Development and Application of an Atomic Absorption Spectrometry-Based Method to Quantify Magnesium in Leaves of Dioscorea polystachya
Abstract
:1. Introduction
2. Results and Discussion
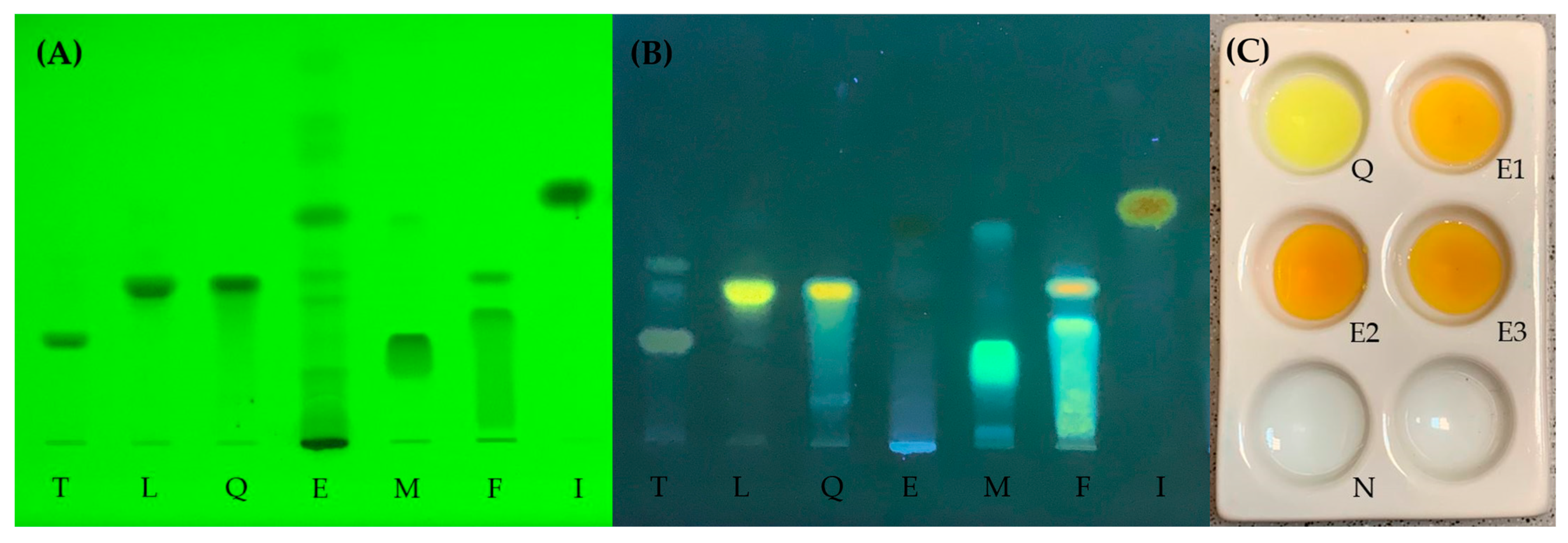
2.1. Extraction and Thin-Layer Chromatography
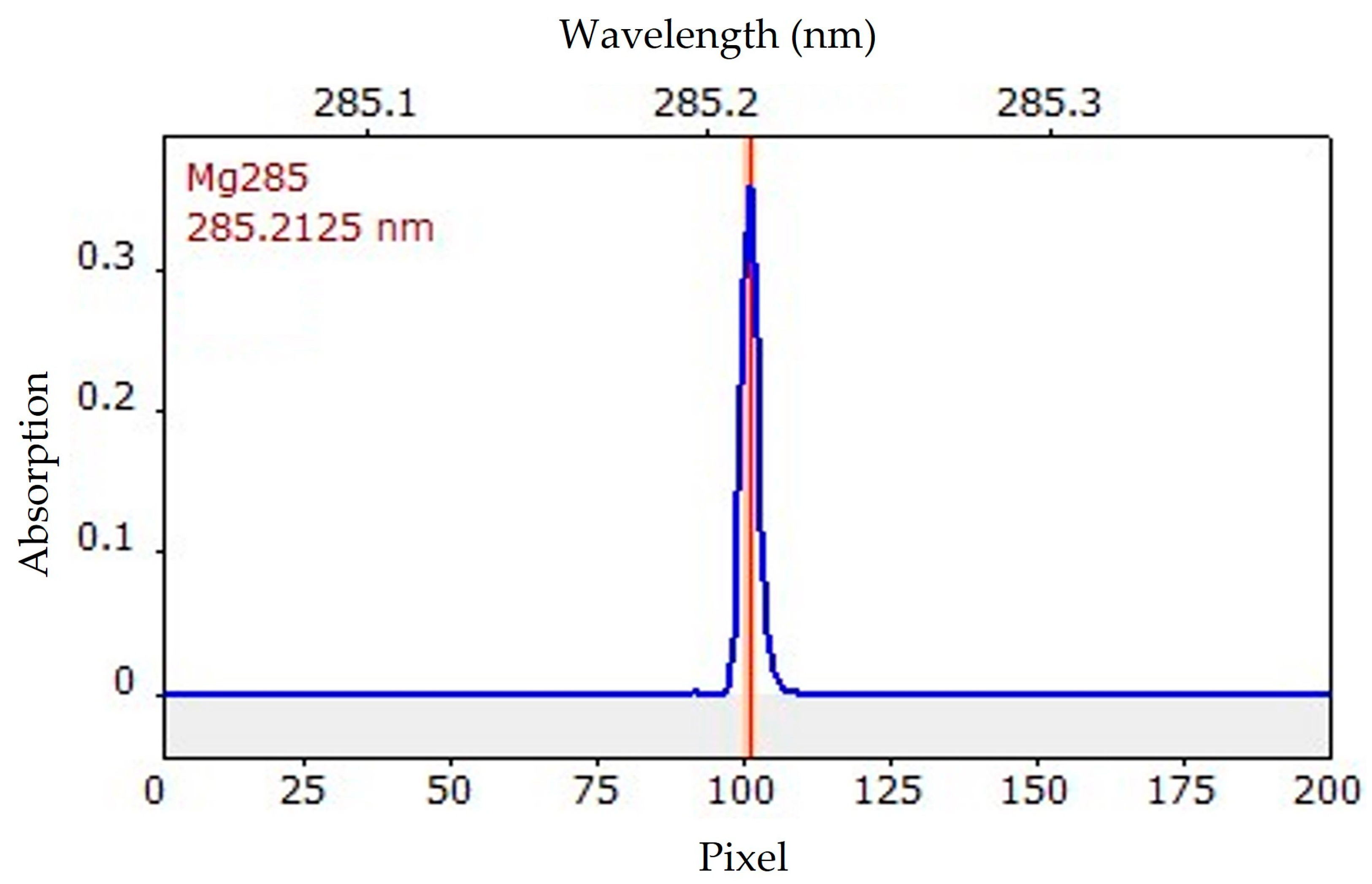
2.2. Selection of the Wavelength
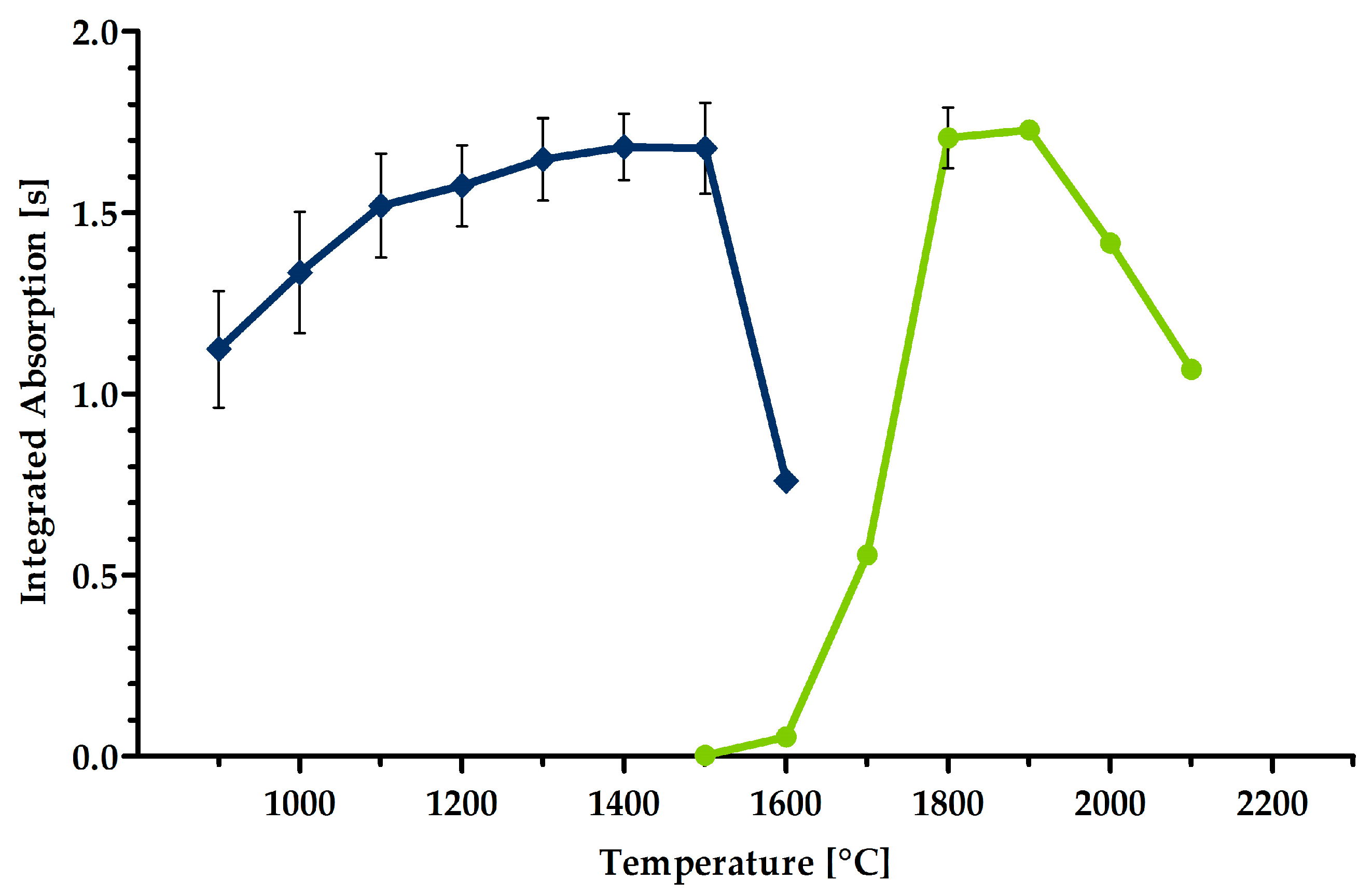
2.3. Optimization of the Graphite Furnace Program
2.4. Calibration and Figures of Merit
2.5. Quantification of the Magnesium Content in Biological Samples
3. Materials and Methods
3.1. Chemicals
3.2. Plant Collection and Extraction
3.3. Thin-Layer Chromatography and Identification Reaction
3.4. Instrumentation
3.5. HR CS AAS Procedure
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Obidiegwu, J.E.; Lyons, J.B.; Chilaka, C.A. The Dioscorea Genus (Yam)—An Appraisal of Nutritional and Therapeutic Potentials. Foods 2020, 9, 1304. [Google Scholar] [CrossRef] [PubMed]
- Epping, J.; Laibach, N. An underutilized orphan tuber crop—Chinese yam: A review. Planta 2020, 252, 58. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Ji, S.; Xu, T.; Zhong, Y.; Xu, M.; Liu, Y.; Li, M.; Fan, B.; Wang, F.; Xiao, J.; et al. Chinese yam (Dioscorea): Nutritional value, beneficial effects, and food and pharmaceutical applications. Trends Food Sci. Technol. 2023, 134, 29–40. [Google Scholar] [CrossRef]
- Alharazi, W.Z.; McGowen, A.; Rose, P.; Jethwa, P.H. Could consumption of yam (Dioscorea) or its extract be beneficial in controlling glycaemia: A systematic review. Br. J. Nutr. 2022, 128, 613–624. [Google Scholar] [CrossRef] [PubMed]
- Jezek, M.; Allan, A.C.; Jones, J.J.; Geilfus, C.-M. Why do plants blush when they are hungry? New Phytol. 2023, 239, 494–505. [Google Scholar] [CrossRef] [PubMed]
- Ishfaq, M.; Wang, Y.; Yan, M.; Wang, Z.; Wu, L.; Li, C.; Li, X. Physiological Essence of Magnesium in Plants and Its Widespread Deficiency in the Farming System of China. Front. Plant Sci. 2022, 13, 802274. [Google Scholar] [CrossRef] [PubMed]
