11B NMR of the Morphological Evolution of Traditional Chinese Medicine Borax
Abstract
:1. Introduction
2. Results
2.1. Factors Affecting Chemical Shift
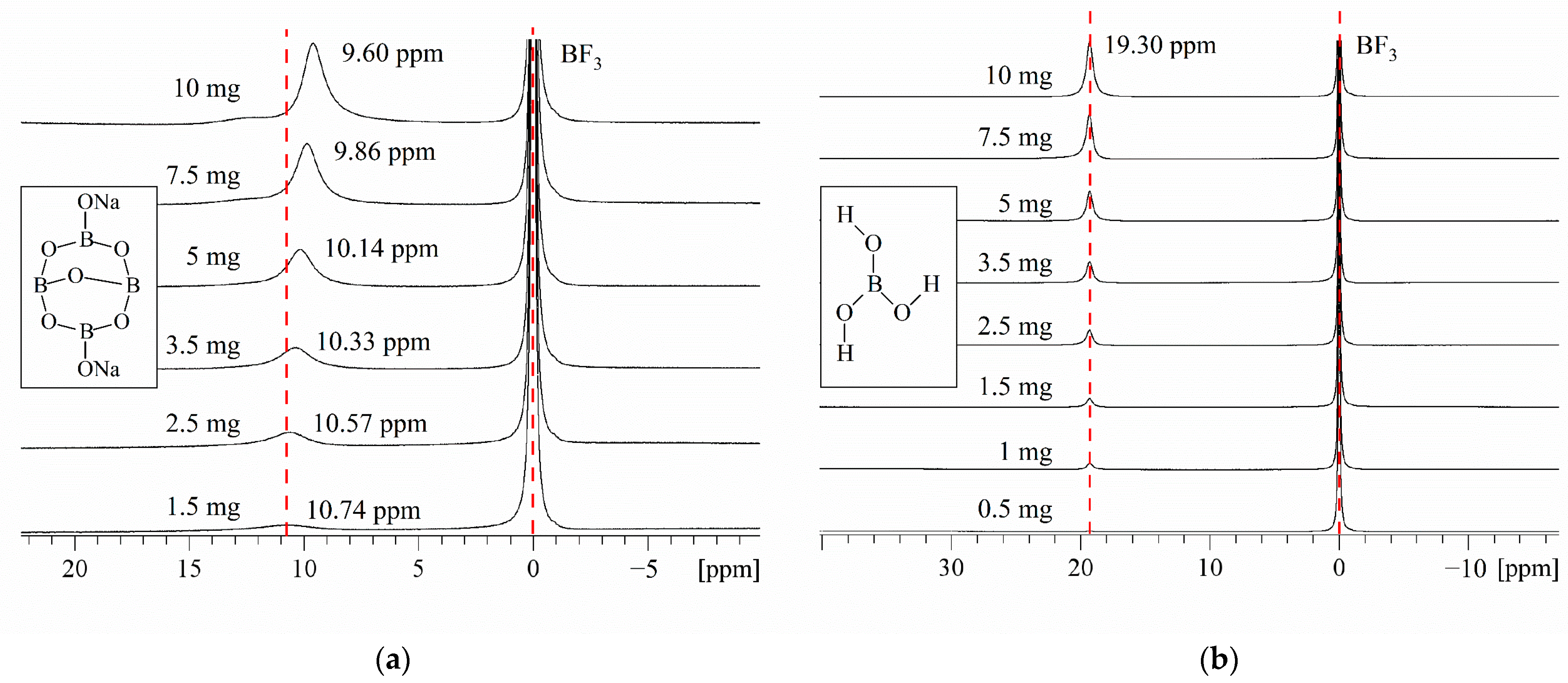
2.1.1. 11B NMR Study of Borax Solutions at Various Concentrations
2.1.2. 11B NMR Study of Borax Solutions at Various pHs
 |
 |
 |
2.1.3. 11B NMR Study of Borax Solutions at Various Temperature
2.2. Borax Conversion to Boric Acid Studies Vinegar Processing of Borax Studies
2.3. 11B NMR of Boron-Containing TCM
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Methods
4.2.1. 11B NMR Analysis
4.2.2. NMR Sample Preparation of Boron-Containing TCMs
4.2.3. NMR Sample Preparation of Boric Acid and Borax
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhai, W.; Ma, F.; Zhao, F. Research progress in traditional Chinese medicine borax. Prog. Vet. Med. 2007, 28, 87–91. [Google Scholar]
- Yin, L.; Xu, L.; Hu, G.; Suo, Z.; Duan, H.; Yang, Z.; Gao, L.; Mu, X. Current research status of anti-tumor traditional Chinese medicine and its effective ingredients. Prog. Vet. Med. 2006, 27, 39–43. [Google Scholar]
- Wang, Y.; Hou, X. Clinical Observation on the Treatment of Simple Candida Vaginitis with Nystatin Combined with Bingboron Powder for Vaginal Administration. Med. Forum 2006, 10, 1079–1080. [Google Scholar]
- Balakrishnan, B.; Mohanty, M.; Pr, U.; Athipettah, J. Evaluation of an in Situ Forming Hydrogel Wound Dressing Based on Oxidized Alginate and Gelatin. Biomaterials 2005, 26, 6335–6342. [Google Scholar] [CrossRef] [PubMed]
- Shang, C.; Gu, Y.; Mo, J.; Xu, H. The effect of boron on 10 physiological and biochemical indicators in chickens. Chin. J. Vet. Sci. 2007, 27, 221–223. [Google Scholar]
- Wang, J.; Liu, H.; Ma, J.; Wang, J. Fluorine poisoning and related research techniques. Prog. Vet. Med. 2006, 27, 51–54. [Google Scholar]
- Avdeeva, V.V.; Privalov, V.I.; Kubasov, A.S.; Nikiforova, S.E.; Malinina, E.A.; Kuznetsov, N.T. 2D COSY 11B NMR Spectroscopy in the Interpretation of the Structures of Iso and Trans Isomers of the Macropolyhedral Boron Cluster [B20H18]2–. Inorg. Chim. Acta 2023, 555, 121564. [Google Scholar] [CrossRef]
- Park, Y.; Davis, M.E. Local Geometry of Tetrahedrally Coordinated Boron Correlates with 11B NMR Chemical Shifts in Borosilicate Minerals. J. Phys. Chem. C 2023, 127, 6578–6585. [Google Scholar] [CrossRef]
- Ksenofontov, A.A.; Isaev, Y.I.; Lukanov, M.M.; Makarov, D.M.; Eventova, V.A.; Khodov, I.A.; Berezin, M.B. Accurate Prediction of 11B NMR Chemical Shift of BODIPYs via Machine Learning. Phys. Chem. Chem. Phys. PCCP 2023, 25, 9472–9481. [Google Scholar] [CrossRef]
