Cyclopropane-Containing Specialized Metabolites from the Marine Cyanobacterium cf. Lyngbya sp.
Abstract
:1. Introduction
2. Results and Discussion
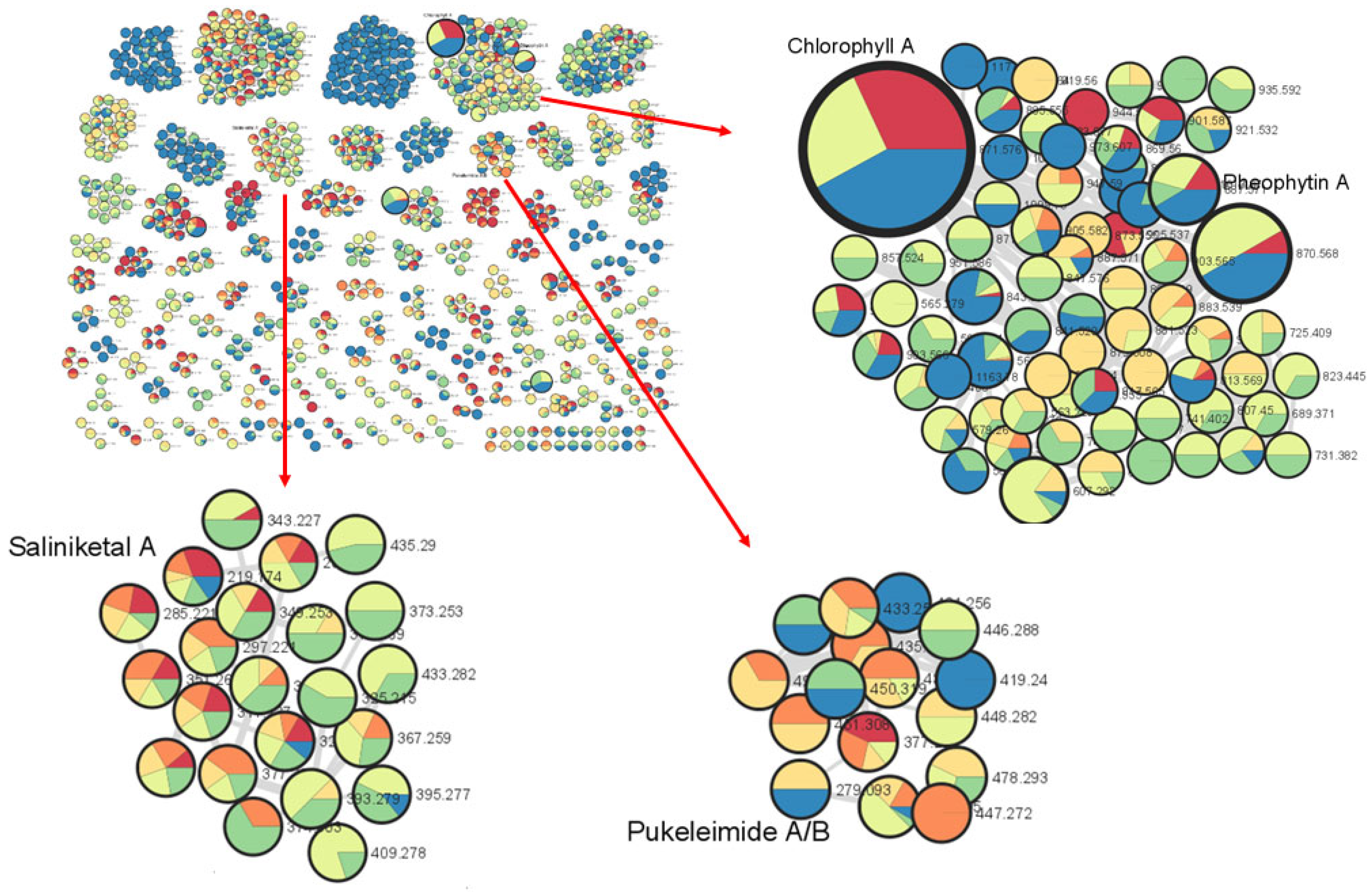
2.1. Extraction, Fractionation, Bioassay Evaluation and Preliminary Dereplication of Marine Cyanobacterial Specialized Metabolites
2.2. Isolation and Structural Elucidation of Cyclopropane-Containing Specialized Metabolites
2.3. Biological Activity of Cyclopropane-Containing Metabolites
2.4. Molecular Docking of Marine Cyanobacterial Cyclopropane-Containing Metabolites
2.5. Ecological Significance of Marine Cyanobacterial Lyngbyoic Acid and Other Modified Fatty Acids
| Marine Cyanobacterial Modified Lipid Acid | Organism/Highest Reported Amount | Biological Activity of Lipid Acid/ # of Analogs Containing a Lipid Acid Tail |
|---|---|---|
| Lyngbic acids 7-Methoxydodec-4(E)-enoic acid (3) 7(S)-Methoxytetradec-4(E)-enoic acid (4) 7-Methoxy-9-methylhexadeca-4(E),8(E)-dienoic acid (5) | Lyngbya majuscula/75 mg L. majuscula; Okeania hirsute; Moorea producens; cyanobacterial mat from black-band consortium/>200 mg in a report by Soares and co-workers [53] L. majuscula/10 mg | Not tested/6 Interfered quorum sensing in the Vibrio harveyi QS reporters and luminescence in native coral Vibrio spp./32 Not tested/2 |
| Malyngic acid (6) | L. majuscula/766 mg from two collections | Not tested/0 |
| (2R)-2,5-Dimethyldodecanoic acid (7) | L. aestuarii/54 mg | Strongly inhibited the growth of the common duckweed Lemna minor/1 |
| Pitinoic acid A (8) | Lyngbya sp./0.3% of total dry weight | Interfered quorum sensing in P. aeruginosa by reducing the transcript levels of lasB and the pyocyanin biosynthetic member phzG1/1 |
| Lyngbyoic acid (1) | L. cf. majuscula/42.4 mg | Strongly affected the AHL receptor LasR and reduces pyocyanin and elastase (LasB), both on the protein and transcript level in wild-type P. aeruginosa; inhibited fungal growth and herbivore feeding/5 |
| Dysidazirine carboxylic acid (9) | Caldora sp./3 mg | Anti-inflammatory/0 |
| Puna’auic acid (10) | Pseudanabaena sp./3.8 mg | Not tested/0 |
| 11-Oxopalmitelaidic acid (11) | Leibleinia gracilis/3.5 mg | Not tested/0 |
| 2-Methyldecanoic acid (12) and 2-methyldodecanoic acid (13) | Trichodesmium erythraeum/comprising up to 75% of the total fatty acid pool | Not tested/0 |
3. Materials and Methods
3.1. General Experimental Procedures
3.2. Sample Collection
3.3. Extraction and Isolation of Compounds
3.4. Compound Characterization Data
3.5. Quorum-Sensing Inhibitory Assay
3.6. Anti-Biofilm Assay
