Insights into Preparation Methods and Functions of Carbon-Based Solid Acids
Abstract
1. Introduction
- Rich acidic groups, such as sulfonic acid groups, hydroxyl groups, and carboxyl groups;
- Good biocompatibility via reduced toxic residuals compared with metal-based catalysts;
- Rich in raw materials and low in cost (carbon precursors are often animal wastes or plant wastes);
- Easy recycling and minimal corrosion of equipment.
2. Research on Different Carbonization Methods for Carbon-Based Solid Acids
2.1. One-Step Carbonization
2.2. Precise Targeting Methods
2.2.1. Preparation of Carbon-Based Solid Acid by Hydrothermal Carbonization
2.2.2. Template Carbonization
3. Different Acidification Methods of Carbon-Based Solid Acids
3.1. Preparation of Carbon-Based Solid Acid via Sulfonating Agent Sulfonation
3.2. Preparation of Carbon-Based Solid Acid via Acidification of Phosphoric Acid
3.3. Preparation of Carbon-Based Solid Acid via Acidification of Heteropoly Acid
3.4. Preparation of Carbon-Based Solid Acid by Nitric Acid Acidification
4. Summary and Outlook
Author Contributions
Funding
Conflicts of Interest
References
- Anastas, P.T.; Heine, L.G.; Williamson, T.C. Green chemical syntheses and processes: Introduction. ACS Symp. Ser. 2000, 767, 1–6. [Google Scholar]
- Sheldon, R.A. Atom utilisation, E factors and the catalytic solution. Comptes Rendus L’académie Sci.-Ser. IIC-Chem. 2000, 3, 541–551. [Google Scholar] [CrossRef]
- Li, S.; Wu, Y.; Dao, M.U.; Dragoi, E.-N.; Xia, C. Spotlighting of the role of catalysis for biomass conversion to green fuels towards a sustainable environment: Latest innovation avenues, insights, challenges, and future perspectives. Chemosphere 2023, 318, 137954. [Google Scholar] [CrossRef]
- Maddila, S.; Kerru, N.; Jonnalagadda, S.B. Recent Progress in the Multicomponent Synthesis of Pyran Derivatives by Sustainable Catalysts under Green Conditions. Molecules 2022, 27, 6347. [Google Scholar] [CrossRef]
- Rezayan, A.; Nie, R.; Wang, J.; Lu, T.; Xu, C.C.; Zhang, Y. Efficient one-pot synthesis of 5-hydroxymethylfurfural from functionalized microcrystalline cellulose: An alternative approach to strong acidic/basic catalysis. Chem. Eng. J. 2023, 462, 142219. [Google Scholar] [CrossRef]
- Xiong, S.S.; Ling, Y.L.; Tan, J.Y.; Han, Y.F.; Chao, L.U.O.; Zhu, L.J.; Wang, S.R. Preparation of platform compounds by hydrothermal conversion of lemon peel under the catalysis of sulfuric acid. J. Fuel Chem. Technol. 2021, 49, 1883–1888. [Google Scholar] [CrossRef]
- Fu, P.; Zhang, D.; Tang, B.; Lin, X.; Cai, H. Targeted production of aromatics from corn stover and high-density polyethylene composite pyrolysis over nitric acid modified biochar catalyst. J. Energy Inst. 2024, 112, 101447. [Google Scholar] [CrossRef]
- Jiang, C.-X.; Di, J.-H.; Su, C.; Yang, S.-Y.; Ma, C.-L.; He, Y.-C. One-pot co-catalysis of corncob with dilute hydrochloric acid and tin-based solid acid for the enhancement of furfural production. Bioresour. Technol. 2018, 268, 315–322. [Google Scholar] [CrossRef]
- Guan, C.-Y.; Chen, S.S.; Lee, T.-H.; Yu, C.-P.; Tsang, D.C. Valorization of biomass from plant microbial fuel cells into levulinic acid by using liquid/solid acids and green solvents. J. Clean. Prod. 2020, 260, 121097. [Google Scholar] [CrossRef]
- Zailan, Z.; Tahir, M.; Jusoh, M.; Zakaria, Z.Y. A review of sulfonic group bearing porous carbon catalyst for biodiesel production. Renew. Energy 2021, 175, 430–452. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, H.; Wang, Q.; An, X.; Ji, X.; Tian, Z.; Liu, S.; Yang, G. Recent advances in sustainable preparation of cellulose nanocrystals via solid acid hydrolysis: A mini-review. Int. J. Biol. Macromol. 2023, 253, 127353. [Google Scholar] [CrossRef]
- Gupta, P.; Paul, S. Solid acids: Green alternatives for acid catalysis. Catal. Today 2014, 236, 153–170. [Google Scholar] [CrossRef]
- Esmi, F.; Borugadda, V.B.; Dalai, A.K. Heteropoly acids as supported solid acid catalysts for sustainable biodiesel production using vegetable oils: A Review. Catal. Today 2022, 404, 19–34. [Google Scholar] [CrossRef]
- Mahajan, A.; Gupta, P.L. Carbon-based solid acids: A review. Environ. Chem. Lett. 2020, 18, 299–314. [Google Scholar] [CrossRef]
- Zou, R.; Qian, M.; Wang, C.; Mateo, W.; Wang, Y.; Dai, L.; Lin, X.; Zhao, Y.; Huo, E.; Wang, L. Biochar: From by-products of agro-industrial lignocellulosic waste to tailored carbon-based catalysts for biomass thermochemical conversions. Chem. Eng. J. 2022, 441, 135972. [Google Scholar] [CrossRef]
- Pengfei, Y.; Kobayashi, H.; Fukuoka, A. Recent developments in the catalytic conversion of cellulose into valuable chemicals. Chin. J. Catal. 2011, 32, 716–722. [Google Scholar] [CrossRef]
- Liang, X.Z. Novel magnetic carbon based solid acid for alkylation of benzene and dodecene. Chem. Eng. J. 2015, 264, 251–257. [Google Scholar] [CrossRef]
- Abbas, Z.; Ghani, K.; Shokrolahi, A.; Keshavarz, M. Carbon-based solid acid as an efficient and reusable catalyst for cross-aldol condensation of ketones with aromatic aldehydes under solvent-free conditions. Chin. J. Catal. 2008, 29, 602–606. [Google Scholar] [CrossRef]
