Abstract
Laportea bulbifera (Sieb. et Zucc.) Wedd. (L. bulbifera) is a significant plant in the Laportea genus. Traditionally, it has been used in ethnomedicine for treating various conditions such as rheumatic arthralgia, fractures, falling injuries, nephritis dropsy, limb numbness, pruritus, fatigue-induced internal imbalances, and irregular menstruation. Modern pharmacological studies have confirmed its therapeutic potential, including anti-inflammatory, immunosuppressive, analgesic, and anti-rheumatoid arthritis properties. To gather comprehensive information on L. bulbifera, a thorough literature search was conducted using databases like Web of Science, PubMed, ProQuest, and CNKI. This review aims to provide a comprehensive understanding of L. bulbifera, covering various aspects such as ethnomedicinal uses, geographical distribution, botanical description, phytochemistry, pharmacology, and quality control. The goal is to establish a solid foundation and propose new research avenues for exploring and developing potential applications of L. bulbifera. So far, a total of one hundred and eighty-nine compounds have been isolated and identified from L. bulbifera, including flavonoids, phenolics, nitrogen compounds, steroids, terpenoids, coumarins, phenylpropanoids, fatty acids and their derivatives, and other compounds. Notably, flavonoids and fatty acids have demonstrated remarkable antioxidant and anti-inflammatory properties. Additionally, these compounds show promising potential in activities such as analgesia, hypoglycemia, and hypolipidemia, as well as toxicity. Despite extensive fundamental studies on L. bulbifera, further research is still needed to enhance our understanding of its mechanism of action and improve quality control. This requires more comprehensive investigations to explore the specific material basis, uncover new mechanisms of action, and refine quality control methods related to L. bulbifera. By doing so, we could contribute to the further development and utilization of this plant.
1. Introduction
Laportea bulbifera (Sieb. et Zucc.) Wedd. (L. bulbifera) (Figure 1) is an important plant in the Laportea genus. It is referred to by various names, including Laportea elevata, Laportea terminalis, and Laportea sinensis. Currently, a variety of active ingredients have been isolated from L. bulbifera, such as flavonoids [1,2,3], coumarins [1,4,5], phenolic acids [6], phenylpropanoids [7,8], steroids [1,9,10], aliphatic acids [5,8], nitrogen compounds [8,11], and other compounds. Modern pharmacological studies have demonstrated that extracts and monomeric compounds from L. bulbifera possess anti-inflammatory [12,13], immunosuppressive [14], analgesic [15], and anti-rheumatoid arthritis properties [16], with particular emphasis on its anti-inflammatory and anti-rheumatoid arthritis effects.

Figure 1.
Morphology of Laportea bulbifera: aboveground part (A) and root (B).
Among the ethnic medicines in Guizhou Province in southwest China that have been incorporated into the national drug standards, various preparations containing L. bulbifera have been developed. These include Runzao Antipruritic Capsules, Liuwei Shangfuning Ointments, Fufang Shangfuning Ointments, and Tongluo Guzhining Ointments. The cultivation and utilization of L. bulbifera have become crucial endeavors in Guizhou’s ethnic medicine pillar industry, possessing distinctive regional resource advantages and development potential [16]. Runzao Antipruritic Capsules, in particular, have gained significant popularity in the Chinese market due to their unique therapeutic effect in treating skin itching caused by blood deficiencies in the elderly [17]. This inclusion in the Report on the Scientific and Technological Competitiveness of Large Varieties of Traditional Chinese Medicine showcases their popularity [18]. Additionally, the young leaves of L. bulbifera are edible, and the stem fibers are durable and suitable for use in textile production [19].
Despite existing research that has summarized the phytochemistry and pharmacology of L. bulbifera [20], there are significant gaps in the coverage. These gaps include the incomplete classification of components, a partial listing of constituents, and the lack of information concerning the chemical structure, exact theoretical molecular weight, and characterization method for these components. Furthermore, the mechanisms underlying the pharmacological effects are often insufficiently detailed and clarified.
In contrast, our review addresses these deficiencies by reporting a total of one hundred and eighty-nine components and providing structural information for each compound, including the name, formula, exact theoretical molecular weight, characterization method, references, and source. Additionally, our review introduces a different classification of pharmacological research compared to the previous report. Importantly, we incorporate the latest research findings on L. bulbifera, resulting in an up-to-date and comprehensive perspective.
Therefore, the objective of our review is to bridge these gaps by providing a comprehensive assessment of the ethnomedicinal uses, geographical distribution, botanical description, phytochemistry, pharmacology, and quality control of L. bulbifera. This review aims to serve as a valuable reference for future investigations into L. bulbifera, as well as offering new insights into the rational utilization of L. bulbifera resources and the efficient development of related products.
2. Ethnomedicinal Uses
L. Bulbifera, also known as “reib ndad gunb” or “uab detdend” in the Miao language, is widely used as a traditional medicine by ethnic minorities in Guizhou Province, Hubei Province, and Guangxi Zhuang Autonomous Region, China. These communities include the Miao, Buyi, Tujia, Zhuang, and Yao. During the autumn season, the roots are harvested and then sun-dried after removing the stems, leaves, and soil. L. Bulbifera has a pungent flavor and a hot nature, making it suitable for treating conditions related to the cold meridian [21]. Its primary functions include clearing the blood network and nervous network [6]. For internal use, it is typically decocted with water at a dosage of 9–15 g. When using fresh products, the dosage should be doubled. Alternatively, it can be soaked in Chinese Baijiu. For external application, an appropriate amount can be used for washing or applied externally after being mashed. Its effects encompass dispelling wind and dampness, promoting blood circulation, and removing stasis. It is particularly effective in clearing the food channel, strengthening the spleen, and eliminating accumulated food. Common applications include the treatment of rheumatic arthralgia, fractures, falling injuries, nephritis dropsy, limb numbness, pruritus, fatigue-induced internal imbalances, and irregular menstruation. Additionally, Zhuang doctors often use it to address infantile malnutrition in children and urinary tract stones. The following are some specific prescriptions that involve L. Bulbifera: (1) To treat rheumatism and numbness, decoct 15 g of L. Bulbifera with water, take the water decoction orally, and use the water decoction to wash the affected area. (2) For rheumatic arthralgia, soak 15 g of L. Bulbifera and 9 g of Acanthopanacis gracilistylus in Chinese Baijiu before consuming. (3) For falling injuries, grind the dried roots into powder and take 6 g of Chinese Baijiu before bedtime. (4) To treat urticaria, decoct 6–9 g of L. Bulbifera with water and take the water decoction orally. For pediatric use, the dosage should be appropriately reduced. (5) To alleviate body deficiency and swelling, take 9–15 g of L. Bulbifera and 250 g of pork. Stew them together and consume the soup and meat once a day for 2–3 days. (6) For cough, decoct 20–30 g of L. Bulbifera with water and take the water decoction orally. (7) For anemofrigid cold and cough, decoct 30 g of L. Bulbifera with water and take the water decoction orally [21].
3. Geographical Distribution
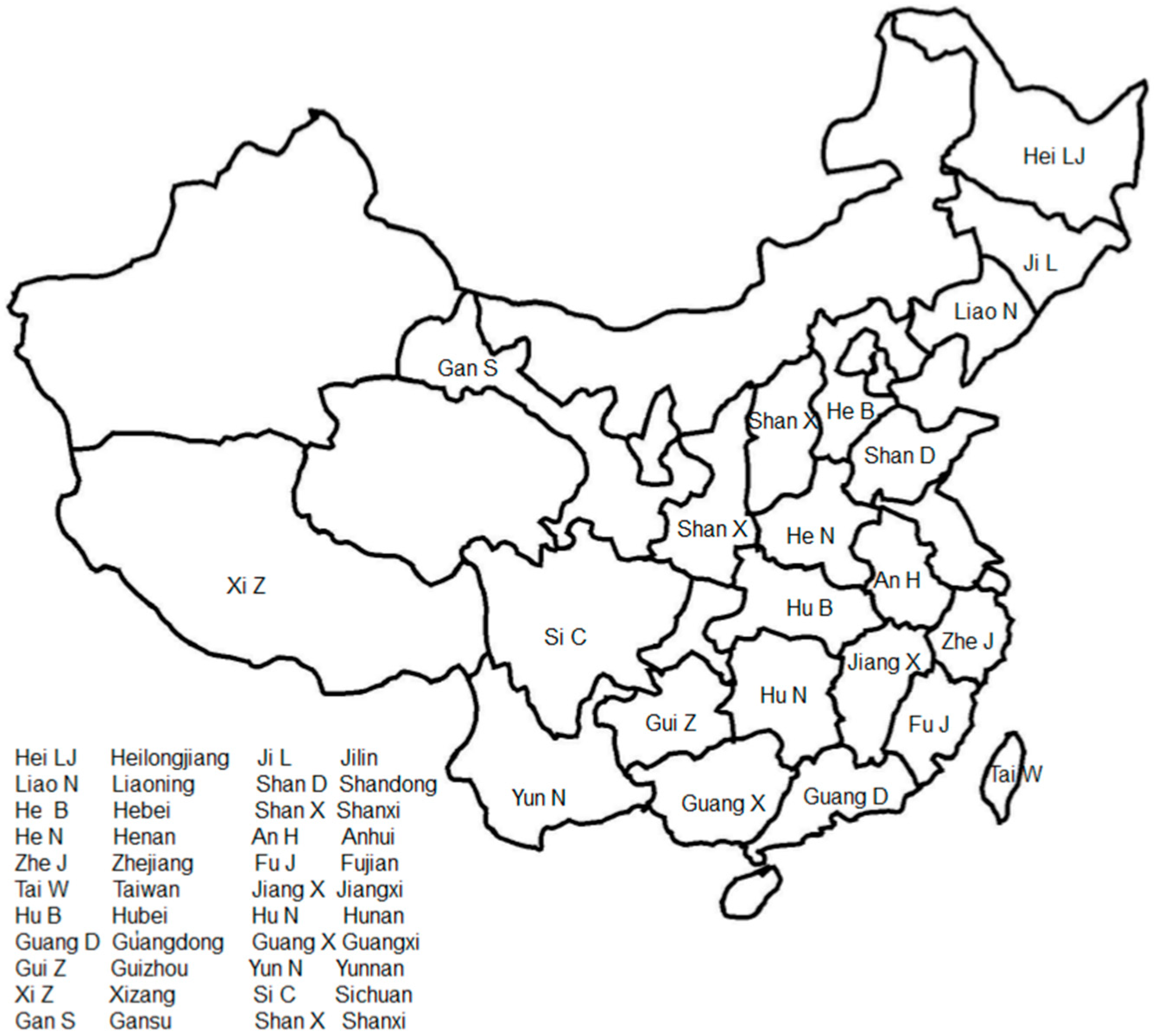
L. bulbifera is distributed across various regions in China, including Heilongjiang, Jilin, Liaoning, Shandong, Hebei, Shanxi, Henan, Anhui, Zhejiang, Fujian, Taiwan, Jiangxi, Hubei, Hunan, northern Guangdong, Guangxi, Guizhou, Yunnan, Xizang, Sichuan, Gansu, and Shaanxi. Figure 2 illustrates the general geographical distribution of L. bulbifera in China. It can also be found in Japan, North Korea, Russia, Sikkim, India, Sri Lanka, and Java Island in Indonesia. This plant grows in hillside forests and on semi-shady slopes at altitudes of 1000–2400 m [22].
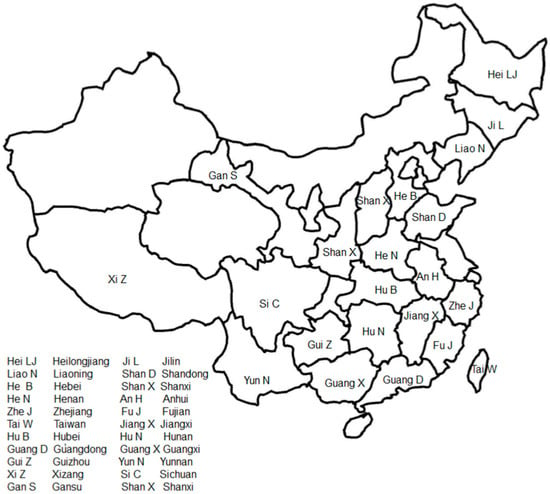
Figure 2.
The general geographical distribution of Laportea bulbifera in China.
