Study on Extraction Valuable Metal Elements by Co-Roasting Coal Gangue with Coal Gasification Coarse Slag
Abstract
:1. Introduction
2. Results and Discussion
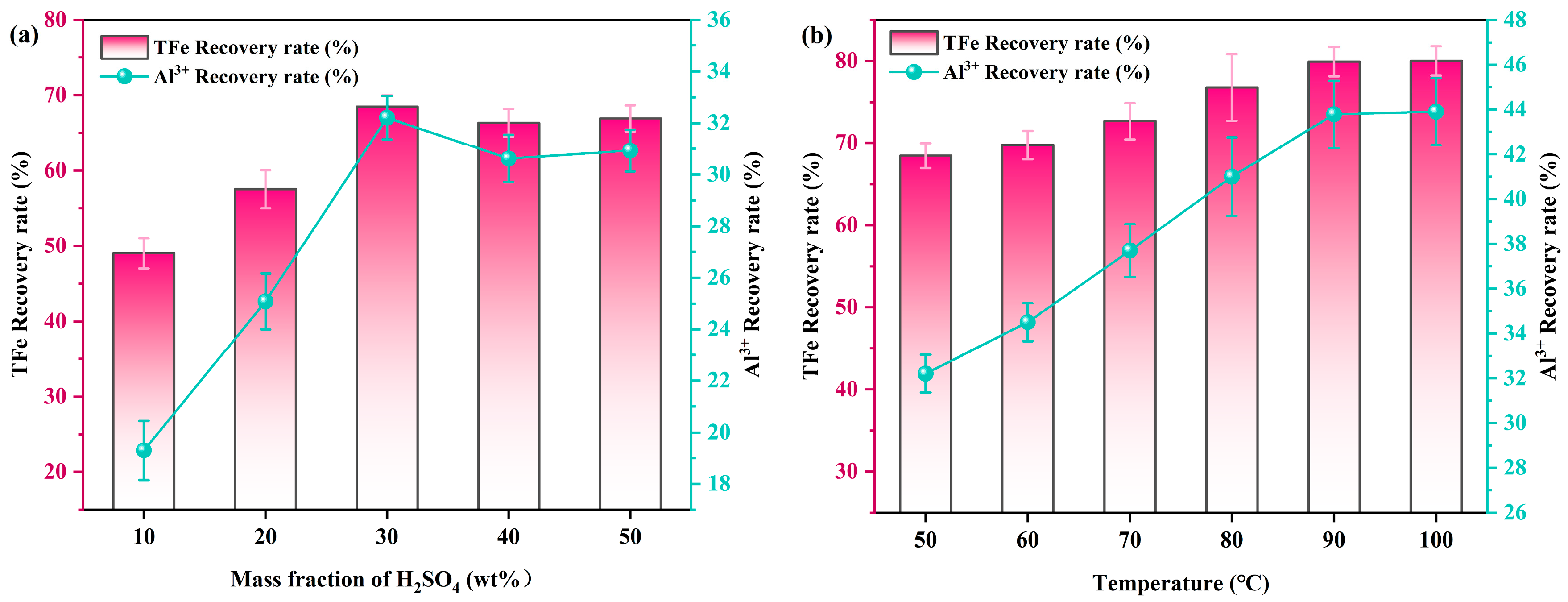
2.1. Optimization of Co-Roasting Parameter
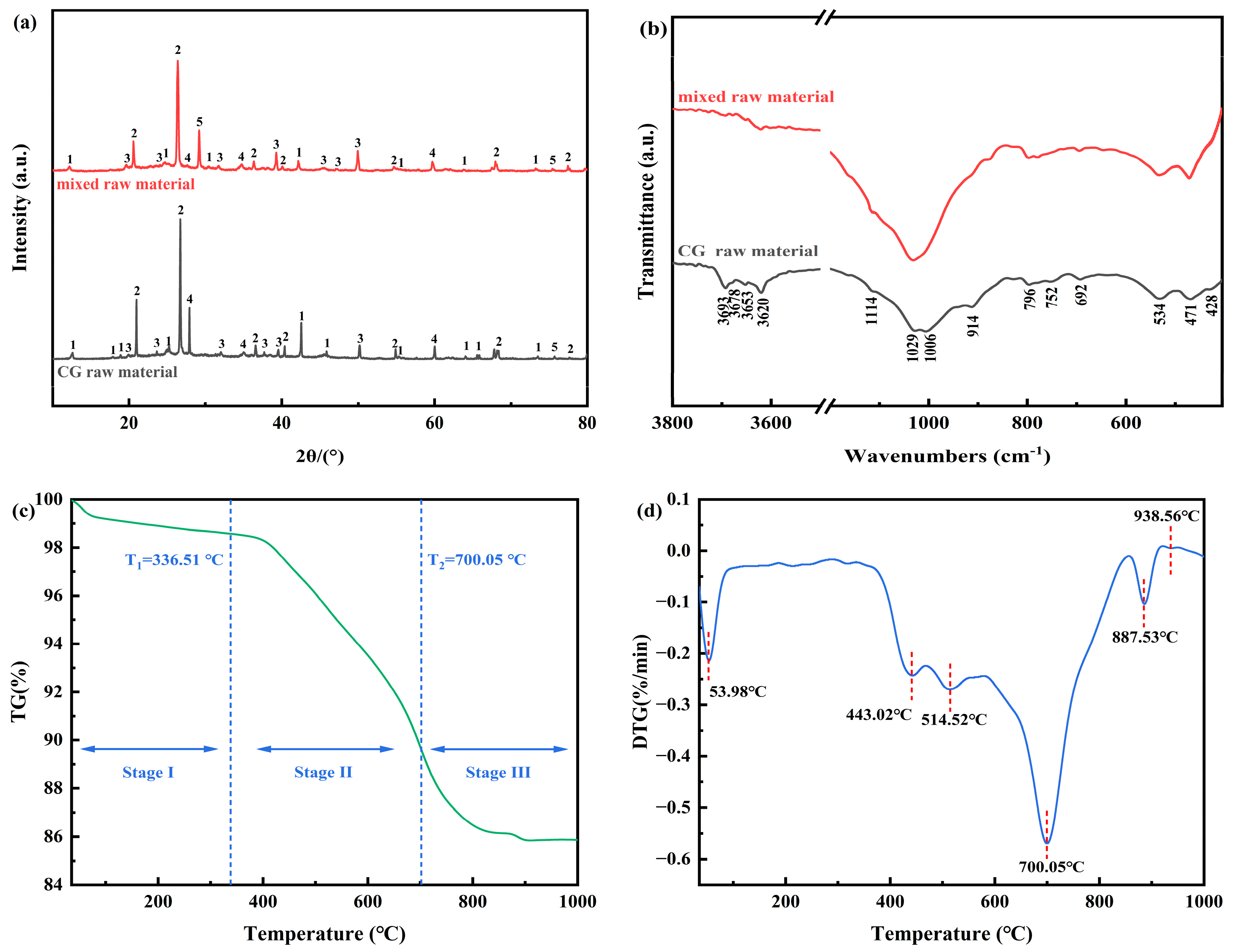
2.2. Raw Material Characterization
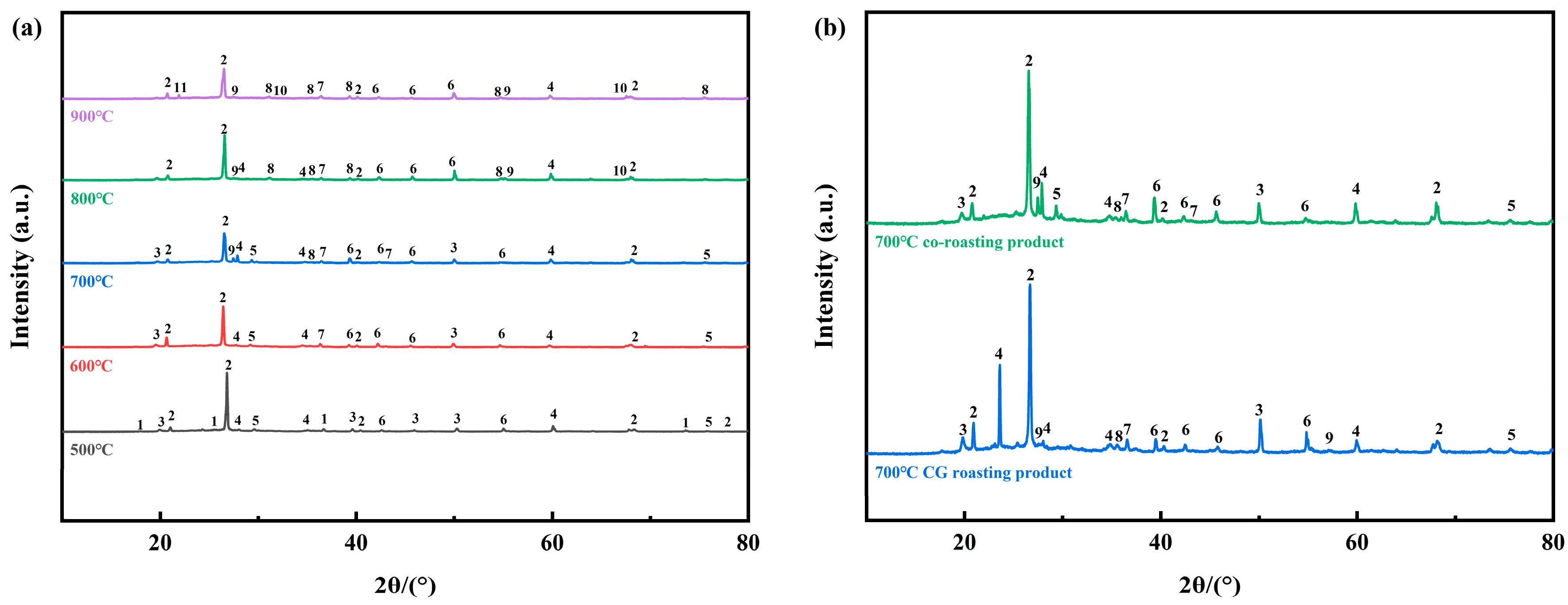
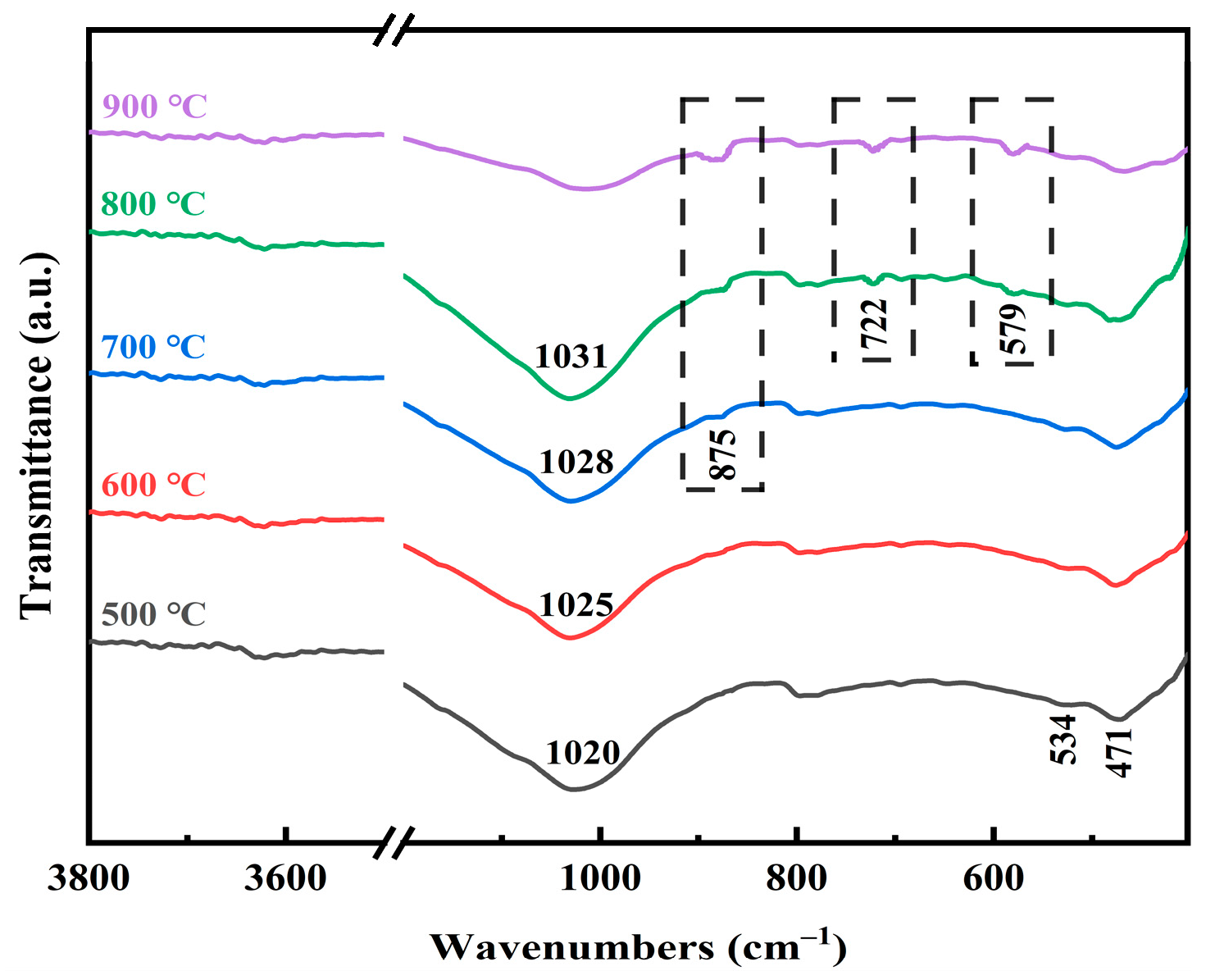
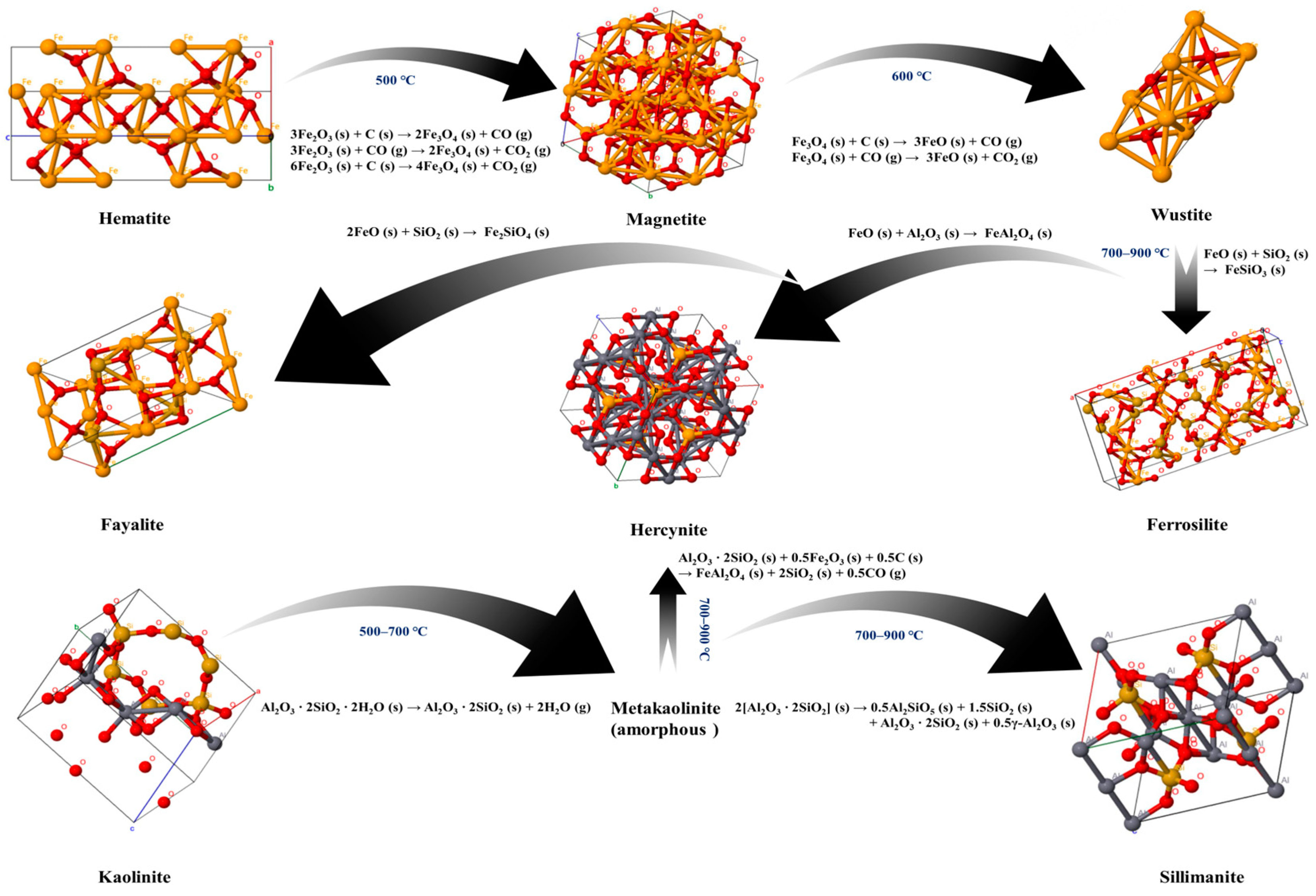
2.3. Phase Transformation during Co-Roasting Process
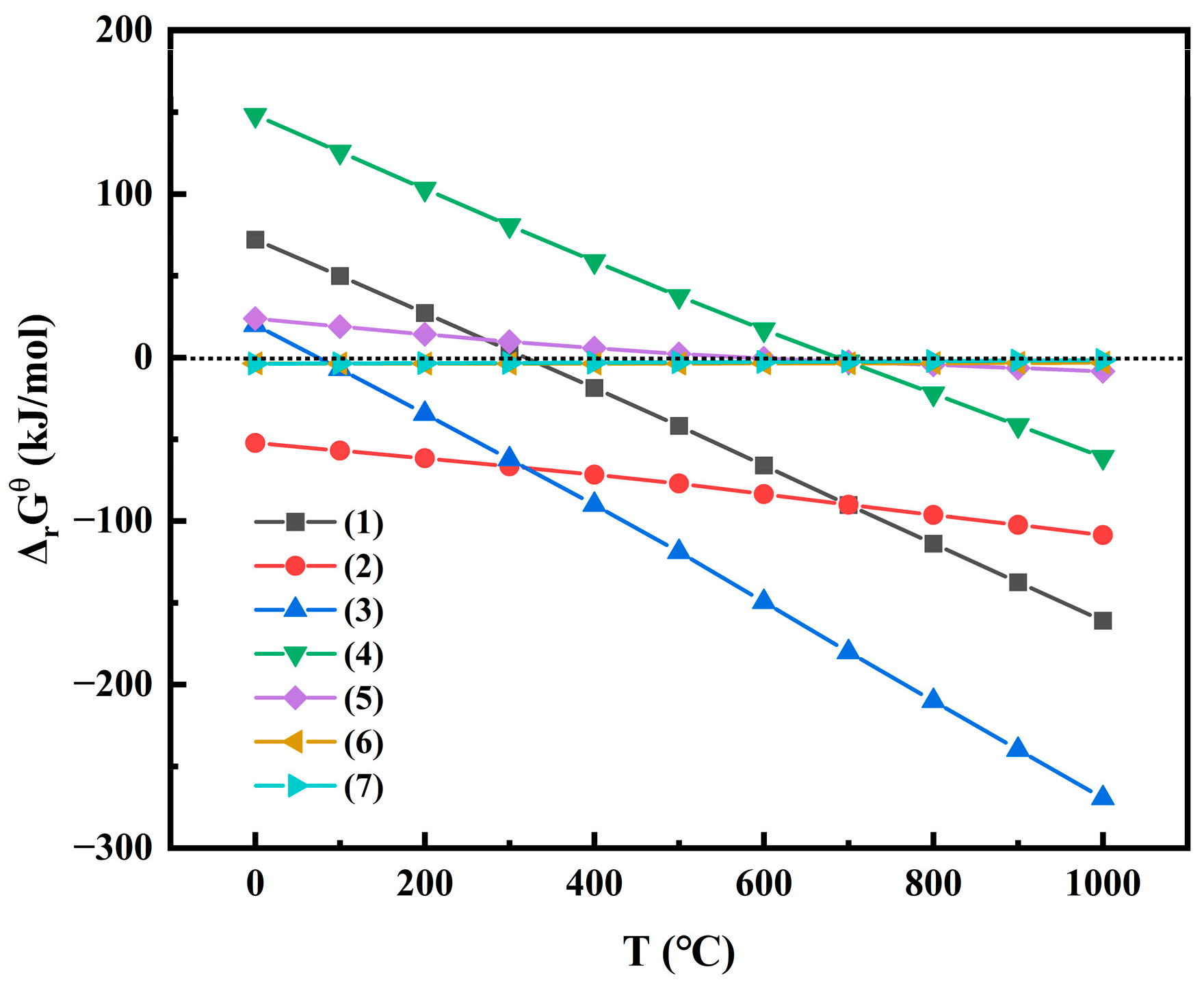