- Horst, R.; Farley, K.A.; Kormos, B.L.; Withka, J.M. NMR Spectroscopy: The Swiss Army Knife of Drug Discovery. J. Biomol. NMR 2020, 74, 509–519. [Google Scholar] [CrossRef]
- Lin, W.; Lu, H.; Lee, M.; Yang, S.; Chen, H.; Chang, Y.; Chang, W. Evaluation of the Cultivation Age of Dried Ginseng Radix and Its Commercial Products by Using 1H-NMR Fingerprint Analysis. Am. J. Chin. Med. 2010, 38, 205–218. [Google Scholar] [CrossRef] [PubMed]
- Keiffer, S.; Carneiro, M.G.; Hollander, J.; Kobayashi, M.; Pogoryelev, D.; Ab, E.; Theisgen, S.; Müller, G.; Siegal, G. NMR in Target Driven Drug Discovery: Why Not? J. Biomol. NMR 2020, 74, 521–529. [Google Scholar] [CrossRef] [PubMed]
- Uluisik, I.; Karakaya, H.C.; Koc, A. The Importance of Boron in Biological Systems. J. Trace Elem. Med. Biol. 2018, 45, 156–162. [Google Scholar] [CrossRef] [PubMed]
- Turner, C.L.; Taylor, R.E.; Kaner, R.B. 10B and 11B NMR Study of Elemental Boron. J. Phys. Chem. C 2015, 119, 13807–13813. [Google Scholar] [CrossRef]
- Patel, I.; Venkatramani, C.J.; Stumpf, A.; Wigman, L.; Yehl, P. Trace Analysis of Potentially Mutagenic Boronic Acids and Esters in Drug Substance by ICP-MS. Org. Process Res. Dev. 2017, 21, 182–186. [Google Scholar] [CrossRef]
- Baldwin, A.F.; North, R.; Eisenbeis, S. Trace Level Quantification of Derivatized Boronic Acids by LC/MS/MS. Org. Process Res. Dev. 2019, 23, 88–92. [Google Scholar] [CrossRef]
- Yousef, T.A.; Alrabiah, H.; Al-Agamy, M.H.; Al-Salahi, R.; Ali, E.A.; Mostafa, G.A.E. Synthesis of (R)-(6-Methoxyquinolin-4-Yl)[(1S,2S,4S,5R)-5-Vinylquinuclidin-2-Yl]Methanol Tetraphenylborate Ion-Pair Complex: Characterization, Antimicrobial, and Computational Study. Molecules 2023, 28, 6974. [Google Scholar] [CrossRef]
- Diaw-Ndiaye, F.; Sanz Miguel, P.J.; Rodríguez, R.; Macías, R. The Synthesis, Characterization, and Fluxional Behavior of a Hydridorhodatetraborane. Molecules 2023, 28, 6462. [Google Scholar] [CrossRef]
- Beckett, M.A.; Horton, P.N.; Hursthouse, M.B.; Timmis, J.L. Synthesis and Thermal Studies of Two Phosphonium Tetrahydroxidohexaoxidopentaborate(1-) Salts: Single-Crystal XRD Characterization of [iPrPPh3][B5O6(OH)4]·3.5H2O and [MePPh3][B5O6(OH)4]·B(OH)3·0.5H2O. Molecules 2023, 28, 6867. [Google Scholar] [CrossRef]
- Ban, H.S.; Nakamura, H. Boron-Based Drug Design: Boron-Based Drug Design. Chem. Rec. 2015, 15, 616–635. [Google Scholar] [CrossRef]
- Woods, W.G. An Introduction to Boron: History, Sources, Uses, and Chemistry. Environ. Health Perspect. 1994, 102, 5. [Google Scholar] [PubMed]
- Jacobs, R.T.; Plattner, J.J.; Keenan, M. Boron-Based Drugs as Antiprotozoals. Curr. Opin. Infect. Dis. 2011, 24, 586–592. [Google Scholar] [CrossRef] [PubMed]
