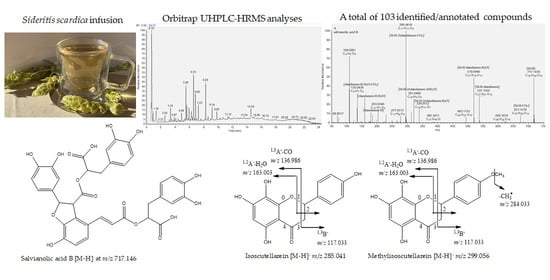
A Comprehensive Phytochemical Analysis of Sideritis scardica Infusion Using Orbitrap UHPLC-HRMS
Abstract
:1. Introduction
2. Results and Discussion
2.1. Metabolite Profiling of S. scardica Lyophilized Infusion
2.1.1. Sugar Acids and Saccharides
2.1.2. Carboxylic, Hydroxybenzoic, Hydroxycinnamic Acids and Their Derivatives
2.1.3. Caffeic Acids Oligomers
2.1.4. Acylquinic Acid
2.1.5. Acylhexaric Acids
2.1.6. Phenylethanoid Glycosides
2.1.7. Iridoid Glycosides
2.1.8. Flavonoids
2.1.9. Fatty Acids and Organosulfur Compounds
| No | Identified/Tentatively Annotated Compound | Molecular Formula | Exact Mass [M−H]− | Fragmentation Pattern in (-) ESI-MS/MS | tR (min) | Δ ppm | Level of Confidence [29] | Content [mg/g li] Mean ± SD |
|---|---|---|---|---|---|---|---|---|
| Sugar acids and saccharides | ||||||||
| 1. | Xylonic acid | C5H10O6 | 165.0405 | 165.0395 (60.5), 147.0288 (8.8), 129.0181 (12.3), 111.0073 (0.3), 105.0179 (33.1), 101.0228 (2.7), 87.0073 (43.1), 75.0072 (100) | 0.68 | −6.006 | 2 | - |
| 2. | Hexose | C6H12O6 | 179.0561 | 179.0541 (68.6), 161.0444 (11.1), 143.0338 (10.1), 125.0231 (1.53), 99.0437 (1.71), 81.0331 (4.8), 75.0072 (100) | 0.70 | −4.975 | 2 | - |
| 3. | Gluconic acid | C6H12O7 | 195.0510 | 195.0503 (96.1), 177.0396 (18.6), 159.0288 (14.7), 147.0287 (15.5), 141.0184 (5.9), 129.0180 (48.3), 111.0073 (5.3), 105.0179 (332.8), 75.0072 (100) | 0.72 | −3.619 | 2 | - |
| 4. | Pentose | C5H10O5 | 149.0456 | 149.0444 (21.6), 131.0334 (5.2), 101.0224 (0.8), 89.0229 (17.8), 75.0072 (100) | 0.74 | −7.358 | 2 | - |
| 5. | Asystoside | C25H44O15 | 583.2607 | 583.2618 (100), 421.2090 (4.9), 289.1662 (15.4), 161.0445 (15.8), 101.0230 (21.3), 71.0123 (30.6) | 6.34 | 1.880 | 2 | - |
| Carboxylic acids | ||||||||
| 6. | Quinic acid | C7H12O6 | 191.0561 | 191.0553 (100), 173.0446 (1.8), 155.0340 (0.2), 137.022 (0.2), 127.0387 (3.3), 99.0438 (0.6), 93.0331 (5.6), 85.0279 (18.2), 71.0123 (1.6), 59.0123 (1.37) | 0.69 | −4.194 | 2 | - |
| 7. | Oxaloglutaric acid | C7H8O7 | 203.0197 | 203.0191 (100), 159.0292 (2.3), 141.0181 (27.8), 115.0022 (11.7), 97.0279 (97.1), 95.0123 (14.2), 79.0174 (11.2), 72.9915 (7.1), 71.0123 (50.6), 69.0330 (66.2) | 0.88 | −2.984 | 2 | - |
| 8. | Citric acid | C6H8O7 | 191.0197 | 191.0191 (2.6), 173.0084 (1.43), 154.9979 (0.7), 147.0286 (0.4), 129.0181 (6.1), 111.0074 (100), 101.0231 (0.7), 87.0073 (43.8), 85.0280 (27.0) | 0.90 | −3.119 | 2 | - |
| Hydroxybenzoic, hydroxycinnamic acids, and their derivatives | ||||||||
| 9. | Gallic acid | C7H6O5 | 169.0143 | 169.0133 (37.5), 125.0231 (100), 97.0280 (3.8), 69.0330 (4.9) | 1.13 | −5.660 | 1 | 3.45 ± 0.51 |
| 10. | Hydroxybenzoic acid O-pentosylhexoside a | C18H24O12 | 431.1194 | 431.1200 (69.3), 299.0776 (2.3), 137.0232 (100), 93.0331 (73.0) | 1.78 | 1.231 | 2 | 1.32 ± 0.13 |
| 11. | Vanillic acid O-pentosylhexoside a | C19H26O13 | 461.1301 | 461.1310 (76.2), 329.0879 (1.3), 167.0340 (100), 152.0104 (52.4), 123.0438 (11.3), 108.0203 (27.4) | 1.99 | 2.052 | 2 | 2.27 ± 0.35 |
| 12. | Protocatechuic acid a | C7H6O4 | 153.0193 | 153.0182 (15.9), 123.0439 (0.1), 109.0281 (100), 81.0330 (1.4), 65.0380 (0.38) | 2.01 | −7.397 | 1 | 7.98 ± 0.54 |
| 13. | Caffeic acid O-rutinoside (swertiamacroside) | C21H28O13 | 487.1457 | 487.1465 (28.9), 179.0342 (100), 161.0234 (14.4), 135.0439 (45.5), 113.0232 (2.9) | 2.80 | 1.572 | 2 | 4.68 ± 0.45 |
| 14. | Caffeic acid O-dihexoside a | C21H28O14 | 503.1406 | 503.1414 (34.7), 341.0878 (42.9), 179.0341 (100), 135.0438 (77.1) | 2.82 | 1.513 | 2 | - |
| 15. | 2,3-Dihydroxybenzoic acid a | C7H6O4 | 153.0193 | 153.0182 (51.9), 123.0074 (26.7), 108.0203 (100), 95.0124 (32.1), 85.0280 (33.1) | 2.95 | −7.267 | 2 | - |
| 16. | p-Hydroxybenzoic acid a | C7H6O3 | 137.0244 | 137.0231 (35.2), 108.0200 (3.5), 93.0331 (100) | 2.97 | −9.614 | 1 | - |
| 17. | Dihydrocaffeic acid a | C9H10O4 | 181.0506 | 181.0499 (52.1), 163.0377 (0.4), 137.0596 (100), 135.0439 (19.6), 123.0436 (58.0), 121.0282 (24.9), 119.0489 (15.1), 109.0281 (27.6), 93.0332 (2.5), 59.0124 (86.3) | 3.33 | −4.154 | 2 | - |
