1. Introduction
Tau, a microtubule-associated protein, is enriched in the brain and mainly localized in neuronal axons. It plays an important role in microtubule assembly and stabilization. There are six major isoforms of tau in the human brain (including N-terminal 0–2 N and C-terminal 3/4R), which are formed by alternative splicing of the MAPT gene in chromosome 17 (17q21-q22) [
1] In physiological conditions, the native tau is hypophosphorylated and shows little tendency for accumulation. However, hyperphosphorylated tau is the primary component of paired helical filaments (PHFs) and neurofibrillary tangles (NFTs), characterizing a wide range of neurodegenerative diseases known as tauopathies [
2].
Tauopathies are consistent with a variety of neurodegenerative diseases including frontotemporal dementia, Parkinson’s disease, and Alzheimer’s disease (AD), the most common form of dementia. In neurodegenerative diseases with tauopathy, the oligomeric tau aggregates are believed to act as the most neurotoxic species and dominate the major aspects of tau-induced neuropathology. In wild-type mice, subcortical stereotaxic injection of recombinant tau oligomers impairs the memory and induces synaptic and mitochondrial dysfunctions [
3]. In mice overexpressing mutated α-synuclein (A53T mice), treatment with a tau oligomer-specific monoclonal antibody efficiently protects the mice from cognitive and motor deficits with decreased toxic tau oligomers levels. Moreover, tau oligomer depletion also protects against dopamine and synaptic protein loss [
4]. However, experimental evidences from post-mortem human brain and various animal and cell models have suggested that oligomeric tau undergoes cell-to-cell transmission, which raises concern about the long-term beneficial effect of anti-tau antibody immunotherapy [
5]. In order to reduce tau pathology in AD, a variety of small molecules have been described, including modulators of post-translational modifications and aggregation inhibitors, most of which are in the preclinical stage [
6].
Flavonoids are types of polyphenolic phytochemicals mostly found in fruits, vegetables, and nuts, presenting multiple pleiotropic properties (they act on different cellular targets and biochemical mechanisms). Many studies describe the multiple mechanisms of the neuroprotective activity of flavonoids [
7]. Quercetagitrin, also known as quercetagetin-7-O-glucoside, is a flavonoid derivative extracted from marigold (
Tagetes erecta). The anti-inflammatory effects of the compound have been reported in multiple inflammatory diseases. In rat neutrophils, quercetagitrin yielded more than 60% of inhibition on lysosomal enzyme secretion and arachidonic acid release [
8]. In HaCaT human keratinocytes, quercetagetin effectively blocked IFN-γ and TNF-α-induced chemokines CCL17 and CCL22 expression [
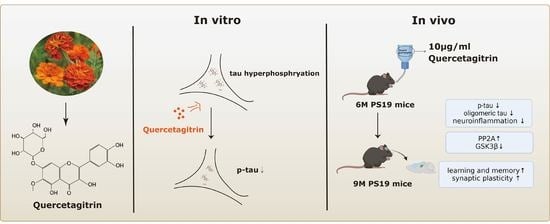
9]. However, the role of quercetagitrin in neurodegenerative diseases, especially tauopathy, remains unclear. Here, for the first time, we investigate the therapeutic effects of quercetagitrin on tau pathology. Our results show that quercetagetin could inhibit tau aggregation and tau phosphorylation both in vivo and in vitro, with attenuated NF-κB activation, neuroinflammation, neuronal loss, synaptic impairments, and cognitive deficits in the P301S-tau transgenic mouse model. The results here provide in vivo evidence for further development of quercetagetin as potential drug candidate for AD and other tauopathies.
3. Discussion
Tau pathology is characterized by intracellular neurofibrillary tangles composed of hyperphosphorylated tau protein, which is identified as a leading cause of multiple neurodegenerative diseases, including the most common form of dementia, Alzheimer’s disease. The hyperphosphorylated tau protein promotes its detachment from microtubules, increases cytoplasmic tau levels, and boosts PHFs and NFTs formation [
13]. Hyperphosphorylation of tau at Thr181, Ser202, Thr205, Thr212, Ser214, and Thr231 is found in the degenerating neurons of the AD brain during embryonic and early postnatal periods [
1]. In the current study, we demonstrate that quercetagitrin could reduce tau phosphorylation at these sites in HEK293 cells and N2a cells and in P301S-tau transgenic mice. The underlying mechanism of quercetagitrin-mediated tau-aggregation inhibition could be attributed to the interaction between its aromatic structure and the hydrophobic β-sheet secondary structure of tau aggregates. Our previous studies have demonstrated that another structurally similar flavonoid, iso·bavachalcone, could directly interact with tau-K18 through binding sites I278 and V309 positioned in the R2 (VQIINK) and R3 (VQIVYK) of the protein and therefore exhibit an anti-aggregation effect [
14,
15]. Interestingly, we also noticed that quercetagitrin treatment could reduce tau acetylation at Lys174 in the P301S-tau transgenic mice. This is in accordance with previous studies showing that increased tau acetylation at Lys174 or Lys280 could promote tau accumulation and aggravate tau-mediated neurodegeneration and cognitive impairments [
16,
17].