2.4. Co-Roasting Reaction Mechanism
2.5. Optimization of H2SO4 Leaching Parameter
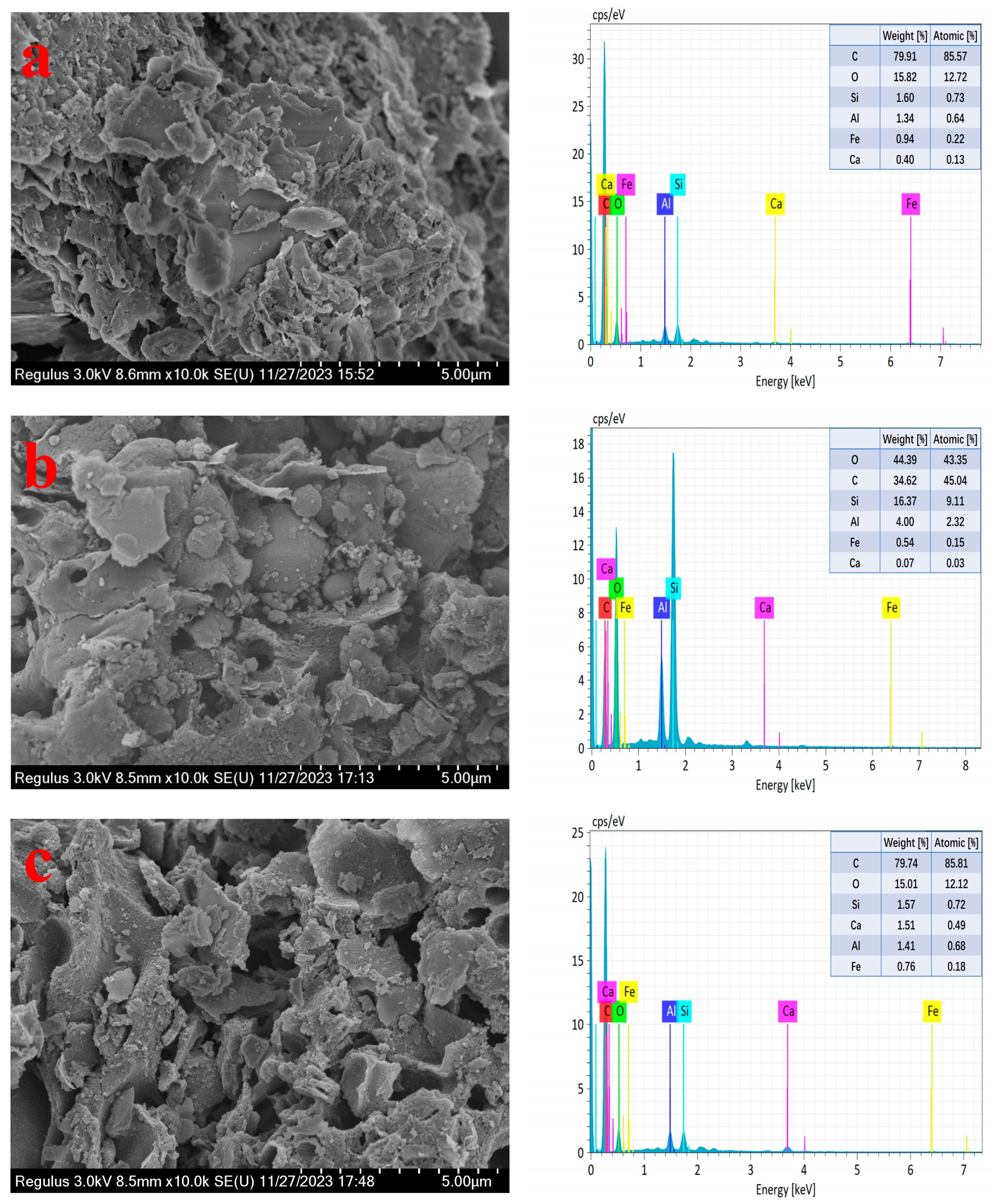
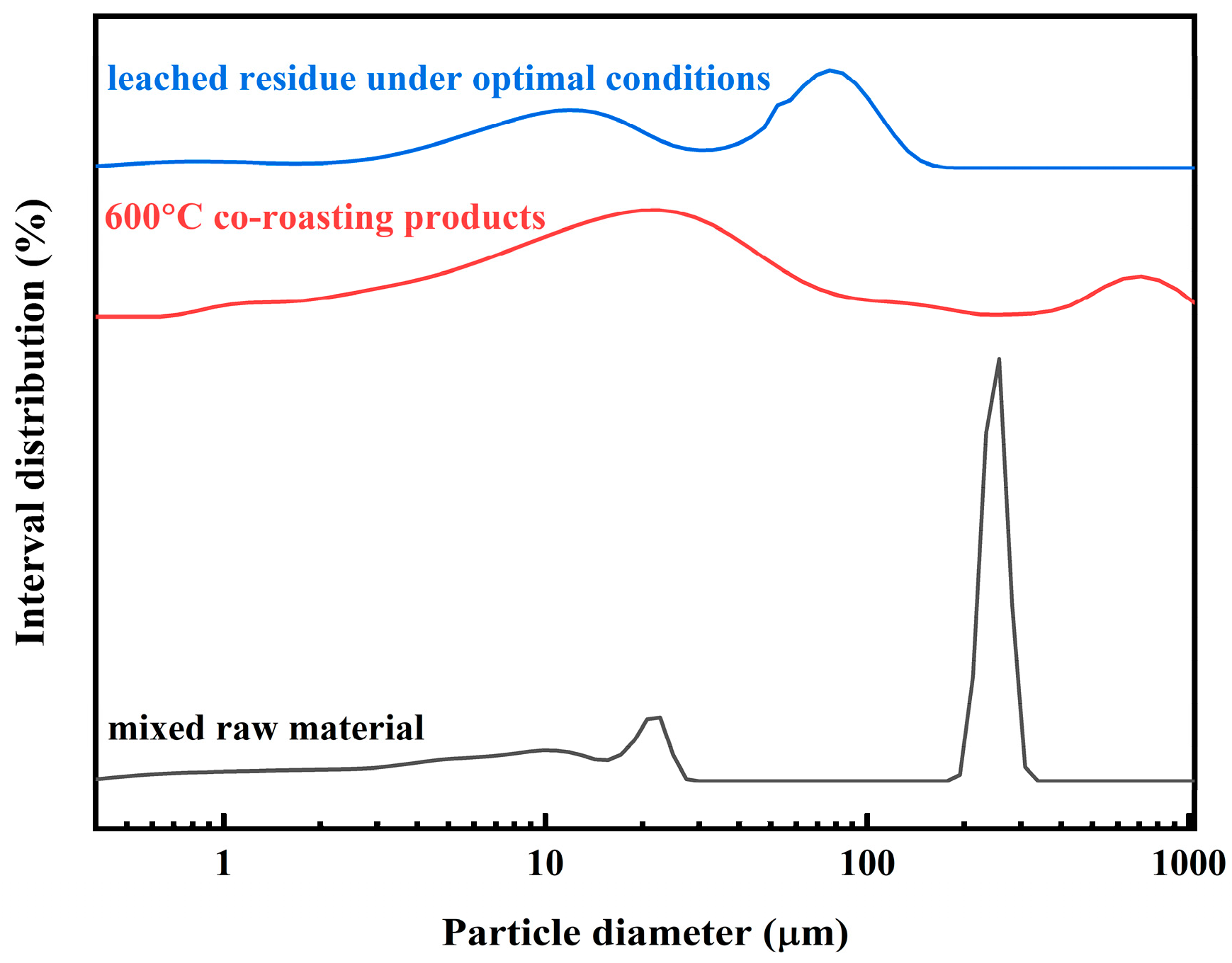
2.6. Analysis of Mixed Raw Material, Co-Roasting Product and Leached Residue
2.7. Prospects and Challenges in the Application of Acid Solution and Leached Residue
3. Materials and Methods
3.1. Materials
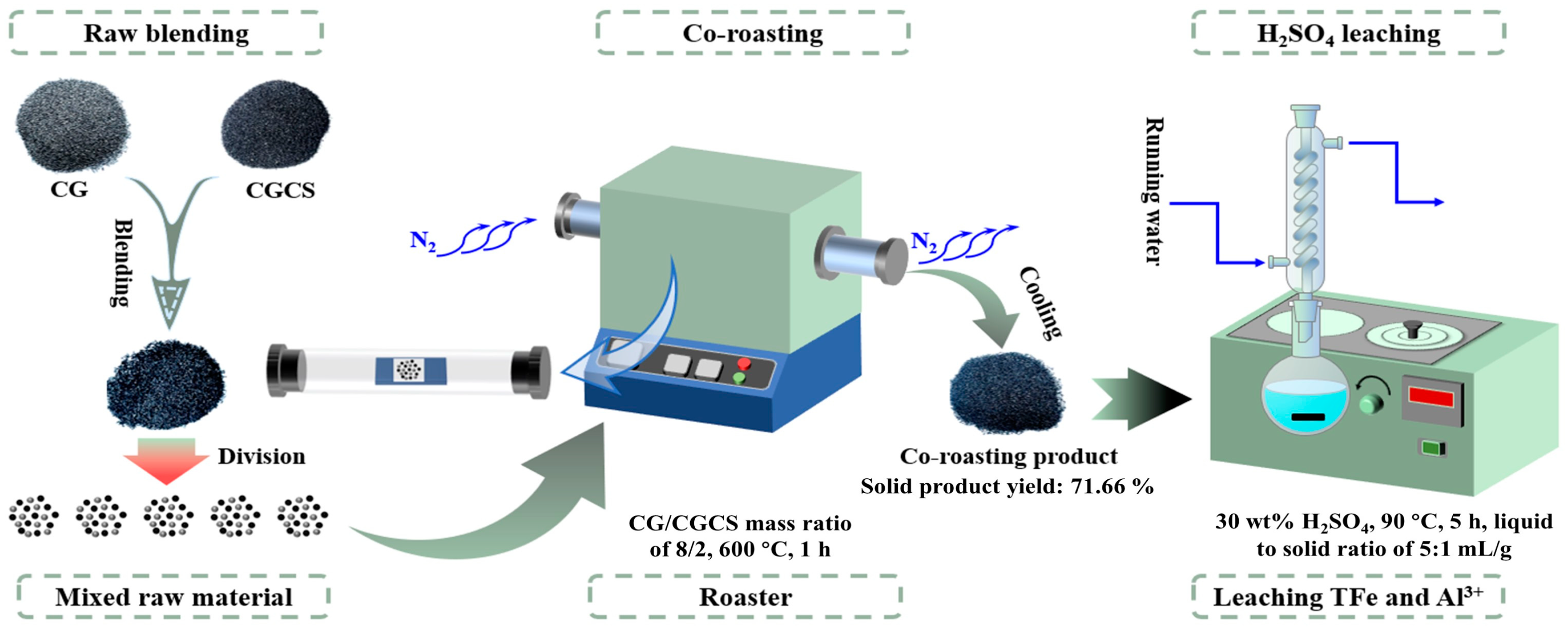
3.2. Experimental Procedures
3.3. Characterization Methods
4. Conclusions
- (1)
