Preparation of Aliphatic Hydroxamic Acid from Litsea cubeba Kernel Oil and Its Application to Flotation of Fe(III)-Activated Wolframite
Abstract
:1. Introduction
2. Results and Discussion
2.1. Preparation and Characterization of AHA
2.1.1. Preparation of AHA
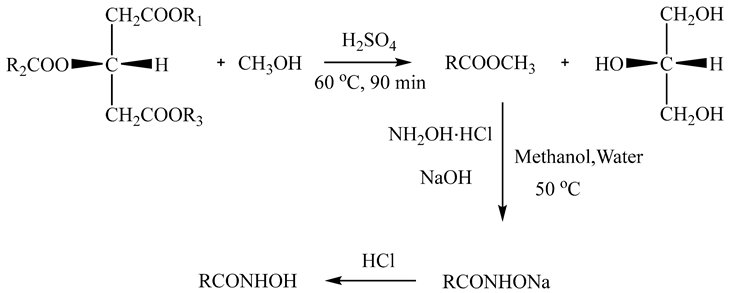
2.1.2. Characterization of AHA
2.2. Micro-Flotation
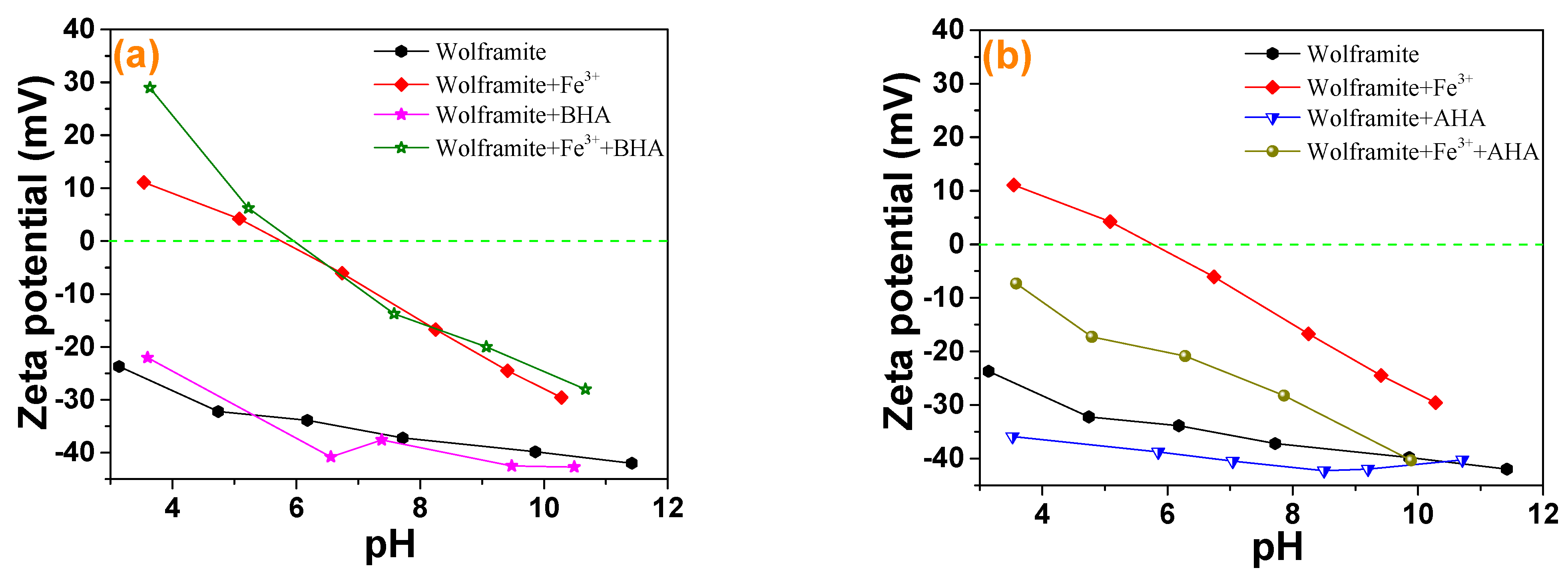
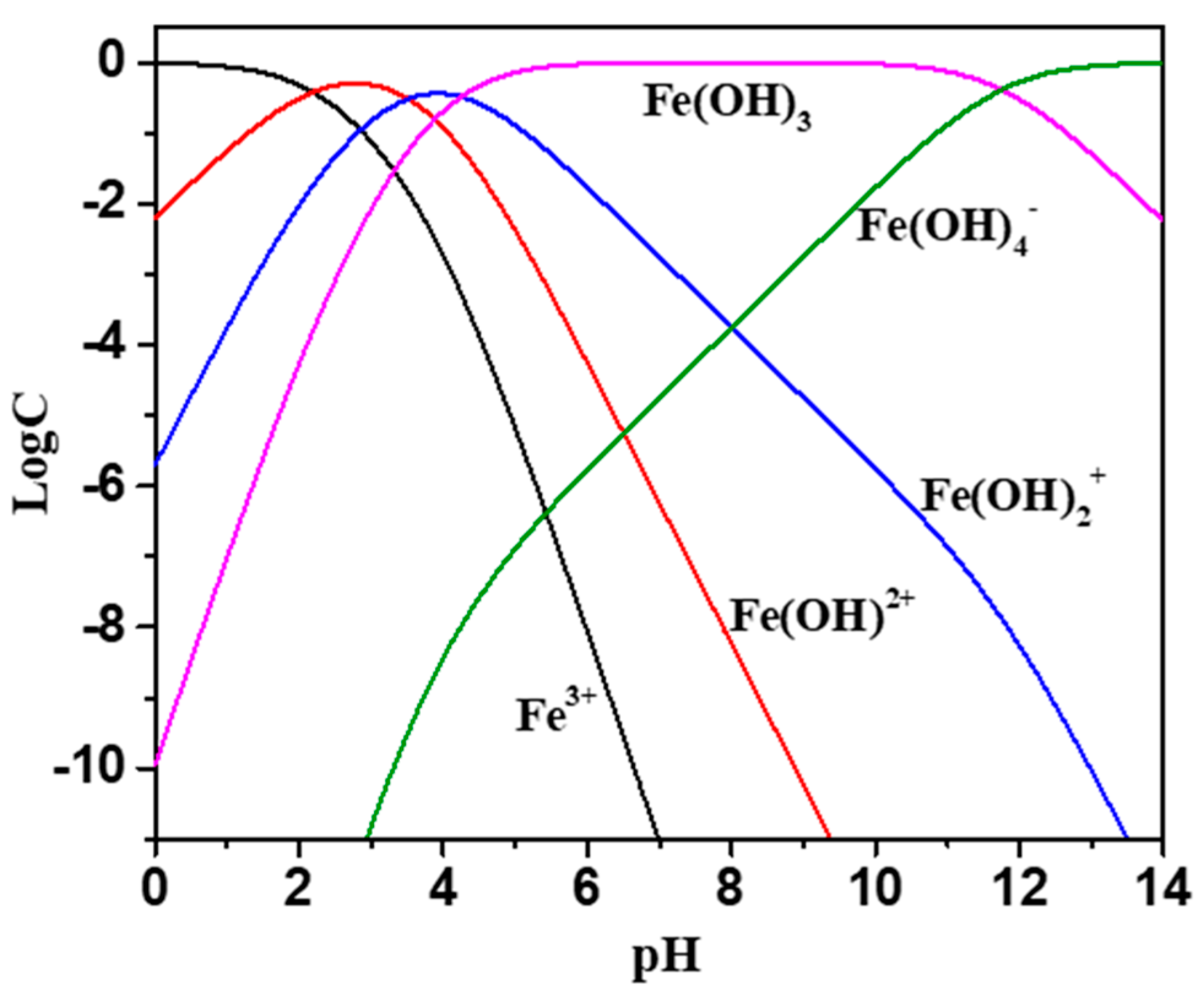
2.3. Zeta Potential
2.4. Contact Angle