Neuroinflammation and gliosis occur in early AD and other tau pathology-related neurodegenerative diseases and persist throughout the disease development. Those pathologies would encourage the formation of an inflammatory neuron–glia microenvironment and exacerbate tau-mediated synaptic impairment and neurodegeneration. Our results suggest that quercetagitrin treatment leads to attenuated hippocampal astro- and microgliosis, inhibited NF-κB signaling activation, and reduced the production of the inflammatory cytokines Il-1α and Il-6 in P301S-tau transgenic mice. NF-κB signaling activation is closely associated with neuroinflammation both in astrocytes and microglia, and it plays a critical role in the progression of tauopathy [
11,
18]. Quercetagitrin, isolated from the flowers of the African marigold (
Tagetes erecta), has been reported to have an anti-inflammation effect in neutrophils [
8,
19]. Moreover, studies have indicated that quercetagetin, a similar flavonoid also derived from marigolds, could suppress NF-κB activation via the inhibition of c-Jun NH
2-terminal kinases [
20]. The inactivation of NF-κB signaling reduces astrogliosis, microgliosis, and inflammatory responses, and it protects against spatial memory deficits in WT and P301S-tau transgenic mice [
11,
18]. Thus, the reversed gliosis and inflammatory responses observed in P301S-tau transgenic mice might be attributable to the inhibitory effect of quercetagetin on NF-κB signaling.
Synaptic impairment is closely correlated with cognitive deficits in tau pathology [
12,
21]. Synaptophysin is a presynaptic vesicle membrane protein with major functions in regulating vesicle formation and release [
22]. Reduced synaptophysin protein levels have been implicated in AD, Parkinson’s disease, and frontotemporal dementia. Impaired synaptic plasticity with decreased synaptophysin expression in the hippocampal CA3 subset has been observed in P301S-tau transgenic mice [
23]. Our results revealed that quercetagitrin significantly attenuated neuronal loss and cognitive deficits, with restored synaptophysin level in the brains of P301S-tau transgenic mice.
In recent years, much of the literature has identified the tight correlation between tau pathology and the clinical progression of AD, and therapeutic strategies aimed at targeting toxic tau proteins are increasingly recognized as promising approaches [
24,
25,
26]. Our findings suggest that quercetagitrin could lessen tau pathology by restoring memory function by blocking toxic tau formation and inhibiting NF-κB-related inflammatory responses. Other flavonoids such as quercetin and myricetin have also been found to have the potential to improve AD. A study showed that quercetin protects cognitive and emotional function in aged 3xTg-AD mice [
27]. Myricetin and dihydromyricetin have been demonstrated to reverse AD pathologies by interacting with Aβ [
28,
29]. However, their pharmacological properties and bioavailability might place limitations on their application. Low bioavailability has always been one of the main limitations in developing flavonoids as oral medications. Research has illustrated that the glycoside form of flavonoid compounds has better absorption when taken orally [
30]. With regard to its various neuroprotective effects, we believe that quercetagitrin is a potential drug candidate for AD and that further investigation in amyloidosis will be beneficial.
4. Materials and Methods
4.1. Expression and Purification of Tau-K18 Protein
Recombinant human tau-K18 (Q244-E372) proteins were extracted from
E. coli BL-21 cells [
14]. Briefly, BL-21 cells transfected with recombinant vector (tau-K18) were cultured for 12–16 h in LB medium containing 100 μg/mL ampicillin at 180 rpm, at 37 °C. Then, the cells were collected by centrifugation (6000×
g rpm, 15 min), and were lysed by sonication (5 s sonication, 2 s interval, total 15 min) in bacterial lysate with 1 mM PMSF. The tau-K18 protein was purified by anion exchange chromatography (HiPrep CM FF 16/10, Uppsala, Sweden) and agarose chromatography (Hiload 16/600 Superdex 75 pg, Uppsala, Sweden) sequentially. A fast protein liquid chromatography system (AKTA, GE Healthcare, Boston, MA, USA) was used in this study. Purified fractions were lyophilized and identified by Coomassie brilliant blue staining. For anion exchange chromatography, start buffer: 25 mM Tris-HCl, 20 mM NaCl, pH 8; elution buffer: 25 mM Tris-HCl, 1 M NaCl, pH 8. For agarose chromatography, running buffer: 0.05 M NaPO
4, 0.15 M NaCl, pH 7.2.