- Wei, W.; Wu, J.; Shao, Q.; Yu, Z.; Yu, H.; Zhao, G.J.W.; Valorization, B. Biodiesel production using a biomass-based solid acid catalyst synthesized from agricultural residue garlic peel. Waste Biomass Valorization 2022, 13, 3597–3609. [Google Scholar] [CrossRef]
- Orege, J.I.; Oderinde, O.; Kifle, G.A.; Ibikunle, A.A.; Raheem, S.A.; Ejeromedoghene, O.; Okeke, E.S.; Olukowi, O.M.; Orege, O.B.; Fagbohun, E.O.; et al. Recent advances in heterogeneous catalysis for green biodiesel production by transesterification. Energy Convers. Manag. 2022, 258, 115406. [Google Scholar] [CrossRef]
- Kalemba-Jaje, Z.; Drelinkiewicz, A.; Lalik, E.; Konyushenko, E.; Stejskal, J. Bio-esters formation in transesterification and esterification reactions on carbon and silica supported organo-sulfonic acids-polyaniline solid catalysts. Fuel 2014, 135, 130–145. [Google Scholar] [CrossRef]
- Xu, H.; Li, X.; Hu, W.; Lu, L.; Chen, J.; Zhu, Y.; Zhou, H.; Si, C. Recent advances on solid acid catalyic systems for production of 5-Hydroxymethylfurfural from biomass derivatives. Fuel Process. Technol. 2022, 234, 107338. [Google Scholar] [CrossRef]
- Pan, H.; Xia, Q.; Wang, Y.; Shen, Z.; Huang, H.; Ge, Z.; Li, X.; He, J.; Wang, X.; Li, L. Recent advances in biodiesel production using functional carbon materials as acid/base catalysts. Fuel Process. Technol. 2022, 237, 107421. [Google Scholar] [CrossRef]
- Guo, Y.; Delbari, S.A.; Namini, A.S.; Van Le, Q.; Park, J.Y.; Kim, D.; Varma, R.S.; Jang, H.W.; Ali, T.; Shokouhimehr, M. Recent developments in solid acid catalysts for biodiesel production. Mol. Catal. 2023, 547, 113362. [Google Scholar] [CrossRef]
- Guo, M.-L.; Yin, X.-Y.; Huang, J. Preparation of novel carbonaceous solid acids from rice husk and phenol. Mater. Lett. 2017, 196, 23–25. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, L.; Sun, W.; Zhang, M.; Yu, S. One-step preparation of sulfonated carbon-based solid acid from distillers’ grain for esterification. Res. Chem. Intermed. 2017, 43, 5917–5932. [Google Scholar] [CrossRef]
- Leesing, R.; Siwina, S.; Fiala, K. Yeast-based biodiesel production using sulfonated carbon-based solid acid catalyst by an integrated biorefinery of durian peel waste. Renew. Energy 2021, 171, 647–657. [Google Scholar] [CrossRef]
- Chen, N.; Zhang, G.; Zhang, P.; Tao, X.; Wu, Y.; Wang, S.; Nabi, M. Rice husk-based solid acid for efficient hydrolysis and saccharification of corncob. Bioresour. Technol. 2019, 292, 121915. [Google Scholar] [CrossRef]
- Souza, M.; Batista, A.; Cuevas, R.; da Silva Filho, W.; Balanta, M.; Champi, A.; de Assunção, R. Simultaneous carbonization and sulfonation of microcrystalline cellulose to obtain solid acid catalyst and carbon quantum dots. Bioresour. Technol. Rep. 2022, 19, 101193. [Google Scholar] [CrossRef]
- Hara, M.; Yoshida, T.; Takagaki, A.; Takata, T.; Kondo, J.N.; Hayashi, S.; Domen, K. A carbon material as a strong protonic acid. Angew. Chem. 2004, 116, 3015–3018. [Google Scholar] [CrossRef]
- Kang, S.; Chang, J.; Fan, J. One Step Preparation of Sulfonated Solid Catalyst and Its Effect in Esterification Reaction. Chin. J. Chem. Eng. 2014, 22, 392–397. [Google Scholar] [CrossRef]
- Mateo, W.; Lei, H.; Villota, E.; Qian, M.; Zhao, Y.; Huo, E.; Zhang, Q.; Lin, X.; Wang, C. One-step synthesis of biomass-based sulfonated carbon catalyst by direct carbonization-sulfonation for organosolv delignification. Bioresour. Technol. 2021, 319, 124194. [Google Scholar] [CrossRef]
- Liang, X.; Zeng, M.; Qi, C. One-step synthesis of carbon functionalized with sulfonic acid groups using hydrothermal carbonization. Carbon 2010, 48, 1844–1848. [Google Scholar] [CrossRef]
- Zhang, S.; Li, J.; Ji, G.; Liang, X. Catalysis. One-pot synthesis of a novel magnetic carbon based solid acid for alkylation. Kinet. Catal. 2017, 58, 414–421. [Google Scholar] [CrossRef]
- Zhang, B.; Gao, M.; Geng, J.; Cheng, Y.; Wang, X.; Wu, C.; Wang, Q.; Liu, S.; Cheung, S. Catalytic performance and deactivation mechanism of a one-step sulfonated carbon-based solid-acid catalyst in an esterification reaction. Renew. Energy 2021, 164, 824–832. [Google Scholar] [CrossRef]
- Cao, M.; Peng, L.; Xie, Q.; Xing, K.; Lu, M.; Ji, J. Sulfonated Sargassum horneri carbon as solid acid catalyst to produce biodiesel via esterification. Bioresour. Technol. 2021, 324, 124614. [Google Scholar] [CrossRef]
- Roy, M.; Mohanty, K. Valorization of de-oiled microalgal biomass as a carbon-based heterogeneous catalyst for a sustainable biodiesel production. Bioresour. Technol. 2021, 337, 125424. [Google Scholar] [CrossRef]
- Wang, W.; Yang, D.; Mou, L.; Wu, M.; Wang, Y.; Cai, W.; Tan, F. Preparation of the porous carbon-based solid acid from starch for efficient degradation of chitosan to D-glucosamine. Int. J. Biol. Macromol. 2022, 209, 1629–1637. [Google Scholar] [CrossRef]