| 18. | 2,4-Dihydroxybenzoic acid a | C7H6O4 | 153.0193 | 153.0182 (76.8), 135.0075 (29.7), 123.0439 (0.26), 109.0281 (100), 108.0201 (0.2), 91.0174 (5.6), 81.0333 (0.3), 65.0381 (14.5) | 3.47 | −7397 | 1 | 4.77 ± 0.74 |
| 19. | p-Hydroxyphenyl acetic acid a | C8H8O3 | 151.0400 | 151.0389 (100), 136.0156 (20.1), 123.0075 (4.6), 109.0283 (11.5), 107.0489 (2.3) | 3.49 | −7.133 | 1 | - |
| 20. | Caffeic acid | C9H8O4 | 179.0350 | 179.0341 (21.3), 135.0439 (100), 117.0335 (0.7), 107.0487 (1.35), 91.0537 (0.5) | 3.54 | −4.759 | 1 | 87.25 ± 6.54 |
| 21. | Gentisic acid | C7H6O4 | 153.0193 | 153.0182 (45.9), 109.0281 (100), 91.0175 (1.1), 108.0203 (8.7), 81.0330 (1.9), 65.0382 (0.1) | 3.65 | −7.397 | 1 | 0.42 ± 0.05 |
| 22. | Ferulic acid O-rutinoside a,b | C22H30O13 | 501.1614 | 501.1618 (10.2), 193.0500 (100), 175.0393 (8.4), 160.0157 (9.7), 134.0361 (43.4), 113.0230 (5.5) | 3.78 | 3.125 | 2 | - |
| 23. | p-Coumaric acid O-hexoside a | C15H18O8 | 325.0928 | 163.0391 (100), 145.0288 (4.14), 119.0488 (35.7) | 3.81 | 3.197 | 2 | - |
| 24. | Syringic acid a | C9H10O5 | 197.0455 | 197.0449 (40.0), 182.0213 (48.9), 153.0547 (100), 138.0311 (11.5), 123.0075 (26.7), 121.0281 (85.6), 106.0046 (9.67), 95.0123 (8.1), 89.0018 (14.97) | 4.34 | −3.231 | 2 | 1.69 ± 0.26 |
| 25. | O-coumaric acid a | C9H8O3 | 163.0401 | 163.0390 (9.7), 135.0075 (0.2), 119.0489 (100) | 4.55 | −6.363 | 1 | - |
| 26. | Ferulic acid | C10H10O4 | 193.0506 | 193.0499 (100), 178.0260 (14.1), 165.0545 (13.5), 149.0600 (21.4), 134.0358 (12.3), 123.0438 (92.0), 79.0538 (4.1) | 5.17 | −3.637 | 1 | - |
| Caffeic acid oligomers | ||||||||
| 27. | Lithospermic acid a | C27H22O12 | 537.1038 | 537.1035 (7.1), 493.1142 (30.4), 339.0515 (100), 313.0726 (8.8), 295.0613 (23.6), 267.0671 (10.9), 179.0345 (12.4), 135.0440 (49.3) | 4.97 | −0.743 | 2 | 0.51 ± 0.05 |
| 28. | Salvianolic acid B a | C36H30O16 | 717.1460 | 717.1478 (49.4), 673.1570 (7.7), 537.1055 (28.0), 519.0946 (47.9), 493.1153 (5.9), 339.0512 (13.0), 321.0409 (26.0), 313.0717 (11.5), 295.0616 (100), 277.0513 (6.7), 229.0141 (8.7), 203.0346 (13.9), 197.0447 (2.5), 179.0340 (11.3), 161.0237 (1.1), 135.0439 (32.2), 109.0281 (71.9) | 6.06 | 2.401 | 2 | 1.42 ± 0.01 |
| 29. | Rosmarinic acid | C18H16O8 | 359.0772 | 359.0782 (14.1), 197.0449 (26.0), 179.0342 (11.3), 161.0233 (100), 135.0439 (14.64), 133.0282 (19.8), 109.0275 (0.4) | 6.33 | 2.56 | 1 | 6.07 ± 0.46 |
| 30. | Isosalvianolic acid C a | C26H20O10 | 491.0983 | 491.0991 (100), 311.0567 (98.4), 267.0666 (39.3), 265.0508 (3.9), 249.0559 (1.5), 197.0454 (2.2), 179.0339 (1.8), 135.0440 (48.7) | 7.29 | 1.466 | 2 | 0.42 ± 0.03 |
| 31. | Didehydrosalvianolic acid B a | C36H28O16 | 715.1305 | 715.1324 (52.4), 535.0894 (20.2), 517.0786 (5.4), 337.0357 (8.1), 319.0241 (12.4), 311.0575 (7.6), 293.0461 (100), 265.0503 (7.1), 197.0446 (8.9), 135.0438 (10.5), 109.0279 (5.5) | 7.76 | 2.786 | 2 | 0.19 ± 0.03 |
| Acylquinic acids | ||||||||
| 32. | (Neo)chlorogenic acid O-hexoside a | C22H28O14 | 515.1406 | 515.1414 (52.2), 353.0883 (5.6), 191.0553 (100), 179.0351 (3.4), 135.0441 (4.0), 93.0332 (11.0) | 2.13 | 1.536 | 2 | - |
| 33. | Neochlorogenic acid | C16H18O9 | 353.0877 | 353.0883 (40.7), 191.0555 (100), 179.0341 (61.2), 173.0451 (3.1), 161.0235 (3.5), 135.0439 (48.7), 93.0331 (4.5) | 2.36 | 0.575 | 1 | - |
| 34. | Chlorogenic acid O-hexoside a | C22H28O14 | 515.1406 | 515.1414 (100), 323.0767(51.9), 191.0554 (94.8), 179.0327 (4.5), 161.0238 (33.5), 135.0434 (6.4), 111.0435 (4.0) | 2.85 | 2.487 | 1 | - |
| 35. | 3-p-coumaroylquinic acid a | C16H18O8 | 337.0928 | 337.0932 (6.9), 191.0555 (6.9), 173.0449 (3.5), 163.0390 (100), 135.0437 (0.5), 119.0488 (26.5), 111.0438 (0.7), 93.0332 (0.8) | 2.99 | 0.918 | 2 | - |
| 36. | Chlorogenic acid | C16H18O9 | 353.0877 | 353.0879 (4.6), 191.0554 (100), 179.0380 (0.9), 161.0235 (1.9), 111.0437 (0.8), 93.0331 (2.7), 85.0280 (7.5) | 3.17 | 0.325 | 1 | 5.22 ± 0.21 |
| 37. | 5-Syringoylquinic acid a | C16H20O10 | 371.0983 | 371.0987 (36.1), 197.0448 (4.9), 191.0554 (100), 173.0443 (14.6), 153.0538 (2.3), 121.0279 (9.4), 111.0435 (2.8), 93.0331 (31.6), 85.0279 (3.7) | 3.30 | 0.862 | 3 | - |
