Experimental and Theoretical Investigations of the Fragmentation of Ethylenediamine Induced by Low-Energy (<10 eV) Electrons
Abstract
:1. Introduction
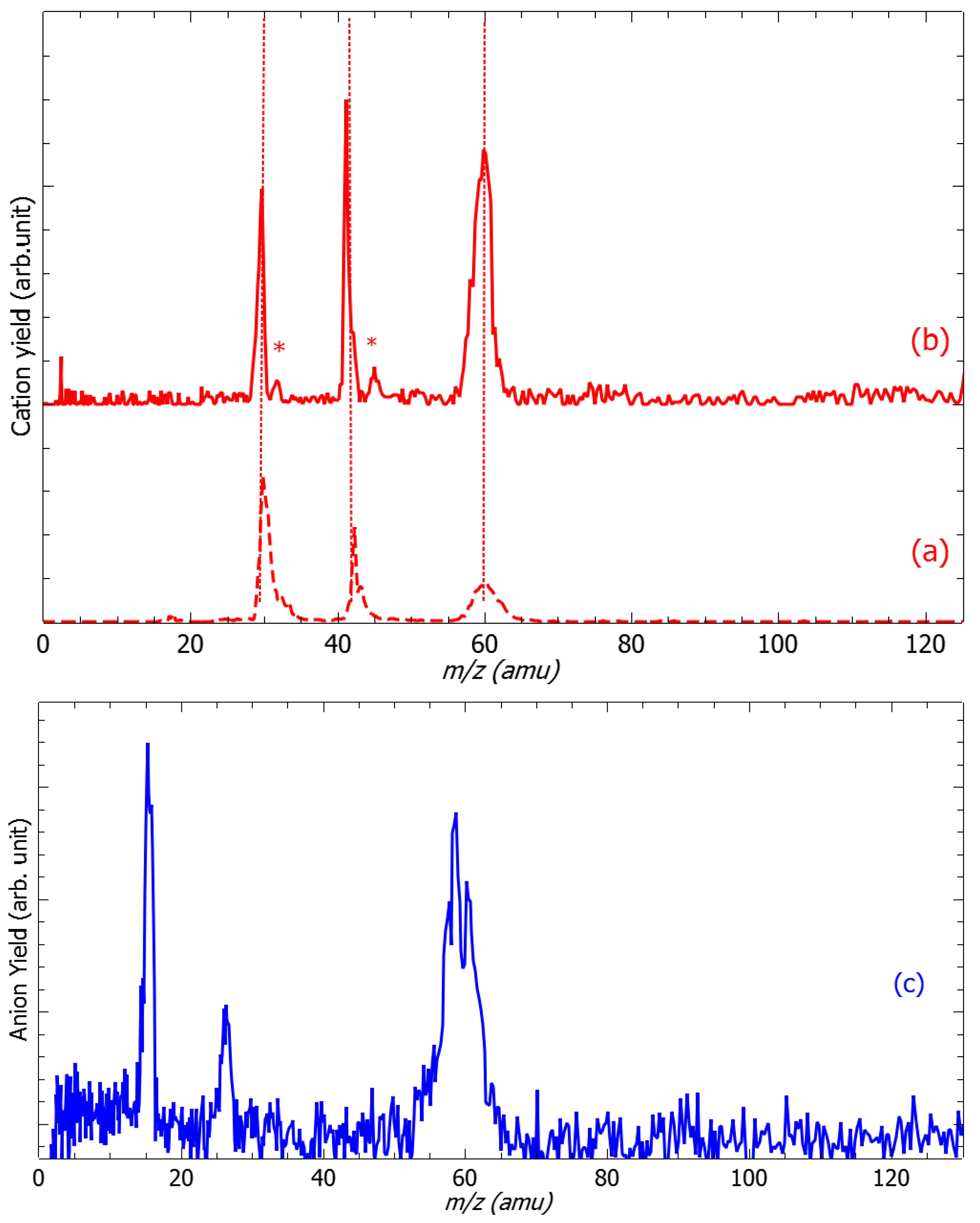
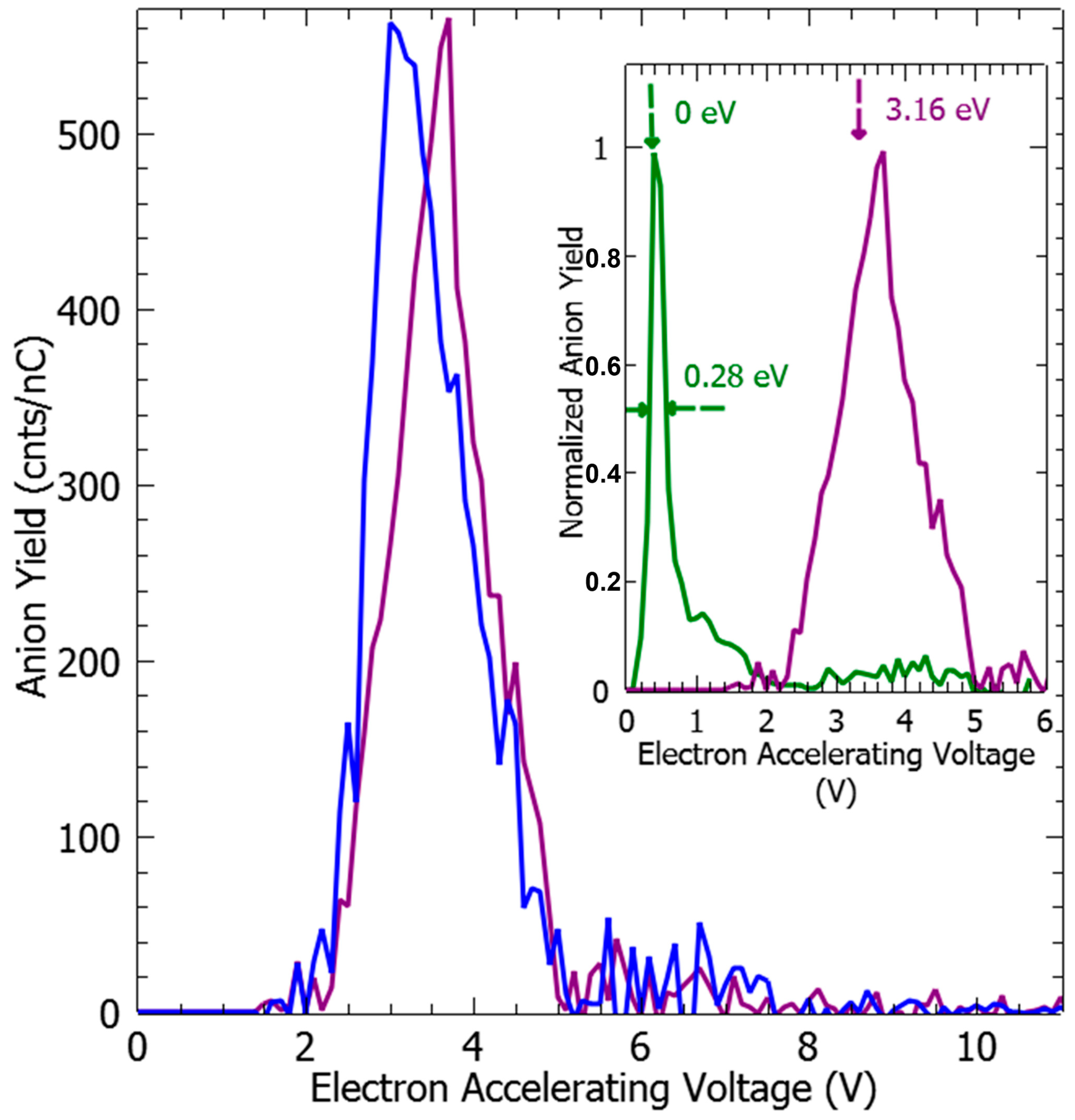
2. Results and Discussion
3. Methods
3.1. Experimental Method
3.2. Theoretical Method
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Eller, K.; Henkes, E.; Rossbacher, R.; Höke, H. Amines aliphatic. In Ullmann’s Encyclopedia of Industrial Chemistry; Wiley-VCH Verlag: Weinheim, Germany, 2005. [Google Scholar] [CrossRef]
- The Global Ethylenediamine (EDA) market size increase from 520 to 627 Kilo Tons from 2017 to 2022 and It Is Expected to Reach 831 Kilo Tons in 2027. Available online: https://www.hdinresearch.com/news/257 (accessed on 25 December 2023).
