Photocatalytic Degradation of Gaseous Benzene Using Cu/Fe-Doped TiO2 Nanocatalysts under Visible Light
Abstract
:1. Introduction
2. Experimental Part
2.1. Chemicals and Materials
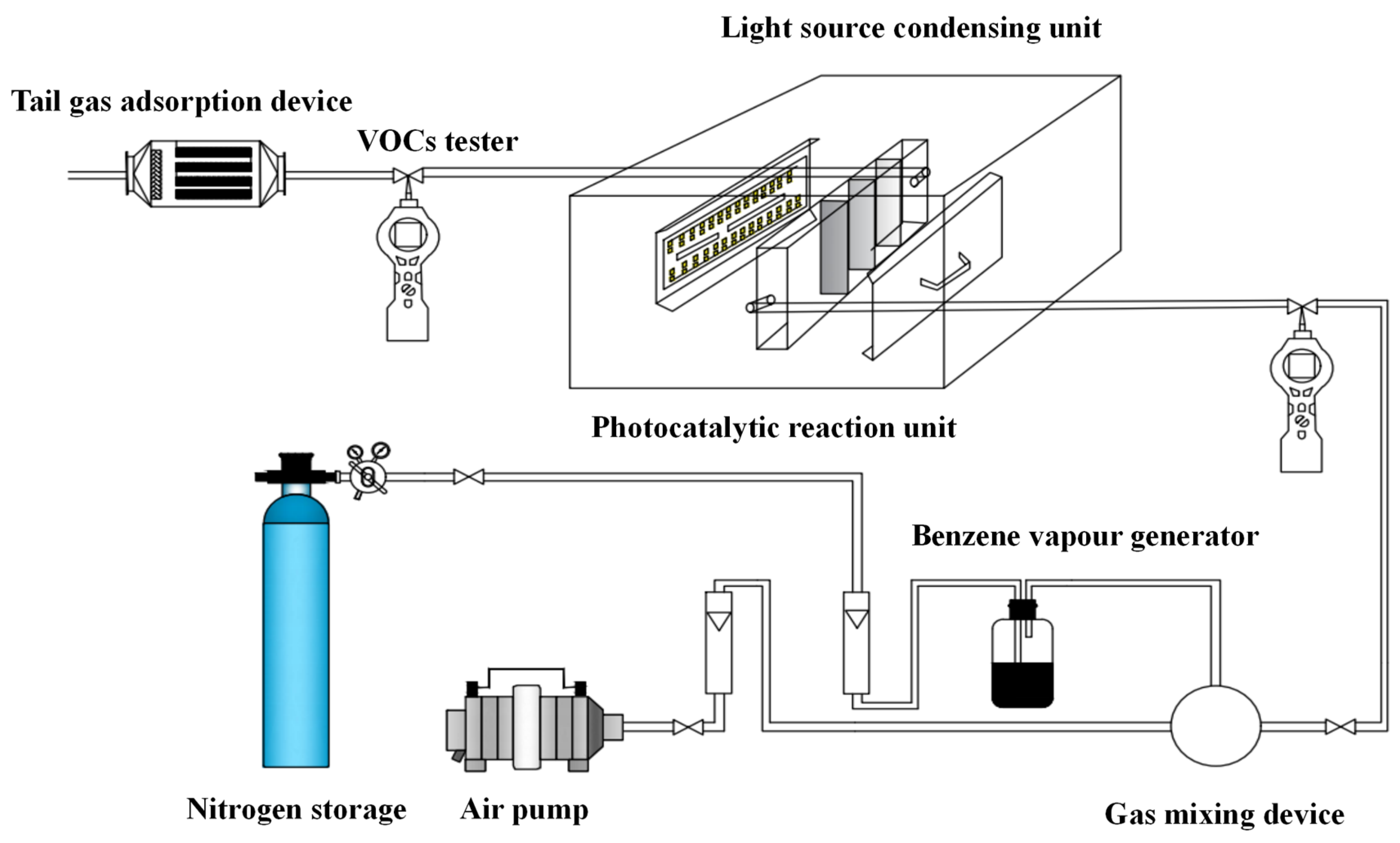
2.2. Photocatalysis Experimental Setups
2.3. Synthesis of Photocatalysts
2.4. Characterization Instrumentation
2.5. Assessment of Photocatalytic Benzene Degradation
3. Results and Discussion
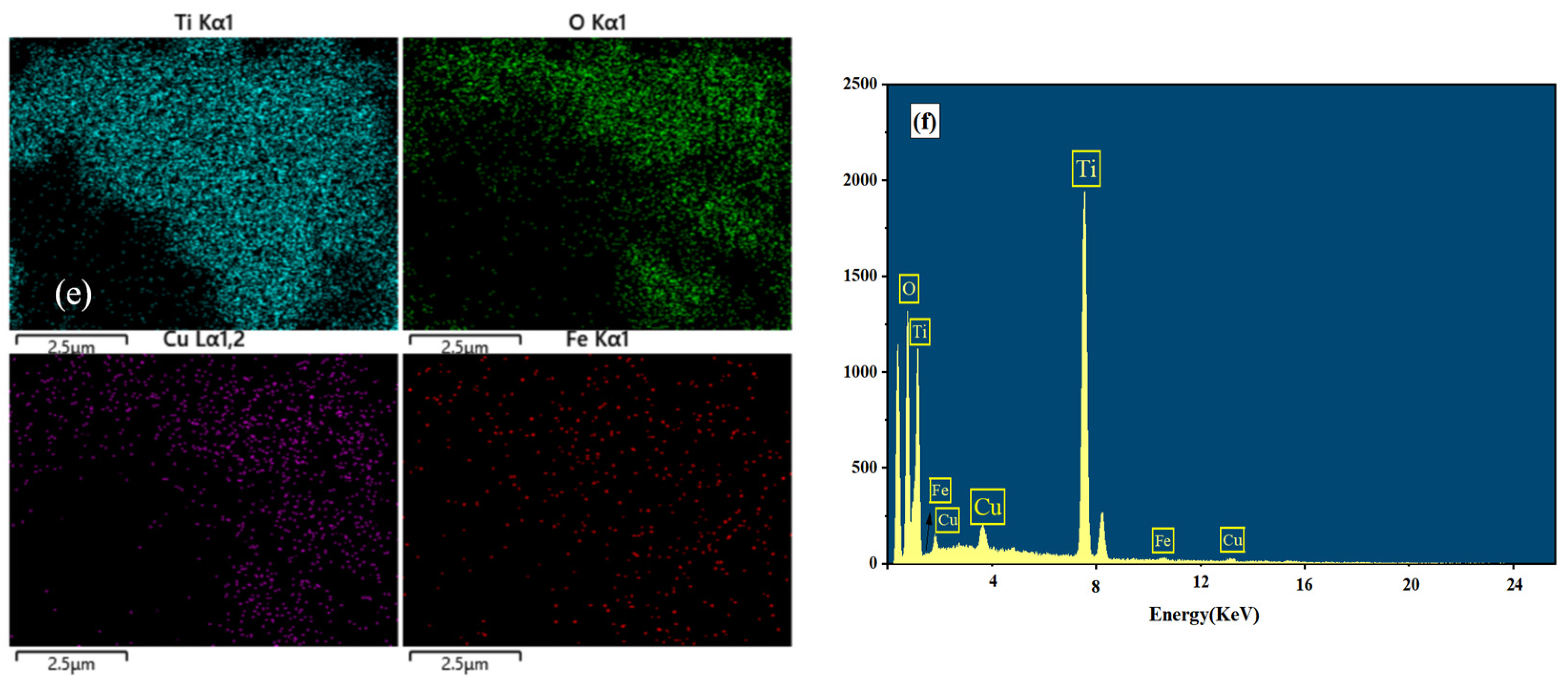
3.1. SEM and EDS Analysis
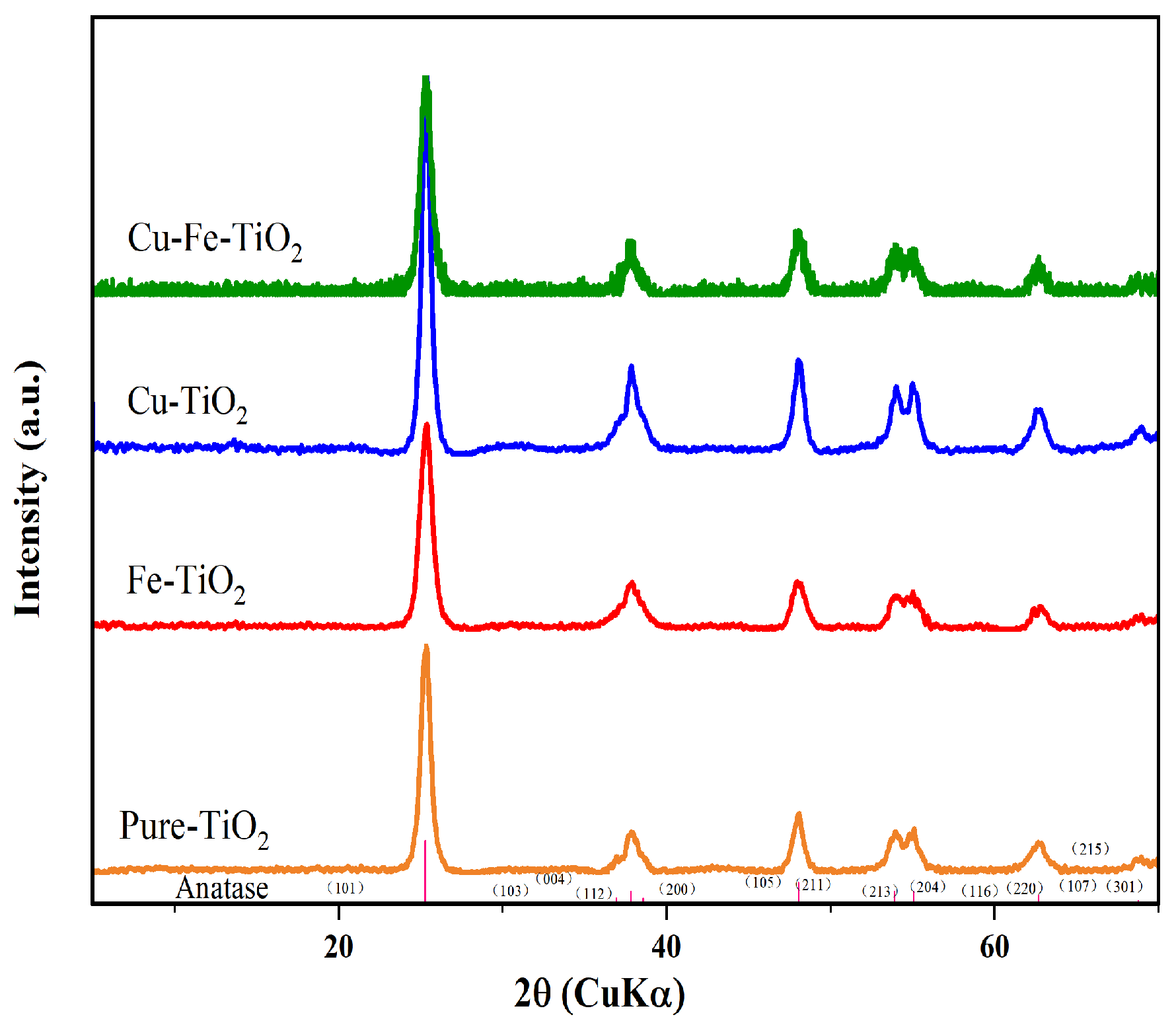
3.2. XRD and BET Analysis
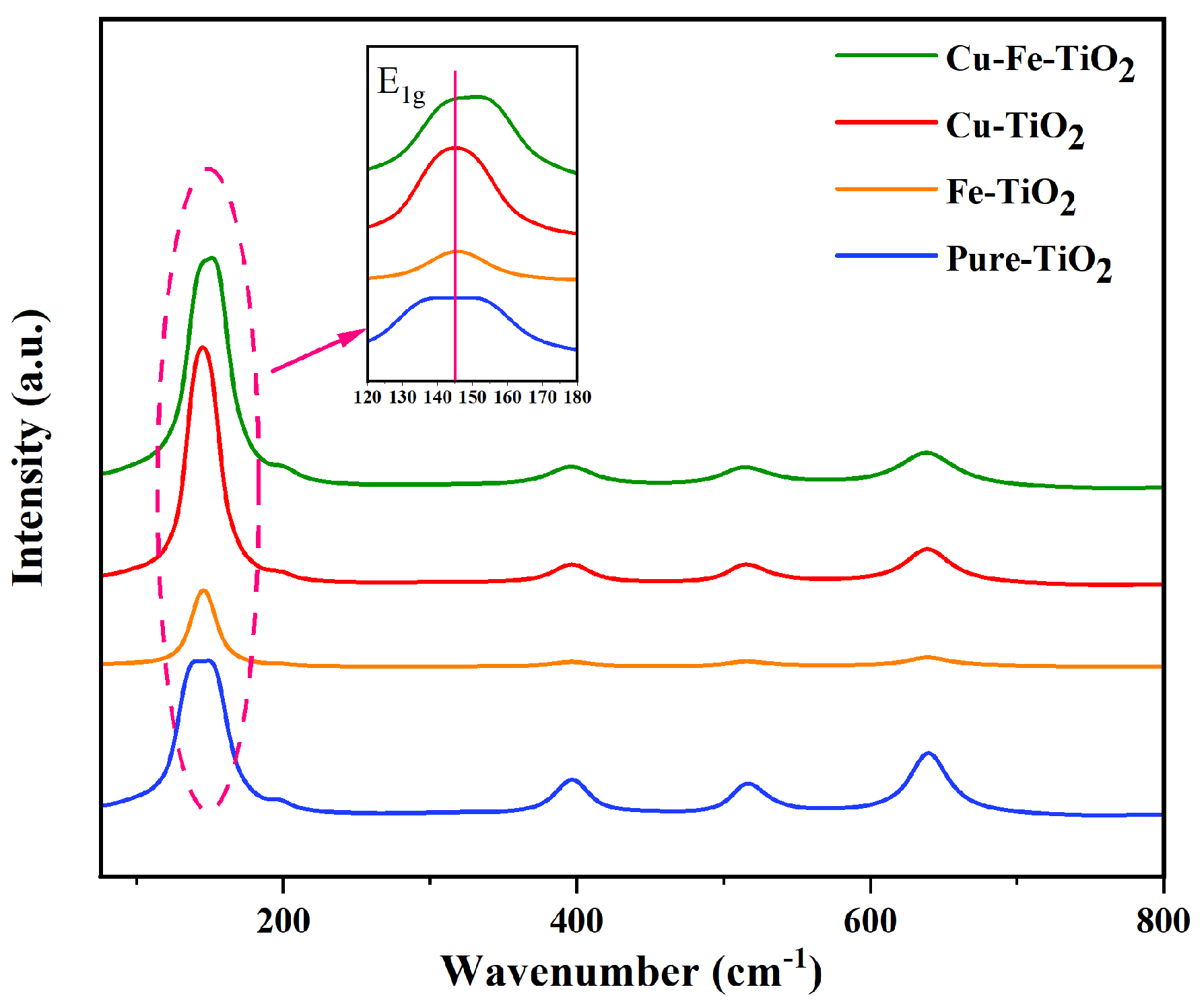
3.3. Raman Analysis
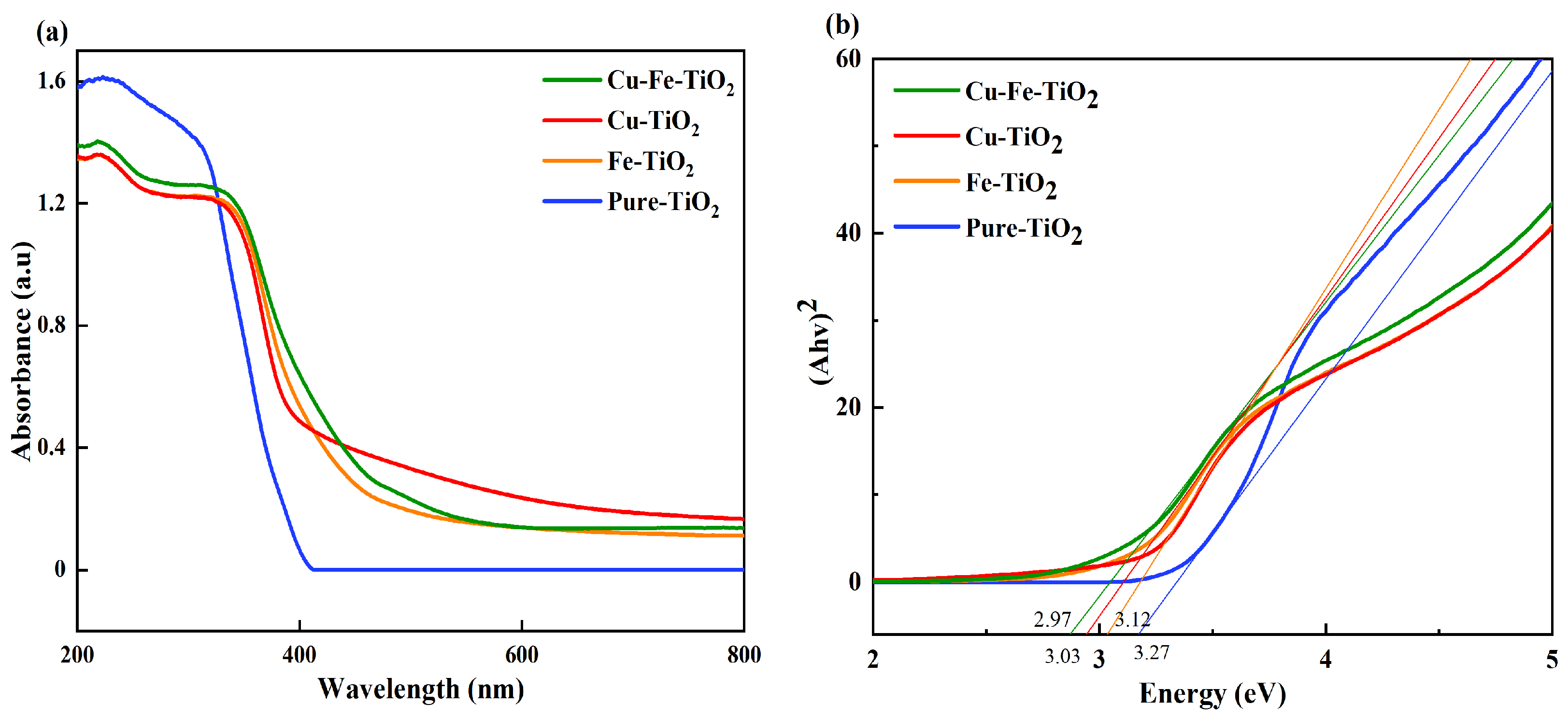
3.4. UV-Vis-DRS Analysis
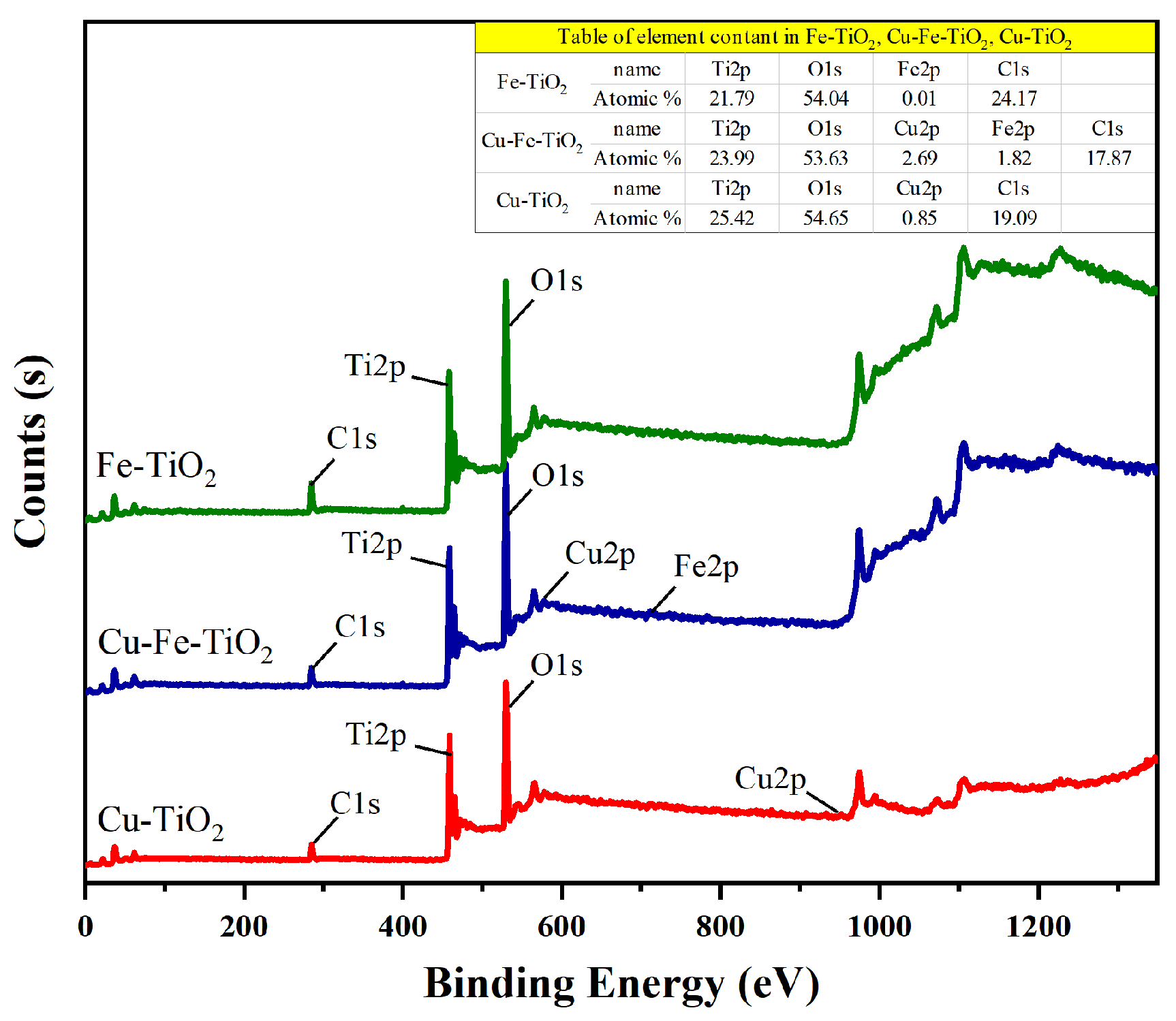
3.5. XPS Analysis
3.6. Catalytic Activity
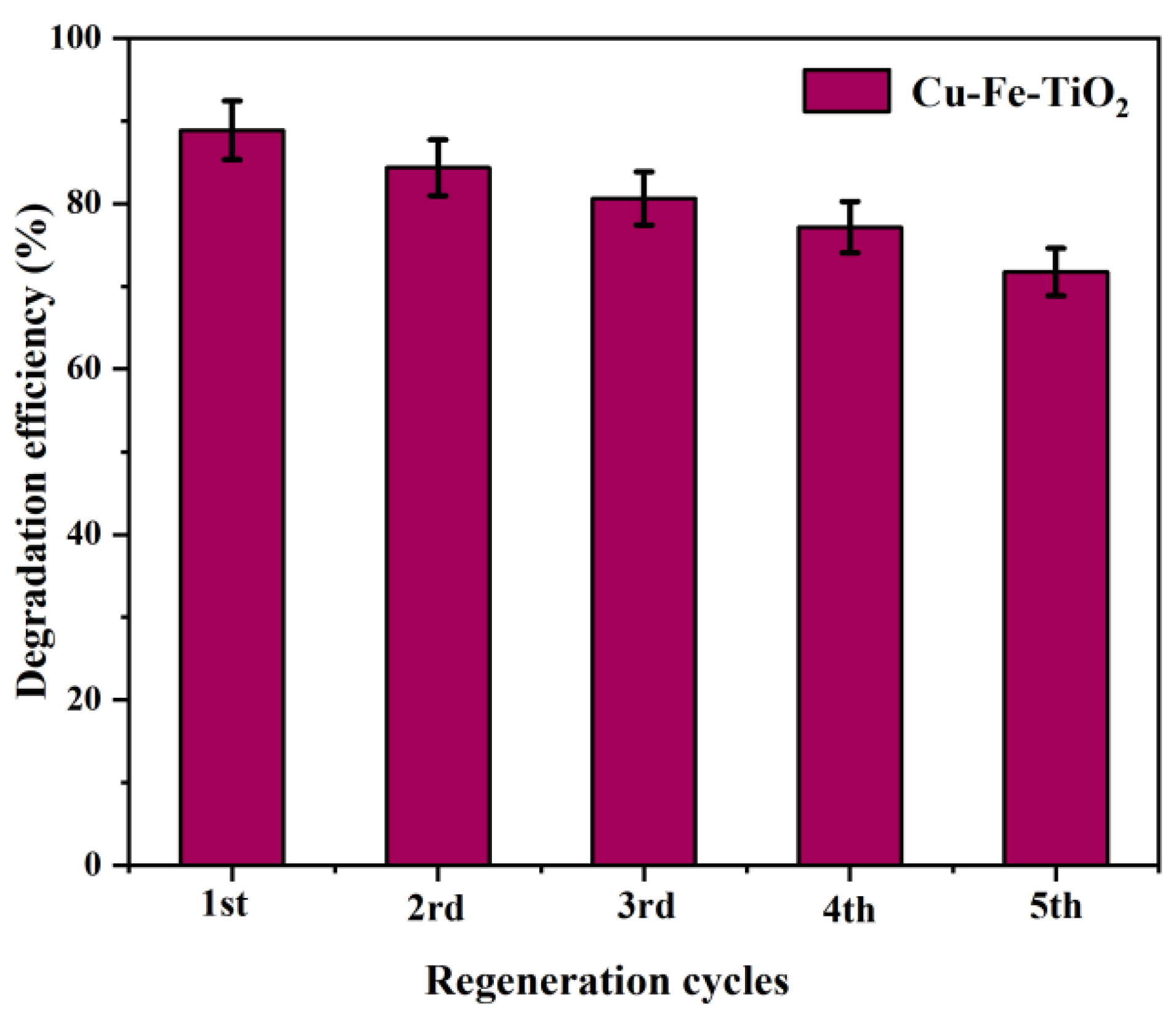
3.7. Reusability Tests
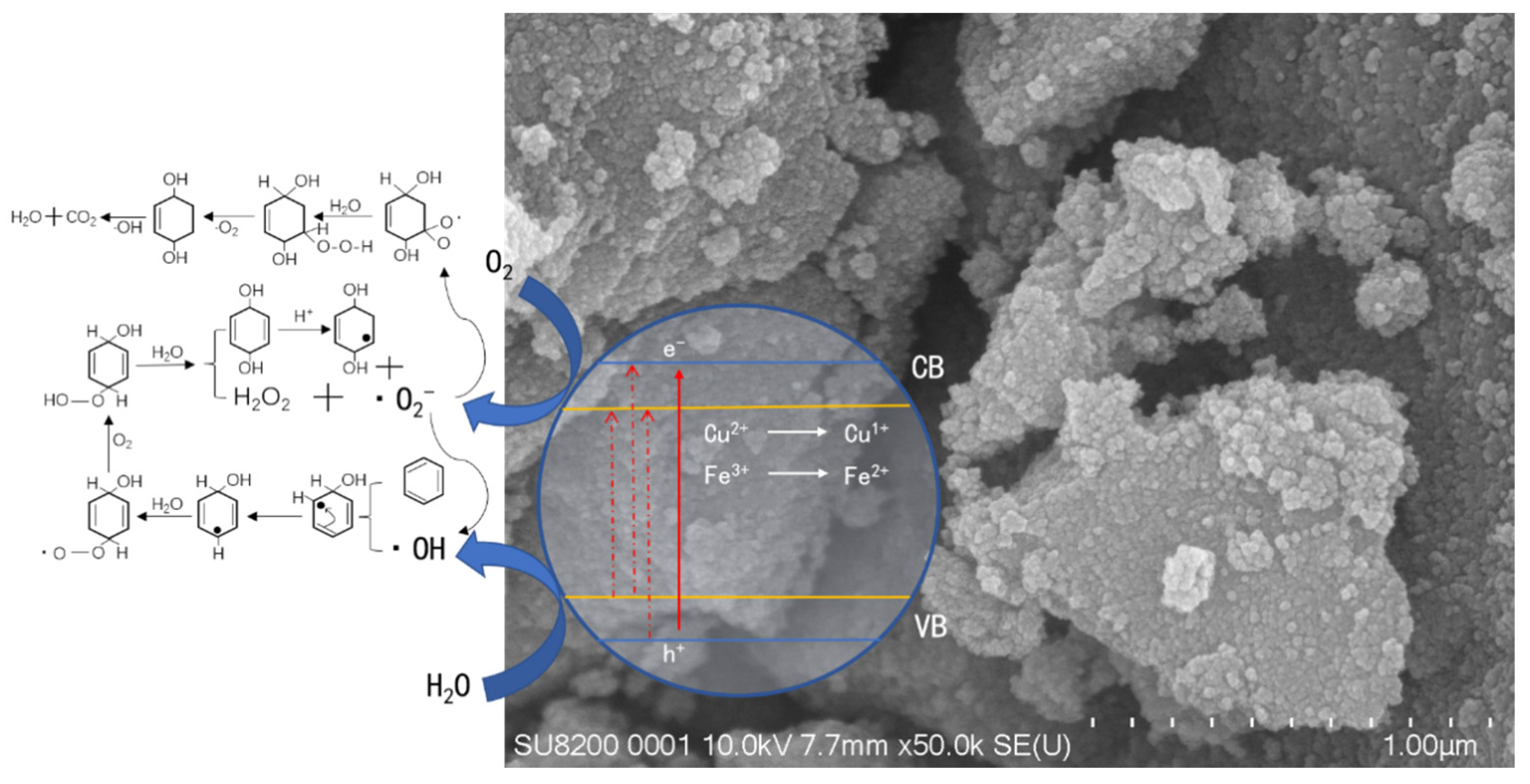
3.8. Reaction Mechanism
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Guo, Y.L.; Wen, M.C.; Li, G.Y.; An, T.C. Recent advances in VOC elimination by catalytic oxidation technology onto various nanoparticles catalysts: A critical review. Appl. Catal. B 2020, 281, 119447. [Google Scholar] [CrossRef]
- Li, X.Q.; Zhang, L.; Yang, Z.Q.; Wang, P.; Yan, Y.F.; Ran, J.Y. Adsorption materials for volatile organic compounds (VOCs) and the key factors for VOCs adsorption process: A review. Sep. Purif. Technol. 2020, 235, 116213. [Google Scholar] [CrossRef]
- Zhu, L.; Shen, D.; Luo, K.H. A critical review on VOCs adsorption by different porous materials: Species, mechanisms and modification methods. J. Hazard. Mater. 2020, 389, 122102. [Google Scholar] [CrossRef] [PubMed]