| 38. | 4-Caffeoylquinic acid a | C16H18O9 | 353.0877 | 353.0883 (32.7), 191.0555 (39.50), 179.0342 (75.3), 173.0447 (100), 135.0439 (52.1), 111.0436 (3.3), 93.0331 (21.3) | 3.36 | 0.515 | 2 | 7.65 ± 0.96 |
| 39. | 3-Feruloylquinic acid | C17H20O9 | 367.1034 | 367.1036 (19.5), 193.0500 (100), 173.0447 (4.7), 137.0226 (3.4), 134.0361 (56.7) | 3.42 | 0.339 | 2 | 0.63 ± 0.04 |
| 40. | 4-Syringoylquinic acid a | C16H20O10 | 371.0983 | 371.0978 (9.8), 197.0456 (11.5), 191.0554 (100), 173.0443 (14.6), 153.0538 (2.3), 121.0283 (4.9), 111.0435 (2.8), 93.0331 (14.5) | 3.43 | −1.509 | 3 | - |
| 41. | 5-p-Coumaroylquinic acid a | C16H18O8 | 337.0928 | 337.0940 (8.9), 191.0554 (100), 173.0446 (6.6), 163.0390 (5.31), 119.0488 (4.8), 111.0437 (2.0), 93.0331 (16.7) | 3.96 | 3.172 | 2 | 1.85 ± 0.03 |
| 42. | 4-p-Coumaroylquinic acid a | C16H18O8 | 337.0928 | 337.0941 (8.6), 191.0555 (2.7), 173.0445 (100), 163.0390 (18.5), 119.0488 (9.0), 111.0436 (3.1), 93.0330 (22.2) | 4.02 | 3.558 | 2 | 1.82 ± 0.05 |
| 43. | 5-Feruloylquinic acid | C17H20O9 | 367.1034 | 367.1038 (23.7), 193.0500 (16.9), 191.0554 (100), 173.0446 (81.4), 155.0340 (3.8), 134.0361 (19.2), 111.0438 (4.9), 93.0331 (39.7) | 4.38 | 0.912 | 2 | 5.13 ± 0.08 |
| 44. | 1-p-Coumaroylquinic acid a | C16H18O8 | 337.0928 | 337.0932 (7.2), 191.0554 (100), 173.0445 (100), 135.0447 (2.3), 163.0393 (0.5), 119.0487 (1.1), 111.0437 (0.5), 93.0331 (5.3) | 4.60 | 1.007 | 2 | 0.56 ± 0.04 |
| 45. | 1-Feruloylquinic acid | C17H20O9 | 367.1034 | 367.1039 (10.2), 191.0554 (100), 179.0340 (0.5), 173.0448 (2.3), 161.0239 (0.3), 134.0360 (3.1), 111.0440 (1.41), 93.0331 (5.15) | 4.90 | −2.996 | 2 | 1.12 ± 0.16 |
| 46. | Syringoyl–caffeoylquinic acid a | C25H26O13 | 533.1300 | 533.1305 (14.5), 335.0777 (44.6), 291.0875 (20.7), 197.0450 (100), 153.0546 (12.6), 137.0232 (38.4), 123.0073 (17.0), 111.0439 (10.4), 93.0331 (49.0) | 6.90 | 0.799 | 2 | 0.81 ± 0.08 |
| Acylhexaric acids | ||||||||
| 47. | Hydroxydihydrocaffeoyl–hexaric acid a,b | C15H18O12 | 389.0725 | 389.0730 (16.8), 371.0631 (0.8), 209.0297 (16.8), 191.0192 (34.2), 153.0539 (1.0), 147.0286 (17.9), 129.0180 (10.8), 85.0280 (100) | 1.65 | 1.236 | 3 | 1.12 ± 0.16 |
| 48. | Hydroxydihydrocaffeoyl–hexaric acid isomer a,b | C15H18O12 | 389.0725 | 389.0728 (16.8), 371.0621 (2.7), 209.0294 (17.2), 197.0442 (3.7), 191.0191 (45.3), 173.0087 (3.1), 147.0286 (20.6), 129.0180 (13.5), 111.0079 (4.5), 85.0280 (100) | 2.60 | 0.773 | 3 | 0.81 ± 0.08 |
| 49. | Hydroxydihydrocaffeoyl–siryngoyl–hexaric acid a,b | C24H26O16 | 569.1148 | 569.1159 (49.1), 389.0726 (8.8), 371.0627 (52.7), 327.0726 (13.8), 209.0299 (1.4), 197.0450 (39.1), 191.0186 (3.7), 182.0211 (3.3), 173.0084 (18.4), 166.9975 (1.6), 147.0285 (10.0), 138.0309 (1.7), 129.0181 (54.5), 123.0072 (1.6), 121.0282 (17.5), 111.0073 (14.3), 97.6908 (1.7), 85.0280 (100) | 3.53 | 1.937 | 3 | 1.77 ± 0.15 |
| 50. | Hydroxydihydrocaffeoyl–siryngoyl–hexaric acid isomer a,b | C24H26O16 | 569.1148 | 569.1158 (43.9), 389.0731 (7.6), 371.0625 (42.8), 327.0728 (12.2), 209.0295 (1.13), 197.0450 (40.8), 191.0186 (3.8), 182.0214 (4.3), 173.0084 (15.4), 166.9978 (1.0), 153.0548 (10.2), 147.0288 (8.0), 138.0315 (2.2), 129.0181 (59.1), 123.0074 (3.6), 121.0281 (16.5), 111.0073 (12.5), 85.0280 (100) | 3.77 | 2.148 | 3 | 3.53 ± 0.237 |
| Phenylethanoid glycosides | ||||||||
| 51. | Decaffeoyl aceteoside/verbasoside | C20H30O12 | 461.1664 | 461.1673 (100), 315.1085 (5.2), 297.0984 (2.6), 135.0439 (29.4), 113.0230 (46.0), 85.0280 (20.6), 71. 0123 (20.7) | 2.57 | 0.881 | 2 | 35.09 ± 2.46 |
| 52. | Hydroxyverbascoside | C29H36O16 | 639.1931 | 639.1946 (86.8), 621.1832 (5.3), 459.1533 (1.9), 179.0342 (36.0), 161.0232 (5.3), 135.0440 (22.3), 133.0283 (37.6), 113.0231 (8.6) | 4.49 | 2.381 | 2 | 13.45 ± 0.47 |
| 53. | Verbascoside | C29H36O15 | 623.1987 | 623.1996 (60.3), 461.1674 (9.3), 315.1078 (1.80), 179.0340 (2.4), 161.0234 (100), 135.0440 (8.7), 133.0283 (23.9) | 5.48 | 2.305 | 2 | 151.54 ± 10.86 |
| 54. | Echinacoside | C35H46O20 | 785.2509 | 785.2529 (72.5), 623.2175 (7.7), 461.1666 (6.3), 179.0349 (3.6), 161.0234 (100), 135.0440 (24.9), 133.0282 (47.0) | 5.23 | 2.424 | 2 | 0.74 ± 0.005 |