- Fang, L.J.; Li, Y.H.; Liu, P.F.; Wang, D.P.; Zeng, H.D.; Wang, X.L.; Yang, H.G. Facile fabrication of large aspect ratio g-C3N4 nanosheets for enhanced photocatalytic hydrogen evolution. ACS Sustain. Chem. Eng. 2017, 5, 2039–2043. [Google Scholar] [CrossRef]
- Chen, M.; Xie, Y.; Chen, H.; Qiao, Z.; Qian, Y. Preparation and characterization of metal sulfides in ethylendiamine under ambient condition through a γ-irradiation route. J. Colloid Interf. Sci. 2001, 237, 47–53. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.; Je, M.; Ton, N.N.T.; Lei, W.; Taniike, T.; Yanagida, S.; Ogawa, D.; Suzuki, N.; Terashima, C.; Fujishima, A.; et al. C-doped ZnS-ZnO/Rh nanosheets as multijunctioned photocatalysts for effective H2 generation from pure water under solar simulating light. Appl. Catal. B Environ. 2021, 297, 120473. [Google Scholar] [CrossRef]
- Ali, H.E.; Nasef, S.M.; Gad, Y.H. Remediation of Astrazon blue and Lerui acid brilliant blue dyes from waste solution using amphoteric supraparamagnetic nanocomposite hydrogen based on chitosan prepared by gamma rays. Carbohydr. Polym. 2022, 283, 119149. [Google Scholar] [CrossRef] [PubMed]
- Editorial, Site selective reactions: Nature chemistry. Nat. Chem. 2012, 4, 955.
- Brixner, T.; Pfeifer, T.; Gerber, G.; Wollenhaupt, M.; Baumert, T. Optimal control of atomic, molecular and electron dynamics with tailored femtosecond laser pulses. In Femtosecond Laser Spectroscopy; Hannaford, P., Ed.; Springer Science: New York, NY, USA, 2005; Chapter 9; pp. 225–263. [Google Scholar]
- Decker, C. Photoinitiated cross-linking polymerization. Prog. Polymer. Sci. 1996, 21, 593–650. [Google Scholar] [CrossRef]
- Peiffer, R.W. Photopolymerization: Fundamentals and Applications; Scranton, A.B., Bowman, C.N., Peiffer, R.W., Eds.; American Chemical Society: Washington, DC, USA, 1997; pp. 1–14. [Google Scholar]
- Sullivan, K.K.; Boamah, M.D.; Shulenberger, K.E.; Chapman, S.; Atkinson, K.E.; Boyer, M.C.; Arumainayagam, C.R. Low energy (<20 eV) and high-energy (1000 eV) electron- induced methanol radiolysis of astronomical interest. Mon. Not. R. Astron. Soc. 2016, 460, 664–672. [Google Scholar]
- Böhler, E.; Warneke, J.; Swiderek, P. Control of chemical and synthesis by low energy electrons. Chem. Soc. Rev. 2013, 42, 9219–9231. [Google Scholar] [CrossRef]
- Abdoul-Carime, H.; Bald, I.; Illenberger, E.; Kopyra, J. Selective synthesis of ethylene and acetylene from dimethyl sulfide cold films controlled by slow electrons. J. Phys. Chem. C 2018, 122, 24137–24142. [Google Scholar] [CrossRef]
- Hla, S.-W.; Rieder, K.-H. STM control of chemical reaction: Single molecule synthesis. Annu. Rev. Phys. Chem. 2003, 54, 307–330. [Google Scholar] [CrossRef]