- Hadrup, N.; Frederiksen, M.; Sharma, A.K. Toxicity of Boric Acid, Borax and Other Boron Containing Compounds: A Review. Regul. Toxicol. Pharmacol. 2021, 121, 104873. [Google Scholar] [CrossRef] [PubMed]
- Das, B.C.; Nandwana, N.K.; Das, S.; Nandwana, V.; Shareef, M.A.; Das, Y.; Saito, M.; Weiss, L.M.; Almaguel, F.; Hosmane, N.S.; et al. Boron Chemicals in Drug Discovery and Development: Synthesis and Medicinal Perspective. Molecules 2022, 27, 2615. [Google Scholar] [CrossRef] [PubMed]
- Tülüce, Y.; Masseh, H.D.I.; Koyuncu, İ.; Kiliç, A.; Durgun, M.; Özkol, H. Novel Fluorine Boron Hybrid Complex as Potential Antiproliferative Drugs on Colorectal Cancer Cell Line. Anti-Cancer Agents Med. Chem. 2019, 19, 627–637. [Google Scholar] [CrossRef] [PubMed]
- Kilic, A.; Emin KARATAS, M.; Beyazsakal, L.; Okumus, V. Preparation and Spectral Studies of Boronate Ester Modified Magnetite Iron Nanoparticles (Fe3O4@APTES-B) as a New Type of Biological Agents. J. Mol. Liq. 2022, 361, 119602. [Google Scholar] [CrossRef]
- Kilic, A.; Alshhab, A.; Okumus, V. Preparation and Spectroscopic Properties of Bioactive 1, 2, 3-Triazole-Linked Boronate Esters for Use in Antioxidant, Antimicrobial, and DNA Binding Studies. J. Organomet. Chem. 2023, 993, 122707. [Google Scholar] [CrossRef]
- Erkmen, T.; Serdar, B.S.; Ateş, H.; Korkmaz, M.; Koçtürk, S. Borax Pentahydrate and Disodium Pentaborate Decahydrate Are Candidates as Anti-Leukemic Drug Components by Inducing Apoptosis and Changing Bax/Bcl-2 Ratio in HL-60 Cell Line. Biol. Trace Elem. Res. 2022, 200, 1608–1616. [Google Scholar] [CrossRef]
- Fernandes, G.F.S.; Denny, W.A.; Dos Santos, J.L. Boron in Drug Design: Recent Advances in the Development of New Therapeutic Agents. Eur. J. Med. Chem. 2019, 179, 791–804. [Google Scholar] [CrossRef]
- Rock, F.L.; Mao, W.; Yaremchuk, A.; Tukalo, M.; Crépin, T.; Zhou, H.; Zhang, Y.-K.; Hernandez, V.; Akama, T.; Baker, S.J.; et al. An Antifungal Agent Inhibits an Aminoacyl-tRNA Synthetase by Trapping tRNA in the Editing Site. Science 2007, 316, 1759–1761. [Google Scholar] [CrossRef]
- O’Donovan, M.R.; Mee, C.D.; Fenner, S.; Teasdale, A.; Phillips, D.H. Boronic Acids—A Novel Class of Bacterial Mutagen. Mutat. Res./Genet. Toxicol. Environ. Mutagen. 2011, 724, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Messner, K.; Vuong, B.; Tranmer, G.K. The Boron Advantage: The Evolution and Diversification of Boron’s Applications in Medicinal Chemistry. Pharmaceuticals 2022, 15, 264. [Google Scholar] [CrossRef] [PubMed]
- Sabuncuoglu, B.T.; Kocaturk, P.A.; Yaman, O.; Kavas, G.O.; Tekelioglu, M. Effects of Subacute Boric Acid Administration on Rat Kidney Tissue. Clin. Toxicol. 2006, 44, 249–253. [Google Scholar] [CrossRef] [PubMed]