3.7. Mass Spectrometric-Based Molecular Network of Marine Cyanobacterial VLC-Derived Fractions
3.8. LC-MS of Selected VLC-Derived Marine Cyanobacterial Fractions
3.9. Molecular Docking of Marine Cyanobacterial Metabolites
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Hoffman, L. Marine cyanobacteria in tropical regions: Diversity and ecology. Eur. J. Phycol. 1999, 34, 371–379. [Google Scholar] [CrossRef]
- Tan, L.T.; Phyo, M.Y. Marine cyanobacteria: A source of lead compounds and their clinically-relevant molecular targets. Molecules 2020, 25, 2197. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.B. Discovery and development of dolastatin 10-derived antibody drug conjugate anticancer drugs. J. Nat. Prod. 2022, 85, 666–687. [Google Scholar] [CrossRef] [PubMed]
- Berger, G.K.; McBride, A.; Lawson, S.; Royball, K.; Yun, S.; Gee, K.; Bin Riaz, I.; Saleh, A.A.; Puvvada, S.; Answer, F. Brentuximab vedotin for treatment of non-Hodgkin lymphomas: A systematic review. Crit. Rev. Oncol. Hematol. 2017, 109, 42–50. [Google Scholar] [CrossRef]
- Youssef, D.T.A.; Mufti, S.J.; Badiab, A.A.; Shaala, L.A. Anti-infective secondary metabolites of the marine cyanobacterium Lyngbya morphotype between 1979 and 2022. Mar. Drugs 2022, 20, 768. [Google Scholar] [CrossRef]
- Barbosa Da Silva, E.; Sharma, V.; Hernandez-Alvarez, L.; Tang, A.H.; Stoye, A.; O’Donoghue, A.J.; Gerwick, W.H.; Payne, R.J.; McKerrow, J.H.; Podust, L.M. Intramolecular interactions enhance the potency of gallinamide A analogues against Trypanosoma cruzi. J. Med. Chem. 2022, 65, 4255–4269. [Google Scholar] [CrossRef]
- Ashhurst, A.S.; Tang, A.H.; Fajtová, P.; Yoon, M.C.; Aggarwal, A.; Bedding, M.J.; Stoye, A.; Beretta, L.; Pwee, D.; Drelich, A.; et al. Potent anti-SARS-CoV-2 activity by the natural product gallinamide A and analogues via inhibition of cathepsin L. J. Med. Chem. 2022, 65, 2956–2970. [Google Scholar] [CrossRef]
- Gerwick, W.H.; Proteau, P.J.; Nagle, D.G.; Hamel, E.; Blokhin, A.; Slate, D.L. Structure of curacin A, a novel antimitotic, antiproliferative and brine shrimp toxic natural product from the marine cyanobacterium Lyngbya majuscula. J. Org. Chem. 1994, 59, 1243–1245. [Google Scholar] [CrossRef]
- Yoo, H.-D.; Gerwick, W.H. Curacins B and C, new antimitotic natural products from the marine cyanobacterium Lyngbya majuscula. J. Nat. Prod. 1995, 58, 1961–1965. [Google Scholar] [CrossRef]
- Márquez, B.; Verdier-Pinard, P.; Hamel, E.; Gerwick, W.H. Curacin D, an antimitotic agent from the marine cyanobacterium Lyngbya majuscula. Phytochemistry 1998, 49, 2387–2389. [Google Scholar] [CrossRef]