| 55. | Forsythoside B/samioside/lavandulifolioside | C34H44O19 | 755.2403 | 755.2424 (80.5), 593.2080 (8.2), 461.1675 (8.7), 267.1614 (1.5), 179.0341 (6.8), 161.0234 (100), 135.0439 (23.5), 133.0282 (45.8), 113.0231 (9.4) | 5.38 | 2.606 | 2 | 6.57 ± 0.103 |
| 56. | Alyssonoside | C35H46O19 | 769.2560 | 769.2578 (100), 593.2076 (8.5), 461.1658 (8.9), 193.05 (20.9), 175.0393 (44.1), 161.0236 (10.2), 160.0156 (44.4), 135.0442 (15.4), 134.0362 (20.9), 132.0206 (14.9), 123.0439 (7.4), 113.023 (15.1), 85.0281 (9.3), 71.0124 (10.0) | 6.07 | 22.207 | 2 | 2.16 ± 0.14 |
| 57. | Leucoseptoside A | C30H38O15 | 637.2138 | 637.2154 (100), 461.1669 (16.2), 315.1091 (3.8), 193.0501 (13.00), 175.0392 (67.8), 161.0233 (109), 160.0155 (60.1), 113.0230 (18.9) | 6.25 | 2.505 | 2 | 22.80 ± 0.82 |
| 58. | Leontoside B/stachyoside D | C36H48O19 | 783.2716 | 783.2734 (100), 193.0501 (34.7), 175.0392 (92.4), 167.0700 (1.5), 160.0156 (73.8), 132.0205 (26.8) | 6.97 | 2.142 | 2 | 0.90 ± 0.05 |
| 59. | Acetylverbascoside | C31H38O16 | 665.2087 | 665.2103 (65.0), 503.1778 (3.3), 461.1676 (3.1), 161.0234 (100), 179.0339 (2.9), 135.0440 (10.4), 133.0283 (38.4), 113.0229 (1.9) | 6.99 | 2.348 | 2 | 0.90 ± 0.01 |
| 60. | Martynoside | C31H40O15 | 651.2294 | 651.2317 (95.8), 475.1836 (1.2), 329.1252 (1.1), 193.0500 (13.6), 175.0392 (100), 160.0156 (69.1), 132.0204 (25.1), 113.0230 (14.0) | 7.21 | 3.450 | 2 | 11.35 ± 0.75 |
| Iridoid glycosides | ||||||||
| 61. | Melittoside | C21H32O15 | 523.1668 | 523.1676 (10.9), 361.1139 (4.5), 343.1040 (5.4), 325.0919 (1.3), 313.9523 (0.4), 283.0820 (0.5), 253.0722 (1.3), 223.0613 (1.7), 205.0506 (1.6), 179.0553 (100), 161.0447 (16.8), 119.0337 (34.9), 101.0230 (30.8), 89.0229 (80.9) | 1.33 | 1.446 | 2 | 13.22 ± 1.36 |
| 62. | p-Coumaroylmelittoside | C30H38O17 | 669.2035 | 669.2049 (100), 489.1423 (5.4), 325.0932 (28.3), 307.0823 (5.0), 265.0729 (3.2), 235.0605 (3.4), 205.0499 (16.9), 163.0390 (71.9), 145.0283 (84.4), 119.0488 (46.4), 93.0330 (4.9), 89.0230 (6.4) | 3.98 | 1.909 | 2 | 3.71 ± 0.06 |
| 63. | Caffeoylmelittoside a,b | C30H38O18 | 685.1974 | 685.1998 (100), 649.1036 (1.3), 523.1473 (1.9), 187.0396 (1.1), 181.0498 (24.9), 179.0342 (61.7), 163.0391 (39.9), 161.0235 (12.7), 135.0440 (78.4), 93.0331 (2.4), 89.0227 (2.3) | 4.79 | 1.799 | 2 | 0.70 ± 0.01 |
| 64. | Feruloylmelittoside a,b | C31H40O18 | 699.2142 | 699.2161 (75.9), 519.1519 (8.4), 357.0992 (54.0), 193.0500 (100), 163.0389 (30.6), 135.0435 (16.10), 134.0361 (70.0) | 5.56 | 2.778 | 2 | - |
| Flavonoids | ||||||||
| 65. | Apigenin 6,8-C-hexosyl hexoside | C27H30O15 | 593.1522 | 593.1522 (100), 503.1212 (4.8), 473.1094 (14.5), 413.0891 (2.0), 395.0770 (0.8), 383.0776 (18.0), 353.0672 (30.1), 325.0729 (2.8), 297.0770 (10.7), 161.0235 (2.2), 117.0333 (3.3) | 4.05 | 1.630 | 2 | 1.59 ± 0.18 |
| 66. | Luteolin 7-O-dihexoside | C27H30O16 | 609.1461 | 609.1473 (100), 447.0949 (4.7), 285.0408 (61.9), 284.0331 (18.1), 256.0380 (1.0), 151.0025 (3.8), 133.0281 (3.4), 107.0121 (2.1) | 4.94 | 2.023 | 2 | 0.58 ± 0.01 |
| 67. | Hypolaetin 7-O-hexosyl (1→2)-hexoside | C27H30O17 | 625.1410 | 625.1422 (100), 463.0883 (6.6), 445.0775 (6.5), 301.0356 (88.8), 300.0279 (30.4), 283.0244 (0.7), 255.0292 (3.6), 227.0350 (2.3), 166.9975 (3.3), 163.0029 (1.1), 137.0232 (4.0), 133.0280 (7.5) | 5.08 | 1.900 | 1 | 5.30 ± 0.09 |
| 68. | Naringenin 7-O-dihexoside | C27H32O15 | 595.1668 | 595.1658 (100), 475.1172 (4.6), 355.0674 (5.6), 271.0619 (54.8), 270.0215 (1.8), 269.0457 (38.2), 151.0027 (43.4), 119.0488 (21.5), 107.0123 (18.3) | 5.45 | −1.703 | 2 | - |
| 69. | Apigenin 7-O-allosyl (1→2) glucoside | C27H30O15 | 593.1512 | 593.1522 (64.7), 431.0986 (3.0), 269.0459 (100), 225.0546 (0.7), 151.0027 (1.4), 161.0232 (1.7), 117.0333 (3.3), 107.0125 (1.8) | 5.50 | 1.731 | 2 | 1.93 ± 0.04 |
| 70. | Isoscutellarein 7-O-pentosyl–hexoside a,b | C26H28O15 | 579.1356 | 579.1366 (38.9), 461.0086 (0.4), 285.0408 (100), 257.0451 (1.7), 241.0506 (1.6), 229.0497 (1.5), 213.0554 (5.1), 187.0393 (4.4), 136.9867 (0.9), 117.0330 (0.5) | 5.51 | 1.825 | 2 | 1.01 ± 0.05 |