- Trapani, V.; Farruggia, G.; Marraccini, C.; Iotti, S.; Cittadini, A.; Wolf, F.I. Intracellular magnesium detection: Imaging a brighter future. Analyst 2010, 135, 1855–1866. [Google Scholar] [CrossRef] [PubMed]
- Resano, M.; Garcia-Ruiz, E.; Aramendia, M.; Belarra, M.A. Quo vadis high-resolution continuum source atomic/molecular absorption spectrometry? J. Anal. At. Spectrom. 2019, 34, 59–80. [Google Scholar] [CrossRef]
- Bartz, F.-M.; Beirow, K.; Wurm, K.; Baecker, D.; Link, A.; Bednarski, P.J. A graphite furnace-atomic absorption spectrometry-based rubidium efflux assay for screening activators of the Kv7.2/3 channel. Arch. Pharm. 2023, 356, e2200585. [Google Scholar] [CrossRef]
- Ma, B.N.; Baecker, D.; Descher, H.; Brandstaetter, P.; Hermann, M.; Kircher, B.; Gust, R. Synthesis and biological evaluation of salophen nickel(II) and cobalt(III) complexes as potential anticancer compounds. Arch. Pharm. 2023, 356, 2200655. [Google Scholar] [CrossRef]
- Baecker, D.; Sesli, Ö.; Knabl, L.; Huber, S.; Orth-Höller, D.; Gust, R. Investigating the antibacterial activity of salen/salophene metal complexes: Induction of ferroptosis as part of the mode of action. Eur. J. Med. Chem. 2021, 209, 112907. [Google Scholar] [CrossRef] [PubMed]
- Baecker, D.; Obermoser, V.; Kirchner, E.A.; Hupfauf, A.; Kircher, B.; Gust, R. Fluorination as tool to improve bioanalytical sensitivity and COX-2-selective antitumor activity of cobalt alkyne complexes. Dalton Trans. 2019, 48, 15856–15868. [Google Scholar] [CrossRef] [PubMed]
- Baecker, D.; Guenther, S. General Applicability of High-Resolution Continuum-Source Graphite Furnace Molecular Absorption Spectrometry to the Quantification of Oligopeptides Using the Example of Glutathione. Analytica 2022, 3, 24–35. [Google Scholar] [CrossRef]