4. Botanical Description
The female perianth has four segments, and the male perianth has 4–5 segments. The ovary has a pistil stalk, and the stigma is filiform, measuring 2–4 mm in length. Initially, the ovary is upright and later becomes oblique. The achenes are round and obovate or nearly semicircular, oblique, flat, and 2–3 mm long with purplish-brown spots. The pistil stalk is retroflex, and two persistent perianth segments extend to the middle of the fruit. The fruit stalk has membranous wings, and sometimes, the fruit inflorescence is branched and winged, spoon-shaped, with a concave top. The flowering period is from June to August, and the fruiting period is from August to December [22,23].
L. bulbifera is a perennial herb. The root of L. bulbifera is long and conical or slender, spindle-shaped, and twisted, with a length ranging from 6 to 20 cm and a diameter of 3–6 mm. The surface has a grayish-brown to reddish-brown color, with fine longitudinal wrinkles and slender fibrous roots or fibrous root scars. It has a hard texture and is not easily broken, with a fibrous cross-section and a light reddish-brown color [21]. The stem is 0.4–1.5 m tall, with short hairs and a few stinging hairs. The bulbils are almost spherical, with a diameter of 3–6 mm. The leaves are alternate, ovate, elliptical, or lanceolate, measuring 8–16 cm in length and 3–6 cm in width. The apex is acuminate, the base is broadly cuneate or circular, and the margin is densely toothed. The lower surface is sparsely covered with short hairs and stinging hairs. Cystoliths are punctate, with three basal veins and 4–6 pairs of lateral veins. The petiole is 1.5–6 cm long, and the stipules are oblong-lanceolate, measuring 0.5–1 cm in length and being two-lobed. The inflorescence is paniculate, and the plant is monoecious. The male inflorescence is located in the upper leaf axil of the stem and measures 3–10 cm in length, while the female inflorescence is located at or near the top leaf axil, measuring 10–25 cm in length with a peduncle of 5–12 cm. The female perianth has 4 segments, and the male perianth has 4–5 segments. The ovary has a pistil stalk, and the stigma is filiform, measuring 2–4 mm in length. Initially, the ovary is upright and later becomes oblique. The achenes are round, obovate, or nearly semicircular, oblique, flat, and 2–3 mm long with purplish-brown spots. The pistil stalk is retroflex, and two persistent perianth segments extend to the middle of the fruit. The fruit stalk has membranous wings, and sometimes, the fruit inflorescence is branched and winged, spoon-shaped, with a concave top. The flowering period is from June to August, and the fruiting period is from August to December [22,23].
5. Phytochemistry
Over the years, numerous active compounds have been isolated and identified from the aerial parts or roots of L. bulbifera, particularly in recent times. As the importance and utilization of this plant increase, research on its components has also expanded. According to reports, a total of one hundred and eighty-nine compounds have been isolated or identified from L. bulbifera. These compounds can be categorized into nine groups, including flavonoids, phenolics, nitrogen compounds, steroids, terpenoids, coumarins, phenylpropanoids, fatty acids and their derivatives, as well as other compounds. This remarkable abundance of bioactive ingredients in L. bulbifera highlights its potential as a source for drug development and clinical applications.
5.1. Flavonoids
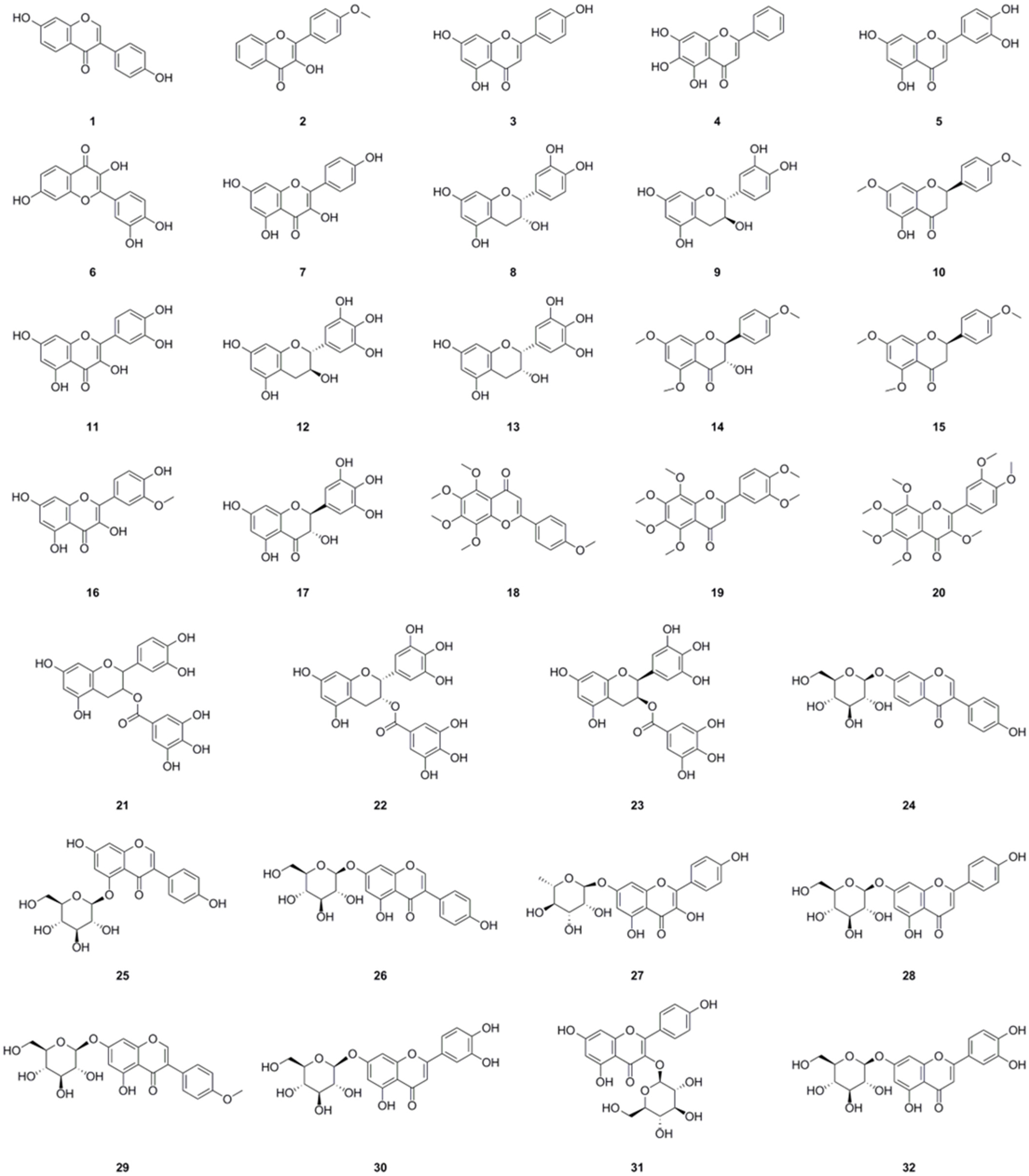
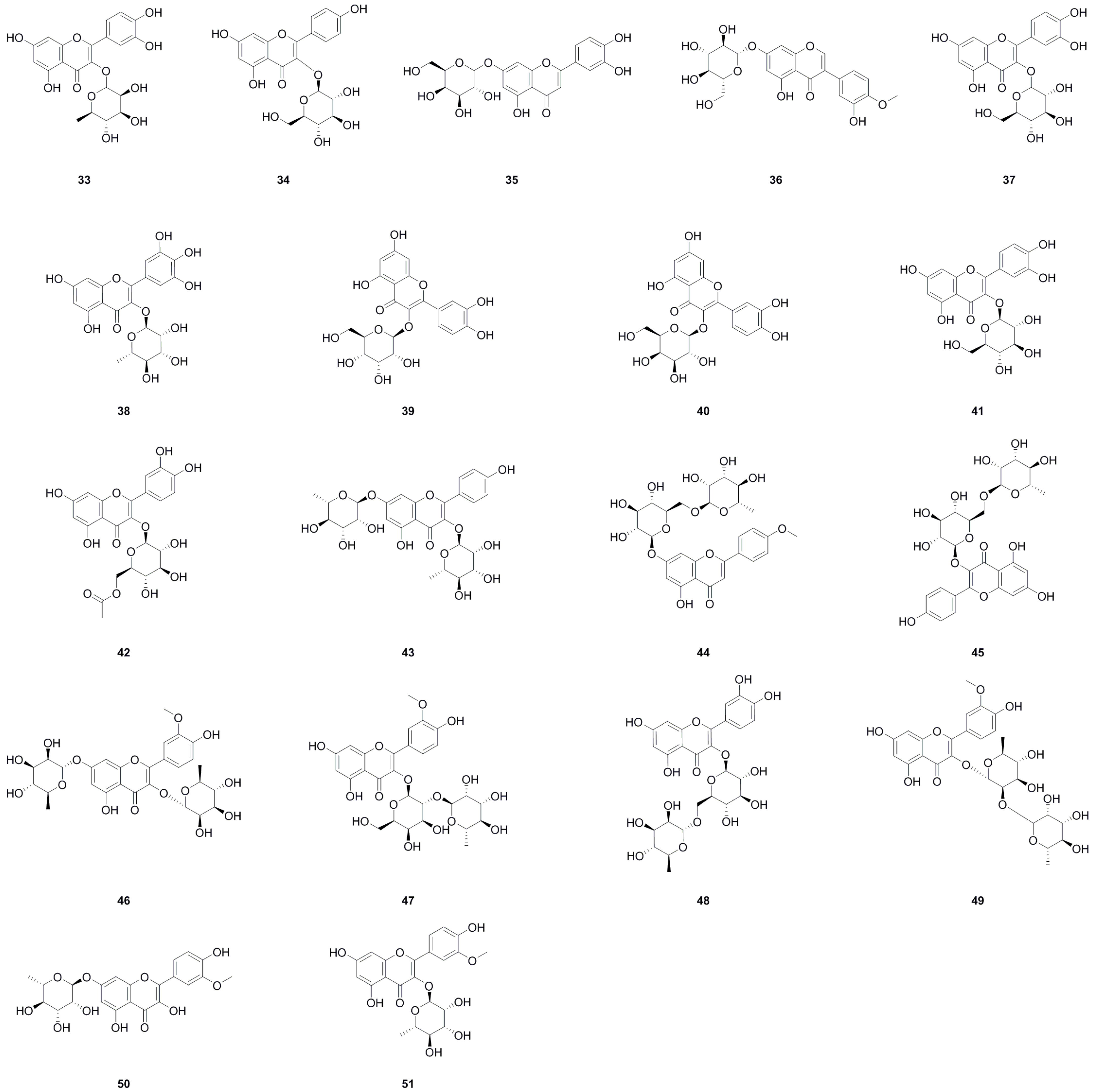
Among the compounds derived from L. bulbifera, flavonoids have received the most extensive research and were the earliest reported type. A total of fifty-one flavonoid components have been identified, consisting of twenty-three flavonoids and twenty-eight flavonoid glycosides (Table 1, Figure 3). The team from Dalian University isolated nine flavonoids and their glycosides from the aerial parts and the whole herb of L. bulbifera, respectively [2,24]. Additionally, five flavonoids were isolated from the aerial parts [11], while twenty-six flavonoids and their glycosides were obtained from the roots of L. bulbifera through bioassay-guided isolation [1]. Furthermore, HPLC-MS technology was used to identify seven flavonoids and their glycosides from the 95% ethanol extract of the roots [2]. Epigallocatechin was isolated from the whole herb [25], and rutin was isolated from the aerial parts [26]. Two flavonoid glycosides were also obtained from the whole herb [10], and four flavonoids and their glycosides were identified through UHPLC-ESI-Q-TOF-MS technology from the 70% ethanol extract [7]. Similarly, We employed the same technique to identify two flavonoid glycosides from the methanol extract of the roots [27].

Table 1.
Flavonoids isolated from Laportea bulbifera.
