Polysaccharides from Brasenia schreberi with Great Antioxidant Ability and the Potential Application in Yogurt
Abstract
:1. Introduction
2. Results and Discussions
2.1. Chemical Properties of Polysaccharides Extracted from Different Conditions
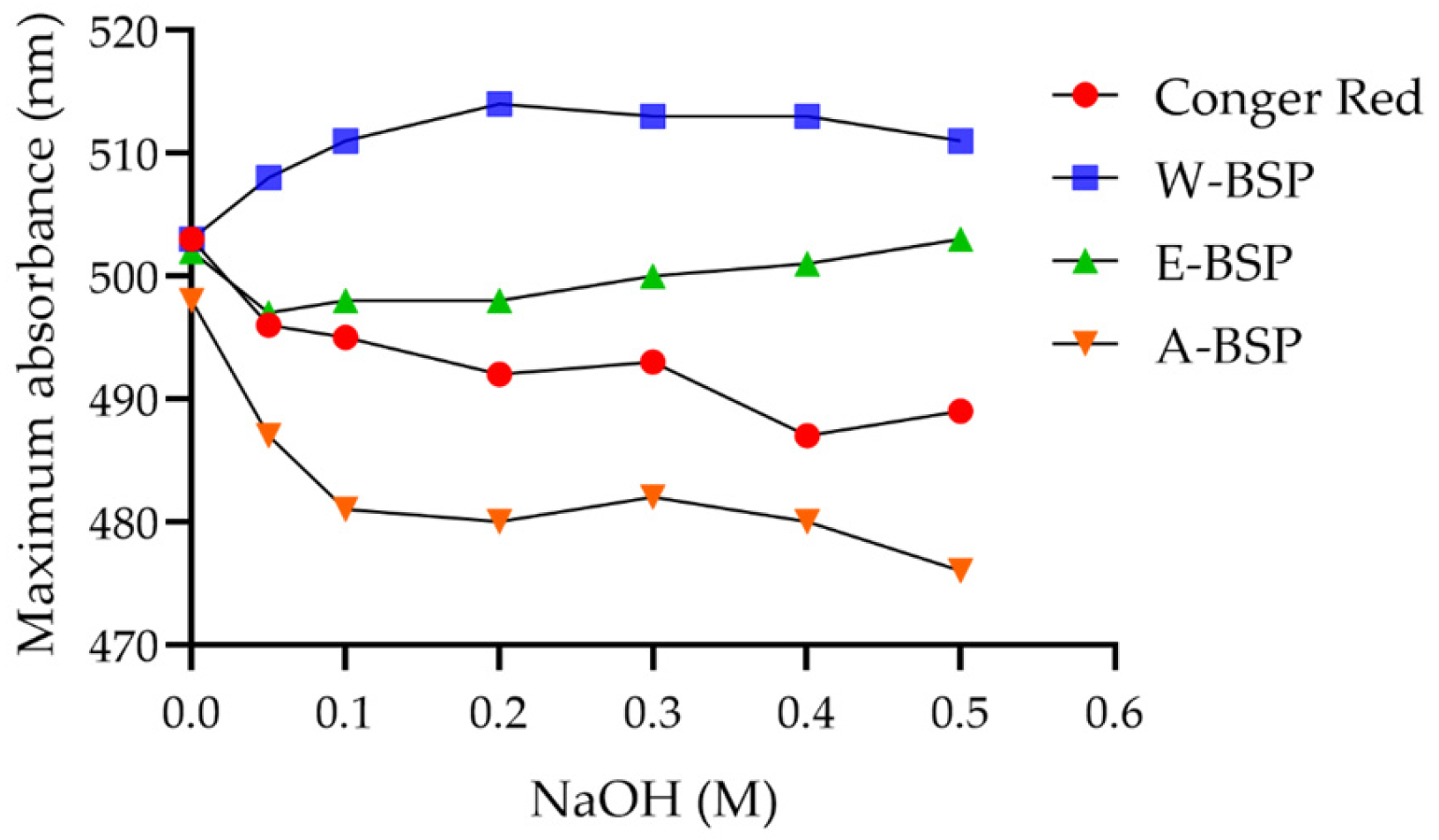
2.2. Triple Helix Structure of Different Polysaccharides
2.3. The Antioxidant Capacity of Different Polysaccharides
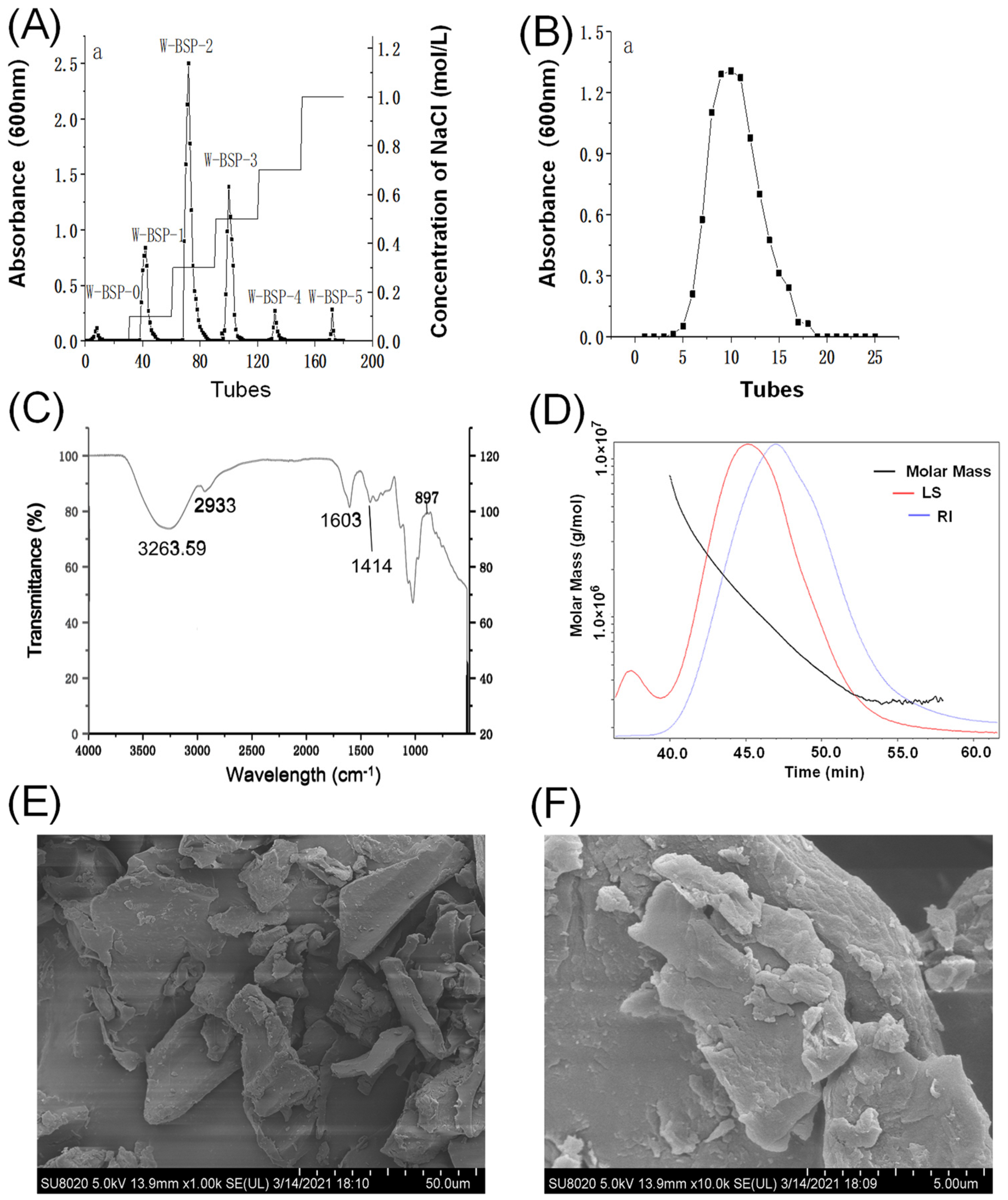
2.4. Characteristics of Polysaccharides
2.5. Optimization of Enzymatic Hydrolysis
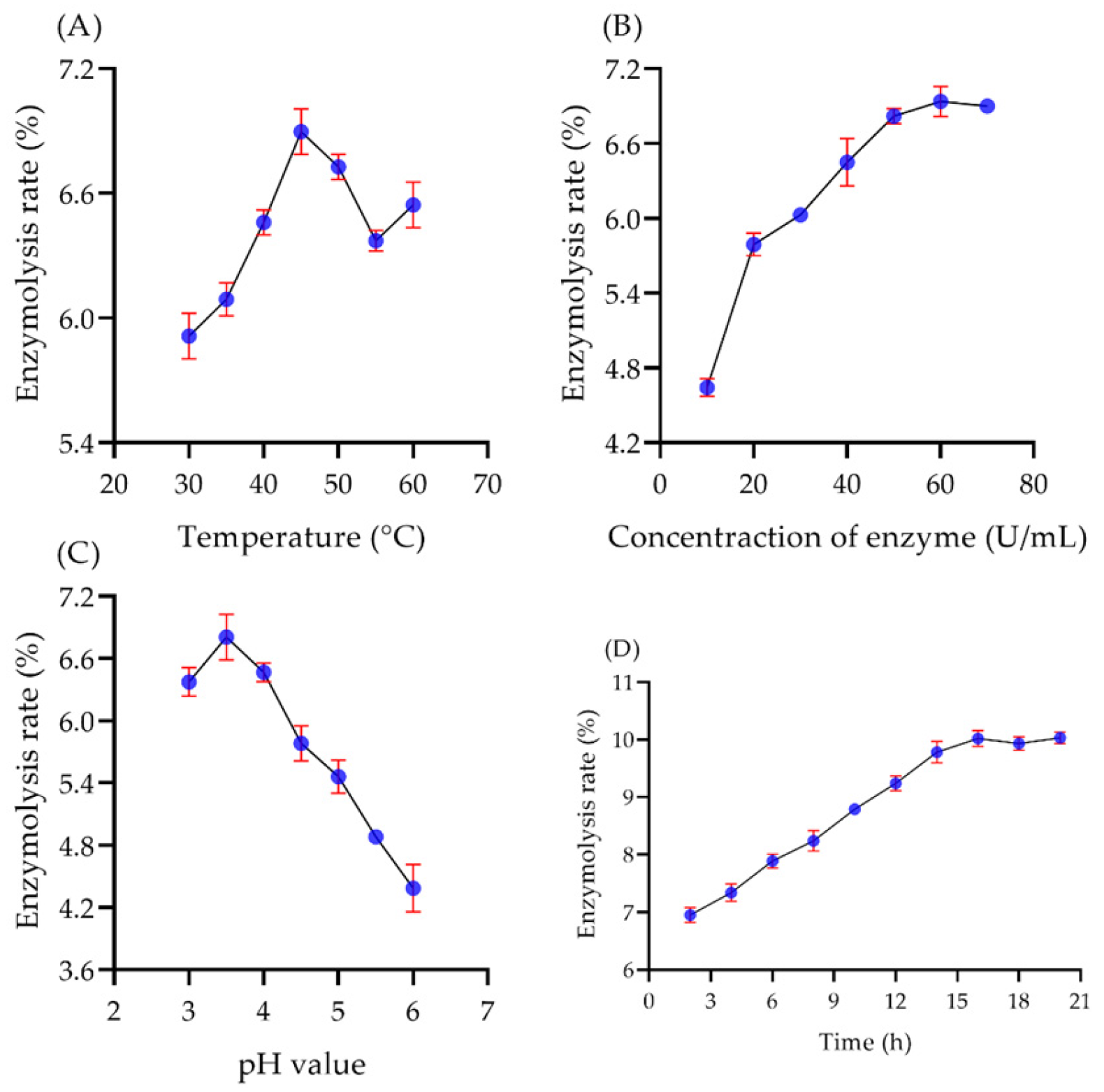
2.5.1. The Effect of Single Factors on Enzymatic Hydrolysis
2.5.2. RSM Analysis
2.6. The Antioxidant Ability of Polysaccharides In Vitro and In Vivo
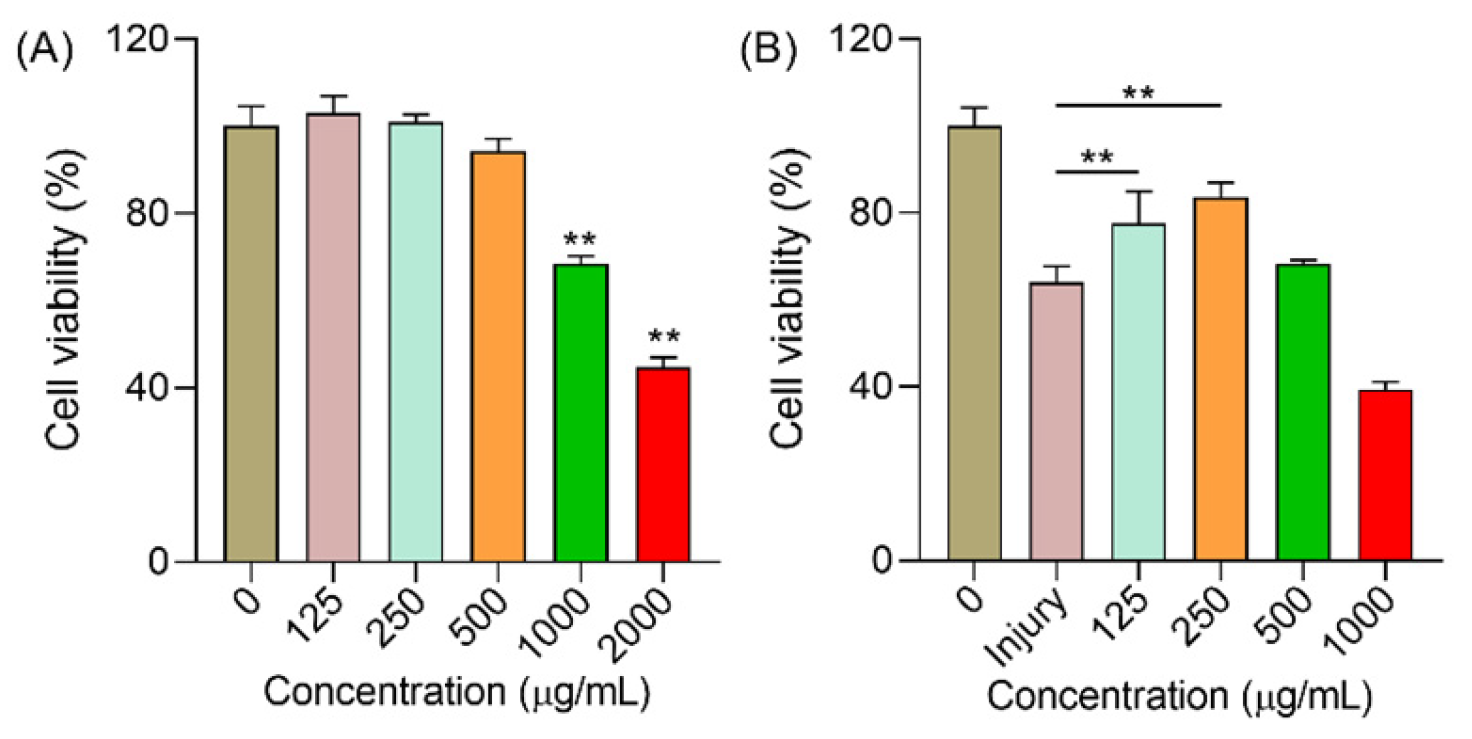
2.6.1. Polysaccharides Increased the Cell Viability under H2O2
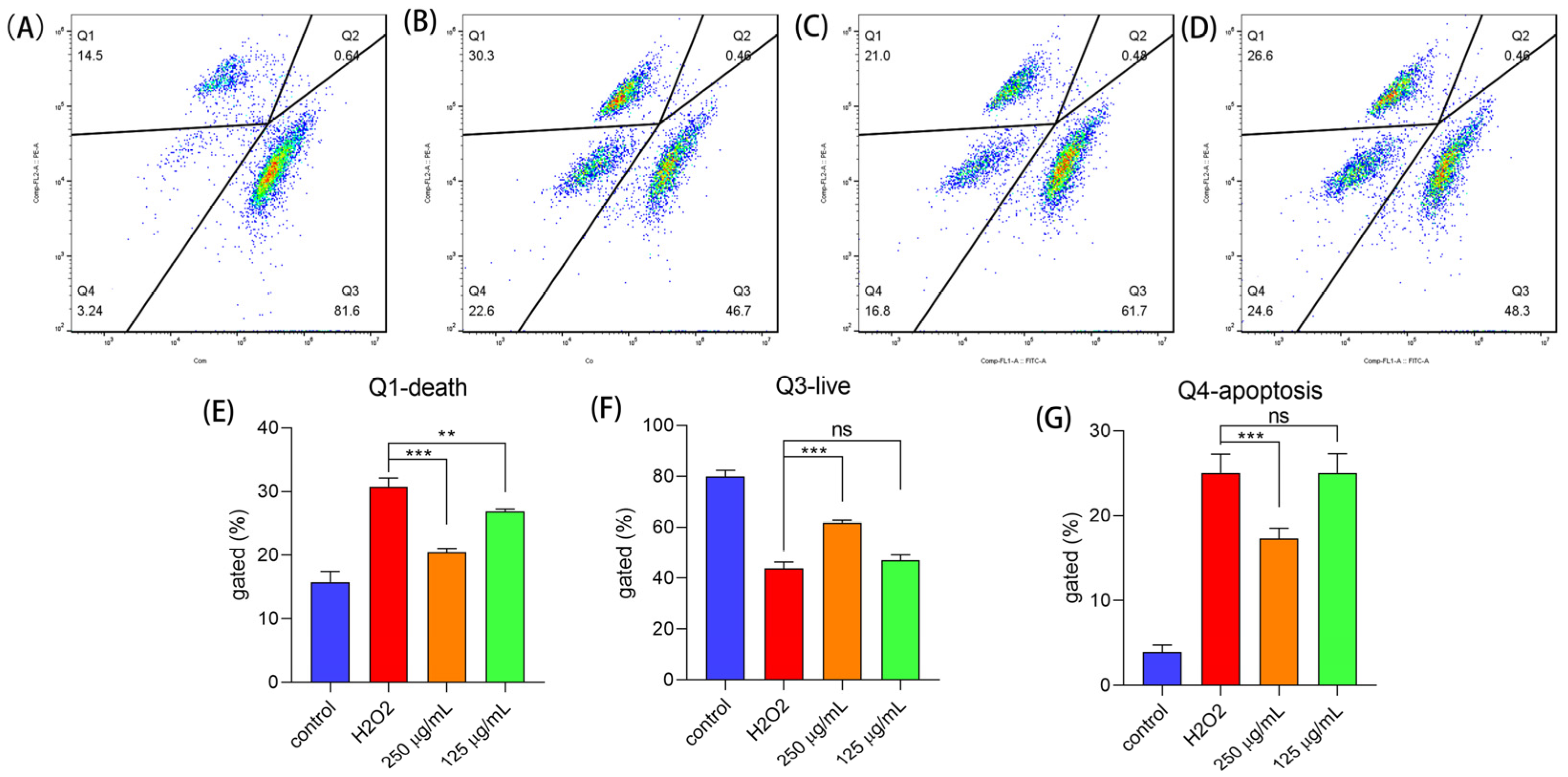
2.6.2. Polysaccharides Reduced Cell Apoptosis under H2O2
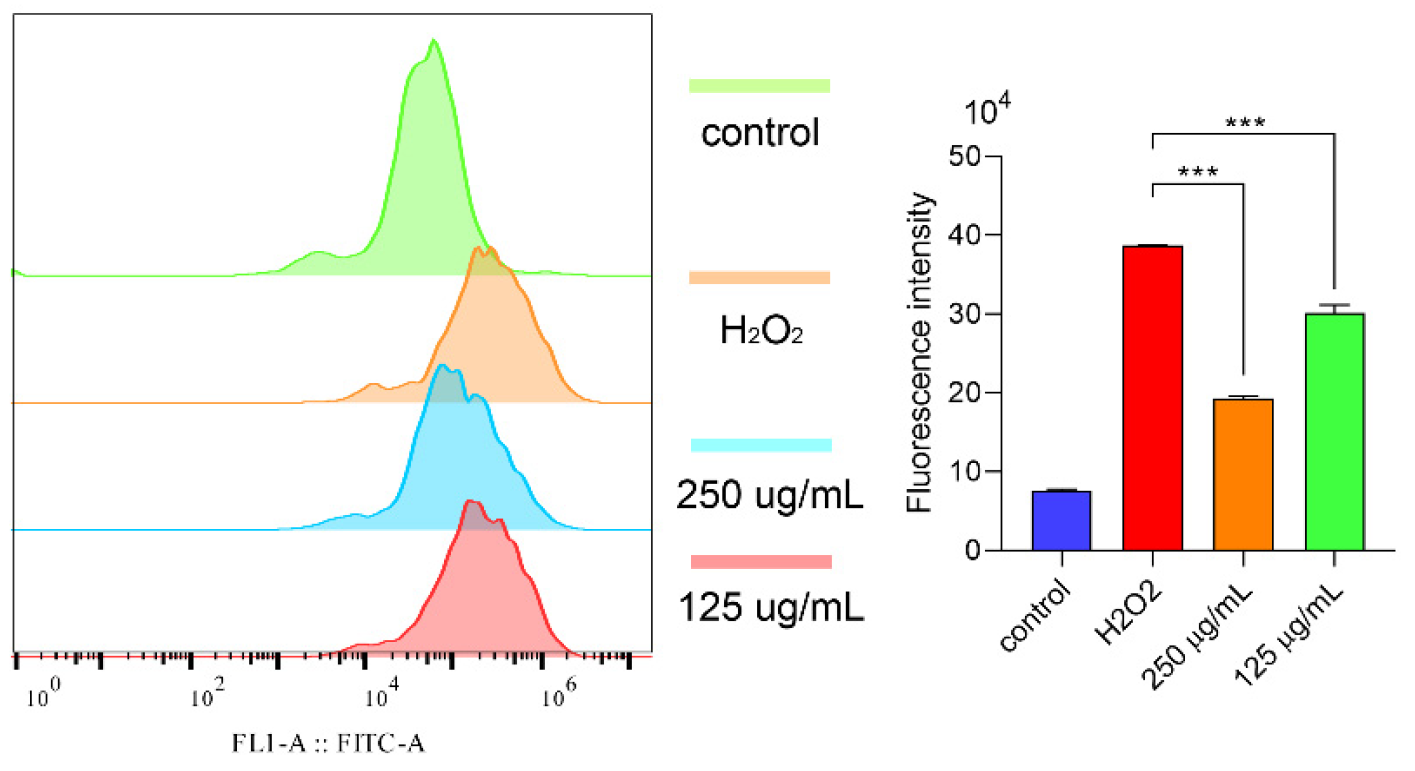
2.6.3. Polysaccharides Reduced ROS under H2O2
2.6.4. Polysaccharides Increased C. elegans’ Survival under Thermal Stress
2.6.5. Polysaccharides Reduced the ROS Level under Thermal Stress
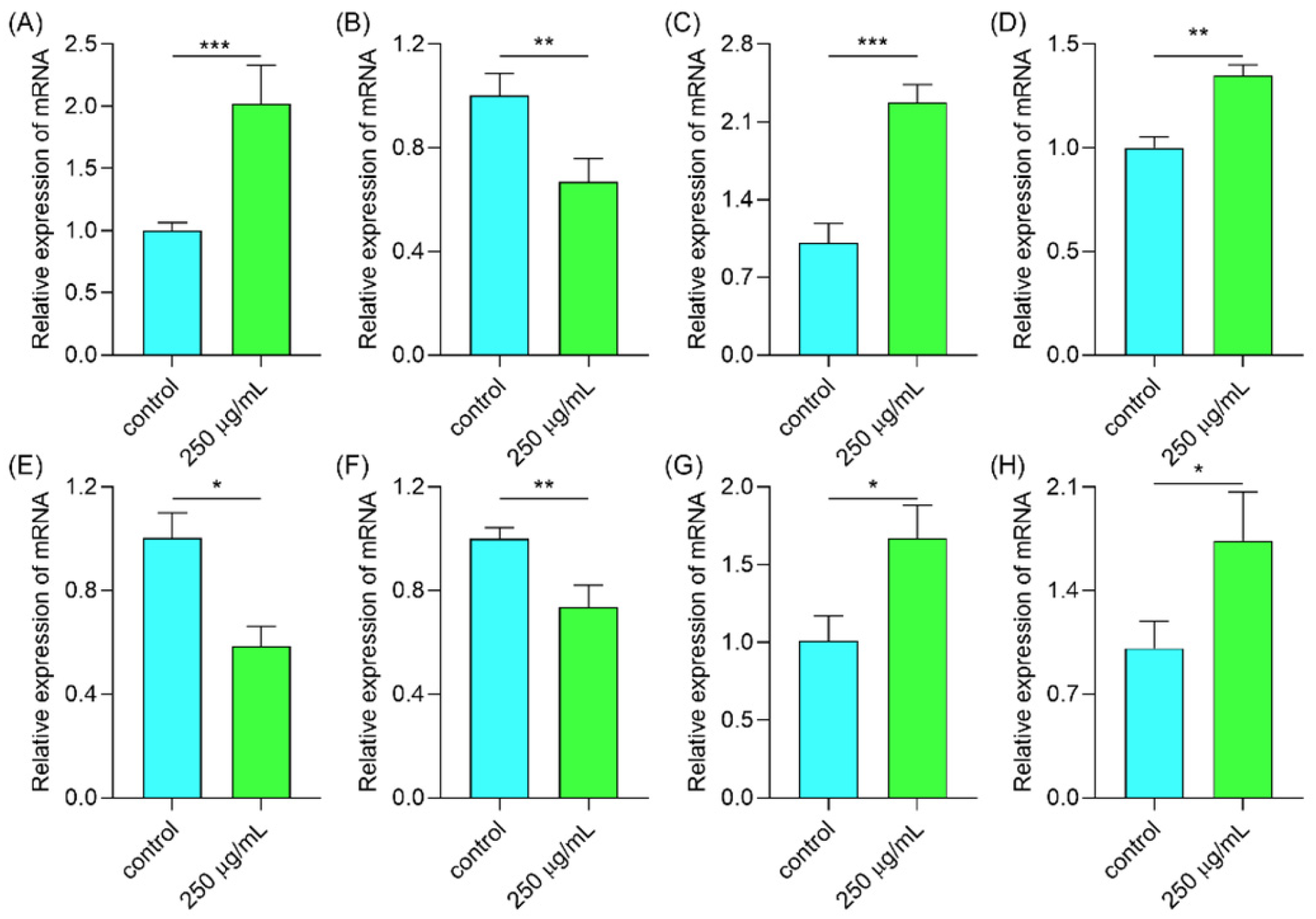
2.6.6. Polysaccharides Regulated Gene Expression in the IIS/MAPK/mTOR Signal Pathway
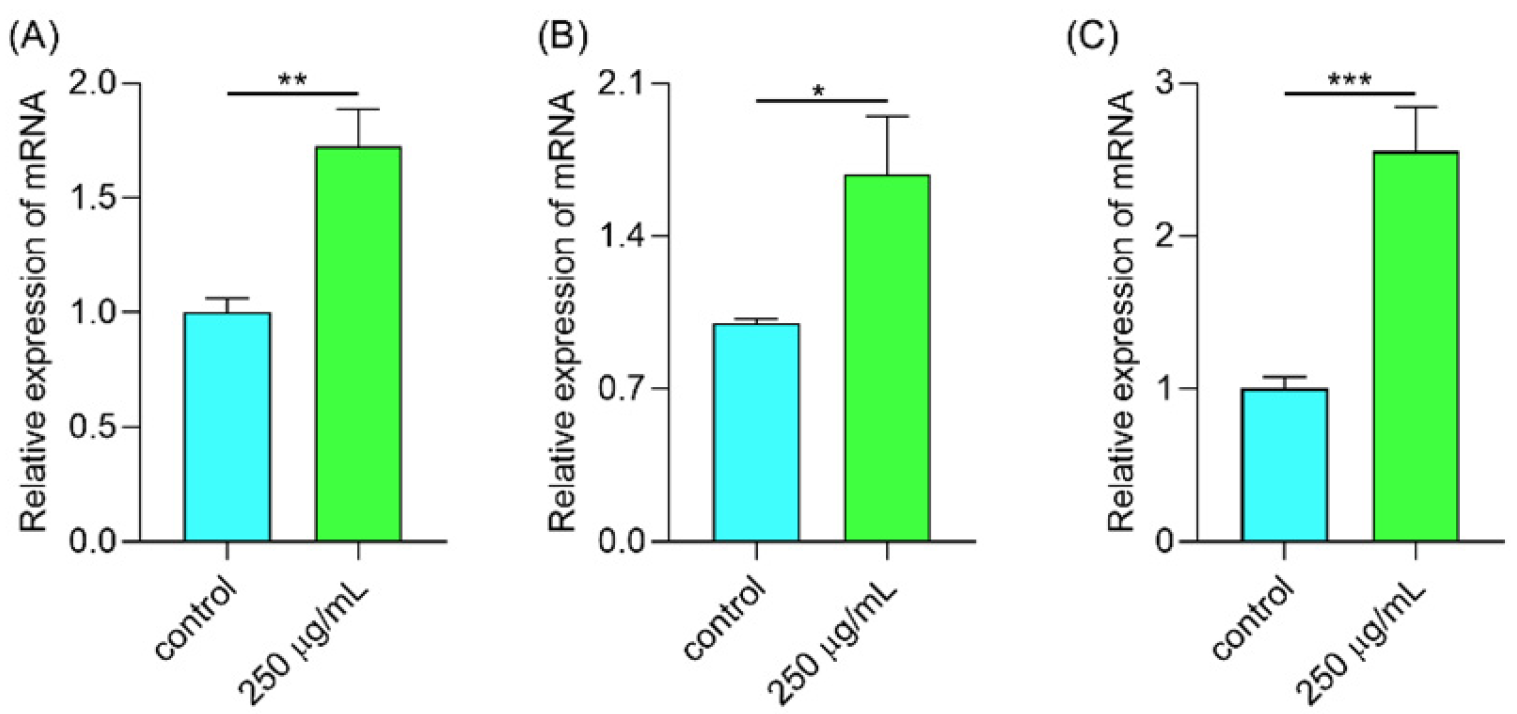
2.6.7. Polysaccharides Up-Regulated Gene Expression Involved in the Antioxidant System
2.7. The Effect of Polysaccharides on Yogurt
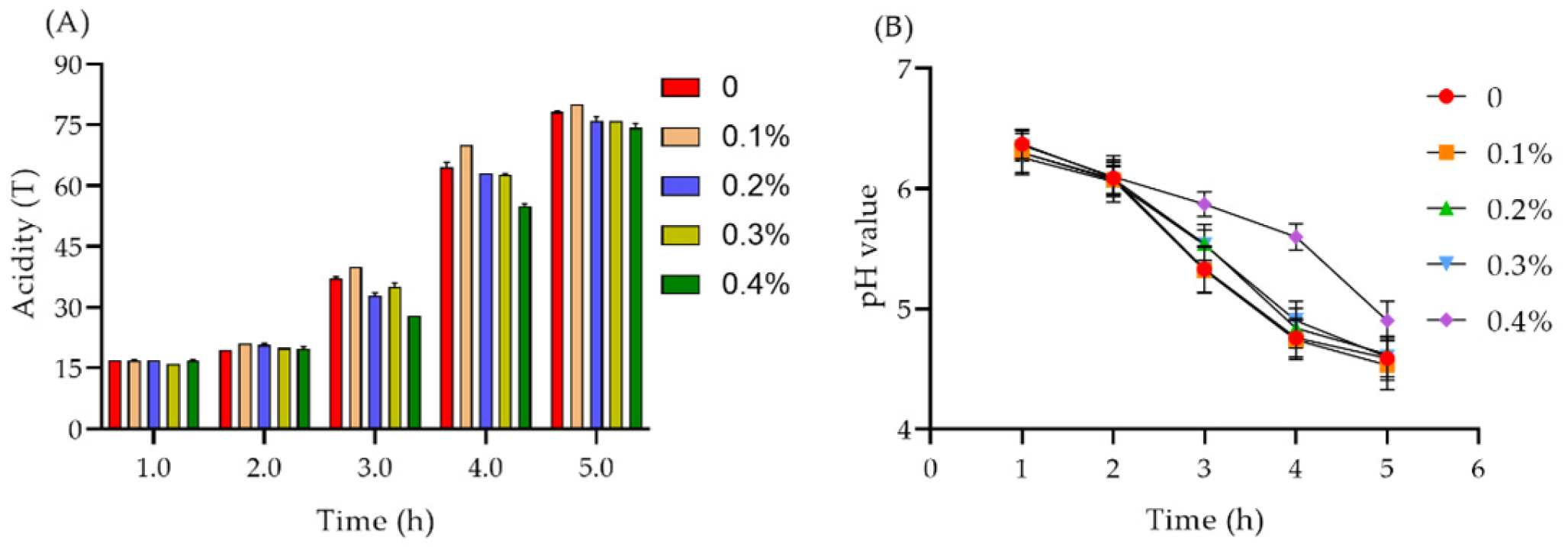
2.7.1. Acidity and pH Value of Yogurt
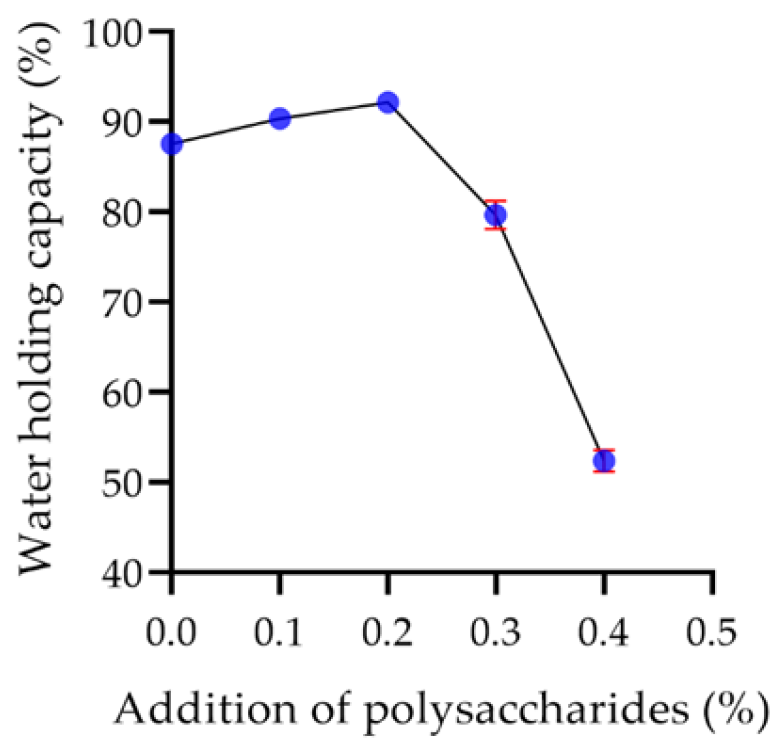
2.7.2. Water Holding Capacity of Yogurt
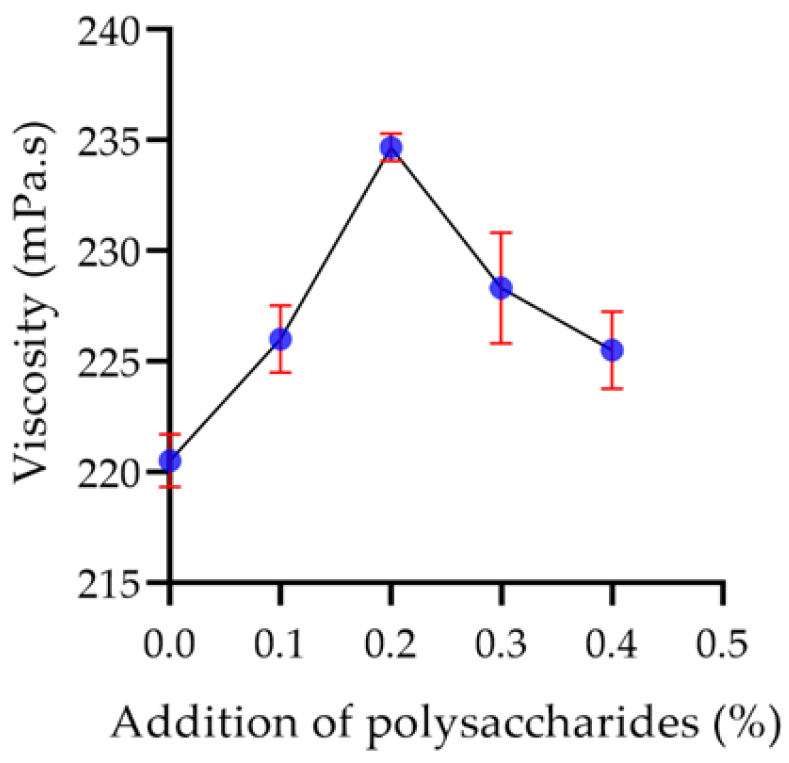
2.7.3. Viscosity of Yogurt
2.7.4. Texture Property of Yogurt
2.7.5. Viable Lactic Acid Bacteria of Yogurt
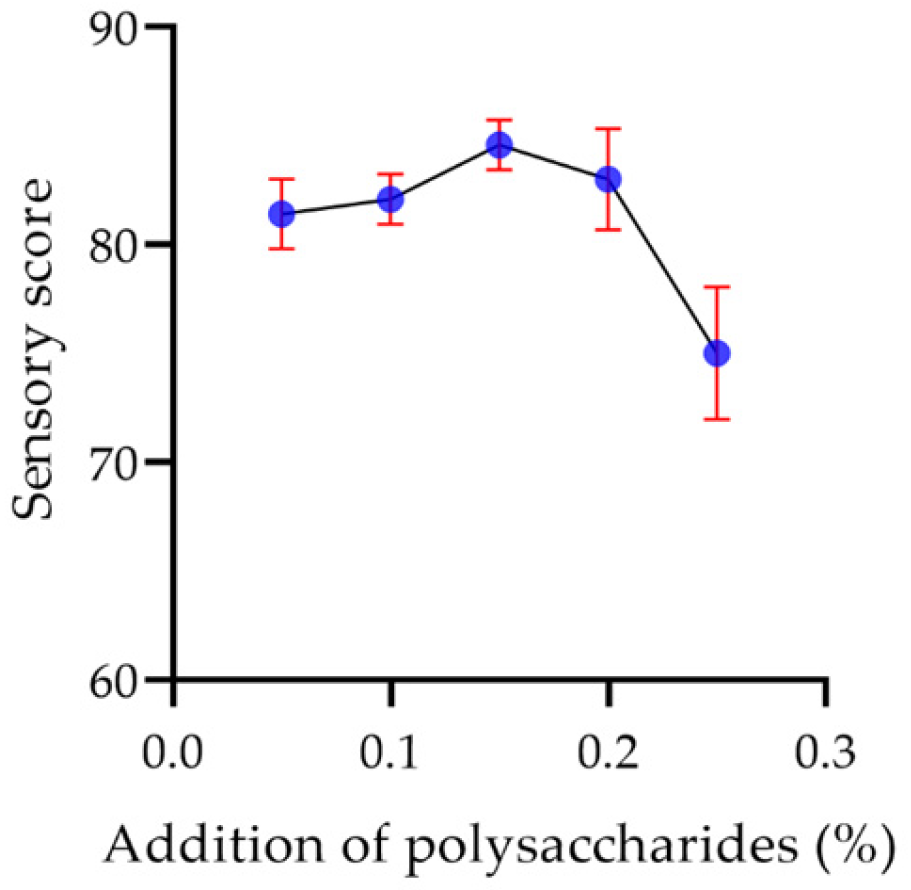
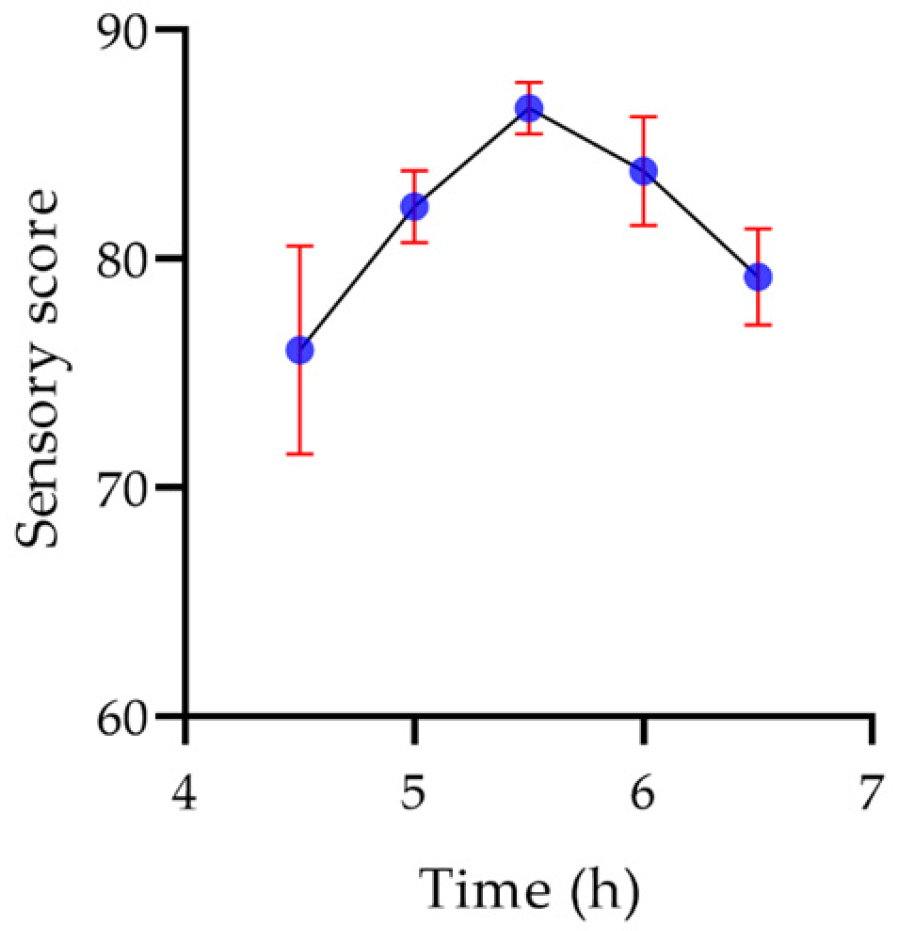
2.7.6. Sensory Evaluation of Yogurt
2.8. The Effect of Single Factors on Yogurt Quality
2.8.1. Fermentation Temperature
2.8.2. Inoculation Amount
2.8.3. Polysaccharide Concentration
2.8.4. Fermentation Time
2.8.5. Sucrose Concentration
2.9. Orthogonal Optimization of Fermentation
2.10. Quality of Polysaccharide Yogurt after Orthogonal Optimization
2.11. Simulated Digestion of Polysaccharide Yogurt In Vitro
3. Materials and Methods
3.1. Materials and Reagents
3.2. Polysaccharides from B. schreberi through Different Extraction Methods
3.3. Assessment of Chemical Property
3.4. Detection of Triple Helix Structure
3.5. Determination of the Antioxidant Ability
3.6. Characterization of the Polysaccharide
3.7. Preparation of Enzymolysis Polysaccharides
3.7.1. Detection of Enzymatic Hydrolysis Rate
3.7.2. Single Factor Experiment on Enzymatic Hydrolysis of Polysaccharides
3.7.3. Response Surface Methodology for Enzymatic Hydrolysis of Polysaccharides
3.8. Cellular Assays
3.8.1. Cell Culture
3.8.2. Determination of Cell Toxicity
3.8.3. Determination of Cell Protection
3.8.4. Determination of Cell Apoptosis and ROS Level
3.9. In Vivo Assays
3.9.1. C. elegans Maintenance
3.9.2. Determination of Survival under Thermal Stress