- Makarishcheva, D.D.; Kolesnikova, O.N.; Tregubova, V.E.; Ustinnikova, O.B. Development of a Quantitative Determination Method for Aluminum Ions in Adsorbed Drugs Using Atomic Absorption Spectrometry with Electrothermal Atomization. Pharm. Chem. J. 2022, 56, 527–531. [Google Scholar] [CrossRef] [PubMed]
- Kantor, T.; Bezur, L.; Pungor, E. Volatilization studies of magnesium compounds by a graphite furnace and flame combined atomic absorption method. The use ofa halogenating atmosphere. Spectrochim. Acta B 1983, 38, 581–607. [Google Scholar] [CrossRef]
- Wang, Z.; Zhao, S.; Tao, S.; Hou, G.; Zhao, F.; Tan, S.; Meng, Q. Dioscorea spp.: Bioactive Compounds and Potential for the Treatment of Inflammatory and Metabolic Diseases. Molecules 2023, 28, 2878. [Google Scholar] [CrossRef]
- Gamela, R.R.; Barrera, E.G.; Duarte, A.T.; Boschetti, W.; da Silva, M.M.; Vale, M.G.R.; Dessuy, M.B. Fast Sequential Determination of Zn, Fe, Mg, Ca, Na, and K in Infant Formulas by High-Resolution Continuum Source Flame Atomic Absorption Spectrometry Using Ultrasound-Assisted Extraction. Food Anal. Methods 2019, 12, 1420–1428. [Google Scholar] [CrossRef]
- Anal, J.M.H.; Chase, P. Trace elements analysis in some medicinal plants using graphite furnace-atomic absorption spectroscopy. Environ. Eng. Res. 2016, 21, 247–255. [Google Scholar] [CrossRef]
- Prkić, A.; Giljanovic, J.; Petricevic, S.; Brkljaca, M.; Bralic, M. Determination of Cadmium, Chromium, Copper, Iron, Lead, Magnesium, Manganese, Potassium, and Zinc in Mint Tea Leaves by Electrothermal Atomizer Atomic Absorption Spectrometry in Samples Purchased at Local Supermarkets and Marketplaces. Anal. Lett. 2013, 46, 367–378. [Google Scholar] [CrossRef]
- Welz, B.; Sperling, M. Atomabsorptionsspektrometrie, 4th ed.; Wiley-VHS: Weinheim, Germany, 1997; pp. 393–394. [Google Scholar]