- Bolt, H.M.; Başaran, N.; Duydu, Y. Human Environmental and Occupational Exposures to Boric Acid: Reconciliation with Experimental Reproductive Toxicity Data. J. Toxicol. Environ. Health Part A 2012, 75, 508–514. [Google Scholar] [CrossRef] [PubMed]
- Scialli, A.R.; Bonde, J.P.; Brüske-Hohlfeld, I.; Culver, B.D.; Li, Y.; Sullivan, F.M. An Overview of Male Reproductive Studies of Boron with an Emphasis on Studies of Highly Exposed Chinese Workers. Reprod. Toxicol. 2010, 29, 10–24. [Google Scholar] [CrossRef]
- Duydu, Y.; Başaran, N.; Üstündağ, A.; Aydın, S.; Yalçın, C.Ö.; Anlar, H.G.; Bacanlı, M.; Aydos, K.; Atabekoğlu, C.S.; Golka, K.; et al. Birth Weights of Newborns and Pregnancy Outcomes of Environmentally Boron-Exposed Females in Turkey. Arch. Toxicol. 2018, 92, 2475–2485. [Google Scholar] [CrossRef] [PubMed]
- Bolt, H.M.; Başaran, N.; Duydu, Y. Effects of Boron Compounds on Human Reproduction. Arch. Toxicol. 2020, 94, 717–724. [Google Scholar] [CrossRef]
- Park, E.H.; Uk Jeong, S.; Ho Jung, U.; Kim, S.H.; Lee, J.; Woo Nam, S.; Hoon Lim, T.; Jun Park, Y.; Ho Yu, Y. Recycling of Sodium Metaborate to Borax. Int. J. Hydrogen Energy 2007, 32, 2982–2987. [Google Scholar] [CrossRef]
- Islam, T.M.B.; Yoshino, K.; Sasane, A. 11B NMR Study of P-Carboxybenzeneboronic Acid Ions for Complex Formation with Some Monosaccharides. Anal. Sci. Int. J. Jpn. Soc. Anal. Chem. 2003, 19, 455–460. [Google Scholar] [CrossRef]
- Kui, X.; Zhao, C.; Zhou, P.; Zhang, Q.; Wang, B. Literature research on the preparation methods of traditional Chinese medicine borax. J. Chin. Med. Mater. 2012, 35, 322–325. [Google Scholar]
- Jackman, L.M. Nuclear Magnetic Resonance. Nature 1960, 187, 358. [Google Scholar] [CrossRef]
- Macho, J.M.; Blue, R.M.; Lee, H.-W.; MacMillan, J.B. Boron NMR as a Method to Screen Natural Product Libraries for B-Containing Compounds. Org. Lett. 2022, 24, 3161–3166. [Google Scholar] [CrossRef] [PubMed]
- Lewiński, J.; Kubicki, D. NMR Spectroscopy, Heteronuclei, B., Al, Ga, In, Tl☆. In Encyclopedia of Spectroscopy and Spectrometry, 3rd ed.; Lindon, J.C., Tranter, G.E., Koppenaal, D.W., Eds.; Academic Press: Oxford, UK, 2017; pp. 318–329. ISBN 978-0-12-803224-4. [Google Scholar]
- Yinghuai, Z.; Hosmane, N.S. Applications and Perspectives of Boron-Enriched Nanocomposites in Cancer Therapy. Future Med. Chem. 2013, 5, 705–714. [Google Scholar] [CrossRef] [PubMed]
- Pizzorno, L. Nothing Boring About Boron. Integr. Med. 2015, 14, 35–48. [Google Scholar]