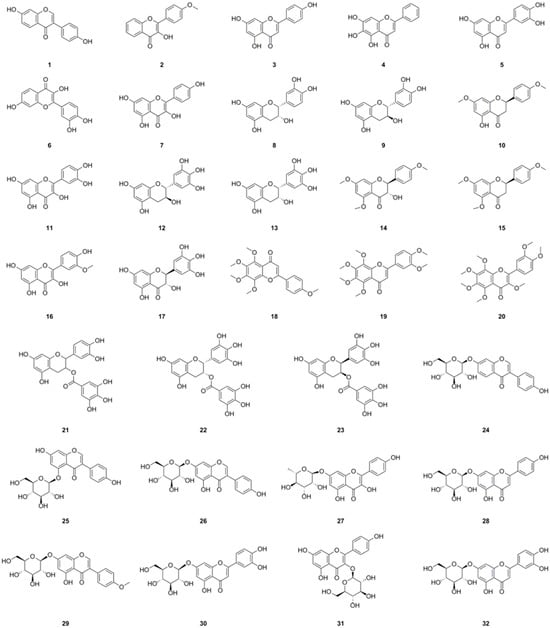
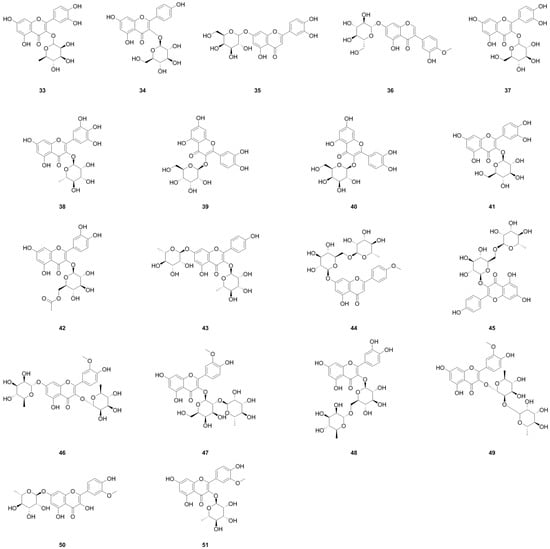
Figure 3.
Chemical structures of flavonoids isolated from Laportea bulbifera. Chemical structures were drawn using ChemDraw Professional 15.0 software.
Flavonoids are natural polyphenolic substances and secondary metabolites of plants. They possess remarkable antioxidant activity, which has been extensively investigated. This antioxidant activity aids in the prevention of damage caused by free radicals through scavenging reactive oxygen species (ROS), activating antioxidant enzymes, and inhibiting oxidases. Moreover, flavonoids elevate uric acid levels and exhibit metal-chelating activity to alleviate oxidative stress [28]. Studies have also indicated that flavonoids activate antioxidant pathways, thereby contributing to their anti-inflammatory effects. They inhibit the secretion of enzymes such as lysozymes and β-glucuronidase, as well as the secretion of arachidonic acid, thus reducing inflammatory reactions. Flavonoids such as apigenin (3), kaempferol (7), and (−)-epigallocatechin 3-O-gallate (22) play a role in modulating the expression and activation of various cytokines, including interleukin-1beta (IL-1β), tumor necrosis factor-alpha (TNF-α), interleukin-6 (IL-6), and interleukin-8 (IL-8). They also regulate the gene expression of several pro-inflammatory molecules, such as nuclear factor-kappaB (NF-κB), activator protein-1 (AP-1), and intercellular adhesion molecule-1 (ICAM). Additionally, they inhibit pro-inflammatory enzymes such as inducible nitric oxide (NO) synthase, cyclooxygenase-2, and lipoxygenase [29].
5.2. Phenolics
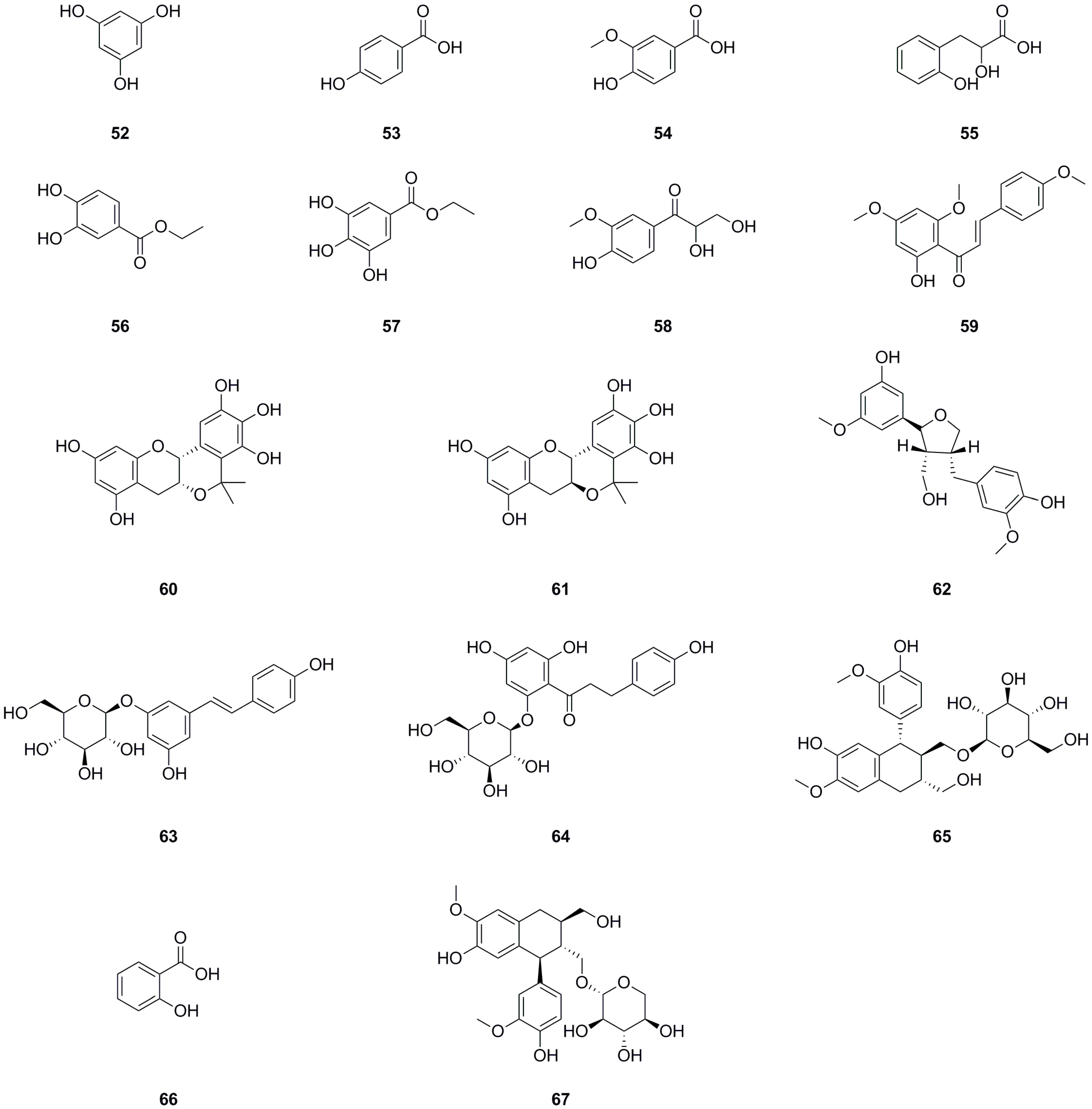
A total of sixteen phenolics have been isolated and identified from different parts of L. bulbifera, including the roots, aerial parts, and the whole herb (Table 2, Figure 4). Nine phenolics were obtained from the roots using bioassay-guided isolation [1]. Phloroglucinol (52) was isolated from the whole herb [25], C-veratroylglycol (58) was isolated from the roots [8], and vanillic acid (54) was also isolated from the roots [30]. Another study identified phenolics such as ethyl 3,4-dihydroxybenzoate (56), ethyl gallate (57), and (+)-isolariciresinol 9′-O-glucoside (65). Two phenolics, salicylic acid (66) and schizandriside (67), were identified from the methanol extract of the roots using UHPLC-ESI-Q-TOF-MS technology. Phenolics have demonstrated potent antioxidant, anti-inflammatory, and immunomodulatory activities [31], as well as hypolipidemic, hypoglycemic, and antihypertensive properties [32].

Table 2.
Phenolics isolated from Laportea bulbifera.
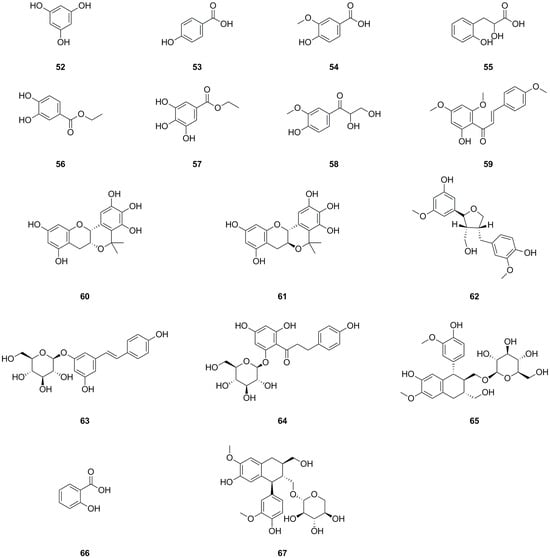
Figure 4.
Chemical structures of phenolics isolated from Laportea bulbifera. Chemical structures were drawn using ChemdDaw Professional 15.0 software.
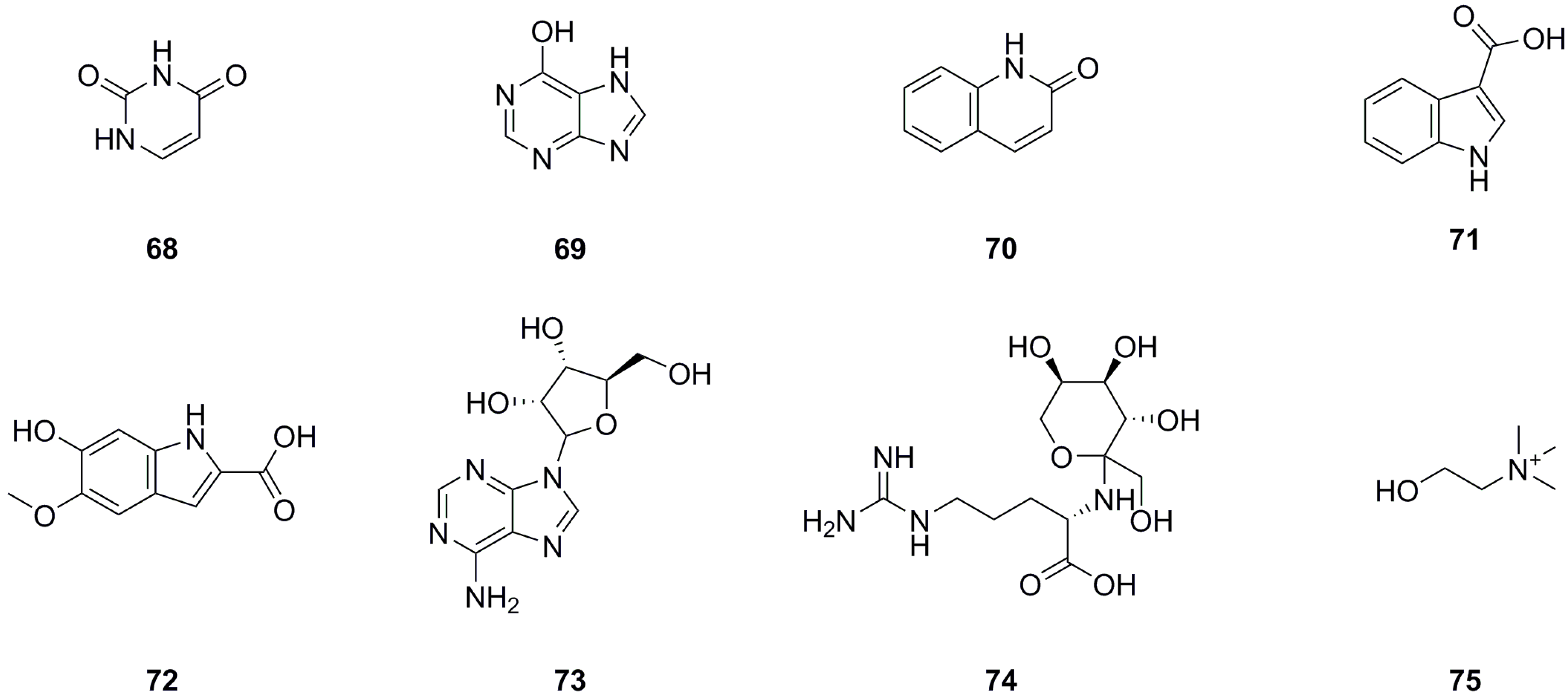
5.3. Nitrogen Compounds
Currently, eight nitrogen compounds have been isolated and identified from various parts of L. bulbifera, including the roots, aerial parts, and the whole herb (Table 3, Figure 5). Uracil (68), 6-hydroxypurine (69), 1H-indole-3-carboxylic acid (71), and 9-ribofuranosyladenine (73) were isolated from the aerial parts [11]. Quinolin-2(1H)-one (70) was identified from the aerial parts [5], and 6-hydroxy-5-methoxy-1H-indole-2-carboxylic acid (72) was identified from the 70% ethanol extract of the roots. N2-Fructopyranosylarginine (74) and choline were identified from the methanol extract of the roots using UHPLC-ESI-Q-TOF-MS [27].