3.9.3. Determination of Reactive Oxygen Species (ROS)
3.9.4. Determination of Gene Expression
3.10. Preparation of Polysaccharide Yogurt
3.10.1. Determination of Acidity in the Yogurt Fermentation Process
3.10.2. Determination of the pH Value in the Yogurt Fermentation Process
3.10.3. Determination of the Water Holding Capacity of Yogurt
3.10.4. Measurement of Viscosity
3.10.5. Determination of the Yogurt Textural Property
3.10.6. Determination of Viable Count of Lactic Acid Bacteria
3.10.7. Sensory Evaluation Score
3.11. Optimization of the Fermentation Conditions
3.11.1. Single Factor Assays
3.11.2. Optimization of Fermentation Conditions of Polysaccharide Yogurt by the Orthogonal Experiment
3.11.3. Determination of the Indicators of the Polysaccharide Yogurt
3.12. Simulated Digestion of Polysaccharide Yogurt In Vitro
3.12.1. Production of Polysaccharide Yogurt
3.12.2. Simulated Gastric Juice Digestion In Vitro
3.12.3. Simulated Intestinal Fluid Digestion In Vitro
3.13. Data Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Legault, J.; Perron, T.; Mshvildadze, V.; Girard-Lalancette, K.; Perron, S.; Laprise, C.; Sirois, P.; Pichette, A. Antioxidant and anti-inflammatory activities of quercetin 7-O-β-D-glucopyranoside from the leaves of Brasenia schreberi. J. Med. Food 2011, 14, 1127–1134. [Google Scholar] [CrossRef] [PubMed]
- Hisayoshi, T.; Shinomura, M.; Yokokawa, K.; Kuze, I.; Konishi, A.; Kawaji, K.; Kodama, E.N.; Hata, K.; Takahashi, S.; Nirasawa, S.; et al. Inhibition of the DNA polymerase and RNase H activities of HIV-1 reverse transcriptase and HIV-1 replication by Brasenia schreberi (Junsai) and Petasites japonicus (Fuki) components. J. Nat. Med. 2015, 69, 432–440. [Google Scholar] [CrossRef] [PubMed]
- Misaki, A.; Smith, F. Food plant polysaccharides, structure of the polysaccharide of the Japanese water-plant, Brasenia schreberi J. F. Gmel. composition of the polysaccharide and isolation of 2-O-(β-D-glucopyranosyluronic acid)-D-mannose. J. Agric. Food Chem. 1962, 10, 104–108. [Google Scholar] [CrossRef]
- Kakuta, M.; Misaki, A. The polysaccharide of “Junsai (Brasenia schreberi J. F. Gmel)” Mucilage; Fragmentation Analysis by Successive Smith Degradations and Partial Acid Hydrolysis. Agric. Biol. Chem. 1979, 43, 1269–1276. [Google Scholar] [CrossRef]
- Xiao, H.; Cai, X.; Fan, Y.; Luo, A. Antioxidant activity of water-soluble polysaccharides from Brasenia schreberi. Pharmacogn. Mag. 2016, 12, 193–197. [Google Scholar] [CrossRef]
- Kim, H.; Wang, Q.; Shoemaker, C.F.; Zhong, F.; Bartley, G.E.; Yokoyama, W.H. Polysaccharide gel coating of the leaves of Brasenia schreberi lowers plasma cholesterol in hamsters. J. Tradit. Complement. Med. 2015, 5, 56–61. [Google Scholar] [CrossRef]
- Khursheed, R.; Singh, S.K.; Wadhwa, S.; Gulati, M.; Kapoor, B.; Jain, S.K.; Gowthamarajan, K.; Zacconi, F.; Chellappan, D.K.; Gupta, G.; et al. Development of mushroom polysaccharide and probiotics based solid self-nanoemulsifying drug delivery system loaded with curcumin and quercetin to improve their dissolution rate and permeability: State of the art. Int. J. Biol. Macromol. 2021, 189, 744–757. [Google Scholar] [CrossRef]