- Blackwell, D.D.; Chen, F.F. Time resolved measurements of the electron energy distribution function in helicon plasma. Plasma Sources Sci. Technol. 2001, 10, 226–235. [Google Scholar] [CrossRef]
- Szczerbinski, J.; Gyr, L.; Kaeslin, J.; Zenobi, R. Plasmon driven photocatalysis leads to products known from E-beam and X-ray induced surface chemistry. Nano Lett. 2018, 18, 6740–6749. [Google Scholar] [CrossRef] [PubMed]
- Bernardi, M.; Mustafa, J.; Neaton, J.B.; Louie, S.G. Theory and computation of hot carriers generated by surface plasmon polartitons in noble metal. Nat. Comm. 2015, 6, 7044. [Google Scholar] [CrossRef]
- Illenberger, E.; Momigny, J. Gaseous Molecular Ions: An Introduction to Elementary Processes Induced by Ionization; Baümgartel, H., Franck, E.U., Grünbein, W., Eds.; Springer: New York, NY, USA, 1992. [Google Scholar]
- Fabrikant, I.I.; Eden, S.; Mason, N.J.; Fedor, J. Recent progress in dissociative electron attachment: From diatomic to biomolecule. Adv. Atom. Mol. Opt. Phys. 2017, 66, 545–657. [Google Scholar]
- Abdoul-Carime, H.; Thiam, G.; Rabilloud, F.; Charlieux, F.; Kopyra, J. Chemistry in acetonitrile-water films induced by slow (<15 eV) electrons; application to the Eath and space chemistry. ACS Earth Space Chem. 2022, 6, 1126–1132. [Google Scholar]
- NIST, Webbook of Chemistry. Available online: https://webbook.nist.gov/cgi/cbook.cgi?ID=107-15-3&Units=SI (accessed on 25 December 2023).
- Matsuura, K.; Muto, H. Electronic structure of acetylene radical anion with a trans-bent form. J. Phys. Chem. 1993, 97, 8842–8844. [Google Scholar] [CrossRef]
- Ha, T.-K.; Suter, H.U.; Nguyen, M.T. Is acetylene radical anion with a tran-bent form observed in matrix experiment? An ab initio study. J. Chem. Phys. 1996, 105, 6385–6387. [Google Scholar] [CrossRef]
- Chandrasekhar, J.; Kahn, R.A.; von Ragué Schleyer, P. The preferred structure of C2H2−. Chem. Phys. Lett. 1982, 85, 493–495. [Google Scholar] [CrossRef]
- Matejcik, S.; Kiendler, A.; Stamatovic, A.; Märk, T.D. A crossed beam high resolution study of dissociative electron attachment to CCl4. Int. J. Mass. Spectrom. Ion Proc. 1995, 149, 311–319. [Google Scholar] [CrossRef]
- Desfrançois, C.; Bouteiller, Y.; Schermann, J.P.; Radisic, D.; Stokes, S.T.; Bowen, K.H.; Hammer, N.I.; Compton, R.N. Long-Range Electron Binding to Quadrupolar Molecules. Phys. Rev. Lett. 2004, 92, 083003. [Google Scholar] [CrossRef]
- Abdoul-Carime, H.; Mounier, F.; Charlieux, F.; André, H. Correlated ion-ion/neutral time of flight mass spectrometer. Rev. Sci. Instrum. 2023, 94, 045104. [Google Scholar] [CrossRef] [PubMed]
