Enhancing Lithium-Sulfur Battery Performance by MXene, Graphene, and Ionic Liquids: A DFT Investigation
Abstract
:1. Introduction
2. Results and Discussion
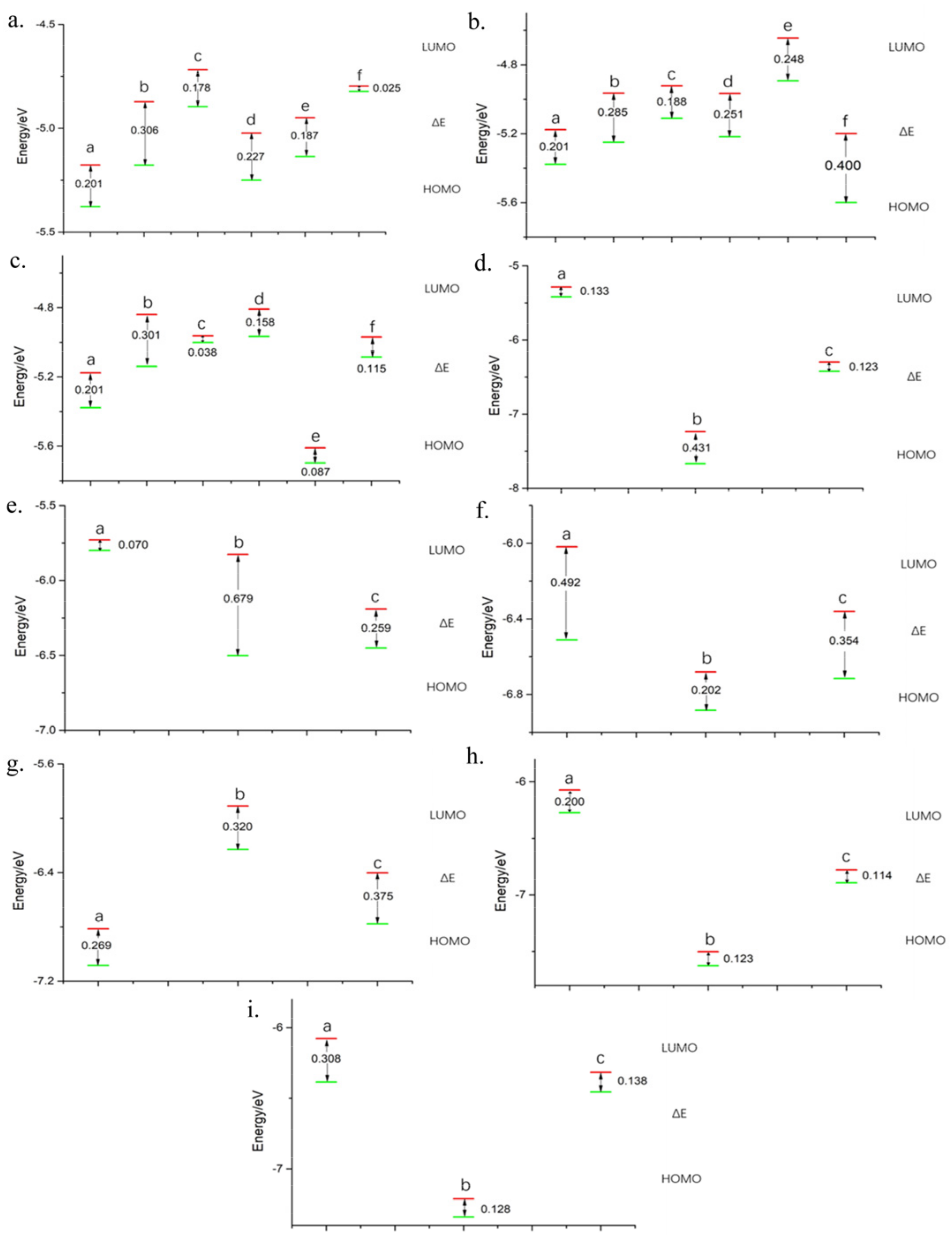
2.1. Graphite Oxide Materials Frontier Molecular Orbital Analysis
2.2. Ionic Liquid Frontier Molecular Orbital Analysis
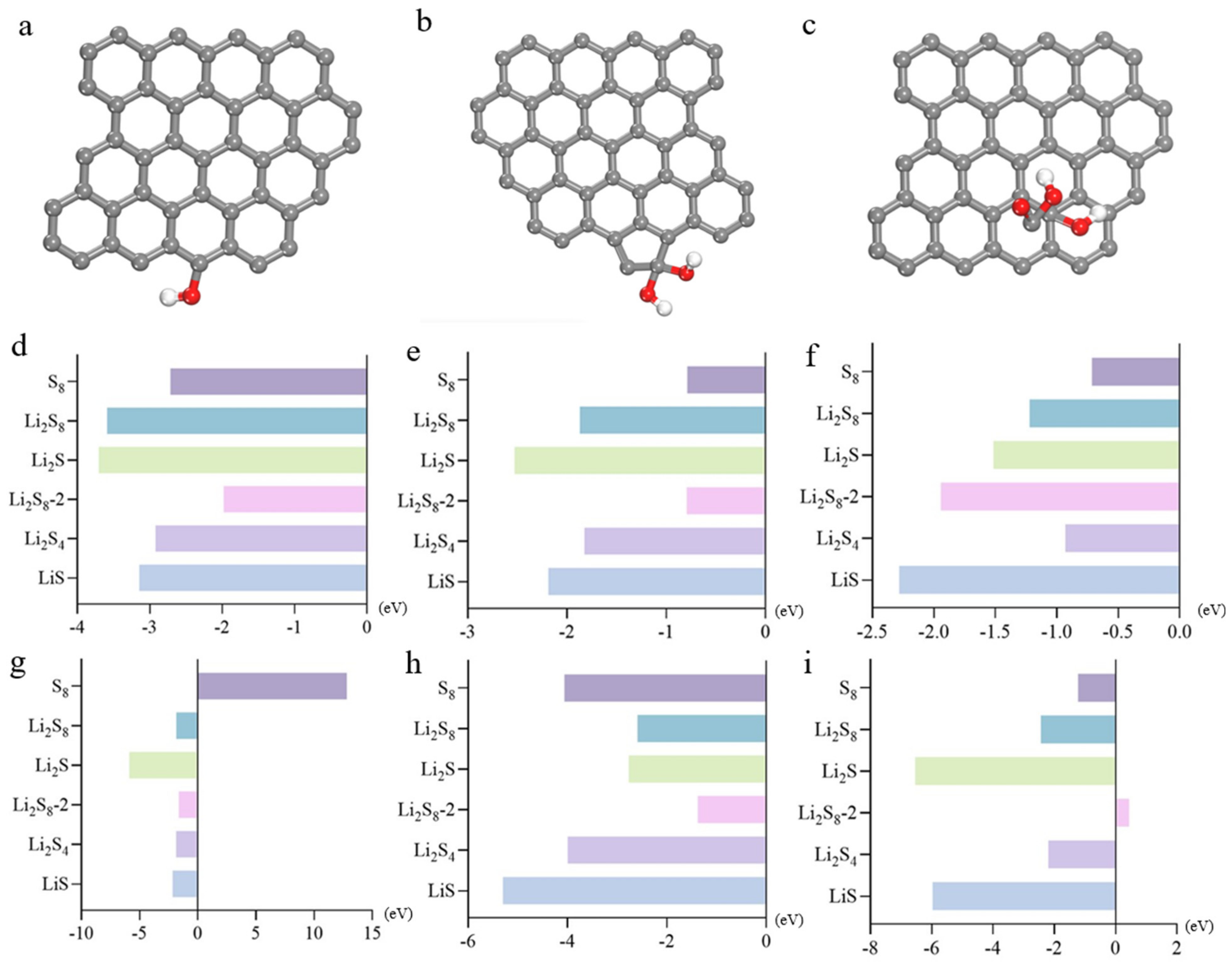
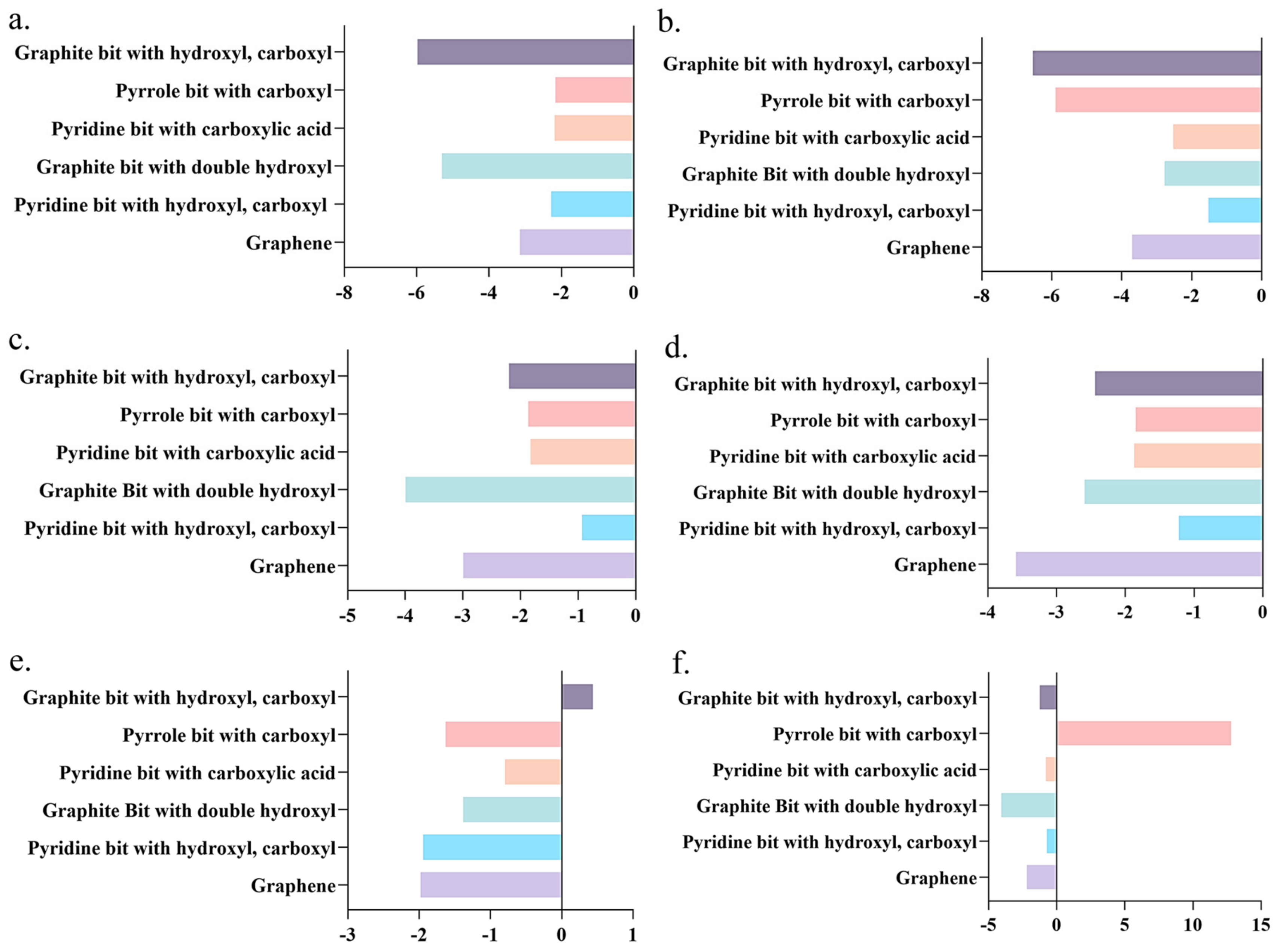
2.3. Adsorption of Polysulfide with Graphite Oxide
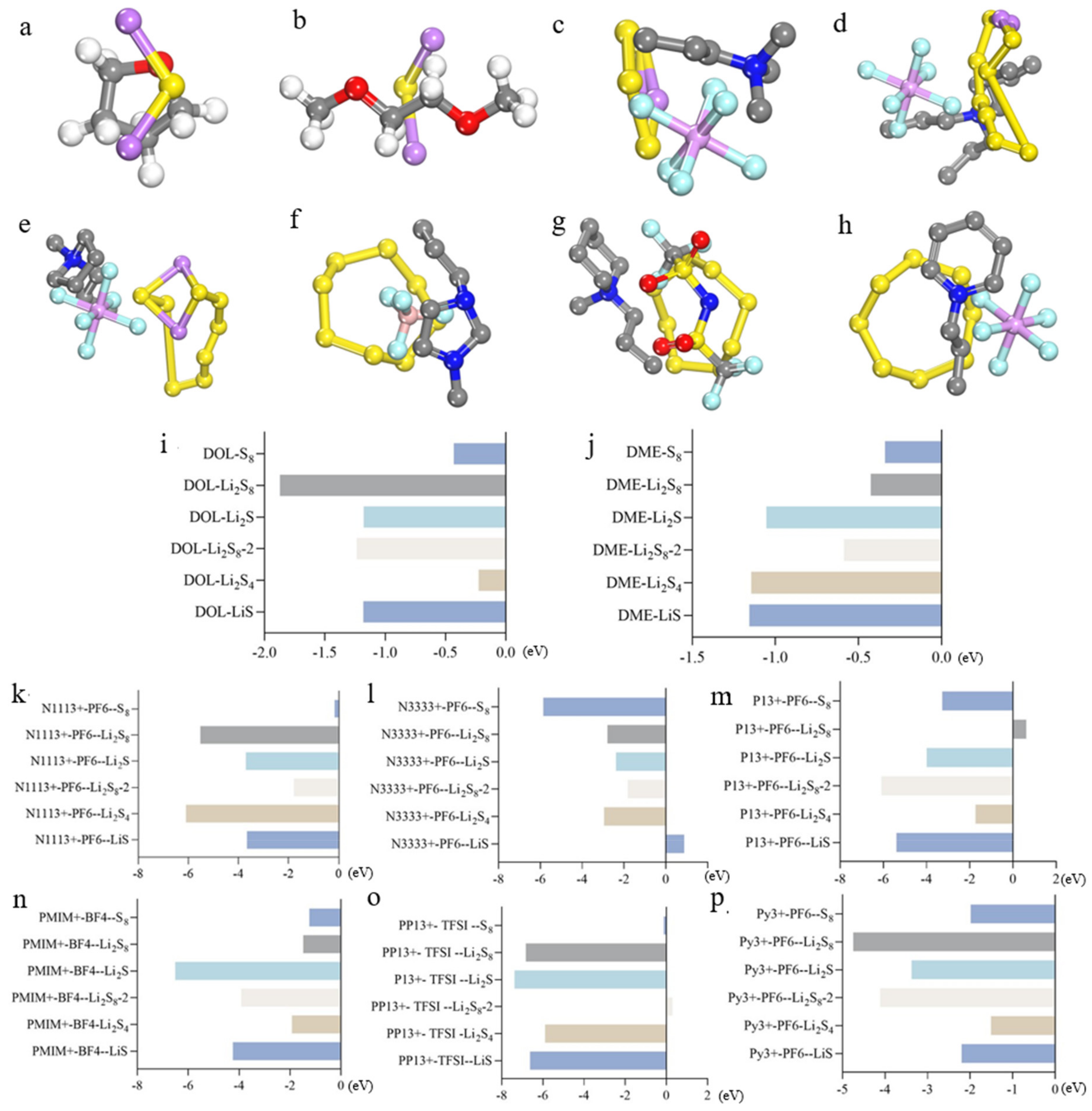
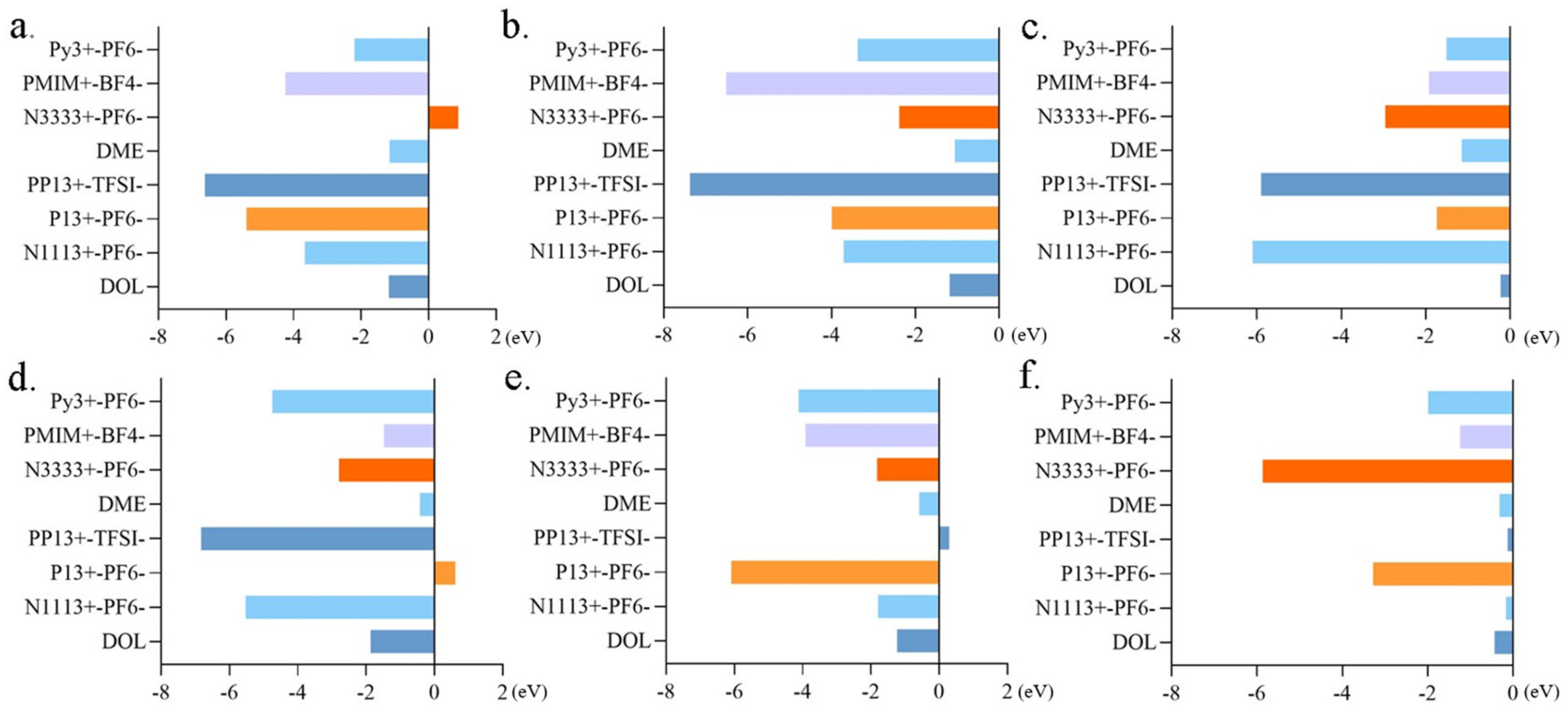
2.4. Dissolution Behavior of Polysulfides in Electrolyte Solutions
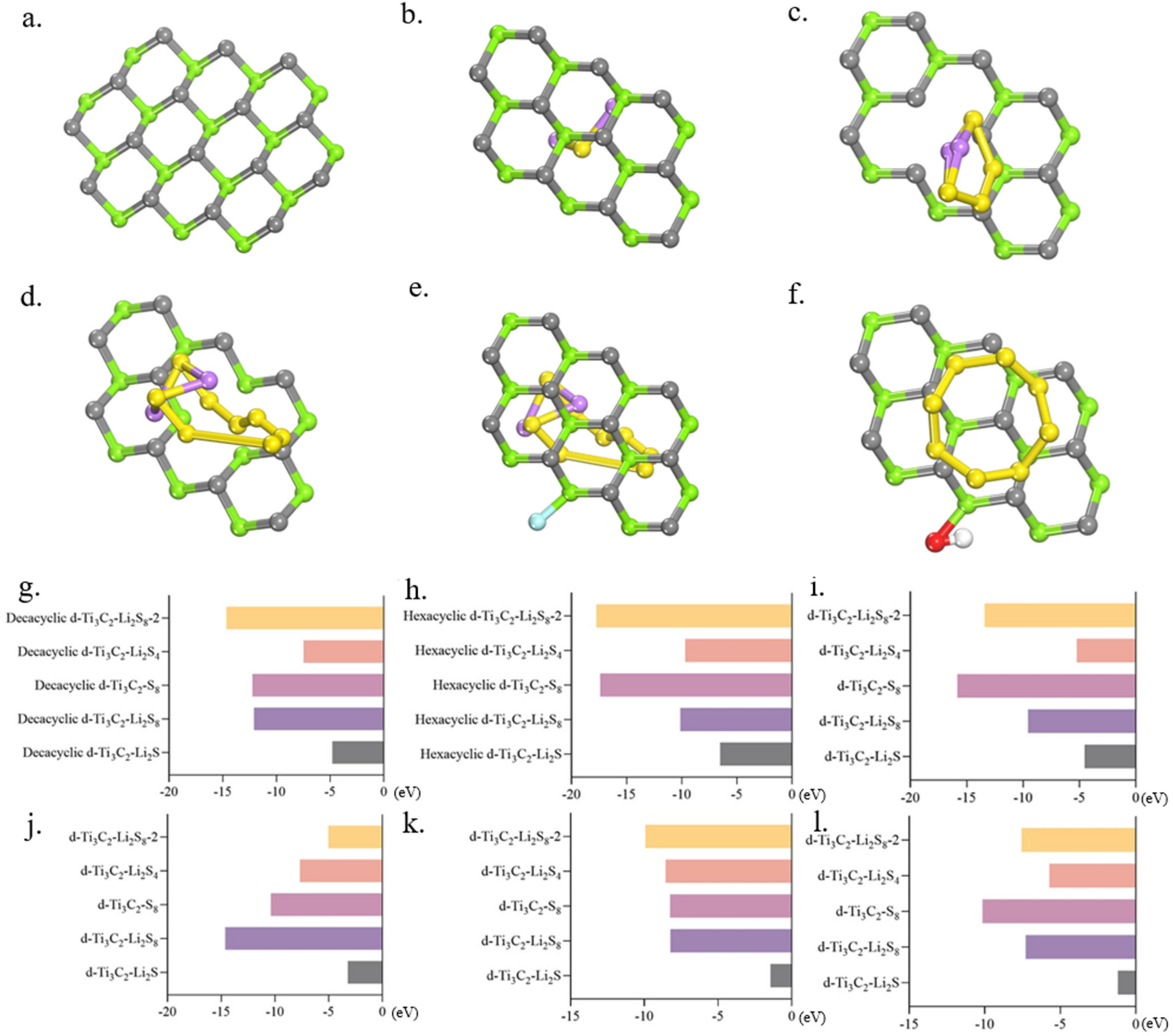
2.5. Adsorption Behaviour of d-Ti3C2-MXene Monolayer for Polysulfide

3. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Liu, J.; Zheng, Q.; Goodman, M.D.; Zhu, H.; Kim, J.; Krueger, N.A.; Ning, H.; Huang, X.; Liu, J.; Terrones, M.; et al. Graphene Sandwiched Mesostructured Li-ion Battery Electrodes. Adv. Mater. 2016, 28, 7696–7702. [Google Scholar] [CrossRef] [PubMed]
- Liu, R. Li-ion Battery. In Battery Technologies: Materials and Components; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2022; pp. 1–46. [Google Scholar]
- Zhou, L.; Pan, F.; Zeng, S.; Li, Q.; Bai, L.; Liu, Y.; Nie, Y. Ionic liquid assisted fabrication of cellulose-based conductive films for Li-ion battery. J. Appl. Polym. Sci. 2020, 137, 49430. [Google Scholar] [CrossRef]
- Zhu, F.; Liu, G.; Tao, C.; Wang, K.; Jiang, K. Battery management system for Li-ion battery. J. Eng. 2017, 2017, 1437–1440. [Google Scholar] [CrossRef]
- Manthiram, A.; Yu, X.; Wang, S. Lithium battery chemistries enabled by solid-state electrolytes. Nat. Rev. Mater. 2017, 2, 16103. [Google Scholar] [CrossRef]
