal Helminths; Springer: Berlin/Heidelberg, Germany, 1985; pp. 125–181. [Google Scholar]
- Lacey, E. Mode of Action of Benzimidazoles. Parasitol. Today 1990, 6, 112–115. [Google Scholar] [CrossRef]
- McCracken, R.O.; Stillwell, W.H. A Possible Biochemical Mode of Action for Benzimidazole Anthelmintics. Int. J. Parasitol. 1991, 21, 99–104. [Google Scholar] [CrossRef]
- Baudoin, O.; Guéritte, F. Natural Bridged Biaryls with Axial Chirality and Antimitotic Properties. Stud. Nat. Prod. Chem. 2003, 29, 355–417. [Google Scholar] [CrossRef]
- Sun, X.; Zhao, Y.; Huang, J. Interactions between Benzimidazole Fungicides and Β2-Tubulin of Fusarium Graminearum. Mycosystema 2022, 41, 492–500. [Google Scholar] [CrossRef]
- Thiabendazole: Uses, Interactions, Mechanism of Action|DrugBank Online. Available online: https://go.drugbank.com/drugs/DB00730 (accessed on 13 January 2023).
- Marzano, C.; Pellei, M.; Tisato, F.; Santini, C. Copper Complexes as Anticancer Agents. Anticancer Agents Med. Chem. 2012, 9, 185–211. [Google Scholar] [CrossRef]
- Tocco, D.J.; Rosenblum, C.; Martin, C.M.; Robinson, H.J. Absorption, Metabolism, and Excretion of Thiabendazole in Man and Laboratory Animals. Toxicol. Appl. Pharmacol. 1966, 9, 31–39. [Google Scholar] [CrossRef]
- Fujitani, T.; Yoneyama, M.; Ogata, A.; Ueta, T.; Mori, K.; Ichikawa, H. New Metabolites of Thiabendazole and the Metabolism of Thiabendazole by Mouse Embryo in Vivo and in Vitro. Food Chem. Toxicol. 1991, 29, 265–274. [Google Scholar] [CrossRef] [PubMed]
- Thiabendazole: Indications, Side Effects, Warnings—Drugs.Com. Available online: https://www.drugs.com/cdi/thiabendazole.html (accessed on 16 January 2023).
- Abacavir: Uses, Interactions, Mechanism of Action|DrugBank Online. Available online: https://go.drugbank.com/drugs/DB01048 (accessed on 16 January 2023).
- Abametapir: Uses, Interactions, Mechanism of Action|DrugBank Online. Available online: https://go.drugbank.com/drugs/DB11932 (accessed on 16 January 2023).
- Aspirin: Uses, Interactions, Mechanism of Action|DrugBank Online. Available online: https://go.drugbank.com/drugs/DB00945 (accessed on 16 January 2023).
- Gomaa, A.A. The Effect of Thiabendazole on Pain Threshold. Acta Pharmacol. Toxicol. (Copenh) 1985, 57, 18–22. [Google Scholar] [CrossRef] [PubMed]
- Rajzman, A. Determination of Thiabendazole in Citrus Fruits by Ultraviolet Spectrophotometry. Analyst 1974, 99, 120–127. [Google Scholar] [CrossRef] [PubMed]
- Altunay, N.; Ülüzger, D.; Gürkan, R. Simple and Fast Spectrophotometric Determination of Low Levels of Thiabendazole Residues in Fruit and Vegetables after Pre-Concentration with Ionic Liquid Phase Microextraction. Food Addit. Contam.-Part A Chem. Anal. Control Expo. Risk Assess. 2018, 35, 1139–1154. [Google Scholar] [CrossRef] [PubMed]
- Tuzen, M.; Altunay, N.; Elik, A.; Afshar Mogaddam, M.R.; Katin, K. Experimental and Theoretical Investigation for the Spectrophotometric Determination of Thiabendazole in Fruit Samples. Microchem. J. 2021, 168, 106488. [Google Scholar] [CrossRef]
- Müller Molnár, C.; Leontin, D.; Pinzaru, S.C. Detection of Thiabendazole Applied to Organic Fruit by near Infrared Surface-Enhanced Raman Spectroscopy. Spectrosc. Eur. 2013, 25, 6–11. [Google Scholar]
- He, L.; Chen, T.; Labuza, T.P. Recovery and Quantitative Detection of Thiabendazole on Apples Using a Surface Swab Capture Method Followed by Surface-Enhanced Raman Spectroscopy. Food Chem. 2014, 148, 42–46. [Google Scholar] [CrossRef]
- Luo, H.; Huang, Y.; Lai, K.; Rasco, B.A.; Fan, Y. Surface-Enhanced Raman Spectroscopy Coupled with Gold Nanoparticles for Rapid Detection of Phosmet and Thiabendazole Residues in Apples. Food Control 2016, 68, 229–235. [Google Scholar] [CrossRef]
- Hong, J.; Kawashima, A.; Hamada, N. A Simple Fabrication of Plasmonic Surface-Enhanced Raman Scattering (SERS) Substrate for Pesticide Analysis via the Immobilization of Gold Nanoparticles on UF Membrane. Appl. Surf. Sci. 2017, 407, 440–446. [Google Scholar] [CrossRef]
- Feng, J.; Hu, Y.; Grant, E.; Lu, X. Determination of Thiabendazole in Orange Juice Using an MISPE-SERS Chemosensor. Food Chem. 2018, 239, 816–822. [Google Scholar] [CrossRef]
- Lin, L.; Dong, T.; Nie, P.; Qu, F.; He, Y.; Chu, B.; Xiao, S. Rapid Determination of Thiabendazole Pesticides in Rape by Surface Enhanced Raman Spectroscopy. Sensors 2018, 18, 1082. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.M.; Roy, P.K.; Juluri, B.K.; Chattopadhyay, S. A SERS Tattoo for in Situ, Ex Situ, and Multiplexed Detection of Toxic Food Additives. Sens. Actuators B Chem. 2018, 261, 218–225. [Google Scholar] [CrossRef]
- Nie, P.; Dong, T.; Xiao, S.; Lin, L.; He, Y.; Qu, F. Quantitative Determination of Thiabendazole in Soil Extracts by Surface-Enhanced Raman Spectroscopy. Molecules 2018, 23, 1949. [Google Scholar] [CrossRef] [PubMed]
- Kang, Y.; Wu, T.; Chen, W.; Li, L.; Du, Y. A Novel Metastable State Nanoparticle-Enhanced Raman Spectroscopy Coupled with Thin Layer Chromatography for Determination of Multiple Pesticides. Food Chem. 2019, 270, 494–501. [Google Scholar] [CrossRef]
- Fu, G.; Sun, D.W.; Pu, H.; Wei, Q. Fabrication of Gold Nanorods for SERS Detection of Thiabendazole in Apple. Talanta 2019, 195, 841–849. [Google Scholar] [CrossRef]
- Xuan, T.; Gao, Y.; Cai, Y.; Guo, X.; Wen, Y.; Yang, H. Fabrication and Characterization of the Stable Ag-Au-Metal-Organic-Frameworks: An Application for Sensitive Detection of Thiabendazole. Sens. Actuators B Chem. 2019, 293, 289–295. [Google Scholar] [CrossRef]
- Mekonnen, M.L.; Chen, C.H.; Osada, M.; Su, W.N.; Hwang, B.J. Dielectric Nanosheet Modified Plasmonic-Paper as Highly Sensitive and Stable SERS Substrate and Its Application for Pesticides Detection. Spectrochim. Acta-Part A Mol. Biomol. Spectrosc. 2020, 225, 117484. [Google Scholar] [CrossRef]
- Teixeira, C.A.; Poppi, R.J. Paper-Based SERS Substrate and One-Class Classifier to Monitor Thiabendazole Residual Levels in Extracts of Mango Peels. Spectrochim. Acta-Part A Mol. Biomol. Spectrosc. 2020, 229, 117913. [Google Scholar] [CrossRef]
- Wang, K.; Sun, D.W.; Pu, H.; Wei, Q. Two-Dimensional Au@Ag Nanodot Array for Sensing Dual-Fungicides in Fruit Juices with Surface-Enhanced Raman Spectroscopy Technique. Food Chem. 2019, 310, 125923. [Google Scholar] [CrossRef]
- Oliveira, M.J.S.; Rubira, R.J.G.; Furini, L.N.; Batagin-Neto, A.; Constantino, C.J.L. Detection of Thiabendazole Fungicide/Parasiticide by SERS: Quantitative Analysis and Adsorption Mechanism. Appl. Surf. Sci. 2020, 517, 145786. [Google Scholar] [CrossRef]
- Hu, B.; Sun, D.W.; Pu, H.; Wei, Q. Rapid Nondestructive Detection of Mixed Pesticides Residues on Fruit Surface Using SERS Combined with Self-Modeling Mixture Analysis Method. Talanta 2020, 217, 120998. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Mehedi Hassan, M.; Wang, J.; Wei, W.; Zou, M.; Ouyang, Q.; Chen, Q. Investigation of Nonlinear Relationship of Surface Enhanced Raman Scattering Signal for Robust Prediction of Thiabendazole in Apple. Food Chem. 2021, 339, 127843. [Google Scholar] [CrossRef] [PubMed]
- Hussain, A.; Pu, H.; Hu, B.; Sun, D.W. Au@Ag-TGANPs Based SERS for Facile Screening of Thiabendazole and Ferbam in Liquid Milk. Spectrochim. Acta-Part A Mol. Biomol. Spectrosc. 2021, 245, 118908. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Sun, Y.; Shi, J.; Zhang, W.; Zhang, X.; Huang, X.; Zou, X.; Li, Z.; Wei, R. Facile Synthesis of Au@Ag Core–Shell Nanorod with Bimetallic Synergistic Effect for SERS Detection of Thiabendazole in Fruit Juice. Food Chem. 2022, 370, 131276. [Google Scholar] [CrossRef] [PubMed]
- Pan, H.; Ahmad, W.; Jiao, T.; Zhu, A.; Ouyang, Q.; Chen, Q. Label-Free Au NRs-Based SERS Coupled with Chemometrics for Rapid Quantitative Detection of Thiabendazole Residues in Citrus. Food Chem. 2022, 375, 131681. [Google Scholar] [CrossRef]
- Huang, Y.; Wang, J.; Xue, S.F.; Tao, Z.; Zhu, Q.J.; Tang, Q. Determination of Thiabendazole in Aqueous Solutions Using a Cucurbituril-Enhanced Fluorescence Method. J. Incl. Phenom. Macrocycl. Chem. 2011, 72, 397–404. [Google Scholar] [CrossRef]
- García Sánchez, F.; Cruces Blanco, C. Spectrofluorometric Determination of Pesticide Residue Mixtures by Isodifferential Derivative Spectroscopy. Anal. Chem. 1988, 60, 323–328. [Google Scholar] [CrossRef]
- González Díaz, V.; González Armas, A.; Ossuna Martí, C.; Pérez Pont, M.L. Spectrofluorimetric Determination of Thiabendazole in a Micellar Medium. Mikrochim. Acta 1993, 111, 113–118. [Google Scholar] [CrossRef]
- Capitán, F.; Alonso, E.; Avidad, R.; Capitán-Vallvey, L.F.; Vilchez, J.L. Determination of Thiabendazole Residues in Waters by Solid-Phase Spectrofluorometry. Anal. Chem. 1993, 65, 1336–1339. [Google Scholar] [CrossRef]
- Garcia, L.F.; Aaron, J.J. Flow Injection Analysis for the Determination of Thiabendazole and Fuberidazole in Water by Spectrofluorimetry. Microchim. Acta 1997, 126, 289–294. [Google Scholar] [CrossRef]
- Picón Zamora, D.; Martínez Galera, M.; Garrido Frenich, A.; Martínez Vidal, J.L. Trace Determination of Carbendazim, Fuberidazole and Thiabendazole in Water by Application of Multivariate Calibration to Cross-Sections of Three-Dimensional Excitation–Emission Matrix Fluorescence. Analyst 2000, 125, 1167–1174. [Google Scholar] [CrossRef]
- Rodríguez-Cuesta, M.J.; Boqué, R.; Rius, F.X.; Picón Zamora, D.; Martínez Galera, M.; Garrido Frenich, A. Determination of Carbendazim, Fuberidazole and Thiabendazole by Three-Dimensional Excitation-Emission Matrix Fluorescence and Parallel Factor Analysis. Anal. Chim. Acta 2003, 491, 47–56. [Google Scholar] [CrossRef]
- de Armas, G.; Becerra, E.; Cladera, A.; Estela, J.M.; Cerdà, V. Sequential Injection Analysis for the Determination of Fuberidazole and Thiabendazole by Variable-Angle Scanning Fluorescence Spectrometry. Anal. Chim. Acta 2001, 427, 83–92. [Google Scholar] [CrossRef]
- de Armas, G.; Miró, M.; Estela, J.M.; Cerdà, V. Simultaneous Determination of Fluorophores with Overlapped Spectra by Sequential Injection Analysis Coupled to Variable Angle Scanning Fluorescence Spectrometry and Multivariate Linear Regression Algorithms. Anal. Chim. Acta 2002, 471, 173–186. [Google Scholar] [CrossRef]
- Ruedas Rama, M.J.; Ruiz Medina, A.; Molina Díaz, A. Use of a Solid Sensing Zone Implemented with Unsegmented Flow Analysis for Simultaneous Determination of Thiabendazole and Warfarin. Anal. Chim. Acta 2002, 459, 235–243. [Google Scholar] [CrossRef]
- García Reyes, J.F.; Llorent Martínez, E.J.; Ortega Barrales, P.; Molina Díaz, A. Continuous-Flow Separation and Pre-Concentration Coupled on-Line to Solid-Surface Fluorescence Spectroscopy for the Simultaneous Determination of o-Phenylphenol and Thiabendazole. Anal. Bioanal. Chem. 2004, 378, 429–437. [Google Scholar] [CrossRef] [PubMed]
- García-Reyes, J.F.; Ortega-Barrales, P.; Molina-Díaz, A. Development of a Single Fluorescence-Based Optosensor for Rapid Simultaneous Determination of Fungicides Benomyl and Thiabendazole in Waters and Commercial Formulations. J. Agric. Food Chem. 2004, 52, 2197–2202. [Google Scholar] [CrossRef]
- García-Reyes, J.F.; Llorent-Martínez, E.J.; Ortega-Barrales, P.; Molina-Díaz, A. Determination of Thiabendazole Residues in Citrus Fruits Using a Multicommuted Fluorescence-Based Optosensor. Anal. Chim. Acta 2006, 557, 95–100. [Google Scholar] [CrossRef]
- Piccirilli, G.N.; Escandar, G.M. A Novel Flow-through Fluorescence Optosensor for the Determination of Thiabendazole. Anal. Chim. Acta 2007, 601, 196–203. [Google Scholar] [CrossRef]
- López Flores, J.; Fernández de Córdova, M.L.; Molina Díaz, A. Simultaneous Flow-Injection Solid-Phase Fluorometric Determination of Thiabendazole and Metsulfuron Methyl Using Photochemical Derivatization. Anal. Sci. Int. J. Japan Soc. Anal. Chem. 2009, 25, 681–686. [Google Scholar] [CrossRef]
- Llorent-Martínez, E.J.; Fernández-de Córdova, M.L.; Ruiz-Medina, A.; Ortega-Barrales, P. Fluorimetric Determination of Thiabendazole Residues in Mushrooms Using Sequential Injection Analysis. Talanta 2012, 96, 190–194. [Google Scholar] [CrossRef] [PubMed]
- Zhong, X.-D.; Fu, D.-S.; Wu, P.-P.; Liu, Q.; Lin, G.-C.; Cao, S.-H.; Li, Y.-Q. Rapid Fluorescence Spectroscopic Screening Method for the Sensitive Detection of Thiabendazole in Red Wine. Anal. Methods 2014, 6, 7260–7267. [Google Scholar] [CrossRef]
- Li, W.; Wang, Y.; Huang, L.; Wu, T.; Hu, H.; Du, Y. Rapid Determination of Trace Thiabendazole in Apple Juice Utilizing Dispersive Liquid-Liquid Microextraction Combined with Fluorescence Spectrophotometry. Luminescence 2015, 30, 872–877. [Google Scholar] [CrossRef]
- Chen, Q.; Zuo, J.; He, X.; Mo, X.; Tong, P.; Zhang, L. Enhanced Fluorescence of Terbium with Thiabendazole and Application in Determining Trace Amounts of Terbium and Thiabendazole. Talanta 2017, 162, 540–546. [Google Scholar] [CrossRef]
- Murillo Pulgarín, J.A.; García Bermejo, L.F.; Becedas Rodríguez, S. Simultaneous Determination of 1-Naphthylacetic Acid and Thiabendazole in Strawberry Tree Berries and Citrus Fruits by Fluorescence Spectrometry. Food Anal. Methods 2018, 11, 394–402. [Google Scholar] [CrossRef]
- Kaur, N.; Raj, P.; Singh, A.; Singh, N.; Kim, D.Y. A Facile Route to Ionic Liquids-Functionalized ZnO Nanorods for the Fluorometric Sensing of Thiabendazole Drug. J. Mol. Liq. 2018, 261, 137–145. [Google Scholar] [CrossRef]
- Pagani, A.P.; Ibañez, G.A. Pesticide Residues in Fruits and Vegetables: High-Order Calibration Based on Spectrofluorimetric/PH Data. Microchem. J. 2019, 149, 104042. [Google Scholar] [CrossRef]
- Kazemifard, N.; Ensafi, A.A.; Rezaei, B. Green Synthesized Carbon Dots Embedded in Silica Molecularly Imprinted Polymers, Characterization and Application as a Rapid and Selective Fluorimetric Sensor for Determination of Thiabendazole in Juices. Food Chem. 2020, 310, 125812. [Google Scholar] [CrossRef]
- Peng, X.X.; Bao, G.M.; Zhong, Y.F.; Zhang, L.; Zeng, K.B.; He, J.X.; Xiao, W.; Xia, Y.F.; Fan, Q.; Yuan, H.Q. Highly Sensitive and Rapid Detection of Thiabendazole Residues in Oranges Based on a Luminescent Tb3+-Functionalized MOF. Food Chem. 2021, 343, 128504. [Google Scholar] [CrossRef]
- Chen, Y.; Wu, H.L.; Wang, T.; Sun, X.D.; Liu, B.B.; Chang, Y.Y.; Chen, J.C.; Ding, Y.J.; Yu, R.Q. Quantitative Analysis of Carbaryl and Thiabendazole in Complex Matrices Using Excitation-Emission Fluorescence Matrices with Second-Order Calibration Methods. Spectrochim. Acta-Part A Mol. Biomol. Spectrosc. 2022, 264, 120267. [Google Scholar] [CrossRef]
- Parker, R.T.; Freedlander, R.S.; Bruce Dunlap, R. The Development of Room Temperature Phosphorescence into a New Technique for Chemical Determinations: Part 2. Analytical Considerations of Room Temperature Phosphorimetry. Anal. Chim. Acta 1980, 120, 1–17. [Google Scholar] [CrossRef]