4.2. Thioflavin T (ThT) Fluorescence Experiments
To evaluate the effect of quercetagitrin on tau aggregation in vitro, heparin sodium was used as the inducer, and ThT was used as the detection reagent. In brief, tau-K18 (50 μM), DTT (2 μM), heparin sodium (12.5 μM), ThT (60 μM), and different concentrations of quercetagitrin (0, 3.12, 6.25, 12.5, 25, 50, and 100 μM) were mixed in a 96-well plate and incubated at 37 °C in a microplate reader (Synergy H1, Biotek, Winooski, VT, USA), and the fluorescence value was monitored every half an hour for 48 h (λex: 440 nm, λem: 485 nm). Quercetagitrin was firstly dissolved in DMSO at 10 mM and then diluted to the working concentration with Tris-HCl buffer (50 mM Tris, 100 mM NaCl, pH 7.4). Other components were diluted with Tris-HCl buffer. DTT and protein were premixed in advance before use.
4.3. Transmission Electron Microscopy (TEM)
The effect of quercetagitrin on tau-K18 fibrilization morphologies was investigated by performing TEM. In brief, the protein samples from the end of the ThT fluorescence experiments were diluted to 5 μM with ddH2O and spotted onto a 230-mesh carbon-coated copper grid (BZ1102, EMCN, Beijing, China). After incubation for 30 min, the residual solution on the surface of the grid was removed by using filter paper. The grids were washed with ddH2O and mixed with 1% uranium acetate for 30 s. The morphology of tau-K18 fibrils was observed using TEM (JEM1230, Tokyo, Japan).
4.4. Animals and Drug Treatment
The P301S-tau transgenic mice expressing human P301S mutant 1N4R tau were purchased from the Jackson Laboratory. Adult C57BL/6 mice were purchased from the Guangdong medical laboratory animal center. In this study, P301S-tau transgenic mice were randomly assigned to drug or vehicle groups. For the drug groups, the drinking water contained 10 μg/mL quercetagitrin (HY-N4150, MedChemExpress LLC, Shanghai, China) and 0.05% DMSO (v/v). P301S-tau transgenic mice-vehicle group and age-matched C57 mice were given water containing 0.05% DMSO (v/v). Because of the photolysis of quercetagitrin, only 100 mL of the drug solution was given to the mice each time, and the container was wrapped with tinfoil to avoid light. All mice were housed in standard conditions with free access to food and water. All animal protocols were approved by the Ethics Committee of Shenzhen University in Animal Experimentation (permit number AEWC-20140615-002).
4.5. Behavioral Test
In this study, the mice’s movements were recorded using the Smart V3.0 computerized tracking system.
4.6. Y-Maze
The Y-maze apparatus used in this study consisted of three opaque arms spaced at angles of 120° from each other. The three arms were interconnected in the middle to form a Y shape. The experimental procedure was adapted from published protocols [
31]. Briefly, each mouse was placed at the distal end of one of the arms and allowed to explore the maze freely for 5 min. The camera recorded the movement traces of each mouse. An alternation was defined as occurring when the mice entered three different arms in succession. The alternation rate (%) was calculated using the following formula: Alternation (%) = [Number of alternation/(Total number of arm entries-2)] × 100%.
4.7. Morris Water Maze (MWM)
In this study, the MWM test was conducted in a circular pool with a diameter of 160 cm and a depth of 50 cm. The pool was divided into four quadrants by two diameters perpendicular to each other. The pool was filled with opaque water up to 20 cm from the top of the wall. An escape platform was placed in one quadrant of the pool. The height of the escape platform was 1 cm below the surface of the water. The MWM test was divided into two stages: a training period and a testing period. In the training period, the mice were placed in the water at one quadrant without a platform, facing towards the wall of the maze. Then, they were allowed to explore the pool freely to find the platform. If the mice found the platform within 60 s, they could reside there for 15 s. The time taken to find the platform was recorded. If not, the mice were guided to the platform and resided there for 15 s. In this case, the time taken to find the platform was recorded as 60 s. The training process was repeated three times a day starting in different quadrants without the platform. The above training process was executed for four consecutive days, and the detection was performed 24 h after the last round of training. During the testing period, the hidden platform was removed, and the mice were placed into the pool at the quadrant opposite the platform. The mice were allowed to explore the pool freely, and their movements were recorded for one minute [
32].