- Frith, J.T.; Lacey, M.J.; Ulissi, U. A non-academic perspective on the future of lithium-based batteries. Nat. Commun. 2023, 14, 420. [Google Scholar] [CrossRef] [PubMed]
- Moeremans, B.; Cheng, H.-W.; Hu, Q.; Garces, H.F.; Padture, N.P.; Renner, F.U.; Valtiner, M. Lithium-ion battery electrolyte mobility at nano-confined graphene interfaces. Nat. Commun. 2016, 7, 12693. [Google Scholar] [CrossRef] [PubMed]
- Manthiram, A.; Fu, Y.; Su, Y.-S. Challenges and Prospects of Lithium–Sulfur Batteries. Acc. Chem. Res. 2013, 46, 1125–1134. [Google Scholar] [CrossRef]
- Zhu, K.; Wang, C.; Chi, Z.; Ke, F.; Yang, Y.; Wang, A.; Wang, W.; Miao, L. How far away are lithium-sulfur batteries from commercialization? Front. Energy Res. 2019, 7, 123. [Google Scholar] [CrossRef]
- Kim, K.R.; Lee, K.-S.; Ahn, C.-Y.; Yu, S.-H.; Sung, Y.-E. Discharging a Li-S battery with ultra-high sulfur content cathode using a redox mediator. Sci. Rep. 2016, 6, 32433. [Google Scholar] [CrossRef]
- Zhao, Q.; Zhu, Q.; Liu, Y.; Xu, B. Status and Prospects of MXene-Based Lithium–Sulfur Batteries. Adv. Funct. Mater. 2021, 31, 2100457. [Google Scholar] [CrossRef]
- Kim, J.T.; Rao, A.; Nie, H.Y.; Hu, Y.; Li, W.; Zhao, F.; Deng, S.; Hao, X.; Fu, J.; Luo, J.; et al. Manipulating Li(2)S(2)/Li(2)S mixed discharge products of all-solid-state lithium sulfur batteries for improved cycle life. Nat. Commun. 2023, 14, 6404. [Google Scholar] [CrossRef]
- Qu, H.; Zhang, J.; Du, A.; Chen, B.; Chai, J.; Xue, N.; Wang, L.; Qiao, L.; Wang, C.; Zang, X.; et al. Multifunctional Sandwich-Structured Electrolyte for High-Performance Lithium–Sulfur Batteries. Adv. Sci. 2018, 5, 1700503. [Google Scholar] [CrossRef]