- Soriano-Ursúa, M.A.; Das, B.C.; Trujillo-Ferrara, J.G. Boron-Containing Compounds: Chemico-Biological Properties and Expanding Medicinal Potential in Prevention, Diagnosis and Therapy. Expert Opin. Ther. Pat. 2014, 24, 485–500. [Google Scholar] [CrossRef]




| No. | Proprietary Chinese Medicine Preparation | The Number of 11B Signal Peaks |
|---|---|---|
| 1 | Compound Borax Solution | 3 |
| 2 | BingPengHanPian | 3 |
| 3 | ZhuHuangChuiHouSan | 3 |
| 4 | Laryngitis Pill | 3 |
| 5 | DingPeng Cream | 3 |
| 6 | Boric Acid Ointment | 2 |
| 7 | ZhenZhuBingPengSan | 1 |
| 8 | QingYanWan | 1 |
| 9 | Boric Acid Ear Drops | 1 |
| 10 | Lotion Boracic Acid | 1 |
| 11 | BeiLing Capsules | 1 |
| 12 | BingPengYanHouSan | 1 |
| 13 | QingYanPian | 1 |
| 14 | BingPengSan | 1 |
| 15 | MaYingLongBaBaoYanGao | — |
| 16 | LuPaoSan | — |
| 17 | ShuJinDingTong Capsules | — |
| 18 | HouKangSan | — |
| 19 | BoYunDing | — |
| 20 | Naphazoline Hydrochloride | — |
| No. | Proprietary Chinese Medicine Preparation | Borax Peak | Boric Acid Peak | δB 1–2 ppm | δB 5.6–6.2 ppm | δB = 15.4 ppm | δB 18–19 ppm | δB = 23.3 ppm |
|---|---|---|---|---|---|---|---|---|
| 1 | QingYanWan | √ | ||||||
| 2 | Boric Acid Ear Drops | √ | ||||||
| 3 | Lotion Boracic Acid | √ | ||||||
| 4 | QingYanPian | √ | ||||||
| 5 | ZhenZhuBingPengSan | √ | ||||||
| 6 | BingPengSan; | √ | ||||||
| 7 | DingPeng Cream | √ | √ | √ | ||||
| 8 | Compound Borax Solution | √ | √ | √ | ||||
| 9 | Laryngitis Pill | √ | √ | √ | ||||
| 10 | BingPengHanPian | √ | √ | √ | ||||
| 11 | ZhuHuangChuiHouSan | √ | √ | √ | ||||
| 12 | BingPengYanHouSan | √ | ||||||
| 13 | BeiLing Capsules | √ | ||||||
| 14 | Boric Acid Ointment | √ | √ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Q.; Yang, Y.; Wang, Q.; Han, X.; Zhu, J.; Zhang, N.; Wang, Q.; Li, K.; Gong, P.; Chen, F. 11B NMR of the Morphological Evolution of Traditional Chinese Medicine Borax. Molecules 2024, 29, 251. https://doi.org/10.3390/molecules29010251
Li Q, Yang Y, Wang Q, Han X, Zhu J, Zhang N, Wang Q, Li K, Gong P, Chen F. 11B NMR of the Morphological Evolution of Traditional Chinese Medicine Borax. Molecules. 2024; 29(1):251. https://doi.org/10.3390/molecules29010251
Chicago/Turabian StyleLi, Qiulin, Yawen Yang, Qingfeng Wang, Xiang Han, Junfeng Zhu, Nan Zhang, Qiuhong Wang, Kanshe Li, Pin Gong, and Fuxin Chen. 2024. "11B NMR of the Morphological Evolution of Traditional Chinese Medicine Borax" Molecules 29, no. 1: 251. https://doi.org/10.3390/molecules29010251
APA StyleLi, Q., Yang, Y., Wang, Q., Han, X., Zhu, J., Zhang, N., Wang, Q., Li, K., Gong, P., & Chen, F. (2024). 11B NMR of the Morphological Evolution of Traditional Chinese Medicine Borax. Molecules, 29(1), 251. https://doi.org/10.3390/molecules29010251