| 71. | Isoscutellarein 7-O-hexosyl (1→2)-hexoside | C27H30O16 | 609.1461 | 609.1472 (87.2), 447.0912 (0.4), 429.0833(6.8), 285.0409 (100), 284.0328 (9.5), 255.0303 (2.2), 167.0496 (0.5), 163.0028 (2.1), 136.9868 (1.7), 117.0333 (1.8) | 5.62 | 1.826 | 2 | 47.47 ± 0.95 |
| 72. | Isoscutellarein 7-O-hexoside | C21H20O11 | 447.0933 | 447.0941 (26.4), 285.0408 (100), 229.0509 (1.0), 136.9868 (2.3), 117.0332 (1.1) | 5.81 | 1.801 | 2 | 0.58 ± 0.01 |
| 73. | Hypolaetin 7-O-acetylhexosiyl–hexoside | C29H32O18 | 667.1516 | 625.1406 (3.2), 463.0898 (3.2), 445.079 (6.6), 301.0357 (76.0), 300.0278 (30.8), 283.0285 (1.1), 255.0298 (1.4), 227.0347 (1.0), 166.9973 (1.9), 163.0020 (1.0), 137.0232 (4.0), 133.0284 (8.3), 109.0282 (1.0) | 5.93 | 3.152 | 2 | 19.17 ± 1.07 |
| 74. | Methylhypolaetin 7-O-dihexoside | C28H32O17 | 639.1567 | 639.1579 (75.3), 315.0516 (100), 300.0279 (40.7), 271.0264 (1.2), 243.0296 (2.5), 165.9901 (0.7), 136.9871 (6.2), 117.1944 (0.5), 133.0283 (2.5) | 5.96 | 1.920 | 2 | 17.33 ± 0.25 |
| 75. | Apigenin 7-O-glucoside | C21H20O10 | 431.0984 | 431.0988 (100), 269.0453 (24.9), 268.0381 (54.3), 211.0395 (1.6), 151.0025 (3.2), 117.0330 (1.8), 170.0124 (2.0) | 6.06 | 1.044 | 1 | 0.46 ± 0.02 |
| 76. | Methylhypolaetin 7-O-hexoside | C22H22O12 | 477.1039 | 477.1045 (30.5), 315.0515 (100), 300.0278 (32.0), 227.0350 (1.6), 136.9870 (6.2) | 6.13 | 1.406 | 2 | 0.26 ± 0.01 |
| 77. | Apigenin 7-O-[6‴-O-acetyl]-hexosyl(1→2)-hexoside | C29H32O16 | 635.1618 | 635.1629 (60.6), 593.1563 (1.1), 431.0981 (2.3), 269.0458 (100), 225.0560 (12.4), 151.0024 (1.7), 117.0332 (5.1), 107.0126 (2.6) | 6.44 | 1.798 | 2 | 2.18 ± 0.003 |
| 78. | Isoscutellarein 7-O-hexosyl-(1→2)-[6″-O-acetyl]-hexoside | C29H32O17 | 651.1567 | 651.1581 (69.3), 429.0831 (8.9), 285.0408 (100), 255.0285 (1.0), 239.0344 (1.1), 163.0026 (1.5), 136.9863 (0.8), 117.0334 (1.1) | 6.58 | 2.161 | 2 | 151.70 ± 14.79 |
| 79. | 4′-Methylhypolaetin 7-O-acetyl–hexosyl–hexoside | C30H34O18 | 681.1672 | 681.1688 (89.6), 639.1533 (1.2), 357.0594 (0.9), 315.0516 (100), 300.0279 (44.2), 271.0248 (1.6), 243.0291 (1.4), 136.9868 (7.9), 133.0283 (4.1) | 6.83 | 2.236 | 2 | 78.33 ± 3.29 |
| 80. | 4′-Methylisoscutellarein 7-O-dihexoside | C28H32O16 | 623.1618 | 623.1630 (100), 461.1117 (0.6), 299.0565 (83.6), 284.0330 (39.2), 255.0299 (3.0), 117.0330 (0.7) | 7.23 | 1.929 | 2 | 24.20 ± 0.98 |
| 81. | Tremasperin | C30H34O16 | 649.1774 | 649.1786 (5.4), 607.1672 (2.6), 283.0616 (100), 268.0381 (55.5), 284.0649 (5.8), 240.0431 (2.2), 151.0024 (0.5) | 8.21 | 1.913 | 2 | 0.55 ± 0.04 |
| 82. | 4′-O-methylisoscutellarein 7-O-[6‴-O-acetyl]hexosyl-(1→2)hexoside | C30H34O17 | 665.1723 | 665.1740 (84.8), 299.0565 (100), 284.0330 (30.6), 255.0293 (2.5), 240.0429 (2.5), 227.0343 (2.5), 163.0025 (1.1), 136.9867 (9.2), 117.0338 (1.9) | 8.24 | 2.447 | 2 | 107.44 ± 9.07 |
| 83. | Isoscutellarein 7-O-acetylhexosyl-O-acetylhexoside | C31H34O18 | 693.1672 | 693.1663 (85.8), 471.0903 (7.4), 285.0407 (100), 213.0551 (6.0), 163.0022 (4.5), 136.9864 (1.9), 117.0331 (3.4) | 8.26 | −1.323 | 2 | - |
| 84. | Methylhypolaetin 7-O-acetylhexosyl-O-acetylhexoside | C32H36O19 | 723.1778 | 723.1794 (89.7), 315.0515 (100), 300.0280 (44.9), 271.0255 (1.1), 243.0298 (1.4), 199.0390 (4.6), 136.9866 (9.5), 133.0284 (5.9) | 8.47 | 2.182 | 2 | 0.31 ± 0.02 |
| 85. | Naringenin | C15H12O5 | 271.0612 | 271.0615 (100), 227.0701 (0.7), 165.0180 (2.5), 151.0025 (65.7), 125.0228 (1.3), 119.0489 (52.3), 107.0124 (15.5), 93.0331 (11.9) | 8.60 | 1.193 | 2 | - |
| 86. | Apigenin 7-O-p-coumaroyl-O-hexoside | C30H26O12 | 577.1352 | 577.1360 (100), 431.0990 (13.4), 413.0890 (7.9), 269.0459 (77.0), 145.0283 (83.4), 163.0391 (4.4), 117.0332 (38.3), 107.0121 (1.1), 151.0026 (2.1) | 9.06 | 1.457 | 2 | 0.75 ± 0.01 |
| 87. | Naringenin 7-O-coumaroylhexoside a | C30H28O12 | 579.1508 | 579.1514 (100), 415.1033 (3.9), 307.0829 (12.0), 271.0616 (79.4), 151.0026 (40.9), 163.0391 (15.7), 145.0283 (57.4), 119.0489 (40.9), 107.0125 (15.2), 117.0332 (21.9) | 9.15 | 0.985 | 2 | 1.09 ± 0.09 |