- The effects of various factors on the extraction efficiency of valuable metal elements (Al, Fe) were investigated through single factor experiments. Under optimal conditions including 20 wt% CGCS content, 600 °C co-roasting temperature for 1 h followed by leaching at 90 °C with a liquid to solid ratio of 5:1 mL/g using a H2SO4 concentration of 30 wt%, a TFe leaching rate of 79.93% was achieved along with an Al3+ leaching rate of 43.78% after a leaching time of 5 h. Furthermore, we found that acid solution and leached residue both have broad application prospects.
- (2)
- The activation behavior and phase transformation mechanism during the co-roasting process were investigated through Gibbs free energy calculation, as well as XRD, FTIR, and XPS characterization analysis. Inert kaolinite in CG and CGCS was converted to active metakaolinite after co-roasting. The reaction of metakaolinite with Si and Al elements from the mixed raw material produced sillimanite (Al2SiO5) and hercynite (FeAl2O4). Hematite (Fe2O3) was reduced to magnetite (Fe3O4) and wustite (FeO) by fixed carbon in CG and CGCS. Subsequently, wustite reacted with Si and Al elements from the mixed raw material to form iron phases such as ferrosilite (FeSiO3), hercynite (FeAl2O4), and fayalite (Fe2SiO4).
- (3)
- Co-roasting+H2SO4 leaching provides a novel method for extensive utilization of CG and CGCS while also offering a new approach for treating two or more types of coal-based solid waste that can alleviate industry development’s pressure on the environment.