- Shayegan, Z.; Lee, C.S.; Haghighat, F. TiO2 photocatalyst for removal of volatile organic compounds in gas phase—A review. Chem. Eng. J. 2018, 334, 2408–2439. [Google Scholar] [CrossRef]
- Patil, S.B.; Shivaraj, B.P.; Nagaraju, G.; Kakarla, R.R. Recent progress in metal-doped TiO2 non-metal doped/codoped TiO2 and TiO2 nanostructured hybrids for enhanced photocatalysis. Int. J. Hydrogen Energy 2019, 45, 7764–7778. [Google Scholar]
- Dagmawi, M.D.; Liao, Z.Y. Photocatalytic degradation of volatile organic compounds using nanocomposite of P-type and N-type transition metal semiconductors. J. Sol-Gel Sci. Technol. 2021, 98, 605–614. [Google Scholar]
- Liu, H.L.; Ma, Y.P.; Chen, J.Y.; Wen, M.C.; Li, G.Y.; An, T.C. Highly efficient visible-light-driven photocatalytic degradation of VOCs by CO2 assisted synthesized mesoporous carbon confined mixed-phase TiO2 nanocomposites derived from MOFs. Appl. Catal. B 2019, 250, 337–346. [Google Scholar] [CrossRef]
- Xu, J.J.; Chen, Y.F.; Dong, Z.Y.; Peng, Y.N.; Situ, Y.; Huang, H. Strong effect of multi-electron oxygen reduction reaction on photocatalysis through the promotion of interfacial charge transfer. Appl. Catal. B Environ. 2019, 252, 41–46. [Google Scholar] [CrossRef]
- Bai, W.L.; Pakdel, E.; Wang, Q.M.; Tang, B.; Wang, J.F.; Chen, Z.Q.; Zhang, Y.H.; Hurren, C.; Wang, X.G. Synergetic adsorption-photocatalysis process of titania-silica photocatalysts and their immobilization on PEEK nonwoven filter for VOC removal. J. Environ. Chem. Eng. 2022, 10, 108920. [Google Scholar] [CrossRef]
- Mina, S.; Habibi, Y.J.; Chand, H.S.; Venkata, K. Activation of persulfate by novel TiO2/FeOCl photocatalyst under visible light: Facile synthesis and high photocatalytic performance. Sep. Purif. Technol. 2020, 250, 117268. [Google Scholar]