Table 3.
Nitrogen compounds isolated from Laportea bulbifera.
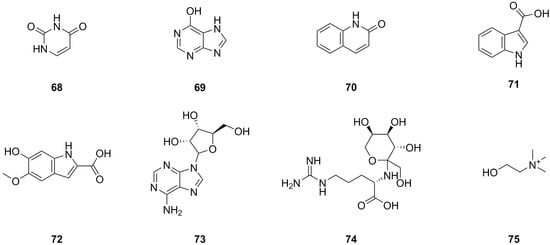
Figure 5.
Chemical structures of nitrogen compounds isolated from Laportea bulbifera. Chemical structures were drawn using ChemDraw Professional 15.0 software.
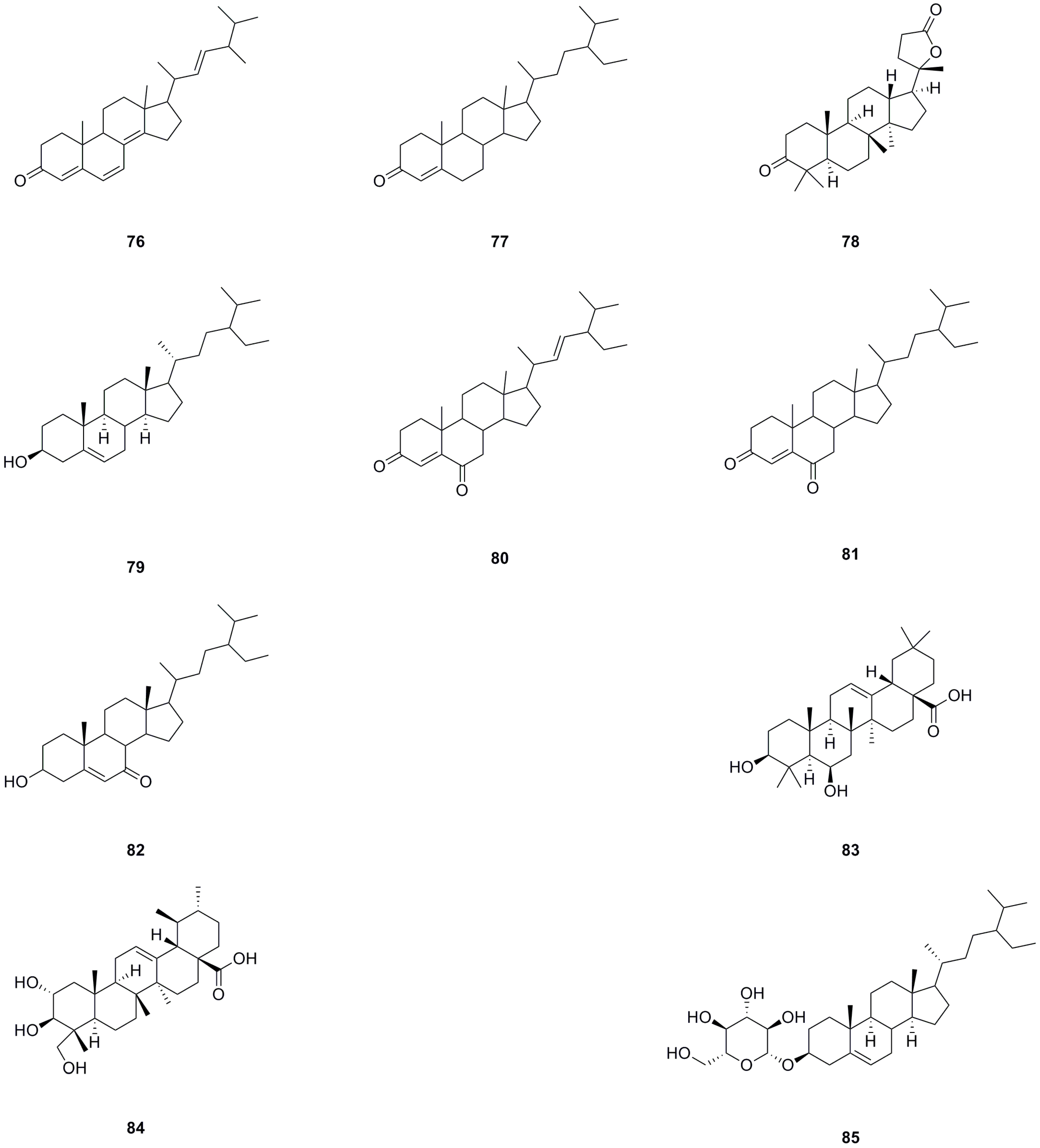
5.4. Steroids
A total of ten steroids have been isolated and identified from different parts of L. bulbifera, including the roots, aerial parts, and the whole herb (Table 4, Figure 6). Ergosta-4,6,8(14),22-tetraen-3-one (76), sitostenone (77), stigmasta-4,22-diene-3,6-dione (80), and stigmast-4-ene-3,6-dione (81) were isolated from the whole herb [10]. (+)-Cabralealactone (78) and 7-keto-β-sitosterol (82) were isolated from the roots [1], while β-sitosterol (79) and β-daucosterol (85) were isolated from both the roots and aerial parts [9]. Sumaresinolic acid (83) and asiatic acid (84) were identified from the 70% ethanol extract of the roots through HPLC-MS analysis [8]. Among these steroids, compound 79 is the major compound and displays various biological activities, including immunomodulatory, anti-inflammatory, lipid-lowering, hepatoprotective, antioxidant, and anti-diabetic effects [33].

Table 4.
Steroids isolated from Laportea bulbifera.
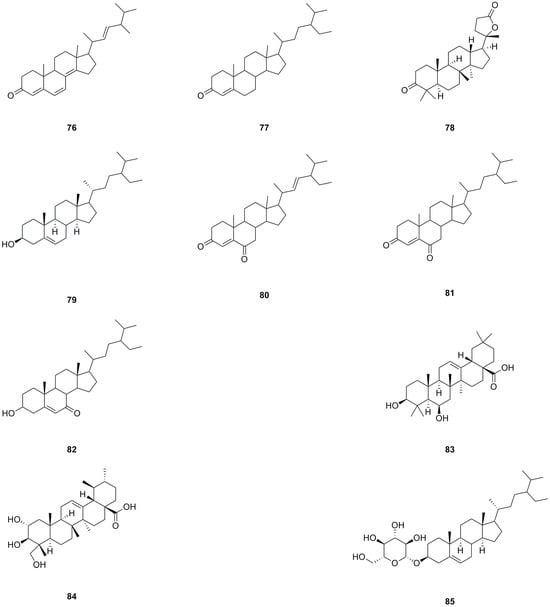
Figure 6.
Chemical structures of steroids isolated from Laportea bulbifera. Chemical structures were drawn using ChemDraw Professional 15.0 software.
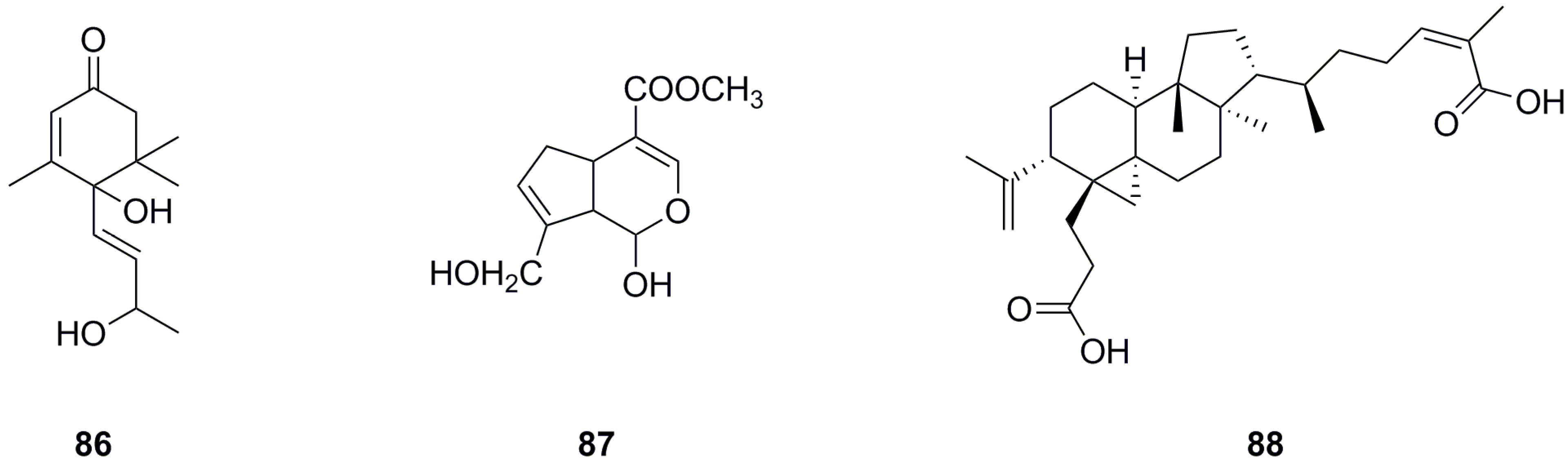
5.5. Terpenoids
Only three terpenoids have been isolated and identified from the roots and aerial parts of L. bulbifera so far (Table 5, Figure 7). α-Ionol (86) was isolated from the aerial parts [11], while genipin (87) and nigranoic acid (88) were identified from the roots.

Table 5.
Terpenoids isolated from Laportea bulbifera.
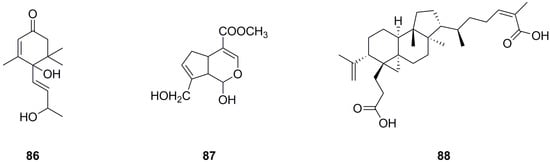
Figure 7.
Chemical structures of terpenoids isolated from Laportea bulbifera. Chemical structures were drawn using ChemDraw Professional 15.0 software.