4.8. Cell Culture, Transfection, and Drug Treatment
Human embryonic kidney 293 (HEK293) cells were cultured in high-glucose DMEM supplemented with 10% fetal bovine serum (FBS) and 1% penicillin/streptomycin solution. Mouse neuroblastoma (Neuro2a, N2a) cells were cultured in the medium with 45% Opti-MEM, 50% DMEM, 5% FBS, and 1% penicillin/streptomycin solution. The cells were kept in a cell incubator containing 5% CO2 at 37 °C. Transfection of P301S-tau plasmids was performed when the cells reached 75% confluence using the LipoFectMax™ Transfection Reagent (FP310, ABP Biosciences, San Diego, CA, USA). At 24 h after transfection, the culture medium was changed to a fresh culture medium containing different concentrations of quercetagitrin and 0.01% DMSO. For HEK293 cells, 0, 10, 20, or 40 μM quercetagitrin was used, and for N2a cells, 0, 5, 10, or 15 μM quercetagitrin was used. The cells were collected using a cell scraper 24 h after the quercetagitrin treatment.
4.9. Cytotoxicity Testing
HEK293 cells or N2a cells were cultured in a 96-well plate. The culture medium was changed to a medium containing different concentrations of quercetagitrin (0, 2, 5, 10, 20, 30, 40, 60, 80, and 100 μM) with 0.1% DMSO when the cells reached 70–80% confluence. After 24 h, the culture medium was changed to a fresh medium containing 10% cck-8 reagent (MF128-01, Mei5bio, Beijing, China). After a 2-h incubation, the absorbance at 450 nm (Synergy H1, Biotek, Winooski, VT, USA) was measured, and the cell viability was calculated using the following formula: cell viability (%) = [(As − Ab)/(Ac − Ab)] × 100%. As refers to the absorbance of the sample containing cells, drug, and reagent; Ab is the absorbance of the blank control containing 10% cck-8 reagent in the absence of the cells and drug; and Ac is the absorbance of the negative control containing cells and 10% cck-8 reagent in the absence of the drug.
4.10. Protein Extraction
For tissue samples, mice hippocampus or cortex tissues were lysed in RIPA lysate (50 mM Tris, 150 mM NaCl, 1% Triton X-100, 1% sodium deoxycholate, and 0.1% SDS, pH 7.4) and homogenized by using a freeze homogenizer at 4 °C (65 Hz, 10 min). Protease phosphatase inhibitor (P002, NCM Biotech, Shanghai, China) was added into RIPA lysate at the ratio of 1:100 before use. The tissue homogenate was centrifuged at 12,000× g for 25 min at 4 °C. The supernatant was collected and sonicated for 1 min (15% ultrasound power, 2 s sonication, 2 s interval). For cell samples, the RIPA lysate was added to the cell culture wells after they were washed with PBS buffer once. The cells were scraped off using a cell scraper and collected in a sample tube. The samples were placed on ice for 30 min and then sonicated for 1 min (15% ultrasound power, 2 s sonication, 2 s interval). For both tissue samples and cell samples, protein concentrations were determined using a BCA assay kit (P0009, Beyotime, Shanghai, China) and homogenized with RIPA lysates.
4.11. Western Blotting
The protein samples in the loading buffer (50 mM Tris-HCL, 2% SDS, 10% glycerol, and 0.1% bromophenol blue) were boiled for 8 min at 95 °C. The proteins were dispersed by performing SDS-PAGE gel electrophoresis (PowerPac Basic, BioRad, Hercules, CA, USA). After electrophoresis, the proteins were transferred to nitrocellulose membrane from the SDSPAGE gels (DYCZ-40D, BioRad, USA). The membranes were blocked in blocking solution (Beyotime, P0252, Shanghai, China) for 20 min and then incubated with primary antibody at 4 °C overnight. The following primary antibodies were used in this study: AT8 (MN1020, Invitrogen, Carlsbad, CA, USA,1:1000), AT270 (MN1050, Invitrogen, Carlsbad, CA, USA, 1:1000), AT180 (MN1040, Invitrogen, Carlsbad, CA, USA, 1:1000), AT100 (MN1060, Invitrogen, Carlsbad, CA, USA, 1:1000), Tau13 (835201, Biolegend, San Diego, CA, USA, 1:1000), Actin (3700S, CST, Danvers, MA, USA, 1:1000), GAPDH (ab8245, abcam, Cambridge, UK, 1:1000), Ace-tau (K174) (HW181, SAB, Los Angeles, CA, USA, 1:1000), T22 (ABN45S, EMD Millipore, Darmstadt, Germany), PP2A (2259T, CST, Danvers, MA, USA), GSK3β (93926, abcam, Cambridge, UK, 1:1000), p-GSK3β (75745, abcam, Cambridge, UK, 1:1000), NF-κB (8242S, CST, Danvers, MA, USA, 1:1000), p-NF-κB (3033S, CST, Danvers, MA, USA, 1:1000), IL1α (50794S, CST, Danvers, MA, USA, 1:1000), and IL6 (12912S, CST, Danvers, MA, USA, 1:1000). The membrane was washed two times with TBST buffer and incubated with horseradish peroxidase (HRP)-conjugated secondary antibody for 1 h at room temperature. The secondary antibodies were diluted in TBST buffer at a ratio of 1:1000. At the end, the membrane was washed three times with TBST buffer at room temperature. Immunoreactive bands were visualized using an enhanced chemiluminescence kit (P0018FM, Beyotime, Shanghai, China). The image was then imported into ImageJ software to detect the grayscale values of the bands. To compare the relative expression levels between different samples, the grayscale values of the target protein were divided by the grayscale values of the loading control. Then, the values were imported into GraphPad Prism 8.0 for the analysis of significant differences.