- Nasrin, S.; Aziz, H.Y.; Mahsa, P.; Soheila, A.K.; Srabanti, G. Integration of BiOI and Ag3PO4 nanoparticles onto oxygen vacancy rich-TiO2 for efficient visible-light photocatalytic decontaminations. J. Photochem. Photobiol. A Chem. 2020, 400, 112659. [Google Scholar]
- Song, G.X.; Gao, R.; Zhao, Z.; Zhang, Y.J.; Tan, H.Q.; Li, H.B.; Wang, D.D.; Sun, Z.C.; Feng, M. High-spin state Fe(III) doped TiO2 for electrocatalytic nitrogen fixation induced by surface F modification. Appl. Catal. B Environ. 2022, 301, 120809. [Google Scholar] [CrossRef]
- Jia, M.Y.; Yang, Z.H.; Xu, H.Y.; Song, P.P.; Xiong, W.P.; Cao, J.; Zhang, Y.R.; Xiang, Y.P.; Hu, J.H.; Zhou, C.Y.; et al. Integrating N and F co-doped TiO2 nanotubes with ZIF-8 as photoelectrode for enhanced photo-electrocatalytic degradation of sulfamethazine. Chem. Eng. J. 2020, 388, 124388. [Google Scholar] [CrossRef]
- Jo, W.K.; Kim, J.T. Application of visible-light photocatalysis with nitrogen-doped or unmodified titanium dioxide for control of indoor-level volatile organic compounds. J. Hazard. Mater. 2009, 164, 360–366. [Google Scholar] [CrossRef] [PubMed]
- Jo, W.K.; Kang, H.J. Aluminum sheet-based S-doped TiO2 for photocatalytic decomposition of toxic organic vapors. Chin. J. Catal. 2014, 35, 1189–1195. [Google Scholar] [CrossRef]
- Thalgaspitiya, W.R.K.; Kapuge, T.K.; He, J.K.; Deljoo, B.; Meguerdichian, A.G.; Aindow, M.; Suib, S.L. Multifunctional transition metal doped titanium dioxide reduced graphene oxide composites as highly efficient adsorbents and photocatalysts. Microporous Mesoporous Mater. 2020, 307, 110521. [Google Scholar] [CrossRef]