- Segura Carretero, A.; Cruces Blanco, C.; Estrada Fernández, R.; Fernández Gutiérrez, A. Micellar-Stabilized Room-Temperature Phosphorimetric Determination of the Fungicide Thiabendazole in Canned Pineapple Samples. Fresenius. J. Anal. Chem. 1998, 360, 605–608. [Google Scholar] [CrossRef]
- Segura-Carretero, A.; Cruces-Blanco, C.; Cañabate-Díaz, B.; Fernández-Sánchez, J.F.; Fernández-Gutiérrez, A. Heavy-Atom Induced Room-Temperature Phosphorescence: A Straightforward Methodology for the Determination of Organic Compounds in Solution. Anal. Chim. Acta 2000, 417, 19–30. [Google Scholar] [CrossRef]
- Cañabate Díaz, B.; Segura Carretero, A.; Cruces Blanco, C.; Fernandez Gutierrez, A. Simultaneous Determination of the Pesticides Carbaryl and Thiabendazole in Environmental Samples by a Three-Dimensional Derivative Variable-Angle and a Synchronous Room-Temperature Phosphorescence Spectroscopy. Appl. Spectrosc. 2003, 57, 1585–1591. [Google Scholar] [CrossRef] [PubMed]
- Tang, B.; Wang, X.; Wang, G.; Wang, Y.; Chen, Z. Highly Sensitive and Selective Room-Temperature Phosphorescence Determination of Thiabendazole by the Supramolecular Interaction of Thiabendazole/β-Cyclodextrin/Triton X-100. Analyst 2005, 130, 1038–1045. [Google Scholar] [CrossRef] [PubMed]
- Correa, R.A.; Escandar, G.M. A New Analytical Application of Nylon-Induced Room-Temperature Phosphorescence: Determination of Thiabendazole in Water Samples. Anal. Chim. Acta 2006, 571, 58–65. [Google Scholar] [CrossRef] [PubMed]
- Piccirilli, G.N.; Escandar, G.M. Flow Injection Analysis with On-Line Nylon Powder Extraction for Room-Temperature Phosphorescence Determination of Thiabendazole. Anal. Chim. Acta 2009, 646, 90–96. [Google Scholar] [CrossRef]
- Dinh, T.V.; Winefordner, J.D. Room Temperature Phosphorimetry as a New Spectrochemical Method of Analysis. Appl. Spectrosc. Rev. 1977, 13, 261–294. [Google Scholar] [CrossRef]
- Pawliszyn, J.; Bayona, J.M.; Dugo, P.; Le, X.C.; Lee, H.K.; Li, X.-F.; Lord, H.L. Comprehensive Sampling and Sample Preparation: Analytical Techniques for Scientists, 1st ed.; Academic Press: Cambridge, MA, USA, 2012; ISBN 0123813735. [Google Scholar]
- Asghar, M.; Yaqoob, M.; Munawar, N.; Nabi, A. Flow-Injection Determination of Thiabendazole Fungicide in Water Samples Using a Diperiodatocuprate(III)-Sulfuric Acid-Chemiluminescence System. Anal. Sci. 2016, 32, 337–342. [Google Scholar] [CrossRef]
- Harvey, D. Modern Analytical Chemestry; Smith, J.M., Ed.; McGraw-Hill: Boston, MA, USA, 2000; ISBN 0071169539. [Google Scholar]
- Tanaka, A.; Fujimoto, Y. Gas Chromatographic Determination of Thiabendazole in Fruits as Its Methyl Derivative. J. Chromatogr. A 1976, 117, 149–160. [Google Scholar] [CrossRef]
- Nose, N.; Kobayashi, S.; Tanaka, A.; Hirose, A.; Watanabe, A. Determination of Thiabendazole by Electron-Capture Gas-Liquid Chromatography after Reaction with Pentafluorobenzoyl Chloride. J. Chromatogr. A 1977, 130, 410–413. [Google Scholar] [CrossRef] [PubMed]
- Maštovská, K.; Lehotay, S.J.; Hajšlová, J. Optimization and Evaluation of Low-Pressure Gas Chromatography-Mass Spectrometry for the Fast Analysis of Multiple Pesticide Residues in a Food Commodity. J. Chromatogr. A 2001, 926, 291–308. [Google Scholar] [CrossRef] [PubMed]
- Walorczyk, S. Development of a Multi-Residue Screening Method for the Determination of Pesticides in Cereals and Dry Animal Feed Using Gas Chromatography–Triple Quadrupole Tandem Mass Spectrometry. J. Chromatogr. A 2007, 1165, 200–212. [Google Scholar] [CrossRef] [PubMed]
- Walorczyk, S. Development of a Multi-Residue Method for the Determination of Pesticides in Cereals and Dry Animal Feed Using Gas Chromatography-Tandem Quadrupole Mass Spectrometry II. Improvement and Extension to New Analytes. J. Chromatogr. A 2008, 1208, 202–214. [Google Scholar] [CrossRef] [PubMed]
- Walorczyk, S.; Drodyński, D.; Gnusowski, B. Multiresidue Determination of 160 Pesticides in Wines Employing Mixed-Mode Dispersive-Solid Phase Extraction and Gas Chromatography–Tandem Mass Spectrometry. Talanta 2011, 85, 1856–1870. [Google Scholar] [CrossRef]
- Silva, M.G.D.; Aquino, A.; Dórea, H.S.; Navickiene, S. Simultaneous Determination of Eight Pesticide Residues in Coconut Using MSPD and GC/MS. Talanta 2008, 76, 680–684. [Google Scholar] [CrossRef]
- Lesueur, C.; Knittl, P.; Gartner, M.; Mentler, A.; Fuerhacker, M. Analysis of 140 Pesticides from Conventional Farming Foodstuff Samples after Extraction with the Modified QuECheRS Method. Food Control 2008, 19, 906–914. [Google Scholar] [CrossRef]
- Menezes Filho, A.; dos Santos, F.N.; de Paula Pereira, P.A. Development, Validation and Application of a Methodology Based on Solid-Phase Micro Extraction Followed by Gas Chromatography Coupled to Mass Spectrometry (SPME/GC-MS) for the Determination of Pesticide Residues in Mangoes. Talanta 2010, 81, 346–354. [Google Scholar] [CrossRef]
- Machado, I.; Gérez, N.; Pistón, M.; Heinzen, H.; Cesio, M.V. Determination of Pesticide Residues in Globe Artichoke Leaves and Fruits by GC–MS and LC–MS/MS Using the Same QuEChERS Procedure. Food Chem. 2017, 227, 227–236. [Google Scholar] [CrossRef]
- Gionfriddo, E.; Gruszecka, D.; Li, X.; Pawliszyn, J. Direct-Immersion SPME in Soy Milk for Pesticide Analysis at Trace Levels by Means of a Matrix-Compatible Coating. Talanta 2020, 211, 120746. [Google Scholar] [CrossRef]
- Lees, M.; Humber Institute of Food and Fisheries. Food Authenticity and Traceability; CRC Press: Boca Raton, FL, USA, 2003; ISBN 9781855735262. [Google Scholar]
- Authority, E.F.S. Conclusion on the Peer Review of the Pesticide Risk Assessment of the Active Substance Thiabendazole. EFSA J. 2014, 12, 3880. [Google Scholar] [CrossRef]
- Long, A.R.; Hsieh, L.C.; Malbrough, M.S.; Short, C.R.; Barker, S.A. Matrix Solid Phase Dispersion (MSPD) Extraction and Liquid Chromatographic Determination of Five Benzimidazole Anthelmintics in Pork Muscle Tissue. J. Food Compos. Anal. 1990, 3, 20–26. [Google Scholar] [CrossRef]
- García Sánchez, F.; Navas Díaz, A.; García Pareja, A.G. Ion-Pair Reversed-Phase Liquid Chromatography with Fluorimetric Detection of Pesticides. J. Chromatogr. A 1994, 676, 347–354. [Google Scholar] [CrossRef]
- Cannavan, A.; Haggan, S.A.; Kennedy, D.G. Simultaneous Determination of Thiabendazole and Its Major Metabolite, 5-Hydroxythiabendazole, in Bovine Tissues Using Gradient Liquid Chromatography with Thermospray and Atmospheric Pressure Chemical Ionisation Mass Spectrometry. J. Chromatogr. B Biomed. Appl. 1998, 718, 103–113. [Google Scholar] [CrossRef]
- Lacassie, E.; Dreyfuss, M.F.; Daguet, J.L.; Vignaud, M.; Marquet, P.; Lachâtre, G. Liquid Chromatography–Electrospray Mass Spectrometry Multi-Residue Determination of Pesticides in Apples and Pears. J. Chromatogr. A 1999, 830, 135–143. [Google Scholar] [CrossRef]
- Balizs, G. Determination of Benzimidazole Residues Using Liquid Chromatography and Tandem Mass Spectrometry. J. Chromatogr. B Biomed. Sci. Appl. 1999, 727, 167–177. [Google Scholar] [CrossRef]
- Thiabendazole|C10H7N3S—PubChem. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Thiabendazole (accessed on 27 April 2023).
- Fernández-Alba, A.R.; Tejedor, A.; Agüera, A.; Contreras, M.; Garrido, J. Determination of Imidacloprid and Benzimidazole Residues in Fruits and Vegetables by Liquid Chromatography-Mass Spectrometry after Ethyl Acetate Multiresidue Extraction. J. AOAC Int. 2000, 83, 748–755. [Google Scholar] [CrossRef]
- Fernández, M.; Rodríguez, R.; Picó, Y.; Mañes, J. Liquid Chromatographic–Mass Spectrometric Determination of Post-Harvest Fungicides in Citrus Fruits. J. Chromatogr. A 2001, 912, 301–310. [Google Scholar] [CrossRef]
- Pous, X.; Ruíz, M.J.; Picó, Y.; Font, G. Determination of Imidacloprid, Metalaxyl, Myclobutanil, Propham, and Thiabendazole in Fruits and Vegetables by Liquid Chromatography-Atmospheric Pressure Chemical Ionization-Mass Spectrometry. Fresenius. J. Anal. Chem. 2001, 371, 182–189. [Google Scholar] [CrossRef]
- Yoshioka, N.; Akiyama, Y.; Teranishi, K. Rapid Simultaneous Determination of O-Phenylphenol, Diphenyl, Thiabendazole, Imazalil and Its Major Metabolite in Citrus Fruits by Liquid Chromatography-Mass Spectrometry Using Atmospheric Pressure Photoionization. J. Chromatogr. A 2004, 1022, 145–150. [Google Scholar] [CrossRef]
- Yoshioka, N.; Akiyama, Y.; Matsuoka, T.; Mitsuhashi, T. Rapid Determination of Five Post-Harvest Fungicides and Metabolite in Citrus Fruits by Liquid Chromatography/Time-of-Flight Mass Spectrometry with Atmospheric Pressure Photoionization. Food Control 2010, 21, 212–216. [Google Scholar] [CrossRef]
- Agüera, A.; López, S.; Fernández-Alba, A.R.; Contreras, M.; Crespo, J.; Piedra, L. One-Year Routine Application of a New Method Based on Liquid Chromatography–Tandem Mass Spectrometry to the Analysis of 16 Multiclass Pesticides in Vegetable Samples. J. Chromatogr. A 2004, 1045, 125–135. [Google Scholar] [CrossRef] [PubMed]
- Zamora, T.; Pozo, O.J.; López, F.J.; Hernández, F. Determination of Tridemorph and Other Fungicide Residues in Fruit Samples by Liquid Chromatography-Electrospray Tandem Mass Spectrometry. J. Chromatogr. A 2004, 1045, 137–143. [Google Scholar] [CrossRef] [PubMed]
- Dowling, G.; Cantwell, H.; O’Keeffe, M.; Smyth, M.R. Multi-Residue Method for the Determination of Benzimidazoles in Bovine Liver. Anal. Chim. Acta 2005, 529, 285–292. [Google Scholar] [CrossRef]
- Msagati, T.A.M.; Nindi, M.M. Comparative Study of Sample Preparation Methods; Supported Liquid Membrane and Solid Phase Extraction in the Determination of Benzimidazole Anthelmintics in Biological Matrices by Liquid Chromatography-Electrospray-Mass Spectrometry. Talanta 2006, 69, 243–250. [Google Scholar] [CrossRef]
- García-Reyes, J.F.; Gilbert-López, B.; Molina-Díaz, A.; Fernández-Alba, A.R. Determination of Pesticide Residues in Fruit-Based Soft Drinks. Anal. Chem. 2008, 80, 8966–8974. [Google Scholar] [CrossRef]
- Gilbert-López, B.; García-Reyes, J.F.; Mezcua, M.; Ramos-Martos, N.; Fernández-Alba, A.R.; Molina-Díaz, A. Multi-Residue Determination of Pesticides in Fruit-Based Soft Drinks by Fast Liquid Chromatography Time-of-Flight Mass Spectrometry. Talanta 2010, 81, 1310–1321. [Google Scholar] [CrossRef]
- Gilbert-López, B.; Jaén-Martos, L.; García-Reyes, J.F.; Villar-Pulido, M.; Polgar, L.; Ramos-Martos, N.; Molina-Díaz, A. Study on the Occurrence of Pesticide Residues in Fruit-Based Soft Drinks from the EU Market and Morocco Using Liquid Chromatography–Mass Spectrometry. Food Control 2012, 26, 341–346. [Google Scholar] [CrossRef]
- Gilbert-López, B.; García-Reyes, J.F.; Molina-Díaz, A. Determination of Fungicide Residues in Baby Food by Liquid Chromatography-Ion Trap Tandem Mass Spectrometry. Food Chem. 2012, 135, 780–786. [Google Scholar] [CrossRef]
- Moral, A.; Sicilia, M.D.; Rubio, S. Determination of Benzimidazolic Fungicides in Fruits and Vegetables by Supramolecular Solvent-Based Microextraction/Liquid Chromatography/Fluorescence Detection. Anal. Chim. Acta 2009, 650, 207–213. [Google Scholar] [CrossRef]
- Moral, A.; Sicilia, M.D.; Rubio, S. Supramolecular Solvent-Based Extraction of Benzimidazolic Fungicides from Natural Waters Prior to Their Liquid Chromatographic/Fluorimetric Determination. J. Chromatogr. A 2009, 1216, 3740–3745. [Google Scholar] [CrossRef] [PubMed]
- Economou, A.; Botitsi, H.; Antoniou, S.; Tsipi, D. Determination of Multi-Class Pesticides in Wines by Solid-Phase Extraction and Liquid Chromatography-Tandem Mass Spectrometry. J. Chromatogr. A 2009, 1216, 5856–5867. [Google Scholar] [CrossRef] [PubMed]
- Barahona, F.; Gjelstad, A.; Pedersen-Bjergaard, S.; Rasmussen, K.E. Hollow Fiber-Liquid-Phase Microextraction of Fungicides from Orange Juices. J. Chromatogr. A 2010, 1217, 1989–1994. [Google Scholar] [CrossRef] [PubMed]
- Dreassi, E.; Zanfini, A.; Zizzari, A.T.; La Rosa, C.; Botta, M.; Corbini, G. Lc/Esi/Ms/Ms Determination of Postharvest Fungicide Residues in Citrus Juices. LWT-Food Sci. Technol. 2010, 43, 1301–1306. [Google Scholar] [CrossRef]
- Cho, S.K.; Abd El-Aty, A.M.; Park, K.H.; Park, J.H.; Assayed, M.E.; Jeong, Y.M.; Park, Y.S.; Shim, J.H. Simple Multiresidue Extraction Method for the Determination of Fungicides and Plant Growth Regulator in Bean Sprouts Using Low Temperature Partitioning and Tandem Mass Spectrometry. Food Chem. 2013, 136, 1414–1420. [Google Scholar] [CrossRef]
- Reichert, B.; Pizzutti, I.R.; Costabeber, I.H.; Uclés, A.; Herrera, S.; Fernández-Alba, A.R. Validation and Application of Micro Flow Liquid Chromatography-Tandem Mass Spectrometry for the Determination of Pesticide Residues in Fruit Jams. Talanta 2015, 134, 415–424. [Google Scholar] [CrossRef]
- Vissers, J.P.C.; Claessens, H.A.; Cramers, C.A. Microcolumn Liquid Chromotography: Instrumentation, Detection and Applications. J. Chromatogr. A 1997, 779, 1–28. [Google Scholar] [CrossRef]
- Uclés, A.; Herrera López, S.; Dolores Hernando, M.; Rosal, R.; Ferrer, C.; Fernández-Alba, A.R. Application of Zirconium Dioxide Nanoparticle Sorbent for the Clean-up Step in Post-Harvest Pesticide Residue Analysis. Talanta 2015, 144, 51–61. [Google Scholar] [CrossRef]
- Dasenaki, M.E.; Thomaidis, N.S. Multi-Residue Determination of 115 Veterinary Drugs and Pharmaceutical Residues in Milk Powder, Butter, Fish Tissue and Eggs Using Liquid Chromatography-Tandem Mass Spectrometry. Anal. Chim. Acta 2015, 880, 103–121. [Google Scholar] [CrossRef]
- Han, Y.; Zou, N.; Song, L.; Li, Y.; Qin, Y.; Liu, S.; Li, X.; Pan, C. Simultaneous Determination of 70 Pesticide Residues in Leek, Leaf Lettuce and Garland Chrysanthemum Using Modified QuEChERS Method with Multi-Walled Carbon Nanotubes as Reversed-Dispersive Solid-Phase Extraction Materials. J. Chromatogr. B 2015, 1005, 56–64. [Google Scholar] [CrossRef]
- Boix, C.; Ibáñez, M.; Fabregat-Safont, D.; Morales, E.; Pastor, L.; Sancho, J.V.; Sánchez-Ramírez, J.E.; Hernández, F. Analytical Methodologies Based on LC–MS/MS for Monitoring Selected Emerging Compounds in Liquid and Solid Phases of the Sewage Sludge. MethodsX 2016, 3, 333–342. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.G.; Park, D.W.; Kang, G.R.; Kim, T.S.; Yang, Y.; Moon, S.J.; Choi, E.A.; Ha, D.R.; Kim, E.S.; Cho, B.S. Simultaneous Determination of Plant Growth Regulator and Pesticides in Bean Sprouts by Liquid Chromatography-Tandem Mass Spectrometry. Food Chem. 2016, 208, 239–244. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, J.A.; Ferreira, J.M.S.; Talamini, V.; Facco, J.d.F.; Rizzetti, T.M.; Prestes, O.D.; Adaime, M.B.; Zanella, R.; Bottoli, C.B.G. Determination of Pesticides in Coconut (Cocos Nucifera Linn.) Water and Pulp Using Modified QuEChERS and LC-MS/MS. Food Chem. 2016, 213, 616–624. [Google Scholar] [CrossRef] [PubMed]
