A Review: Subcritical Water Extraction of Organic Pollutants from Environmental Matrices
Abstract
:1. Introduction
2. Types of Analytes Extracted
2.1. PAHs
2.2. PCBs
2.3. Pesticides
2.4. Other OPs
2.5. Comparison of SBWE with Other Conventional Techniques
3. Conclusions
4. Future Developments
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kaleem, M.; Mumtaz, A.S.; Hashmi, M.Z.; Saeed, A.; Inam, F.; Waqar, R.; Jabeen, A. Myco-and phyco-remediation of polychlorinated biphenyls in the environment: A review. Environ. Sci. Pollut. Res. 2023, 30, 13994–14007. [Google Scholar] [CrossRef] [PubMed]
- Eldos, H.I.; Zouari, N.; Saeed, S.; Al-Ghouti, M.A. Recent advances in the treatment of PAHs in the environment: Application of nanomaterial-based technologies. Arab. J. Chem. 2022, 15, 103918. [Google Scholar] [CrossRef]
- Karimi-Maleh, H.; Ghalkhani, M.; Dehkordi, Z.S.; Singh, J.; Wen, Y.; Baghayeri, M.; Rouhi, J.; Fu, L.; Rajendran, S. MOF-enabled pesticides as developing approach for sustainable agriculture and reducing environmental hazards. J. Ind. Eng. Chem. 2023, 129, 105–123. [Google Scholar] [CrossRef]
- Alharbi, O.M.L.; Khattab, R.A.; Ali, I. Health and environmental effects of persistent organic pollutants. J. Mol. Liq. 2018, 263, 442–453. [Google Scholar] [CrossRef]
- Negrete-Bolagay, D.; Zamora-Ledezma, C.; Chuya-Sumba, C.; De Sousa, F.B.; Whitehead, D.; Alexis, F.; Guerrero, V.H. Persistent organic pollutants: The trade-off between potential risks and sustainable remediation methods. J. Environ. Manag. 2021, 300, 113737. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Wang, X.; Cai, Z. Analytical chemistry of the persistent organic pollutants identified in the Stockholm Convention: A review. Anal. Chim. Acta 2013, 790, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Cabral, C.M.; Mielke, K.C.; Pires, F.R.; Dos Santos, J.B. Organic Pollutants Threatening Human Health. In Nanotechnology For Environmental Pollution Decontamination; Apple Academic Press: New York, NY, USA, 2023; pp. 3–37. [Google Scholar]
- Manousi, N.; Rosenberg, E.; Deliyanni, E.; Zachariadis, G.A.; Samanidou, V. Magnetic solid-phase extraction of organic compounds based on graphene oxide nanocomposites. Molecules 2020, 25, 1148. [Google Scholar] [CrossRef]
- Albero, B.; Tadeo, J.L.; Pérez, R.A. Ultrasound-assisted extraction of organic contaminants. TrAC Trends Anal. Chem. 2019, 118, 739–750. [Google Scholar] [CrossRef]
- Andreu, V.; Picó, Y. Pressurized liquid extraction of organic contaminants in environmental and food samples. TrAC Trends Anal. Chem. 2019, 118, 709–721. [Google Scholar] [CrossRef]
- Llompart, M.; Celeiro, M.; Dagnac, T. Microwave-assisted extraction of pharmaceuticals, personal care products and industrial contaminants in the environment. TrAC Trends Anal. Chem. 2019, 116, 136–150. [Google Scholar] [CrossRef]
- Akvan, N.; Azimi, G.; Parastar, H. Chemometric assisted determination of 16 PAHs in water samples by ultrasonic assisted emulsification microextraction followed by fast high-performance liquid chromatography with diode array detector. Microchem. J. 2019, 150, 104056. [Google Scholar] [CrossRef]
- Al-Marzouqi, A.H.; Zekri, A.Y.; Azzam, A.A.; Alraeesi, A.Y. Optimization of supercritical fluid extraction of hydrocarbons from a contaminated soil: An experimental approach. Int. J. Environ. Sci. Dev. 2019, 10, 301–309. [Google Scholar] [CrossRef]
- Hawthorne, S.B.; Yang, Y.; Miller, D.J. Extraction of organic pollutants from environmental solids with sub-and supercritical water. Anal. Chem. 1994, 66, 2912–2920. [Google Scholar] [CrossRef]
- Costa, J.M.; Strieder, M.M.; Saldaña, M.D.A.; Rostagno, M.A.; Forster-Carneiro, T. Recent Advances in the Processing of Agri-food By-products by Subcritical Water. Food Bioproc. Technol. 2023, 16, 2705–2724. [Google Scholar] [CrossRef]
- Cheng, Y.; Xue, F.; Yu, S.; Du, S.; Yang, Y. Subcritical water extraction of natural products. Molecules 2021, 26, 4004. [Google Scholar] [CrossRef]
- Atanasova, A.; Petrova, A.; Teneva, D.; Ognyanov, M.; Georgiev, Y.; Nenov, N.; Denev, P. Subcritical water extraction of rosmarinic acid from lemon balm (Melissa officinalis L.) and its effect on plant cell wall constituents. Antioxidants 2023, 12, 888. [Google Scholar] [CrossRef] [PubMed]
- Park, J.-S.; Han, J.-M.; Shin, Y.-N.; Park, Y.-S.; Shin, Y.-R.; Park, S.-W.; Roy, V.C.; Lee, H.-J.; Kumagai, Y.; Kishimura, H. Exploring Bioactive Compounds in Brown Seaweeds Using Subcritical Water: A Comprehensive Analysis. Mar. Drugs 2023, 21, 328. [Google Scholar] [CrossRef]
- Claux, O.; Santerre, C.; Abert-Vian, M.; Touboul, D.; Vallet, N.; Chemat, F. Alternative and sustainable solvents for green analytical chemistry. Curr. Opin. Green Sustain. Chem. 2021, 31, 100510. [Google Scholar] [CrossRef]
- Breynaert, E.; Houlleberghs, M.; Radhakrishnan, S.; Grübel, G.; Taulelle, F.; Martens, J.A. Water as a tuneable solvent: A perspective. Chem. Soc. Rev. 2020, 49, 2557–2569. [Google Scholar] [CrossRef]
- Cvjetko Bubalo, M.; Vidović, S.; Radojčić Redovniković, I.; Jokić, S. Green solvents for green technologies. J. Chem. Technol. Biotechnol. 2015, 90, 1631–1639. [Google Scholar] [CrossRef]
- Gbashi, S.; Madala, N.E.; Adebo, O.A.; Piater, L.; Phoku, J.Z.; Njobeh, P.B. Subcritical water extraction and its prospects for aflatoxins extraction in biological materials. In Aflatoxin-Control, Analysis, Detection and Health Risks; InTech: Rijeka, Croatia, 2017; pp. 229–250. [Google Scholar]
- Yang, Y.; Hawthorne, S.B.; Miller, D.J. Class-selective extraction of polar, moderately polar, and nonpolar organics from hydrocarbon wastes using subcritical water. Environ. Sci. Technol. 1997, 31, 430–437. [Google Scholar] [CrossRef]
- Yang, Y.; Belghazi, M.; Lagadec, A.; Miller, D.J.; Hawthorne, S.B. Elution of organic solutes from different polarity sorbents using subcritical water. J. Chromatogr. A 1998, 810, 149–159. [Google Scholar] [CrossRef]