- Maria, S.; Robina, N.; Jehangir, K.; Muhammad, Z.; Riaz, U.; Rizwan, K.; Sumaira, N.; Hesham, S.A.; Sulaiman, A.A. Metal doped titania nanoparticles as efficient photocatalyst for dyes degradation. J. King Saud Univ.-Sci. 2021, 33, 101312. [Google Scholar]
- Arora, I.; Chawla, H.; Chandra, A.; Sagadevan, S.; Garg, S. Advances in the strategies for enhancing the photocatalytic activity of TiO2: Conversion from UV-light active to visible-light active photocatalyst. Inorg. Chem. Commun. 2022, 143, 109700. [Google Scholar] [CrossRef]
- Naghibi, S.; Gharagozlou, M. Solvothermal synthesis of M-doped TiO2 nanoparticles for sonocatalysis of methylene blue and methyl orange (M = Cd, Ag, Fe, Ce, and Cu). J. Chin. Chem. Soc. 2017, 64, 640–650. [Google Scholar] [CrossRef]
- Li, J.P.; Ren, D.J.; Wu, Z.X.; Xu, J.; Bao, Y.J.; He, S.; Chen, Y.H. Flame retardant and visible light-activated Fe-doped TiO2 thin films anchored to wood surfaces for the photocatalytic degradation of gaseous formaldehyde. J. Colloid Interface Sci. 2018, 530, 78–87. [Google Scholar] [CrossRef]
- Moretti, E.; Cattaruzza, E.; Flora, C.; Talon, A.; Casini, E.; Vomiero, A. Photocatalytic performance of Cu-doped titania thin films under UV light irradiation. Appl. Surf. Sci. 2021, 553, 149535. [Google Scholar] [CrossRef]
- Ambrozova, N.; Reli, M.; Sihor, M.; Kustrowski, P.; Wu, J.C.S.; Koci, K. Copper and platinum doped titania for photocatalytic reduction of carbon dioxide. Appl. Surf. Sci. 2018, 430, 475–487. [Google Scholar] [CrossRef]
- Saeid, M.P.; Radhakrishnan, K.; Tan, H.R.; Yi, R.; Wong, T.I.; Dalapati, G.K. Titanium doped cupric oxide for photovoltaic application. Sol. Energy Mater. Sol. Cells 2015, 140, 266–274. [Google Scholar]