- Arias, J.L.d.O.; Schneider, A.; Batista-Andrade, J.A.; Vieira, A.A.; Caldas, S.S.; Primel, E.G. Chitosan from Shrimp Shells: A Renewable Sorbent Applied to the Clean-up Step of the QuEChERS Method in Order to Determine Multi-Residues of Veterinary Drugs in Different Types of Milk. Food Chem. 2018, 240, 1243–1253. [Google Scholar] [CrossRef]
- da Costa Morais, E.H.; Collins, C.H.; Jardim, I.C.S.F. Pesticide Determination in Sweet Peppers Using QuEChERS and LC–MS/MS. Food Chem. 2018, 249, 77–83. [Google Scholar] [CrossRef]
- Cerqueira, M.B.R.; Soares, K.L.; Caldas, S.S.; Primel, E.G. Sample as Solid Support in MSPD: A New Possibility for Determination of Pharmaceuticals, Personal Care and Degradation Products in Sewage Sludge. Chemosphere 2018, 211, 875–883. [Google Scholar] [CrossRef]
- Colazzo, M.; Alonso, B.; Ernst, F.; Cesio, M.V.; Perez-Parada, A.; Heinzen, H.; Pareja, L. Determination of Multiclass, Semi-Polar Pesticide Residues in Fatty Fish Muscle Tissue by Gas and Liquid Chromatography Mass Spectrometry. MethodsX 2019, 6, 929–937. [Google Scholar] [CrossRef]
- Martínez-Piernas, A.B.; Plaza-Bolaños, P.; Gilabert, A.; Agüera, A. Application of a Fast and Sensitive Method for the Determination of Contaminants of Emerging Concern in Wastewater Using a Quick, Easy, Cheap, Effective, Rugged and Safe-Based Extraction and Liquid Chromatography Coupled to Mass Spectrometry. J. Chromatogr. A 2021, 1653, 462396. [Google Scholar] [CrossRef]
- Fares, N.V.; Hassan, Y.A.A.; Hussein, L.A.; Ayad, M.F. Determination of Fungicides’ Residues and Their Degradation Kinetics in Orange Tree Fruits Using Liquid Chromatography—Tandem Mass Spectrometry Coupled with QuEChERS Method. Microchem. J. 2021, 168, 106376. [Google Scholar] [CrossRef]
- Zheng, D.; Hu, X.; Fu, X.; Xia, Z.; Zhou, Y.; Peng, L.; Yu, Q.; Peng, X. Flowerlike Ni–NiO Composite as Magnetic Solid-Phase Extraction Sorbent for Analysis of Carbendazim and Thiabendazole in Edible Vegetable Oils by Liquid Chromatography-Mass Spectrometry. Food Chem. 2022, 374, 131761. [Google Scholar] [CrossRef]
- Watts, M.T.; Raisys, V.A.; Bauer, L.A. Determination of Thiabendazole and 5-Hydroxythiabendazole in Human Serum by Fluorescence-Detected High-Performance Liquid Chromatography. J. Chromatogr. 1982, 230, 79–86. [Google Scholar] [CrossRef] [PubMed]
- Collinge, A.; Noirfalise, A. Determination of Thiabendazole Residues in Marmalades by High-Performance Liquid Chromatography. J. Chromatogr. A 1983, 257, 416–418. [Google Scholar] [CrossRef] [PubMed]
- Sannino, A. Investigation into Contamination of Processed Fruit Products by Carbendazim, Methyl Thiophanate and Thiabendazole. Food Chem. 1995, 52, 57–61. [Google Scholar] [CrossRef]
- Bushway, R.J. Complementation of Direct-Injection High-Performance Liquid Chromatography and Enzyme-Linked Immunosorbent Assay for the Analysis of Thiabendazole in Fruit Juices and Concentrates. J. Chromatogr. A 1996, 754, 431–435. [Google Scholar] [CrossRef] [PubMed]
- Tharsis, N.; Portillo, J.L.; Broto-Puig, F.; Comellas, L. Simplified Reversed-Phase Conditions for the Determination of Benzimidazole Fungicides in Fruits by High-Performance Liquid Chromatography with UV Detection. J. Chromatogr. A 1997, 778, 95–101. [Google Scholar] [CrossRef]
- Bushway, R.J.; Perkins, L.B.; Larkin, K.L.; Fan, T.S. A Modified High Performance Liquid Chromatographic Analysis of Thiabendazole in Fruits and Vegetables with ELISA Confirmation. J. Liq. Chromatogr. Relat. Technol. 1998, 21, 1217–1226. [Google Scholar] [CrossRef]
- Ito, Y.; Ikai, Y.; Oka, H.; Hayakawa, J.; Kagami, T. Application of Ion-Exchange Cartridge Clean-up in Food Analysis. I. Simultaneous Determination of Thiabendazole and Imazalil in Citrus Fruit and Banana Using High-Performance Liquid Chromatography with Ultraviolet Detection. J. Chromatogr. A 1998, 810, 81–87. [Google Scholar] [CrossRef]
- Di Muccio, A.; Girolimetti, S.; Attard Barbini, D.; Pelosi, P.; Generali, T.; Vergori, L.; De Merulis, G.; Leonelli, A.; Stefanelli, P. Selective Clean-up Applicable to Aqueous Acetone Extracts for the Determination of Carbendazim and Thiabendazole in Fruits and Vegetables by High-Performance Liquid Chromatography with UV Detection. J. Chromatogr. A 1999, 833, 61–65. [Google Scholar] [CrossRef]
- De Ruyck, H.; Van Renterghem, R.; De Ridder, H.; De Brabander, D. Determination of Anthelmintic Residues in Milk by High Performance Liquid Chromatography. Food Control 2000, 11, 165–173. [Google Scholar] [CrossRef]
- Su, S.C.; Chang, C.L.; Chang, P.C.; Chou, S.S. Simultaneous Determination of Albendazole, Thiabendazole, Mebendazole and Their Metabolites in Livestock by High Performance Liquid Chromatography. J. Food Drug Anal. 2020, 11, 307–319. [Google Scholar] [CrossRef]
- Prousalis, K.P.; Polygenis, D.A.; Syrokou, A.; Lamari, F.N.; Tsegenidis, T. Determination of Carbendazim, Thiabendazole, and o-Phenylphenol Residues in Lemons by HPLC Following Sample Clean-up by Ion-Pairing. Anal. Bioanal. Chem. 2004, 379, 458–463. [Google Scholar] [CrossRef] [PubMed]
- Halko, R.; Padron Sanz, C.; Sosa Ferrera, Z.; Santana Rodríguez, J.J. Determination of Benzimidazole Fungicides by HPLC with Fluorescence Detection after Micellar Extraction. Chromatographia 2004, 60, 151–156. [Google Scholar] [CrossRef]
- Nozal, M.J.; Bernal, J.L.; Jiménez, J.J.; Martín, M.T.; Bernal, J. Determination of Azolic Fungicides in Wine by Solid-Phase Extraction and High-Performance Liquid Chromatography–Atmospheric Pressure Chemical Ionization–Mass Spectrometry. J. Chromatogr. A 2005, 1076, 90–96. [Google Scholar] [CrossRef] [PubMed]
- Turiel, E.; Tadeo, J.L.; Cormack, P.A.G.; Martin-Esteban, A. HPLC Imprinted-Stationary Phase Prepared by Precipitation Polymerisation for the Determination of Thiabendazole in Fruit. Analyst 2005, 130, 1601–1607. [Google Scholar] [CrossRef] [PubMed]
- López Monzón, A.; Vega Moreno, D.; Torres Padrón, M.E.; Sosa Ferrera, Z.S.; Santana Rodríguez, J.J. Solid-Phase Microextraction of Benzimidazole Fungicides in Environmental Liquid Samples and HPLC-Fluorescence Determination. Anal. Bioanal. Chem. 2007, 387, 1957–1963. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Yang, X.; Wang, C.; Zhao, J.; Li, W.; Wang, Z. A Sensitive Determination Method for Carbendazim and Thiabendazole in Apples by Solid-Phase Microextraction−high Performance Liquid Chromatography with Fluorescence Detection. Food Addit. Contam.-Part A Chem. Anal. Control. Expo. Risk Assess. 2008, 25, 314–319. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; Li, Y.; Wang, C.; Liu, Z.; Zang, X.; Zhou, X.; Wang, Z. Dispersive Liquid–Liquid Microextraction Combined with High Performance Liquid Chromatography–Fluorescence Detection for the Determination of Carbendazim and Thiabendazole in Environmental Samples. Anal. Chim. Acta 2009, 638, 139–145. [Google Scholar] [CrossRef]
- Barahona, F.; Turiel, E.; Martín-Esteban, A. Supported Liquid Membrane-Protected Molecularly Imprinted Fibre for Solid-Phase Microextraction of Thiabendazole. Anal. Chim. Acta 2011, 694, 83–89. [Google Scholar] [CrossRef]
- Asensio-Ramos, M.; Hernández-Borges, J.; Borges-Miquel, T.M.; Rodríguez-Delgado, M.Á. Ionic Liquid-Dispersive Liquid-Liquid Microextraction for the Simultaneous Determination of Pesticides and Metabolites in Soils Using High-Performance Liquid Chromatography and Fluorescence Detection. J. Chromatogr. A 2011, 1218, 4808–4816. [Google Scholar] [CrossRef]
- Han, D.; Tang, B.; Tian, M.; Row, K.H. Solid-Phase Extraction Combined with Dispersive Liquid-Liquid Microextraction for the Determination of Three Benzimidazole Pesticides (Carbendazim, Thiabendazole, and Thiophanate-Methyl) in Tomatoes. Anal. Lett. 2013, 46, 557–568. [Google Scholar] [CrossRef]
- Lin, L.; Yang, C.L.; Peng, Z.; Wang, M.Y.; Zeng, Z.Q.; Guo, H. Bin Determination of Imidacloprid, Acetamiprid, Thiabendazole and Carbendazim Residues in Edible Fungi by HPLC. Adv. Mater. Res. 2013, 781–784, 99–103. [Google Scholar] [CrossRef]
- Boeris, V.; Arancibia, J.A.; Olivieri, A.C. Determination of Five Pesticides in Juice, Fruit and Vegetable Samples by Means of Liquid Chromatography Combined with Multivariate Curve Resolution. Anal. Chim. Acta 2014, 814, 23–30. [Google Scholar] [CrossRef] [PubMed]
- Vichapong, J.; Santaladchaiyakit, Y.; Burakham, R.; Kanchanamayoon, W.; Srijaranai, S. Determination of Benzimidazole Anthelmintics Using HPLC after Vortex-Assisted Mixed Anionic–Cationic Surfactant-Enhanced Emulsification Microextraction with Solidification of Floating Organic Droplet Procedure. J. Food Compos. Anal. 2015, 37, 30–37. [Google Scholar] [CrossRef]
- Vichapong, J.; Santaladchaiyakit, Y.; Srijaranai, S.; Burakham, R. Cationic Micellar Precipitation for Simultaneous Preconcentration of Benzimidazole Anthelmintics in Milk Samples by High-Performance Liquid Chromatography. J. Braz. Chem. Soc. 2016, 28, 724–730. [Google Scholar] [CrossRef]
- Golge, O.; Kabak, B. Determination of 115 Pesticide Residues in Oranges by High-Performance Liquid Chromatography–Triple-Quadrupole Mass Spectrometry in Combination with QuEChERS Method. J. Food Compos. Anal. 2015, 41, 86–97. [Google Scholar] [CrossRef]
- Xu, X.; Long, N.; Lv, J.; Wang, L.; Zhang, M.; Qi, X.; Zhang, L. Functionalized Multiwalled Carbon Nanotube as Dispersive Solid-Phase Extraction Materials Combined with High-Performance Liquid Chromatography for Thiabendazole Analysis in Environmental and Food Samples. Food Anal. Methods 2016, 9, 30–37. [Google Scholar] [CrossRef]
- Amelin, V.G.; Andoralov, A.M. High-Performance Liquid Chromatography–Time-of-Flight Mass Spectrometry in the Identification and Determination of 111 Pesticides in Food, Feed, Water, and Soil. J. Anal. Chem. 2015, 71, 82–93. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, M.; Zhang, D.; Zhang, L. New Approach for the Simultaneous Determination Fungicide Residues in Food Samples by Using Carbon Nanofiber Packed Microcolumn Coupled with HPLC. Food Control 2016, 60, 1–6. [Google Scholar] [CrossRef]
- Zhang, P.; Tian, J.; Rustum, A. Development and Validation of a Fast Stability-Indicating Ion-Paired Reversed-Phase HPLC Method for the Assay of Thiabendazole and Estimation of Its Related Compounds. J. AOAC Int. 2017, 100, 74–81. [Google Scholar] [CrossRef]
- Yu, Q.W.; Sun, H.; Wang, K.; He, H.B.; Feng, Y.Q. Monitoring of Carbendazim and Thiabendazole in Fruits and Vegetables by SiO2@NiO-Based Solid-Phase Extraction Coupled to High-Performance Liquid Chromatography-Fluorescence Detector. Food Anal. Methods 2017, 10, 2892–2901. [Google Scholar] [CrossRef]
- García-Fernández, M.; Díaz-Álvarez, M.; Martín-Esteban, A. Molecularly Imprinted Magnetic Nanoparticles for the Micro Solid-Phase Extraction of Thiabendazole from Citrus Samples. J. Sep. Sci. 2017, 40, 2638–2644. [Google Scholar] [CrossRef] [PubMed]
- Díaz-Álvarez, M.; Martín-Esteban, A. Hollow Fiber Membrane-Protected Molecularly Imprinted Microspheres for Micro Solid-Phase Extraction and Clean-up of Thiabendazole in Citrus Samples. J. Chromatogr. A 2018, 1531, 39–45. [Google Scholar] [CrossRef] [PubMed]
- Díaz-Álvarez, M.; Turiel, E.; Martín-Esteban, A. Molecularly Imprinted Polymer Monolith Containing Magnetic Nanoparticles for the Stir-Bar Sorptive Extraction of Thiabendazole and Carbendazim from Orange Samples. Anal. Chim. Acta 2019, 1045, 117–122. [Google Scholar] [CrossRef] [PubMed]
- Liang, G.; Guo, X.; Tan, X.; Mai, S.; Chen, Z.; Zhai, H. Molecularly Imprinted Monolithic Column Based on Functionalized β-Cyclodextrin and Multi-Walled Carbon Nanotubes for Selective Recognition of Benzimidazole Residues in Citrus Samples. Microchem. J. 2019, 146, 1285–1294. [Google Scholar] [CrossRef]
- Lu, S.H.; Li, S.S.; Yin, B.; Mi, J.Y.; Zhai, H.L. The Rapid Quantitative Analysis of Three Pesticides in Cherry Tomatoes and Red Grape Samples with Tchebichef Image Moments. Food Chem. 2019, 290, 72–78. [Google Scholar] [CrossRef] [PubMed]
- Sutcharitchan, C.; Miao, S.; Li, W.; Liu, J.; Zhou, H.; Ma, Y.; Ji, S.; Cui, Y. High Performance Liquid Chromatography-Tandem Mass Spectrometry Method for Residue Determination of 39 Plant Growth Regulators in Root and Rhizome Chinese Herbs. Food Chem. 2020, 322, 126766. [Google Scholar] [CrossRef]
- Zhao, W.; Wang, X.; Guo, J.; Guo, Y.; Lan, C.; Xie, F.; Zong, S.; He, L.; Zhang, S. Evaluation of Sulfonic Acid Functionalized Covalent Triazine Framework as a Hydrophilic-Lipophilic Balance/Cation-Exchange Mixed-Mode Sorbent for Extraction of Benzimidazole Fungicides in Vegetables, Fruits and Juices. J. Chromatogr. A 2020, 1618, 460847. [Google Scholar] [CrossRef]
- Moreno-González, D.; Cutillas, V.; Hernando, M.D.; Alcántara-Durán, J.; García-Reyes, J.F.; Molina-Díaz, A. Quantitative Determination of Pesticide Residues in Specific Parts of Bee Specimens by Nanoflow Liquid Chromatography High Resolution Mass Spectrometry. Sci. Total Environ. 2020, 715, 137005. [Google Scholar] [CrossRef]
- Choi, S.-I.; Han, X.; Lee, S.-J.; Men, X.; Oh, G.; Lee, D.-S.; Lee, O.-H. Validation of an Analytical Method for the Determination of Thiabendazole in Various Food Matrices. Separations 2022, 9, 135. [Google Scholar] [CrossRef]
- Rathod, R.H.; Chaudhari, S.R.; Patil, A.S.; Shirkhedkar, A.A. Ultra-High Performance Liquid Chromatography-MS/MS (UHPLC-MS/MS) in Practice: Analysis of Drugs and Pharmaceutical Formulations. Futur. J. Pharm. Sci. 2019, 5, 6. [Google Scholar] [CrossRef]
- Romero-González, R.; Frenich, A.G.; Vidal, J.L.M. Multiresidue Method for Fast Determination of Pesticides in Fruit Juices by Ultra Performance Liquid Chromatography Coupled to Tandem Mass Spectrometry. Talanta 2008, 76, 211–225. [Google Scholar] [CrossRef] [PubMed]
- Lehotay, S.J.; Maštovská, K.; Lightfield, A.R. Use of Buffering and Other Means to Improve Results of Problematic Pesticides in a Fast and Easy Method for Residue Analysis of Fruits and Vegetables. J. AOAC Int. 2005, 88, 615–629. [Google Scholar] [CrossRef] [PubMed]
- Garrido Frenich, A.; Aguilera-Luiz, M.d.M.; Martínez Vidal, J.L.; Romero-González, R. Comparison of Several Extraction Techniques for Multiclass Analysis of Veterinary Drugs in Eggs Using Ultra-High Pressure Liquid Chromatography-Tandem Mass Spectrometry. Anal. Chim. Acta 2010, 661, 150–160. [Google Scholar] [CrossRef] [PubMed]
- Romero-González, R.; Aguilera-Luiz, M.M.; Plaza-Bolaños, P.; Frenich, A.G.; Vidal, J.L.M. Food Contaminant Analysis at High Resolution Mass Spectrometry: Application for the Determination of Veterinary Drugs in Milk. J. Chromatogr. A 2011, 1218, 9353–9365. [Google Scholar] [CrossRef]
