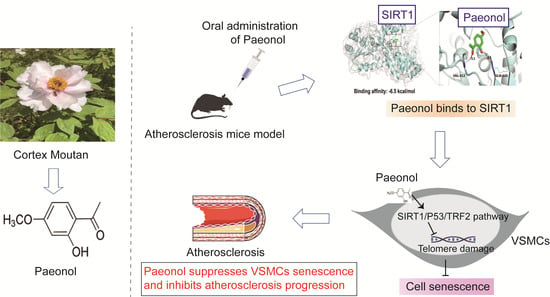
Paeonol Attenuates Atherosclerosis by Inhibiting Vascular Smooth Muscle Cells Senescence via SIRT1/P53/TRF2 Signaling Pathway
Abstract
:1. Introduction
2. Results
2.1. Paeonol Attenuates the Development of HFD-Induced Atherosclerosis in ApoE−/− Mice
2.2. Paeonol Prevents Vascular Senescence in ApoE−/− Mice
2.3. Paeonol Attenuates VSMCs Senescence and Regulates the SIRT1 Pathway in ApoE−/− Mice
2.4. Paeonol Delays the Senescence of t-BHP-Injured VSMCs
2.5. Paeonol Inhibits SIRT1-Mediated Telomere Damage and Partially Suppresses Inflammation in Senescent VSMCs
2.6. SIRT1 Is a Potential Cellular Target for Paeonol to Delay the Senescence of VSMCs
2.7. Paeonol Attenuates t-BHP-Stimulated VSMCs Senescence by Activating the SIRT1/P53/TRF2 Pathway
3. Discussion
4. Materials and Methods
4.1. Reagents and Antibodies
4.2. Animal Experiments
4.3. Cell Culture and Treatment
4.4. Analysis of Atherosclerotic Lesions
4.5. Micro-Ultrasound
4.6. Cell Counting Kit-8 (CCK-8) Assay
4.7. ELISA Assay
4.8. Immunofluorescence Staining
4.9. Quantitative Real-Time PCR Assay
4.10. EdU Staining
4.11. Cellular Thermal Shift Assay
4.12. Drug Affinity Responsive Target Stability
4.13. SiRNA Silencing SIRT1
4.14. Western Blotting
4.15. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Wolf, D.; Ley, K. Immunity and inflammation in atherosclerosis. Circ. Res. 2019, 44, 107–120. [Google Scholar] [CrossRef] [PubMed]
- Basatemur, G.; Jørgensen, H.; Clarke, M.; Bennett, M.; Mallat, Z. Vascular smooth muscle cells in atherosclerosis. Nat. Rev. Cardiol. 2019, 16, 727–744. [Google Scholar] [CrossRef] [PubMed]
- Grootaert, M.; Moulis, M.; Roth, L.; Martinet, W.; Vindis, C.; Bennett, M.; De Meyer, G. Vascular smooth muscle cell death, autophagy and senescence in atherosclerosis. Cardiovasc. Res. 2018, 114, 622–634. [Google Scholar] [CrossRef] [PubMed]
- Grootaert, M.; Finigan, A.; Figg, N.; Uryga, A.; Bennett, M. SIRT6 Protects Smooth Muscle Cells From Senescence and Reduces Atherosclerosis. Circ. Res. 2021, 128, 474–491. [Google Scholar] [CrossRef] [PubMed]
- Matthews, C.; Gorenne, I.; Scott, S.; Figg, N.; Kirkpatrick, P.; Ritchie, A.; Goddard, M.; Bennett, M. Vascular smooth muscle cells undergo telomere-based senescence in human atherosclerosis: Effects of telomerase and oxidative stress. Circ. Res. 2006, 99, 156–164. [Google Scholar] [CrossRef] [PubMed]
- Duan, J.; Ruan, B.; Song, P.; Fang, Z.; Yue, Z.; Liu, J.; Dou, G.; Han, H.; Wang, L. Shear stress-induced cellular senescence blunts liver regeneration through Notch-sirtuin 1-P21/P16 axis. Hepatology 2022, 75, 584–599. [Google Scholar] [CrossRef]
- Fu, G.; Chen, S.; Liang, L.; Li, X.; Tang, P.; Rao, X.; Pan, M.; Xu, X.; Li, Y.; Yao, Y.; et al. SIRT1 inhibitors mitigate radiation-induced GI syndrome by enhancing intestinal-stem-cell survival. Cancer Lett. 2021, 501, 20–30. [Google Scholar] [CrossRef]
- Kadono, K.; Kageyama, S.; Nakamura, K.; Hirao, H.; Ito, T.; Kojima, H.; Dery, K.; Li, X.; Kupiec-Weglinski, J. Myeloid Ikaros-SIRT1 signaling axis regulates hepatic inflammation and pyroptosis in ischemia-stressed mouse and human liver. J. Hepatol. 2022, 76, 896–909. [Google Scholar] [CrossRef]
- Chen, H.; Wang, F.; Gao, P.; Pei, J.; Liu, Y.; Xu, T.; Tang, X.; Fu, W.; Lu, J.; Yan, Y.; et al. Age-Associated Sirtuin 1 Reduction in Vascular Smooth Muscle Links Vascular Senescence and Inflammation to Abdominal Aortic Aneurysm. Circ. Res. 2016, 119, 1076–1088. [Google Scholar] [CrossRef]