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Li, M.; Zhang, J.; Li, A.; Zhou, N. Reutilisation of coal gangue and fly ash as underground backfill materials for surface subsidence control. J. Clean. Prod. 2020, 254, 120113. [Google Scholar] [CrossRef]
- Shao, S.; Ma, B.; Wang, C.; Chen, Y. Extraction of valuable components from coal gangue through thermal activation and HNO3 leaching. J. Ind. Eng. Chem. 2022, 113, 564–574. [Google Scholar] [CrossRef]
- Li, J.; Wang, J. Comprehensive utilization and environmental risks of coal gangue: A review. J. Clean. Prod. 2019, 239, 117946. [Google Scholar] [CrossRef]
- Wang, H.; Fang, X.; Du, F.; Tan, B.; Zhang, L.; Li, Y.; Xu, C. Three-dimensional distribution and oxidation degree analysis of coal gangue dump fire area: A case study. Sci. Total Environ. 2021, 772, 145606. [Google Scholar] [CrossRef] [PubMed]
- Shu, Q.; Sun, Z.; Zhu, G.; Wang, C.; Li, H.; Qi, F.; Zhang, Q.; Li, S. Highly efficient synthesis of ZSM-5 zeolite by one-step microwave using desilication solution of coal gasification coarse slag and its application to VOCs adsorption. Process Saf. Environ. Prot. 2022, 167, 173–183. [Google Scholar] [CrossRef]
- Zhu, D.; Zuo, J.; Jiang, Y.; Zhang, J.; Zhang, J.; Wei, C. Carbon-silica mesoporous composite in situ prepared from coal gasification fine slag by acid leaching method and its application in nitrate removing. Sci. Total Environ. 2020, 707, 136102. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Li, A.; Zhang, J.; Huang, Y.; Li, J. Effects of particle sizes on compressive deformation and particle breakage of gangue used for coal mine goaf backfill. Powder Technol. 2020, 360, 493–502. [Google Scholar] [CrossRef]
- Mjonono, D.; Harrison, S.; Kotsiopoulos, A. Supplementing structural integrity of waste rock piles through improved packing protocols to aid acid rock drainage prevention strategies. Miner. Eng. 2019, 135, 13–20. [Google Scholar] [CrossRef]
- Wu, Y.; Yu, X.; Hu, S.; Shao, H.; Liao, Q.; Fan, Y. Experimental study of the effects of stacking modes on the spontaneous combustion of coal gangue. Process Saf. Environ. Prot. 2019, 123, 39–47. [Google Scholar] [CrossRef]
- Shi, S.; Ma, B.; Zhao, D.; Li, X.; Shao, S.; Wang, C.; Chen, Y. Thermal Behavior of Al(NO3)3·9H2O and its Application in preparing Al2O3 and Regenerating HNO3. Process Saf. Environ. Prot. 2023, 178, 728–738. [Google Scholar] [CrossRef]
- Chen, Y.; Zhou, X.; Wan, S.; Zheng, R.; Tong, J.; Hou, H.; Wang, T. Synthesis and characterization of geopolymer composites based on gasification coal fly ash and steel slag. Constr. Build. Mater. 2019, 211, 646–658. [Google Scholar] [CrossRef]
- Li, Z.; Zhang, Y.; Zhao, H.; Chen, H.; He, R. Structure characteristics and composition of hydration products of coal gasification slag mixed cement and lime. Constr. Build. Mater. 2019, 213, 265–274. [Google Scholar] [CrossRef]
- Liu, T.; Awasthi, M.K.; Awasthi, S.K.; Ren, X.; Liu, X.; Zhang, Z. Influence of fine coal gasification slag on greenhouse gases emission and volatile fatty acids during pig manure composting. Bioresour. Technol. 2020, 316, 123915. [Google Scholar] [CrossRef] [PubMed]
- Yuan, N.; Zhao, A.; Hu, Z.; Tan, K.; Zhang, J. Preparation and application of porous materials from coal gasification slag for wastewater treatment: A review. Chemosphere 2021, 287, 132227. [Google Scholar] [CrossRef] [PubMed]
- Xiao, J.; Li, F.; Zhong, Q.; Bao, H.; Wang, B.; Huang, J.; Zhang, Y. Separation of aluminum and silica from coal gangue by elevated temperature acid leaching for the preparation of alumina and SiC. Hydrometallurgy 2015, 155, 118–124. [Google Scholar] [CrossRef]
- Yang, Q.; Zhang, F.; Deng, X.; Guo, H.; Zhang, C.; Shi, C.; Zeng, M. Extraction of alumina from alumina rich coal gangue by a hydro-chemical process. R. Soc. Open Sci. 2020, 7, 192132. [Google Scholar] [CrossRef] [PubMed]
- Dong, L.; Li, Y.; Yan, J.; Shu, X.Q. Efficient Extraction of SiO2 and Al2O3 from Coal Gangue by Means of Acidic Leaching. Adv. Mater. Res. 2014, 878, 149–156. [Google Scholar] [CrossRef]
- Cheng, F.; Cui, L.; Miller, J.D.; Wang, X. Aluminum Leaching from Calcined Coal Waste Using Hydrochloric Acid Solution. Miner. Process. Extr. Metall. Rev. 2012, 33, 391–403. [Google Scholar] [CrossRef]
- Gaviría, J.; Bohé, A.; Pasquevich, A.; Pasquevich, D. Hematite to magnetite reduction monitored by Mössbauer spectroscopy and X-ray diffraction. Phys. B Condens. Matter 2007, 389, 198–201. [Google Scholar] [CrossRef]
- Pineau, A.; Kanari, N.; Gaballah, I. Kinetics of reduction of iron oxides by H2: Part I: Low temperature reduction of hematite. Thermochim. Acta 2006, 447, 89–100. [Google Scholar] [CrossRef]
- Strangway, P.; Ross, H. The Kinetics of Hematite Reduction by Partially Reformed Natural Gas. Can. Metall. Q. 1966, 5, 221–235. [Google Scholar] [CrossRef]
- Han, R.; Guo, X.; Guan, J.; Yao, X.; Hao, Y. Activation Mechanism of Coal Gangue and Its Impact on the Properties of Geopolymers: A Review. Polymers 2022, 14, 3861. [Google Scholar] [CrossRef] [PubMed]
- Qin, Q.; Deng, J.; Geng, H.; Bai, Z.; Gui, X.; Ma, Z.; Miao, Z. An exploratory study on strategic metal recovery of coal gangue and sustainable utilization potential of recovery residue. J. Clean. Prod. 2022, 340, 130765. [Google Scholar] [CrossRef]
- Guan, X.; Chen, J.; Zhu, M.; Gao, J. Performance of microwave-activated coal gangue powder as auxiliary cementitious material. J. Mater. Res. Technol. 2021, 14, 2799–2811. [Google Scholar] [CrossRef]
- Guo, Y.; Yan, K.; Cui, L.; Cheng, F. Improved extraction of alumina from coal gangue by surface mechanically grinding modification. Powder Technol. 2016, 302, 33–41. [Google Scholar] [CrossRef]
- Li, C.; Wan, J.; Sun, H.; Li, L. Investigation on the activation of coal gangue by a new compound method. J. Hazard. Mater. 2010, 179, 515–520. [Google Scholar] [CrossRef]
- Han, L.; Ren, W.; Wang, B.; He, X.; Ma, L.; Huo, Q.; Wang, J.; Bao, W.; Chang, L. Extraction of SiO2 and Al2O3 from coal gangue activated by supercritical water. Fuel 2019, 253, 1184–1192. [Google Scholar] [CrossRef]