- Chen, Z.; Zheng, Z.; He, C.; Liu, J.; Zhang, R.; Chen, Q. Oily sludge treatment in subcritical and supercritical water: A review. J. Hazard. Mater. 2022, 433, 128761. [Google Scholar] [CrossRef] [PubMed]
- Yabalak, E.; Akay, S.; Kayan, B.; Gizir, A.M.; Yang, Y. Solubility and Decomposition of Organic Compounds in Subcritical Water. Molecules 2023, 28, 1000. [Google Scholar] [CrossRef] [PubMed]
- Shitu, A.; Izhar, S.; Tahir, T.M. Sub-critical water as a green solvent for production of valuable materials from agricultural waste biomass: A review of recent work. Glob. J. Environ. Sci. Manag. 2015, 1, 255–264. [Google Scholar]
- Carr, A.G.; Mammucari, R.; Foster, N.R. A review of subcritical water as a solvent and its utilisation for the processing of hydrophobic organic compounds. Chem. Eng. J. 2011, 172, 1–17. [Google Scholar] [CrossRef]
- Zhang, J.; Wen, C.; Zhang, H.; Duan, Y.; Ma, H. Recent advances in the extraction of bioactive compounds with subcritical water: A review. Trends Food Sci. Technol. 2020, 95, 183–195. [Google Scholar] [CrossRef]
- Kronholm, J.; Hartonen, K.; Riekkola, M.-L. Analytical extractions with water at elevated temperatures and pressures. TrAC Trends Anal. Chem. 2007, 26, 396–412. [Google Scholar] [CrossRef]
- Zango, Z.U.; Sambudi, N.S.; Jumbri, K.; Ramli, A.; Abu Bakar, N.H.H.; Saad, B.; Rozaini, M.N.; Isiyaka, H.A.; Osman, A.M.; Sulieman, A. An overview and evaluation of highly porous adsorbent materials for polycyclic aromatic hydrocarbons and phenols removal from wastewater. Water 2020, 12, 2921. [Google Scholar] [CrossRef]
- Bertoz, V.; Purcaro, G.; Conchione, C.; Moret, S. A review on the occurrence and analytical determination of PAHs in olive oils. Foods 2021, 10, 324. [Google Scholar] [CrossRef]
- Premnath, N.; Mohanrasu, K.; Rao, R.G.R.; Dinesh, G.H.; Prakash, G.S.; Ananthi, V.; Ponnuchamy, K.; Muthusamy, G.; Arun, A. A crucial review on polycyclic aromatic Hydrocarbons-Environmental occurrence and strategies for microbial degradation. Chemosphere 2021, 280, 130608. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Shafy, H.I.; Mansour, M.S.M. A review on polycyclic aromatic hydrocarbons: Source, environmental impact, effect on human health and remediation. Egypt. J. Pet. 2016, 25, 107–123. [Google Scholar] [CrossRef]
- Rengarajan, T.; Rajendran, P.; Nandakumar, N.; Lokeshkumar, B.; Rajendran, P.; Nishigaki, I. Exposure to polycyclic aromatic hydrocarbons with special focus on cancer. Asian Pac. J. Trop. Biomed. 2015, 5, 182–189. [Google Scholar] [CrossRef]
- Sampaio, G.R.; Guizellini, G.M.; da Silva, S.A.; de Almeida, A.P.; Pinaffi-Langley, A.C.C.; Rogero, M.M.; de Camargo, A.C.; Torres, E.A.F.S. Polycyclic aromatic hydrocarbons in foods: Biological effects, legislation, occurrence, analytical methods, and strategies to reduce their formation. Int. J. Mol. Sci. 2021, 22, 6010. [Google Scholar] [CrossRef] [PubMed]
- Valavanidis, A.; Fiotakis, K.; Vlachogianni, T. The role of stable free radicals, metals and PAHs of airborne particulate matter in mechanisms of oxidative stress and carcinogenicity. In Urban Airborne Particulate Matter: Origin, Chemistry, Fate and Health Impacts; Springer: Berlin/Heidelberg, Germany, 2011; pp. 411–426. [Google Scholar]
- Khairy, M.A.; Kolb, M.; Mostafa, A.R.; Anwar, E.-F.; Bahadir, M. Risk assessment of polycyclic aromatic hydrocarbons in a Mediterranean semi-enclosed basin affected by human activities (Abu Qir Bay, Egypt). J. Hazard. Mater. 2009, 170, 389–397. [Google Scholar] [CrossRef] [PubMed]
- Kocbach Bølling, A.; Pagels, J.; Yttri, K.E.; Barregard, L.; Sallsten, G.; Schwarze, P.E.; Boman, C. Health effects of residential wood smoke particles: The importance of combustion conditions and physicochemical particle properties. Part Fibre Toxicol. 2009, 6, 29. [Google Scholar] [CrossRef]
- Kocak, T.K.; Kocak, G.O.; Stuart, A.L. Polycyclic aromatic hydrocarbons in aquatic media of Turkey: A systematic review of cancer and ecological risk. Mar. Pollut. Bull. 2023, 188, 114671. [Google Scholar] [CrossRef]
- Lee, M.L.; Bartle, K.D.; Novotny, M.V. Analytical Chemistry of Polycyclic Aromatic Hydrocarbons; Academic Press: Cambridge, MA, USA, 1981. [Google Scholar]
- Asfaram, A.; Dil, E.A.; Arabkhani, P.; Sadeghfar, F.; Ghaedi, M. Magnetic Cu: CuO-GO nanocomposite for efficient dispersive micro-solid phase extraction of polycyclic aromatic hydrocarbons from vegetable, fruit, and environmental water samples by liquid chromatographic determination. Talanta 2020, 218, 121131. [Google Scholar] [CrossRef]
- de Barros Caetano, V.C.L.; da Costa Cunha, G.; Oliveira, R.V.M.; da Rosa Alexandre, M.; Romão, L.P.C. Magnetic hybrid support for ultrasound-assisted magnetic solid-phase extraction of polycyclic aromatic hydrocarbons from produced water. Microchem. J. 2019, 146, 1195–1203. [Google Scholar] [CrossRef]
- Shamsipur, M.; Hashemi, B. Extraction and determination of polycyclic aromatic hydrocarbons in water samples using stir bar sorptive extraction (SBSE) combined with dispersive liquid–liquid microextraction based on the solidification of floating organic drop (DLLME-SFO) followed by HPLC-UV. RSC Adv. 2015, 5, 20339–20345. [Google Scholar]
- Sun, T.; Wang, D.; Tang, Y.; Xing, X.; Zhuang, J.; Cheng, J.; Du, Z. Fabric-phase sorptive extraction coupled with ion mobility spectrometry for on-site rapid detection of PAHs in aquatic environment. Talanta 2019, 195, 109–116. [Google Scholar] [CrossRef] [PubMed]
- Latawiec, A.E.; Reid, B.J. Sequential extraction of polycyclic aromatic hydrocarbons using subcritical water. Chemosphere 2010, 78, 1042–1048. [Google Scholar] [CrossRef] [PubMed]
- Kariyawasam, T.; Doran, G.S.; Howitt, J.A.; Prenzler, P.D. Optimization and Comparison of Microwave-Assisted Extraction, Supercritical Fluid Extraction, and Eucalyptus Oil–Assisted Extraction of Polycyclic Aromatic Hydrocarbons from Soil and Sediment. Environ. Toxicol. Chem. 2023, 42, 982–994. [Google Scholar] [CrossRef] [PubMed]