- Martin-Benlloch, X.; Lanfranchi, D.A.; Haid, S.; Pietschmann, T.; Davioud-Charvet, E.; Elhabiri, M. Magnesium Complexes of Ladanein: A Beneficial Strategy for Stabilizing Polyphenolic Antivirals. Eur. J. Inorg. Chem. 2021, 27, 2764–2772. [Google Scholar] [CrossRef]
- Malacaria, L.; Bijlsma, J.; Hilgers, R.; de Bruijn, W.J.C.; Vincken, J.-P.; Furia, E. Insights into the complexation and oxidation of quercetin and luteolin in aqueous solutions in presence of selected metal cations. J. Mol. Liq. 2023, 369, 120840. [Google Scholar] [CrossRef]
- Ghosh, N.; Chakraborty, T.; Mallick, S.; Mana, S.; Singha, D.; Ghosh, B.; Roy, S. Synthesis, characterization and study of antioxidant activity of quercetin–magnesium complex. Spectrochim. Acta A 2015, 151, 807–813. [Google Scholar] [CrossRef] [PubMed]
- Seeger, T.S.; Muller, E.I.; Mesko, M.F.; Duarte, F.A. Magnesium and calcium determination in desalted crude oil by direct sampling graphite furnace atomic absorption spectrometry. Fuel 2019, 236, 1483–1488. [Google Scholar] [CrossRef]
- Schlemmer, G.; Welz, B. Palladium and magnesium nitrates, a more universal modifier for graphite furnace atomic absorption spectrometry. Spectrochim. Acta B 1986, 41, 1157–1165. [Google Scholar] [CrossRef]
- Adelantado, J.V.G.; Martinez, V.P.; Garcia, A.P.; Reig, F.B. Atomic-absorption spectrometric determination of calcium, magnesium and potassium in leaf samples after decomposition with molten sodium hydroxide. Talanta 1991, 38, 959–963. [Google Scholar] [CrossRef] [PubMed]
- Bradfield, E.G.; Spincer, D. Leaf analysis as a guide to the nutrition of fruit crops. VI—Determination of magnesium, zinc and copper by atomic absorption spectroscopy. J. Sci. Food Agric. 1965, 16, 33–38. [Google Scholar] [CrossRef]
- Prkić, A.; Juric, A.; Giljanovic, J.; Politeo, N.; Sokol, V.; Boskovic, P.; Brkljaca, M.; Stipisic, A.; Fernandez, C.; Vukusic, T. Monitoring content of cadmium, calcium, copper, iron, lead, magnesium and manganese in tea leaves by electrothermal and flame atomizer atomic absorption spectrometry. Open Chem. 2017, 15, 200–207. [Google Scholar] [CrossRef]