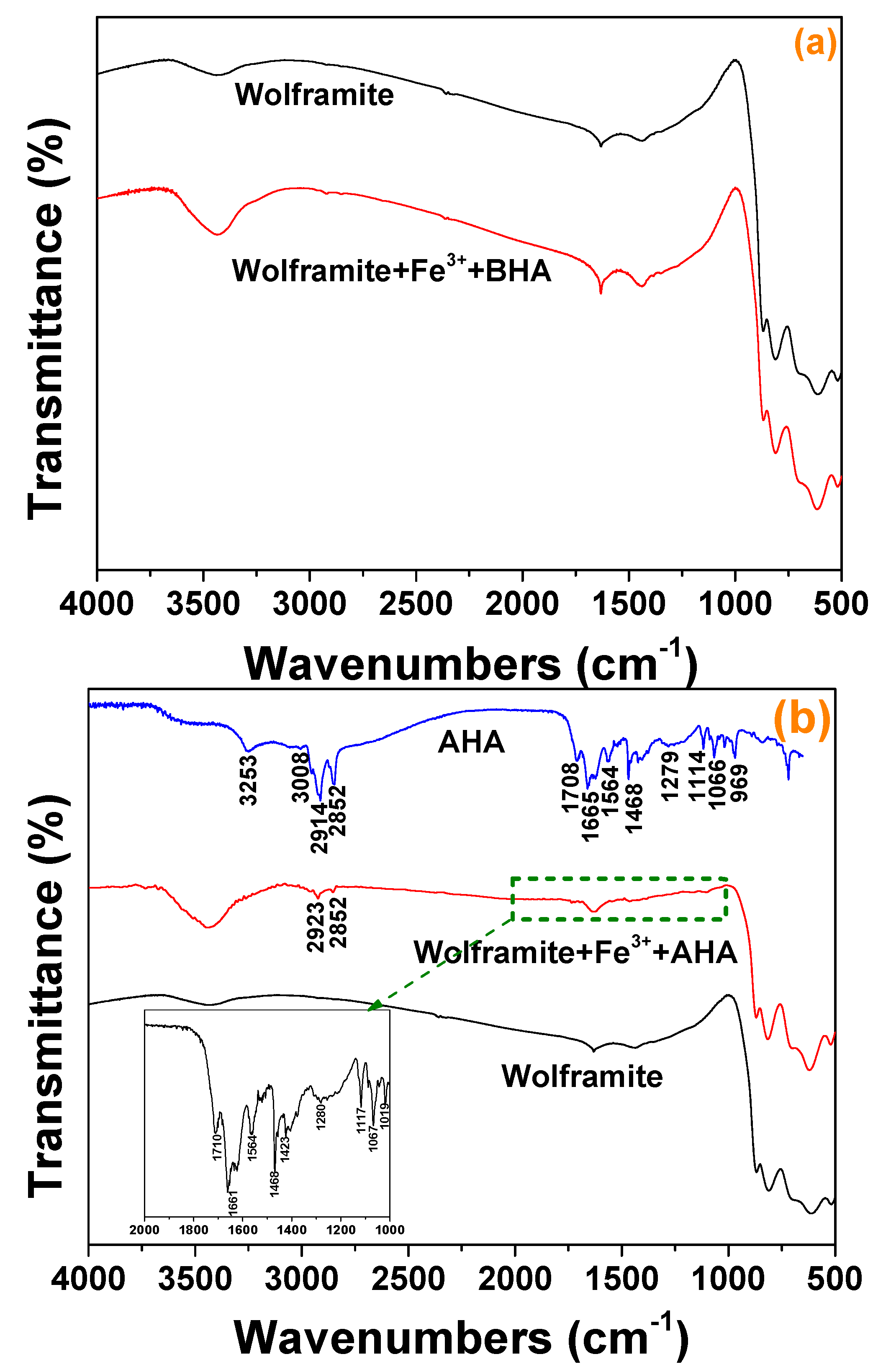
2.5. FTIR Spectra Analysis
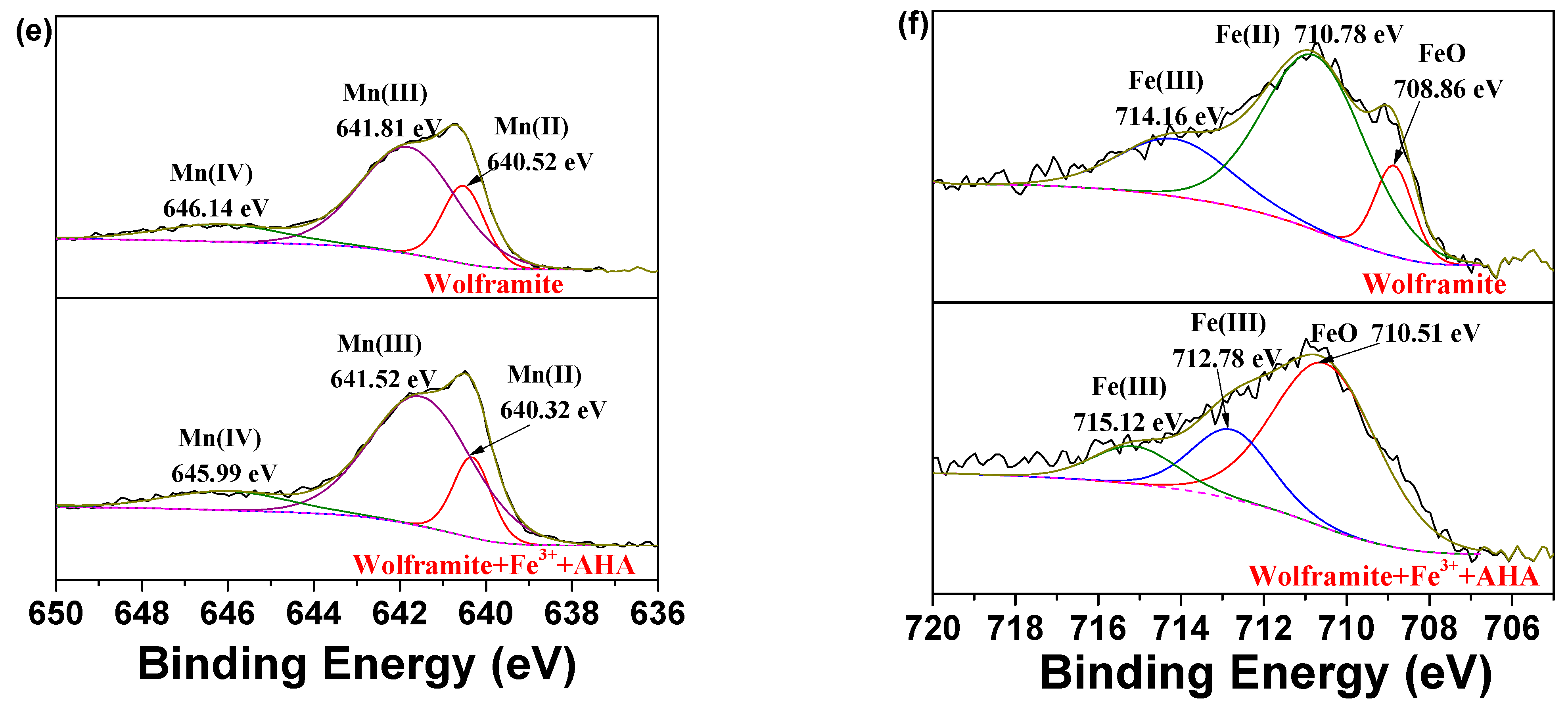
2.6. XPS Analysis
2.7. DFT Calculation