- Sangar, S.K.; Syazwani, O.N.; Farabi, M.A.; Razali, S.; Shobhana, G.; Teo, S.H.; Taufiq-Yap, Y.H. Effective biodiesel synthesis from palm fatty acid distillate (PFAD) using carbon-based solid acid catalyst derived glycerol. Renew. Energy 2019, 142, 658–667. [Google Scholar] [CrossRef]
- Hussein, M.F.; El Naga, A.O.A.; El Saied, M.; AbuBaker, M.M.; Shaban, S.A.; El Kady, F.Y. Potato peel waste-derived carbon-based solid acid for the esterification of oleic acid to biodiesel. Environ. Technol. Innov. 2021, 21, 101355. [Google Scholar] [CrossRef]
- Sangar, S.K.; Lan, C.S.; Razali, S.; Farabi, M.A.; Taufiq-Yap, Y.H. Methyl ester production from palm fatty acid distillate (PFAD) using sulfonated cow dung-derived carbon-based solid acid catalyst. Energy Convers. Manag. 2019, 196, 1306–1315. [Google Scholar] [CrossRef]
- Higai, D.; Lee, C.; Lang, J.; Qian, E.W. Saccharification of cellulose using biomass-derived activated carbon-based solid acid catalysts. Fuel Process. Technol. 2021, 215, 106738. [Google Scholar] [CrossRef]
- Zhao, Y.; Lei, H.; Liu, Y.; Ruan, R.; Qian, M.; Huo, E.; Zhang, Q.; Huang, Z.; Lin, X.; Wang, C. Microwave-assisted synthesis of bifunctional magnetic solid acid for hydrolyzing cellulose to prepare nanocellulose. Sci. Total Environ. 2020, 731, 138751. [Google Scholar] [CrossRef]
- Ning, Y.; Niu, S. Preparation and catalytic performance in esterification of a bamboo-based heterogeneous acid catalyst with microwave assistance. Energy Convers. Manag. 2017, 153, 446–454. [Google Scholar] [CrossRef]
- Ibrahim, N.A.; Rashid, U.; Taufiq-Yap, Y.H.; Yaw, T.C.S.; Ismail, I. Synthesis of carbonaceous solid acid magnetic catalyst from empty fruit bunch for esterification of palm fatty acid distillate (PFAD). Energy Convers. Manag. 2019, 195, 480–491. [Google Scholar] [CrossRef]
- Shi, Y.; Liang, X. Novel carbon microtube based solid acid from pampas grass stick for biodiesel synthesis from waste oils. J. Saudi Chem. Soc. 2019, 23, 515–524. [Google Scholar] [CrossRef]
- Ma, Z.; Xing, X.; Qu, Z.; Sun, Y.; Sun, G.; Wang, X.; Han, Y. Activity of microporous lignin-derived carbon-based solid catalysts used in biodiesel production. Int. J. Biol. Macromol. 2020, 164, 1840–1846. [Google Scholar] [CrossRef]
- Liang, X.; Yang, J. Synthesis of a Novel Carbon Based Strong Acid Catalyst through Hydrothermal Carbonization. Catal. Lett. 2009, 132, 460–463. [Google Scholar] [CrossRef]
- Memon, S.S.; Memon, N.; Memon, S.; Lachgar, A. An excellent sulfonated hydrothermal carbon catalyst from Mangifera indica L.(mango peels) for biodiesel production: Preparation, characterization, optimization, and kinetic study. Biomass Convers. Biorefinery 2022, 12, 141–151. [Google Scholar] [CrossRef]
- Goscianska, J.; Malaika, A. A facile post-synthetic modification of ordered mesoporous carbon to get efficient catalysts for the formation of acetins. Catal. Today 2020, 357, 84–93. [Google Scholar] [CrossRef]
- Zhao, Z.; Wang, X. Supported phosphotungstic acid catalyst on mesoporous carbon with bimodal pores: A superior catalyst for Friedel-Crafts alkenylation of aromatics with phenylacetylene. Appl. Catal. A Gen. 2016, 526, 139–146. [Google Scholar] [CrossRef]
- Sun, X.; Li, Y. Colloidal carbon spheres and their core/shell structures with noble-metal nanoparticles. Angew. Chem. Int. Ed. 2004, 43, 597–601. [Google Scholar] [CrossRef] [PubMed]
- Gan, L.; Zhu, J.; Lv, L. Cellulose hydrolysis catalyzed by highly acidic lignin-derived carbonaceous catalyst synthesized via hydrothermal carbonization. Cellulose 2017, 24, 5327–5339. [Google Scholar] [CrossRef]
- Jiang, W.; Yu, X.; Yuan, H.; Zhang, M.; Shen, B.; Pan, Z.; Zhou, H. Alcoholysis approach for an efficient and cleaner production of diosgenin by low cost and green carbon based solid acids from biomass residues. J. Clean. Prod. 2022, 331, 129974. [Google Scholar] [CrossRef]
- Fu, X.-b.; Chen, J.; Song, X.-l.; Zhang, Y.-m.; Zhu, Y.; Yang, J.; Zhang, C. Biodiesel production using a carbon solid acid catalyst derived from β-cyclodextrin. J. Am. Oil Chem. Soc. 2015, 92, 495–502. [Google Scholar] [CrossRef]
- Wang, S.; Xue, Y.; Zhao, X.; Yuan, H. Preparation of a carbon microsphere–based solid acid application to waste frying oil transesterification. Diam. Relat. Mater. 2021, 116, 108420. [Google Scholar] [CrossRef]
- Chen, C.; Sun, X.; Jiang, X.; Niu, D.; Yu, A.; Liu, Z.; Li, J. A two-step hydrothermal synthesis approach to monodispersed colloidal carbon spheres. Nanoscale Res. Lett. 2009, 4, 971–976. [Google Scholar] [CrossRef] [PubMed]
- Pan, H.; Sun, J.; Liu, J.; Zhang, Y.; Zhou, S. Preparation of sulfonated carbon derived from orange peel and its application in esterification. Chem. Phys. Lett. 2021, 770, 138395. [Google Scholar] [CrossRef]
- Yang, H.; Lei, S.; Xu, K.; Fang, Y.; Chen, X.; Chen, Y.; Wang, X.; Chen, H. Catalytic pyrolysis of cellulose with sulfonated carbon catalyst to produce levoglucosenone. Fuel Process. Technol. 2022, 234, 107323. [Google Scholar] [CrossRef]
- Zhao, G.; Liu, M.; Xia, X.; Li, L.; Xu, B. Conversion of furfuryl alcohol into ethyl levulinate over glucose-derived carbon-based solid acid in ethanol. Molecules 2019, 24, 1881. [Google Scholar] [CrossRef]