- Prkić, A.; Politeo, N.; Giljanovic, J.; Sokol, V.; Boskovic, P.; Brkljaca, M.; Stipisic, A. Survey of content of cadmium, calcium, chromium, copper, iron, lead, magnesium, manganese, mercury, sodium and zinc in chamomile and green tea leaves by electrothermal or flame atomizer atomic absorption spectrometry. Open Chem. 2018, 16, 228–237. [Google Scholar] [CrossRef]
- Tanoi, K.; Kobayashi, N.I. Leaf Senescence by Magnesium Deficiency. Plants 2015, 4, 756–772. [Google Scholar] [CrossRef]
- Kaiser, B.N.; Gridley, K.L.; Brady, J.N.; Phillips, T.; Tyerman, S.D. The Role of Molybdenum in Agricultural Plant Production. Ann. Bot. 2005, 96, 745–754. [Google Scholar] [CrossRef]
- Yu, M.; Hu, C.-X.; Wang, Y.-H. Effects of Molybdenum on the Intermediates of Chlorophyll Biosynthesis in Winter Wheat Cultivars Under Low Temperature. Agric. Sci. China 2006, 5, 670–677. [Google Scholar] [CrossRef]
- Jia, B.; Waldo, P.; Conner, R.L.; Moumen, I.; Khan, N.; Xia, X.; Hou, A.; You, F.M. Marsh spot disease and its causal factor, manganese deficiency in plants: A historical and prospective review. Agric. Sci. 2021, 12, 928–948. [Google Scholar] [CrossRef]
- Schmidt, S.B.; Husted, S. The Biochemical Properties of Manganese in Plants. Plants 2019, 8, 381. [Google Scholar] [CrossRef] [PubMed]
- Rufa’i, F.A.; Baecker, D.; Mukhtar, M.D. Phytochemical Screening, GC-MS Analysis, and Evaluating In Vivo Antitrypanosomal Effects of a Methanolic Extract of Garcinia kola Nuts on Rats. Antibiotics 2023, 12, 713. [Google Scholar] [CrossRef]

| Operation | Temperature (°C) | Heating Rate (°C s−1) | Holding Time (s) | Argon Flow |
|---|---|---|---|---|
| Drying 1 | 90 | 10 | 10 | Maximal |
| Drying 2 | 100 | 5 | 10 | Maximal |
| Drying 3 | 120 | 5 | 15 | Maximal |
| Pyrolysis | 1500 | 150 | 15 | Maximal |