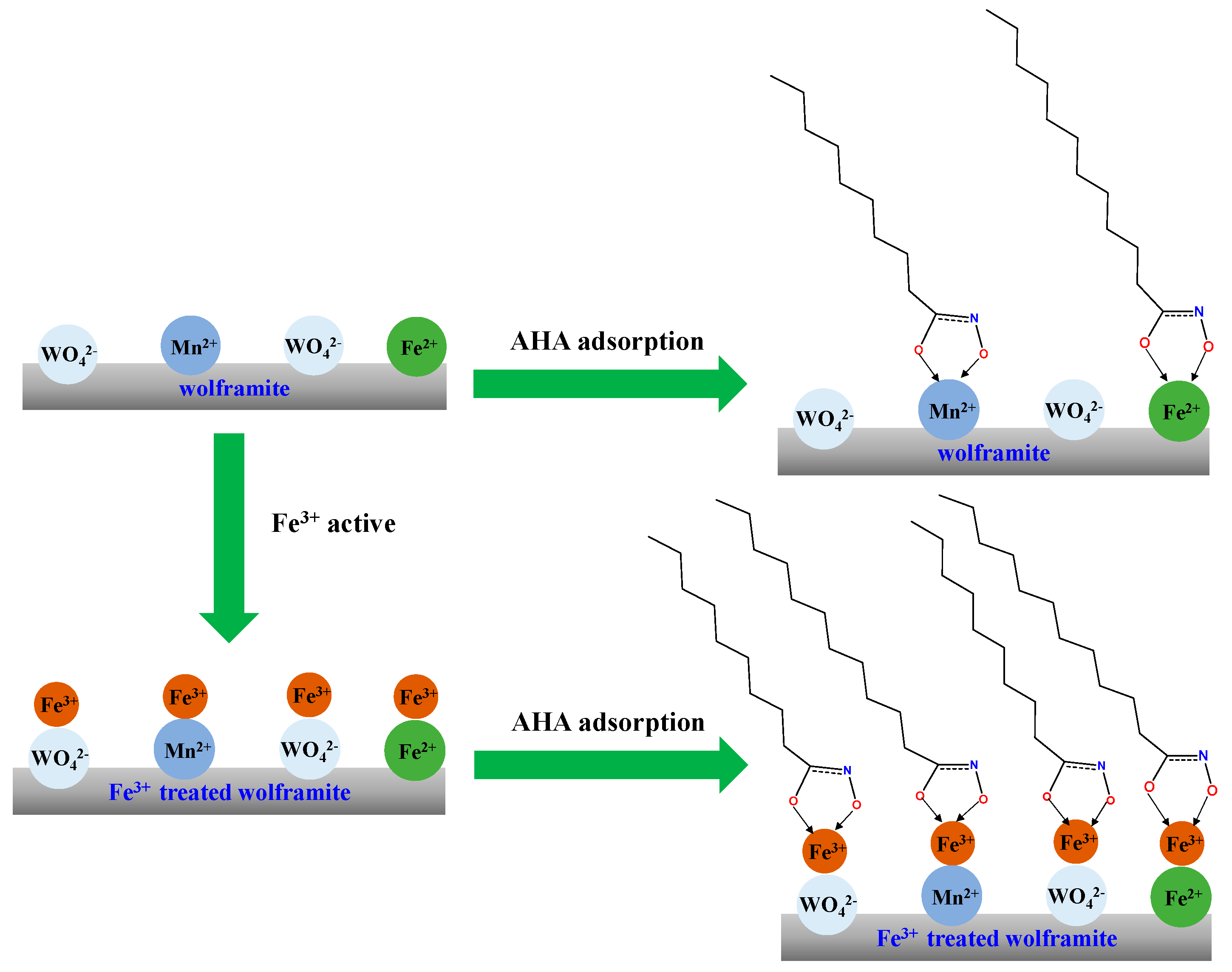
2.8. Discussion
 ) were generated. DFT calculations further elucidated that AHA and Fe3+ ions can form a more stable chelate.