- Lagadec, A.J.M.; Miller, D.J.; Lilke, A.V.; Hawthorne, S.B. Pilot-scale subcritical water remediation of polycyclic aromatic hydrocarbon-and pesticide-contaminated soil. Environ. Sci. Technol. 2000, 34, 1542–1548. [Google Scholar] [CrossRef]
- Dean, J.R. Tutorial review. Extraction of polycyclic aromatic hydrocarbons from environmental matrices: Practical considerations for supercritical fluid extraction. Analyst 1996, 121, 85R–89R. [Google Scholar] [CrossRef]
- Leo, C.H.; Ong, E.S. Recent advances in the combination of organic solvent-free extraction, chemical standardization, antioxidant assay, and cell culture metabolomics for functional food and its by-product. Crit. Rev. Food Sci. Nutr. 2023, 1–15. [Google Scholar] [CrossRef]
- Hawthorne, S.B.; Trembley, S.; Moniot, C.L.; Grabanski, C.B.; Miller, D.J. Static subcritical water extraction with simultaneous solid-phase extraction for determining polycyclic aromatic hydrocarbons on environmental solids. J. Chromatogr. A 2000, 886, 237–244. [Google Scholar] [CrossRef]
- Hageman, K.J.; Mazeas, L.; Grabanski, C.B.; Miller, D.J.; Hawthorne, S.B. Coupled subcritical water extraction with solid-phase microextraction for determining semivolatile organics in environmental solids. Anal. Chem. 1996, 68, 3892–3898. [Google Scholar] [CrossRef]
- Fernandez-Perez, V.; de Castro, M.D.L. Micelle formation for improvement of continuous subcritical water extraction of polycyclic aromatic hydrocarbons in soil prior to high-performance liquid chromatography–fluorescence detection. J. Chromatogr. A 2000, 902, 357–367. [Google Scholar] [CrossRef]
- McGowin, A.E.; Adom, K.K.; Obubuafo, A.K. Screening of compost for PAHs and pesticides using static subcritical water extraction. Chemosphere 2001, 45, 857–864. [Google Scholar] [CrossRef]
- Islam, M.N.; Jo, Y.-T.; Park, J.-H. Remediation of PAHs contaminated soil by extraction using subcritical water. J. Ind. Eng. Chem. 2012, 18, 1689–1693. [Google Scholar] [CrossRef]
- Islam, M.N.; Jo, Y.-T.; Jung, S.-K.; Park, J.-H. Thermodynamic and kinetic study for subcritical water extraction of PAHs. J. Ind. Eng. Chem. 2013, 19, 129–136. [Google Scholar] [CrossRef]
- Islam, M.N.; Jo, Y.-T.; Park, J.-H. Subcritical water remediation of petroleum and aromatic hydrocarbon-contaminated soil: A semi-pilot scale study. Water Air Soil. Pollut. 2014, 225, 1–8. [Google Scholar] [CrossRef]
- Khanjari, Y.; Eikani, M.H.; Rowshanzamir, S. Experimental and theoretical investigation of the removal organic pollutants from contaminated soils using superheated water. J. Supercrit. Fluids 2015, 103, 55–60. [Google Scholar] [CrossRef]
- Sushkova, S.; Minkina, T.; Mandzhieva, S.; Tjurina, I.; Bolotova, O.; Vasilyeva, G.; Orlović-Leko, P.; Varduni, T.; Kızılkaya, R.; Akca, I. Solubility of benzo [a] pyrene and organic matter of soil in subcritical water. Croat. Chem. Acta 2015, 88, 247–253. [Google Scholar] [CrossRef]
- Moreno, E.; Reza, J.; Trejo, A. Extraction of polycyclic aromatic hydrocarbons from soil using water under subcritical conditions. Polycycl. Aromat. Compd. 2007, 27, 239–260. [Google Scholar] [CrossRef]
- Wang, X.; Lin, L.; Luan, T.; Yang, L.; Tam, N.F.Y. Determination of hydroxylated metabolites of polycyclic aromatic hydrocarbons in sediment samples by combining subcritical water extraction and dispersive liquid–liquid microextraction with derivatization. Anal. Chim. Acta 2012, 753, 57–63. [Google Scholar] [CrossRef]
- Teoh, W.H.; Mammucari, R.; Vieira de Melo, S.A.B.; Foster, N.R. Solubility and solubility modeling of polycyclic aromatic hydrocarbons in subcritical water. Ind. Eng. Chem. Res. 2013, 52, 5806–5814. [Google Scholar] [CrossRef]
- Erickson, M.D.; Kaley, R.G. Applications of polychlorinated biphenyls. Environ. Sci. Pollut. Res. 2011, 18, 135–151. [Google Scholar] [CrossRef]
- Steliga, T.; Wojtowicz, K.; Kapusta, P.; Brzeszcz, J. Assessment of biodegradation efficiency of polychlorinated biphenyls (PCBs) and petroleum hydrocarbons (TPH) in soil using three individual bacterial strains and their mixed culture. Molecules 2020, 25, 709. [Google Scholar] [CrossRef]
- Ravanipour, M.; Nabipour, I.; Yunesian, M.; Rastkari, N.; Mahvi, A.H. Exposure sources of polychlorinated biphenyls (PCBs) and health risk assessment: A systematic review in Iran. Environ. Sci. Pollut. Res. 2022, 29, 55437–55456. [Google Scholar] [CrossRef]
- Montano, L.; Pironti, C.; Pinto, G.; Ricciardi, M.; Buono, A.; Brogna, C.; Venier, M.; Piscopo, M.; Amoresano, A.; Motta, O. Polychlorinated biphenyls (PCBs) in the environment: Occupational and exposure events, effects on human health and fertility. Toxics 2022, 10, 365. [Google Scholar] [CrossRef] [PubMed]
- Othman, N.; Ismail, Z.; Selamat, M.I.; Sheikh Abdul Kadir, S.H.; Shibraumalisi, N.A. A Review of Polychlorinated Biphenyls (PCBs) Pollution in the Air: Where and How Much Are We Exposed to? Int. J. Environ. Res. Public Health 2022, 19, 13923. [Google Scholar] [CrossRef] [PubMed]
- Baker Jr, E.L.; Landrigan, P.J.; Glueck, C.J.; Zack Jr, M.M.; Liddle, J.A.; Burse, V.W.; Housworth, W.J.; Needham, L.L. Metabolic consequences of exposure to polychlorinated biphenyls (PCB) in sewage sludge. Am. J. Epidemiol. 1980, 112, 553–563. [Google Scholar] [CrossRef] [PubMed]
- Baqar, M.; Sadef, Y.; Ahmad, S.R.; Mahmood, A.; Qadir, A.; Aslam, I.; Li, J.; Zhang, G. Occurrence, ecological risk assessment, and spatio-temporal variation of polychlorinated biphenyls (PCBs) in water and sediments along River Ravi and its northern tributaries, Pakistan. Environ. Sci. Pollut. Res. 2017, 24, 27913–27930. [Google Scholar] [CrossRef] [PubMed]
- Mao, S.; Liu, S.; Zhou, Y.; An, Q.; Zhou, X.; Mao, Z.; Wu, Y.; Liu, W. The occurrence and sources of polychlorinated biphenyls (PCBs) in agricultural soils across China with an emphasis on unintentionally produced PCBs. Environ. Pollut. 2021, 271, 116171. [Google Scholar] [CrossRef]