- Wang, S.; Eberhardt, T.L.; Pan, H. Efficient dehydration of fructose into 5-HMF using a weakly-acidic catalyst prepared from a lignin-derived mesoporous carbon. Fuel 2022, 316, 123255. [Google Scholar] [CrossRef]
- Malaika, A.; Kozłowski, M. Glycerol conversion towards valuable fuel blending compounds with the assistance of SO3H-functionalized carbon xerogels and spheres. Fuel Process. Technol. 2019, 184, 19–26. [Google Scholar] [CrossRef]
- Yang, Y.; Chiang, K.; Burke, N. Porous carbon-supported catalysts for energy and environmental applications: A short review. Catal. Today 2011, 178, 197–205. [Google Scholar] [CrossRef]
- Wang, B.; Ang, T.P.; Borgna, A. A rapid hard template method for the synthesis of N-doped mesoporous carbon replicated from TUD-1. Microporous Mesoporous Mater. 2012, 158, 99–107. [Google Scholar] [CrossRef]
- Yang, L.; Yuan, H.; Wang, S. Preparation and application of ordered mesoporous carbon-based solid acid catalysts for transesterification and epoxidation. J. Porous Mater. 2019, 26, 1435–1445. [Google Scholar] [CrossRef]
- Zhong, R.; Sels, B. Sulfonated mesoporous carbon and silica-carbon nanocomposites for biomass conversion. Appl. Catal. B Environ. 2018, 236, 518–545. [Google Scholar] [CrossRef]
- Guan, L.; Hu, H.; Teng, X.-l.; Zhu, Y.-f.; Zhang, Y.-l.; Chao, H.-x.; Yang, H.; Wang, X.-s.; Wu, M.-b. Templating synthesis of porous carbons for energy-related applications: A review. New Carbon Mater. 2022, 37, 25–45. [Google Scholar] [CrossRef]
- Saleem, A.; Zhang, Y.; Usman, M.; Haris, M.; Li, P. Tailored architectures of mesoporous carbon nanostructures: From synthesis to applications. Nano Today 2022, 46, 101607. [Google Scholar] [CrossRef]
- Elaigwu, S.E.; Kyriakou, G.; Prior, T.J.; Greenway, G.M. Microwave-assisted hydrothermal synthesis of carbon monolith via a soft-template method using resorcinol and formaldehyde as carbon precursor and pluronic F127 as template. Mater. Lett. 2014, 123, 198–201. [Google Scholar] [CrossRef]
- Kevin, M.-W.; Liang, X. Synthesis of a Novel Porous Carbon Based Solid Acid and Its Catalytic Activities for Biodiesel Synthesis from Waste Oils. Kinet. Catal. 2020, 61, 486–493. [Google Scholar] [CrossRef]
- Wang, S.; Lyu, L.; Sima, G.; Cui, Y.; Li, B.; Zhang, X.; Gan, L. Optimization of fructose dehydration to 5-hydroxymethylfurfural catalyzed by SO3H-bearing lignin-derived ordered mesoporous carbon. Korean J. Chem. Eng. 2019, 36, 1042–1050. [Google Scholar] [CrossRef]
- Deng, J.; Xiong, T.; Wang, H.; Zheng, A.; Wang, Y. Effects of Cellulose, Hemicellulose, and Lignin on the Structure and Morphology of Porous Carbons. ACS Sustain. Chem. Eng. 2016, 4, 3750–3756. [Google Scholar] [CrossRef]
- Hu, S.; Meng, F.; Huang, D.; Huang, J.; Lou, W. Hydrolysis of corn stover pretreated by DESs with carbon-based solid acid catalyst. SN Appl. Sci. 2020, 2, 1240. [Google Scholar] [CrossRef]
- Mallick, A.; Mukhopadhyay, M.; Ash, S. Synthesis, characterization and performance evaluation of a solid acid catalyst prepared from coconut shell for hydrolyzing pretreated Acacia nilotica heartwood. J. Inst. Eng. (India) Ser. E 2020, 101, 69–76. [Google Scholar] [CrossRef]
- Rangarajan, G.; Farnood, R. Role of persistent free radicals and lewis acid sites in visible-light-driven wet peroxide activation by solid acid biochar catalysts–A mechanistic study. J. Hazard. Mater. 2022, 438, 129514. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Ao, Z.; Wu, H.; Zhang, S.; Chi, C.; Hou, C.; Qian, L. Waste paper-derived magnetic carbon composite: A novel eco-friendly solid acid for the synthesis of n-butyl levulinate from furfuryl alcohol. Renew. Energy 2020, 146, 477–483. [Google Scholar] [CrossRef]
- Dai, Y.; Yang, S.; Wang, T.; Tang, R.; Wang, Y.; Zhang, L. High conversion of xylose to furfural over corncob residue-based solid acid catalyst in water-methyl isobutyl ketone. Ind. Crops Prod. 2022, 180, 114781. [Google Scholar] [CrossRef]
- Nie, Y.; Hou, Q.; Qian, H.; Bai, X.; Xia, T.; Lai, R.; Yu, G.; Rehman, M.L.U.; Ju, M. Synthesis of mesoporous sulfonated carbon from chicken bones to boost rapid conversion of 5-hydroxymethylfurfural and carbohydrates to 5-ethoxymethylfurfural. Renew. Energy 2022, 192, 279–288. [Google Scholar] [CrossRef]
- Wang, W.; Cao, X.; Guo, H.; Yang, X.; Guo, N.; Ma, Y. Carbon-based solid acid derived from lignin and polyvinyl chloride for conversion of xylose and crop wastes to furfural. Mol. Catal. 2022, 524, 112329. [Google Scholar] [CrossRef]
- Xu, H.; Xiong, S.; Zhao, Y.; Zhu, L.; Wang, S. Conversion of xylose to furfural catalyzed by carbon-based solid acid prepared from pectin. Energy Fuels 2021, 35, 9961–9969. [Google Scholar] [CrossRef]
- Pi, Y.; Liu, W.; Wang, J.; Peng, G.; Jiang, D.; Guo, R.; Yin, D. Preparation of Activated Carbon-Based Solid Sulfonic Acid and Its Catalytic Performance in Biodiesel Preparation. Front. Chem. 2022, 10, 944398. [Google Scholar] [CrossRef] [PubMed]