- Denifl, S.; Ptasinska, S.; Probst, M.; Hrusak, J.; Scheier, P.; Märk, T.D. Alectron attachment to the gas phase DNA base cytosine and thymine. J. Phys. Chem. A 2004, 108, 6562–6569. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian 16, Revision C.01; Gaussian, Inc.: Wallingford, CT, USA, 2016. [Google Scholar]
- Chai, J.; Head-Gordon, M. Systematic optimization of long-range corrected hybrid density functionals. J. Chem. Phys. 2008, 128, 084106. [Google Scholar] [CrossRef] [PubMed]
- Kendall, R.A.; Dunning, T.H.J.; Harrison, R.J. Electron affinities of the first-row atoms revisited. Systematic basis sets and wave functions. J. Chem. Phys. 1992, 96, 6796–6806. [Google Scholar] [CrossRef]
- Thiam, G.; Rabilloud, F. Multi-basis-set (TD-)DFT methods for predicting electron attachment energies. J. Phys. Chem. Lett. 2021, 12, 9995–10001. [Google Scholar] [CrossRef]
- Allouche, A.-R.; Gabedi, A. User Interface for Computational Chemistry Softwares. J. Comput. Chem. 2011, 32, 174–182. [Google Scholar] [CrossRef]
- Vinogradoff, V.; Duvernay, F.; Danger, G.; Theulé, P.; Borget, F.; Chiavassa, T. Formaldehyde and methylamine reactivity in interstellar ices analogues as a source of molecular complexity at low temperature. Astron. Astrophys. 2013, 549, A40. [Google Scholar] [CrossRef]


| Reaction | Fragmentation Energy (0 K) | Gibbs Free Energy (298 K) |
|---|---|---|
| EDA → (EDA-H)− + H | ||
| 4.68 | 3.31 |
| 4.05 | 3.30 |
| 5.26 | 3.96 |
| 4.63 | 3.89 |
| 7.91 | 6.50 |
| 3.24 | 2.55 |
| 8.61 | 5.94 |
| 3.32 | 1.42 |
| 3.74 | 1.93 |
| 2.95 | 1.83 |
| Anion Fragment | Peak Position (±0.15 eV) | Calculated EDA#− Resonance State (eV) | EDA#− Molecular Orbital (Figure 4) |
|---|---|---|---|
| EDA− | 2.72 | 2.81 | 24 |
| (EDA-H)− | 3.16 (29%) | 3.01 | 25 |
| CN− | 3.07 (15%) | 3.01 | 25 |
| 4.23 (21%) | 4.36, 4.46 | 26, 27 | |
| NH− | 2.94 (56%) | 3.01 | 25 |
| 3.93 (79%) | 4.36, 4.46 | 26, 27 | |
| 4.80 | |||
| 5.92 | 5.75 | 28 | |
| 6.99 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abdoul-Carime, H.; Lys, E.; Gipouloux, J.; Rabilloud, F. Experimental and Theoretical Investigations of the Fragmentation of Ethylenediamine Induced by Low-Energy (<10 eV) Electrons. Molecules 2024, 29, 191. https://doi.org/10.3390/molecules29010191
Abdoul-Carime H, Lys E, Gipouloux J, Rabilloud F. Experimental and Theoretical Investigations of the Fragmentation of Ethylenediamine Induced by Low-Energy (<10 eV) Electrons. Molecules. 2024; 29(1):191. https://doi.org/10.3390/molecules29010191
Chicago/Turabian StyleAbdoul-Carime, Hassan, Elena Lys, Jeanne Gipouloux, and Franck Rabilloud. 2024. "Experimental and Theoretical Investigations of the Fragmentation of Ethylenediamine Induced by Low-Energy (<10 eV) Electrons" Molecules 29, no. 1: 191. https://doi.org/10.3390/molecules29010191