- Cosma, D.; Urda, A.; Radu, T.; Rosu, M.C.; Mihet, M.; Socaci, C. Evaluation of the Photocatalytic Properties of Copper Oxides/Graphene/TiO2 Nanoparticles Composites. Molecules 2022, 27, 5803. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Ran, M.J.; Zhou, Z.Y.; Han, X.Y.; Zhu, H.L.; Gu, J.C. Lanthanum/titanium dioxide immobilized onto industrial waste with enhanced photocatalytic activity, and the degradation of dimethyl phthalate. J. Clean. Prod. 2021, 321, 129014. [Google Scholar] [CrossRef]
- Yan, X.Q.; Xue, C.; Yang, B.L.; Yang, G.D. Novel three-dimensionally ordered macroporous Fe3+-doped TiO2 photocatalysts for H2 production and degradation applications. Appl. Surf. Sci. 2017, 394, 248–257. [Google Scholar] [CrossRef]
- Jiang, Z.C.; Long, Q.; Cheng, B.; He, R.A.; Wang, L.X. 3D ordered macroporous sulfur-doped g-C3N4/TiO2 S-scheme photocatalysts for efficient H2O2 production in pure water. J. Mater. Sci. Technol. 2023, 162, 1–10. [Google Scholar] [CrossRef]
- Reda, M.M.; Delft, W.B.; Amal, S.B.; Razan, H.Q. Photo-catalytic destruction of acetaldehyde using cobalt, copper co-doped titania dioxide nanoparticles beneath Visible light. Appl. Nanosci. 2019, 10, 931–939. [Google Scholar]
- Dai, X.N.; Liu, Z.J.; Wang, J.; Zhang, K.; Ma, D.D.; Chen, W.; Zheng, H.L. Bimetallic CuMo@TiO2 activating peroxymonosulfate for micropollutants degradation: Synergy and mechanism. J. Environ. Chem. Eng. 2023, 11, 109304. [Google Scholar] [CrossRef]
- Zhang, M.; Li, W.M.; Wu, X.F.; Zhao, F.; Wang, D.D.; Zha, X.C.; Li, S.D.; Liu, H.D.; Chen, Y.F. Low-temperature catalytic oxidation of benzene over nanocrystalline Cu–Mn composite oxides by facile sol–gel synthesis. New J. Chem. 2020, 44, 2442–2451. [Google Scholar] [CrossRef]