- Peng, X.; Shen, S.; Wang, C.; Li, T.; Li, Y.; Yuan, S.; Wen, X. Influence of relative proportions of cellulose and lignin on carbon-based solid acid for cellulose hydrolysis. Mol. Catal. 2017, 442, 133–139. [Google Scholar] [CrossRef]
- Alvear-Daza, J.J.; Pasquale, G.A.; Rengifo-Herrera, J.A.; Romanelli, G.P.; Pizzio, L.R. Mesoporous activated carbon from sunflower shells modified with sulfonic acid groups as solid acid catalyst for itaconic acid esterification. Catal. Today 2021, 372, 51–58. [Google Scholar] [CrossRef]
- Deshan, A.D.K.; Forero, J.J.; Bartley, J.P.; Marasinghege, C.; Tuiatua, K.; Beltramini, J.; Doherty, W.O. Structural features of cotton gin trash derived carbon material as a catalyst for the dehydration of fructose to 5-hydroxymethylfurfural. Fuel 2021, 306, 121670. [Google Scholar] [CrossRef]
- Maneechakr, P.; Karnjanakom, S. Catalytic transformation of furfural into bio-based succinic acid via ultrasonic oxidation using β-cyclodextrin-SO3H carbon catalyst: A liquid biofuel candidate. Energy Convers. Manag. 2017, 154, 299–310. [Google Scholar] [CrossRef]
- Nata, I.F.; Irawan, C.; Mardina, P.; Lee, C.-K. Carbon-based strong solid acid for cornstarch hydrolysis. J. Solid State Chem. 2015, 230, 163–168. [Google Scholar] [CrossRef]
- Chen, G.; Wang, X.; Jiang, Y.; Mu, X.; Liu, H. Insights into deactivation mechanism of sulfonated carbonaceous solid acids probed by cellulose hydrolysis. Catal. Today 2019, 319, 25–30. [Google Scholar] [CrossRef]
- Shen, F.; Guo, T.; Bai, C.; Qiu, M.; Qi, X. Hydrolysis of cellulose with one-pot synthesized sulfonated carbonaceous solid acid. Fuel Process. Technol. 2018, 169, 244–247. [Google Scholar] [CrossRef]
- Balasubramaniam, S.; Ninomiya, S.; Sasaki, M.; Quitain, A.; Kida, T.; Saldaña, M. Carbon-based solid acid catalyst derived from Undaria pinnatifida and its application in esterification. Algal Res. 2021, 55, 102272. [Google Scholar] [CrossRef]
- Li, C.; Zhang, J.; Shan, R.; Yuan, H.; Chen, Y. Kinetic study for thermocatalytic degradation of waste mixed cloth over antibiotic residue derived carbon-based solid acids. Fuel 2023, 331, 125797. [Google Scholar] [CrossRef]
- Yuan, H.; Li, C.; Shan, R.; Zhang, J.; Zhu, L.; Chen, Y. Municipal sludge derived solid acids for levoglucosenone production via cellulose fast pyrolysis. J. Anal. Appl. Pyrolysis 2022, 167, 105663. [Google Scholar] [CrossRef]
- Ye, X.-n.; Lu, Q.; Wang, X.; Guo, H.-q.; Cui, M.-s.; Dong, C.-q.; Yang, Y.-p. Catalytic fast pyrolysis of cellulose and biomass to selectively produce levoglucosenone using activated carbon catalyst. ACS Sustain. Chem. Eng. 2017, 5, 10815–10825. [Google Scholar] [CrossRef]
- Zhurinsh, A.; Dobele, G.; Pomilovskis, R.; Volperts, A.; Jurkjane, V. Evolution of 1, 6-anhydrosugars in the pyrolysis of biomass with phosphoric acid and P-containing activated carbon. Catal. Today 2021, 367, 51–57. [Google Scholar] [CrossRef]
- Grünert, A.; Schmidt, W.; Schüth, F. Carbon Supported Phosphoric Acid Catalysts for Gas-Phase Synthesis of Diesel Additives. Catal. Lett. 2020, 150, 2951–2958. [Google Scholar] [CrossRef]
- Obalı, Z.; Doğu, T. Activated carbon–tungstophosphoric acid catalysts for the synthesis of tert-amyl ethyl ether (TAEE). Chem. Eng. J. 2008, 138, 548–555. [Google Scholar] [CrossRef]
- Kumar, V.B.; Pulidindi, I.N.; Gedanken, A. Selective conversion of starch to glucose using carbon based solid acid catalyst. Renew. Energy 2015, 78, 141–145. [Google Scholar] [CrossRef]
- Yang, X.; Li, X.; Liu, J.; Rong, L. Ni/phosphomolybdic acid immobilized on carbon nanotubes for catalytic cracking of Jatropha oil. Chem. Phys. Lett. 2019, 720, 42–51. [Google Scholar] [CrossRef]
- da Costa, N.L.; Pereira, L.G.; Resende, J.V.M.; Mendoza, C.A.D.; Ferreira, K.K.; Detoni, C.; Souza, M.M.; Gomes, F.N. Phosphotungstic acid on activated carbon: A remarkable catalyst for 5-hydroxymethylfurfural production. Mol. Catal. 2021, 500, 111334. [Google Scholar] [CrossRef]
- Hu, S.; Huang, J.; Huang, D.; Li, P.; Tang, J.; Meng, F. Preparation of a bagasse-based solid catalyst loaded with phosphotungstic acid and its catalytic activity in the conversion of monosaccharide. Chem. Pap. 2022, 76, 157–167. [Google Scholar] [CrossRef]
- Guo, L.; Xie, W.; Gao, C. Heterogeneous H6PV3MoW8O40/AC-Ag catalyst for biodiesel production: Preparation, characterization and catalytic performance. Fuel 2022, 316, 123352. [Google Scholar] [CrossRef]
- Toda, M.; Takagaki, A.; Okamura, M.; Kondo, J.N.; Hayashi, S.; Domen, K.; Hara, M. Biodiesel made with sugar catalyst. Nature 2005, 438, 178. [Google Scholar] [CrossRef] [PubMed]
- Pandurangappa, M.; Lawrence, N.S.; Compton, R.G. Homogeneous chemical derivatisation of carbon particles: A novel method for funtionalising carbon surfaces. Analyst 2002, 127, 1568–1571. [Google Scholar] [CrossRef] [PubMed]
- Tang, Z.-E.; Lim, S.; Pang, Y.-L.; Ong, H.-C.; Lee, K.-T. Synthesis of biomass as heterogeneous catalyst for application in biodiesel production: State of the art and fundamental review. Renew. Sustain. Energy Rev. 2018, 92, 235–253. [Google Scholar] [CrossRef]