| 88. | 4′-Methylisoscutellarein 7-O-(6‴-acetyl)-hexosyl(1→2)-[6′-O-acetyl]hexoside | C32H36O18 | 707.1829 | 707.1844 (12.1), 299.0565 (100), 284.0330 (31.9), 300.0598 (6.8), 298.0496 (8.2), 255.0292 (4.7), 240.0424 (3.2), 227.0341 (1.7), 163.0023 (3.5), 136.9867 (10.7), 117.0332 (1.5) | 9.89 | 2.125 | 2 | 0.09 ± 0.003 |
| 89. | Pectolinarigenin | C17H14O6 | 313.0718 | 313.0721 (100), 298.0485 (53.9), 283.0251 (52.0), 269.0468 (2.4), 255.0302 (14.4), 227.0342 (2.17), 211.0386 (1.3), 183.0446 (2.1), 178.9918 (3.0), 163.0031 (11.8), 135.0075 (2.8), 117.0331 (13.1) | 10.36 | 0.922 | 1 | 3.11 ± 0.50 |
| 90. | Eupatilin | C18H16O7 | 343.0823 | 343.0826 (85.2), 328.0594 (100), 313.0360 (54.6), 298.0125 (13.3), 285.0412 (2.0), 270.0174 (42.6), 257.0095 (2.6), 133.0282 (3.7), 123.0439 (4.6) | 11.05 | 0.915 | 2 | 0.47 ± 0.07 |
| 91. | 8-Methoxycirsilineol | C18H16O7 | 343.0823 | 343.0826 (100), 328.0594 (48.7), 313.0360 (71.8), 299.0952 (0.7), 298.0124 (209), 270.0175 (10.6), 242.0220 (4.2), 161.0233 (0.8), 117.0333 (8.9) | 11.24 | 0.828 | 2 | 14.15 ± 2.30 |
| 92. | Genkwanin | C16H12O5 | 283.0611 | 283.0615 (100), 268.0379 (67.4), 240.0428 (6.2), 239.0352 (1.8), 178.9915 (1.2), 151.0025 (4.1), 107.0125 (3.3) | 11.42 | 1.036 | 2 | - |
| Fatty acids | ||||||||
| 93. | Trihydroxyoctadecadienoic acid | C18H32O5 | 327.2177 | 327.2166 (100), 309.2069 (0.8), 291.1971 (3.5), 229.1443 (12.5), 211.1334 (16.2), 183.1383 (1.6), 171.1015 (6.0), 85.0280 (2.5), 57.0329 (0.9) | 9.15 | 0.986 | 2 | - |
| 94. | Trihydroxyoctadecenoic acid | C18H34O5 | 329.2334 | 329.2338 (100), 311.2232 (1.4), 293.2119 (0.4), 229.1442 (17.2), 211.1335 (23.2), 183.1381 (2.7), 171.1020 (4.4), 127.1115 (1.6) | 9.80 | 1.466 | 2 | - |
| 95. | Dihydroxyoctadecatrienoic acid | C18H30O4 | 309.2071 | 309.2076 (100), 291.1972 (54.3), 247.2075 (1.0), 185.1179 (5.4), 137.0959 (17.9), 97.0645 (4.1) | 10.90 | 1.641 | 2 | - |
| 96. | Dihydroxyoctadecadienoic acid | C18H32O4 | 311.2228 | 311.2233 (100), 293.2132 (7.2), 275.2029 (6.4), 201.1128 (61.0), 183.1387 (1.6), 171.1015 (11.2), 127.1114 (4.3)12.62 | 12.62 | 1.662 | 2 | - |
| 97. | Dihydroxyoctadecenoic acid | C18H34O4 | 313.2384 | 313.2389 (100), 295.2266 (5.8), 277.2166 (4.9), 201.1126 (42.8), 171.1012 (5.11), 127.1116 (4.8), 125.0960 (3.3) | 13.75 | 1.460 | 2 | - |
| 98. | Dihydroxyoctadecanoic acid | C18H36O4 | 315.2541 | 315.2544 (100), 297.2450 (4.3), 287.2241 (4.3), 171.1380 (0.6), 141.1272 (3.2), 127.1116 (0.6), 89.0230 (0.5) | 14.87 | 1.101 | 2 | - |
| 99. | Hydroxylinoleic acid | C18H32O3 | 295.2279 | 295.2282 (100), 277.2175 (14.6), 195.1384 (17.5), 113.0960 (1.1) | 15.98 | 1.056 | 2 | - |
| Organosulfur compounds | ||||||||
| 100. | Dodecyl sulfate | C12H26O4S | 265.1479 | 265.1482 (100), 96.9586 (66.4), 79.9558 (1.6) | 14.55 | 1.081 | 2 | - |
| 101. | Lauryl ether sulfate | C14H30O5S | 309.1741 | 309.1746 (100), 122.9746 (1.9), 104.9527 (0.2), 96.9586 (54.3), 79.9558 (6.6) | 16.10 | 1.624 | 2 | - |
| 102. | 4-Dodecylbenenesulfonic acid | C18H30O3S | 325.1842 | 325.1846 (100), 216.0095 (0.2), 183.0113 (46.5), 197.0272 (0.8), 184.0147 (1.9) | 17.44 | 0.957 | 2 | - |
| 103. | Myristyl sulfate | C14H30O4S | 293.1792 | 293.1796 (100), 96.9586 (73.6), 79.9558 (2.2) | 17.89 | 1.455 | 2 | - |
2.2. Quantitative Determination
2.3. Study Strength, Limitation and Future Direction
3. Materials and Methods
3.1. Chemicals
3.2. Plant Material
3.3. Sample Extraction
3.4. UHPLC–HRMS Dereplication/Annotation
3.5. UHPLC–HRMS Quantification
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Petrova, A.; Vladimirov, V. Balkan Endemics in the Bulgarian Flora. Phytol. Balcan. 2010, 16, 293–311. [Google Scholar]
- Assenov, I.; Ganchev, G. Morphological Characteristics of Sideritis scardica Griseb. Pharmacia 1978, 2, 29–32. [Google Scholar]
- Evstatieva, L. Sideritis scardica Griseb. In Red Data Book of the Republic of Bulgaria; Peev, D., Ed.; Joint Edition of the Bulgarian Academy of Sciences & Ministry of Environment and Water of Bulgaria, Digital Edition; Bulgarian Academy of Sciences: Sofia, Bulgaria, 2011; Volume 1, Available online: http://e-ecodb.bas.bg/rdb/en/ (accessed on 1 December 2023).