- Parke, C.D.; Subramaniam, A.; Subramanian, V.R.; Schwartz, D.T. Realigning the Chemistry and Parameterization of Lithium-sulfur Battery Models to Accommodate Emerging Experimental Evidence and Cell Configurations. ChemElectroChem 2021, 8, 1098–1106. [Google Scholar] [CrossRef]
- Zhou, L.; Danilov, D.L.; Eichel, R.-A.; Notten, P.H.L. Host Materials Anchoring Polysulfides in Li–S Batteries Reviewed. Adv. Energy Mater. 2021, 11, 2001304. [Google Scholar] [CrossRef]
- Tao, X.; Wang, J.; Liu, C.; Wang, H.; Yao, H.; Zheng, G.; Seh, Z.W.; Cai, Q.; Li, W.; Zhou, G.; et al. Balancing surface adsorption and diffusion of lithium-polysulfides on nonconductive oxides for lithium-sulfur battery design. Nat. Commun. 2016, 7, 11203. [Google Scholar] [CrossRef]
- Chen, M.; Wang, N.; Zhou, W.; Zhu, X.; Wu, Q.; Lee, M.-H.; Zhao, D.; Ning, S.; An, M.; Li, L. N-Doping Induced Lattice Expansion of 1D Template Confined Ultrathin MoS2 Sheets to Significantly Enhance Lithium Polysulfides Redox Kinetics for Li–S Battery. Small 2023, 19, 2303015. [Google Scholar] [CrossRef]
- Liu, J.; Zhu, M.; Shen, Z.; Han, T.; Si, T.; Hu, C.; Zhang, H. A Polysulfides-Confined All-in-One Porous Microcapsule Lithium–Sulfur Battery Cathode. Small 2021, 17, 2103051. [Google Scholar] [CrossRef]
- Mo, Y.-X.; Wu, Y.-J.; Yin, Z.-W.; Ren, W.-F.; Gao, Z.-G.; Zhang, P.-F.; Lin, J.-X.; Zhou, Y.; Li, J.-T.; Huang, L.; et al. High Cycling Performance Li-S Battery via Fenugreek Gum Binder through Chemical Bonding of the Binder with Polysulfides in Nanosulfur@CNFs Cathode. ChemistrySelect 2020, 5, 8969–8979. [Google Scholar] [CrossRef]
- Patel, M.U.M.; Arčon, I.; Aquilanti, G.; Stievano, L.; Mali, G.; Dominko, R. X-ray Absorption Near-Edge Structure and Nuclear Magnetic Resonance Study of the Lithium–Sulfur Battery and its Components. Chemphyschem 2014, 15, 894–904. [Google Scholar] [CrossRef]