- Sitachitta, N.; Gerwick, W.H. Grenadadiene and grenadamide, cyclopropyl-containing fatty acid metabolites from the marine cyanobacterium Lyngbya majuscula. J. Nat. Prod. 1998, 61, 681–684. [Google Scholar] [CrossRef] [PubMed]
- Ogawa, H.; Iwasaki, A.; Sumimoto, S.; Iwatsuki, M.; Ishiyama, A.; Hokari, R.; Otoguro, K.; Omura, S.; Suenaga, K. Isolation and total synthesis of hoshinolactam, an antitrypanosomal lactam from a marine cyanobacterium. Org. Lett. 2017, 19, 890–893. [Google Scholar] [CrossRef] [PubMed]
- Gunasekera, S.P.; Li, Y.; Ratnayake, R.; Luo, D.; Lo, J.; Reibenspies, J.H.; Xu, Z.; Clare-Salzler, M.J.; Ye, T.; Paul, V.J.; et al. Discovery, total synthesis and key structural elements for the immunosuppressive activity of cocosolide, a symmetrical glycosylated macrolide dimer from marine cyanobacteria. Chemistry 2016, 22, 8158–8166. [Google Scholar] [CrossRef] [PubMed]
- Balunas, M.J.; Grosso, M.F.; Villa, F.A.; Engene, N.; McPhail, K.L.; Tidgewell, K.; Pineda, L.M.; Gerwick, L.; Spadafora, C.; Kyle, D.E.; et al. Coibacins A-D, antileishmanial marine cyanobacterial polyketides with intriguing biosynthetic origins. Org. Lett. 2012, 14, 3878–3881. [Google Scholar] [CrossRef] [PubMed]
- Carneiro, V.M.; Avila, C.M.; Balunas, M.J.; Gerwick, W.H.; Pilli, R.A. Coibacins A and B: Total synthesis and stereochemical revision. J. Org. Chem. 2014, 79, 630–642. [Google Scholar] [CrossRef]
- Macmillan, J.B.; Molinski, T.F. Majusculoic acid, a brominated cyclopropyl fatty acid from a marine cyanobacterial mat assemblage. J. Nat. Prod. 2005, 68, 604–606. [Google Scholar] [CrossRef]
- Kwan, J.C.; Meickle, T.; Ladwa, D.; Teplitski, M.; Paul, V.; Luesch, H. Lyngbyoic acid, a “tagged” fatty acid from a marine cyanobacterium, disrupts quorum sensing in Pseudomonas aeruginosa. Mol. Biosyst. 2011, 7, 1205–1216. [Google Scholar] [CrossRef]
- Ueoka, R.; Hitora, Y.; Ito, A.; Yoshida, M.; Okada, S.; Takada, K.; Matsunaga, S. Curacin E from the brittle star Ophiocoma scolopendrina. J. Nat. Prod. 2016, 79, 2754–2757. [Google Scholar] [CrossRef]
- Kumar, M.; Singh, P.; Tripathi, J.; Srivastava, A.; Tripathi, M.K.; Ravi, A.K.; Asthana, R.K. Identification and structure elucidation of antimicrobial compounds from Lyngbya aestuarii and Aphanothece bullosa. Cell. Mol. Biol. 2014, 60, 82–89. [Google Scholar]
- Fan, Y.Y.; Gao, X.H.; Yue, J.M. Attractive natural products with strained cyclopropane and/or cyclobutane ring systems. Sci. China Chem. 2016, 59, 1126–1141. [Google Scholar] [CrossRef]