) were generated. DFT calculations further elucidated that AHA and Fe3+ ions can form a more stable chelate.3. Experimental Section
3.1. Materials
3.2. Gas Chromatography
3.3. Micro-Flotation Tests
3.4. Zeta Potential Tests
3.5. Contact Angle Measurements
3.6. FTIR Spectroscopy and XPS
3.7. Theoretical Calculation Methods
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Liu, S.; Xie, L.; Liu, J.; Liu, G.; Zhong, H.; Wang, Y.; Zeng, H. Probing the interactions of hydroxamic acid and mineral surfaces: Molecular mechanism underlying the selective separation. Chem. Eng. J. 2019, 374, 123–132. [Google Scholar] [CrossRef]
- Huang, H.; Qiu, T.; Ren, S.; Qiu, X. Research on flotation mechanism of wolframite activated by Pb(II) in neutral solution. Appl. Surf. Sci. 2020, 530, 147036. [Google Scholar] [CrossRef]
- Kupka, N.; Rudolph, M. Froth flotation of scheelite—A review. Int. J. Min. Sci. Technol. 2018, 28, 373–384. [Google Scholar] [CrossRef]
- Liu, C.; Zhang, W.; Song, S.; Li, H. Study on the activation mechanism of lead ions in wolframite flotation using benzyl hydroxamic acid as the collector. Miner. Eng. 2019, 141, 105859. [Google Scholar] [CrossRef]
- Lu, Y.; Wang, S.; Zhong, H. Study on the role of a hydroxamic acid derivative in wolframite flotation: Selective separation and adsorption mechanism. Appl. Surf. Sci. 2021, 550, 149223. [Google Scholar] [CrossRef]
- Zhao, G.; Zhou, X.; Li, F.; Fu, G.; Shang, X. Flotation performance of anisic hydroxamic acid as new collector for tungsten and tin minerals. J. Cent. South Univ. 2022, 29, 3645–3655. [Google Scholar] [CrossRef]
- Meng, Q.; Yuan, Z.; Feng, Q.; Ou, L. The Effect of Quartz on the Flotation of Fine Wolframite with Octyl Hydroxamic Acid. Minerals 2017, 7, 186. [Google Scholar] [CrossRef]
- Meng, Q.; Yuan, Z.; Li, L.; Lu, J.; Yang, J. Modification mechanism of lead ions and its response to wolframite flotation using salicylhydroxamic acid. Powder Technol. 2020, 366, 477–487. [Google Scholar] [CrossRef]
- Huang, Z.; Shuai, S.; Burov, V.E.; Poilov, V.Z.; Li, F.; Wang, H.; Liu, R.; Zhang, S.; Cheng, C.; Li, W.; et al. Application of a new amidoxime surfactant in flotation separation of scheelite and calcite: Adsorption mechanism and DFT calculation. J. Mol. Liq. 2022, 364, 120036. [Google Scholar] [CrossRef]
- Zhao, G.; Wang, S.; Zhong, H. Study on the Activation of Scheelite and Wolframite by Lead Nitrate. Minerals 2015, 5, 247–258. [Google Scholar] [CrossRef]
- Yang, X. Beneficiation studies of tungsten ores—A review. Miner. Eng. 2018, 125, 111–119. [Google Scholar] [CrossRef]
- Zhang, S.; Huang, Z.; Wang, H.; Liu, R.; Cheng, C.; Guo, Z.; Yu, X.; He, G.; Fu, W. Separation of wolframite ore by froth flotation using a novel “crab” structure sebacoyl hydroxamic acid collector without Pb(NO3)2 activation. Powder Technol. 2021, 389, 96–103. [Google Scholar] [CrossRef]