- Tehmina, A.; Habib, N.; Effat, S.; Syeda, A.B.B.; Sharif, U.; Rana, M.A.I. Efficient photocatalytic degradation of nitrobenzene by copper-doped TiO2: Kinetic study, degradation pathway, and mechanism. Environ. Sci. Pollut. Res. 2022, 29, 49925–49936. [Google Scholar]
- Chen, F.L.; Tian, L.J.; Liu, B.K.; Sun, Y.; Ge, S.J.; Hou, J. Investigation on the gaseous benzene removed by photocatalysis employing TiO2 modified with cobalt and iodine as photocatalysts under visible light. Environ. Technol. 2022, 43, 2990–2999. [Google Scholar] [CrossRef]
- Zhang, X.Y.; Chen, Y.N.; Shang, Q.K.; Guo, Y.N. Copper doping and organic sensitization enhance photocatalytic activity of titanium dioxide: Efficient degradation of phenol and tetrabromobisphenol A. Sci. Total Environ. 2020, 716, 137144. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.L.; Bielan, Z.; Endo-Kimura, M.; Janczarek, M.; Zhang, D.; Kowalski, D.; Zieliska-Jurek, A.; Markowska-Szczupak, A.; Ohtani, B.; Kowalska, E. On the mechanism of photocatalytic reactions on CuxO@TiO2 core-shell photocatalysts. J. Mater. Chem. A 2021, 9, 10135–10145. [Google Scholar] [CrossRef]
- Gu, X.L.; Wang, Y.F.; Zhang, T.; Liu, D.T.; Zhang, R.; Zhang, P.; Wu, J.; Chen, D.Z.; Li, S.B. Enhanced electronic transport in Fe3+-doped TiO2 for high efficiency perovskite solar cells. J. Mater. Chem. C 2017, 5, 10754–10760. [Google Scholar] [CrossRef]
- Kaveh, K.; Mansour, K.; Morteza, S.; Sayed, J.R. Enhancing the photocatalytic oxidation of dibenzothiophene using visible light responsive Fe and N co-doped TiO2 nanoparticles. Ceram. Int. 2017, 43, 973–981. [Google Scholar]