- Ma, S.; Mandalapu, D.; Wang, S.; Zhang, Q. Biosynthesis of cyclopropane in natural products. Nat. Prod. Rep. 2022, 39, 926–945. [Google Scholar] [CrossRef] [PubMed]
- Talele, T.T. The “cyclopropyl fragment” is a versatile player that frequently appears in preclinical/clinical drug molecules. J. Med. Chem. 2016, 59, 8712–8756. [Google Scholar] [CrossRef] [PubMed]
- Sun, M.R.; Li, H.L.; Ba, M.Y.; Cheng, W.; Zhu, H.L.; Duan, Y.T. Cyclopropyl scaffold: A generalist for marketed drugs. Mini Rev. Med. Chem. 2021, 21, 150–170. [Google Scholar] [CrossRef] [PubMed]
- Wessjohann, L.A.; Brandt, W.; Thiemann, T. Biosynthesis and metabolism of cyclopropane rings in natural compounds. Chem. Rev. 2003, 103, 1625–1648. [Google Scholar] [CrossRef] [PubMed]
- Stuart, K.A.; Welsh, K.; Walker, M.C.; Edrada-Ebel, R. Metabolomic tools used in marine natural product drug discovery. Expert Opin. Drug Discov. 2020, 15, 499–522. [Google Scholar] [CrossRef]
- Yang, J.Y.; Sanchez, L.M.; Rath, C.M.; Liu, X.; Boudreau, P.D.; Bruns, N.; Glukhov, E.; Wodtke, A.; de Felicio, R.; Fenner, A.; et al. Molecular networking as a dereplication strategy. J. Nat. Prod. 2013, 76, 1686–1699. [Google Scholar] [CrossRef]
- Cardellina, J.H.; Moore, R.E. The structures of pukeleimides A, B, D, E, F, and G. Tetrahedron Lett. 1979, 20, 2007–2010. [Google Scholar] [CrossRef]
- Ohta, S.; Ono, F.; Shiomi, Y.; Nakao, T.; Aozasa, O.; Nagate, T.; Kitamura, K.; Yamaguchi, S.; Nishi, M.; Miyata, H. Anti-Herpes Simplex Virus substances produced by the marine green alga, Dunaliella primolecta. J. Appl. Phycol. 1998, 10, 349–356. [Google Scholar] [CrossRef]
- Ichikawa, N.; Naya, Y.; Enomoto, S. New halogenated monoterpenes from Desmia (Chondrococcus) hornemanni. Chem. Lett. 1974, 31, 1333–1336. [Google Scholar] [CrossRef]
- Sakuragi, Y.; Kolter, R. Quorum-sensing regulation of the biofilm matrix genes (pel) of Pseudomonas aeruginosa. J. Bacteriol. 2007, 189, 5383–5386. [Google Scholar] [CrossRef]
- Meng, X.Y.; Zhang, H.X.; Mezei, M.; Cui, M. Molecular docking: A powerful approach for structure-based drug discovery. Curr. Comput. Aided Drug. Des. 2011, 7, 146–157. [Google Scholar] [CrossRef] [PubMed]
- Clark, B.R.; Engene, N.; Teasdale, M.E.; Rowley, D.C.; Matainaho, T.; Valeriote, F.A.; Gerwick, W.H. Natural products chemistry and taxonomy of the marine cyanobacterium Blennothrix cantharidosmum. J. Nat. Prod. 2008, 71, 1530–1537. [Google Scholar] [CrossRef] [PubMed]