- Lopes, R.P.; Reyes, R.C.; Romero-González, R.; Frenich, A.G.; Vidal, J.L.M. Development and Validation of a Multiclass Method for the Determination of Veterinary Drug Residues in Chicken by Ultra High Performance Liquid Chromatography-Tandem Mass Spectrometry. Talanta 2012, 89, 201–208. [Google Scholar] [CrossRef]
- Aguilera-Luiz, M.M.; Martínez Vidal, J.L.; Romero-González, R.; Garrido Frenich, A. Multiclass Method for Fast Determination of Veterinary Drug Residues in Baby Food by Ultra-High-Performance Liquid Chromatography–Tandem Mass Spectrometry. Food Chem. 2012, 132, 2171–2180. [Google Scholar] [CrossRef]
- Lopes, R.P.; Reyes, R.C.; Romero-González, R.; Vidal, J.L.M.; Frenich, A.G. Multiresidue Determination of Veterinary Drugs in Aquaculture Fish Samples by Ultra High Performance Liquid Chromatography Coupled to Tandem Mass Spectrometry. J. Chromatogr. B 2012, 895–896, 39–47. [Google Scholar] [CrossRef]
- Martínez-Domínguez, G.; Nieto-García, A.J.; Romero-González, R.; Frenich, A.G. Application of QuEChERS Based Method for the Determination of Pesticides in Nutraceutical Products (Camellia Sinensis) by Liquid Chromatography Coupled to Triple Quadrupole Tandem Mass Spectrometry. Food Chem. 2015, 177, 182–190. [Google Scholar] [CrossRef]
- Whelan, M.; Kinsella, B.; Furey, A.; Moloney, M.; Cantwell, H.; Lehotay, S.J.; Danaher, M. Determination of Anthelmintic Drug Residues in Milk Using Ultra High Performance Liquid Chromatography–Tandem Mass Spectrometry with Rapid Polarity Switching. J. Chromatogr. A 2010, 1217, 4612–4622. [Google Scholar] [CrossRef]
- Xia, X.; Dong, Y.; Luo, P.; Wang, X.; Li, X.; Ding, S.; Shen, J. Determination of Benzimidazole Residues in Bovine Milk by Ultra-High Performance Liquid Chromatography-Tandem Mass Spectrometry. J. Chromatogr. B 2010, 878, 3174–3180. [Google Scholar] [CrossRef]
- Shang, N.; Zhang, J.; Shao, B. Simultaneous Determination of Azoles Antifungal Drugs in Chicken Tissues by Ultra-Performance Liquid Chromatography Tandem Mass Spectrometry. Anal. Sci. 2013, 29, 1183–1188. [Google Scholar] [CrossRef] [PubMed]
- Hou, X.L.; Chen, G.; Zhu, L.; Yang, T.; Zhao, J.; Wang, L.; Wu, Y.L. Development and Validation of an Ultra High Performance Liquid Chromatography Tandem Mass Spectrometry Method for Simultaneous Determination of Sulfonamides, Quinolones and Benzimidazoles in Bovine Milk. J. Chromatogr. B 2014, 962, 20–29. [Google Scholar] [CrossRef] [PubMed]
- Sivaperumal, P.; Anand, P.; Riddhi, L. Rapid Determination of Pesticide Residues in Fruits and Vegetables, Using Ultra-High-Performance Liquid Chromatography/Time-of-Flight Mass Spectrometry. Food Chem. 2015, 168, 356–365. [Google Scholar] [CrossRef] [PubMed]
- Hanot, V.; Goscinny, S.; Deridder, M. A Simple Multi-Residue Method for the Determination of Pesticides in Fruits and Vegetables Using a Methanolic Extraction and Ultra-High-Performance Liquid Chromatography-Tandem Mass Spectrometry: Optimization and Extension of Scope. J. Chromatogr. A 2015, 1384, 53–66. [Google Scholar] [CrossRef]
- Haroune, L.; Cassoulet, R.; Lafontaine, M.P.; Bélisle, M.; Garant, D.; Pelletier, F.; Cabana, H.; Bellenger, J.P. Liquid Chromatography-Tandem Mass Spectrometry Determination for Multiclass Pesticides from Insect Samples by Microwave-Assisted Solvent Extraction Followed by a Salt-out Effect and Micro-Dispersion Purification. Anal. Chim. Acta 2015, 891, 160–170. [Google Scholar] [CrossRef]
- Chitescu, C.L.; Kaklamanos, G.; Nicolau, A.I.; Stolker, A.A.M.L. High Sensitive Multiresidue Analysis of Pharmaceuticals and Antifungals in Surface Water Using U-HPLC-Q-Exactive Orbitrap HRMS. Application to the Danube River Basin on the Romanian Territory. Sci. Total Environ. 2015, 532, 501–511. [Google Scholar] [CrossRef]
- Wang, Y.; Li, X.; Zhang, Z.; Ding, S.; Jiang, H.; Li, J.; Shen, J.; Xia, X. Simultaneous Determination of Nitroimidazoles, Benzimidazoles, and Chloramphenicol Components in Bovine Milk by Ultra-High Performance Liquid Chromatography–Tandem Mass Spectrometry. Food Chem. 2016, 192, 280–287. [Google Scholar] [CrossRef]
- Rizzetti, T.M.; Kemmerich, M.; Martins, M.L.; Prestes, O.D.; Adaime, M.B.; Zanella, R. Optimization of a QuEChERS Based Method by Means of Central Composite Design for Pesticide Multiresidue Determination in Orange Juice by UHPLC-MS/MS. Food Chem. 2016, 196, 25–33. [Google Scholar] [CrossRef]
- Viera, M.S.; Rizzetti, T.M.; de Souza, M.P.; Martins, M.L.; Prestes, O.D.; Adaime, M.B.; Zanella, R. Multiresidue Determination of Pesticides in Crop Plants by the Quick, Easy, Cheap, Effective, Rugged, and Safe Method and Ultra-High-Performance Liquid Chromatography Tandem Mass Spectrometry Using a Calibration Based on a Single Level Standard Addition I. J. Chromatogr. A 2017, 1526, 119–127. [Google Scholar] [CrossRef]
- Rizzetti, T.M.; de Souza, M.P.; Prestes, O.D.; Adaime, M.B.; Zanella, R. Optimization of Sample Preparation by Central Composite Design for Multi-Class Determination of Veterinary Drugs in Bovine Muscle, Kidney and Liver by Ultra-High-Performance Liquid Chromatographic-Tandem Mass Spectrometry. Food Chem. 2018, 246, 404–413. [Google Scholar] [CrossRef]
- Li, X.; Guo, P.; Shan, Y.; Ke, Y.; Li, H.; Fu, Q.; Wang, Y.; Liu, T.; Xia, X. Determination of 82 Veterinary Drugs in Swine Waste Lagoon Sludge by Ultra-High Performance Liquid Chromatography–Tandem Mass Spectrometry. J. Chromatogr. A 2017, 1499, 57–64. [Google Scholar] [CrossRef] [PubMed]
- Campos-Mañas, M.C.; Plaza-Bolaños, P.; Sánchez-Pérez, J.A.; Malato, S.; Agüera, A. Fast Determination of Pesticides and Other Contaminants of Emerging Concern in Treated Wastewater Using Direct Injection Coupled to Highly Sensitive Ultra-High Performance Liquid Chromatography-Tandem Mass Spectrometry. J. Chromatogr. A 2017, 1507, 84–94. [Google Scholar] [CrossRef] [PubMed]
- da Silva, G.R.; Lima, J.A.; de Souza, L.F.; Santos, F.A.; Lana, M.A.G.; de Assis, D.C.S.; Cançado, S.d.V. Multiresidue Method for Identification and Quantification of Avermectins, Benzimidazoles and Nitroimidazoles Residues in Bovine Muscle Tissue by Ultra-High Performance Liquid Chromatography Tandem Mass Spectrometry (UHPLC-MS/MS) Using a QuEChERS Approach. Talanta 2017, 171, 307–320. [Google Scholar] [CrossRef]
- López, A.; Coscollà, C.; Yusà, V. Evaluation of Sampling Adsorbents and Validation of a LC-HRMS Method for Determination of 28 Airborne Pesticides. Talanta 2018, 189, 211–219. [Google Scholar] [CrossRef]
- Yao, L.; Lv, Y.Z.; Zhang, L.J.; Liu, W.R.; Zhao, J.L.; Liu, Y.S.; Zhang, Q.Q.; Ying, G.G. Determination of 24 Personal Care Products in Fish Bile Using Hybrid Solvent Precipitation and Dispersive Solid Phase Extraction Cleanup with Ultrahigh Performance Liquid Chromatography-Tandem Mass Spectrometry and Gas Chromatography-Mass Spectrometry. J. Chromatogr. A 2018, 1551, 29–40. [Google Scholar] [CrossRef]
- Álvarez-Muñoz, D.; Rambla-Alegre, M.; Carrasco, N.; Lopez de Alda, M.; Barceló, D. Fast Analysis of Relevant Contaminants Mixture in Commercial Shellfish. Talanta 2019, 205, 119884. [Google Scholar] [CrossRef]
- Liang, Y.; Liu, J.; Zhong, Q.; Yu, D.; Yao, J.; Huang, T.; Zhu, M.; Zhou, T. A Fully Automatic Cross Used Solid-Phase Extraction Online Coupled with Ultra-High Performance Liquid Chromatography–Tandem Mass Spectrometry System for the Trace Analysis of Multi-Class Pharmaceuticals in Water Samples. J. Pharm. Biomed. Anal. 2019, 174, 330–339. [Google Scholar] [CrossRef]
- Qie, M.; Zhao, Y.; Yang, S.; Wang, W.; Xu, Z. Rapid Simultaneous Determination of 160 Drugs in Urine and Blood of Livestock and Poultry by Ultra-High-Performance Liquid Chromatography-Tandem Mass Spectrometry. J. Chromatogr. A 2019, 1608, 460423. [Google Scholar] [CrossRef]
- Pugajeva, I.; Ikkere, L.E.; Judjallo, E.; Bartkevics, V. Determination of Residues and Metabolites of More than 140 Pharmacologically Active Substances in Meat by Liquid Chromatography Coupled to High Resolution Orbitrap Mass Spectrometry. J. Pharm. Biomed. Anal. 2019, 166, 252–263. [Google Scholar] [CrossRef]
- Li, Y.; Gan, Z.; Liu, Y.; Chen, S.; Su, S.; Ding, S.; Tran, N.H.; Chen, X.; Long, Z. Determination of 19 Anthelmintics in Environmental Water and Sediment Using an Optimized PLE and SPE Method Coupled with UHPLC-MS/MS. Sci. Total Environ. 2020, 719, 137516. [Google Scholar] [CrossRef]
- Zhan, J.; Shi, X.-Z.; Xu, X.-W.; Cao, G.-Z.; Chen, X.-F. Generic and Rapid Determination of Low Molecular Weight Organic Chemical Contaminants in Protein Powder by Using Ultra-Performance Liquid Chromatography-Tandem Mass Spectrometry. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2020, 1138, 121967. [Google Scholar] [CrossRef] [PubMed]
- Tomai, P.; Gentili, A.; Fanali, S.; Picó, Y. Multi-Residue Determination of Organic Micro-Pollutants in River Sediment by Stir-Disc Solid Phase Extraction Based on Oxidized Buckypaper. J. Chromatogr. A 2020, 1621, 461080. [Google Scholar] [CrossRef] [PubMed]
- Castilla-Fernández, D.; Moreno-González, D.; Bouza, M.; Saez-Gómez, A.; Ballesteros, E.; García-Reyes, J.F.; Molina-Díaz, A. Assessment of a Specific Sample Cleanup for the Multiresidue Determination of Veterinary Drugs and Pesticides in Salmon Using Liquid Chromatography/Tandem Mass Spectrometry. Food Control 2021, 130, 108311. [Google Scholar] [CrossRef]
- Poole, C.F.; Cooke, M.; Wilson, I.D. Encyclopedia of Separation Science, Ten-Volume Set, 1st ed.; Wilson, I., Poole, C., Cooke, M., Eds.; Academic Press: Cambridge, MA, USA, 2000; ISBN 9780080917795. [Google Scholar]
- Peris-Vicente, J.; Marzo-Mas, A.; Roca-Genovés, P.; Carda-Broch, S.; Esteve-Romero, J. Use of Micellar Liquid Chromatography for Rapid Monitoring of Fungicides Post Harvest Applied to Citrus Wastewater. J. Environ. Sci. 2016, 42, 284–292. [Google Scholar] [CrossRef]
- Peris-Vicente, J.; Roca-Genovés, P.; Tayeb-Cherif, K.; Esteve-Romero, J. Development and Validation of a Method to Determine Thiabendazole and O-phenylphenol in Wastewater Using Micellar Liquid Chromatography-fluorescence Detection. Electrophoresis 2016, 37, 2517–2521. [Google Scholar] [CrossRef]
- Rodríguez, R.; Picó, Y.; Font, G.; Mañes, J. Analysis of Post-Harvest Fungicides by Micellar Electrokinetic Chromatography. J. Chromatogr. A 2001, 924, 387–396. [Google Scholar] [CrossRef]
- Bol’Shakov, D.S.; Amelin, V.G.; Tret’Yakov, A.V. Determination of Polar Pesticides in Soil by Micellar Electrokinetic Chromatography Using QuEChERS Sample Preparation. J. Anal. Chem. 2014, 69, 89–97. [Google Scholar] [CrossRef]
- Fanali, S.; Haddad, P.R.; Poole, C.F.; Riekkola, M.-L. Liquid Chromatography: Fundamentals and Instrumentation, 2nd ed.; Fanali, S., Haddad, P.R., Poole, C., Riekkola, M.-L., Eds.; Elsevier: Amsterdam, The Netherlands, 2017; Volume 1, ISBN 9780128093450. [Google Scholar]
- Cacho, C.; Schweitz, L.; Turiel, E.; Pérez-Conde, C. Molecularly Imprinted Capillary Electrochromatography for Selective Determination of Thiabendazole in Citrus Samples. J. Chromatogr. A 2008, 1179, 216–223. [Google Scholar] [CrossRef]
- Boyer, R.F. Modern Experimental Biochemistry, 3rd ed.; Benjamin Cummings: San Francisco, CA, USA, 2000; ISBN 9780805331110. [Google Scholar]
- Plummer, D.T. An Introduction to Practical Biochemistry; McGraw-Hill: Boston, MA, USA, 1987; ISBN 0070841659. [Google Scholar]
- Rodríguez, R.; Picó, Y.; Font, G.; Mañes, J. Analysis of Thiabendazole and Procymidone in Fruits and Vegetables by Capillary Electrophoresis-Electrospray Mass Spectrometry. J. Chromatogr. A 2002, 949, 359–366. [Google Scholar] [CrossRef]
- Hu, X.Z.; Chen, M.L.; Gao, Q.; Yu, Q.W.; Feng, Y.Q. Determination of Benzimidazole Residues in Animal Tissue Samples by Combination of Magnetic Solid-Phase Extraction with Capillary Zone Electrophoresis. Talanta 2012, 89, 335–341. [Google Scholar] [CrossRef]
- Xu, L.; Luan, F.; Liu, H.; Gao, Y. Dispersive Liquid-Liquid Microextraction Combined with Non-Aqueous Capillary Electrophoresis for the Determination of Imazalil, Prochloraz and Thiabendazole in Apples, Cherry Tomatoes and Grape Juice. J. Sci. Food Agric. 2015, 95, 745–751. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, A.M.; Loureiro, H.C.; de Jesus, F.F.S.; de Jesus, D.P. Electromembrane Extraction and Preconcentration of Carbendazim and Thiabendazole in Water Samples before Capillary Electrophoresis Analysis. J. Sep. Sci. 2017, 40, 1532–1539. [Google Scholar] [CrossRef] [PubMed]
- Tejada-Casado, C.; Moreno-González, D.; Lara, F.J.; García-Campaña, A.M.; del Olmo-Iruela, M. Determination of Benzimidazoles in Meat Samples by Capillary Zone Electrophoresis Tandem Mass Spectrometry Following Dispersive Liquid–Liquid Microextraction. J. Chromatogr. A 2017, 1490, 212–219. [Google Scholar] [CrossRef] [PubMed]
- Witkowski, D.; Łysko, J.; Karczemska, A. Joule Heating Effects in Capillary Electrophoresis—Designing Electrophoretic Microchips. J. Achiev. Mater. Manuf. Eng. 2009, 37, 592–597. [Google Scholar]
- Msagati, T.; Ngila, J. Voltammetric Determination of a Benzimidazole Anthelmintic Mixture at a Poly(3-Methylthiophene)-Modified Glassy Carbon Electrode. S. Afr. J. Chem. 2003, 56, 5–9. [Google Scholar]
- Yang, J.; Zhang, D.; Wang, L.; Long, N.; Zhang, M.; Zhang, L. An Electrochemical Method for High Sensitive Detection of Thiabendazole and Its Interaction with Human Serum Albumin. Food Anal. Methods 2015, 8, 507–514. [Google Scholar] [CrossRef]
- Dong, Y.; Yang, L.; Zhang, L. Simultaneous Electrochemical Detection of Benzimidazole Fungicides Carbendazim and Thiabendazole Using a Novel Nanohybrid Material-Modified Electrode. J. Agric. Food Chem. 2017, 65, 727–736. [Google Scholar] [CrossRef]
- Ribeiro, F.W.P.; de Oliveira, R.C.; de Oliveira, A.G.; Nascimento, R.F.; Becker, H.; de Lima-Neto, P.; Correia, A.N. Electrochemical Sensing of Thiabendazole in Complex Samples Using Boron-Doped Diamond Electrode. J. Electroanal. Chem. 2020, 866, 114179. [Google Scholar] [CrossRef]
- Volnyanska, O.V.; Labyak, O.V.; Blazheyevskiy, M.Y.; Brizitskiy, O.A.; Tkach, V.I. Amperometric and Spectrophotometric Determination of Food Additive Thiabendazole (E-233) in Bananas. Int. J. Adv. Pharm. Biol. Chem. 2016, 5, 271–281. [Google Scholar]
- Volnyanska, O.V.; Mironyak, M.O.; Manzuk, M.V.; Labyak Oksana, V.; Nikolenko, N.V.; Kovalenko, V.L.; Kotok, V.A. The Potentiometric Sensor for Determination of Thiabendazole. J. Eng. Appl. Sci. 2020, 15, 1088–1093. [Google Scholar]
- Budetić, M.; Samardžić, M.; Bubnjar, K.; Dandić, A.; Živković, P.; Széchenyi, A.; Kiss, L. A New Sensor for Direct Potentiometric Determination of Thiabendazole in Fruit Peels Using the Gran Method. Food Chem. 2022, 392, 133290. [Google Scholar] [CrossRef] [PubMed]