- Zhao, W.; Yang, B.; Yi, Y.; Feng, Q.; Liu, D. Synergistic activation of smithsonite with copper-ammonium species for enhancing surface reactivity and xanthate adsorption. Int. J. Min. Sci. Technol. 2023, 33, 519–527. [Google Scholar] [CrossRef]
- Shen, Z.; Wen, S.; Wang, H.; Miao, Y.; Wang, X.; Meng, S.; Feng, Q. Effect of dissolved components of malachite and calcite on surface properties and flotation behavior. Int. J. Miner. Metall. Mater. 2023, 30, 1297–1309. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, Y.; Zhou, Q.; Zhang, Y.; Sun, J. Thermal kinetics analysis of coal-gangue selected from Inner Mongolia in China. J. Therm. Anal. Calorim. 2018, 131, 1835–1843. [Google Scholar] [CrossRef]
- Xu, B.; Liu, Q.; Ai, B.; Ding, S.; Frost, R.L. Thermal decomposition of selected coal gangue. J. Therm. Anal. Calorim. 2018, 131, 1413–1422. [Google Scholar] [CrossRef]
- Madejová, J. FTIR techniques in clay mineral studies. Vib. Spectrosc. 2003, 31, 1–10. [Google Scholar] [CrossRef]
- Hu, H.Y.P. Controlled coating of antimony-doped tin oxide nanoparticles on kaolinite particles. Appl. Clay Sci. 2010, 48, 368–374. [Google Scholar] [CrossRef]
- Ion, R.M.; Radovici, C.; Fierascu, R.C.; Fierascu, I. Thermal and mineralogical investigations of iron archaeological materials. J. Therm. Anal. Calorim. 2015, 121, 1247–1253. [Google Scholar] [CrossRef]
- Cheng, H.; Li, K.; Liu, Q.; Zhang, S.; Li, X.; Frost, R.L. Insight into the thermal decomposition of kaolinite intercalated with potassium acetate: An evolved gas analysis. J. Therm. Anal. Calorim. 2014, 117, 1231–1239. [Google Scholar] [CrossRef]
- Shao, S.; Ma, B.; Wang, C.; Chen, Y. Thermal behavior and chemical reactivity of coal gangue during pyrolysis and combustion. Fuel 2023, 331. [Google Scholar] [CrossRef]
- Bi, H.; Wang, C.; Lin, Q.; Jiang, X.; Jiang, C.; Bao, L. Pyrolysis characteristics, artificial neural network modeling and environmental impact of coal gangue and biomass by TG-FTIR. Sci. Total Environ. 2021, 751, 142293. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Zhao, Q.; Yan, K.; Cheng, F.; Lou, H.H. Novel Process for Alumina Extraction via the Coupling Treatment of Coal Gangue and Bauxite Red Mud. Ind. Eng. Chem. Res. 2014, 53, 4518–4521. [Google Scholar] [CrossRef]
- Magdy, A.; Fouad, Y.; Abdel-Aziz, M.; Konsowa, A. Synthesis and characterization of Fe3O4/kaolin magnetic nanocomposite and its application in wastewater treatment. J. Ind. Eng. Chem. 2017, 56, 299–311. [Google Scholar] [CrossRef]
- Yuan, S.; Zhou, W.; Han, Y.; Li, Y. Selective enrichment of iron particles from complex refractory hematite-goethite ore by coal-based reduction and magnetic separation. Powder Technol. 2020, 367, 305–316. [Google Scholar] [CrossRef]
- Zhu, D.; Cui, Y.; Vining, K.; Hapugoda, S.; Douglas, J.; Pan, J.; Zheng, G. Upgrading low nickel content laterite ores using selective reduction followed by magnetic separation. Int. J. Miner. Process. 2012, 106–109, 1–7. [Google Scholar] [CrossRef]
- Yuan, S.; Zhou, W.; Han, Y.; Li, Y. Efficient enrichment of low-grade refractory rhodochrosite by preconcentration-neutral suspension roasting-magnetic separation process. Powder Technol. 2020, 361, 529–539. [Google Scholar] [CrossRef]
- Yuan, S.; Zhou, W.; Han, Y.; Li, Y. Selective enrichment of iron from fine-grained complex limonite using suspension magnetization roasting followed by magnetic separation. Sep. Sci. Technol. 2019, 55, 3427–3437. [Google Scholar] [CrossRef]
- Li, X.-B.; Xiao, W.; Liu, W.; Liu, G.-H.; Peng, Z.-H.; Zhou, Q.-S.; Qi, T.-G. Recovery of alumina and ferric oxide from Bayer red mud rich in iron by reduction sintering. Trans. Nonferrous Met. Soc. China 2009, 19, 1342–1347. [Google Scholar] [CrossRef]
- Qiao, X.C.; Si, P.; Yu, J.G. A Systematic Investigation into the Extraction of Aluminum from Coal Spoil through Kaolinite. Environ. Sci. Technol. 2008, 42, 8541–8546. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Liu, X.; Hu, Y. Investigation of the thermal behaviour and decomposition kinetics of kaolinite. Clay Miner. 2015, 50, 199–209. [Google Scholar] [CrossRef]
- Omsinsombon, J.; Chaiyasat, A.; Busabok, C.; Chaiyasat, P. A novel iron aluminate composite polymer particle for high-efficiency self-coating solar heat reflection. Sol. Energy Mater. Sol. Cells 2021, 230, 111248. [Google Scholar] [CrossRef]
- Jin, J.; Liu, X.; Yuan, S.; Gao, P.; Li, Y.; Zhang, H.; Meng, X. Innovative utilization of red mud through co-roasting with coal gangue for separation of iron and aluminum minerals. J. Ind. Eng. Chem. 2021, 98, 298–307. [Google Scholar] [CrossRef]
- Yamashita, T.; Hayes, P. Analysis of XPS spectra of Fe2+ and Fe3+ ions in oxide materials. Appl. Surf. Sci. 2008, 254, 2441–2449. [Google Scholar] [CrossRef]
- Fang, S.; Xu, L.; Wu, H.; Xu, Y.; Wang, Z.; Shu, K.; Hu, Y. Influence of surface dissolution on sodium oleate adsorption on ilmenite and its gangue minerals by ultrasonic treatment. Appl. Surf. Sci. 2020, 500, 144038. [Google Scholar] [CrossRef]
- Omran, M.; Fabritius, T.; Elmahdy, A.M.; Abdel-Khalek, N.A.; El-Aref, M.; Elmanawi, A.E.-H. XPS and FTIR spectroscopic study on microwave treated high phosphorus iron ore. Appl. Surf. Sci. 2015, 345, 127–140. [Google Scholar] [CrossRef]
- Salama, W.; El Aref, M.; Gaupp, R. Spectroscopic characterization of iron ores formed in different geological environments using FTIR, XPS, Mössbauer spectroscopy and thermoanalyses. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2015, 136, 1816–1826. [Google Scholar] [CrossRef]
- Dong, H.; Du, W.; Dong, J.; Che, R.; Kong, F.; Cheng, W.; Ma, M.; Gu, N.; Zhang, Y. Depletable peroxidase-like activity of Fe3O4 nanozymes accompanied with separate migration of electrons and iron ions. Nat. Commun. 2022, 13, 5365. [Google Scholar] [CrossRef] [PubMed]
- Grosvenor, A.; Kobe, B.; McIntyre, N. Examination of the oxidation of iron by oxygen using X-ray photoelectron spectroscopy and QUASESTM. Surf. Sci. 2004, 565, 151–162. [Google Scholar] [CrossRef]
- Liu, H.; Zhang, S.; Yang, W.; Yu, N.; Liu, C.; Chai, Y.; Dong, B. Directional Reconstruction of Iron Oxides to Active Sites for Superior Water Oxidation. Adv. Funct. Mater. 2023, 33, 2303776. [Google Scholar] [CrossRef]