- Habibullah-Al-Mamun, M.; Ahmed, M.K.; Islam, M.S.; Tokumura, M.; Masunaga, S. Occurrence, distribution and possible sources of polychlorinated biphenyls (PCBs) in the surface water from the Bay of Bengal coast of Bangladesh. Ecotoxicol. Environ. Saf. 2019, 167, 450–458. [Google Scholar] [CrossRef]
- Sethi, S.; Morgan, R.K.; Feng, W.; Lin, Y.; Li, X.; Luna, C.; Koch, M.; Bansal, R.; Duffel, M.W.; Puschner, B. Comparative analyses of the 12 most abundant PCB congeners detected in human maternal serum for activity at the thyroid hormone receptor and ryanodine receptor. Environ. Sci. Technol. 2019, 53, 3948–3958. [Google Scholar] [CrossRef]
- Yang, Y.; Bowadt, S.; Hawthorne, S.B.; Miller, D.J. Subcritical water extraction of polychlorinated biphenyls from soil and sediment. Anal. Chem. 1995, 67, 4571–4576. [Google Scholar] [CrossRef]
- Hartonen, K.; Inkala, K.; Kangas, M.; Riekkola, M.-L. Extraction of polychlorinated biphenyls with water under subcritical conditions. J. Chromatogr. A 1997, 785, 219–226. [Google Scholar] [CrossRef]
- Li, B.; Yang, Y.; Gan, Y.; Eaton, C.D.; He, P.; Jones, A.D. On-line coupling of subcritical water extraction with high-performance liquid chromatography via solid-phase trapping. J. Chromatogr. A 2000, 873, 175–184. [Google Scholar] [CrossRef]
- Islam, M.N.; Park, J.-H.; Shin, M.-S.; Park, H.-S. Decontamination of PCBs-containing soil using subcritical water extraction process. Chemosphere 2014, 109, 28–33. [Google Scholar] [CrossRef] [PubMed]
- Pross, S.; Gau, W.; Wenclawiak, B.W. Extraction of polychlorinated biphenyl with supercritical carbon dioxide, sulfur hexafluoride and subcritical water. Fresenius J. Anal. Chem. 2000, 367, 89–90. [Google Scholar] [CrossRef] [PubMed]
- Hawthorne, S.B.; Grabanski, C.B.; Hageman, K.J.; Miller, D.J. Simple method for estimating polychlorinated biphenyl concentrations on soils and sediments using subcritical water extraction coupled with solid-phase microextraction. J. Chromatogr. A 1998, 814, 151–160. [Google Scholar] [CrossRef]
- Yang, Y.; Li, B. Subcritical water extraction coupled to high-performance liquid chromatography. Anal. Chem. 1999, 71, 1491–1495. [Google Scholar] [CrossRef]
- Lopes-Ferreira, M.; Maleski, A.L.A.; Balan-Lima, L.; Bernardo, J.T.G.; Hipolito, L.M.; Seni-Silva, A.C.; Batista-Filho, J.; Falcao, M.A.P.; Lima, C. Impact of pesticides on human health in the last six years in Brazil. Int. J. Environ. Res. Public Health 2022, 19, 3198. [Google Scholar] [CrossRef]
- Centanni, M.; Ricci, G.F.; De Girolamo, A.M.; Romano, G.; Gentile, F. A review of modeling pesticides in freshwaters: Current status, progress achieved and desirable improvements. Environ. Pollut. 2023, 316, 120553. [Google Scholar] [CrossRef]
- Dardiotis, E.; Skouras, P.; Varvarelis, O.-P.; Aloizou, A.-M.; Hernández, A.F.; Liampas, I.; Rikos, D.; Dastamani, M.; Golokhvast, K.S.; Bogdanos, D.P. Pesticides and tremor: An overview of association, mechanisms and confounders. Environ. Res. 2023, 2023, 115442. [Google Scholar] [CrossRef]
- Sharma, A.; Kumar, V.; Shahzad, B.; Tanveer, M.; Sidhu, G.P.S.; Handa, N.; Kohli, S.K.; Yadav, P.; Bali, A.S.; Parihar, R.D. Worldwide pesticide usage and its impacts on ecosystem. SN Appl. Sci. 2019, 1, 1–16. [Google Scholar] [CrossRef]
- De, A.; Bose, R.; Kumar, A.; Mozumdar, S. Targeted Delivery of Pesticides Using Biodegradable Polymeric Nanoparticles; Springer: Berlin/Heidelberg, Germany, 2014. [Google Scholar]
- Rajmohan, K.S.; Chandrasekaran, R.; Varjani, S. A review on occurrence of pesticides in environment and current technologies for their remediation and management. Indian J. Microbiol. 2020, 60, 125–138. [Google Scholar] [CrossRef]
- Hashimi, M.H.; Hashimi, R.; Ryan, Q. Toxic effects of pesticides on humans, plants, animals, pollinators and beneficial organisms. APRJ 2020, 5, 37–47. [Google Scholar] [CrossRef]
- Silva, V.; Mol, H.G.J.; Zomer, P.; Tienstra, M.; Ritsema, C.J.; Geissen, V. Pesticide residues in European agricultural soils–A hidden reality unfolded. Sci. Total Environ. 2019, 653, 1532–1545. [Google Scholar] [CrossRef] [PubMed]
- Aktaş, Ş.; Aminzai, M.T.; Tegin, İ.; Yabalak, E.; Acar, O. Determination of pesticide residues in varieties of pepper sold at different periods and provinces in Turkey and investigation of their adverse effects on human health and the environment. Int. J. Environ. Health Res. 2023, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Toptanci, İ.; Kiralan, M.; Ramadan, M.F. Levels of pesticide residues in fruits and vegetables in the Turkish domestic markets. Environ. Sci. Pollut. Res. 2021, 28, 39451–39457. [Google Scholar] [CrossRef] [PubMed]
- Kazar, S.D.; Turgut, N.; Yalçın, M.; Turgut, C.; Karakuş, P.B.K. Evaluation of pesticide residues in fruits and vegetables from the Aegean region of Turkey and assessment of risk to consumers. Environ. Sci. Pollut. Res. 2021, 28, 27511–27519. [Google Scholar] [CrossRef] [PubMed]
- Zhang, A.A.; Sutar, P.P.; Bian, Q.; Fang, X.M.; Ni, J.B.; Xiao, H.W. Pesticide residue elimination for fruits and vegetables: The mechanisms, applications, and future trends of thermal and non-thermal technologies. J. Future Foods. 2022, 2, 223–240. [Google Scholar] [CrossRef]
- Gilliom, R.J.; Barbash, J.E.; Crawford, C.G.; Hamilton, P.A.; Martin, J.D.; Nakagaki, N.; Nowell, L.H.; Scott, J.C.; Stackelberg, P.E.; Thelin, G.P. Pesticides in the Nation’s Streams and Ground Water, 1992–2001; US Geological Survey: Reston, VA, USA, 2006. [Google Scholar]
- Farcas, A.; Matei, A.V.; Florian, C.; Badea, M.; Coman, G. Health effects associated with acute and chronic exposure to pesticides. In Environmental Security Assessment and Management of Obsolete Pesticides in Southeast Europe; Springer: Berlin/Heidelberg, Germany, 2013; pp. 103–110. [Google Scholar]
- Richter, P.; Sepulveda, B.; Oliva, R.; Calderon, K.; Seguel, R. Screening and determination of pesticides in soil using continuous subcritical water extraction and gas chromatography–mass spectrometry. J. Chromatogr. A 2003, 994, 169–177. [Google Scholar] [CrossRef]