- Meng, Q.; Du, Y.; Xu, Y.; Yuan, Z.; Li, H. Investigations on the effect of Fe2+ and Mn2+ in the flotation of fine wolframite with octyl hydroxamic acid. Miner. Eng. 2022, 183, 107626. [Google Scholar] [CrossRef]
- Ren, L.; Qiu, H.; Zhang, M.; Feng, K.; Liu, P.; Guo, J.; Feng, J. Behavior of Lead Ions in Cassiterite Flotation Using Octanohydroxamic Acid. Ind. Eng. Chem. Res. 2017, 56, 8723–8728. [Google Scholar] [CrossRef]
- Ren, L.; Qiu, H.; Qin, W.; Zhang, M.; Li, Y.; Wei, P. Inhibition mechanism of Ca2+, Mg2+ and Fe3+ in fine cassiterite flotation using octanohydroxamic acid. R. Soc. Open Sci. 2018, 5, 180158. [Google Scholar] [CrossRef]
- Meng, Q.; Feng, Q.; Ou, L. Flotation behavior and adsorption mechanism of fine wolframite with octyl hydroxamic acid. J. Cent. South Univ. 2016, 23, 1339–1344. [Google Scholar] [CrossRef]
- Qi, J.; Liu, S.; Qiu, X.; Liu, G. Enhanced flotation of Pb(II)-activated wolframite using a novel collector. Min. Proc. Ext. Met. Rev. 2022, 44, 577–583. [Google Scholar] [CrossRef]
- Sun, Q.; Ma, X.; Lu, Y.; Wang, S.; Zhong, H. Insights into the selective adsorption mechanism of a multifunctional thioether-containing hydroxamic acid on separation of wolframite from fluorite. Powder Technol. 2021, 380, 421–429. [Google Scholar] [CrossRef]
- Chen, Q.; Tian, M.; Kasomo, R.M.; Li, H.; Zheng, H.; Song, S.; Luo, H.; He, D. Depression effect of Al(III) and Fe(III) on rutile flotation using dodecylamine polyxyethylene ether as collector. Colloids Surf. A 2020, 603, 125269. [Google Scholar] [CrossRef]
- Yao, X.; Yu, X.; Wang, L.; Zeng, Y.; Mao, L.; Liu, S.; Xie, H.; He, G.; Huang, Z.; Zhang, S. Preparation of cinnamon hydroxamic acid and its flotation characteristics and mechanism to fine-grained wolframite. J. Mol. Liq. 2022, 362, 119721. [Google Scholar] [CrossRef]
- Deng, L.; Zhong, H.; Wang, S.; Liu, G. A novel surfactant N-(6-(hydroxyamino)-6-oxohexyl)octanamide: Synthesis and flotation mechanisms to wolframite. Sep. Purif. Technol. 2015, 145, 8–16. [Google Scholar] [CrossRef]
- Li, F.; Zhao, G.; Zhong, H.; Wang, S.; Liu, G. A novel activation system for wolframite flotation by using Cu(II) ion and α-hydroxyoctyl phosphinic acid. Chem. Eng. J. Adv. 2022, 9, 100234. [Google Scholar] [CrossRef]
- Cao, Y.; Xie, X.; Tong, X.; Feng, D.; Lv, J.; Chen, Y.; Song, Q. The activation mechanism of Fe(II) ion-modified cassiterite surface to promote salicylhydroxamic acid adsorption. Miner. Eng. 2021, 160, 106707. [Google Scholar] [CrossRef]
- Jin, S.; Zhang, P.; Ou, L.; Zhang, Y.; Chen, J. Flotation of cassiterite using alkyl hydroxamates with different carbon chain lengths: A theoretical and experimental study. Miner. Eng. 2021, 170, 107025–107034. [Google Scholar] [CrossRef]
- GB/T GB5009.168-2016; Determination of Fatty Acids in Food. National Standards of the People’s Republic of China: Beijing, China, 2016.