- Wang, X.; Liu, R.; Waje, M.M.; Chen, Z.; Yan, Y.; Bozhilov, K.N.; Feng, P. Sulfonated ordered mesoporous carbon as a stable and highly active protonic acid catalyst. Chem. Mater. 2007, 19, 2395–2397. [Google Scholar] [CrossRef]
- Araujo, R.O.; da Silva Chaar, J.; Queiroz, L.S.; da Rocha Filho, G.N.; da Costa, C.E.F.; da Silva, G.C.; Landers, R.; Costa, M.J.; Gonçalves, A.A.; de Souza, L.K.; et al. Low temperature sulfonation of acai stone biomass derived carbons as acid catalysts for esterification reactions. Energy Convers. Manag. 2019, 196, 821–830. [Google Scholar] [CrossRef]
- Balasubramanian, S.; Venkatachalam, P.J.C. Green synthesis of carbon solid acid catalysts using methane sulfonic acid and its application in the conversion of cellulose to platform chemicals. Cellulose 2022, 29, 1509–1526. [Google Scholar] [CrossRef]
- Farabi, M.A.; Ibrahim, M.L.; Rashid, U.; Taufiq-Yap, Y.H. Esterification of palm fatty acid distillate using sulfonated carbon-based catalyst derived from palm kernel shell and bamboo. Energy Convers. Manag. 2019, 181, 562–570. [Google Scholar] [CrossRef]
- Puziy, A.; Poddubnaya, O.; Gawdzik, B.; Tascón, J. Phosphorus-containing carbons: Preparation, properties and utilization. Carbon 2020, 157, 796–846. [Google Scholar] [CrossRef]
- Wang, L.; Dong, X.; Jiang, H.; Li, G.; Zhang, M. Phosphorylated ordered mesoporous carbon as a novel solid acid catalyst for the esterification of oleic acid. Catal. Commun. 2014, 56, 164–167. [Google Scholar] [CrossRef]
- Fulvio, P.F.; Mayes, R.T.; Bauer, J.C.; Wang, X.; Mahurin, S.M.; Veith, G.M.; Dai, S. “One-pot” synthesis of phosphorylated mesoporous carbon heterogeneous catalysts with tailored surface acidity. Catal. Today 2012, 186, 12–19. [Google Scholar] [CrossRef]
- Hu, R.; Xiao, J.; Wang, T.; Chen, G.; Chen, L.; Tian, X. Engineering of phosphate-functionalized biochars with highly developed surface area and porosity for efficient and selective extraction of uranium. Chem. Eng. J. 2020, 379, 122388. [Google Scholar] [CrossRef]
- Sun, Y.; Kang, Y.; Zhong, W.; Liu, Y.; Dai, Y. A simple phosphorylation modification of hydrothermally cross-linked chitosan for selective and efficient removal of U (VI). J. Solid State Chem. 2020, 292, 121731. [Google Scholar] [CrossRef]
- Sun, Y.; Zhang, H.; Yuan, N.; Ge, Y.; Dai, Y.; Yang, Z.; Lu, L. Phosphorylated biomass-derived porous carbon material for efficient removal of U (VI) in wastewater. J. Hazard. Mater. 2021, 413, 125282. [Google Scholar] [CrossRef] [PubMed]
- Cai, Y.; Wu, C.; Liu, Z.; Zhang, L.; Chen, L.; Wang, J.; Wang, X.; Yang, S.; Wang, S. Fabrication of a phosphorylated graphene oxide–chitosan composite for highly effective and selective capture of U (VI). Environ. Sci. Nano 2017, 4, 1876–1886. [Google Scholar] [CrossRef]
- Kirpsza, A.; Lalik, E.; Mordarski, G.; Micek-Ilnicka, A. Catalytic properties of carbon nanotubes-supported heteropolyacids in isopropanol conversion. Appl. Catal. A Gen. 2018, 549, 254–262. [Google Scholar] [CrossRef]
- Ghubayra, R.; Nuttall, C.; Hodgkiss, S.; Craven, M.; Kozhevnikova, E.F.; Kozhevnikov, I.V. Oxidative desulfurization of model diesel fuel catalyzed by carbon-supported heteropoly acids. Appl. Catal. B Environ. 2019, 253, 309–316. [Google Scholar] [CrossRef]
- Ghubayra, R.; Yahya, R.; Kozhevnikova, E.F.; Kozhevnikov, I.V. Oxidative desulfurization of model diesel fuel catalyzed by carbon-supported heteropoly acids: Effect of carbon support. Fuel 2021, 301, 121083. [Google Scholar] [CrossRef]
- Chen, T.; Fan, C. One-pot generation of mesoporous carbon supported nanocrystalline H3PW12O40 heteropoly acid with high performance in microwave esterification of acetic acid and isoamyl alcohol. J. Porous Mater. 2013, 20, 1225–1230. [Google Scholar] [CrossRef]
- Prado, R.G.; Bianchi, M.L.; da Mota, E.G.; Brum, S.S.; Lopes, J.H.; da Silva, M.J. H3PMo12O40/Agroindustry Waste Activated Carbon-Catalyzed Esterification of Lauric Acid with Methanol: A Renewable Catalytic Support. Waste Biomass Valorization 2017, 9, 669–679. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhao, M.; Wang, H.; Hu, H.; Liu, R.; Huang, Z.; Chen, C.; Chen, D.; Feng, Z. Damaged starch derived carbon foam-supported heteropolyacid for catalytic conversion of cellulose: Improved catalytic performance and efficient reusability. Bioresour. Technol. 2019, 288, 121532. [Google Scholar] [CrossRef]
- Van Pelt, A.H.; Simakova, O.A.; Schimming, S.M.; Ewbank, J.L.; Foo, G.S.; Pidko, E.A.; Hensen, E.J.; Sievers, C. Stability of functionalized activated carbon in hot liquid water. Carbon 2014, 77, 143–154. [Google Scholar] [CrossRef]
- Woodhead, A.L.; de Souza, M.L.; Church, J.S. An investigation into the surface heterogeneity of nitric acid oxidized carbon fiber. Appl. Surf. Sci. 2017, 401, 79–88. [Google Scholar] [CrossRef]
- Xia, W.; Jin, C.; Kundu, S.; Muhler, M. A highly efficient gas-phase route for the oxygen functionalization of carbon nanotubes based on nitric acid vapor. Carbon 2009, 47, 919–922. [Google Scholar] [CrossRef]