| Auto-zero | 1500 | 0 | 5 | Stop |
| Atomization | 1800 | 1500 | 5 | Stop |
| Cleaning | 2200 | 500 | 5 | Maximal |
| Parameter | Value |
|---|---|
| Linear working range | 1–10 µg L−1 |
| Correlation coefficient of calibration (R2) | 0.9975 |
| LOD | 0.23 µg L−1 |
| LOQ | 2.00 µg L−1 |
| 0.027 pg | |
| Recovery/precision (3 µg L−1) | 99.9%/11.9% |
| Recovery/precision (6 µg L−1) | 102.0%/3.1% |
| Recovery/precision (9 µg L−1) | 96.7%/2.2% |
| Coloration of Leaves | Extraction Material | Name | Magnesium Content (g kg−1) | Mean (g kg−1) | Standard Deviation (%) |
|---|---|---|---|---|---|
| Normal coloration | Dried leaves | NL-DE 1 | 7.75 | 7.61 | 1.74 |
| NL-DE 2 | 7.48 | ||||
| Fresh leaves | NL-FE | 7.42 | - | - | |
| Discoloration | Dried leaves | DL-DE 1 | 18.54 | 20.75 | 10.65 |
| DL-DE 2 | 22.96 | ||||
| Fresh leaves | DL-FE | 19.28 | - | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Krüger, D.; Weng, A.; Baecker, D. Development and Application of an Atomic Absorption Spectrometry-Based Method to Quantify Magnesium in Leaves of Dioscorea polystachya. Molecules 2024, 29, 109. https://doi.org/10.3390/molecules29010109
Krüger D, Weng A, Baecker D. Development and Application of an Atomic Absorption Spectrometry-Based Method to Quantify Magnesium in Leaves of Dioscorea polystachya. Molecules. 2024; 29(1):109. https://doi.org/10.3390/molecules29010109
Chicago/Turabian StyleKrüger, David, Alexander Weng, and Daniel Baecker. 2024. "Development and Application of an Atomic Absorption Spectrometry-Based Method to Quantify Magnesium in Leaves of Dioscorea polystachya" Molecules 29, no. 1: 109. https://doi.org/10.3390/molecules29010109
APA StyleKrüger, D., Weng, A., & Baecker, D. (2024). Development and Application of an Atomic Absorption Spectrometry-Based Method to Quantify Magnesium in Leaves of Dioscorea polystachya. Molecules, 29(1), 109. https://doi.org/10.3390/molecules29010109