- Ramos, L.; Kristenson, E.M.; Brinkman, U.A.T. Current use of pressurised liquid extraction and subcritical water extraction in environmental analysis. J. Chromatogr. A 2002, 975, 3–29. [Google Scholar] [CrossRef]
- Miller, D.J.; Hawthorne, S.B. Method for determining the solubilities of hydrophobic organics in subcritical water. Anal. Chem. 1998, 70, 1618–1621. [Google Scholar] [CrossRef]
- Jimenez-Carmona, M.M.; Manclus, J.J.; Montoya, A.; de Castro, M.D.L. Sub-and supercritical fluid extraction of trichloropyridinol from soil prior to immunoassay. J. Chromatogr. A 1997, 785, 329–336. [Google Scholar] [CrossRef]
- Crescenzi, C.; D’Ascenzo, G.; Di Corcia, A.; Nazzari, M.; Marchese, S.; Samperi, R. Multiresidue herbicide analysis in soil: Subcritical water extraction with an on-line sorbent trap. Anal. Chem. 1999, 71, 2157–2163. [Google Scholar] [CrossRef] [PubMed]
- Lou, X.; Miller, D.J.; Hawthorne, S.B. Static subcritical water extraction combined with anion exchange disk sorption for determining chlorinated acid herbicides in soil. Anal. Chem. 2000, 72, 481–488. [Google Scholar] [CrossRef] [PubMed]
- Krieger, M.S.; Cook, W.L.; Kennard, L.M. Extraction of tricyclazole from soil and sediment with subcritical water. J. Agric. Food Chem. 2000, 48, 2178–2183. [Google Scholar] [CrossRef] [PubMed]
- Luque-Garcıa, J.L.; de Castro, M.D.L. Coupling continuous subcritical water extraction, filtration, preconcentration, chromatographic separation and UV detection for the determination of chlorophenoxy acid herbicides in soils. J. Chromatogr. A 2002, 959, 25–35. [Google Scholar] [CrossRef]
- Eskilsson, C.S.; Hartonen, K.; Mathiasson, L.; Riekkola, M. Pressurized hot water extraction of insecticides from process dust–comparison with supercritical fluid extraction. J. Sep. Sci. 2004, 27, 59–64. [Google Scholar] [CrossRef]
- Chienthavorn, O.; Su-In, P. Modified superheated water extraction of pesticides from spiked sediment and soil. Anal. Bioanal. Chem. 2006, 385, 83–89. [Google Scholar] [CrossRef]
- Islam, M.N.; Jo, Y.-T.; Jung, S.-K.; Park, J.-H. Evaluation of subcritical water extraction process for remediation of pesticide-contaminated soil. Water Air Soil. Pollut. 2013, 224, 1652. [Google Scholar] [CrossRef]
- Zhao, F.; Wang, S.; She, Y.; Zhang, C.; Zheng, L.; Jin, M.; Shao, H.; Jin, F.; Du, X.; Wang, J. Subcritical water extraction combined with molecular imprinting technology for sample preparation in the detection of triazine herbicides. J. Chromatogr. A 2017, 1515, 17–22. [Google Scholar] [CrossRef]
- Konda, L.N.; Füleky, G.; Morovján, G. Subcritical water extraction to evaluate desorption behavior of organic pesticides in soil. J. Agric. Food Chem. 2002, 50, 2338–2343. [Google Scholar] [CrossRef]
- Di Corcia, A.; Caracciolo, A.B.; Crescenzi, C.; Giuliano, G.; Murtas, S.; Samperi, R. Subcritical water extraction followed by liquid chromatography mass spectrometry for determining terbuthylazine and its metabolites in aged and incubated soils. Environ. Sci. Technol. 1999, 33, 3271–3277. [Google Scholar] [CrossRef]
- Krieger, M.S.; Wynn, J.L.; Yoder, R.N. Extraction of cloransulam-methyl from soil with subcritical water and supercritical CO2. J. Chromatogr. A 2000, 897, 405–413. [Google Scholar] [CrossRef] [PubMed]
- Rodil, R.; Popp, P. Development of pressurized subcritical water extraction combined with stir bar sorptive extraction for the analysis of organochlorine pesticides and chlorobenzenes in soils. J. Chromatogr. A 2006, 1124, 82–90. [Google Scholar] [CrossRef] [PubMed]
- Vaudin, P.; Augé, C.; Just, N.; Mhaouty-Kodja, S.; Mortaud, S.; Pillon, D. When pharmaceutical drugs become environmental pollutants: Potential neural effects and underlying mechanisms. Environ. Res. 2022, 205, 112495. [Google Scholar] [CrossRef] [PubMed]
- Ríos, A.L.M.; Gutierrez-Suarez, K.; Carmona, Z.; Ramos, C.G.; Oliveira, L.F.S. Pharmaceuticals as emerging pollutants: Case naproxen an overview. Chemosphere 2022, 291, 132822. [Google Scholar] [CrossRef] [PubMed]
- He, P.; Wu, J.; Peng, J.; Wei, L.; Zhang, L.; Zhou, Q.; Wu, Z. Pharmaceuticals in drinking water sources and tap water in a city in the middle reaches of the Yangtze River: Occurrence, spatiotemporal distribution, and risk assessment. Environ. Sci. Pollut. Res. 2022, 29, 2365–2374. [Google Scholar] [CrossRef] [PubMed]
- Deo, R.P. Pharmaceuticals in the surface water of the USA: A review. Curr. Environ. Health Rep. 2014, 1, 113–122. [Google Scholar] [CrossRef]
- Sanusi, I.O.; Olutona, G.O.; Wawata, I.G.; Onohuean, H. Occurrence, environmental impact and fate of pharmaceuticals in groundwater and surface water: A critical review. Environ. Sci. Pollut. Res. 2023, 30, 90595–90614. [Google Scholar] [CrossRef]
- Long, B.M.; Harriage, S.; Schultz, N.L.; Sherman, C.D.H.; Thomas, M. Pharmaceutical pollution in marine waters and benthic flora of the southern Australian coastline. Environ. Chem. 2023, 19, 375–384. [Google Scholar] [CrossRef]
- Thiele-Bruhn, S. Pharmaceutical antibiotic compounds in soils–a review. J. Plant Nutr. Soil Sci. 2003, 166, 145–167. [Google Scholar] [CrossRef]
- Kunene, P.N.; Mahlambi, P.N. Case study on antiretroviral drugs uptake from soil irrigated with contaminated water: Bio-accumulation and bio-translocation to roots, stem, leaves, and fruits. Environ. Pollut. 2023, 319, 121004. [Google Scholar] [CrossRef]
- Jayampathi, T.; Atugoda, T.; Jayasinghe, C. Uptake and accumulation of pharmaceuticals and personal care products in leafy vegetables. In Pharmaceuticals and Personal Care Products: Waste Management and Treatment Technology; Elsevier: Amsterdam, The Netherlands, 2019; pp. 87–113. [Google Scholar]
- Coimbra, R.N.; Escapa, C.; Otero, M. Removal of pharmaceuticals from water: Conventional and alternative treatments. Water 2021, 13, 487. [Google Scholar] [CrossRef]