- Mohanta, S.; Singh, S.K.; Kumar, B.; Gulati, M.; Kumar, R.; Yadav, A.K.; Wadhwa, S.; Jyoti, J.; Som, S.; Dua, K.; et al. Efficacy of co-administration of modified apple polysaccharide and probiotics in guar gum-Eudragit S100 based mesalamine mini tablets: A novel approach in treating ulcerative colitis. Int. J. Biol. Macromol. 2019, 126, 427–435. [Google Scholar] [CrossRef]
- Khan, I.; Huang, G.; Li, X.; Leong, W.; Xia, W.; Hsiao, W.W.L. Mushroom polysaccharides from Ganoderma lucidum and Poria cocos reveal prebiotic functions. J. Funct. Foods 2018, 41, 191–201. [Google Scholar] [CrossRef]
- Xu, K.; Guo, M.; Du, J.; Zhang, Z. Okra polysaccharide: Effect on the texture and microstructure of set yoghurt as a new natural stabilizer. Int. J. Biol. Macromol. 2019, 133, 117–126. [Google Scholar] [CrossRef]
- Jonga, S.D.; Velde, F.V.D. Charge density of polysaccharide controls microstructure and large deformation properties of mixed gels. Food Hydrocoll. 2007, 21, 1172–1187. [Google Scholar] [CrossRef]
- Li, S.; Nagendra, S.P. Effects of Pleurotus eryngii polysaccharides on bacterial growth, texture properties, proteolytic capacity, and angiotensin-I-converting enzyme-inhibitory activities of fermented milk. J. Dairy Sci. 2015, 98, 2949–2961. [Google Scholar] [CrossRef] [PubMed]
- Saeedeh, S.; Shima, Y.; Alireza, F. Developing an innovative textural structure for semi-volume breads based on interaction of spray-dried yogurt powder and jujube polysaccharide. Int. J. Biol. Macromol. 2017, 104, 992–1002. [Google Scholar] [CrossRef]
- Qiu, J.; Zhang, H.; Wang, Z. Ultrasonic degradation of Polysaccharides from Auricularia auricula and the antioxidant activity of their degradation products. LWT-Food Sci. Technol. 2019, 113, 108266. [Google Scholar] [CrossRef]
- Zhang, H.; Cai, X.T.; Tian, Q.H.; Xiao, L.X.; Zeng, Z.; Cai, X.T.; Yan, J.Z.; Li, Q.Y. Microwave-assisted degradation of polysaccharide from Polygonatum sibiricum and antioxidant activity. J. Food Sci. 2019, 84, 754–761. [Google Scholar] [CrossRef]
- Feng, S.; Luan, D.; Ning, K.; Shao, P.; Sun, P. Ultrafiltration isolation, hypoglycemic activity analysis and structural characterization of polysaccharides from Brasenia schreberi. Int. J. Biol. Macromol. 2019, 135, 141–151. [Google Scholar] [CrossRef]
- Xu, X.; Yan, H.; Zhang, X. Structure and immuno-stimulating activities of a new heteropolysaccharide from Lentinula edodes. J. Agric. Food Chem. 2012, 60, 11560–11566. [Google Scholar] [CrossRef]
- Ma, G.; Yang, W.; Mariga, A.M.; Fang, Y.; Ma, N.; Pei, F.; Hu, Q. Purification, characterization and antitumor activity of polysaccharides from Pleurotus eryngii residue. Carbohydr. Polym. 2014, 114, 297–305. [Google Scholar] [CrossRef]
- Yang, B.; Wang, J.; Zhao, M.; Liu, Y.; Wang, W.; Jiang, Y. Identification of polysaccharides from pericarp tissues of litchi (Litchi chinensis Sonn.) fruit in relation to their antioxidant activities. Carbohydr. Res. 2006, 341, 634–638. [Google Scholar] [CrossRef]