- Huang, Y.; Liu, G.; Liu, J.; Yang, X.; Zhang, Z. Thiadiazole-thione surfactants: Preparation, flotation performance and adsorption mechanism to malachite. J. Ind. Eng. Chem. 2018, 67, 99–108. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A.; et al. Gaussian 09, Revision D. 01; Gaussian, Inc.: Wallingford, CT, USA, 2013. [Google Scholar]
- Marenich, A.V.; Cramer, C.J.; Truhlar, D.G. Universal solvation model based on solute electron density and on a continuum model of the solvent defined by the bulk dielectric constant and atomic surface tensions. J. Phys. Chem. B 2009, 113, 6378–6396. [Google Scholar] [CrossRef]
- Chen, X.K.; Zhang, E.M.; Wu, D.; Chen, K.Q. Strain-Induced Medium-Temperature Thermoelectric Performance of Cu 4 Ti Se 4: The Role of Four-Phonon Scattering. Phys. Rev. Appl. 2023, 19, 044052. [Google Scholar] [CrossRef]






| Serial Number | Retention Time /min | Retention Index | Component | Content | Name |
|---|---|---|---|---|---|
| 1 | 21.174 | 1308 | C10:0 | 16.433% | capric acid |
| 2 | 24.593 | 1510 | C12:0 | 66.893% | lauric acid |
| 3 | 28.065 | 1710 | C14:0 | 2.148% | myristic acid |
| 4 | 31.506 | 1912 | C16:0 | 1.095% | palmitic acid |
| 5 | 35.665 | 2113 | C18:0 | 0.561% | stearic acid |
| 6 | 37.025 | 2084 | C18:1 | 9.534% | oleic acid |
| 7 | 39.408 | 2079 | C18:2 | 2.995% | 1inoleic acid |
| 8 | 40.989 | 2309 | C20:0 | 0.085% | arachidic acid |
| 9 | 42.517 | 2072 | C18:3 | 0.077% | 1inolenic acid |
| 10 | 42.703 | 2281 | C20:1 | 0.179% | eicosenoic acid |
| Complexes | EC | EM | ES | EB |
|---|---|---|---|---|
| Collector-Fe2+ | −597.672161 | −123.5570888 | −721.27406 | −0.0448102 |
| Collector-Mn2+ | −103.9976303 | −701.794163 | −0.1243717 | |
| Collector-Fe3+ | −123.1967865 | −721.071508 | −0.2025605 |
| Composition | WO3 | MnO | Fe2O3 | Al2O3 | NiO | Ta2O5 | CuO | TiO2 | Others |
|---|---|---|---|---|---|---|---|---|---|
| Wolframite (%) | 74.56 | 14.31 | 9.45 | 0.74 | 0.18 | 0.17 | 0.15 | 0.15 | 0.29 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xiao, J.; Li, P.; Liu, R.; Deng, Q.; Liu, X.; Li, C.; Xiao, Z. Preparation of Aliphatic Hydroxamic Acid from Litsea cubeba Kernel Oil and Its Application to Flotation of Fe(III)-Activated Wolframite. Molecules 2024, 29, 217. https://doi.org/10.3390/molecules29010217
Xiao J, Li P, Liu R, Deng Q, Liu X, Li C, Xiao Z. Preparation of Aliphatic Hydroxamic Acid from Litsea cubeba Kernel Oil and Its Application to Flotation of Fe(III)-Activated Wolframite. Molecules. 2024; 29(1):217. https://doi.org/10.3390/molecules29010217
Chicago/Turabian StyleXiao, Jingjing, Peiwang Li, Rukuan Liu, Qi Deng, Xudong Liu, Changzhu Li, and Zhihong Xiao. 2024. "Preparation of Aliphatic Hydroxamic Acid from Litsea cubeba Kernel Oil and Its Application to Flotation of Fe(III)-Activated Wolframite" Molecules 29, no. 1: 217. https://doi.org/10.3390/molecules29010217
APA StyleXiao, J., Li, P., Liu, R., Deng, Q., Liu, X., Li, C., & Xiao, Z. (2024). Preparation of Aliphatic Hydroxamic Acid from Litsea cubeba Kernel Oil and Its Application to Flotation of Fe(III)-Activated Wolframite. Molecules, 29(1), 217. https://doi.org/10.3390/molecules29010217