- Aminzai, M.T.; Azizi, N.; Nural, Y.; Yabalak, E. A Review on Recent Advances in Polymer-Assisted Green and Sustainable Technology for Remediation of Pharmaceuticals from Water and Wastewater. Water Air Soil. Pollut. 2023, 234, 681. [Google Scholar] [CrossRef]
- Richter, P.; Toral, M.I.; Toledo, C. SubcriticalWater Extraction and Determination of Nifedipine in Pharmaceutical Formulations. J. AOAC Int. 2006, 89, 365–368. [Google Scholar] [CrossRef] [PubMed]
- Yabalak, E.; Döndaş, H.A.; Gizir, A.M. Subcritical water oxidation of 6-aminopenicillanic acid and cloxacillin using H2O2, K2S2O8, and O2. J. Environ. Sci. Health Part A 2017, 52, 210–220. [Google Scholar] [CrossRef] [PubMed]
- Emire, Z.; Yabalak, E.; Görmez, Ö.; Gizir, A.M. Solubility and degradation of paracetamol in subcritical water. J. Serbian Chem. Soc. 2017, 82, 99–106. [Google Scholar] [CrossRef]
- Przybylińska, P.A.; Wyszkowski, M. Environmental contamination with phthalates and its impact on living organisms. Ecol. Chem. Eng. S 2016, 23, 347–356. [Google Scholar] [CrossRef]
- Bergé, A.; Cladière, M.; Gasperi, J.; Coursimault, A.; Tassin, B.; Moilleron, R. Meta-analysis of environmental contamination by phthalates. Environ. Sci. Pollut. Res. 2013, 20, 8057–8076. [Google Scholar] [CrossRef]
- Fierens, T.; Servaes, K.; Van Holderbeke, M.; Geerts, L.; De Henauw, S.; Sioen, I.; Vanermen, G. Analysis of phthalates in food products and packaging materials sold on the Belgian market. Food Chem. Toxicol. 2012, 50, 2575–2583. [Google Scholar] [CrossRef]
- Crinnion, W.J. Toxic effects of the easily avoidable phthalates and parabens. Altern. Med. Rev. 2010, 15, 190. [Google Scholar]
- Li, H.-L.; Ma, W.-L.; Liu, L.-Y.; Zhang, Z.; Sverko, E.; Zhang, Z.-F.; Song, W.-W.; Sun, Y.; Li, Y.-F. Phthalates in infant cotton clothing: Occurrence and implications for human exposure. Sci. Total Environ. 2019, 683, 109–115. [Google Scholar] [CrossRef]
- Koniecki, D.; Wang, R.; Moody, R.P.; Zhu, J. Phthalates in cosmetic and personal care products: Concentrations and possible dermal exposure. Environ. Res. 2011, 111, 329–336. [Google Scholar] [CrossRef] [PubMed]
- Lyche, J.L. Phthalates. In Reproductive and Developmental Toxicology; Elsevier: Amsterdam, The Netherlands, 2017; pp. 829–856. [Google Scholar]
- Wang, Y.; Qian, H. Phthalates and their impacts on human health. Healthcare 2021, 9, 603. [Google Scholar] [CrossRef] [PubMed]
- Hlisníková, H.; Petrovičová, I.; Kolena, B.; Šidlovská, M.; Sirotkin, A. Effects and mechanisms of phthalates’ action on neurological processes and neural health: A literature review. Pharmacol. Rep. 2021, 73, 386–404. [Google Scholar] [CrossRef] [PubMed]
- Chang, M.S.; Shen, J.Y.; Yang, S.-H.; Wu, G.J. Subcritical water extraction for the remediation of phthalate ester-contaminated soil. J. Hazard. Mater. 2011, 192, 1203–1209. [Google Scholar] [CrossRef] [PubMed]
- Čolnik, M.; Knez, Ž.; Škerget, M. Sub-and supercritical water for chemical recycling of polyethylene terephthalate waste. Chem. Eng. Sci. 2021, 233, 116389. [Google Scholar] [CrossRef]
- Xiu, F.-R.; Tan, X.; Qi, Y.; Wang, M. Treatment of DEHP-rich PVC waste in subcritical urine wastewater: Efficient dechlorination, denitrification, plasticizer decomposition, and preparation of high-purity phthalic acid crystals. J. Hazard. Mater. 2023, 441, 129820. [Google Scholar] [CrossRef]
- Prince, R.C.; Lessard, R.R. Crude oil releases to the environment: Natural fate and remediation options. Encycl. Energy 2004, 1, 727–736. [Google Scholar]
- Iskander, L.; Khalil, C.A.; Boufadel, M.C. Fate of crude oil in the environment and remediation of oil spills. STEM Fellowsh. J. 2021, 6, 69–75. [Google Scholar] [CrossRef]
- Ahmad, A.A.; Muhammad, I.; Shah, T.; Kalwar, Q.; Zhang, J.; Liang, Z.; Mei, D.; Juanshan, Z.; Yan, P.; Zhi, D.X. Remediation methods of crude oil contaminated soil. World J. Agric. Soil Sci. 2020, 4, 8. [Google Scholar]
- Taki, G.; Islam, M.N.; Park, S.-J.; Park, J.-H. Optimization of operating parameters to remove and recover crude oil from contaminated soil using subcritical water extraction process. Environ. Eng. Res. 2018, 23, 175–180. [Google Scholar] [CrossRef]
- Islam, M.N.; Jo, Y.-T.; Park, J.-H. Remediation of soil contaminated with lubricating oil by extraction using subcritical water. J. Ind. Eng. Chem. 2014, 20, 1511–1516. [Google Scholar] [CrossRef]
- Islam, M.N.; Park, H.-S.; Park, J.-H. Extraction of diesel from contaminated soil using subcritical water. Environ. Earth Sci. 2015, 74, 3059–3066. [Google Scholar] [CrossRef]
- Gunathilake, T.M.S.U.; Ching, Y.C.; Kadokami, K. An overview of organic contaminants in indoor dust, their health impact, geographical distribution and recent extraction/analysis methods. Environ. Geochem. Health 2022, 44, 677–713. [Google Scholar] [CrossRef] [PubMed]
- Nasiri, M.; Ahmadzadeh, H.; Amiri, A. Sample preparation and extraction methods for pesticides in aquatic environments: A review. TrAC Trends Anal. Chem. 2020, 123, 115772. [Google Scholar] [CrossRef]
- Jagirani, M.S.; Ozalp, O.; Soylak, M. New trend in the extraction of pesticides from the environmental and food samples applying microextraction based green chemistry scenario: A review. Crit. Rev. Anal. Chem. 2022, 52, 1343–1369. [Google Scholar] [CrossRef] [PubMed]
- Silalahi, E.T.M.E.; Anita, S.; Teruna, H.Y. Comparison of extraction techniques for the determination of polycyclic aromatic hydrocarbons (PAHs) in soil. J. Phys. Conf. Ser. 2021, 1819, 12061. [Google Scholar] [CrossRef]
- Wang, W.; Meng, B.; Lu, X.; Liu, Y.; Tao, S. Extraction of polycyclic aromatic hydrocarbons and organochlorine pesticides from soils: A comparison between Soxhlet extraction, microwave-assisted extraction and accelerated solvent extraction techniques. Anal. Chim. Acta 2007, 602, 211–222. [Google Scholar] [CrossRef]
- Yuan, X.; You, F.; Yong, L.; Yang, C.; Zhu, L.; Hu, B.; Liu, T. Rapid determination of 16 polycyclic aromatic hydrocarbons in PM2. 5 by microwave assisted extraction-high performance liquid chromatography. Microchem. J. 2019, 144, 391–396. [Google Scholar] [CrossRef]