- Sun, T.; Zhao, X.; Li, B.; Shu, H.; Luo, L.; Xia, W.; Chen, M.; Zeng, P.; Yang, X.; Gao, P.; et al. NiMoO4 Nanosheets Anchored on N-S Doped Carbon Clothes with Hierarchical Structure as a Bidirectional Catalyst toward Accelerating Polysulfides Conversion for Li-S Battery. Adv. Funct. Mater. 2021, 31, 2101285. [Google Scholar] [CrossRef]
- Wang, Q.; Lu, Y.; Jin, J.; Chen, C.; Wen, Z. Li1.5Al0.5Ge1.5(PO4)3 Ceramic Based Lithium-sulfur Batteries with High Cycling Stability Enabled by a Dual Confinement Effect for Polysulfides. ChemElectroChem 2020, 7, 4093–4100. [Google Scholar] [CrossRef]
- Tang, T.; Hou, Y. Chemical Confinement and Utility of Lithium Polysulfides in Lithium Sulfur Batteries. Small Methods 2020, 4, 1900001. [Google Scholar] [CrossRef]
- Yao, Y.; Feng, W.; Chen, M.; Zhong, X.; Wu, X.; Zhang, H.; Yu, Y. Boosting the Electrochemical Performance of Li–S Batteries with a Dual Polysulfides Confinement Strategy. Small 2018, 14, 1802516. [Google Scholar] [CrossRef]
- Deng, S.; Guo, T.; Heier, J.; Zhang, C. Unraveling Polysulfide’s Adsorption and Electrocatalytic Conversion on Metal Oxides for Li-S Batteries. Adv. Sci. 2023, 10, 2204930. [Google Scholar] [CrossRef]
- Lang, S.; Yu, S.H.; Feng, X.; Krumov, M.R.; Abruna, H.D. Understanding the lithium-sulfur battery redox reactions via operando confocal Raman microscopy. Nat. Commun. 2022, 13, 4811. [Google Scholar] [CrossRef]
- Yu, Z.; Shao, Y.; Ma, L.; Liu, C.; Gu, C.; Liu, J.; He, P.; Li, M.; Nie, Z.; Peng, Z.; et al. Revealing the Sulfur Redox Paths in a Li–S Battery by an In Situ Hyphenated Technique of Electrochemistry and Mass Spectrometry. Adv. Mater. 2022, 34, 2106618. [Google Scholar] [CrossRef]