- Kang, E.; Kim, H.; Han, S.; Seo, H. Duck Oil-loaded Nanoemulsion Inhibits Senescence of Angiotensin II-treated Vascular Smooth Muscle Cells by Upregulating SIRT1. Food Sci. Anim. Resour. 2020, 40, 106–117. [Google Scholar] [CrossRef]
- Lin, X.; Li, K.; Yang, Z.; Chen, B.; Zhang, T. Dulcitol suppresses proliferation and migration of hepatocellular carcinoma via regulating SIRT1/p53 pathway. Phytomed. Int. J. Phytother. Phytopharm. 2020, 66, 153112. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Uryga, A.; Reinhold, J.; Figg, N.; Baker, L.; Finigan, A.; Gray, K.; Kumar, S.; Clarke, M.; Bennett, M. Vascular Smooth Muscle Cell Senescence Promotes Atherosclerosis and Features of Plaque Vulnerability. Circulation 2015, 132, 1909–1919. [Google Scholar] [CrossRef] [PubMed]
- Dou, Y.; Kong, P.; Li, C.; Sun, H.; Li, W.; Yu, Y.; Nie, L.; Zhao, L.; Miao, S.; Li, X.; et al. Smooth muscle SIRT1 reprograms endothelial cells to suppress angiogenesis after ischemia. Theranostics 2020, 10, 1197–1212. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Li, D.; Liu, L. Paeonol: Pharmacological effects and mechanisms of action. Int. Immunopharmacol. 2019, 72, 413–421. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Han, Y.; Gu, X.; Li, M.; Du, Y.; Feng, N.; Li, J.; Zhang, S.; Maslov, L.; Wang, G.; et al. Paeonol promotes Opa1-mediated mitochondrial fusion via activating the CK2α-Stat3 pathway in diabetic cardiomyopathy. Redox Biol. 2021, 46, 102098. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Song, A.; Hu, W.; Dai, M. The Anti-atherosclerotic Effect of Paeonol against Vascular Smooth Muscle Cell Proliferation by Up-regulation of Autophagy via the AMPK/mTOR Signaling Pathway. Front. Pharmacol. 2017, 8, 948. [Google Scholar] [CrossRef] [PubMed]
- Shi, X.; Wu, H.; Liu, Y.; Huang, H.; Liu, L.; Yang, Y.; Jiang, T.; Zhou, M.; Dai, M. Inhibiting vascular smooth muscle cell proliferation mediated by osteopontin regulating gut microbial lipopolysaccharide: A novel mechanism for paeonol in atherosclerosis treatment. Front. Pharmacol. 2022, 13, 936677. [Google Scholar] [CrossRef]
- Jamal, J.; Mustafa, M.; Wong, P. Paeonol protects against premature senescence in endothelial cells by modulating Sirtuin 1 pathway. J. Ethnopharmacol. 2014, 154, 428–436. [Google Scholar] [CrossRef]
- Chi, C.; Li, D.; Jiang, Y.; Tong, J.; Fu, H.; Wu, Y.; Shen, F. Vascular smooth muscle cell senescence and age-related diseases: State of the art. Biochim. Biophys. Acta Mol. Basis Dis. 2019, 1865, 1810–1821. [Google Scholar] [CrossRef]
- Zha, Y.; Zhuang, W.; Yang, Y.; Zhou, Y.; Li, H.; Liang, J. Senescence in Vascular Smooth Muscle Cells and Atherosclerosis. Front.Cardiovasc. Med. 2022, 9, 910580. [Google Scholar] [CrossRef]
- Zhang, S.; Liu, W.; Wang, P.; Hu, B.; Lv, X.; Chen, S.; Wang, B.; Shao, Z. Activation of HSP70 impedes tert-butyl hydroperoxide (t-BHP)-induced apoptosis and senescence of human nucleus pulposus stem cells via inhibiting the JNK/c-Jun pathway. Mol. Cell. Biochem. 2021, 476, 1979–1994. [Google Scholar] [CrossRef] [PubMed]
- Jia, R.; Yang, F.; Yan, P.; Ma, L.; Yang, L.; Li, L. Paricalcitol inhibits oxidative stress-induced cell senescence of the bile duct epithelium dependent on modulating Sirt1 pathway in cholestatic mice. Free Radic. Biol. Med. 2021, 169, 158–168. [Google Scholar] [CrossRef] [PubMed]
- Valerio, D.; Luddi, A.; De Leo, V.; Labella, D.; Longobardi, S.; Piomboni, P. SA1/SA2 cohesion proteins and SIRT1-NAD+ deacetylase modulate telomere homeostasis in cumulus cells and are eligible biomarkers of ovarian aging. Hum. Reprod. 2018, 33, 887–894. [Google Scholar] [CrossRef] [PubMed]