- Barco-Bonilla, N.; Vidal, J.L.M.; Frenich, A.G.; Romero-González, R. Comparison of ultrasonic and pressurized liquid extraction for the analysis of polycyclic aromatic compounds in soil samples by gas chromatography coupled to tandem mass spectrometry. Talanta 2009, 78, 156–164. [Google Scholar] [CrossRef]
- Numata, M.; Yarita, T.; Aoyagi, Y.; Takatsu, A. Evaluation of a microwave-assisted extraction technique for the determination of polychlorinated biphenyls and organochlorine pesticides in sediments. Anal. Sci. 2004, 20, 793–798. [Google Scholar] [CrossRef]
- Sułkowski, W.; Rosińska, A. Comparison of the efficiency of extraction methods for polychlorinated biphenyls from environmental wastes. J. Chromatogr. A 1999, 845, 349–355. [Google Scholar] [CrossRef]
- Fenoll, J.; Hellín, P.; Martínez, C.M.; Flores, P.; Navarro, S. Determination of 48 pesticides and their main metabolites in water samples by employing sonication and liquid chromatography–tandem mass spectrometry. Talanta 2011, 85, 975–982. [Google Scholar] [CrossRef] [PubMed]
- Drljača, D.; Ašperger, D.; Ferenčak, M.; Gavranić, M.; Babić, S.; Mikac, I.; Ahel, M. Comparison of four extraction methods for the determination of veterinary pharmaceuticals in sediment. Chromatographia 2016, 79, 209–223. [Google Scholar] [CrossRef]
- Ngwenya, N.; Mahlambi, P. Methods optimization and application: Solid phase extraction, ultrasonic extraction and Soxhlet extraction for the determination of antiretroviral drugs in river water, wastewater, sludge, soil and sediment. J. Pharm. Biomed. Anal. 2023, 230, 115358. [Google Scholar] [CrossRef]
- Kumirska, J.; Łukaszewicz, P.; Caban, M.; Migowska, N.; Plenis, A.; Białk-Bielińska, A.; Czerwicka, M.; Qi, F.; Piotr, S. Determination of twenty pharmaceutical contaminants in soil using ultrasound-assisted extraction with gas chromatography-mass spectrometric detection. Chemosphere 2019, 232, 232–242. [Google Scholar] [CrossRef]
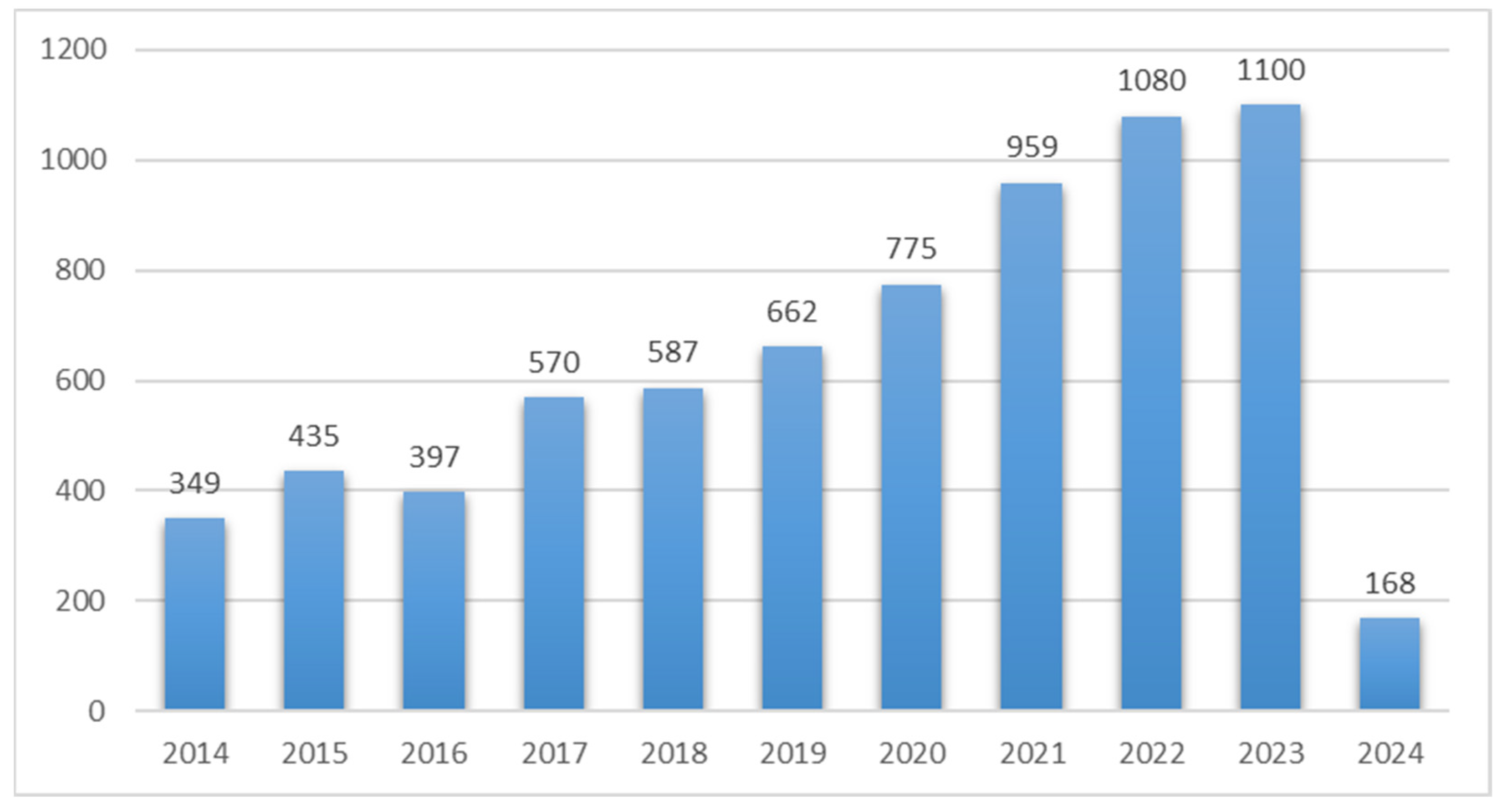
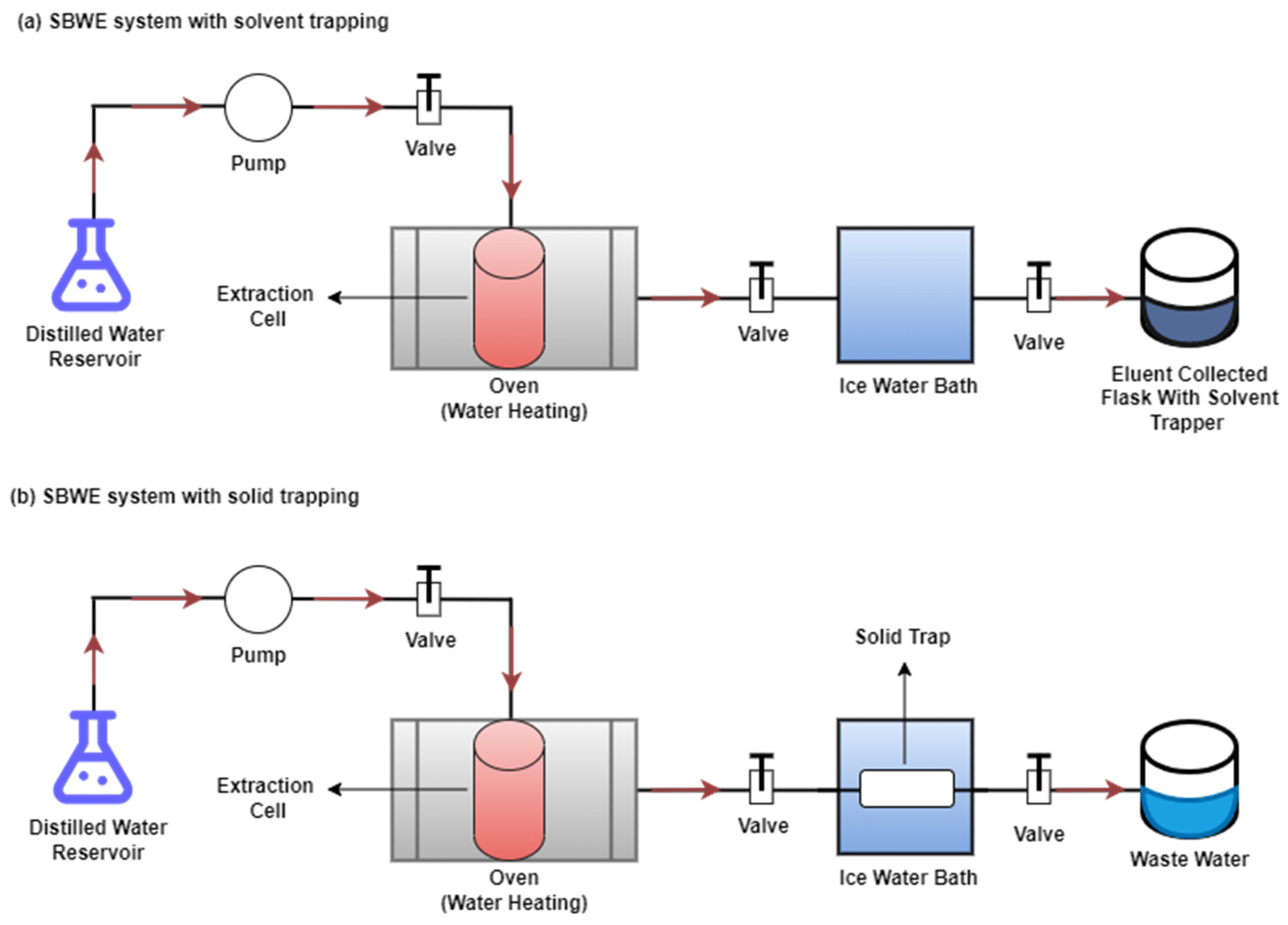
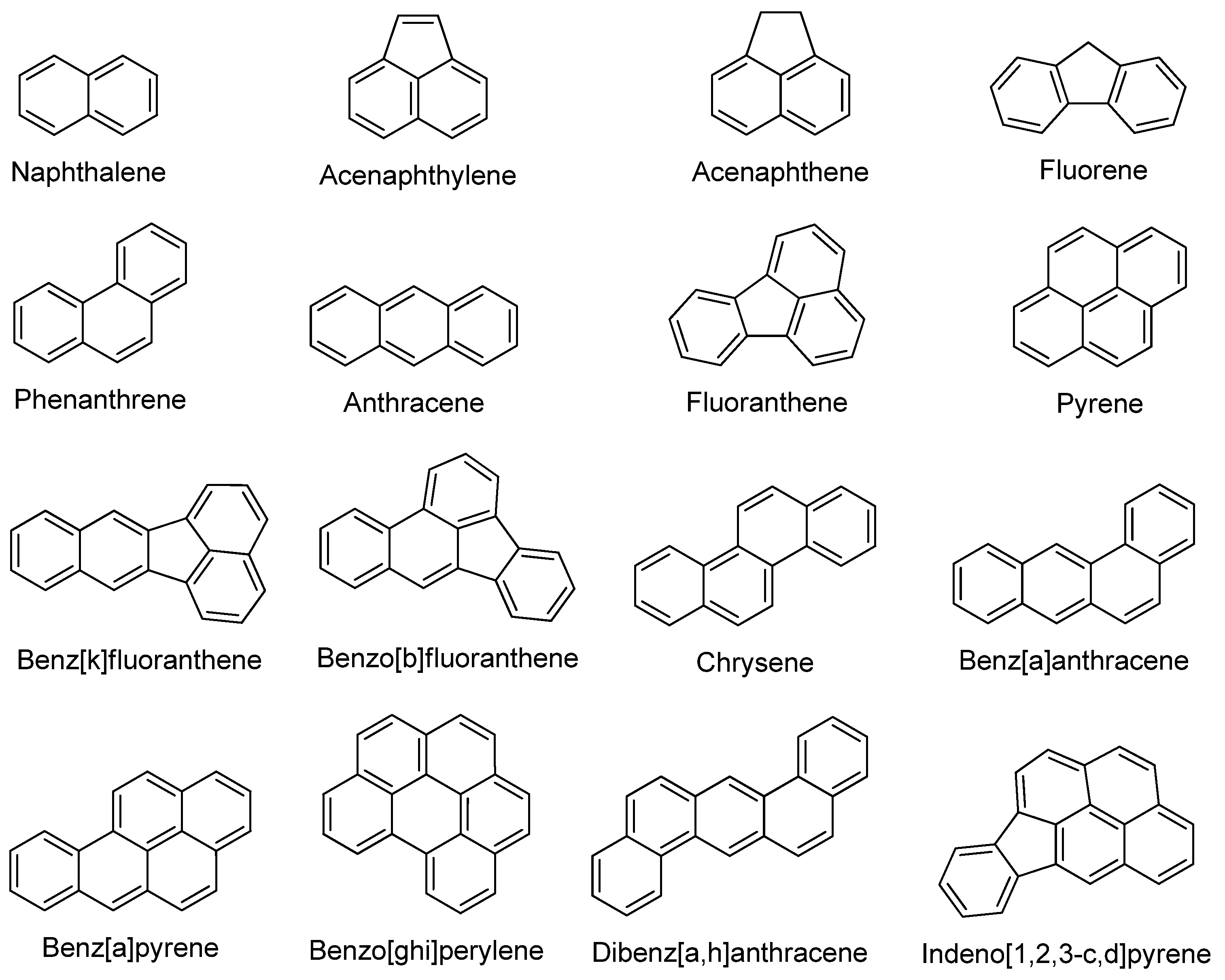
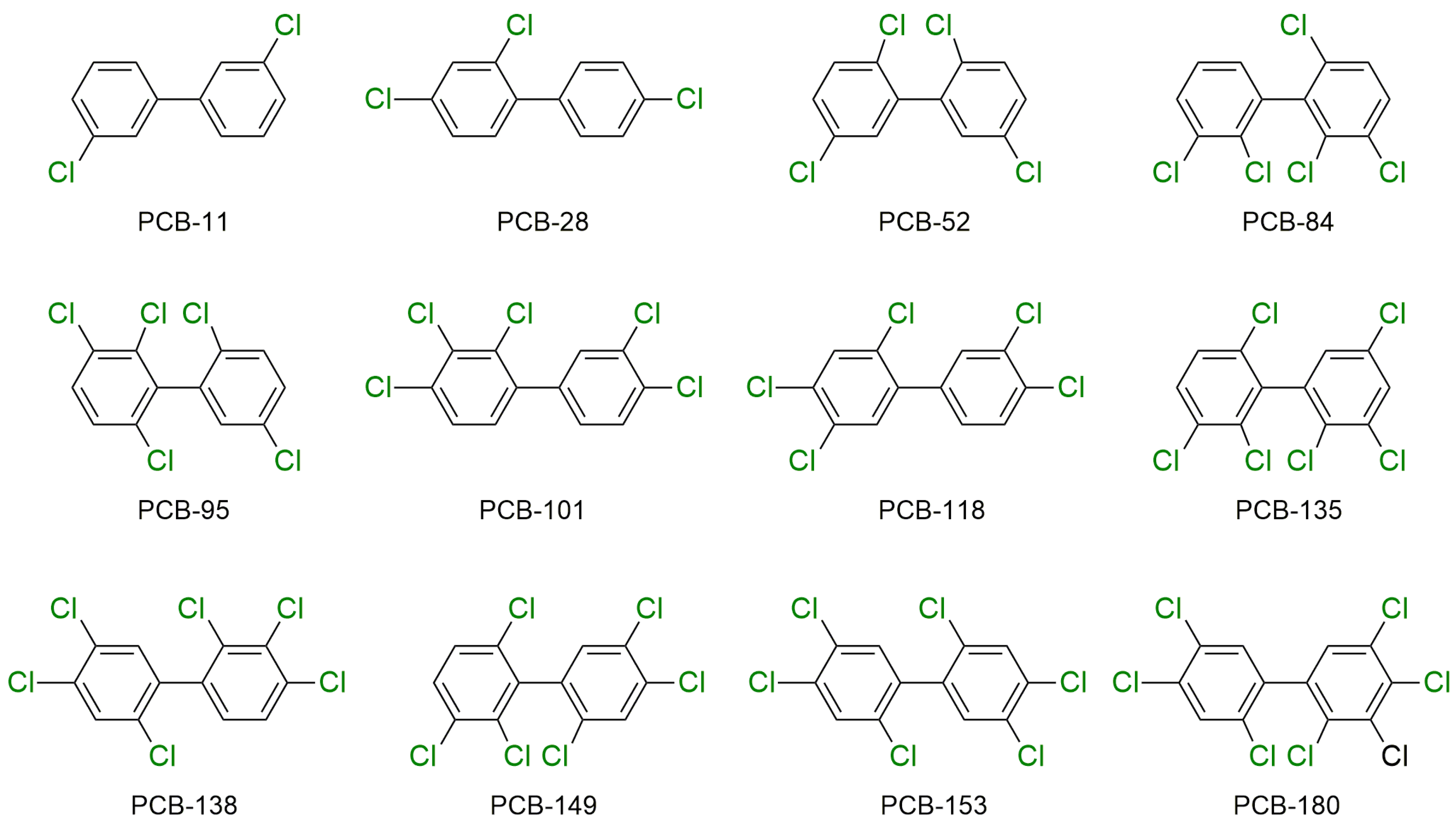
| Environmental Matrices Type | Type of Extracted PAHs | Extraction Conditions | % Recovery (% RSD) | Removal (%) | Ref. | ||
|---|---|---|---|---|---|---|---|
| Temp (°C) | P (bar) | Time (min) | |||||
| Spiked Sand | Naphthalene | 250 | 50 | 15 | >90% | 100 | [14] |
| Phenanthrene | 96 | ||||||
| Anthracene | 95 | ||||||
| Chrysene | 90 | ||||||
| Benzo[a]pyrene | 86 | ||||||
| Benzo[ghi]perylene | 94 | ||||||
| Railroad Bed Soil | Naphthalene | 250 | - | 60 | 278 (7) | - | [52] |
| Acenaphthene | 210 (6) | ||||||
| Phenanthrene | 99 (12) | ||||||
| Anthracene | 324 (14) | ||||||
| Fluoranthene | 91 (10) | ||||||
| Pyrene | 104 (10) | ||||||
| Benz[a]anthracene | 153 (13) | ||||||
| Chrysene | 100 (9) | ||||||
| Benzo[a]pyrene | 98 (22) | ||||||
| Perylene | 161 (11) | ||||||
| Benzo[ghi]perylene | 80 (16) | ||||||
| Petroleum Waste Sludge | Naphthalene | 250 | 50.7 | 60 | 126 (10) | 49 | [23] |
| Phenanthrene | 109 (15) | 77 | |||||
| Pyrene | 82 (31) | 87 | |||||
| Soil | Naphthalene | 275 | 100 | 60 | - | >99 | [48] |
| Acenaphthene | |||||||
| Chrysene | |||||||
| Benz[a]anthracene | |||||||
| Benzo[b+k]fluoranthene | |||||||
| Benzo[e]pyrene | |||||||
| Benzo[a]pyrene | |||||||
| Indeno[1,2,3-cd]pyrene | |||||||
| Benzo[ghi]perylene | |||||||
| Soil | Naphthalene | 250 | - | 60 | 96 | >90% | [51] |
| 2-Methyl naphthalene | 99 | ||||||
| 1-Methyl naphthalene | 99 | ||||||
| Acenaphthene | 93 | ||||||
| Phenanthrene | 94 | ||||||
| Anthracene | 93 | ||||||
| Fluoranthene | 99 | ||||||
| Pyrene | 91 | ||||||
| Benzo[a]anthracene | 94 | ||||||
| Chrysene | 99 | ||||||
| Benzo[b+j+k]fluoranthene | 93 | ||||||
| Benzo[a]pyrene | 93 | ||||||
| Perylene | 93 | ||||||
| Indeno[1,2,3-cd]pyrene | 92 | ||||||
| Benzo[ghi]perylene | 92 | ||||||
| Soil | Pyrene | 150 | 50 | 15 + 10 | 101.8 (10.4) | - | [53] |
| Benzo[a]anthracene | 73.6 (11.5) | ||||||
| Benzo[e]acenaphthen | 96.8 (10.2) | ||||||
| Benzo[k]fluoranthene | 110.4 (7.4) | ||||||
| Benzo[a]pyrene | 106.5 (9.3) | ||||||
| Benzo[ghi]perylene | 104.0 (1.2) | ||||||
| Sediment (CRM 104) | Benz[a]anthracene | 150 | - | 20 | 88 (5) | - | [54] |
| Benzo[b]fluoranthene | 55 (4) | ||||||
| Benzo[k]fluoranthene | 104 (4) | ||||||
| Benzo[ghi]perylene | 95 (1) | ||||||
| Benzo[a]pyrene | 89 (3) | ||||||
| Chrysene | 87 (4) | ||||||
| Fluoranthene | 89 (12) | ||||||
| Indole[1,2,3-cd]pyrene | 106 (1) | ||||||
| Phenanthrene | 87 (6) | ||||||
| Pyrene | 101 (12) | ||||||
| Soil | Naphthalene | 150 | 100 | 30 | - | 99.61 | [55] |
| Phenanthrene | 300 | 98.12 | |||||
| Fluoranthene | 96.24 | ||||||
| Pyrene | 94.05 | ||||||
| Soil | Naphthalene | 200 | - | 60 | - | 100 | [56] |
| Phenanthrene | 250 | 96 | |||||
| Fluoranthene | 96 | ||||||
| Pyrene | 98 | ||||||
| Soil | Phenanthrene | 275 | 40 | 60 | - | 99 | [57] |
| Fluoranthene | 92 | ||||||
| Pyrene | 91 | ||||||
| Soil | Phenanthrene | 165 | 20 | 15 | - | 83.58 | [58] |
| Soil | Benzo[a]pyrene | 250 | 101.3 | 30 | - | 96 | [59] |
| Environmental Matrices Type | Type of Extracted PCBs | Extraction Conditions | Removal (%) | % Recovery (%RSD) | Ref. | ||
|---|---|---|---|---|---|---|---|
| Temp (°C) | P (bar) | Time (min) | |||||
| Sediment | PCB-26 | 250 | 50.7 | 15 | >99 | 74 (11) | [73] |
| PCB-28 | 106 (13) | ||||||
| PCB-44 | 101 (3) | ||||||
| PCB-52 | 100 (15) | ||||||
| PCB-102 | 88 (9) | ||||||
| PCB-118 | 89 (23) | ||||||
| PCB-149 | ND | ||||||
| PCB-153 | ND | ||||||
| PCB-105 | ND | ||||||
| PCB-128 | 73 (18) | ||||||
| PCB-156 | ND | ||||||
| PCB-180 | 73 (25) | ||||||
| Industrial Soil | PCB-28 | 250 | 50.7 | 15 | >99 | 95 (15) | [73] |
| PCB-52 | >99 | 91 (11) | |||||
| PCB-101 | 96 | 92 (9) | |||||
| PCB-118 | 92 | 84 (10) | |||||
| PCB-149 | 92 | 79 (5) | |||||
| PCB-153 | 85 | 81 (9) | |||||
| PCB-105 | 94 | 91 (8) | |||||