- Choi, H.; Mascuch, S.J.; Villa, F.A.; Byrum, T.; Teasdale, M.E.; Smith, J.E.; Preskitt, L.B.; Rowley, D.C.; Gerwick, L.; Gerwick, W.H. Honaucins A–C, potent inhibitors of inflammation and bacterial quorum sensing: Synthetic derivatives and structure-activity relationships. Chem. Biol. 2012, 19, 589–598. [Google Scholar] [CrossRef] [PubMed]
- Kaczmarzyk, D.; Fulda, M. Fatty acid activation in cyanobacteria mediated by acyl-acyl carrier protein synthetase enables fatty acid recycling. Plant Physiol. 2010, 152, 1598–1610. [Google Scholar] [CrossRef]
- Dobretsov, S.; Teplitski, M.; Alagely, A.; Gunasekera, S.P.; Paul, V.J. Malyngolide from the cyanobacterium Lyngbya majuscula interferes with quorum sensing circuitry. Environ. Microbiol. Rep. 2010, 2, 739–744. [Google Scholar] [CrossRef] [PubMed]
- Sneed, J.M.; Meickle, T.; Engene, N.; Reed, S.; Gunasekera, S.; Paul, V.J. Bloom dynamics and chemical defenses of benthic cyanobacteria in the Indian River Lagoon, Florida. Harmful Algae 2017, 69, 75–82. [Google Scholar] [CrossRef]
- Engene, N.; Tronholm, A.; Paul, V.J. Uncovering cryptic diversity of Lyngbya: The new tropical marine cyanobacterial genus Dapis (Oscillatoriales). J. Phycol. 2018, 54, 435–446. [Google Scholar] [CrossRef]
- Cardellina, J.H.; Dalietos, D.; Marner, F.-J.; Mynderse, J.S.; Moore, R.E. (−)-trans-7(S)-Methoxytetradec-4-enoic acid and related amides from the marine cyanophyte Lyngbya majuscula. Phytochemistry 1978, 17, 2091–2095. [Google Scholar] [CrossRef]
- Loui, M.S.M.; Moore, R.E. 7-methoxy-9-methylhexadeca-4(E),8(E)-dienoic acid from Lyngbya majuscula. Phytochemistry 1979, 18, 1733–1734. [Google Scholar] [CrossRef]
- Mesguiche, V.; Valls, R.; Piovetti, L.; Peiffer, G. Characterization and synthesis of (−)-7-methoxydodec-4(E)-enoic acid, a novel fatty acid isolated from Lyngbya majuscula. Tetrahedron Lett. 1999, 40, 7473–7476. [Google Scholar] [CrossRef]
- Cardellina, J.H.; Moore, R.E. Malyngic acid, a new fatty acid from Lyngbya majuscula. Tetrahedron 1980, 36, 993–996. [Google Scholar] [CrossRef]
- Entzeroth, M.; Mead, D.J.; Patterson, G.M.L.; Moore, R.E. A herbicidal fatty acid produced by Lyngbya aestuarii. Phytochemistry 1985, 24, 2875–2876. [Google Scholar] [CrossRef]
- Montaser, R.; Paul, V.J.; Luesch, H. Modular strategies for structure and function employed by marine cyanobacteria: Characterization and synthesis of pitinoic acids. Org. Lett. 2013, 15, 4050–4053. [Google Scholar] [CrossRef] [PubMed]
- Gunasekera, S.P.; Kokkaliari, S.; Ratnayake, R.; Sauvage, T.; Dos Santos, L.A.H.; Luesch, H.; Paul, V.J. Anti-inflammatory dysidazirine carboxylic acid from the marine cyanobacterium Caldora sp. collected from the reefs of Fort Lauderdale, Florida. Molecules 2022, 27, 1717. [Google Scholar] [CrossRef]