- Dandić, A.; Novak, I.; Jozanović, M.; Pukleš, I.; Széchenyi, A.; Budetić, M.; Samardžić, M. A New, MWCNT-Based, Solid-State Thiabendazole-Selective Sensor. Sensors 2022, 22, 3785. [Google Scholar] [CrossRef] [PubMed]
- Brandon, D.L.; Binder, R.G.; Bates, A.H.; Montague, W.C. A Monoclonal Antibody-Based ELISA for Thiabendazole in Liver. J. Agric. Food Chem. 1992, 40, 1722–1726. [Google Scholar] [CrossRef]
- Brandon, D.L.; Binder, R.G.; Wilson, R.E.; Montague, W.C. Analysis of Thiabendazole in Potatoes and Apples by ELISA Using Monoclonal Antibodies. J. Agric. Food Chem. 1993, 41, 996–999. [Google Scholar] [CrossRef]
- Brandon, D.L.; Binder, R.G.; Bates, A.H.; Montague, W.C. Competitive ELISA of Thiabendazole Residues in Produce Using Indirectly Immobilized Monoclonal Antibodies. Food Agric. Immunol. 1995, 7, 99–108. [Google Scholar] [CrossRef]
- Bushway, R.J.; Brandon, D.L.; Bates, A.H.; Li, L.; Larkin, K.A.; Young, B.S. Quantitative Determination of Thiabendazole in Fruit Juices and Bulk Juice Concentrates Using a Thiabendazole Monoclonal Antibody. J. Agric. Food Chem. 1995, 43, 1407–1412. [Google Scholar] [CrossRef]
- Blažková, M.; Rauch, P.; Fukal, L. Strip-Based Immunoassay for Rapid Detection of Thiabendazole. Biosens. Bioelectron. 2010, 25, 2122–2128. [Google Scholar] [CrossRef]



| Molecular weight (g/mol) | 201.25 | |
| LogP | 2.47 | |
| Hydrogen Bond Donor Count | 1 | |
| Hydrogen Bond Acceptor Count | 3 | |
| Exact mass (Da) | 201.03606841 | |
| pKa (at 25 °C) | 4.64 | |
| Solubility (g/L at 25 °C) | n-heptane | 0.1 |
| methanol | 8.28 | |
| acetone | 2.43 | |
| ethyl acetate | 1.49 | |
| Method | Sample | Conditions and Characteristic Method Parameters | Measuring Range (μg/mL) | Correlation Coefficient | LOD (μg/mL) | RSD (%) | Recovery (%) | Reference |
|---|---|---|---|---|---|---|---|---|
| UV-Vis spectroscopy | fruit, vegetables | max. absorption: 340 nm; solvent: 1-butyl-3-methylimidazolium hexafluorophosphate | 3.0 × 10−4–2.8 × 10−1 | 0.9948 | 1.0 × 10−4 | 2.1–5.6 | 95.1–98.2 | [37] |
| UV-Vis spectroscopy | fruit | max. absorption: 305 nm; eutectic solvents: betaine with 2-furoic acid, phenylacetic acid, mandelic acid, and glycolic acid | 4.0 × 10−4–1.5 × 10−1 | 0.9996 | 1.0 × 10−4 | 1.2–3.8 | 91.0–106.0 | [38] |
| SERS | lemon | solvent: water and ethanol; EF: Ag colloids (500 µL); spectral resolution: 10 cm−1; WN: 200–3400 cm−1; Ex: 532 nm; laser power: 40 mW | - | - | 4.0 × 10−6 | - | - | [39] |
| SERS | fruit | solvent: water and ethanol; EF: Ag colloids (500 µL); spectral resolution: 8 cm−1; room temperature; WN: 200–3400 cm−1; Ex: 532 nm; laser power: 40 mW | - | - | 4.0 × 10−6 | - | - | [6] |
| SERS | apple | solvent: methanol; EF: Ag dendrites (5 µL); spectral resolution: 3 cm−1; room temperature; WN: 500–2000 cm−1 | - | 0.9770 | 0.1 | - | 89.2–115.4 | [40] |
| SERS | apple | solvent: 1% acetic acid in acetonitrile; EF: Au nanoparticles (50 µL); WN: 550–2000 cm−1; laser power: 80 mW | - | 0.9630–0.9770 | 2.0 × 10−2 | 3.6–6.7 | >90 | [41] |
| SERS | TBZ solution, orange | solvent: water and ethanol; EF: Au nanoparticles (100 µL); WN: 500–1700 cm−1; Ex: 785 nm; laser power: 15 mW | 1.0 × 10−3–100 (TBZ solution) 0–2.5 1 (orange) | 0.9764 (TBZ solution) 0.9926 (orange) | 1.0 × 10−2 (TBZ solution) 1.3 × 10−2 1 (orange) | 1.4–12.2 (TBZ solution) 2.2–19.4 (orange) | - | [42] |
| SERS | orange | solvent: acetonitrile; EF: Ag colloids (0.24 µL); WN: 400–2000 cm−1; Ex: 785 nm; laser power: 25 mW | - | 0.9900 | 4.0 | - | 87.3–108.8 | [43] |
| SERS | rapeseed | solvent: acetonitrile; EF: Ag nanoparticles (500 µL); spectral resolution: 2 cm−1; WN: 200–3300 cm−1; Ex: 785 nm; source power: 200 mW | - | 0.7400–0.9100 | 0.1 | 0.7–9.9 | 94.7–118.9 | [44] |
| SERS | orange | solvent: ethanol; EF: Au coated paper; spectral resolution: 0.6 cm−1; WN: 400–1800 cm−1; Ex: 632 nm | - | - | 2.0 × 10−2 | 10.0–20.0 | - | [45] |
| SERS | red soil | solvent: acetonitrile; EF: Au nanoparticles; spectral resolution: 2 cm−1; WN: 200–3300 cm−1; Ex: 785 nm; source power: 200 mW | - | 0.9892–0.9948 | 0.1 | 1.4–10.5 | 71.8–116.7 | [46] |
| SERS | lemon, carrot, and mango juices | solvent: acetone–water (50:50); EF: gold nanorods (10 µL); spectral resolution: 6 cm−1; WN: 500–1800 cm−1; Ex: 785 nm; laser power: 20 mW | - | 0.9800–0.9900 | 1.5 × 10−1–1.8 × 10−1 | - | 96.0–99.0 | [3] |
| SERS | cherry | solvent: methanol–dichloromethane (1:1); EF: Ag sol–polyurethane (80 µL); Ex: 785 nm; laser power: 3 mW | - | - | 2.0 × 10−2 | - | 82.0–94.0 | [47] |
| SERS | TBZ in methanol solution, apple | solvent: acetonitrile–water (50:50); EF: Au nanorods; WN: 600–1700 cm−1; Ex: 785 nm; laser power: 50 mW | - | 0.9910 (methanol solution) 0.9800 (apple) | 3.7 × 10−2 (methanol solution) 6.0 × 10−2 (apple) | 15.4 | 76.0–107.0 (apple) | [48] |
| SERS | peach | EF: Au-IP6-Mil-101(Fe); WN: 600–1700 cm−1; Ex: 633 nm | 1.5–7.5 | 5.0 × 10−2 | 0.9860 | 2.7–17.2 | 84.3–113.1 | [49] |
| SERS | TBZ solution, apple surface, apple juice | solvent: water; EF: Ag@SiO2 nanocubes; WN: 600–1200 cm−1; Ex: 633 nm; laser power: 90 mW | 1.0 × 10−3–1.0 | 0.9920 | 1.9 × 10−2 | 8.8 | - | [50] |
| SERS | mango | solvent: water; EF: Au nanoparticles; spectral resolution: 2 cm−1; WN: 200–3200 cm−1; Ex: 785 nm; source power: 250 mW | 3.0 × 10−2–10.0 | - | - | 2.3 | - | [51] |
| SERS | pear, apple, orange | solvent: methanol; EF: Au@Ag nanoparticles; WN: 600–1800 cm−1; Ex: 633 nm; laser power: 4.25 mW | - | 0.9620–0.9800 | 5.1 × 10−2 | 10.5 | 76.0–134.0 | [52] |
| SERS | TBZ solution | solvent: methanol; EF: Ag nanoparticles (980 µL); WN: 200–1800 cm−1; Ex: 514.5 nm; | 1.6 × 10−2–3.2 × 10−2 | - | 1.4 × 10−2 | - | - | [53] |
| SERS | apple, tomato, pear | solvent: methanol; EF: Au nanorods; WN: 543–1683 cm−1; Ex: 633 nm; laser power: 17 mW | 1.0 × 10−2–100.0 | 0.9777–0.9803 | 1.5 × 10−2–1.6 × 10−2 | - | 74.3–127.7 | [54] |
| SERS | apple | solvent: ethanol; EF: Au@Ag nanoparticles; WN: 600–1800 cm−1; Ex: 785 nm | 1.0 × 10−3–1.0 × 103 | 0.9406 | 1.0 × 10−3 | <10 | 83.0–93.5 | [55] |
| SERS | milk | solvent: water; EF: Au@Ag nanoparticles–TGA; WN: 350–1800 cm−1; Ex: 633 nm; laser power: 50 mW | - | 0.9880 | 1.2 × 10−1 | 4.1–9.2 | 88.0–103.0 | [56] |
| SERS | apple, peach | solvent: acetonitrile and methanol; EF: Au@Ag nanoparticles; WN: 500–1800 cm−1; Ex: 785 nm; source power: 140 mW | 0.1–100.0 | 0.9770–0.9980 | 3.2 × 10−2–3.4 × 10−2 | 3.9–4.4 | 95.0–101.0 | [57] |
| SERS | citrus fruit | solvent: methanol; EF: Au nanorods (5.0 µL); WN: 200–3200 cm−1; Ex: 785 nm | - | 0.9737 | 0.33 | <5 | - | [58] |
| fluorimetry | water | solvent: methanol; pH = 2–3; Ex: 300 nm, Em: 340 nm | 5.0 × 10−3–4.0 × 10−2 | >0.9900 | 2.9 × 10−4 | 2.1–5.4 | 97.0–102.0 | [64] |
| fluorimetry | pesticides | solvent: methanol; Ex: 260–306 nm, Em: 310–370 nm | - | 0.9990 | 4.7 × 10−3 | 2.0 | - | [65] |
| fluorimetry combined with SIA | water | solvent: ethanol; pH = 2; FR: 6.6 mL/min; carrier: water; Ex: 301 nm, Em: 355 nm | 8.0 × 10−5–2.0 × 10−2 | - | 2.0 × 10−5 | 0.5 | 96.0–106.0 | [66] |
| fluorimetry combined with SIA | water | solvent: ethanol; pH = 2; FR: 6.7 mL/min; Ex: 301 nm, Em: 355 nm | 1.9 × 10−4–6.0 × 10−2 | 0.9997 | 0.3 × 10−4 | 0.7 | 90.0–104.0 | [67] |
| fluorimetry combined with FIA | water, pharmaceutical preparations, commercial pesticides | solvent: methanol–water (50:50); pH = 2; FR: 1.14 mL/min; Ex: 309 nm, Em: 354 nm | 1.0 × 10−2–0.8 | 0.9997 | 2.4 × 10−3 | 0.9 | 97.1–104.5 | [68] |
| fluorimetry combined with FIA | water | solvent: ethanol; FR: 1.16 mL/min; carrier: methanol–water (20:80); Ex: 305 nm, Em: 358 nm | 5.0 × 10−4–1.6 × 10−2 | 0.9995 | 9.0 × 10−5 | 0.7–2.0 | 97.0–103.0 | [69] |
| fluorimetry combined with FIA | water, commercial pesticides | solvent: ethanol; FR: 1.16 mL/min; carrier: methanol–-water (20:80); Ex: 305 nm, Em: 358 nm | 4.0 × 10−4–2.0 × 10−2 | 0.9996 | 8.0 × 10−5 | 0.2–0.8 | 98.8–102.0 | [70] |
| fluorimetry combined with FIA | citrus fruit | solvent: acetonitrile; FR: 1 mL/min; carrier: methanol–water (25:75); Ex: 305 nm, Em: 358 nm | 0.3–10.0 1 | 0.9995 | 9.0 × 10−2 1 | 1.5–4.0 | 87.1–108.9 | [71] |
| fluorimetry combined with FIA | water | solvent: methanol; FR: 0.9 mL/min; carrier: water; Ex: 300 nm, Em: 340 nm | 8.0 × 10−3–1.2 × 10−1 | - | 2.8 × 10−3 | 0.2–1 | 98.0–105.8 | [72] |
| fluorimetry combined with FIA | water | solvent: methanol, 0.04 M SDS-0.01 M NaOH; pH = 12; FR: 2.4 mL/min; carrier: methanol–water (15:85); Ex: 296 nm, Em: 347 nm | 8.0 × 10−3–0.2 | 0.9950 | 2.5 × 10−3 | 1.1 | 93.2–108.3 | [73] |
| fluorimetry | water | solvent: water; pH = 6.5; Ex: 302 nm, Em: 355 nm, 358 nm | 1.2 × 10−2–1.6 | 0.9998 | 1.1 × 10−3 | 0.5–2.2 | 91.9–103.6 | [59] |
| fluorimetry combined with SIA | mushroom | solvent: acetonitrile; FR: 10 mL/min; carrier: methanol–water (30:70); Ex: 305 nm, Em: 345 nm | 1.6–40.0 1 | 0.9992 | 0.5 1 | 2.0–5.0 | 93.0–110.0 | [74] |
| fluorimetry | red wine | solvent: methanol; Ex: 315 nm, Em: 355–365 nm | 5.0 × 10−2–1.0 | 0.9980 | 7.2 × 10−3 | 1.8–7.8 | 85.9–102.8 | [75] |
| fluorimetry | apple juice | solvent: methanol; pH = 8; Ex: 302 nm, Em: 345 nm | 5.0 × 10−3–5.0 × 10−2 | 0.9987 | 2.2 × 10−3 | 2.1–7.9 | 94.1–108.1 | [76] |
| fluorimetry | orange juice | solvent: acetonitrile; pH = 10; Ex: 320 nm, Em: 546 nm | 1.6–8.1 | 0.9990 | - | 1.6–2.5 | 101.1–104.6 | [77] |
| fluorimetry | strawberry tree berries, citrus fruit | solvent: ethanol; pH = 4.7; Ex: 304 nm, Em: 350 nm | 3.0 × 10−2–1.2 × 10−1 | 0.9920 | 1.7 × 10–4 | 0.4–2.3 | 99.9–102.8 | [78] |
| fluorimetry | TBZ solution | solvent: ionic liquids; Ex: 352 nm, 317 nm, Em: 350 nm, 357 nm | 2.0–16.1 | - | 2.4 × 10–3 | - | - | [79] |
| fluorimetry combined with FIA | fruit, vegetables | solvent: methanol; FR: 0.15 mL/min; carrier: acid sample (in acetic acid); Ex: 210–310 nm, Em: 320–410 nm | 9.0 × 10−3–4.0 × 10−2 | - | 3.0 × 10−3 | - | 82.0–115.0 | [80] |
| fluorimetry | apple, orange, tomato juice | solvent: universal buffer solution (boric, acetic, and phosphoric acid, all 0.04 M and NaOH solution); pH = 7; Ex: 300 nm, Em: 360 nm | 3.0 × 10−2–1.7 | - | 8.0 × 10−3 | 2.6–5.5 | 92.5–105.1 | [81] |
| fluorimetry | orange | solvent: acetonitrile; Ex: 300 nm, Em: 544 nm | 5.5 × 10−2–16.1 | 0.9950 | 5.5 × 10−2 | 1.6–2.7 | 98.4–104.5 | [82] |
| fluorimetry | peach, soil, sewage | solvent: methanol–water (40:60); Ex: 302 nm, Em: 420 nm | - | 0.9930–0.9950 | 1.0 × 10−4 (soil) 1.1 × 10−3 (sewage) 1.2 × 10−3 (peach) | 0.3–2.5 | 86.2–109.2 | [83] |
| RTP | aqueous solution | Ex: 300 nm, Em: 488 nm; in solution; heavy atom salt: KI; deoxygenation: Na2SO3; lifetime: 89 µs | 1.5 × 10−2–1.5 × 10−1 | - | 1.5 × 10−2 | 4.5 | - | [86] |
| RTP | water | Ex: 306 nm, Em: 450 nm; in solution; heavy atom salt: KI; deoxygenation: Na2SO3; lifetime: 100 µs | 0.0–1.8 × 10−1 | - | 1.4 × 10−3 | 1.1–3.0 | 78.8–114.3 | [87] |
| RTP | water, pineapple | Ex: 298 nm, Em: 481 nm; in solution; β-CD, TBZ and Triton X-100 (1:1:1); heavy atom salt: KI; lifetime: 800 µs | 2.0 × 10−2–8.2 × 10−1 | 0.9937 | 2.1 × 10−3 | 1.9 | 95.0–102.0 | [88] |
| RTP | water | Ex: 300 nm, Em: 485 nm; in solid phase; nylon; heavy atom salt: Pb (II) acetate; deoxygenation: N2 | 3.2 × 10−2–2.6 × 10−1 | - | 1.0 × 10−2 | 2.4 | 93.0–118.0 | [89] |
| RTP | water | Ex: 300 nm, Em: 485 nm; in solid phase; nylon; heavy atom salt: KI; deoxygenation: Na2SO3 | 1.3 × 10−2–1.1 × 10−1 | 0.9985 | 4.5 × 10−3 | 3.2 | 97.0–110.0 | [90] |
| CL | water | solvent: ethanol; glass spiral flow cell (1.5 mm I.D.); FR: 2.8 mL/min; reaction time: 8 s | 1.0 × 10−3–2.0 | 0.9999 | 3.0 × 10–4 | 1.1–2.9 | 92.0–108.0 | [93] |
| Method | Sample | Conditions and Characteristic Method Parameters | Measuring Range (μg/mL) | Correlation Coefficient | LOD (μg/mL) | RSD (%) | Recovery (%) | Reference |
|---|---|---|---|---|---|---|---|---|
| LP-GC-MS | carrot extract | solvent: toluene; MP: helium; column: Gas-Chrom G(HP) (Sigma-Aldrich, Darmstadt, Germany) (3 m × 0.15 mm); tR: <20 min; 250 °C, detection: m/z 201, 174; selected ion monitoring mode | - | - | 3.0 × 10−3 1 | - | - | [97] |
| GC-MS/MS | cereals, dry animal feed | solvent: acetonitrile; MP: helium; column: Gas-Chrom G(HP) (Sigma-Aldrich, Darmstadt, Germany) (30 m × 0.25 mm) with 0.5 μm particles; tR: 16.46 min; temperature: 300 °C detection: m/z 201, 174, 130; electron ionization mode | - | - | 4.0 × 10−3 1 | 7.0–10.0 | 103.0–124.0 | [98] |
| GC-MS/MS | cereals, dry animal feed | solvent: 5% formic acid in acetonitrile; MP: helium, FR: 1.2 mL/min; column: J&W Scientific DB-5 MS (Agilent, Santa Clara, CA, USA) (30 m × 0.25 mm) with 0.5 µm particles; tR: 16.31 min; detection: m/z 201, 174, 130; electron ionization mode | 1.0 × 10−2–0.5 | 0.9999 | - | 3.0–9.0 | 92.0–112.0 | [99] |
| GC-MS/MS | wine | solvent: acetonitrile; MP: helium, FR: 1.2 mL/min; column: DB-5 (Agilent, Santa Clara, CA, USA) (30 m × 0.25 mm) with 0.5 µm particles; tR: 16.4 min; temperature: 300 °C; detection: m/z 201, 174, 130; electron impact mode | 1.0 × 10−2–0.2 | 0.9989 | - | 4.0–17.0 | 89.0–95.0 | [100] |
| GC-MS | coconut pulp | solvent: acetonitrile, n-hexane; MP: helium, FR: 1.8 mL/min; column: J&W Scientific DB-5MS (Agilent, Santa Clara, CA, USA) (30 m × 0.25 mm) with 0.25 µm particles; tR: 19.26 min; detection: m/z 201, 174, 129; electron impact ionization | 9.0 × 10−2–10.0 | 0.9979 | 8.0 × 10−2 1 | 4.5–14.7 | 74.1–88.4 | [101] |
| GC-MS | fruit, vegetables | solvent: acetone–ethyl acetate (1:1); MP: helium; column: Agilent Technologies HP 5 MS (Agilent, Santa Clara, CA, USA) (30 m × 0.25 mm) with 0.25 µm particles; temperature: 280 °C; ESI; positive mode | 5.0 × 10−2–10.0 | - | - | 7.3–12.9 | 102.0–119.0 | [102] |
| GC-MS | mango | solvent: methanol; MP: helium, FR: 1.0 mL/min; column: Restek Rtx®-1 MS Crossbond (Restek, Rheinfelden Switzerland) (30 m × 0.25 mm) with 0.25 µm particles; temperature: 250 °C; detection: m/z 201, 174, 129; electron impact mode | 3.3 × 10−2–1.67 | 0.9948 | 1.0 × 10−2 1 | 5.8–15.6 | 78.5–95.1 | [103] |
| GC-MS | artichoke leaves, fruit | solvent: ethyl acetate containing triphenyl phosphate; MP: helium, FR: 1.0 mL/min; column: TR-5MS Thermo (Termo Fisher Scientific, Neuilly-sur-Seine, France) (30 m × 0.25 mm) with 0.25 µm particles; tR: 9.1 min; detection: m/z 202, 175, 131; electron impact mode | - | - | - | 6.7–17.5 | 70.8–71.3 | [104] |
| GC-MS | soy milk | solvent: acetone–water (3:7); MP: helium, FR: 1.5 mL/min; column: Agilent Technologies HP- 5MS (Agilent, Santa Clara, CA, USA) (30 m × 0.25 mm) with 0.25 µm particles; 260 °C, electron ionization | 5.0 × 10−3–1 1 | 0.9972 | 1.0 × 10−3 1 | 4.0–16.0 | 81.0–121.0 | [105] |