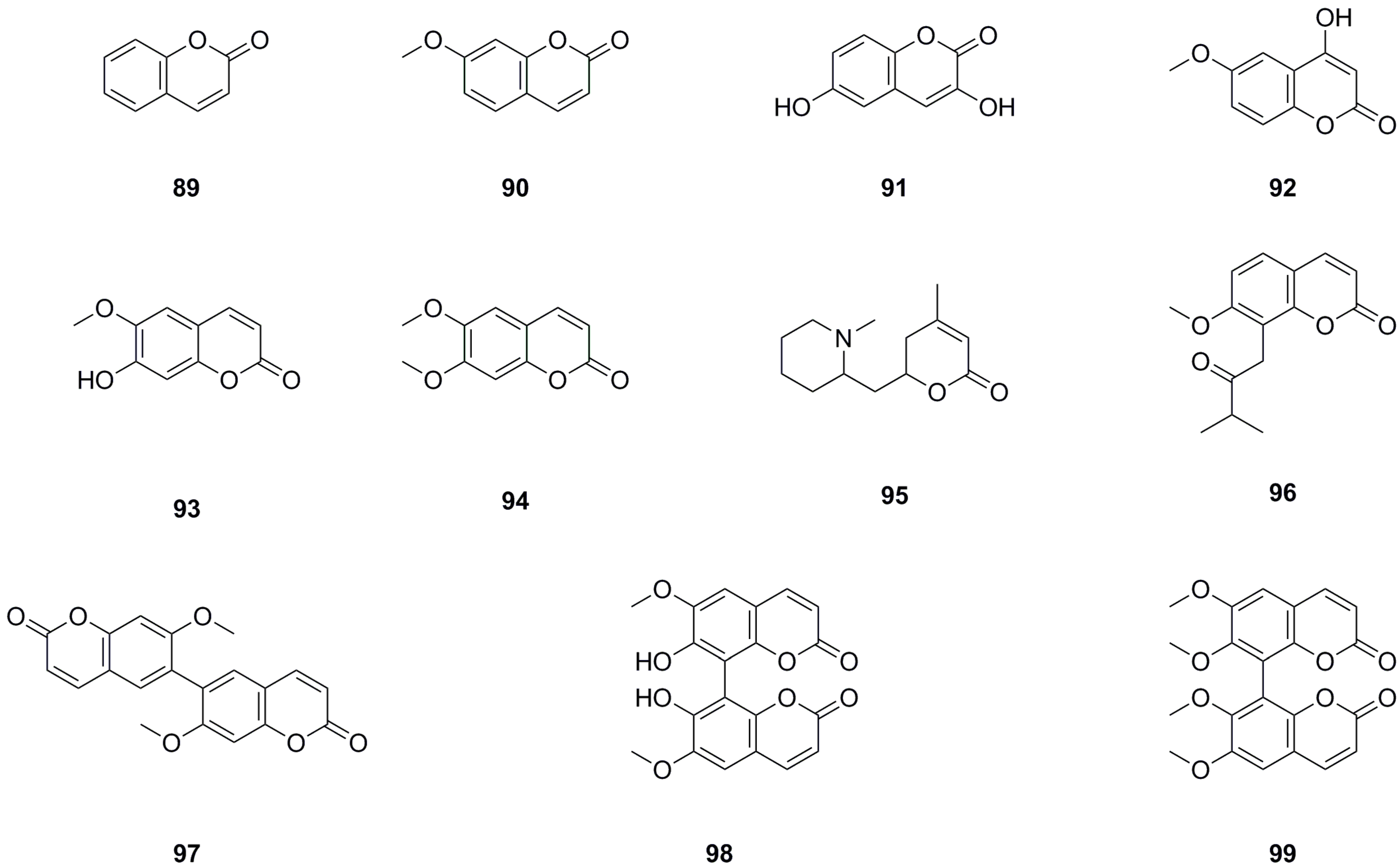
5.6. Coumarins
Eleven coumarins have been isolated and identified from different parts of L. bulbifera, including the roots, aerial parts, and the whole herb (Table 6, Figure 8). The main categories of coumarins are simple coumarins and coumarin dimers. 7-Methoxy-2H-chromen-2-one (90) and scoparone (94) were isolated from the roots [1]. Five coumarins, including coumarin, were identified from the 70% ethanol extract of the whole herb using UHPLC-QTOF-MS/MS [5]. Scoparone (94) and three dimers, 7,7′-dimethoxy-6,6′-biscoumarin (97), 7,7′-dihydroxy-6,6′-dimethoxy-8,8′-biscoumarin (98), and 6,6′,7,7′-tetramethoxyl-8,8′-biscoumarin (99), were isolated from the roots [4]. Scopoletin (93) was isolated from both the aerial parts and the whole herb [6,11], while isomeranzin (96) was isolated from the whole herb [30]. Scopoletin (93) has antioxidant, anti-inflammatory, and neuroprotective properties [34]. Scoparone (94) possesses anti-inflammatory, antioxidant, anti-fibrotic, and hypolipidemic properties [35].

Table 6.
Coumarins isolated from Laportea bulbifera.
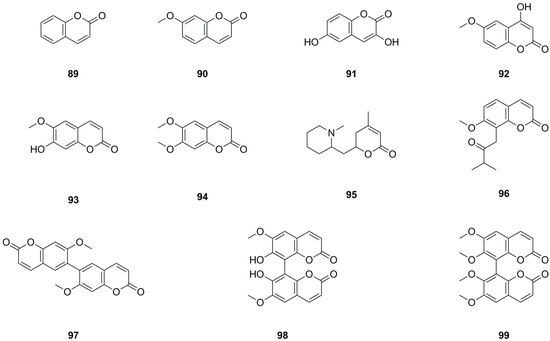
Figure 8.
Chemical structures of coumarins isolated from Laportea bulbifera. Chemical structures were drawn using ChemDraw Professional 15.0 software.
5.7. Phenylpropanoids
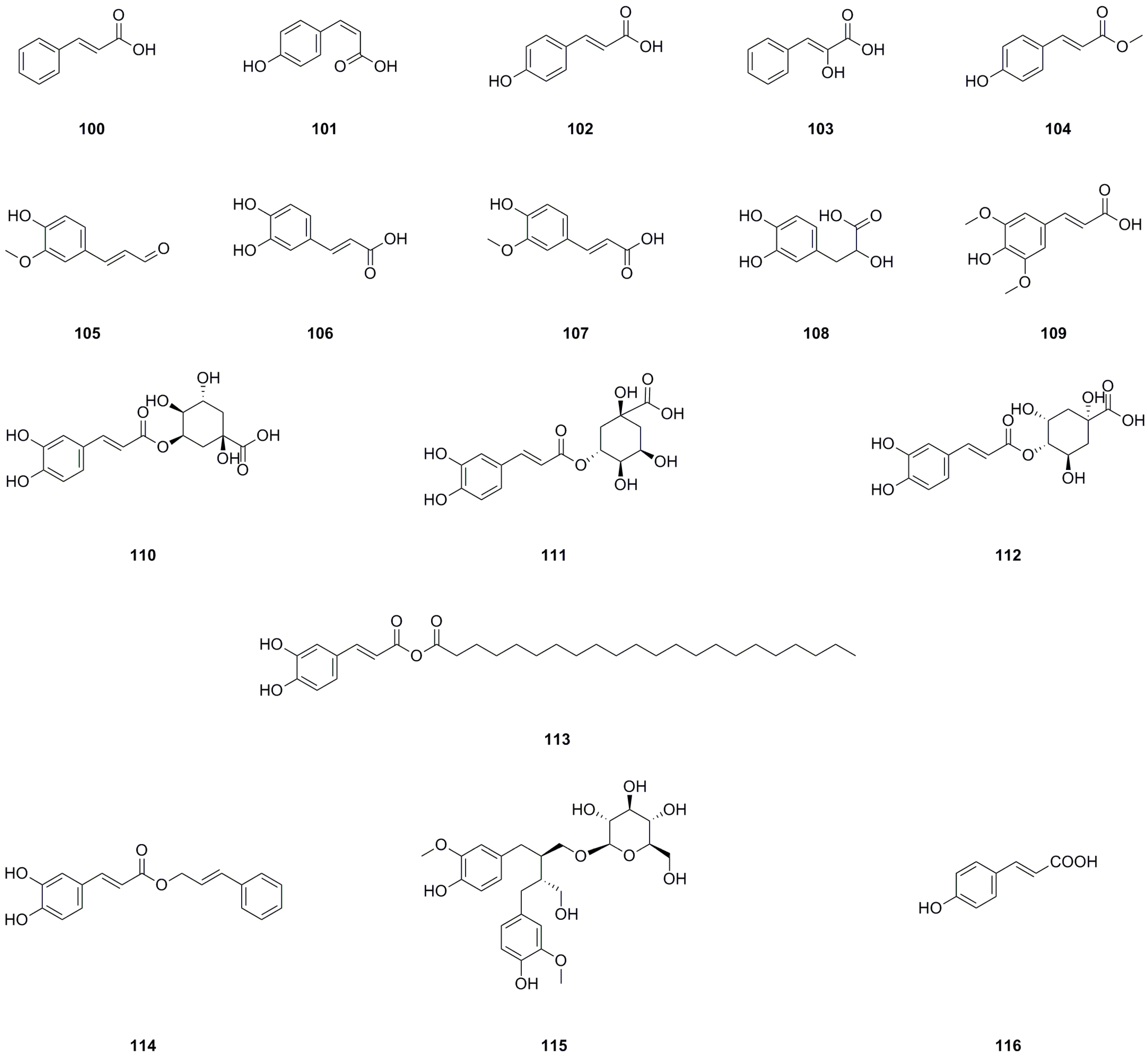
Seventeen phenylpropanoids have been isolated and identified from the roots, aerial parts, or the whole herb of L. bulbifera (Table 7, Figure 9). Seven phenylpropanoids have been isolated and identified from the roots [8]. trans-p-Hydroxycinnamic acid (102), cis-hydroxycinnamic acid (103), and methyl-trans-4-hydroxycinnamate (104) have been isolated from the aerial parts [11].

Table 7.
Phenylpropanoids isolated from Laportea bulbifera.
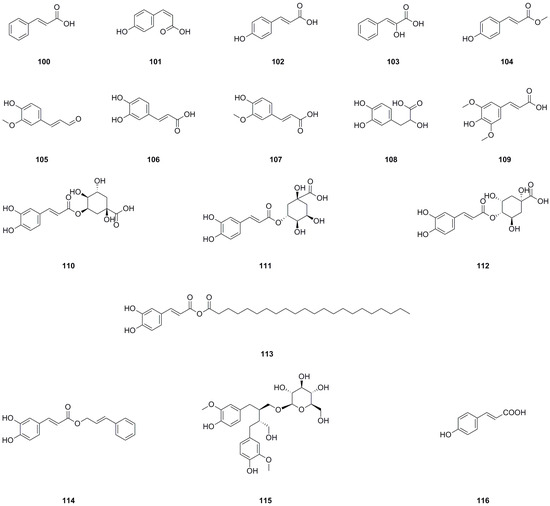
Figure 9.
Chemical structures of phenylpropanoids isolated from Laportea bulbifera. Chemical structures were drawn using ChemDraw Professional 15.0 software.
Neochlorogenic acid (110), chlorogenic acid (111), and 4-O-caffeoylquinic acid have been identified from the 70% ethanol extract of the roots and the whole herb [7,26]. Caffeic acid cinnamyl ester (114), secoisolariciresinol 9-O-β-D-glucopyranoside (115), and (E)-4-coumaric acid (116) have been identified from the methanol extract of the roots by us [27]. Caffeic acid (106) has also been isolated from the roots [30]. Chlorogenic acid (111) is a significant compound with antioxidant, hepatoprotective, cardioprotective, anti-inflammatory, and free radical scavenging activities. Moreover, it has been found to modulate lipid metabolism and glucose levels [36]. Caffeic acid (106) is another important compound known for its antioxidant, immunomodulatory, and anti-inflammatory activities [37]. Danshensu (108) exhibits effects such as antioxidant properties, inflammation regulation, and lipidemia control [38].