- Luo, Q.-L.; Tang, Z.-H.; Zhang, X.-F.; Zhong, O.-H.; Yao, S.-Z.; Wang, L.-S.; Lin, C.-W.; Luo, X. Chemical properties and antioxidant activity of a water-soluble polysaccharide from Dendrobium officinale. Int. J. Biol. Macromol. 2016, 89, 219–227. [Google Scholar] [CrossRef]
- Sthijns, M.M.J.P.E.; Schiffers, P.M.; Janssen, G.M.; Lemmens, K.J.A.; Ides, B.; Vangrieken, P.; Bouwman, F.G.; Mariman, E.C.; Pader, I.; Arnér, E.S.J.; et al. Rutin protects against H2O2-triggered impaired relaxation of placental arterioles and induces Nrf2-mediated adaptation in Human Umbilical Vein Endothelial Cells exposed to oxidative stress. Biochim. Biophys. Acta Gen. Subj. 2017, 1861, 1177–1189. [Google Scholar] [CrossRef] [PubMed]
- Sies, H. Hydrogen peroxide as a central redox signaling molecule in physiological oxidative stress: Oxidative eustress. Redox Biol. 2017, 11, 613–619. [Google Scholar] [CrossRef] [PubMed]
- El-Kenawi, A.; Ruffell, B. Inflammation, ROS, and Mutagenesis. Cancer Cell 2017, 32, 727–729. [Google Scholar] [CrossRef] [PubMed]
- Tafani, M.; Sansone, L.; Limana, F.; Arcangeli, T.; Santis, E.D.; Polese, M.; Fini, M.; Russo, M.A. The interplay of reactive oxygen species, hypoxia, inflammation, and sirtuins in cancer initiation and progression. Oxidative Med. Cell. Longev. 2016, 2016, 3907147. [Google Scholar] [CrossRef] [PubMed]
- Schöttker, B.; Brenner, H.; Jansen, E.H.; Gardiner, J.; Peasey, A.; Kubínová, R.; Pająk, A.; Topor-Madry, R.; Tamosiunas, A.; Saum, K.-U.; et al. Evidence for the free radical/oxidative stress theory of ageing from the CHANCES consortium: A meta-analysis of individual participant data. BMC Med. 2015, 13, 300. [Google Scholar] [CrossRef] [PubMed]
- Nobuhiko, K.; DeLano, F.A.; Schmid-Schönbein, G.W. Oxidative stress promotes endothelial cell apoptosis and loss of microvessels in the spontaneously hypertensive rats. Arter. Thromb. Vasc. Biol. 2005, 25, 2114–2121. [Google Scholar] [CrossRef]
- An, R.; Wang, X.; Yang, L.; Zhang, J.; Wang, N.; Xu, F.; Hou, Y.; Zhang, H.; Zhang, L. Polystyrene microplastics cause granulosa cells apoptosis and fibrosis in ovary through oxidative stress in rats. Toxicology 2021, 449, 152665. [Google Scholar] [CrossRef]
- Zhang, S.; Yi, X.; Su, X.; Jian, Z.; Cui, T.; Guo, S.; Gao, T.; Li, C.; Li, S.; Xiao, Q. Ginkgo biloba extract protects human melanocytes from H2O2-induced oxidative stress by activating Nrf2. J. Cell. Mol. Med. 2019, 23, 5193–5199. [Google Scholar] [CrossRef]
- Jittiporn, K.; Moongkarndi, P.; Samer, J.; Kumphune, S.; Suvitayavat, W. Water extract of mangosteen suppresses H2O2-induced endothelial apoptosis by inhibiting oxidative stress. J. Appl. Pharm. Sci. 2019, 9, 10–16. [Google Scholar] [CrossRef]
- Zhou, L.; Luo, S.; Li, J.; Zhou, Y.; Wang, X.; Kong, Q.; Chen, T.; Feng, S.; Yuan, M.; Ding, C. Optimization of the extraction of polysaccharides from the shells of Camellia oleifera and evaluation on the antioxidant potential in vitro and in vivo. J. Funct. Foods 2021, 86, 104678. [Google Scholar] [CrossRef]