| LC-MS | pear, tomato | solvent: acetonitrile–water (50:50); MP: acetonitrile and ammonium formate in water–acetonitrile (95:5) (gradient), FR: 1 mL/min; column: Zorbax SB-C8 (Agilent, Santa Clara, CA, USA) (150 × 4.6 mm) with 3.5 μm particles; tR: 9 min; vaporization temperature: 400 °C; detection: m/z 202.1; EA; APCI; positive ion mode | 5.0 × 10−2–5.0 1 | 0.9990 | 5.0 × 10−4 | - | 95.0–108.0 | [114] |
| LC-MS | orange | solvent: acetonitrile–2 mM ammonium formate (50:50); MP: methanol and 2 mM ammonium formate in water (gradient), FR: 1 mL/min; column: Zorbax SB-C18 (Agilent, Santa Clara, CA, USA) (30 × 4.6 mm) with 3.5 μm particles; tR: <4 min; vaporization temperature: 325 °C; detection: m/z 202; EA; APCI; positive ion mode; 350 °C | 5.0 × 10−2–5.0 1 | - | 2.0 × 10−2 1 | 7.0 | 75.0 | [115] |
| LC-MS | fruit, vegetables | solvent: methanol; MP: methanol and 50 mM ammonium formate (gradient), FR: 1 mL/min; column: Phenomenex Luna C18 (Phenomenex, Torrance, CA, USA) (150 × 4.6 mm) with a Phenomenax C18 guard cartridge (4 × 2 mm), both with 5 μm particles; tR: <8 min; vaporization temperature: 450 °C; detection: m/z 202.1; APCI; positive ion mode | 5.0 × 10−2–10.0 1 | >0.9940 | 1.0 × 10−2 1 | 10.0–15.0 | 63.0 | [116] |
| LC-MS | citrus fruit | solvent: methanol; MP: methanol and water (gradient), FR: 0.5 mL/min; column: Inertsil ODS-3 (GL Sciences, Tokyo, Japan) (150 × 3 mm) with 5 μm particles; tR: <4 min; vaporization temperature: 450 °C; detection: m/z 200; APPI; negative ion mode | - | - | 1.0 × 10−2 1 | 2.0–12.0 | 82.0–100.0 | [117] |
| LC-MS | citrus fruit | solvent: methanol; MP: methanol and water (gradient), FR: 0.5 mL/min; column: Inertsil ODS-3 (GL Sciences, Tokyo, Japan) (150 × 3 mm) with 5 μm particles; tR: 3.0–4.2 min; vaporization temperature: 350 °C; detection: m/z 200.02–200.04, 173.01–173.03; APPI; negative ion mode | - | - | 2.0 × 10−3 1 | 2.2–5.4 | 82.8–91.7 | [118] |
| LC-MS/MS | vegetables | solvent: methanol; MP: ammonium formate and acetonitrile–methanol (3:1) (gradient); FR: 0.2 mL/min; column: Polaris C18-A (Polaris, Medina, WA, USA) (150 × 2 mm) with 3 μm particles and with a Varian precolumn (30 × 2 mm); tR: 6.53 min; desolvation temperature: 300 °C; detection: m/z 131; ESI; positive ion mode | 1.0 × 10−2–1 | >0.9900 | 1.5 × 10−3 1 | 5.0–28.0 | 83.0–95.0 | [119] |
| LC-MS/MS | banana, orange | solvent: acetone, diluted with methanol (1:5); MP: 2.5 mM ammonium acetate-0.01% formic acid in methanol (gradient); FR: 0.3 mL/min; column: Nucleosil C18 (Macherey-Nagel, Düren, Germany) (125 × 2.1 mm) with 5 μm particles; tR: 12.80 min; desolvation temperature: 350 °C; detection: m/z 174.9, 130.9; ESI; positive ion mode | 1.1 × 10−3–0.1 | >0.9990 | 5.0 × 10−5 (bananas) 0.01 1 (oranges) | 3.0–8.0 | 83.0–88.0 | [120] |
| LC | bovine liver | solvent: methanol–water (50:50); MP: ammonium dihydrogen phosphate buffer pH 6.8-methanol-acetonitrile (gradient); FR: 0.5 mL/min; column: Waters stainless-steel C18 (Waters, Wien, Austria) (150 × 3.0 mm) with a Waters guard column (3 × 2 mm), both with 3.5 μm particles; tR: <13 min; temperature: 40 °C; UV detection (298 nm) | 2.5 × 10−1–15.0 | ≥0.9900 | 1.2 × 10−1 1 | 4.6–24.0 | 56.0–96.0 | [121] |
| LC-MS | water, urine, milk | solvent: methanol–water (1:1) containing 5% formic acid; MP: acetonitrile and acetic acid (gradient), FR: 0.15 mL/min; column: Waters XTerra® C8 (Waters, Wien, Austria) (50 × 2.1 mm) with 3.5 μm particles; tR: <8 min; detection: m/z 201.5–202.5; ES | 1.0 × 10−5–1.0 | 0.9900 | 1.0 × 10−7 | - | 74.0–100.0 | [122] |
| LC-MS | fruit-based soft drinks | solvent: methanol–water (1:2); MP: water with 0.1% formic acid and acetonitrile (gradient), FR: 0.6 mL/min; column: Zorbax Eclipse XDB-C8 (Agilent, Santa Clara, CA, USA) (150 × 4.6 mm) with 5 μm particles; tR: 9.8 min; temperature: 325 °C; detection: m/z 202.04334; ESI; positive ion mode | 1.0 × 10−4–5.0 × 10−2 | 0.9996 | 9.0 × 10−6 | 6.9–10.1 | 95.3–104.6 | [123] |
| LC-MS | fruit-based soft drinks | solvent: methanol–water (1:2); MP: water with 0.1% formic acid and acetonitrile (gradient), FR: 0.5 mL/min; column: Zorbax Rapid Resolution Eclipse XDB-C18 (Agilent, Santa Clara, CA, USA) (50 × 4.6 mm) with 1.8 μm particles; tR: 4.37 min; temperature: 325 °C; detection: m/z 202.0427; ESI; positive ion mode | 1.0 × 10−4–5.0 × 10−2 | 0.9987 | - | 3.4–8.0 | 88.6–104.7 | [124] |
| LC-MS | fruit-based soft drinks | solvent: methanol–water (1:2); MP: water with 0.1% formic acid and acetonitrile (gradient), FR: 0.5 mL/min; column: Zorbax Rapid Resolution Eclipse XDB-C18 (Agilent, Santa Clara, CA, USA) (50 × 4.6 mm) with 1.8 μm particles; tR: 1.5 min; temperature: 325 °C; detection: m/z 202.0433; ESI; positive ion mode | - | - | 1.0 × 10−5 | 6.5 | - | [125] |
| LC-MS/MS | baby food | solvent: 10% methanol; MP: water with 0.1% formic acid and acetonitrile (gradient), FR: 0.6 mL/min; column: Zorbax Eclipse XDB-C8 (Agilent, Santa Clara, CA, USA) (150 × 4.6 mm) with 5 μm particles; tR: 7.6 min; temperature: 350 °C; detection: m/z 202; ESI; positive ion mode | - | 0.9978 | 3.0 × 10−3 1 | - | - | [126] |
| LC | fruit, vegetables | supramolecular solvent: decanoic acid, tetrabutylammonium hydroxide; MP: phosphate buffer pH 4–methanol (60:40); FR: 1 mL/min; column: Kromasil C18 (Eka Chemicals, Bohus, Sweden) (150 × 4.6 mm) with 5 μm particles; tR: <11 min; fluorimetric detection; Ex: 300 nm, Em: 350 nm | 5.0 × 10−5–6.0 × 10−2 | 0.9999 | 4.0 × 10−4 1 | 2.2–3.5 | 95.0–100.0 | [127] |
| LC | water | supramolecular solvent: decanoic acid, tetrabutylammonium chloride, tetrabutylammonium hydroxide; MP: phosphate buffer pH 4-methanol (60:40); FR: 1 mL/min; column: Kromasil C18 (Eka Chemicals, Bohus, Sweden) (150 × 4.6 mm) with 5 μm particles; tR: <11 min; fluorimetric detection; Ex: 300 nm, Em: 350 nm | 1.0 × 10−5–1.6 × 10−2 | 0.9999 | 4.0 × 10−6 | 4.0 | 95.0–102.0 | [128] |
| LC-MS/MS | wine | solvent: methanol; MP: 0.1% formic acid and 0.1% formic acid in acetonitrile (gradient); FR: 0.2 mL/min; column: XTerra C18 (Waters, Wien, Austria) (150 × 2.1 mm) with a XTerra C18 guard column (10 × 2.1 mm), both with 3.5 μm particles; tR: 2.7 min; temperature: 350 °C; detection: m/z 175, 131; ESI; positive ion mode | 1.0 × 10−4–0.1 | 0.9970 (white wine) 0.9950 (red wine) | 4.5 × 10−4 (white wine) 8.8 × 10−4 (red wine) | 6.7–9.2 | 87.0–88.0 | [129] |
| LC-MS | orange juice | solvent: ethanol; MP: 20 mM formic acid and methanol (gradient), FR: 0.05 mL/min; column: BioBasic 8 C8 (Bio Basic, Markham, IL, USA) (50 × 1 mm) with 5 μm particles; tR: <9 min; detection: m/z 202; ESI; positive ion mode | 1.0 × 10−4–1.0 × 10−2 | 0.9990 | 5.0 × 10−5 | 7.9–10.6 | - | [130] |
| LC-MS/MS | citrus juice | solvent: 0.05% formic acid; MP: 0.05% formic acid and acetonitrile (gradient), FR: 0.2 mL/min; column: Varian Pursuit C18 (Varian, Santa Clara, CA, USA) (50 × 2 mm) with 3 μm particles; tR: 4.3 min; desolvation temperature: 300 °C; detection: m/z 174.8, 130.9; ESI; positive ion mode | 5.0 × 10−5–1.5 × 10−2 1 | 0.9970 | 1.7 × 10−5 1 | 4.0–8.9 | 95.3–98.6 | [131] |
| LC-MS/MS | bean sprouts | solvent: acetonitrile; MP: 10 mM ammonium formate and methanol (gradient), FR: 0.5 mL/min; column: YMC Pack Pro C8 (YMC, Kyoto, Japan) (150 × 4.6 mm) with 3 μm particles; tR: 10.02 min; temperature: 350 °C; detection: m/z 175, 131; ESI; positive ion mode | 1.0 × 10−2–1.0 1 | 0.9980 | 2.0 × 10−3 1 | 0.5–1.8 | 88.9–96.3 | [132] |
| LC-MS/MS | fruit jam | solvent: acetonitrile; MP: 0.1% formic acid and acetonitrile with 0.1% formic acid (gradient), FR: 0.03 mL/min; column: Eksigent Halo C18 (Eksigent Technologies, Framingham, MA, USA) (50 × 0.5 mm) with 2.7 μm particles; tR: 0.46 min; temperature: 400 °C; detection: m/z 174.9, 131.1; ESI; positive ion mode | 9.0 × 10−3–0.6 1 | 0.9800 | 9.0 × 10−3 | 2.0–6.0 | 65.0–107.0 | [133] |
| LC-MS/MS | orange, pear | solvent: acetonitrile; MP: 0.1% formic acid and acetonitrile with 0.1% formic acid and 5% of water (gradient), FR: 0.3 mL/min; column: Zorbax Eclipse plus C8 (Agilent, Santa Clara, CA, USA) (100 × 2.1 mm) with 1.8 μm particles; tR: 1.31 min; temperature: 375 °C; detection: m/z 175.0, 131.0; ESI; positive ion mode | 1.0 × 10−3–0.5 1 | 0.9989 (orange) 0.9996 (pear) | - | 0.0–1.0 | 77.0–96.0 | [135] |
| LC-MS/MS | milk powder, butter, fish tissue, egg | solvent: methanol–0.05% formic acid (25:75); MP: 0.01% formic acid and methanol (gradient), FR: 0.1 mL/min; column: Atlantis T3 C18 (Waters, Wien, Austria) (100 × 2.1 mm) with 3 μm particles and guard column; tR: 9.12 min; temperature: 300 °C; detection: m/z 175, 131; ESI; positive ion mode | 1.0 × 10−3–0.2 | >0.9970 | - | 4.0–6.8 | 56.1–101.0 | [136] |
| LC-MS/MS | leek, leaf lettuce and garland chrysanthemum | solvent: acetonitrile; MP: acetonitrile and 0.1% formic acid (gradient), FR: 0.3 mL/min; column: Hypersil GOLD C18 (Termo Fisher Scientific, Waltham, MA, USA) (100 × 2.1 mm) with 3 μm particles; tR: 1.11 min; temperature: 300 °C; detection: m/z 175.1, 131.2; ESI; positive ion mode | 1.0 × 10−2–1.0 | ≥0.9992 | 3.0 × 10−4 1 | 2.1–14.7 | 81.0–109.0 | [137] |
| LC-MS/MS | sewage sludge | solvent: methanol–water (1:9); MP: water and methanol, both with 0.1 mM ammonium acetate and 0.01% formic acid (gradient), FR: 0.3 mL/min; column: Waters Symmetry C18 (Waters, Wien, Austria) (50 × 2.1 mm) with 3.5 μm particles; tR: 4.8 min; temperature: 350 °C; detection: m/z 175.2, 131.2; ESI; positive ion mode | 5.0 × 10−4–0.5 | 0.9990 | - | 1.0–42.0 | 92.0–122.0 | [138] |
| LC-MS/MS | bean sprouts | solvent: acetonitrile–water (12.5:87.5); MP: water–methanol (95:5) with 0.1% formic acid and water–methanol (50:50) (gradient), FR: 0.4 mL/min; column: Acquity UPLC BEH C18 (Waters, Milford, CT, USA) (50 × 2.1 mm) with 1.7 μm particles; tR: 1.58 min; temperature: 300 °C; detection: m/z 174.8, 130.9; ESI; positive ion mode | 2.0 × 10−3–0.2 | 0.9978 | 2.1 × 10−3 1 | 1.9–3.1 | 102.5–103.2 | [139] |
| LC-MS/MS | coconut | solvent: acetonitrile–water; MP: water–methanol (98:2) and methanol, both with 0.1% formic acid and 5 mM ammonium formate (gradient), FR: 0.225 mL/min; column: Acquity UPLC BEH C18 (Waters, Milford, CT, USA) (100 × 2.1 mm) with 1.7 μm particles; tR: 3.45 min; temperature: 500 °C; detection: m/z 175.2, 131.2; ESI; positive ion mode | 2.5 × 10−3–2.5 × 10−1 | ≥0.9984 | 3.0 × 10−3 1 | 1.0–7.0 | 84.0–100.0 | [140] |
| LC-MS/MS | milk | solvent: acetonitrile; MP: 0.1% formic acid and acetonitrile with 0.1% formic acid (gradient), FR: 0.2–0.4 mL/min; column: Waters Xterra C18 (Waters, Wien, Austria) (50 × 3.0 mm) with 3.5 μm particles; tR: 5.22 min; temperature: 400 °C; detection: m/z 175.2, 64.9; ESI; positive ion mode | 5.0 × 10−2–1.0 1 | >0.9900 | 1.7 × 10−2 1 | 5.7–10.9 | 83.0–89.0 | [141] |
| LC-MS/MS | sweet green pepper | solvent: methanol; MP: 0.1% formic acid and methanol (gradient), FR: 0.3 mL/min; column: Waters Nova-Pak C18 (Waters, Wien, Austria) (150 × 3.9 mm) with a guard column (20 × 3.9), both with 4 μm particles; tR: 8.5 min; temperature: 400 °C; detection: m/z 174.8, 130.6; ESI; positive ion mode | 0.2–2.4 1 | 0.9954 | - | - | 71.0–107.0 | [142] |
| LC-MS/MS | sewage sludge | solvent: methanol; MP: acetonitrile and 100 mM ammonium formate (gradient), FR: 0.2 mL/min; column: Waters Atlantis HILIC (Waters, Wien, Austria) (50 × 4.6 mm) with 3 μm particles; ESI; positive and negative ion mode | 1.3 × 10−3–2.5 1 | 0.9966 | - | 3.0–9.0 | 48.0–95.0 | [143] |
| LC-MS/MS | freshwater fatty fish muscle tissue | solvent: acetonitrile; MP: 0.1% formic acid and acetonitrile (gradient), FR: 0.6 mL/min; column: Zorbax Eclipse XDB-C18 (Agilent, Santa Clara, CA, USA) (150 × 4.6 mm) with 5 μm particles; tR: 9.9, 10.2 min; detection: m/z 175.1, 131.1 | - | >0.9900 | - | 2.0–9.0 | 88.0–107.0 | [144] |
| LC-MS/MS | wastewater | solvent: acetonitrile–water (10:90); MP: 0.1% formic acid and methanol (gradient), FR: 0.5 mL/min; column: Phenomenex Kinetex C18 (Phenomenex, Torrance, CA, USA) (150 × 4.6 mm) with 2.6 μm particles; tR: 7.26 min; temperature: 500 °C; detection: m/z 175.1, 131.1; ESI; positive ion mode | 1.0 × 10−5–1.0 × 10−3 | 0.9956 | - | 6.0–8.0 | 79.0–102.0 | [145] |
| LC-MS/MS | orange | solvent: acetonitrile; MP: 10 mM ammonium formate buffer pH 4-methanol (10:90), FR: 0.6 mL/min; column: Zorbax Eclipse plus C18 (Agilent, Santa Clara, CA, USA) (150 × 4.6 mm) with 5 μm particles; tR: 2.66 min; temperature: 450 °C; detection: m/z 175.02; ESI; positive ion mode | 5.0 × 10−3–0.2 1 | 0.9998 | 1.3 × 10−3 1 | 5.0–5.4 | 102.9 | [146] |
| LC-MS/MS | vegetable oil | solvent: methanol–water (10:90); MP: acetonitrile and 2 mM ammonium acetate (gradient), FR: 0.5 mL/min; column: Waters Acquity UPLC BEH C18 (Waters, Milford, CT, USA) (100 × 2.1 mm) with 1.7 μm particles; tR: 3.22 min; temperature: 550 °C; detection: m/z 175.0, 131.0; ESI; positive ion mode | 5.0 × 10−3–0.5 1 | 0.9957 | 1.0 × 10−3 1 | 1.9–10.9 | 95.3–106.2 | [147] |
| HPLC | milk | solvent: acetonitrile–sodium 1-octanesulphonate (1:1), pH 3.5; MP: 0.01 M sodium 1-octanesulphonate, pH = 3.5, acetonitrile–water (gradient); FR: 0.5 mL/min; column: Alltima C18 (Restek, Rheinfelden, Switzerland) (150 × 3.2 mm) with 5 μm particles and 100 Å pore size and a guard column (7.5 × 3.2 mm); temperature: 35 °C; detection: UV PDA, 294 nm | 4.3 × 10−3–1.0 | 0.9999 | 3.4 × 10−3 | 97.9–102.2 | 77.0–80.0 | [156] |
| HPLC | livestock, milk | solvent: acetonitrile– methanol–0.02M sodium dihydrogen phosphate (2:2:6); MP: acetonitrile–methanol–0.02 M NaH2PO4 (gradient); FR: 1.0 mL/min; column: Cosmosil 5 C18 MS-II (Nacalai Tesque, Kyoto, Japan) (250 × 4.6 mm) with 5 µm particles; detection: UV PDA (290–320 nm) and fluorescence (Ex: 290 nm, Em: 320 nm) | 5.0 × 10−2–2.5 | 1.0 | 2.0 × 10−2 (UV) 5.0 × 10−3 (fluorescence) | - | 84.5–97.6 | [157] |
| HPLC | lemon | solvent: 0.5% trifluoroacetic acid in acetonitrile; MP: acetonitrile–water–30% ammonia solution (39:60.5:0.5); FR: 1.0 mL/min; column: Supelcosil LC-18 RP column (Sigma-Aldrich, St. Louis, MO, USA) (250 × 4.6 mm) with 5 μm particles and 100 Å pore size; tR: 5.8 min; room temperature; detection: UV, max. absorption: 254 nm | 0.1–40.0 | 0.9990 | 2.7 × 10−1 | 3.8–5.0 | 96.7–98.3 | [158] |
| HPLC | water | solvent: methanol–water (50:50); MP: methanol–water (40:60) with 0.6% ammonia; FR: 1.0 mL/min; column: Nova-Pack C18 (Waters, Wien, Austria) (150 × 3.9 mm) with a Nova Pack Silica guard column (20 × 3.9), both with 4 μm particles; tR: 6.2 min; room temperature; detection: fluorescence, Ex: 300 nm, Em: 350 nm | 1.0 × 10−3–0.1 | 0.9980 | 0.8 × 10−4–1.3 × 10−4 | 2.3–4.3 | 86.0–89.0 | [159] |