- Elena, C.; Luminita, P.; Irina, A.; Ioana, J.; Elena, M.A.; Veronica, B.; Catalina, G.; Crina, A.; Adriana, R.; Valentin, R.; et al. Fe3+-doped TiO2 nanopowders for photocatalytic mineralization of oxalic acid under solar light irradiation. J. Photochem. Photobiol. A Chem. 2018, 356, 18–28. [Google Scholar]
- Daiane, K.F.; Karina, R.D.F.; Carla, W.S. Ionic liquid/TiO2 nanoparticles doped with non-expensive metals: New active catalyst for phenol photodegradation. ASC Adv. 2022, 12, 2473–2484. [Google Scholar]
- Ma, Y.W.; Lu, Y.F.; Hai, G.T.; Dong, W.J.; Li, R.J.; Liu, J.H.; Wang, G. Bidentate carboxylate linked TiO2 with NH2-MIL-101(Fe) photocatalyst: A conjugation effect platform for high photocatalytic activity under visible light irradiation. Sci. Bull. 2020, 65, 658–669. [Google Scholar] [CrossRef]
- Zhang, Y.; Fan, W.; Du, H.Q.; Zhao, Y.W.; Song, R.G.; Xiang, N. Microstructure and photocatalytic property of TiO2 and Fe3+: TiO2 films produced by micro-arc oxidation. Surf. Coat. Technol. 2017, 315, 196–204. [Google Scholar] [CrossRef]
- Huang, M.; Zhang, S.Y.; Wu, B.; Wei, Y.; Yu, X.; Gan, Y.P.; Lin, T.J.; Yu, F.; Sun, F.F.; Jiang, Z.; et al. Selective Photocatalytic Oxidation of Methane to Oxygenates over Cu-W-TiO2 with Significant Carrier Traps. ACS Catal. 2022, 12, 9515–9525. [Google Scholar] [CrossRef]
- Wang, X.X.; Ni, Q.; Zeng, D.W.; Liao, G.G.; Wen, Y.W.; Shan, B.; Xie, C.S. BiOCl/TiO2 heterojunction network with high energy facet exposed for highly efficient photocatalytic degradation of benzene. Appl. Surf. Sci. 2017, 396, 590–598. [Google Scholar] [CrossRef]