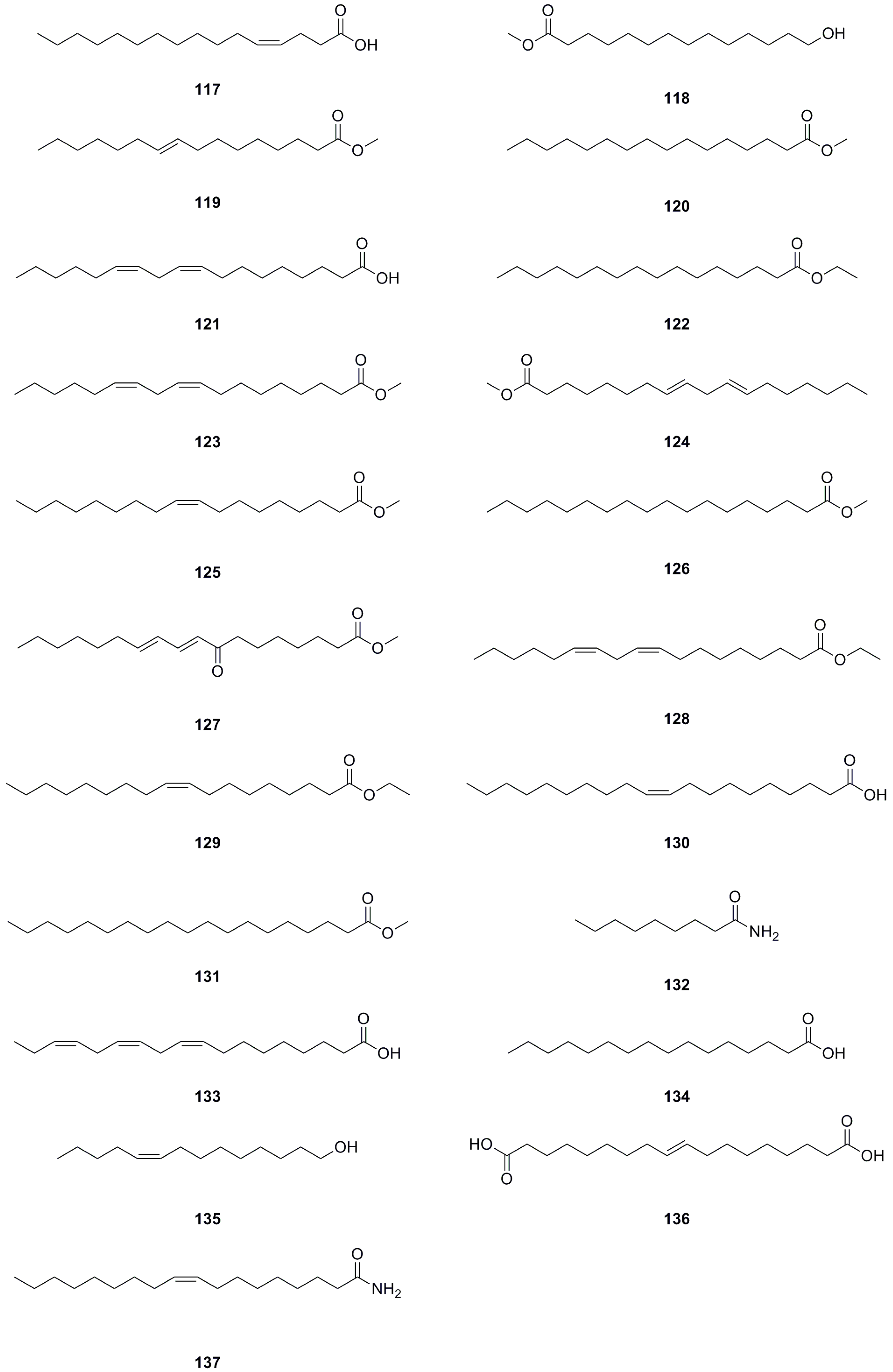
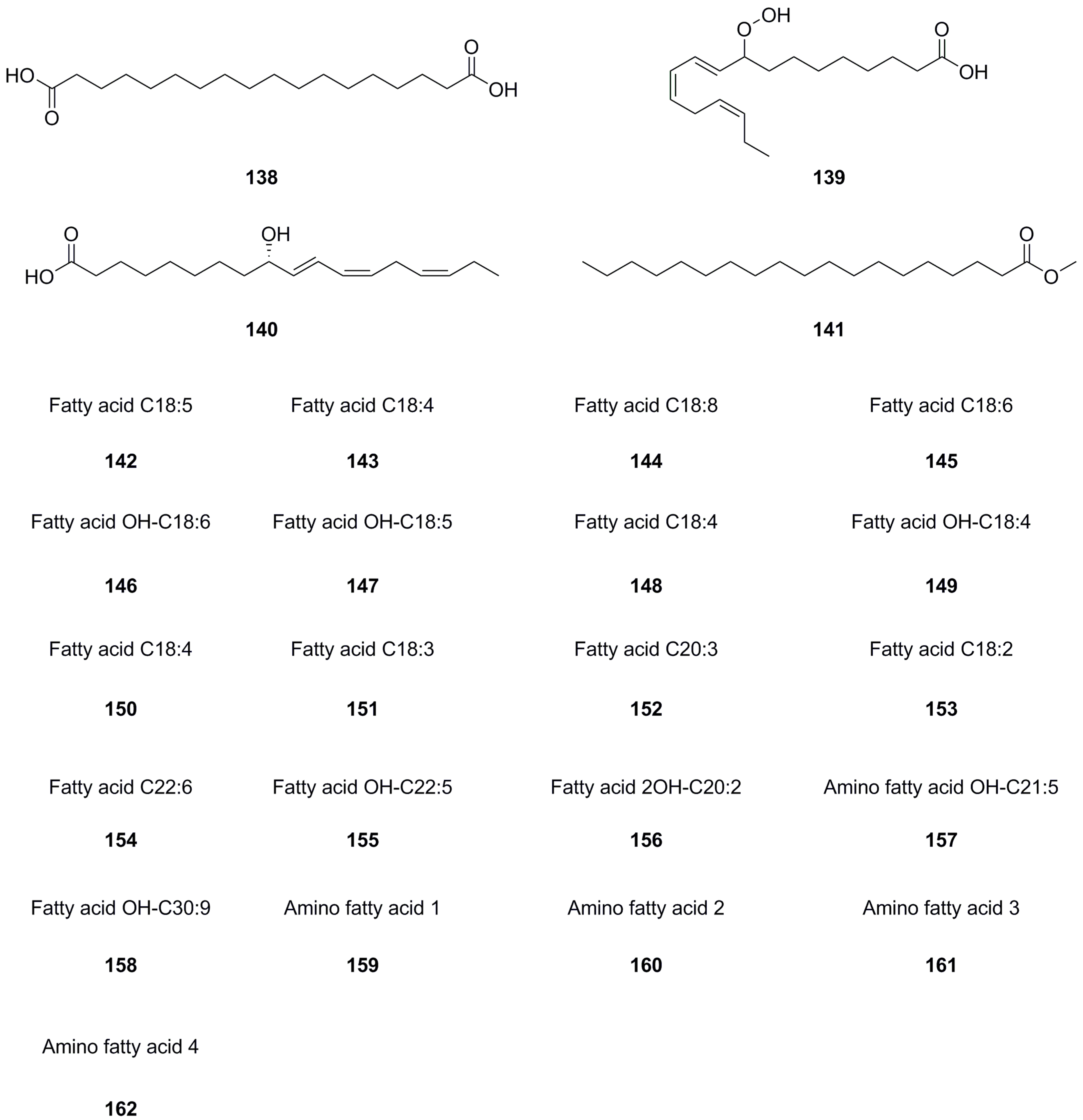
5.8. Fatty Acids and Their Derivatives
A total of forty-five fatty acids and their derivatives were isolated and identified from various parts of L. bulbifera, including the roots, aerial parts, and the whole herb (Table 8, Figure 10). These include saturated and unsaturated fatty acids, hydroxy fatty acids, amino fatty acids, fatty esters, and fatty amides. Fatty acids have shown potential in treating metabolic diseases such as type II diabetes, inflammatory diseases, and cancer [39,40]. Intake of linoleic acid (121) has been found to improve hyperlipidemia and reduce the incidence of type II diabetes [41]. Linolenic acid (133) possesses anti-metabolic syndrome, anticancer, anti-inflammatory, and antioxidant properties [42].

Table 8.
Fatty acids and their derivatives isolated from Laportea bulbifera.
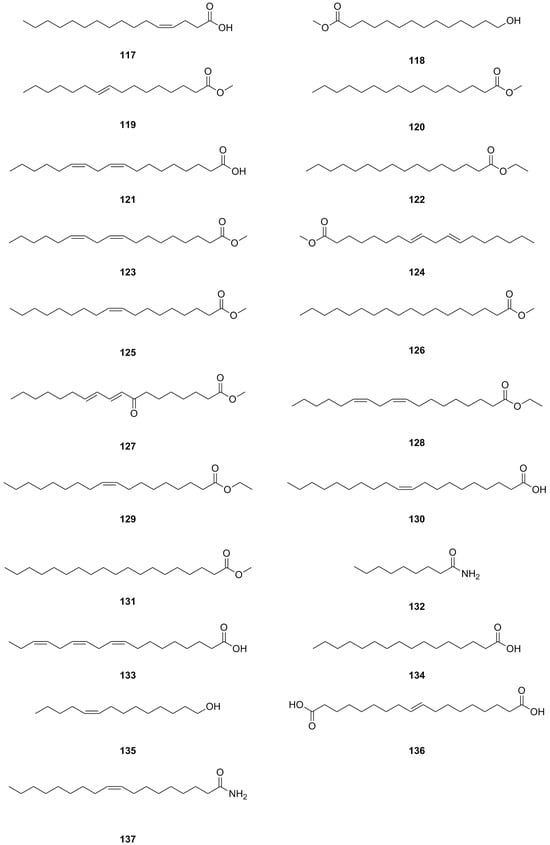
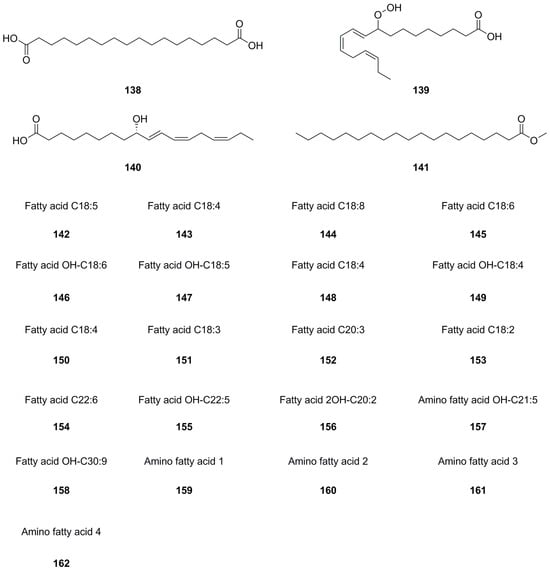
Figure 10.
Chemical structures of fatty acids and their derivatives isolated from Laportea bulbifera. Chemical structures were drawn using ChemDraw Professional 15.0 software.
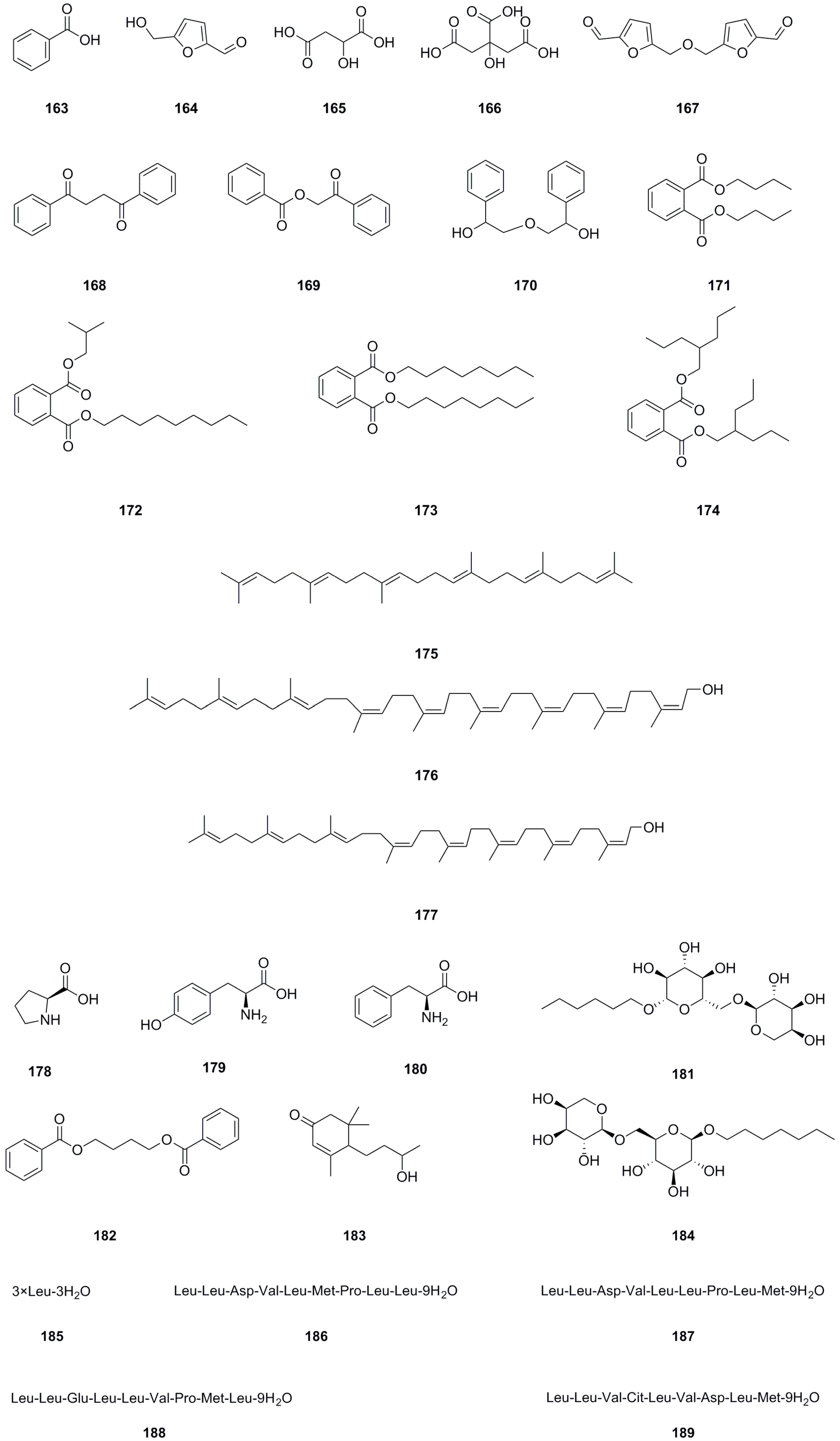
5.9. Others
In addition to the previously mentioned compound types, twenty-seven other compound types have been isolated and identified from different parts of L. bulbifera, including the roots, aerial parts, and the whole herb (Table 9, Figure 11). The roots contain three organic acids: benzoic acid (163), malic acid (165), and citric acid (166) [8]. The whole herb contains four phthalate esters: dibutyl phthalate (171), phthalic acid, isobutyl nonyl ester (172), dioctyl phthalate (173), and bis(2-propylpentyl) phthalate (174) [25,30]. Squalene (175) has been isolated from the roots [1]. Betulaprenol 9 (176) and betulaprenol 8 (177) have been isolated from the whole herb [10]. The roots also contain three amino acids, L-proline (178), L-tyrosine (179), and phenylalanine (180), as well as two alkyl glycosides: creoside IV (181) and heptyl 6-O-α-L-arabinopyranosyl-β-D-glucopyranoside (184) [8]. Additionally, five oligopeptides (185–189) have been identified from the whole herb.