- Zeng, Z.; Liu, X. Sulfur Immobilization by “Chemical Anchor” to Suppress the Diffusion of Polysulfides in Lithium–Sulfur Batteries. Adv. Mater. Interfaces 2018, 5, 1701274. [Google Scholar] [CrossRef]
- Dai, C.; Sun, G.; Hu, L.; Xiao, Y.; Zhang, Z.; Qu, L. Recent progress in graphene-based electrodes for flexible batteries. InfoMat 2020, 2, 509–526. [Google Scholar] [CrossRef]
- Boota, M.; Gogotsi, Y. MXene—Conducting Polymer Asymmetric Pseudocapacitors. Adv. Energy Mater. 2019, 9, 1802917. [Google Scholar] [CrossRef]
- Cui, C.; Cheng, R.; Zhang, H.; Zhang, C.; Ma, Y.; Shi, C.; Fan, B.; Wang, H.; Wang, X. Ultrastable MXene@Pt/SWCNTs’ Nanocatalysts for Hydrogen Evolution Reaction. Adv. Funct. Mater. 2020, 30, 2000693. [Google Scholar] [CrossRef]
- Dekanovsky, L.; Huang, H.; Akir, S.; Ying, Y.; Sofer, Z.; Khezri, B. Light-Driven MXene-Based Microrobots: Mineralization of Bisphenol A to CO2 and H2O. Small Methods 2023, 7, 2201547. [Google Scholar] [CrossRef] [PubMed]
- Fu, H.-C.; Ramalingam, V.; Kim, H.; Lin, C.-H.; Fang, X.; Alshareef, H.N.; He, J.-H. MXene-Contacted Silicon Solar Cells with 11.5% Efficiency. Adv. Energy Mater. 2019, 9, 1900180. [Google Scholar] [CrossRef]
- Fusco, L.; Gazzi, A.; Shuck, C.E.; Orecchioni, M.; Ahmed, E.I.; Giro, L.; Zavan, B.; Yilmazer, A.; Ley, K.; Bedognetti, D.; et al. V4C3 MXene Immune Profiling and Modulation of T Cell-Dendritic Cell Function and Interaction. Small Methods 2023, 7, 2300197. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Zhang, Y.; Sun, H.; Zhou, J.; Yang, F.; Li, H.; Chen, H.; Chen, Y.; Liu, Z.; Qiu, Z.; et al. Progress and Perspective: MXene and MXene-Based Nanomaterials for High-Performance Energy Storage Devices. Adv. Electron. Mater. 2021, 7, 2000967. [Google Scholar] [CrossRef]