- Chen, T.; Luo, S.; Wang, X.; Zhou, Y.; Dai, Y.; Zhou, L.; Feng, S.; Yuan, M.; Ding, C. Polyphenols from Blumea laciniata extended the lifespan and enhanced resistance to stress in Caenorhabditis elegans via the insulin signaling pathway. Antioxidants 2021, 10, 1744. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Luo, S.; Xu, Z.; Liu, L.; Feng, S.; Chen, T.; Zhou, L.; Yuan, M.; Huang, Y.; Ding, C. The potential antioxidant ability of hydroxytyrosol on Caenorhabditis elegans against oxidative damage via the insulin signaling pathway. Arab. J. Chem. 2021, 14, 103149. [Google Scholar] [CrossRef]
- Zhao, D.; Yan, M.; Xu, H.; Liang, H.; Zhang, J.; Li, M.; Wang, C. Antioxidant and antiaging activity of fermented coix seed polysaccharides on Caenorhabditis elegans. Nutrients 2023, 15, 2474. [Google Scholar] [CrossRef] [PubMed]
- Xiao, X.; Zhou, Y.; Tan, C.; Bai, J.; Zhu, Y.; Zhang, J.; Zhou, X.; Zhao, Y. Barley β-glucan resist oxidative stress of Caenorhabditis elegans via daf-2/daf-16 pathway. Int. J. Biol. Macromol. 2021, 193, 1021–1031. [Google Scholar] [CrossRef] [PubMed]
- Arnab, M.; Seung, W.O.; Heidi, T. Worming pathways to and from DAF-16/FOXO. Exp. Gerontol. 2006, 41, 928–934. [Google Scholar] [CrossRef]
- Blackwell, K.T.; Steinbaugh, M.J.; Hourihan, J.M.; Ewald, C.Y.; Isik, M. SKN-1/Nrf, stress responses, and aging in Caenorhabditis elegans. Free Radic. Biol. Med. 2015, 88, 290–301. [Google Scholar] [CrossRef] [PubMed]
- Chatrawee, D.; Panthakarn, R.; Xiaojie, G.; Shaoxiong, Z.; Michael, W.; Tewin, T. Glochidion zeylanicum leaf extracts exhibit lifespan extending and oxidative stress resistance properties in Caenorhabditis elegans via DAF-16/FoxO and SKN-1/Nrf-2 signaling pathways. Phytomedicine 2019, 64, 153061. [Google Scholar] [CrossRef]
- Li, N.; Li, Q.; He, X.; Gao, X.; Wu, L.; Xiao, M.; Cai, W.; Liu, B.; Zeng, F. Antioxidant and anti-aging activities of Laminaria japonica polysaccharide in Caenorhabditis elegans based on metabonomic analysis. Int. J. Biol. Macromol. 2022, 221, 346–354. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhi, L.; Wu, Q.; Yu, Y.; Sun, Q.; Wang, D. p38 MAPK-SKN-1/Nrf signaling cascade is required for intestinal barrier against graphene oxide toxicity in Caenorhabditis elegans. Nanotoxicology 2016, 10, 1469–1479. [Google Scholar] [CrossRef]
- Han, Y.; Song, S.; Wu, H.; Zhang, J.; Ma, E. Antioxidant enzymes and their role in phoxim and carbaryl stress in Caenorhabditis elegans. Pestic. Biochem. Physiol. 2017, 138, 43–50. [Google Scholar] [CrossRef]
- Chen, T.; Wang, X.; Yan, X.; Dai, Y.; Liang, T.; Zhou, L.; Feng, S.; Yuan, M.; Yang, H.; Ding, C. A novel selenium polysaccharide alleviates the manganese (Mn)-induced toxicity in Hep G2 cells and Caenorhabditis elegans. Int. J. Mol. Sci. 2022, 23, 4097. [Google Scholar] [CrossRef] [PubMed]
- MatÉs, J.M.; Pérez-Gómez, C.; Castro, I.N.D. Antioxidant enzymes and human diseases. Clin. Biochem. 1999, 32, 595–603. [Google Scholar] [CrossRef]