- Gu, A.; Chen, K.; Zhou, X.; Gong, C.; Wang, P.; Jiao, Y.; Mao, P.; Chen, K.; Lu, J.; Yang, Y. Trimetallic MOFs-derived Fe-Co-Cu oxycarbide toward peroxymonosulfate activation for efficient trichlorophenol degradation via high-valent metal-oxo species. Chem. Eng. J. 2023, 468, 143444. [Google Scholar] [CrossRef]
- Li, X.; Wang, H.; Zhou, Q.; Qi, T.; Liu, G.; Peng, Z. Efficient separation of silica and alumina in simulated CFB slag by reduction roasting-alkaline leaching process. Waste Manag. 2019, 87, 798–804. [Google Scholar] [CrossRef]
- Zhou, Q.-S.; Li, C.; Li, X.-B.; Peng, Z.-H.; Liu, G.-H.; Qi, T.-G. Reaction behavior of ferric oxide in system Fe2O3–SiO2–Al2O3 during reductive sintering process. Trans. Nonferrous Met. Soc. China 2016, 26, 842–848. [Google Scholar] [CrossRef]
- Zhang, Y.; Xu, L.; Seetharaman, S.; Liu, L.; Wang, X.; Zhang, Z. Effects of chemistry and mineral on structural evolution and chemical reactivity of coal gangue during calcination: Towards efficient utilization. Mater. Struct. 2014, 48, 2779–2793. [Google Scholar] [CrossRef]
- Zhang, C.; Yang, X.Y.; Li, Y.F. Mechanism and Structural Analysis of the Thermal Activation of Coal-Gangue. Adv. Mater. Res. 2011, 356–360, 1807–1812. [Google Scholar] [CrossRef]
- Kong, D.; Zhou, Z.; Song, S.; Feng, S.; Lian, M.; Jiang, R. Preparation of Poly Aluminum-Ferric Chloride (PAFC) Coagulant by Extracting Aluminum and Iron Ions from High Iron Content Coal Gangue. Materials 2022, 15, 2253. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.A.; Han, X.; Lv, G.; Pan, X.; Agajan, S.; Fu, D.; Liu, J.; Zhang, J. Research on Alumina Preparation from Aluminium Chloride Solution by Electrolysis Process. In Light Metals 2018, Proceedings of the TMS 2018, Phoenix, AZ, USA, 11–15 March 2018; Martin, O., Ed.; Springer International Publishing: Cham, Switzerland, 2018; pp. 97–103. [Google Scholar] [CrossRef]
- Asuha, S.; Talintuya, T.; Han, Y.; Zhao, S. Selective extraction of aluminum from coal-bearing kaolinite by room-temperature mechanochemical method for the preparation of γ-Al2O3 powder. Powder Technol. 2018, 325, 121–125. [Google Scholar] [CrossRef]
- Kong, D.; Jiang, R. Preparation of NaA Zeolite from High Iron and Quartz Contents Coal Gangue by Acid Leaching—Alkali Melting Activation and Hydrothermal Synthesis. Crystals 2021, 11, 1198. [Google Scholar] [CrossRef]
- Liu, S.; Chen, X.; Ai, W.; Wei, C. A new method to prepare mesoporous silica from coal gasification fine slag and its application in methylene blue adsorption. J. Clean. Prod. 2019, 212, 1062–1071. [Google Scholar] [CrossRef]
- Li, C.-C.; Qiao, X.-C.; Yu, J.-G. Large surface area MCM-41 prepared from acid leaching residue of coal gasification slag. Mater. Lett. 2016, 167, 246–249. [Google Scholar] [CrossRef]
- GB/T 212-2008; Proximate Analysis of Coal. China Coal Industry Association: Beijing, China, 2008.


| SiO2 | Al2O3 | K2O | Fe (Fe2O3, Fe3O4, FeO, etc.) | S (CaSO4, MgSO4, etc.) | Na2O | Others | |
|---|---|---|---|---|---|---|---|
| The chemical composition (wt%) of leached residue | 74.29 | 13.44 | 3.31 | 1.34 | 0.524 | 0.492 | 6.604 |
| Al2(SO4)3 | FeSO4 | Fe2(SO4)3 | K2SO4 | Na2SO4 | Others | ||
| The chemical content (g/L) of an acid solution | 0.82 | 0.92 | 0.09 | 0.02 | 0.01 | <0.01 | |
| Substance | Methods | Reagents | Extraction | Application | Ref. |
|---|---|---|---|---|---|
| Acid solution (high-iron CG) | Calcination + acid leaching | HCl | Al: 90% Fe: 91% | Preparation of PAFC flocculants | [61] |
| Acid solution (high-alumina fly ash) | Acid leaching + direct-electricity conversion technology + roasting | HCl | No reported | Preparation of Al2O3 | [62] |
| Aacid solution (coal-bearing kaolinite) | Mechanical grinding + acid leaching | H2SO4 | Al: 100% | Preparation of γ-Al2O3 powder | [63] |
| Leached residue (CG) | Calcination + acid leaching | HCl | No reported | Preparation of NaA zeolite | [64] |
| Leached residue (CGFS) | Calcination + acid leaching | HCl | The total leaching rate of all metal oxides: 80% | Preparation of mesoporous silica | [65] |
| Leached residue (CGS) | Non-hydrothermal sol–gel method | No reported | No reported | Preparation of MCM-41 | [66] |
| Acid solution and leached residue (CG) | High temperature acid leaching | HCl | Al: 92.54% SiO2: 96.01% | Preparation of Al2O3 and SiC | [15] |
| Acid solution and leached residue (CG and CGCS) | Co-roasting + acid leaching | H2SO4 | Al: 43.78% Fe: 79.93% | Preparation of aluminium-iron flocculants and mesoporous silica | This work |
| Substance | SiO2 | Al2O3 | Fe2O3 | K2O | MgO | CaO | Others | LOI | |
|---|---|---|---|---|---|---|---|---|---|
| Chemical composition | Content in CG | 58.02 | 20.87 | 10.67 | 4.41 | 2.17 | 1.34 | 2.52 | 20.10 |
| Content in CGCS | 28.80 | 10.51 | 20.23 | 1.37 | 2.36 | 31.93 | 4.80 | 36.75 | |
| Substance | Moisture | Ash | Volatiles | Fixed carbon | |||||
| Proximate analysis | Content in CG | 1.7 | 85.81 | 9.93 | 2.56 | ||||
| Content in CGCS | 2.15 | 74.88 | 5.78 | 17.19 | |||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, J.; Yu, T.; Zhang, H.; Zhang, Y.; Ma, L.; Li, J.; Qu, C.; Wang, T. Study on Extraction Valuable Metal Elements by Co-Roasting Coal Gangue with Coal Gasification Coarse Slag. Molecules 2024, 29, 130. https://doi.org/10.3390/molecules29010130
Zhao J, Yu T, Zhang H, Zhang Y, Ma L, Li J, Qu C, Wang T. Study on Extraction Valuable Metal Elements by Co-Roasting Coal Gangue with Coal Gasification Coarse Slag. Molecules. 2024; 29(1):130. https://doi.org/10.3390/molecules29010130
Chicago/Turabian StyleZhao, Jincheng, Tao Yu, Huan Zhang, Yu Zhang, Lanting Ma, Jinling Li, Chengtun Qu, and Te Wang. 2024. "Study on Extraction Valuable Metal Elements by Co-Roasting Coal Gangue with Coal Gasification Coarse Slag" Molecules 29, no. 1: 130. https://doi.org/10.3390/molecules29010130
APA StyleZhao, J., Yu, T., Zhang, H., Zhang, Y., Ma, L., Li, J., Qu, C., & Wang, T. (2024). Study on Extraction Valuable Metal Elements by Co-Roasting Coal Gangue with Coal Gasification Coarse Slag. Molecules, 29(1), 130. https://doi.org/10.3390/molecules29010130