- Li, W.; Zhang, Y.; Li, H.; Chen, Z.; Shang, T.; Wu, Z.; Zhang, C.; Li, J.; Lv, W.; Tao, Y.; et al. Layered MXene Protected Lithium Metal Anode as an Efficient Polysulfide Blocker for Lithium-sulfur Batteries. Batter. Supercaps 2020, 3, 892–899. [Google Scholar] [CrossRef]
- Wang, C.; Chen, S.; Song, L. Tuning 2D MXenes by Surface Controlling and Interlayer Engineering: Methods, Properties, and Synchrotron Radiation Characterizations. Adv. Funct. Mater. 2020, 30, 2000869. [Google Scholar] [CrossRef]
- Wang, Q.; Liu, A.; Qiao, S.; Zhang, Q.; Huang, C.; Lei, D.; Shi, X.; He, G.; Zhang, F. Mott-Schottky MXene@WS2 Heterostructure: Structural and Thermodynamic Insights and Application in Ultra Stable Lithium-sulfur Batteries. ChemSusChem 2023, 16, e202300507. [Google Scholar] [CrossRef]
- Wang, R.; Li, M.; Zhang, Y.; Sun, K.; Bao, W. Atomic surface modification strategy of MXene materials for high-performance metal sulfur batteries. Int. J. Energy Res. 2022, 46, 11659–11675. [Google Scholar] [CrossRef]
- Wang, X.; Luo, D.; Wang, J.; Sun, Z.; Cui, G.; Chen, Y.; Wang, T.; Zheng, L.; Zhao, Y.; Shui, L.; et al. Strain Engineering of a MXene/CNT Hierarchical Porous Hollow Microsphere Electrocatalyst for a High-Efficiency Lithium Polysulfide Conversion Process. Angew. Chem. 2021, 133, 2401–2408. [Google Scholar] [CrossRef]
- Wei, C.; Xi, B.; Wang, P.; Liang, Y.; Wang, Z.; Tian, K.; Feng, J.; Xiong, S. In Situ Anchoring Ultrafine ZnS Nanodots on 2D MXene Nanosheets for Accelerating Polysulfide Redox and Regulating Li Plating. Adv. Mater. 2023, 35, 2303780. [Google Scholar] [CrossRef]
- Yang, J.; Bao, W.; Jaumaux, P.; Zhang, S.; Wang, C.; Wang, G. MXene-Based Composites: Synthesis and Applications in Rechargeable Batteries and Supercapacitors. Adv. Mater. Interfaces 2019, 6, 1802004. [Google Scholar] [CrossRef]