- González-Burgos, E.; Carretero, M.E.; Gómez-Serranillos, M.P. Sideritis spp.: Uses, Chemical Composition and Pharmacological Activities—A Review. J. Ethnopharmacol. 2011, 135, 209–225. [Google Scholar] [CrossRef] [PubMed]
- Yaneva, I.; Balabanski, V. History of the Uses of Pirin Mountain Tea (Sideritis scardica Griseb.) in Bulgaria. Bulg. J. Public Health 2013, 5, 48–57. [Google Scholar]
- Stanoeva, J.P.; Stefova, M.; Stefkov, G.; Kulevanova, S.; Alipieva, K.; Bankova, V.; Aneva, I.; Evstatieva, L.N. Chemotaxonomic Contribution to the Sideritis species Dilemma on the Balkans. Biochem. Syst. Ecol. 2015, 61, 477–487. [Google Scholar] [CrossRef]
- Irakli, M.; Tsifodimou, K.; Sarrou, E.; Chatzopoulou, P. Optimization Infusions Conditions for Improving Phenolic Content and Antioxidant Activity in Sideritis scardica Tea Using Response Surface Methodology. J. Appl. Res. Med. Aromat. Plants 2018, 8, 67–74. [Google Scholar] [CrossRef]
- Todorova, M.; Trendafilova, A. Sideritis scardica Griseb.; An Endemic Species of Balkan Peninsula: Traditional Uses, Cultivation, Chemical Composition, Biological Activity. J. Ethnopharmacol. 2014, 152, 256–265. [Google Scholar] [CrossRef]
- Jeremic, I.; Petricevic, S.; Tadic, V.; Petrovic, D.; Tosic, J.; Stanojevic, Z.; Petronijevic, M.; Vidicevic, S.; Trajkovic, V.; Isakovic, A. Effects of Sideritis scardica Extract on Glucose Tolerance, Triglyceride Levels and Markers of Oxidative Stress in Ovariectomized Rats. Planta Med. 2019, 85, 465–472. [Google Scholar] [CrossRef]
- Wightman, E.; Jackson, P.A.; Khan, J.; Forster, J.; Heiner, F.; Feistel, B.; Suarez, C.G.; Pischel, I.; Kennedy, D.O. The Acute and Chronic Cognitive and Cerebral Blood Flow Effects of a Sideritis scardica (Greek Mountain Tea) Extract: A Double Blind, Randomized, Placebo Controlled, Parallel Groups Study in Healthy Humans. Nutrients 2018, 10, 955. [Google Scholar] [CrossRef]
- Żyżelewicz, D.; Kulbat-Warycha, K.; Oracz, J.; Żyżelewicz, K. Polyphenols and Other Bioactive Compounds of Sideritis Plants and Their Potential Biological Activity. Molecules 2020, 25, 3763. [Google Scholar] [CrossRef]
- Mróz, M.; Malinowska-Pańczyk, E.; Bartoszek, A.; Kusznierewicz, B. Comparative Study on Assisted Solvent Extraction Techniques for the Extraction of Biologically Active Compounds from Sideritis raeseri and Sideritis scardica. Molecules 2023, 28, 4207. [Google Scholar] [CrossRef] [PubMed]
- Kostadinova, E.; Nikolova, D.; Alipieva, K.; Stefova, M.; Stefkov, G.; Evstatieva, L.; Matevski, V.; Bankova, V. Chemical Constituents of the Essential Oils of Sideritis scardica Griseb. and Sideritis raeseri Boiss and Heldr. from Bulgaria and Macedonia. Nat. Prod. Res. 2007, 21, 819–823. [Google Scholar] [CrossRef] [PubMed]
- Petreska Stanoeva, J.; Stefova, M. Assay of Urinary Excretion of Polyphenols after Ingestion of a Cup of Mountain Tea (Sideritis scardica) Measured by HPLC-DAD-ESI-MS/MS. J. Agric. Food Chem. 2013, 61, 10488–10497. [Google Scholar] [CrossRef] [PubMed]
- Tadić, V.M.; Jeremic, I.; Dobric, S.; Isakovic, A.; Markovic, I.; Trajkovic, V.; Bojovic, D.; Arsic, I. Anti-inflammatory, Gastroprotective, and Cytotoxic Effects of Sideritis scardica extracts. Planta Med. 2012, 78, 415–427. [Google Scholar] [CrossRef] [PubMed]
- Petreska, J.; Stefova, M.; Ferreres, F.; Moreno, D.A.; Tomás-Barberán, F.A.; Stefkov, G.; Kulevanova, S.; Gil-Izquierdo, A. Potential Bioactive Phenolics of Macedonian Sideritis Species Used for Medicinal “Mountain Tea”. Food Chem. 2011, 125, 13–20. [Google Scholar] [CrossRef]
- Alipieva, K.; Petreska, J.; Gil-Izquierdo, Á.; Stefova, M.; Evstatieva, L.; Bankova, V. Influence of the Extraction Method on the Yield of Flavonoids and Phenolics from Sideritis spp. (Pirin Mountain Tea). Nat. Prod. Commun. 2010, 130, 51–54. [Google Scholar] [CrossRef]
- Al Kadhi, O.; Melchini, A.; Mithen, R.; Saha, S. Development of a LC-MS/MS Method for The Simultaneous Detection of Tricarboxylic Acid Cycle Intermediates in A Range of Biological Matrices. J. Anal. Methods Chem. 2017, 2017, 5391832. [Google Scholar] [CrossRef]
- Liu, A.H.; Guo, H.; Ye, M.; Lin, Y.H.; Sun, J.H.; Xu, M.; Guo, D.A. Detection, Characterization and Identification of Phenolic Acids in Danshen Using High-Performance Liquid Chromatography with Diode Array Detection and Electrospray Ionization Mass Spectrometry. J. Chromatogr. A 2007, 1161, 170–182. [Google Scholar] [CrossRef]