- Roulland, E.; Solanki, H.; Calabro, K.; Zubia, M.; Genta-Jouve, G.; Thomas, O.P. Stereochemical study of puna’auic acid, an allenic fatty acid from the Eastern Indo-Pacific cyanobacterium Pseudanabaena sp. Org. Lett. 2018, 20, 2311–2314. [Google Scholar] [CrossRef]
- Solanki, H.; Pierdet, M.; Thomas, O.P.; Zubia, M. Insights into the metabolome of the cyanobacterium Leibleinia gracilis from the lagoon of Tahiti and first inspection of its variability. Metabolites 2020, 10, 215. [Google Scholar] [CrossRef]
- Gosselin, K.M.; Nelson, R.K.; Spivak, A.C.; Sylva, S.P.; Van Mooy, B.A.S.; Aeppli, C.; Sharpless, C.M.; O’Neil, G.W.; Arrington, E.C.; Reddy, C.M.; et al. Production of two highly abundant 2-methyl-branched fatty acids by blooms of the globally significant marine cyanobacteria Trichodesmium erythraeum. ACS Omega 2021, 6, 22803–22810. [Google Scholar] [CrossRef]
- Gerwick, W.H.; Tan, L.T.; Sitachitta, N. Nitrogen-containing metabolites from marine cyanobacteria. In The Alkaloids. Chemistry and Biology: Volume 57; Cordell, G., Ed.; Academic Press: San Diego, CA, USA, 2001; pp. 75–177. [Google Scholar]
- Moss, N.A.; Leão, T.; Rankin, M.R.; McCullough, T.M.; Qu, P.; Korobeynikov, A.; Smith, J.L.; Gerwick, L.; Gerwick, W.H. Ketoreductase domain dysfunction expands chemodiversity: Malyngamide biosynthesis in the cyanobacterium Okeania hirsuta. ACS Chem. Biol. 2018, 13, 3385–3395. [Google Scholar] [CrossRef]
- Meyer, J.L.; Gunasekera, S.P.; Scott, R.M.; Paul, V.J.; Teplitski, M. Microbiome shifts and the inhibition of quorum sensing by Black Band Disease cyanobacteria. ISME J. 2016, 10, 1204–1216. [Google Scholar] [CrossRef]
- LewisOscar, F.; Nithya, C.; Alharbi, S.A.; Alharbi, N.S.; Thajuddin, N. Microfouling inhibition of human nosocomial pathogen Pseudomonas aeruginosa using marine cyanobacteria. Microb. Pathog. 2018, 114, 107–115. [Google Scholar] [CrossRef]
- Cepas, V.; Gutiérrez-Del-Río, I.; López, Y.; Redondo-Blanco, S.; Gabasa, Y.; Iglesias, M.J.; Soengas, R.; Fernández-Lorenzo, A.; López-Ibáñez, S.; Villar, C.J.; et al. Microalgae and cyanobacteria strains as producers of lipids with antibacterial and antibiofilm activity. Mar. Drugs 2021, 19, 675. [Google Scholar] [CrossRef] [PubMed]
- Soares, A.R.; Engene, N.; Gunasekera, S.P.; Sneed, J.M.; Paul, V.J. Carriebowlinol, an antimicrobial tetrahydroquinolinol from an assemblage of marine cyanobacteria containing a novel taxon. J. Nat. Prod. 2015, 78, 534–538. [Google Scholar] [CrossRef] [PubMed]