| PCB-138 | 88 | 74 (9) | |||||
| PCB-128 | 91 | 70 (7) | |||||
| PCB-156 | 82 | 71 (16) | |||||
| PCB-180 | 73 | 70 (19) | |||||
| PCB-170 | 71 | 71 (18) | |||||
| Sea Sand | PCB-101 | 250 | 253.3 | - | - | 87.5 (6) | [74] |
| PCB-138 | 87.9 (5) | ||||||
| PCB-180 | 87.7 (2) | ||||||
| PCB-194 | 90.0 (3) | ||||||
| Sand | PCB-2 | 250 | - | - | - | 102 (12) | [75] |
| PCB-29 | 90 (13) | ||||||
| PCB-52 | 100 (23) | ||||||
| PCB-101 | 95 (30) | ||||||
| PCB-153 | 87 (35) | ||||||
| PCB-180 | 90 (23) | ||||||
| Soil | PCB-118 | 250 | - | 60 | >99.5 | - | [76] |
| PCB-28 | |||||||
| PCB-31 | |||||||
| PCB-44 | |||||||
| PCB-52 | |||||||
| PCB-101 | |||||||
| PCB-118 | |||||||
| PCB-138 | |||||||
| PCB-149 | |||||||
| PCB-153 | |||||||
| PCB-170 | |||||||
| PCB-180 | |||||||
| PCB-194 | |||||||
| PCB-209 | |||||||
| Environmental Matrices Type | Type of Extracted Pesticides | Extraction Conditions | % Recovery (%RSD) | Removal (%) | Ref. | ||
|---|---|---|---|---|---|---|---|
| Temp (°C) | P (bar) | Time (min) | |||||
| Soil | Trichloropyridinol | 250 | 200 | 15 | 99.7 | 100% | [97] |
| Soil | Cynarine | 90 | - | - | 87 | - | [98] |
| Simazine | 89 | ||||||
| Atrazine | 89 | ||||||
| Isoproturon | 88 | ||||||
| Diuron | 86 | ||||||
| Linuron | 84 | ||||||
| Clopyralid | 93 | ||||||
| Picloram | 91 | ||||||
| Dicamba | 90 | ||||||
| Bentazone | 85 | ||||||
| MCPA | 87 | ||||||
| 2,4-D | 85 | ||||||
| Mecoprop | 84 | ||||||
| Diclorprop | 86 | ||||||
| Bromoxynil | 84 | ||||||
| Ioxynil | 84 | ||||||
| 2,4-DB | 63 | ||||||
| MCPB | 62 | ||||||
| Sea Sand | 4-Nitrophenol | 100 | - | 30 | 85 (1.5) | - | [99] |
| Pentacholorphenol | 92 (1.0) | ||||||
| Dinoseb | 42 (12) | ||||||
| 3,5-Dichlorobenzoic acid | 93 (2.5) | ||||||
| Dicamba | 92 (0.5) | ||||||
| 2,4-DP | 99 (0.4) | ||||||
| 2,4-D | 103 (1.2) | ||||||
| 2,4,5-TP | 100 (1.0) | ||||||
| 2,4,5-T | 101 (1.9) | ||||||
| 2,4-DB | 93 (3.9) | ||||||
| Chloramben | 90 (2.2) | ||||||
| Picloram | 69 (9.9) | ||||||
| Acifluorfen | 108 (7.3) | ||||||
| 2,4-Dichlorophenylacetic acid | 92 (2.5) | ||||||
| Agricultural Soil | 4-Nitrophenol | 100 | - | 30 | 90 (6.2) | - | [99] |
| Pentacholorphenol | 88 (7.6) | ||||||
| Dinoseb | 47 (11) | ||||||
| 3,5-Dichlorobenzoic acid | 90 (6.1) | ||||||
| Dicamba | 76 (6.8) | ||||||
| 2,4-DP | 96 (6.1) | ||||||
| 2,4-D | 93 (6.3) | ||||||
| 2,4,5-TP | 92 (6.3) | ||||||
| 2,4,5-T | 92 (7.6) | ||||||
| 2,4-DB | 90 (6.1) | ||||||
| Chloramben | 90 (8.0) | ||||||
| Picloram | 71 (7.6) | ||||||
| Acifluorfen | 95 (9.5) | ||||||
| 2,4-Dichlorophenylacetic acid | 88 (4.9) | ||||||
| Soil and Sediment | Tricyclazole | 150 | - | 30 | 85–100 | - | [100] |
| Soil | Trifluralin | 250 | - | 15 | - | >99 | [48] |
| Atrazine | |||||||
| Alachlor | |||||||
| Metolachlor | |||||||
| Cyanazine | |||||||
| Pendimethalin | |||||||
| Sediment | Ametryne | 110 | - | 20 | 78 (4) | - | [54] |
| Atrazine | 89 (12) | ||||||
| Carbaryl | 74 (5) | ||||||
| Chlorpyrifos | 91 (3) | ||||||
| Trifluralin | 90 (3) | ||||||
| Soil | Bentazone | 85 | - | 60 | 94.2–113.1 | - | [101] |
| 2,4-D | |||||||
| Triclopyr | |||||||
| 2,4,5-T | |||||||
| 2,4,5-Tp | |||||||
| Dust waste | Carbofuran | 100 | 25 | 30 | 81 (2.9) | - | [102] |
| Imidacloprid | 98 (1.0) | ||||||
| Carbosulfan | <1 | ||||||
| Soil | Chlordane | 120 | - | 10 | 99 (13) | - | [103] |
| Malathion | 160 | 87 (12) | |||||
| Heptachlor | 82 (4) | ||||||
| Aldrin | 20 | 89 (2) | |||||
| Dieldrin | 77 (7) | ||||||
| Butachlor | 10 | 82 (11) | |||||
| Metalaxyl | 94 (6) | ||||||
| Propiconazole | 104 (4) | ||||||
| Thiobencarb | 180 | 82 (12) | |||||
| Soil | Diazinon | 150 | 20 | 20 | - | 100 | [104] |
| Parathione | 100 | ||||||
| Phenthoate | 100 | ||||||
| EPN | 99 | ||||||
| Soil | Ametryn | 150 | - | 20 | 78.9–101 | - | [105] |
| Promtryn | |||||||
| Simetryn | |||||||
| Methoprotryn | |||||||
| Simazine | |||||||
| Atrazine | |||||||
| Propazine | |||||||
| Terbuthylazine | |||||||
| Analyzed Compounds | Extraction Method | Extraction Conditions | % Recovery (%RSD) | Ref. | |||
|---|---|---|---|---|---|---|---|
| Temp (°C) | P (bar) | Time (min) | Solvent | ||||
| PAHs | SBWE | 250 | - | 60 | Water | 92–99 (-) | [51] |
| Microwave | - | - | 6 | Acetonitrile | 78.7–115.6 (0.7–7.8) | [147] | |
| Soxhlet | - | - | 960 | DCM/acetone | 57–99 (-) | [145] | |
| Ultrasound | - | - | 45 | n-Hexane | 29.2–82.5 (6.5–47.1) | [148] | |
| PCBs | SBWE | 250 | - | - | Water | 87–102 (12–35) | [75] |
| Microwave | 115 | - | 10 | Hexane/acetone | 81–93 (-) | [149] | |
| Soxhlet | - | - | 540 | Methylene chloride | 64.3–75.2 (-) | [150] | |
| Ultrasound | - | - | 45 | Methylene chloride | 69.2–77.2 (-) | ||
| Pesticides | SBWE | 150 | - | 20 | Water | 78.9–101 | [105] |
| Microwave | 110 | - | 10 | Hexane/acetone | 84.98–104.06 (0.52–9.30) | [146] | |
| Soxhlet | - | - | 900 | Hexane/acetone | 86.79–105.12 (0.61–12.12) | ||
| Ultrasound | - | - | 10 | Acetonitrile | 75–111 (-) | [151] | |
| Pharmaceuticals | SBWE | 150 | - | 20 | Water | 99.2 (1.9) | [121] |
| Microwave | 65 | - | 15 | Methanol/water | 40–100 (<5) | [152] | |
| Soxhlet | - | - | 480 | Acetonitrile | 88–108 (-) | [153] | |
| Ultrasound | - | - | 45 | Ethylacetate/formic acid | >80 (1.1–10) | [154] | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yabalak, E.; Aminzai, M.T.; Gizir, A.M.; Yang, Y. A Review: Subcritical Water Extraction of Organic Pollutants from Environmental Matrices. Molecules 2024, 29, 258. https://doi.org/10.3390/molecules29010258
Yabalak E, Aminzai MT, Gizir AM, Yang Y. A Review: Subcritical Water Extraction of Organic Pollutants from Environmental Matrices. Molecules. 2024; 29(1):258. https://doi.org/10.3390/molecules29010258
Chicago/Turabian StyleYabalak, Erdal, Mohammad Tahir Aminzai, Ahmet Murat Gizir, and Yu Yang. 2024. "A Review: Subcritical Water Extraction of Organic Pollutants from Environmental Matrices" Molecules 29, no. 1: 258. https://doi.org/10.3390/molecules29010258