- Zheleva-Dimitrova, D.; Simeonova, R.; Gevrenova, R.; Savov, Y.; Balabanova, V.; Nasar-Eddin, G.; Bardarov, K.; Danchev, N. In vivo Toxicity Assessment of Clinopodium vulgare L. Water Extract Characterized by UHPLC-HRMS. Food Chem. Toxicol. 2019, 134, 110841. [Google Scholar] [CrossRef]
- Zhu, L.; Ma, S.; Li, K.; Xiong, P.; Qin, S.; Cai, W. Systematic Screening of Chemical Constituents in the Traditional Chinese Medicine Arnebiae Radix by UHPLC-Q-Exactive Orbitrap Mass Spectrometry. Molecules 2022, 27, 2631. [Google Scholar] [CrossRef]
- Gevrenova, R.; Zengin, G.; Sinan, K.I.; Zheleva-Dimitrova, D.; Balabanova, V.; Kolmayer, M.; Voynikov, Y.; Joubert, O. An In-Depth Study of Metabolite Profile and Biological Potential of Tanacetum balsamita L. (Costmary). Plants 2023, 12, 22. [Google Scholar] [CrossRef] [PubMed]
- Gevrenova, R.; Zheleva-Dimitrova, D.; Balabanova, V.; Voynikov, Y.; Sinan, K.I.; Mahomoodally, M.F.; Zengin, G. Integrated phytochemistry, bio-functional potential and multivariate analysis of Tanacetum macrophyllum (Waldst. & Kit.) Sch.Bip. and Telekia speciosa (Schreb.) Baumg. (Asteraceae). Ind. Crops Prod. 2020, 155, 112817. [Google Scholar]
- Clifford, M.N.; Knight, S.; Kuhnert, N. Discriminating between the six isomers of dicaffeoylquinic acid by LC-MS n. J. Agric. Food Chem. 2005, 53, 3821–3832. [Google Scholar] [CrossRef] [PubMed]
- Kleczek, N.; Malarz, J.; Gierlikowska, B.; Kiss, A.; Stojakowska, A. Constituents of Xerolekia speciosissima (L.) Anderb. (Inuleae), and Anti-Inflammatory Activity of 7,10-Diisobutyryloxy-8,9-epoxythymyl Isobutyrate. Molecules 2020, 25, 4913. [Google Scholar] [CrossRef] [PubMed]
- Alipieva, K.; Kostadinova, E.; Evstatieva, L.; Stefova, M.; Bankova, V. An iridoid and a flavonoid from Sideritis lanata L. Fitoterapia 2009, 80, 51–53. [Google Scholar] [CrossRef]
- Kirmizibekmez, H.; Ariburnu, E.; Masullo, M.; Festa, M.; Capasso, A.; Yesilada, E.; Piacente, S. Iridoid, phenylethanoid and flavonoid glycosides from Sideritis trojana. Fitoterapia 2012, 83, 130–136. [Google Scholar] [CrossRef]
- Sinan, K.I.; Zengin, G.; Zheleva-Dimitrova, D.; Etienne, O.K.; Mahomoodally, M.F.; Bouyahya, A.; Lobine, D.; Chiavaroli, A.; Ferrante, C.; Menghini, L.; et al. Qualitative Phytochemical Fingerprint and Network Pharmacology Investigation of Achyranthes aspera Linn. Extracts. Molecules 2020, 25, 1973. [Google Scholar] [CrossRef]
- Sumner, L.W.; Amberg, A.; Barrett, D.; Beale, M.H.; Beger, R.; Daykin, C.A.; Fan, T.W.-M.; Fiehn, O.; Goodacre, R.; Griffin, J.L. Proposed Minimum Reporting Standards for Chemical Analysis: Chemical Analysis Working Group (CAWG) Metabolomics Standards Initiative (MSI). Metabolomics 2007, 3, 211–221. [Google Scholar] [CrossRef]
- Moussavi, N.; Azizullah, H.; Malterud, K.; Inngjerdingen, K. Immunomodulating polyphenols from Sideritis scardica. J. Funct. Food 2022, 96, 105197. [Google Scholar] [CrossRef]
- Assenov, I. Sideritis L. In Flora of PR Bulgaria; Velchev, V., Ed.; Bulgarian Academy of Sciences Publishing House: Sofia, Bulgaria, 1989; Volume 9, pp. 369–374. [Google Scholar]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zheleva-Dimitrova, D.; Voynikov, Y.; Gevrenova, R.; Balabanova, V. A Comprehensive Phytochemical Analysis of Sideritis scardica Infusion Using Orbitrap UHPLC-HRMS. Molecules 2024, 29, 204. https://doi.org/10.3390/molecules29010204
Zheleva-Dimitrova D, Voynikov Y, Gevrenova R, Balabanova V. A Comprehensive Phytochemical Analysis of Sideritis scardica Infusion Using Orbitrap UHPLC-HRMS. Molecules. 2024; 29(1):204. https://doi.org/10.3390/molecules29010204
Chicago/Turabian StyleZheleva-Dimitrova, Dimitrina, Yulian Voynikov, Reneta Gevrenova, and Vessela Balabanova. 2024. "A Comprehensive Phytochemical Analysis of Sideritis scardica Infusion Using Orbitrap UHPLC-HRMS" Molecules 29, no. 1: 204. https://doi.org/10.3390/molecules29010204
APA StyleZheleva-Dimitrova, D., Voynikov, Y., Gevrenova, R., & Balabanova, V. (2024). A Comprehensive Phytochemical Analysis of Sideritis scardica Infusion Using Orbitrap UHPLC-HRMS. Molecules, 29(1), 204. https://doi.org/10.3390/molecules29010204