Table 9.
Others isolated from Laportea bulbifera.
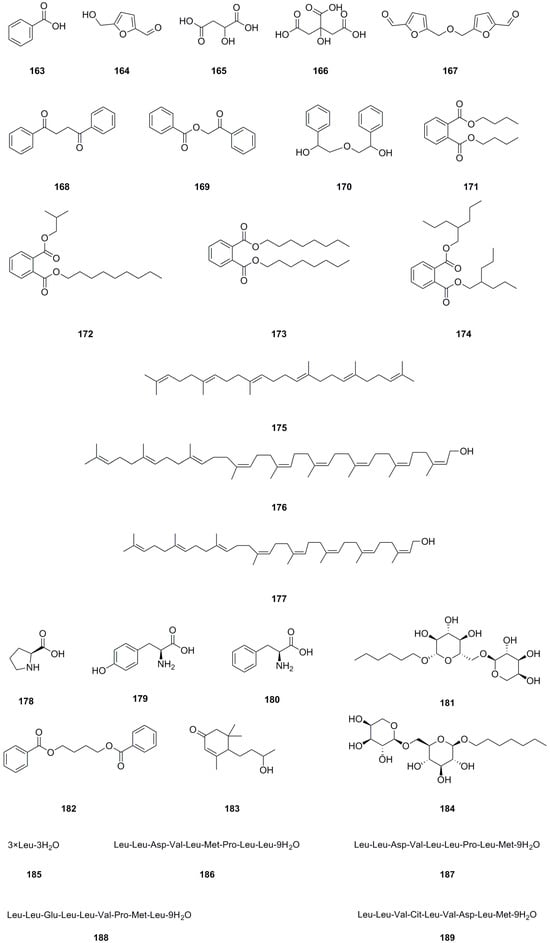
Figure 11.
Chemical structures of others isolated from Laportea bulbifera. Chemical structures were drawn using ChemDraw Professional 15.0 software.
6. Quality Control
For a long time, L. bulbifera has mainly relied on wild resources. However, with the increasing popularity of traditional Chinese medicine based on it, the demand has been growing year by year. Simultaneously, the wild resources have been gradually depleted, and their quality is inconsistent, thus failing to meet the application needs. Therefore, it is crucial to conduct prompt research on quality control. It is worth mentioning that the “Quality Standards for Traditional Chinese Medicine and Ethnomedicine in Guizhou Province” includes documentation on the whole herb of L. bulbifera. This standard only provides information on its name, source, characteristics, identification, nature and flavor, channel tropism, main functions, usage, dosage, and storage. Among these, microscopic identification and thin-layer chromatography (TLC) are used for identification, with β-sitosterol serving as the reference substance [43]. Nevertheless, the level of quality control is relatively low because β-sitosterol is not a characteristic compound and cannot represent the medicinal material’s quality.
Pharmacognostic research on L. bulbifera has been conducted in various studies [44,45]. These studies involve morphological identification; microscopic identification of roots, stems, and leaves; and the use of isorhamnetin-3-O-α-L-rhamnopyranosyl-(1-2)-β-galactopyranoside (47) as a characteristic compound. Furthermore, a characteristic fingerprint of L. bulbifera was established using HPLC to effectively differentiate it from similar varieties [45]. Researchers have also developed TLC and HPLC methods utilizing rutin (48) as the characteristic component. By determining the rutin content in L. bulbifera from different regions, they are able to evaluate the medicinal material’s quality [26]. Additionally, a study has established an HPLC method for the determination of multiple indicators: epicatechin (8), catechin (9), (−)-gallocatechin (12), and epigallocatechin (13) in L. bulbifera. This simple method could be employed for the quality control of L. bulbifera [25]. Furthermore, there are reports on the simultaneous determination of eleven components (flavonoids and phenylpropanoids) in L. bulbifera using UHPLC-ESI-MS, which could be utilized for quality control [46]. This method is currently the most comprehensive for quality control purposes. Researchers have also examined the content of total active ingredients in L. bulbifera, such as total flavonoids [26], total polysaccharides [47], or total coumarins, to evaluate the medicinal material’s quality [48]. Moreover, scholars have investigated quality-related parameters, including water content, total ash content, acid-insoluble ash content, ethanol-soluble extractives, heavy metals, harmful elements, and organochlorine pesticide residues in L. bulbifera [25,26].
Research has shown that L. bulbifera is rich in coumarins and exhibits significant therapeutic effects on arthritis [16]. Moreover, studies indicate a high content of catechins in L. bulbifera, resulting in notable anti-inflammatory effects [1]. Additionally, research findings demonstrate that L. bulbifera has a high flavonoid content and diverse flavonoid types, displaying potent antioxidant activity [11]. Nevertheless, there is significant variation in the results of these studies on active ingredients, with minimal intersections. The underlying reason for this outcome remains unclear and may be attributed to differences in the origin, medicinal parts, and processing methods of L. bulbifera. Future research should focus on strengthening the investigation of its chemical components to elucidate the compounds responsible for its pharmacological effects. Consequently, a correlation model based on spectral efficacy was established to identify quality markers that better reflect the quality of L. bulbifera.
7. Pharmacological Effects
As a medicinal plant, modern pharmacological studies have demonstrated various pharmacological effects of L. bulbifera, including antioxidant, anti-inflammatory, analgesic, hypoglycemic, and hypolipidemic activities, as well as toxicity.
7.1. Antioxidant Activity
Both the water and ethyl acetate extracts (100 μg/mL) of the roots from L. bulbifera, along with the forty-six isolated compounds (10 μM) from the root, were subjected to a 2,2-diphenyl-1-picrylhydrazyl (DPPH) assay, and most of them exhibited good antioxidant activity [1]. The petroleum ether extract, ethyl acetate extract, and water extract (1 g/mL) from forty-three batches of L. bulbifera demonstrated excellent antioxidant activity [13]. A study utilized the DPPH assay to determine the average scavenging rate of different polar extracts (1 mg/mL). The results indicated that the ethyl acetate extract (87.6%) > water extract (63.3%) > petroleum ether extract (36.8%). The ethyl acetate extract was identified as the active antioxidant extract of L. bulbifera using SPSS software (Version 16.0) for variance analysis [26]. Yang et al. isolated five flavonoids, with isorhamnetin-3-O-α-L-rhamnoside (51), isorhamnetin-3,7-O-α-L-dirhamnoside (46), and isorhamnetin-3-O-α-rhamnosyl-(1-2)-rhamnoside (49) showing DPPH scavenging ability (EC50 value) at 45, 20, and 55 μg/mL, respectively, which are comparable to L-ascorbic acid (11 μg/mL) [3]. Our previous research demonstrated that the antioxidant capacity of L. bulbifera root is significantly stronger than that of the aerial part. Through twelve antioxidant experiments, the methanol extract of L. bulbifera root exhibited the best performance among the tested extracts. Additionally, this extract could serve as an oxidative stabilizer for olive oil and sunflower oil, and it also has a protective effect on oxidative imbalance-related liver damage in rats [26].
7.2. Anti-Inflammatory and Analgesic Effects
Inflammation is a common pathological process in clinical practice. It is a defensive response that the body generates after tissue damage or invasion by pathogenic factors. It is essential for the occurrence and development of many diseases. Therefore, research on anti-inflammatory drugs is highly significant [49]. The ethyl acetate extracts (100 μg/mL) derived from the roots of L. bulbifera demonstrated significant inhibitory activity against cyclooxygenase-2 (COX-2), with an inhibitory rate of 60.7%. Out of the forty-six compounds (10 μM) isolated from the ethyl acetate extract, twenty-three compounds exhibited inhibitory rates higher than 50%. Among these, thirteen compounds displayed strong inhibitory activity with IC50 values lower than 1 μM. Notably, compounds such as (−)-epicatechin-3-O-gallate (21), hyperoside (40), rutin (48), quercetin (11), fisetin (6), and luteolin (5) (with IC50 values ranging from 0.13 to 0.24 μM) showed optimal COX-2 inhibitory potency. The inhibitory activity of flavonoids against COX-2 is influenced by the number and position of phenolic hydroxyl groups [1].
In a study using lipopolysaccharide (LPS) to stimulate a mouse macrophage RAW264.7 model, the effects of different extracts from L. bulbifera roots on NO release and their anti-inflammatory activity were examined. Results revealed that the dichloromethane extract, ethyl acetate extract, and n-butanol extract at concentrations of 15.5, 31.25, and 62.5 μg/mL, respectively, exerted a significant impact on NO release, with statistically significant differences observed. At a concentration of 62.5 μg/mL, the inhibitory effects of the petroleum ether extract, dichloromethane extract, ethyl acetate extract, and n-butanol extract on NO release were 11.42%, 21.01%, 33%, and 26.96%, respectively. Specifically, the ethyl acetate extract exhibited the most pronounced effect on NO release, and its impact was dose-dependent, demonstrating excellent anti-inflammatory activity [30]. Inflammatory cell models (RAW264.7) were utilized to evaluate the anti-inflammatory activities. Additionally, the petroleum ether extract (0.2, 2, 20 μg/mL) from L. bulbifera was assessed for its TNF-α inhibition activity. Further analysis is warranted for the thirty-five batches of petroleum ether extract exhibiting therapeutic effects under 2 μg/mL [13]. Several reports have explored the use of total coumarins derived from L. bulbifera roots (20, 40, and 60 mg/kg) to treat type II collagen-induced arthritis in Balb/c mice. The results demonstrated that treatment with total coumarins (60 mg/kg) led to a significant and dose-dependent reduction in clinical arthritis score and paw swelling. Pathological changes indicated that total coumarins protected tissues against bone destruction. This protective effect was associated with a considerable decrease in the production of IFN-γ and IL-2, an increase in IL-10 and TGF-β, and the suppressive expression of T-bet in dendritic cells. Additionally, total coumarins induced the generation of CD4+ CD25+ Treg cells expressing the Foxp3 phenotype. The dendritic cells treated with total coumarins displayed low expression of MHC class II and CD86 molecules, as well as reduced levels of IL-12p70. In summary, total coumarins exhibit significant protective effects and warrant further investigation and development as a potential anti-arthritis drug [16].
To evaluate the anti-rheumatoid arthritis effects of the serum, the human rheumatoid arthritis fibroblast-like synoviocyte line MH7A was cultured and treated with TNF-α (50 ng/mL) in vitro. The serum containing the whole herb of L. bulbifera was used to determine the proliferation and levels of inflammatory cytokines, such as prostaglandin E2 (PGE2), IL-1β, and IL-6, in the MH7A cells. The active components were identified based on the peak areas of common peaks and the results of the anti-rheumatoid arthritis effect test. The serum containing L. bulbifera significantly inhibited the proliferation of TNF-α-activated MH7A cells and the expression of PGE2, IL-6, and IL-1β. Thirty newly generated compounds were detected in the drug-containing serum. Among them, eight components were determined to enter the bloodstream as prototypes, and twelve components showed significant correlation with the pharmaceutical effect. Neochlorogenic acid (110), cryptochlorogenic acid (112), and chlorogenic acid (111) made significant contributions to the anti-rheumatoid arthritis activity [50].