- Ye, Z.; Jiang, Y.; Li, L.; Wu, F.; Chen, R. Self-Assembly of 0D–2D Heterostructure Electrocatalyst from MOF and MXene for Boosted Lithium Polysulfide Conversion Reaction. Adv. Mater. 2021, 33, 2101204. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Luo, C.; Zhou, G.; Pan, Z.-Z.; Ma, J.; Nishihara, H.; He, Y.-B.; Kang, F.; Lv, W.; Yang, Q.-H. Lamellar MXene Composite Aerogels with Sandwiched Carbon Nanotubes Enable Stable Lithium–Sulfur Batteries with a High Sulfur Loading. Adv. Funct. Mater. 2021, 31, 2100793. [Google Scholar] [CrossRef]
- Zhang, C.; Ma, Y.; Zhang, X.; Abdolhosseinzadeh, S.; Sheng, H.; Lan, W.; Pakdel, A.; Heier, J.; Nüesch, F. Two-Dimensional Transition Metal Carbides and Nitrides (MXenes): Synthesis, Properties, and Electrochemical Energy Storage Applications. Energy Environ. Mater. 2020, 3, 29–55. [Google Scholar] [CrossRef]
- Zhang, Y.; Mu, Z.; Yang, C.; Xu, Z.; Zhang, S.; Zhang, X.; Li, Y.; Lai, J.; Sun, Z.; Yang, Y.; et al. Rational Design of MXene/1T-2H MoS2-C Nanohybrids for High-Performance Lithium–Sulfur Batteries. Adv. Funct. Mater. 2018, 28, 1707578. [Google Scholar] [CrossRef]
- Cheviri, M.; Lakshmipathi, S. DFT study of chemical reactivity parameters of lithium polysulfide molecules Li2Sn(1≤ n ≤8) in gas and solvent phase. Comput. Theor. Chem. 2021, 1202, 113323. [Google Scholar] [CrossRef]
- Lu, H.; Chen, Z.; Du, H.; Zhang, K.; Wang, J.; Hou, Z.; Fang, J. The enhanced performance of lithium sulfur battery with ionic liquid-based electrolyte mixed with fluorinated ether. Ionics 2019, 25, 2685–2691. [Google Scholar] [CrossRef]