- Mataragas, M.; Dimitriou, V.; Skandamis, P.N.; Drosinos, E.H. Quantifying the spoilage and shelf-life of yoghurt with fruits. Food Microbiol. 2011, 28, 611–616. [Google Scholar] [CrossRef] [PubMed]
- Gilbert, A.; Turgeon, S.L. Studying stirred yogurt microstructure and its correlation to physical properties: A review. Food Hydrocoll. 2021, 121, 106970. [Google Scholar] [CrossRef]
- Ranasinghe, J.G.S.; Perera, W.T.R. Prevalence of Lactobacillus bulgaricus and Streptococcus thermophilus stability in commercially available yogurts in Sri lanka. Asian J. Med. Sci. 2016, 7, 97–101. [Google Scholar] [CrossRef]
- Bracquart, P. An agar medium for the differential enumeration of Streptococcus thermophilus and Lactobacillus bulgaricus in yoghurt. J. Appl. Bacteriol. 1981, 51, 303–305. [Google Scholar] [CrossRef]
- Sanders, M.E. Probiotics: Considerations for human health. Nutr. Rev. 2003, 61, 91–99. [Google Scholar] [CrossRef]
- Hahn, C.; Sramek, M.; Nöbel, S.; Hinrichs, J. Post-processing of concentrated fermented milk: Influence of temperature and holding time on the formation of particle clusters. Dairy Sci. Technol. 2012, 92, 91–107. [Google Scholar] [CrossRef]
- Miele, N.A.; Cabisidan, E.K.; Blaiotta, G.; Leone, S.; Masi, P.; Monaco, R.D.; Cavella, S. Rheological and sensory performance of a protein-based sweetener (MNEI), sucrose, and aspartame in yogurt. J. Dairy Sci. 2017, 100, 9539–9550. [Google Scholar] [CrossRef]
- Salji, J.P.; Saadi, S.R.; Mashhadi, A. Shelf Life of Plain Liquid Yogurt Manufactured in Saudi Arabia. J. Food Prot. 1987, 50, 123–126. [Google Scholar] [CrossRef]
- GB4789.1-2016; General Provisions for Food Microbiology Examination of National Food Safety Standards. Beijing, China, 2016. Available online: http://down.foodmate.net/standard/sort/3/50366.html (accessed on 23 June 2017). (In Chinese)
- Ying, W.; Yuan, B.; Ji, Y.; Li, H. Hydrolysis of hemicellulose to produce fermentable monosaccharides by plasma acid. Carbohydr. Polym. 2013, 97, 518–522. [Google Scholar] [CrossRef]
- Luo, S.; Jiang, X.; Jia, L.; Tan, C.; Li, M.; Yang, Q.; Du, Y.; Ding, C. In vivo and in vitro antioxidant activities of methanol extracts from olive leaves on Caenorhabditis elegans. Molecules 2019, 24, 704. [Google Scholar] [CrossRef] [PubMed]
- Gaillard, B.D.E.; Thompson, N.S.; Morak, A.J. The interaction of polysaccharides with iodine. Carbohydr. Res. 1969, 11, 509–519. [Google Scholar] [CrossRef]
- Xu, L.; Zhang, Y.; Wang, L. Structure characteristics of a water-soluble polysaccharide purified from dragon fruit (Hylocereus undatus) pulp. Carbohydr. Polym. 2016, 146, 224–230. [Google Scholar] [CrossRef] [PubMed]
- Luo, S.; Li, J.; Zhou, Y.; Liu, L.; Feng, S.; Chen, T.; Zhou, L.; Ding, C. Evaluation on bioactivities of triterpenes from Bergenia emeiensis. Arab. J. Chem. 2021, 14, 103225. [Google Scholar] [CrossRef]
- Zhou, L.; Luo, S.; Wang, X.; Zhou, Y.; Zhang, Y.; Zhu, S.; Chen, T.; Feng, S.; Yuan, M.; Ding, C. Blumea laciniata protected Hep G2 cells and Caenorhabditis elegans against acrylamide-induced toxicity via insulin/IGF-1 signaling pathway. Food Chem. Toxicol. 2021, 158, 112667. [Google Scholar] [CrossRef]