- Cao, H.; Liu, F.Y.; Tai, Y.T.; Wang, W.; Li, X.Y.; Li, P.Y.; Zhao, H.Z.; Xia, Y.Q.; Wang, S.J. Promoting photocatalytic performance of TiO2 nanomaterials by structural and electronic modulation. Chem. Eng. J. 2023, 466, 143219. [Google Scholar] [CrossRef]
- Zhang, H.B.; Wang, Y.; Zuo, S.W.; Zhou, W.; Zhang, J.; Wen, X.; Lou, D. Isolated Cobalt Centers on W18O49Nanowires Perform as a ReactionSwitch for Efficient CO2 Photoreduction. J. Am. Chem. Soc. 2021, 143, 2173–2177. [Google Scholar] [CrossRef] [PubMed]



| Sample | Pore Size (nm) | Lattice Distortion/ε | BET Surface Area (m2/g) |
|---|---|---|---|
| Pure-TiO2 | 43.0 | 0.246 | 32.73 |
| Cu-TiO2 | 18.6 | 0.324 | 83.25 |
| Fe-TiO2 | 20.9 | 0.358 | 62.47 |
| Cu-Fe-TiO2 | 16.4 | 0.625 | 140.71 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tian, T.; Zhang, J.; Tian, L.; Ge, S.; Zhai, Z. Photocatalytic Degradation of Gaseous Benzene Using Cu/Fe-Doped TiO2 Nanocatalysts under Visible Light. Molecules 2024, 29, 144. https://doi.org/10.3390/molecules29010144
Tian T, Zhang J, Tian L, Ge S, Zhai Z. Photocatalytic Degradation of Gaseous Benzene Using Cu/Fe-Doped TiO2 Nanocatalysts under Visible Light. Molecules. 2024; 29(1):144. https://doi.org/10.3390/molecules29010144
Chicago/Turabian StyleTian, Tao, Jie Zhang, Lijiang Tian, Sijie Ge, and Zhenyu Zhai. 2024. "Photocatalytic Degradation of Gaseous Benzene Using Cu/Fe-Doped TiO2 Nanocatalysts under Visible Light" Molecules 29, no. 1: 144. https://doi.org/10.3390/molecules29010144
APA StyleTian, T., Zhang, J., Tian, L., Ge, S., & Zhai, Z. (2024). Photocatalytic Degradation of Gaseous Benzene Using Cu/Fe-Doped TiO2 Nanocatalysts under Visible Light. Molecules, 29(1), 144. https://doi.org/10.3390/molecules29010144