- Dai, S.; Wang, C.; Huang, C.; Li, S.; Xu, Y.; Song, Y.; Zeng, G.; Zhu, J.; Sun, T.; Huang, M. A Polymer Network Layer Containing Dually Anchored Ionic Liquids for Stable Lithium–Sulfur Batteries. Macromol. Rapid Commun. 2023, 44, 2200246. [Google Scholar] [CrossRef]
- Liu, X.; Lu, Y.; Zeng, Q.; Chen, P.; Li, Z.; Wen, X.; Wen, W.; Li, Z.; Zhang, L. Trapping of Polysulfides with Sulfur-Rich Poly Ionic Liquid Cathode Materials for Ultralong-Life Lithium–Sulfur Batteries. ChemSusChem. 2020, 13, 715–723. [Google Scholar] [CrossRef]
- Liang, Q.; Wang, S.; Yao, Y.; Dong, P.; Song, H. Transition Metal Compounds Family for Li–S Batteries: The DFT-Guide for Suppressing Polysulfides Shuttle. Adv. Funct. Mater. 2023, 33, 2300825. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cao, J.; Xue, S.; Zhang, J.; Ren, X.; Gao, L.; Ma, T.; Liu, A. Enhancing Lithium-Sulfur Battery Performance by MXene, Graphene, and Ionic Liquids: A DFT Investigation. Molecules 2024, 29, 2. https://doi.org/10.3390/molecules29010002
Cao J, Xue S, Zhang J, Ren X, Gao L, Ma T, Liu A. Enhancing Lithium-Sulfur Battery Performance by MXene, Graphene, and Ionic Liquids: A DFT Investigation. Molecules. 2024; 29(1):2. https://doi.org/10.3390/molecules29010002
Chicago/Turabian StyleCao, Jianghui, Sensen Xue, Jian Zhang, Xuefeng Ren, Liguo Gao, Tingli Ma, and Anmin Liu. 2024. "Enhancing Lithium-Sulfur Battery Performance by MXene, Graphene, and Ionic Liquids: A DFT Investigation" Molecules 29, no. 1: 2. https://doi.org/10.3390/molecules29010002
APA StyleCao, J., Xue, S., Zhang, J., Ren, X., Gao, L., Ma, T., & Liu, A. (2024). Enhancing Lithium-Sulfur Battery Performance by MXene, Graphene, and Ionic Liquids: A DFT Investigation. Molecules, 29(1), 2. https://doi.org/10.3390/molecules29010002