4.12. Immunohistochemistry Staining
For immunohistochemical staining, a polymer-based detection kit (PV6000, ZSGB-BIO, Beijing, China) was used. In brief, mouse brain slices were immersed in endogenous peroxidase blocker for 10 min after being washed three times in PBS. Then, the slices were mounted onto slides and incubated with primary antibody (AT8, MN1020, Invitrogen, Carlsbad, CA, USA, 1:200) overnight. The slices were washed three times in PBS and incubated with HRP-conjugated secondary antibody for 20 min at 37 °C. A DAB Concentrate Bulk Kit (ZLI9017, ZSGB-BIO, Beijing, China) was used for chromogenic reaction. The slices were immersed in DAB chromogenic agent for 8 min followed by being washed three times in ddH2O. The slides were dehydrated in 50%, 75%, and 95% ethanol, successively, for 8 min each time. The slides were transferred into xylene and sealed with PerMount mounting medium. Histochemical staining images were visualized using a pathological slice scanner (Aperio CS2, LEICA, Wetzlar, Germany). The images were processed using ImageJ to quantify the staining intensity and area. The quantitative analysis method can be summarized as follows: the area and grayscale value of the hippocampus and cortical regions were detected from each brain slice, and the proportion of positively stained areas was calculated. Then, the values were imported into Graph Prism 8.0 for t-test analysis of significant differences.
4.13. Immunofluorescence Staining
After being washed three times with PBS, the brain slices were immersed in Triton-X100 for 15 min and then washed twice with PBS. After that, the slices were blocked in blocking solution for 20 min at room temperature and incubated with primary antibody overnight at 4 °C. The slices were washed three times in PBS, then incubated with Alexa Fluor488/555-conjugated isotype-specific secondary antibody (A0453/A0423, Beyotime, Shanghai, China) for 1 h at 37 °C followed by DAPI staining for 8 min at room temperature. The slices were mounted onto slides after being washed three times in PBS. Labeled sections were visualized using a confocal microscope (LSM880, Zeiss, Oberkochen, Germany). The statistical analysis procedure for this section was similar to that used for the immunohistochemistry staining. The following primary antibodies were used for immunofluorescence in this study: GFAP (ab5541, abcam, Cambridge, UK, 1:500), Iba1(019-19741, Wako, Osaka, Japan, 1:200), NeuN (ab104224, abcam, Cambridge, UK,1:500), and synaptophysin (ab32127, abcam, Cambridge, UK, 1:100).
4.14. Thio-S Staining
Thio-S was used to fluorescently label tau aggregates with a β-sheet structure. In brief, after being washed three times in PBS, the brain slices were incubated in 0.3% Thio-S (Sigma, Darmstadt, Germany, dilution in 50% ethyl alcohol) followed by being washed three times in 50% ethyl alcohol and PBS, respectively. Then, the slices were mounted onto slides and visualized using a confocal microscope (LSM880, Zeiss, Oberkochen, Germany). The statistical analysis procedure for this part was similar to that used for the immunohistochemistry staining.
4.15. Statistical Analysis
Results were shown as mean ± SEM. Statistical analysis was performed using GraphPad Prism 8.0.2 statistical software. Statistical significance was assessed using Student’s
t test, with one-way or two-way ANOVA.
p values of 0.05 or less were considered to denote significance. All the statistical analysis details were summarized in
Table S1.