| HPLC | wine | solvent: methanol; MP: acetonitrile and 10 mM ammonium acetate (gradient); FR: 1.0 mL/min; column: Synergy Max-RP C12 (Phenomenex, Torrance, CA, USA) (250 × 4.6 mm) with 4 µm particles and a guard column Security Guard Max-RP C12 (4.0 mm × 3.0 mm); temperature: 35 °C; vaporization temperature: 300 °C; detection: m/z 202; APCI; positive ion mode | - | 0.9900 | 8.0 × 10−3 | <10 | 94.5–102.4 | [160] |
| HPLC | fruit | solvent: acetonitrile; MP methanol and acetic acid (gradient); FR: 1.0 mL/min; column: polymer particles (50 × 4.6 mm); tR: 7.5 min; temperature: 0–180 °C; detection: fluorescence, Ex: 305 nm, Em: 345 nm | 0.1–5.0 | 0.9990 | 3 × 10−5 | 4.2–7.9 | 88.7–102.4 | [161] |
| HPLC | water | solvent: methanol; MP: methanol–water (45:55); FR: 1.0 mL/min; column: Symmetry C18 (Waters, Wien, Austria) (150 × 3.9 mm) with 8 µm particles; tR: 3.5 min; temperature: 30 °C; detection: fluorescence, Ex: 300 nm, Em: 350 nm | 5.0 × 10−4–0.3 | 0.9990 | 4.0 × 10−5 | 0.2–3.3 | 96.9–115.7 | [162] |
| HPLC | apple | solvent: MP; MP: methanol–water (50:50); FR: 1.0 mL/min; column: Symmetry C18 (Waters, Wien, Austria) (150 × 3.9 mm) with 5 µm particles; tR: <6 min; temperature: 20 °C; detection: fluorescence, Ex: 280 nm, Em: 315 nm | 1.0 × 10−2–1.0 1 | 0.9998 | 3.0 × 10−3 1 | 3.8–4.1 | 92.3–96.1 | [163] |
| HPLC | water, soil | solvent: methanol; MP: methanol–water (60:40), pH 7; FR: 1.0 mL/min; column: Centurysil C18 (Macherey-Nagel, Düren, Ger-many) (250 × 4.6 mm) with 5 µm particles; tR: <8.0 min; room temperature; detection: fluorescence, Ex: 280 nm, Em: 315 nm | 5.0 × 10−3–0.8 (water) 1.0 × 10−2–1.0 1 (soil) | 0.9997 (water) 0.9994 (soil) | 5.0 × 10−4–1.0 × 10−3 (water) 1.0 × 10−3–1.6 × 10−3 1 (soil) | 3.1–5.8 (water) 4.2–6.2 (soil) | 86.0–94.0 (water) 84.0–90.8 (soil) | [164] |
| HPLC | orange juice | solvent: toluene; MP: methanol–water (70:30); FR: 0.6 mL/min; column: Zorbax Eclipse XDB-C8 (Agilent, Santa Clara, CA, USA) (150 × 4.6 mm) with 5 µm particles; tR: <3.0 min; detection: fluorescence (Ex: 305 nm, Em: 345) nm and UV (max. absorption: 290 nm) | 1.0 × 10−2–5.0 | >0.9950 | 4.0 × 10−3 | 6.6–7.6 | 2.1–15.1 | [165] |
| HPLC | soil | solvent: acetonitrile–10 mM phosphate buffer, pH 8.7 (59:41); MP: acetonitrile–10 mM phosphate buffer pH 8.7 (22:78); FR: 1.0 mL/min; column: Nova-Pak C18 (Waters, Wien, Austria) (150 × 3.9 mm) with a Guard-Pak C18 guard column, both with 4 μm particles; tR: 4.65 min; room temperature; detection: fluorescence, Ex: 305 nm, Em: 335 nm | 5.0 × 10−3–5.0 | 0.9970–0.9990 | 1.2 × 10−6–2.8 × 10−6 1 | - | 80.0–117.0 | [166] |
| HPLC | tomato | solvent: acetonitrile; MP: acetonitrile–water (60:40); column: OptimaPak C18 (R Stech, Daejeon, South Korea) (4.6 × 150 mm) with 5 µm particles; tR: <11 min; detection: UV, max. absorption: 245 nm | 5.0 × 10−3–0.2 1 | 0.9990 | 2.4 × 10−4 1 | 4.5–5.3 | 76.0–89.0 | [167] |
| HPLC | edible fungi | solvent: methanol–dichloromethane (5:95); MP: methanol–water (35:65); FR: 1.0 mL/min; column: Centurysil C18 (Macherey-Nagel, Düren, Germany) (250 × 4.6 mm) with 5 µm particles; temperature: 30 °C; detection: UV, max. absorption: 275 nm | 5.0 × 10−2–5.0 | 0.9993 | 3.0 × 10−3 1 | 0.5–3.5 | 86.0–105.0 | [168] |
| HPLC | fruit, vegetables | solvent: MP; MP: methanol–water (50:50); FR: 1.0 mL/min; column: Agilent Sorbax SB C18 (Agilent, Santa Clara, CA, USA) (150 × 4.6 mm) with 5 µm particles; tR: 5.5 min; detection: UV diode array, max. absorption: 280 nm | 2.7 × 10−3–2.0 | 0.9894 | 9.0 × 10−4 | - | - | [169] |
| HPLC | tissue | solvent: 1-octanol; MP: methanol and 0.1% acetic acid (gradient); FR: 1.0 mL/min; column: Atlantis dC18 (Waters, Wien, Austria) (150 × 4.6 mm) with 5 µm particles; tR: 3.3 min; room temperature; detection: UV, max. absorption: 296 nm | 5.0 × 10−3–1.0 | 0.9991–0.9995 | 3.0 × 10−4 | 1.4–3.1 | 89.0–105.0 | [170] |
| HPLC | milk | solvent: 1-octanol; MP: 0.1% acetic acid and methanol (gradient); FR: 1.0 mL/min; column: Atlantis dC18 (Waters, Wien, Austria) (150 × 4.6 mm) with 5 µm particles; tR: <3 min; room; detection: UV, max. absorption: 296 nm | 5.0 × 10−3–1.0 | 0.9990 | 5.0 × 10−3 | 1.2–2.8 | 89.0–105.0 | [171] |
| HPLC | orange | solvent: acetonitrile–acetic acid (99:1); MP: 5 mM ammonium formate and methanol containing 5 mM ammonium formate (gradient); FR: 0.5 mL/min; column: Inertsil ODS-4 (GL Sciences, Tokyo, Japan) (50 × 2.1 mm) with 3 µm particles and a guard column (4 mm × 2 mm) with 4 µm particles; tR: 7.0 min; temperature: 30 °C; source temperature: 500 °C; detection: m/z 175.1; ESI; positive ion mode | 0.1–2.5 × 10−1 1 | 0.9983 | 2.0 × 10−3 1 | 6.9–12.2 | 93.1–92.7 | [172] |
| HPLC | wastewater, fruit juice | solvent: methanol; MP: methanol–water (50:50); FR: 1.0 mL/min; column: Zorbax SB-C18 (Agilent, Santa Clara, CA, USA) (150 × 4.6 mm) with 5 µm particles; tR: 2.2 min; temperature: 30 °C; detection: UV diode array, max. absorption: 298 nm | 1.0 × 10−2–1.0 | 0.9962 | 2.6 × 10−3 | 6.5 | 92.9–103.9 | [173] |
| HPLC | milk, egg, tissue, feed, water, soil | solvent: acetonitrile–HCOOH; MP: 0.1% HCOOH-5 mM HCOONH4–0.1% HCOOH (gradient); FR: 0.3 mL/min; column: Acclaim 120 C18 (Termo Fisher Scientific, Waltham, MA, USA) (150 × 2.1 mm) with 2.2 µm particles; tR: 7.2 min; temperature: 40 °C; source temperature: 250 °C; detection: m/z 202.04; ESI; positive ion mode | 1.0 × 10−6–0.2 1 | - | 1.0 × 10−3 | 5.0 | - | [174] |
| HPLC | lemon, cucumber, apple | solvent: MP; MP: water–methanol (50:50); FR: 1.0 mL/min; column: Zorbax Eclipse XDB-C18 (Agilent, Santa Clara, CA, USA) (150 × 4.6 mm) with 5 µm particles; tR < 5 min; room temperature; detection: UV diode array, max. absorption: 285 nm | 2.0 × 10−3–0.5 | 0.9978 | 5.4 × 10−4 | 1.3–3.4 | 96.9–101.3 | [175] |
| HPLC | TBZ solution | solvent: methanol–water (30:70); MP: 1-octanesulfonic acid sodium salt–0.1% methanesulfonic acid and acetonitrile (gradient); FR: 1.5 mL/min; column: ACE 5 C18 (Advanced Chromatography Technologies, Aberdeen, Scotland) (50 × 4.6 mm) with 5 µm particles; tR: <1.5 min; temperature: 35 °C; detection: UV, max. absorption: 300 nm | 0.1–120.0 | 1.0 | - | 1.3–5.4 | 94.6–99.5 | [176] |
| HPLC | fruit, vegetables | solvent: acetonitrile–water (40:60); MP: acetonitrile–ammonium acetate (40:60); FR: 1.0 mL/min; column: Hisep 5 C18 (Sigma-Aldrich, Darmstadt, Germany) (250 × 4.6 mm) with 5 µm particles; tR: <10 min; temperature: 40 °C; detection: fluorescence, Ex: 285 nm, Em: 315 nm | 2.0 × 10−3–1.0 | 0.9998 | 7.5 × 10−3 1 | 1.3–5.4 | 80.0–99.1 | [177] |
| HPLC | citrus fruit | solvent: acetonitrile; MP: water and methanol (gradient); FR: 1.0 mL/min; column: Kromasil dC18 (Eka Chemicals, Bohus, Sweden) (150 × 4.6 mm) with 5 µm particles; tR: <7 min; temperature: 25 °C; detection: fluorescence, Ex: 280 nm, Em: 315 nm | 0.3–3.0 1 | - | 0.2 1 | <8.0 | 28.0–38.0 | [178] |
| HPLC | citrus fruit | solvent: methanol; MP: water and acetonitrile (gradient); FR: 0.7 mL/min; column: Symmetry C18 (Waters, Wien, Austria) (150 × 3.0 mm) with 3.5 µm particles and a guard cartridge Atlantis T3 with 3 µm particles; tR: <4 min; detection: UV diode array, max. absorption: 286 nm, 300 nm | 2.0 × 10−5–2.0 × 10−3 | 0.9980 | 4.0 × 10−3 1 (orange) 9.0 × 10−3 1 (lemon) | <4.0 | 5.1 (orange) 6.1 (lemon) | [179] |
| HPLC | orange | solvent: MP; MP: 1 × 10−3 M phosphate buffer (pH 7) and methanol (gradient); FR: 0.6 mL/min; column: Zorbax Eclipse XDB-C8 (Agilent, Santa Clara, CA, USA) (150 × 4.6 mm) with 5 µm particles and a guard cartridge Atlantis T3 with 3 µm particles; tR: <8 min; detection: UV diode array, max. absorption: 280 nm, 300 nm | - | 0.9930 | 0.1 1 | 6.0 | 21.0 | [180] |
| HPLC | citrus fruit | solvent: acetonitrile; MP: 1 × 10−2 M acetonitrile–ammonium acetate (35:65); FR: 1.0 mL/min; column: s Epic C18 (Termo Fisher Scientific, Waltham, United States) (150 × 4.6 mm) with 5 µm particles; tR: <8 min; room temperature; detection: fluorescence, Ex: 305 nm, Em: 350 nm | 1.0 × 10−2–1.0 | 0.9990 | 7.2 × 10−4 | 2.6–6.9 | 84.9–98.4 | [181] |
| HPLC | red grape, cherry tomato | solvent: methanol; MP: methanol–water (61:39); FR: 0.8 mL/min; column: Thermo Scientific C18 (Termo Fisher Scientific, Waltham, United States) (250 × 4.6 mm) with 5 µm particles; detection: UV, 200–370 nm | - | 0.9994 | 0.03 | 3.2 | 99.3 | [182] |
| HPLC | Chinese herbs | solvent: acetonitrile; MP: 0.05% formic acid in methanol, 0.05% formic acid and 5 mM ammonium formate in water (gradient); FR: 0.4 mL/min; column: Agilent Poroshell 120 EC-C18 (Agilent, Santa Clara, CA, USA) (150 × 3.0 mm) with 2.7 µm particles; tR: 5.8 min; temperature: 35 °C; source temperature: 500 °C; detection: m/z 202.2; ESI; positive ion mode | 2.0 × 10−3–0.4 1 | 0.9997 | - | 2.3–6.2 | 86.3–99.7 | [183] |
| HPLC | fruit, vegetables, juice | solvent: 0.1 M hydrochloric acid; MP: phosphate buffer–acetonitrile (80:20) adjusted to pH 4; FR: 0.25 mL/min; column: Agilent SB-C18 (Agilent, Santa Clara, CA, USA) (100 × 2.2 mm); tR: <13 min; detection: UV, max. absorption: 285 nm | 4.7 × 10−3–1.0 1 | 0.9930–0.9980 | 1.8 × 10−3–6.1 × 10−3 1 | 3.1–10.6 | 59.3–111.4 | [184] |
| HPLC | specific parts of honeybees | solvent: acetonitrile; MP: water and acetonitrile (gradient); FR: 200 nL/min; column: EASY-Spray PepMap C18 (Termo Fisher Scientific, Waltham, MA, USA) (150 × 75 µm) with 3 µm particles and 100 Å pores; tR: 21 min; temperature: 25 °C; capillary temperature: 250 °C; MS detection, ESI; positive ion mode | - | 0.9950 | 6.0 2 | 9.9 | 75 | [185] |
| HPLC | banana, citrus fruit | solvent: methanol; MP: phosphoric acid buffer (pH 7) –acetonitrile–methanol (70:20:10); FR: 1.0 mL/min; column: Shiseido Capcell Pak C18 (Shiseido, Tokyo, Japan) (250 × 4.6 mm) with 5 µm particles; tR: 12.47 min; temperature: 40 °C; detection: UV photo diode array, max. absorption: 285 nm | 3.1 × 10−1–20.0 | 0.9990 | 9.0 × 10−3–1.7 × 10−2 | 1.3 | 93.6–98.1 | [186] |
| UHPLC-MS/MS | fruit juice | solvent: methanol and dichloromethane; MP: methanol and 0.01% formic acid (gradient), FR: 0.35 mL/min; column; Waters Acquity UPLC BEH C18 column (Waters, Milford, CT, USA) (100 × 2.1 mm) with 1.7 µm particles; tR: 2.72–2.79 min; vaporization temperature: 350 °C; detection: m/z 202, 175, 131; ESI; positive ion mode | - | 0.9981 | 1.8 × 10−3 | 5.0–15.1 | 76.8–87.2 | [188] |
| UHPLC-MS/MS | egg | solvent: methanol–0.05% formic acid (50:50); MP: methanol, 0.05% formic acid, FR: 0.3 mL/min; column: Acquity UPLC BEH C18 column (Waters, Milford, CT, USA) (100 × 2.1 mm) with 1.7 µm particles; tR: 3.09–3.13 min; vaporization temperature: 350 °C; detection: m/z 201.8, 175.2, 131.2; ESI; positive ion mode | - | - | 0.2 1 | 9.1–15.2 | 88.4–97.0 | [190] |
| UHPLC-MS | milk, powdered milk-based infant formulae | solvent: 0.05% formic acid and methanol; MP: 0.05% formic acid and methanol (gradient), FR: 0.3 mL/min; column: Hypersil GOLD aQ C18 column (Termo Fisher Scientific, Waltham, MA, USA) (100 × 2.1 mm) with 1.7 µm particles; tR: 1.40–1.48 min; detection: m/z 202.0434, 175.0324, 131.0604; ESI; positive ion mode | 4.1 × 10−3–8.1 × 10−3 1 | - | 8.1 × 10−3 1 | - | - | [191] |
| UHPLC-MS/MS | chicken meat | solvent: 0.1 % formic acid in acetonitrile–water (50:50); MP: 0.1% formic acid in acetonitrile and 0.1% formic acid (gradient), FR: 0.3 mL/min; column: C18 column (100 × 2.1 mm) with 1.7 µm particles; tR: 1.93–2.13 min; detection: m/z 201.8, 175.2; ESI; positive ion mode | - | - | 6.4 × 10−3 1 | 8.1–20.5 | 91.1–114.2 | [192] |
| UHPLC-MS/MS | meat-based baby food, milk-based infant formulae | solvent: methanol and 0.05% formic acid; MP: methanol and 0.05% formic acid (gradient); FR: 0.3 mL/min; column: UPLC BEH C18 column (Waters, Milford, CT, USA) (100 × 2.1 mm) with 1.7 µm particles; tR: 3.05–3.19 min; detection: m/z 201.8, 175.2; ESI; positive ion mode | 5 × 10−3–0.1 1 | - | 5.0 × 10−4 1 | 1.0–4.0 | 87.2–125.0 | [193] |
| UHPLC-MS/MS | gilthead sea bream | solvent: 0.1% formic acid in acetonitrile and 0.1% formic acid; MP: 0.1% formic acid in acetonitrile and 0.1% formic acid (gradient); FR: 0.3 mL/min; column: BEH C18 column (Waters, Milford, CT, USA) (100 × 2.1 mm) with 1.7 µm particles; tR: 2.04–2.11 min; detection: m/z 201.8, 175.2, 131.2; ESI; positive ion mode | - | - | 3.0 × 10−3 1 | 6.0–17.0 | 76.0–97.0 | [194] |
| UHPLC-MS/MS | green tea tablets and capsules | solvent: acetonitrile and 1% acetic acid; MP: acetonitrile and 0.01% formic acid (gradient); FR: 0.35 mL/min; column: Hypersil GOLD aQ (Termo Fisher Scientific, Waltham, MA, USA) (100 × 2.1 mm) with 1.9 µm particles; tR: 1.93–2.10 min; detection: m/z 202, 175, 131 | - | >0.9800 | 1.0 × 10−3 1 | 7.0–16.0 | 82.0–88.0 | [195] |
| UHPLC-MS/MS | milk | solvent: acetonitrile; MP: 0.01% acetic acid and acetonitrile–water (90:10) (gradient); FR: 0.6 mL/min; column: Acquity stainless steel HSS T3 (Waters, Wien, Austria) (100 × 2.1 mm) with 1.8 µm particles; tR: 3.13 min; detection: m/z 201.9, 130.85, 174.8; ESI; positive ion mode | 1.4 × 10−4–1.9 × 10−3 1 | 0.9980 | 0.1 1 | - | 87.0–101.0 | [196] |
| UHPLC-MS/MS | bovine milk | solvent: 0.1% acetic acid–0.1% formic acid in acetonitrile; MP: 0.1% acetic acid and 0.1% formic acid in acetonitrile (gradient); FR: 0.3 mL/min; column: Acquity BEH C18 column (Waters, Milford, CT, USA) (50 × 2.1 mm) with 1.7 µm particles; tR: 1.23 min; detection: m/z 202, 175 and 131; ESI; positive ion mode | - | - | 3.0 × 10−5 | - | 93.3–95.0 | [197] |
| UHPLC-MS/MS | chicken tissue (egg, liver, muscle) | solvent: methanol–ammonia (99:1); MP: 5% methanol and 95% water containing 0.1% formic acid (gradient); FR: 0.35 mL/min; column: BEH C18 column (Waters, Milford, CT, USA) (100 × 2.1 mm) with 1.7 µm particles; tR: 3.27 min; detection: m/z 202, 175, 131; ESI; positive ion mode | 1.0 × 10−4–0.1 1 | - | 0.1 1 | 3.3–13.7 | 93.6–116.7 | [198] |
| UHPLC-MS/MS | bovine milk | solvent: 0.1% formic acid with 5 mM ammonium acetate–methanol (90:10); MP: 0.1% formic acid with 5 mM ammonium acetate and methanol (gradient); FR: 0.3 mL/min; column: BHE C18 (Waters, Milford, CT, USA) (100 × 2.1 mm) with 1.7 µm particles; detection: m/z 202.1, 175.1, 131.1; ESI; positive ion mode | 1.1 × 10−2–1.1 1 | 0.9991 | 0.1 1 | - | 108.0–112.0 | [199] |
| UHPLC-MS | fruit, vegetables | solvent: methanol; MP: 0.1% formic acid in methanol and 0.1% formic acid (gradient); FR: 1.0 mL/min; column: BEH C18 column (Waters, Milford, CT, USA) (50 × 2.1 mm) with 1.7 µm particles; tR: 1.45 min; detection: m/z 202.0439; ESI; positive ion mode | 1.0 × 10−2–0.8 | 0.9995 | 3.3 × 10−3 1 | 2.5 | 108.0 | [200] |