- Porta-de-la-Riva, M.; Fontrodona, L.; Villanueva, A.; Cerón, J. Basic Caenorhabditis elegans methods: Synchronization and observation. J. Vis. Exp. 2012, 64, e2019. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Çakmakçı, S.; Cetin, B.; Turgut, T.; Gurses, M.; Erdoğan, A. Probiotic properties, sensory qualities, and storage stability of probiotic banana yogurts. Turk. J. Vet. Anim. Sci. 2012, 36, 231–237. [Google Scholar] [CrossRef]
- Wang, J.; Liu, B.; Qi, Y.; Wu, D.; Liu, X.; Liu, C.; Gao, Y.; Shi, J.; Fang, L.; Min, W. Impact of Auricularia cornea var. Li polysaccharides on the physicochemical, textual, flavor, and antioxidant properties of set yogurt. Int. J. Biol. Macromol. 2022, 206, 148–158. [Google Scholar] [CrossRef]






| Chemical Properties | Polysaccharides Extracted Using Different Methods | ||
|---|---|---|---|
| A-BSP | E-BSP | W-BSP | |
| Total sugar content (%) | 35.06 ± 0.12 | 54.45 ± 0.50 | 60.86 ± 0.41 |
| Reducing sugar content (%) | 0.22 ± 0.01 | 1.44 ± 0.02 | 0.36 ± 0.01 |
| Protein content (%) | 9.45 ± 0.18 | 1.56 ± 0.01 | 1.42 ± 0.03 |
| Starch | - | - | - |
| Phenolic hydroxyl | - | - | - |
| Source | Square | Degree of Freedom | F Value | p Value |
|---|---|---|---|---|
| Model | 3.65 | 9 | 31.39 | <0.0001 |
| A | 2.16 | 1 | 167.12 | <0.0001 |
| B | 1.21 × 10−4 | 1 | 9.39 × 10−3 | 0.9255 |
| C | 0.025 | 1 | 1.9 | 0.2104 |
| AB | 4.63 × 10−3 | 1 | 0.36 | 0.5683 |
| AC | 1.93 × 10−4 | 1 | 0.015 | 0.906 |
| BC | 6.90 × 10−6 | 1 | 5.34 × 10−4 | 0.9822 |
| A2 | 0.16 | 1 | 12.6 | 0.0093 |
| B2 | 0.53 | 1 | 40.96 | 0.0004 |
| C2 | 0.63 | 1 | 48.61 | 0.0002 |
| Lack of fit | 0.053 | 3 | 1.85 | 0.2779 |
| Addition of Polysaccharides (%) | Hardness (N) | Elasticity (mm) | Glueyness (N) | Adhesivity (mj) | Adhesion Elongation (mm) |
|---|---|---|---|---|---|
| 0 | 1.19 ± 0.005 a | 6.93 ± 0.38 a | 0.51 ± 0.012 a | 2.68 ± 0.01 a | 10.0 ± 0.089 a |
| 0.1 | 1.15 ± 0.015 b | 8.79 ± 0.08 b | 0.50 ± 0.007 a | 3.02 ± 0.04 b | 5.90 ± 0.030 b |
| 0.2 | 1.11 ± 0.006 c | 8.94 ± 0.07 b | 0.49 ± 0.003 b | 2.82 ± 0.025 c | 4.17 ± 0.032 c |
| 0.3 | 0.98 ± 0.005 d | 7.27 ± 0.16 c | 0.44 ± 0.006 c | 2.30 ± 0.032 d | 3.31 ± 0.036 d |
| 0.4 | 0.89 ± 0.01 e | 5.87 ± 0.10 d | 0.41 ± 0.002 d | 2.18 ± 0.017 e | 2.78 ± 0.043 e |
| Addition of Polysaccharides (%) | Lactic Acid Bacteria (CFU/mL) |
|---|---|
| 0 | 4.63 × 108 ± 0.35 a |
| 0.1 | 6.82 × 108 ± 0.21 b |
| 0.2 | 7.50 × 108 ± 0.10 c |
| 0.3 | 6.20 × 108 ± 0.17 d |
| 0.4 | 5.37 × 108 ± 0.36 e |
| Addition of Polysaccharides (%) | Sensory Evaluation | Scores |
|---|---|---|
| 0 | Uniform coagulation, moderate sweet and sour, milky white color, good taste, smell coordination | 78.6 ± 1.18 a |
| 0.1 | Uniform coagulation, moderate sweet and sour, milky white color, thick taste, smell coordination | 80.67 ± 1.64 a |
| 0.2 | Uniform coagulation, moderate sweet and sour, structure even, light yellow color, delicate and smooth taste, harmonious odor | 81.11 ± 0.90 a |