- Durán-Valle, C.J.; García-Vidal, J.A. Acidic activated carbons: An efficient catalyst for the epoxide ring-opening reaction with ethanol. Catal. Lett. 2009, 130, 37–41. [Google Scholar] [CrossRef]
- Packwood, D.; Nguyen, L.T.H.; Cesana, P.; Zhang, G.; Staykov, A.; Fukumoto, Y.; Nguyen, D.H. Machine Learning in Materials Chemistry: An Invitation. Mach. Learn. Appl. 2022, 8, 100265. [Google Scholar] [CrossRef]
- Pratap, A.; Sardana, N. Machine learning-based image processing in materials science and engineering: A review. Mater. Today Proc. 2022, 62, 7341–7347. [Google Scholar] [CrossRef]
- Tian, X.-l.; Song, S.-w.; Chen, F.; Qi, X.-j.; Wang, Y.; Zhang, Q.-h. Machine learning-guided property prediction of energetic materials: Recent advances, challenges, and perspectives. Energetic Mater. Front. 2022, 3, 177–186. [Google Scholar] [CrossRef]
- Gierlich, C.; Palkovits, S. Featurizing chemistry for machine learning—Methods and a coded example. Curr. Opin. Chem. Eng. 2022, 37, 100840. [Google Scholar] [CrossRef]
- Rajendra, P.; Girisha, A.; Naidu, T.G. Advancement of machine learning in materials science. Mater. Today Proc. 2022, 62, 5503–5507. [Google Scholar] [CrossRef]
- Butler, K.T.; Davies, D.W.; Cartwright, H.; Isayev, O.; Walsh, A. Machine learning for molecular and materials science. Nature 2018, 559, 547–555. [Google Scholar] [CrossRef]
- Selvaratnam, B.; Koodali, R.T. Machine learning in experimental materials chemistry. Catal. Today 2021, 371, 77–84. [Google Scholar] [CrossRef]
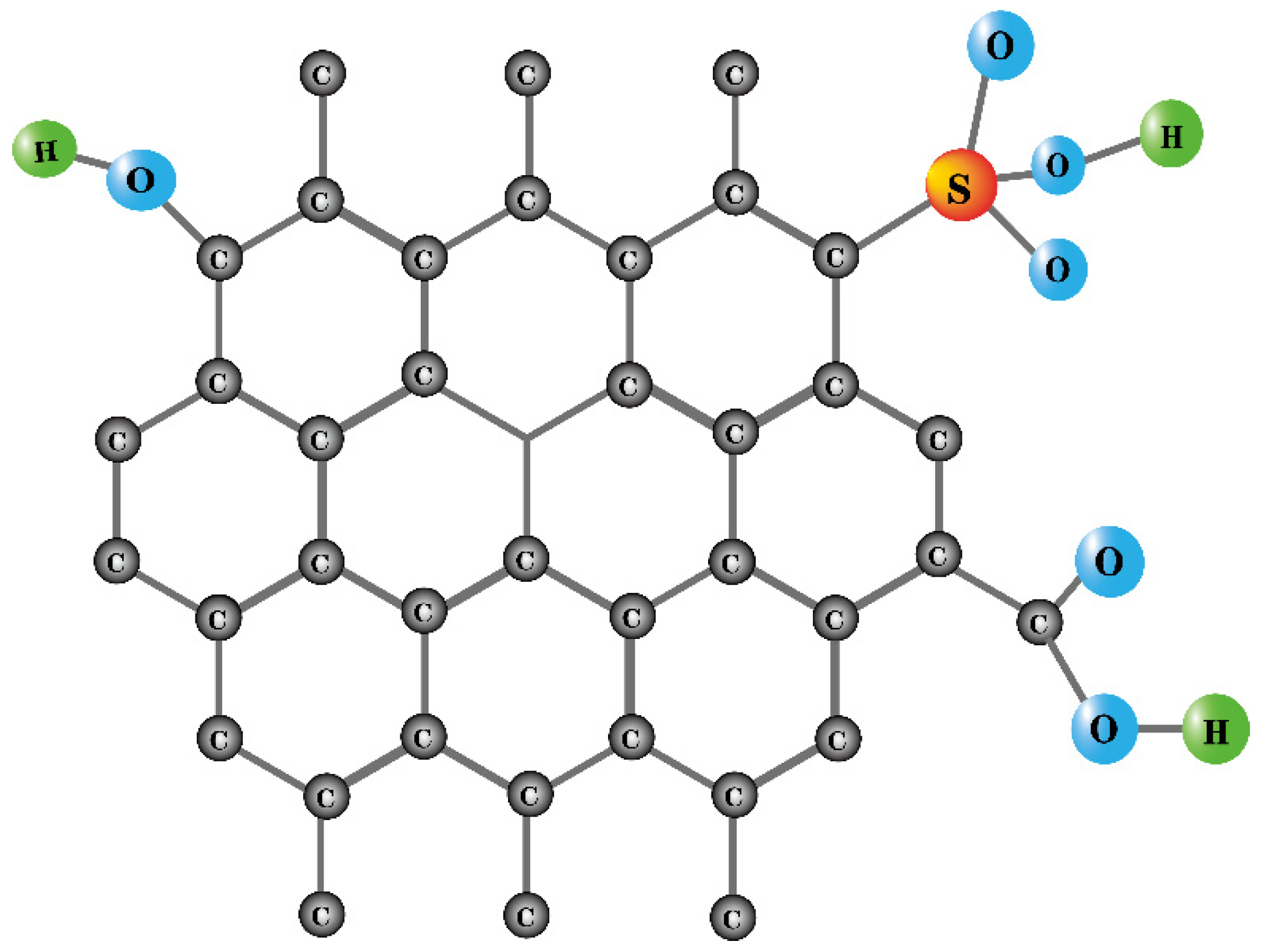
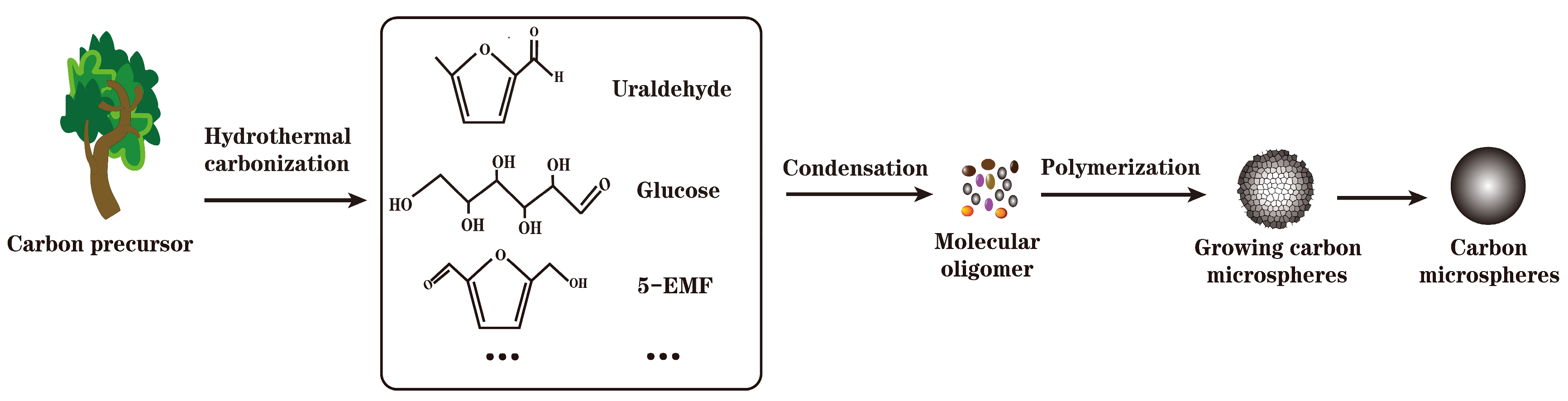
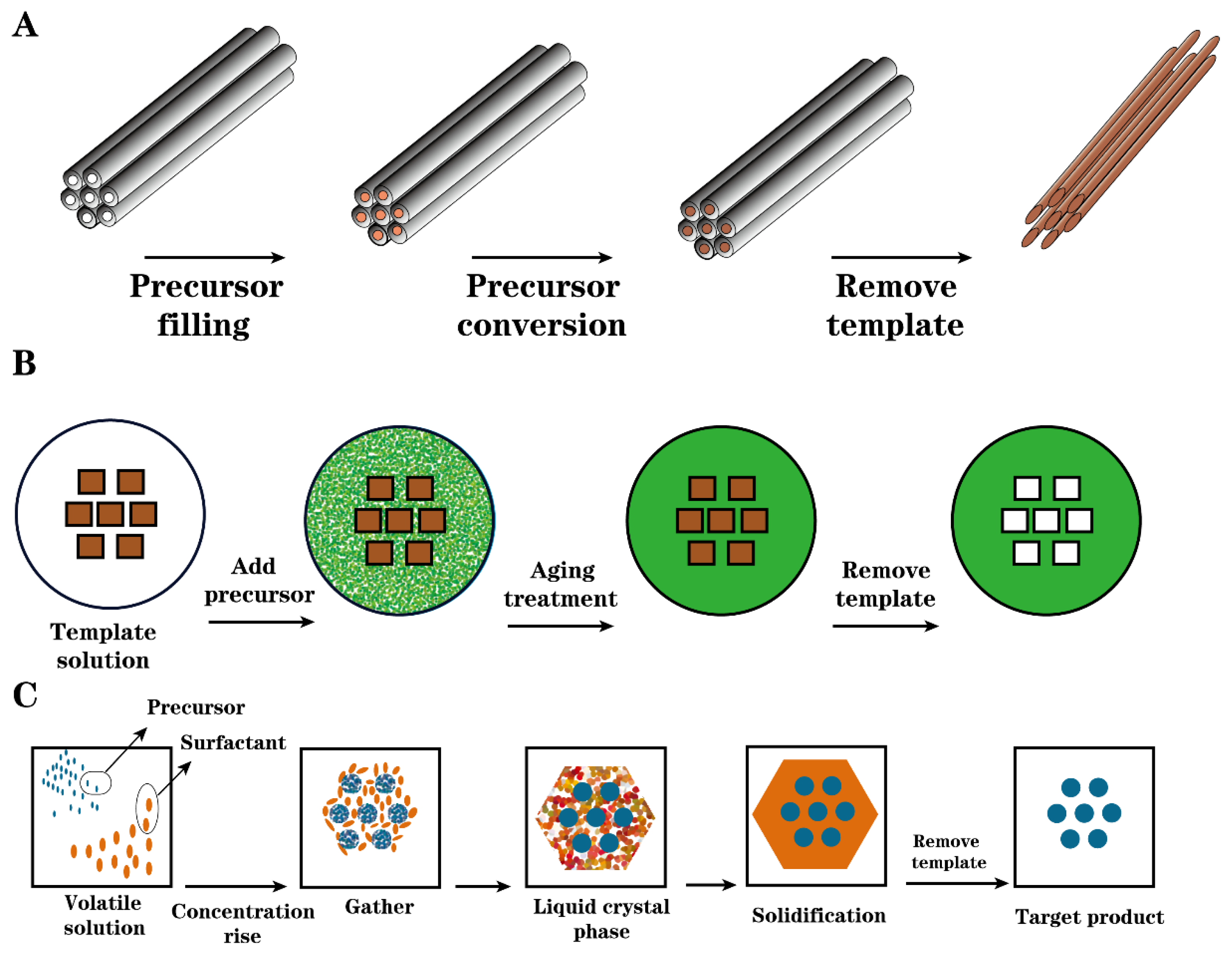
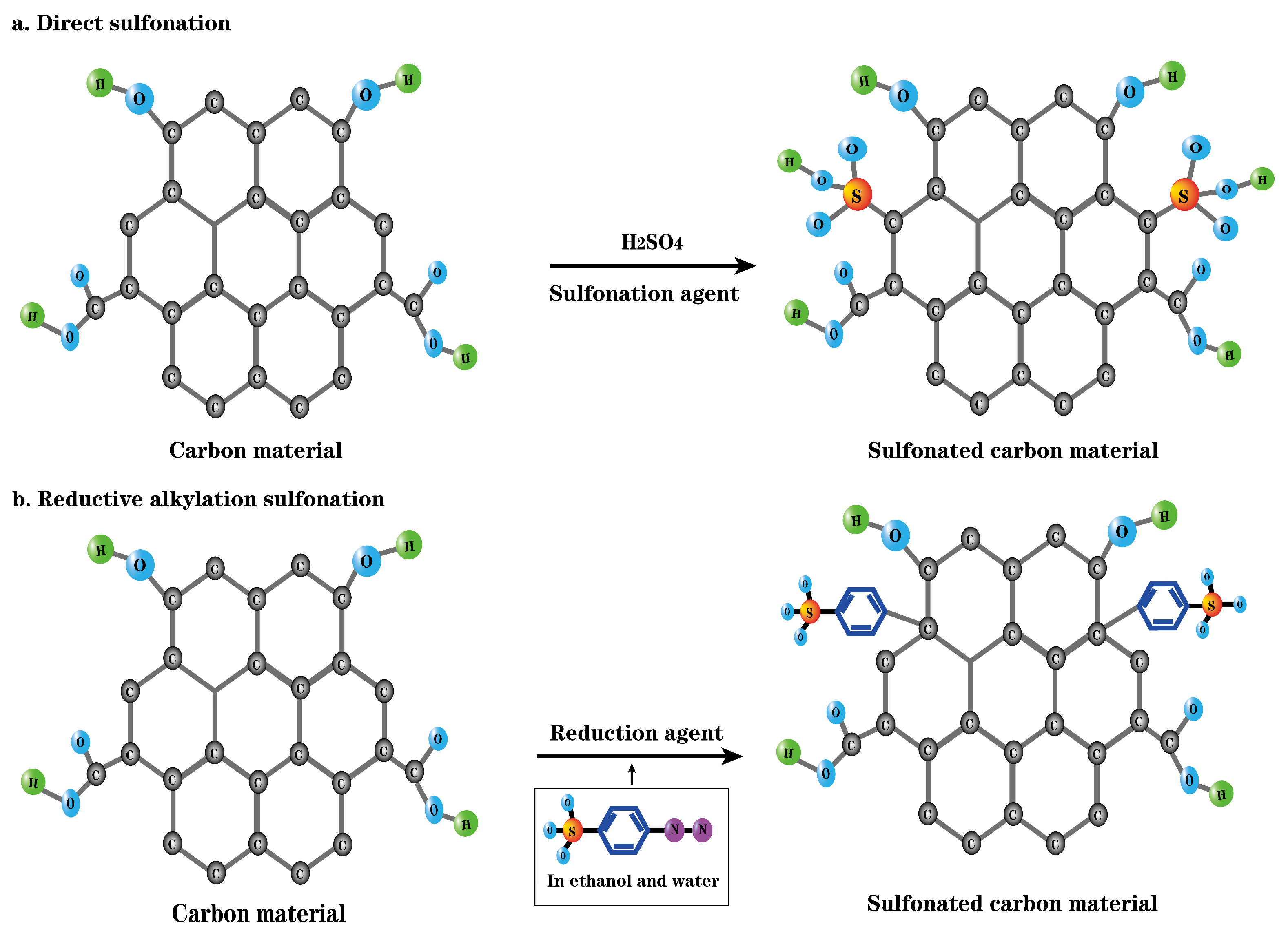

| Raw Material | Preparation Method | Characterization of Solid Acids | Function | Yield | Conversion Rate | Ref | ||
|---|---|---|---|---|---|---|---|---|
| Acid | SBET m2/g | VP m3/g | % | % | ||||
| Group | ||||||||
| mmol/g | ||||||||
| One-Step Direct Carbonization | ||||||||
| Rice husk and sulfuric acid | At 240 °C for 2 h | 1.45 | - | - | Synthetic biodiesel | - | 95.4 | [25] |
| Vinasse and sulfuric acid | At 20 °C for 1.4 h | 0.89 | 26.25 | - | Acetate esterification | - | 97.6 | [26] |
| Durian peel and sulfuric acid | At 100 °C for 2 h | 1.22 | - | - | Synthetic biodiesel | - | 81.67 | [27] |
| Microcrystalline cellulose and sulfuric acid | At 125 °C for 1 h | 1.31 | - | - | Oleic acid esterification | - | 80 | [29] |
| Step carbonization | ||||||||
| Sucralose and sulfuric acid | Carbonization at 300 °C for 0.5 h, sulfonation at 150 °C for 15 h | 0.67 | 0.31 | - | Preparation of lignin and glucose | 33.9 6.9 | - | [73] |
| Coconut shell and sulfuric acid | Carbonization at 450 °C for 1 h, sulfonation at 130 °C for 16 h | 1.27 | 10.16 | - | Preparation of glucose | 91 | - | [74] |
| Waste coffee powder and sulfuric acid | Carbonization at 500 °C for 5 h, sulfonation at 60 °C for 3 h | - | 15.39 | - | Fenton oxidative degradation of methyl orange | - | 70.2 | [75] |
| Paper scraps and sulfuric acid | Carbonization at 400 °C for 6 h, sulfonation at 150 °C for 15 h | 0.67 | 402.1 | - | Synthesis of n-butyl levulinate | 90.6 | - | [76] |
| Corncob residue and sulfuric acid | Carbonization at 400 °C for 3 h, sulfonation at 150 °C for 10 h | 0.68 | 282.94 | - | Preparation of sugar aldehyde | 73.64 | - | [77] |
| Potato skin and sulfuric acid | Carbonization at 450 °C for 1 h, sulfonation at 180 °C for 8 h | 1.6 | 827.7 | 0.92 | Synthetic biodiesel | 97.2 | [40] | |
| Bamboo powder and sulfuric acid | Carbonization at 400 °C for 1 h, sulfonation at 25 °C for 1 h | 1.80 | 1.17 | 0.00061 | Synthetic biodiesel | 97.31 | [44] | |
| Pampas grass and sulfuric acid | Carbonization at 400 °C for 6 h, sulfonation at 150 °C for 12 h | 2.3 | 278 | - | Synthetic biodiesel | 98.9 | 99.1 | [46] |
| Carbonization via precise target method | ||||||||
| Lignin and sulfuric acid | Hydrothermal carbonization at 240 °C for 10 h and sulfonation at 180 °C for 12 h. | 1.2 | - | - | Preparation of glucose | 59.1 | - | [53] |
| Diosgenin by-products and chlorosulfonic acid | Hydrothermal carbonization at 200 °C for 10 h and sulfonation for 4 h. | 1.41 | 5.17 | 0.0125 | Preparation of diosgenin | 22.4 | - | [54] |
| Orange peel and sulfuric acid | Hydrothermal carbonization at 180 °C for 12 h and sulfonation at 80 °C for 2 h. | 1.85 | 4.78 | 0.026 | Esterification of oleic acid and citric acid | - | 92.8 81.3 | [58] |
| Glucose and sulfuric acid | 200 °C hydrothermal carbonization for 6 h, 180 °C sulfonation for 5 h | 2.6 | - | - | Preparation of ethyl levulinate | 67.1 | - | [60] |
| Sucrose and sulfanilic acid | Template carbonization at 160 °C for 6 h and sulfonation at room temperature for 20 h. | 2.18 | 547 | 0.47 | Production of ethyl acetate | - | 95 | [50] |
| Formaldehyde, resorcinol and sulfuric acid | Carbon precursor prepared with soft template method is carbonized at 800 °C for 2 h and sulfonated at 140 °C for 20 h | 0.44 | 444 | 1.46 | Glycerol esterification | - | 95 | [62] |