| UHPLC-MS/MS | fruit, vegetables | solvent: ammonium acetate in methanol/water (95:5); MP: 5 mM ammonium acetate in water–methanol (90:10) and 5 mM ammonium acetate in methanol–water (90:10) (gradient); FR: 0.45 mL/min; column: BEH C18 column (Waters, Milford, CT, USA) (100 × 2.1 mm) with 1.7 µm particles; tR: 4.63 min; detection: m/z 202, 175.1, 131; ESI; positive ion mode | - | - | - | 3.3–10.3 | 81.0–103.1 | [201] |
| UHPLC-MS/MS | insect bolus | solvent: water–methanol (1:1) containing 10 ng/mL triphenyl phosphate; MP: 0.2% formic acid and 0.2% formic acid–methanol–acetonitrile (gradient); FR: 0.4 mL/min; column: BEH C18 column (Waters, Milford, CT, USA) (100 × 2.1 mm) with 1.8 µm particles; tR: 3.5 min; detection: m/z 202, 175, 131; ESI; positive ion mode | 1.0 × 10−4–3.0 × 10−2 | - | 1.6 × 10−4 1 | 13.5 | 82.1 | [202] |
| UHPLC-MS | water | solvent: methanol; MP: 2 mM ammonium formate and 2 mM ammonium formate in methanol (gradient); FR: 0.4 mL/min; column: BEH C18 column (Waters, Milford, CT, USA) (100 × 2.1 mm) with 1.8 µm particles; tR: 5.94 min; detection: m/z 202.0434; ESI; positive ion mode | 0–5.0 × 10−5 1 | 0.9982 | 1.0 × 10−5 | 7.0 | 107.6 | [203] |
| UHPLC-MS/MS | bovine milk | solvent: methanol; MP: 0.1% formic acid and 0.1% formic acid in acetonitrile (gradient), FR: 0.4 mL/min; column: BEH C18 column (Waters, Milford, CT, USA) (50 × 2.1 mm) with 1.7 µm particles; tR: 3.27 min; detection: m/z 201.9, 175, 131.1; ESI; positive ion mode | 0–1.0 × 10−2 1 | 0.9967 | 3.0 × 10−6 1 | - | 52.6–81.7 | [204] |
| UHPLC-MS/MS | orange juice | solvent: acetonitrile; MP: 0.1% formic acid and 5 mM ammonium formate in (a) water–methanol (98:2) and (b) methanol (gradient); FR: 0.25 mL/min; column: BEH C18 column (Waters, Milford, CT, USA) (50 × 2.1 mm) with 1.7 µm particles; tR: 3.04 min; detection: m/z 202; ESI; positive ion mode | - | - | 3.0 × 10−3 | 3.0–11.0 | 80.0–86.0 | [205] |
| UHPLC-MS/MS | vegetables, cereals | solvent: acetonitrile; MP: 0.1% formic acid and 5 mM ammonium formate in water, 0.1 % formic acid and 5 mM ammonium formate in methanol (gradient); FR: 0.25 mL/min; column: BEH C18 column (Waters, Milford, CT, USA) (50 × 2.1 mm) with 1.7 µm particles; ESI; positive ion mode | 2.0 × 10−2–1.6 1 | - | 1.0 × 10−2 1 | 3.0–13.0 | 73.0–74.0 | [206] |
| UHPLC-MS/MS | bovine tissue (liver, kidney, muscle) | solvent: solution of trichloroacetic acid; MP: 10 mM ammonium formate in water–acetonitrile (98:2) and 0.1% formic acid in methanol–acetonitrile (75:25) (gradient); FR: 0.4 mL/min; column: Waters Acquity UPLC column (Waters, Wien, Austria) (100 × 2.1 mm) with 1.8 µm particles; ESI; positive and negative ion mode | - | - | 0.1 1 | 2.0–15.0 | 90.0–109.0 | [207] |
| UHPLC-MS/MS | swine waste lagoon sludge | solvent: 0.2% formic acid in methanol; MP: 0.1% formic acid and 0.1% formic acid in methanol–acetonitrile (20:80) (gradient); FR: 0.4 mL/min; column: BEH C18 column (Waters, Milford, CT, USA) (50 × 2.1 mm) with 1.7 µm particles; detection: m/z 202, 175, 131; ESI; positive ion mode | 1.0 × 10−3–0.5 1 | - | 3.0 × 10−4 1 | - | 70.8–83.1 | [208] |
| UHPLC-MS/MS | wastewater | solvent: acetonitrile; MP: 0.1% formic acid and acetonitrile (gradient); FR: 0.4 mL/min; column: Zorbax Eclipse XDB C18 (Agilent, Santa Clara, CA, USA) (50 × 4.6 mm) with 1.8 µm particles; ESI; positive ion mode | 2.0 × 10−5–5.0 × 10−3 | 0.9979 | 5.0 × 10−6 | 1.0–11.0 | 101.0–115.0 | [209] |
| UHPLC-MS/MS | bovine muscle tissue | solvent: acetonitrile; MP: 0.1% formic acid–5 mM ammonium formate and 0.1% formic acid in acetonitrile–water (95:5) (gradient); FR: 0.4 mL/min; column: BEH C18 column (Waters, Milford, CT, USA) (50 × 2.1 mm) with 1.7 µm particles; tR: 2.04 min; detection: m/z 202, 91.9, 131, 175; ESI; positive ion mode | 5.0 × 10−2–0.2 1 | 0.9980 | 1.4 × 10−2 1 | 2.0–10.5 | 93.3–102.5 | [210] |
| UHPLC-MS | air | solvent: water–methanol (70:30); MP: 0.1% formic acid–4 mM ammonium formate and 0.1% formic acid–4 mM ammonium formate in methanol (gradient); FR: 0.3 mL/min; column: Hypersil Gold aQ column (Termo Fisher Scientific, Waltham, MA, USA) (100 × 2.1 mm) with 1.9 µm particles; tR: 5.97 min; detection: m/z 202.04334, 175.09788; ESI; positive ion mode | 5.0 × 10−3–0.2 | - | - | 0.5–4.0 | 92.8–99.0 | [211] |
| UHPLC-MS/MS | fish bile | solvent: methanol–water (50:50), dichloromethane; MP: 0.05% formic acid and 5 mM ammonium acetate in a) ultrapure water, b) methanol (gradient); FR: 0.35 mL/min; column: Agilent SB-C18 column (Agilent, Santa Clara, CA, USA) (100 × 3 mm) with 1.8 µm particles; tR: 3.22 min; detection: m/z 208.1, 136.1, 180.1; ESI; positive ion mode | 1.0 × 10−4–2.0 × 10−2 | 0.9995 | 8.0 × 10−5 | 1.0–4.0 | 86.0–93.0 | [212] |
| UHPLC-MS | shellfish | solvent: acetonitrile–water (50:50); MP: acetonitrile and water (gradient); FR: 0.2 mL/min; column: Purospher STAR RP-18 end-capped column (Sigma-Aldrich, Darmstadt, Germany) (150 × 2.1 mm) with 2 µm particles; tR: 5.1 min; detection: m/z 202.0433; ESI; positive ion mode | 1.0 × 10−5–5.0 × 10−2 1 | - | 2.0 × 10−5 1 | 1.8 | 96.0–107.0 | [213] |
| UHPLC-MS/MS | water | solvent: 0.1% ammonium hydroxide, methanol–acetonitrile–propan-2-ol (1:1:1); MP: 0.1% formic acid and acetonitrile–methanol (2:1) (gradient); FR: 0.4 mL/min; column: Acquity BEH C18 (Waters, Milford, CT, USA) (150 × 3 mm) with 1.7 µm particles; tR: 6.54 min; ESI; positive ion mode | 4.0 × 10−7–4.0 × 10−4 | 0.9994 | 7.7 × 10−8 | - | 94.7–104.4 | [214] |
| UHPLC-MS/MS | urine and blood of livestock and poultry | solvent: dichloromethane; MP: 0.2% formic acid–2 mM ammonium acetate, 0.2% formic acid in methanol (gradient); FR: 0.4 mL/min; column: Agilent Zorbax Eclipse Plus C18 (Agilent, Santa Clara, CA, USA) (150 × 3 mm) with 1.8 µm particles; tR: 9.15 min; detection: m/z 202, 175, 131; ESI; positive ion mode | 1.0 × 10−3–0.1 | 0.9998 | 3.0 × 10−4 | 4.0–6.0 | 84.0–90.0 | [215] |
| UHPLC-MS | meat (bovine, chicken, porcine) | solvent: 0.01% acetic acid and 5 mmol/L ammonium formate in water–acetonitrile (2:1); MP: 0.1% formic acid, 0.1% formic acid in acetonitrile, and 0.1% formic acid in methanol (gradient); column: Phenomenex Luna Omega (Phenomenex, Torrance, CA, USA) (100 × 2.1 mm) with 1.6 µm particles; tR: 6.6 min; ESI; positive ion mode | - | - | 6.7 × 10−2 1 | 7.9–8.7 | 90.0–108.0 | [216] |
| UHPLC-MS/MS | water, sediment | solvent: methanol–water (1:1); MP: methanol (10–100%, gradient); FR: 0.4 mL/min; column: BEH C18 (Waters, Milford, CT, USA) (100 × 2.1 mm) with 1.7 µm particles; tR: 3.19 min; detection: m/z 202.1, 175, 130.9; ESI; positive ion mode | - | 0.9995 | 2.0 × 10−2 | 1.7–8.9 | 66.0–93.0 | [217] |
| UHPLC-MS/MS | protein powder | solvent: methanol–water (1:5); MP: 0.1% formic acid–0.5 mM ammonium acetate, 0.1% formic acid in methanol, 2 mM ammonium acetate, and methanol (gradient); FR: 0.25 mL/min; column: Acquity HSS-T3 (Waters, Wien, Austria) (100 × 2.1 mm) with 1.8 µm particles; tR: 4.7 min; detection: m/z 202.1, 175, 131; ESI; positive ion mode | - | 0.9970 | - | 1.3–3.2 | 85.6–111.3 | [218] |
| UHPLC-MS/MS | river sediment | solvent: methanol–ultrapure water; MP: water and methanol (gradient); FR: 0.2 mL/min; column: Kinetex XB-C18 (Phenomenex, Torrance, CA, USA) (50 × 2.1 mm) with 1.7 µm particles; tR: 1.19 min; detection: m/z 202.1, 175, 131; ESI; positive ion mode | 7.0 × 10−4–1.0 1 | 0.9775 | 2.0 × 10−4 1 | 6.0–20.0 | 63.0–70.0 | [219] |
| UHPLC-MS/MS | salmon | solvent: water–acetonitrile; MP: 0.1% formic acid and 0.1% formic acid in acetonitrile (gradient), FR: 0.4 mL/min; column: Zorbax RRHD Eclipse Plus C18 (Agilent, Santa Clara, CA, USA) (100 × 2.1 mm) with 1.8 µm particles; tR: 3.1 min; ESI; positive ion mode | - | - | - | 10.0 | 96.0 | [220] |
| MLC | wastewater | solvent: water; MP: 0.07 mol/L SDS, 5% pentan-1-ol, and 0.01 M phosphate buffer; FR: 0.95–1.05 mL/min; column: Kromasil C18 (Eka Chemicals, Bohus, Sweden) (150 × 4.6 mm) with 5 µm particles; tR: 3.5 min; detection: UV/Vis; max. absorption: 305 nm | 0.5–15 | 0.9999 | 0.2 | 1.3–8.1 | 93.9–103.7 | [222] |
| MLC | wastewater | solvent: water; MP: 0.1 mol/L SDS, 6% pentan-1-ol and 0.01 M phosphate buffer; FR: 1 mL/min; column: Kromasil C18 (Eka Chemicals, Bohus, Sweden) (150 × 4.6 mm) with 5 µm particles; detection: UV/Vis; max. absorption: 305 nm | 1.0 × 10−2–2.0 | 0.9995 | 5.0 × 10−3 | - | 92.1–104.2 | [223] |
| MEKC | grape, lettuce, orange, tomato | solvent: hydrochloric acid; buffer: 4 mM borate (pH 9.2) containing sodium cholate; MP: methanol–electrophoretic buffer (20:80); capillary: fused-silica (50 cm effective length × 75 µm inner diameter); tm: 11.6 min; temperature: 25 °C; electrokinetic injection: 5 s at 10 kV; detection: α-diode array, max. absorption: 210 nm; separation voltage: 15 kV | 1.0–100.0 | 0.9960 | 0.1–1.0 1 | 4.0–10.0 | 53.0–56.0 | [224] |
| MEKC | soil | solvent: water; electrolyte: 15 mM sodium tetraborate, 30 mM SDS, 10 mM tetrabutylammonium phosphate, and 10% acetonitrile; capillary: fused-silica (50 cm effective length × 75 µm inner diameter); tm: <7 min; temperature: 20 °C; voltage: 25 kV; detection: UV, max. absorption: 190 nm; separation voltage: 25 kV | 2.5 × 10−1–5.0 | ≥0.9970 | 5.7 × 10−2 1 | <10.0 | 28.0–46.0 | [225] |
| CEC | lemon, orange | solvent: acetonitrile; MP: acetonitrile and 0.02 M sodium dihydrogen phosphate; column: long fused-silica capillary with UV-transparent protecting polymer coating (150 mm, 100 µm I.D.) with TBZ imprinted monolith; 60 °C; tR: <6 min; detection: UV/Vis, max. absorption: 305 nm | 5.0 × 10−2–20.0 | 0.9940–0.9998 | 4.0 × 10−2–4.5 × 10−2 | 4.1–6.9 | 85.0–105.0 | [227] |
| Method | Sample | Conditions and Characteristic Method Parameters | Measuring Range (μg/mL) | Correlation Coefficient | LOD (μg/mL) | RSD (%) | Recovery (%) | Reference |
|---|---|---|---|---|---|---|---|---|
| CE | fruit, vegetables | solvent: dichloromethane; capillary column: fused-silica capillary (150 cm × 75 µm); detection: m/z 202; ESI; positive ion mode | 1.0–10.0 | 0.9889 | 1.0 × 10−2 | 7.0–12.0 | 61.0–68.0 | [230] |
| CE | swine muscle, swine liver | solvent: acetonitrile, trifluoroacetic acid; type: CZE; capillary column: fused-silica capillary (75.5 cm × 75 µm); detection: UV/Vis; max. absorption: 292 nm | 5.0 × 10−2–2.0 1 | 0.9972 (muscle) 0.9929 (liver) | 1.0 × 10−3 1 | 0.3–7.4 | 87.9–104.9 | [231] |
| CE | fruit, fruit juice | solvent: tetrahydrofurane, chloroform; type: NACE; capillary column: fused-silica capillary (60 cm × 75 µm); detection: UV/Vis; max. absorption: 204 nm | 2.5 × 10−2–0.5 | 0.9991 | 5.1 × 10−4 | 1.4–3.2 | 79.9–101.1 | [232] |
| CE | water | solvent: 1 M hydrochloric acid; capillary column: bare silica capillary (60 cm × 50 µm); detection: UV/Vis; max. absorption: 210 nm | 3.7 × 10−3–0.5 | 0.9952 | 1.1 × 10−3 | 2.8 | 96.0–103.0 | [233] |
| CE | meat | solvent: acetonitrile–water (30:70); type: CZE; capillary column: fused-silica capillary (100 cm × 50 µm); detection: m/z 202; ESI; positive ion mode | 4.0 × 10−3–0.1 1 | 0.9987 | 1.0 × 10−3 1 | 2.8–8.5 | 76.7–87.7 | [234] |
| voltammetry | TBZ solution | solvent: methanol–formic acid (95:5); buffer: phosphoric acid–glacial acetic acid–boric acid; pH 2.55–9.54; type: SWV; WE: glassy carbon rotating-disk electrode modified with poly(3-methylthiophene); RE: Ag/AgCl; AE: platinum wire; potential range: −4.0–4.0 V | - | 0.9913 | 6.8 × 10−2 | 1.9 | - | [236] |
| voltammetry | orange, apple, pear | solvent: ethanol; buffer: phosphate (pH 6); type: CV; WE: MWCNT-COOH glassy carbon; RE: saturated calomel; AE: platinum wire; scan rate: 0.05–0.2 V | - | 0.9950 | 6.0 × 10−2 | 4.2 | 95.0–105.0 | [237] |
| voltammetry | apple, leek, tomato | solvent: ethanol; buffer: phosphate (pH 7); type: DPV; WE: glassy carbon modified with ZnFe2O4/SWCNTs; RE: Ag/AgCl; AE: platinum wire; potential range: 0.2–1.0 V | 0.1–20.1 | 0.9970 | 1.0 × 10−2 | 3.3 | 88.0–104.0 | [238] |
| voltammetry | mango, sugar cane, river water, pharmaceutical formulation | solvent: ethanol; buffer: Britton–Robinson (2.2–12); type: SWV; WE: boron-doped diamond; RE: Ag/AgCl; AE: platinum wire; scan rate: 10–150 mVs−1 | 0.1–2.3 | 0.9996 | 2.6 × 10−2 | 0.9–5.3 | 71.0–103.3 | [239] |
| potentiometry | banana | solvent: water, pH 4 with HCl; ISE: liquid membrane (ion-pair: (TBZH2)3(PMo12O40)2; plasticizer: tricresyl phosphate; PVC; solvent: cyclohexanone); reference electrode: Ag/AgCl; slope: 30 mV/decade; response time: 2–3 min (low c), 40–50 s (high c); life time: 2 months | 2.0–2012.5 | - | - | - | 99.0–99.6 | [240] |
| potentiometry | orange, lemon, banana | solvent: water, pH 2.6 with HCl; ISE: liquid membrane (ion-pair: TBZ and 5-sulfosalicylate (1%); plasticizer: dibutyl sebacate; PVC; solvent: tetrahydrofuran); reference electrode: Ag/AgCl; slope: 62.2 mV/decade; response time: 8 s; life time: 1 month | 0.2–201.3 | 0.9990 | 6.4 × 10−2 | - | 102.4–107.0 | [242] |
| potentiometry | orange, lemon, banana, clementine, lime | solvent: water, pH 2.6 with HCl; solid-state ISE: liquid membrane (ion-pair: MWCNT-OSO3−TBZ+ (1%); plasticizer: dibutyl sebacate; PVC; solvent: tetrahydrofuran); reference electrode: Ag/AgCl; slope: 60.4 mV/decade; response time: 8 s; life time: 3 months | 0.1–201.3 | 0.9991 | 1.2 × 10−1 | - | 95.1–110.0 | [243] |
| immunoassay | fruit juice | conjugate: TBZ-ovalbumin; label: carbon nanoparticles; detection complex: carbon-labelled secondary antibodies and anti-TBZ antibodies; TBZ detection: visual and scanning densitometry | 1.1 × 10−4–4.1 × 10−3 | - | 8.0 × 10−5 | - | 81.9–123.6 | [248] |
| immunoassay | orange | conjugate: TBZ hapten-BSA; SPR biosensor using gold surfaces; TBZ detection: SPR: polarized light: 670 nm, flow speed: 20 µL/min | 2.4 × 10−4–1.7 × 10−3 | - | 1.3 × 10−4 | - | 105.1–110.9 | [4] |
| immunoassay | red and white wine | enzyme: HRP; conjugate: TBZ-BSA; absorption: 405 nm | 1.0 × 10−5–2.0 × 10−3 | 0.9985 | 5.0 × 10−6 | - | 90.6–108.0 | [7] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Budetić, M.; Kopf, D.; Dandić, A.; Samardžić, M. Review of Characteristics and Analytical Methods for Determination of Thiabendazole. Molecules 2023, 28, 3926. https://doi.org/10.3390/molecules28093926
Budetić M, Kopf D, Dandić A, Samardžić M. Review of Characteristics and Analytical Methods for Determination of Thiabendazole. Molecules. 2023; 28(9):3926. https://doi.org/10.3390/molecules28093926
Chicago/Turabian StyleBudetić, Mateja, Doris Kopf, Andrea Dandić, and Mirela Samardžić. 2023. "Review of Characteristics and Analytical Methods for Determination of Thiabendazole" Molecules 28, no. 9: 3926. https://doi.org/10.3390/molecules28093926
APA StyleBudetić, M., Kopf, D., Dandić, A., & Samardžić, M. (2023). Review of Characteristics and Analytical Methods for Determination of Thiabendazole. Molecules, 28(9), 3926. https://doi.org/10.3390/molecules28093926