- Hentzer, M.; Riedel, K.; Rasmussen, T.B.; Heydorn, A.; Andersen, J.B.; Parsek, M.R.; Rice, S.A.; Eberl, L.; Molin, S.; Høiby, N.; et al. Inhibition of quorum sensing in Pseudomonas aeruginosa biofilm bacteria by a halogenated furanone compound. Microbiology 2002, 148, 87–102. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Rybtke, M.T.; Jakobsen, T.H.; Hentzer, M.; Bjarnsholt, T.; Givskov, M.; Tolker-Nielsen, T. Computer-aided identification of recognized drugs as Pseudomonas aeruginosa quorum-sensing inhibitors. Antimicrob. Agents Chemother. 2009, 53, 2432–2443. [Google Scholar] [CrossRef] [PubMed]








| C Atom | δH (m, J in Hz) | δC | HMBC |
|---|---|---|---|
| 1′ | 4.70 (d, 5.4) | 63.0 | 131.2, 130.6, 173.2 |
| 2′ | 6.16 (dt, 15.8, 11.6) | 130.6 | 128.7, 131.2, 63.0 |
| 3′ | 6.27 (d, 16.0) | 131.1 | 128.7, 130.6, 63.0 |
| 4′ | 128.5 | ||
| 5′ | 5.84 (brs) | 120.8 | 128.7 |
| 5.67 (brs) | 128.7 | ||
| 1 | 173.1 | 63.0, 34.2 | |
| 2 | 2.42 (t, 16.7) | 34.2 | 173.2, 29.4, 18.7 |
| 3 | 1.54 (m) | 29.4 | 34.2 |
| 4 | 0.43 (m) | 18.7 | 34.2, 29.4, 11.6 |
| 5 | 0.20 (m) | 11.6 | 34.2, 29.4 |
| 6 | 0.43 (m) | 17.9 | |
| 7 | 1.22 (m) | 33.8 | 29.2 |
| 8 | 1.26 (m) | 29.2 | |
| 9–11 | 1.25 (m) | 31.6 | |
| 12 | 1.28 (m) | 22.4 | |
| 13 | 0.87 (t, 13.7) | 13.9 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Salleh, N.F.; Wang, J.; Kundukad, B.; Oluwabusola, E.T.; Goh, D.X.Y.; Phyo, M.Y.; Tong, J.J.L.; Kjelleberg, S.; Tan, L.T. Cyclopropane-Containing Specialized Metabolites from the Marine Cyanobacterium cf. Lyngbya sp. Molecules 2023, 28, 3965. https://doi.org/10.3390/molecules28093965
Salleh NF, Wang J, Kundukad B, Oluwabusola ET, Goh DXY, Phyo MY, Tong JJL, Kjelleberg S, Tan LT. Cyclopropane-Containing Specialized Metabolites from the Marine Cyanobacterium cf. Lyngbya sp. Molecules. 2023; 28(9):3965. https://doi.org/10.3390/molecules28093965
Chicago/Turabian StyleSalleh, Nurul Farhana, Jiale Wang, Binu Kundukad, Emmanuel T. Oluwabusola, Delia Xin Yin Goh, Ma Yadanar Phyo, Jasmine Jie Lin Tong, Staffan Kjelleberg, and Lik Tong Tan. 2023. "Cyclopropane-Containing Specialized Metabolites from the Marine Cyanobacterium cf. Lyngbya sp." Molecules 28, no. 9: 3965. https://doi.org/10.3390/molecules28093965
APA StyleSalleh, N. F., Wang, J., Kundukad, B., Oluwabusola, E. T., Goh, D. X. Y., Phyo, M. Y., Tong, J. J. L., Kjelleberg, S., & Tan, L. T. (2023). Cyclopropane-Containing Specialized Metabolites from the Marine Cyanobacterium cf. Lyngbya sp. Molecules, 28(9), 3965. https://doi.org/10.3390/molecules28093965