- Shi, X.; Xie, X.; Sun, Y.; He, H.; Huang, H.; Liu, Y.; Wu, H.; Dai, M. Paeonol inhibits NLRP3 mediated inflammation in rat endothelial cells by elevating hyperlipidemic rats plasma exosomal miRNA-223. Eur. J. Pharmacol. 2020, 885, 173473. [Google Scholar] [CrossRef] [PubMed]
- Tyrrell, D.; Goldstein, D. Ageing and atherosclerosis: Vascular intrinsic and extrinsic factors and potential role of IL-6. Nat. Rev. Cardiol. 2021, 18, 58–68. [Google Scholar] [CrossRef] [PubMed]
- Koutsaliaris, I.; Moschonas, I.; Pechlivani, L.; Tsouka, A.; Tselepis, A. Inflammation, Oxidative Stress, Vascular Aging and Atherosclerotic Ischemic Stroke. Curr. Med. Chem. 2022, 29, 5496–5509. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Song, X.; Wang, X.; Zheng, L.; Ma, G.; Liu, W.; Su, H.; Liu, X.; Liu, T.; Cao, L.; et al. Oxidative stress impairs the Nur77-Sirt1 axis resulting in a decline in organism homeostasis during aging. Aging Cell 2023, 22, e13812. [Google Scholar] [CrossRef]
- Karnewar, S.; Pulipaka, S.; Katta, S.; Panuganti, D.; Neeli, P.; Thennati, R.; Jerald, M.; Kotamraju, S. Mitochondria-targeted esculetin mitigates atherosclerosis in the setting of aging via the modulation of SIRT1-mediated vascular cell senescence and mitochondrial function in Apoe mice. Atherosclerosis 2022, 356, 28–40. [Google Scholar] [CrossRef]
- Ahamad, S.; Hema, K.; Gupta, D. Identification of Novel Tau-Tubulin Kinase 2 Inhibitors Using Computational Approaches. ACS Omega 2023, 8, 13026–13037. [Google Scholar] [CrossRef]
- Ahamad, S.; Kanipakam, H.; Kumar, V.; Gupta, D. A molecular journey to check the conformational dynamics of tau tubulin kinase 2 mutations associated with Alzheimer’s disease. RSC Adv. 2021, 11, 1320–1331. [Google Scholar] [CrossRef]
- Ahamad, S.; Hema, K.; Kumar, V.; Gupta, D. The structural, functional, and dynamic effect of Tau tubulin kinase1 upon a mutation: A neuro-degenerative hotspot. J. Cell. Biochem. 2021, 122, 1653–1664. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Zhu, X.; Tang, L.; Shen, N.; Cheng, F.; Zou, P.; You, Y.; Yuan, G.; Li, Q.; Zhu, X. ACSL1 promotes imatinib-induced chronic myeloid leukemia cell senescence by regulating SIRT1/p53/p21 pathway. Sci. Rep. 2022, 12, 17990. [Google Scholar] [CrossRef] [PubMed]
- Fujita, K.; Horikawa, I.; Mondal, A.; Jenkins, L.; Appella, E.; Vojtesek, B.; Bourdon, J.; Lane, D.; Harris, C. Positive feedback between p53 and TRF2 during telomere-damage signalling and cellular senescence. Nat. Cell Biol. 2010, 12, 1205–1212. [Google Scholar] [CrossRef] [PubMed]







Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, M.; Ma, X.; Gao, M.; Wu, H.; Liu, Y.; Shi, X.; Dai, M. Paeonol Attenuates Atherosclerosis by Inhibiting Vascular Smooth Muscle Cells Senescence via SIRT1/P53/TRF2 Signaling Pathway. Molecules 2024, 29, 261. https://doi.org/10.3390/molecules29010261
Zhou M, Ma X, Gao M, Wu H, Liu Y, Shi X, Dai M. Paeonol Attenuates Atherosclerosis by Inhibiting Vascular Smooth Muscle Cells Senescence via SIRT1/P53/TRF2 Signaling Pathway. Molecules. 2024; 29(1):261. https://doi.org/10.3390/molecules29010261
Chicago/Turabian StyleZhou, Min, Xiaolin Ma, Menglong Gao, Hongfei Wu, Yarong Liu, Xiaoyan Shi, and Min Dai. 2024. "Paeonol Attenuates Atherosclerosis by Inhibiting Vascular Smooth Muscle Cells Senescence via SIRT1/P53/TRF2 Signaling Pathway" Molecules 29, no. 1: 261. https://doi.org/10.3390/molecules29010261
APA StyleZhou, M., Ma, X., Gao, M., Wu, H., Liu, Y., Shi, X., & Dai, M. (2024). Paeonol Attenuates Atherosclerosis by Inhibiting Vascular Smooth Muscle Cells Senescence via SIRT1/P53/TRF2 Signaling Pathway. Molecules, 29(1), 261. https://doi.org/10.3390/molecules29010261