| 0.3 | Coagulation milk, moderate acidity and sweetness, slightly yellow color, good taste, harmonious odor | 65.89 ± 1.10 b |
| 0.4 | No coagulation, sweet and sour, slightly yellow in color, poor taste | 43.89 ± 3.54 c |
| Strains | Standards | Determination |
|---|---|---|
| Lactic acid bacteria | ≥1 × 106 CFU/g | 6.9 × 108 |
| Escherichia coli | n = 5, c = 2, m = 1 CFU/g, M = 5 CFU/g | 0 |
| Staphylococcus aureus | 0/25 g | 0 |
| Salmonella | 0/25 g | 0 |
| Mold | ≤30 CFU/g | 8 |
| Yeast | ≤100 CFU/g | 35 |
| Properties | Polysaccharides Yogurt | Ordinary Yogurt |
|---|---|---|
| Protein (g/100 g) | 2.82 ± 0.04 ns | 2.61 ± 0.03 |
| Fat (g/100 g) | 3.35 ± 0.02 ns | 3.32 ± 0.02 |
| Water holding capacity | 91.67 ± 0.24% * | 88.90 ± 0.4% |
| Acidity (°T) | 80.15 ± 2.17 * | 74.32 ± 1.21 |
| Sensory score | 85.23 ± 2.37 * | 81.48 ± 1.04 |
| pH Value | Time (h) | Lactic Acid Bacteria (CFU/mL) | |
|---|---|---|---|
| Ordinary Yogurt | Polysaccharide Yogurt | ||
| 1.5 | 0 | 3.23 × 108 ± 0.22 | 3.61 × 108 ± 0.09 |
| 1 | 3.01 × 108 ± 0.30 | 3.44 × 108 ± 0.14 | |
| 2 | 1.38 × 108 ± 0.15 | 2.42 × 108 ± 0.12 | |
| 3 | 1.19 × 108 ± 0.21 | 2.09 × 108 ± 0.45 | |
| 2.5 | 0 | 4.17 × 108 ± 0.33 | 5.11 × 108 ± 0.27 |
| 1 | 3.64 × 108 ± 0.54 | 3.82 × 108 ± 0.15 | |
| 2 | 4.02 × 108 ± 0.19 | 4.51 × 108 ± 0.39 | |
| 3 | 5.09 × 108 ± 0.60 | 5.64 × 108 ± 0.25 | |
| 3.5 | 0 | 4.24 × 108 ± 0.22 | 5.00 × 108 ± 0.65 |
| 1 | 3.67 × 108 ± 0.53 | 4.39 × 108 ± 0.38 | |
| 2 | 4.52 × 108 ± 0.27 | 4.63 × 108 ± 0.13 | |
| 3 | 4.81 × 108 ± 0.16 | 5.11 × 108 ± 0.26 | |
| Time (h) | Lactic Acid Bacteria (CFU/mL) | |
|---|---|---|
| Ordinary Yogurt | Polysaccharide Yogurt | |
| 0 | 6.11 × 108 ± 0.37 | 6.53 × 108 ± 0.25 |
| 1 | 5.93 × 108 ± 0.54 | 6.32 × 108 ± 0.18 |
| 2 | 5.62 × 108 ± 0.41 | 6.38 × 108 ± 0.28 |
| 3 | 5.69 × 108 ± 0.12 | 6.41 × 108 ± 0.45 |
| 4 | 5.31 × 108 ± 0.14 | 6.51 × 108 ± 0.30 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Zou, Y.; Fang, Q.; Feng, R.; Zhang, J.; Zhou, W.; Wei, Q. Polysaccharides from Brasenia schreberi with Great Antioxidant Ability and the Potential Application in Yogurt. Molecules 2024, 29, 150. https://doi.org/10.3390/molecules29010150
Wang Y, Zou Y, Fang Q, Feng R, Zhang J, Zhou W, Wei Q. Polysaccharides from Brasenia schreberi with Great Antioxidant Ability and the Potential Application in Yogurt. Molecules. 2024; 29(1):150. https://doi.org/10.3390/molecules29010150
Chicago/Turabian StyleWang, Yujie, Yue Zou, Qiong Fang, Ruizhang Feng, Jihong Zhang, Wanhai Zhou, and Qin Wei. 2024. "Polysaccharides from Brasenia schreberi with Great Antioxidant Ability and the Potential Application in Yogurt" Molecules 29, no. 1: 150. https://doi.org/10.3390/molecules29010150
APA StyleWang, Y., Zou, Y., Fang, Q., Feng, R., Zhang, J., Zhou, W., & Wei, Q. (2024). Polysaccharides from Brasenia schreberi with Great Antioxidant Ability and the Potential Application in Yogurt. Molecules, 29(1), 150. https://doi.org/10.3390/molecules29010150