| Lignin and acrylic acid | Template carbonization at 900 °C for 2 h and sulfonation at 190 °C for 16 h | - | 1197.1 | 0.63 | Preparation of 5-hydroxymethylfurfural | 96 | - | [61] |
| Raw Material | Acidizing Method | Acidity of Solid Acids | Function | Yield | Conversion Rate | Ref | ||
|---|---|---|---|---|---|---|---|---|
| SO3H Group mmol/g | PO3H2 Group mmol/g | Heteropoly Acid mmol/g | % | % | ||||
| Sulfonic Acid Acidification | ||||||||
| Chicken bone and p-aminobenzenesulfonic acid | P-aminobenzenesulfonic acid sulfonated at 80 °C for 12 h | 2.33 | Synthesis of 5-ethoxymethylfurfural | 68.6 | - | [78] | ||
| Lignin, PVC and p-aminobenzenesulfonic acid | P-aminobenzenesulfonic acid sulfonated at 80 °C for 0.5 h | 0.86 | Synthetic sugar alcohol | 84.3 | - | [79] | ||
| Orange peel pectin and sulfuric acid | Sulfuric acid sulfonation at 170 °C for 6 h | 1.35 | - | - | Synthetic sugar aldehyde | 80.4 | 100 | [80] |
| Activated carbon and sulfuric acid | Sulfuric acid sulfonation at 150 °C for 4 h | 0.64 | Synthetic biodiesel | 94.2 | 95.2 | [81] | ||
| Cellulose, lignin and sulfuric acid | Sulfuric acid sulfonation at 150 °C for 6 h | 0.74 | Preparation of reducing sugar | 60 | [82] | |||
| Sunflower seed hull and chlorosulfonic acid | Sulfonation in chlorosulfonic acid at 25 °C for 2 h | - | - | - | Itaconic acid esterification | 77 | 100 | [83] |
| Cotton ginning waste and chlorosulfonic acid | Sulfonation of chlorosulfonic acid for 9 h at room temperature | 1.89 | Preparation of 5-hydroxymethylfurf-ural | 71.7 | 76.74 | [84] | ||
| β-Cyclodextrin and 2-hydroxyethanesulp-honic acid | 2-hydroxyethanesulp-honic acidsulfonated at 180 °C for 4 h | - | Preparation of succinic acid | 81.2 | [85] | |||
| Glucose and 2-hydroxyethanesulp-honic acid | 2-hydroxyethanesulp-honic acidsulfonated at 180 °C for 4 h | 2.1 | Preparation of reducing sugar | 99.8 | [86] | |||
| Glucose and p-toluenesulfonic acid | p-toluenesulfonic acid sulfonated at 180 °C for 24 h | 0.68 | Hydrolyzed microcrystalline cellulose | 30.9 | [87] | |||
| Sucralose and p-toluenesulfonic acid | p-toluenesulfonic acid sulfonated at 180 °C for 24 h | - | Preparation of reducing sugar | 67.6 | [88] | |||
| Undaria pinnatifida and p-toluenesulfonic acid | p-toluenesulfonic acid sulfonated at 200 °C for 3 h | 0.234 | - | - | Acetate esterification | 81.9 | 98.2 | [89] |
| Acidification of phosphoric acid | ||||||||
| Antibiotic residues and phosphoric acid | Ultrasonic immersion with phosphoric acid for 6 h | 3.05 | Pyrolysis of catalytic waste mixed cloth | - | - | [90] | ||
| Municipal sludge and phosphoric acid | Ultrasonic immersion with phosphoric acid for 3 h | 0.82 | Production of levoglucone | 19.6 | [91] | |||
| Bagasse and phosphoric acid | Soak in phosphoric acid for 2 h | - | - | - | Production of levoglucone | 18.1 | - | [92] |
| Birch and phosphoric acid | Soak in phosphoric acid for 6 h | - | - | - | Production of levoglucone | 20 | - | [93] |
| Activated carbon and phosphoric acid | Phosphoric acid impregnation | - | 0.385 | - | Synthetic dimethyl ether | - | 47 | [94] |
| Heteropoly acid acidification | ||||||||
| Activated carbon and phosphotungstic acid | Phosphotungstic acid impregnation | - | - | 31 | Synthetic tert-myl thyl ether | - | 18.8 | [95] |
| Activated carbon and silicotungstic acid | Silicotungstic acid immersion 72 h | - | - | 20 | Preparation of glucose | 94 | - | [96] |
| Carbon nanotubes and Phosphomolybdic Acid | Phosphomolybdic acid impregnation | - | - | 15 | Synthetic biodiesel | 86.7 | 98.2 | [97] |
| Activated carbon and12- tungstophosphoric acid | 12-tungstenphosphoric acid immersion for 12 h | - | - | 50 | Synthesis of 5-hydroxymethylfurf-ural | 99.3 | 100 | [98] |
| Bagasse and phosphotungstic acid | Phosphotungstic acid impregnation | - | - | 20 | Preparation of fructose | 56.2 | - | [99] |
| Activated carbon and H6PV3MoW8O40 | H6PV3MoW8O40 impregnation | - | - | 35 | Synthetic biodiesel | - | 91.3 | [100] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shu, D.; Zhang, J.; Ruan, R.; Lei, H.; Wang, Y.; Moriko, Q.; Zou, R.; Huo, E.; Duan, D.; Gan, L.; et al. Insights into Preparation Methods and Functions of Carbon-Based Solid Acids. Molecules 2024, 29, 247. https://doi.org/10.3390/molecules29010247
Shu D, Zhang J, Ruan R, Lei H, Wang Y, Moriko Q, Zou R, Huo E, Duan D, Gan L, et al. Insights into Preparation Methods and Functions of Carbon-Based Solid Acids. Molecules. 2024; 29(1):247. https://doi.org/10.3390/molecules29010247
Chicago/Turabian StyleShu, Dong, Jian Zhang, Roger Ruan, Hanwu Lei, Yunpu Wang, Qian Moriko, Rongge Zou, Erguang Huo, Dengle Duan, Lu Gan, and et al. 2024. "Insights into Preparation Methods and Functions of Carbon-Based Solid Acids" Molecules 29, no. 1: 247. https://doi.org/10.3390/molecules29010247
APA StyleShu, D., Zhang, J., Ruan, R., Lei, H., Wang, Y., Moriko, Q., Zou, R., Huo, E., Duan, D., Gan, L., Zhou, D., Zhao, Y., & Dai, L. (2024). Insights into Preparation Methods and Functions of Carbon-Based Solid Acids. Molecules, 29(1), 247. https://doi.org/10.3390/molecules29010247