The results of the experiment on anti-inflammatory activity showed that the swelling inhibition rate in mice treated with the 70% ethanol extract (20 g raw medicine/kg) of the whole herb from L. bulbifera was comparable to that of the positive group, with an inhibition rate greater than 50%. This inhibitory effect was better than that of the water extract. The test on analgesic activity showed that both the 70% ethanol extract group (20 g raw medicine/kg) and the water extract group (20 g raw medicine/kg) from the whole herb of L. bulbifera had an inhibitory effect on the number of twisting times in mice, but the former had a better effect. The experimental results also demonstrated that the pain threshold of mice increased by 34.2% after administration of the 70% ethanol extract (20 g raw medicine/kg), indicating its superior central analgesic effect caused by thermal stimulation compared to that of the water extract [25]. Studies also revealed that the ethyl acetate extract of L. bulbifera obtained similar results. It was found that the ethyl acetate extract could dose-dependently inhibit the proliferation of splenic T lymphocytes and the secretion of IL-2 and IFN-γ in the cell culture supernatant. These findings indicate that the ethyl acetate extract has a certain immunosuppressive effect and serves as the material basis for L. bulbifera’s anti-rheumatoid arthritis effect [15].
A study investigated the differences in intestinal absorption characteristics of L. bulbifera extract between normal and rheumatoid arthritis pathological states in rats. The absorption concentration of L. bulbifera extract was 5.0 mg/mL, and the UHPLC-MS/MS technique was used to detect the content of eight indicator components in the extract. The results revealed that all eight indicator components in the extract could be absorbed into the intestinal sac in a linear manner. The cumulative absorption time curve for each component showed a progressive increase without reaching saturation, suggesting a zero-order absorption rate process. It is suggested that the possible absorption mode for each component is passive diffusion, which provides a theoretical foundation for the development of oral dosage forms. Under normal conditions, the ileum (except for chlorogenic acid) exhibited the highest absorption of various components, while under pathological conditions, the duodenum showed the highest absorption. Additionally, the overall absorption of the eight components in each intestinal segment of rats with rheumatoid arthritis was higher than that of normal rats, suggesting that rheumatoid arthritis may alter the specific site of drug absorption [51].
In another study, the inhibitory effect of four isolated steroids from the whole herb of L. bulbifera on NO activity was evaluated using a mouse RAW264.7 cell model. The results indicated that the four steroid compounds (50 μg/mL) significantly reduced the production of NO in the model cells, with inhibition rates ranging from 27.41% to 40.10%. Among them, ergosterone exhibited the highest efficacy, suggesting that steroids may contribute to the anti-inflammatory properties of L. bulbifera [10].
A study utilized the LPS assay to determine the average anti-inflammatory activity of different polar extracts (1 mg/mL). The results showed that the petroleum ether extract (15.38%) exhibited the highest anti-inflammatory activity, followed by the ethyl acetate extract (7.91%) and the water extract (2.60%). The petroleum ether extract was identified as the active anti-inflammatory extract of L. bulbifera using SPSS software (Version 16.0) for variance analysis [26]. Another report also confirmed the potent anti-inflammatory effects of the petroleum ether extract [13]. Research findings suggest that (E)-4-coumaric acid (116) and caffeic acid (106) in L. bulbifera possess anti-inflammatory activity and can be absorbed into the bloodstream. These components are likely to be the effective anti-inflammatory compounds of L. bulbifera [8].
The results of a different study demonstrated that the ethyl acetate extract from L. bulbifera (at concentrations of 0.5, 1.0, and 1.5 mg/10 g) effectively inhibited the onset of inflammation and joint tissue lesions. It exhibited a favorable therapeutic effect on rheumatoid arthritis, as evidenced by the arthritis index, arthritis incidence rate, spleen index, toe swelling, and pathological photos. The ethyl acetate extract (at concentrations of 0.5, 1.0, and 1.5 mg/10 g) did not influence changes in surface antigens of dendritic cells, but it reduced the expression of T-bet and inhibited IFN-γ secretion while promoting IL-10 secretion. It also affected T cells by inhibiting T-bet expression and promoting GATA-3 expression, thereby enhancing the secretion of IL-4 and IL-10 while inhibiting the expression of IFN-γ and IL-2 to prevent the onset of rheumatoid arthritis [52].
In a study investigating the effects of total coumarins from L. bulbifera on mice with dextran sulfate sodium-induced colitis, it was found that intervention with different doses of total coumarins (37.5, 75, 150 mg/kg) significantly improved colitis symptoms. This improvement was characterized by stable weight gain, reduced damage to the intestinal mucosa, decreased infiltration of inflammatory cells, and the absence of diarrhea or bloody stools. Further research revealed that total coumarins were able to regulate the expression of pro-inflammatory and anti-inflammatory cytokines, as well as reduce the levels of TLR4 and NF-κB in colon tissue. Moreover, no common adverse reactions such as weight loss, infection, or organ damage were observed during the administration of total coumarins. Therefore, this study provides a theoretical foundation for the development and usage of total coumarins of L. bulbifera as immunosuppressants [12].
The immunosuppressive activity of various compounds was assessed using the Cell Counting Kit-8 assay, and the results showed that 6,6′,7,7′-tetramethoxyl-8,8′-biscoumarin (99), 7,7′-dihydroxy-6,6′-dimethoxy-8,8′-biscoumarin (98), 7,7′-dimethoxy-6,6′-biscoumarin (97), and scoparone (94) exhibited immunosuppressive activity, with compound 99 showing particularly strong effects. Additionally, compound 99 (IC50, 5.19 × 10−4 mol/L) significantly enhanced the differentiation of CD4+CD25+Foxp3+ T regulatory cells compared to the normal control, as evidenced by FACS analysis. Therefore, compound 99 possesses specific immunosuppressive properties and holds potential as a therapeutic strategy for autoimmune diseases [4].
In another study, the immunosuppressive effects of the ethyl acetate extract from L. bulbifera were investigated in a murine model of skin allograft rejection. The model involved transplanting skin allografts from C57BL/6 mice onto the wound bed of Balb/c mice. The results demonstrated a significant dose-dependent prolongation of skin allograft survival in animals treated with the ethyl acetate extract. FACS analysis revealed that treatment with the extract (200 mg/kg) led to an immature state of dendritic cells and stimulated the differentiation of CD4+CD25+ Tregs. Moreover, the extract efficiently reduced T-bet gene expression and spleen lymphocyte proliferation in treated mice. In comparison to the model control, recipients treated with the extract exhibited significant downregulation of Th1 cytokines (IL-2, IFN-γ) and a notable increase in Th2 cytokine (IL-10) levels in the serum, with a dose-related pattern. These findings suggest that the ethyl acetate extract has anti-allograft rejection properties by promoting CD4+CD25+ Tregs differentiation and maintaining the immaturity of dendritic cells, thereby inducing a stable immunological tolerance state. This highlights its potential for the treatment of autoimmune diseases [14].
7.3. Hypoglycemic and Hypolipidemic Activity
To investigate the effects of total coumarins on diabetes, eight-week-old non-obese diabetic (NOD) mice were divided into four groups: a control group and low-dose (37.5 mg/kg), middle-dose (75 mg/kg), and high-dose (150 mg/kg) total coumarin treatment groups. The results demonstrated that treatment with total coumarins for four weeks significantly inhibited insulitis, increased pancreatic islet number, delayed the onset, and reduced the development of diabetes by twenty-six weeks of age in NOD mice compared to untreated control mice. Total coumarins also suppressed spleen T-lymphocyte proliferation, induced a Th2-biased cytokine response, promoted the generation of CD4+CD25+Foxp3+ Tregs, and increased Foxp3 mRNA expression. Furthermore, dendritic cells treated with total coumarins exhibited low expression of MHC class II and CD86 molecules. The expressions of the TLR4 gene and protein expressions in the spleen, thymus, and pancreas were downregulated in the groups treated with total coumarins. Key molecules involved in the downstream signaling cascades of TLR4, such as myeloid differentiation factor 88 (MyD88), NF-κB, IL-1β, TRIF, TRAM, IRF-3, and IFN-β, all showed significant decreases in the total coumarins groups. This suggests that total coumarins inhibit both MyD88-dependent and -independent pathways of TLR4. At the cellular level, TLR4 protein expression was found to be downregulated by total coumarins in dendritic cells but not in Tregs. Furthermore, total coumarins enhanced the role of dendritic cells, rather than Tregs, in negative immune regulation in vitro. This effect on dendritic cell immune function was reversed by anti-TLR4 antibody. Therefore, the total coumarins from L. bulbifera can prevent autoimmune diabetes in mice by inhibiting the TLR4 signaling pathway [53].
To establish a model of insulin resistance type II diabetes, BALB/c mice were fed a high-fat diet and injected with small doses of STZ. The effects of different concentrations of total flavonoids of L. bulbifera (25, 50, 100 mg/kg) on the blood glucose concentration of the diabetic model were observed through daily intragastric administration. The results indicated that the total flavonoids group significantly reduced blood sugar levels in mice compared to the model group. Pancreatic HE staining showed no significant difference between the groups. The low-dose group demonstrated a significant effect in reducing triglycerides, total cholesterol, and the insulin resistance index. It also improved glucose tolerance in insulin-resistant mice. Insulin measurement results showed a significant increase in insulin levels only in the high-dose group. SOD and MDA levels did not show significant changes in any of the groups. Additionally, immunoblotting results for insulin receptors and PPAR-γ showed that the low-dose group of total flavonoids increased the expression of insulin receptor levels. These results demonstrate that total flavonoids exert a hypoglycemic and hypolipidemic effect by upregulating insulin receptor levels and increasing insulin sensitivity rather than affecting the free radical pathway [54].
In another study, male Kunming mice were fed a high-fat diet for two weeks to establish a model of hypercholesterolemia. L. bulbifera was extracted and separated using macroporous resin to obtain four fractions: water fraction, 30% ethanol fraction, 70% ethanol fraction, and 95% ethanol fraction. Each fraction was administered by gavage at a dose of 40 mg/g, and serum biochemical indicators were measured after four weeks. Liver sections were stained for observation. The experimental results showed that both the 30% ethanol fraction and 70% ethanol fraction significantly reduced body weight and serum levels of total cholesterol, low-density lipoprotein cholesterol, and MDA in hypercholesterolemic mice. They also increased the levels of SOD in experimental hypercholesterolemic mice. Staining results of mouse liver cells revealed that the liver tissue sections of mice treated with the 30% ethanol fraction and 70% ethanol fraction showed normal liver cells around the central vein, indicating that these fractions could protect and repair the liver tissue of hypercholesterolemic mice. In summary, the 30% ethanol fraction and 70% ethanol fraction of L. bulbifera could regulate blood lipid metabolism in experimental hypercholesterolemic mice and significantly reduce their blood lipid levels [5].
7.4. Other Pharmacological Effects
The inhibitory effect of seventeen isolated compounds on human steroid 5α-reductase 2 (SRD5α2) was evaluated using molecular docking methods. The findings revealed that the compound with the most significant inhibition at the active sites of SRD5α2 was 5,7,3′-trihydroxy-4-methoxyisoflavone-7-O-β-D-glucopyranoside (29), followed by 5,7,4-trihydroxy-isoflavone-5-O-β-D-glucopyranoside (25), kaemferitrin (43), genistin (26), and apigenin (3). These results provide theoretical evidence supporting the application of L. bulbifera in the treatment of benign prostatic hyperplasia [11].
Thirteen flavonoids isolated from the aerial parts of L. bulbifera were evaluated for their inhibitory activity against N1 neuraminidase. Among them, kaempferol-3-O-β-D-glucopyranoside (31), kaemferitrin (43), and quercetin-3-O-β-D-6″-acetylglucopyranoside (42) (at concentrations of 50, 100, and 200 μmol/L) exhibited significantly stronger inhibitory effects compared to the other ten compounds. This suggests that the activity of flavonols surpasses that of flavonoids and isoflavones [2].
7.5. Toxicity
There are records indicating that L. Bulbifera has minor toxicity, although ethnic doctors generally consider it non-toxic [55]. Research reports have demonstrated that the oral administration of a water decoction and powder suspension of L. bulbifera to mice exhibited a minimum lethal dose greater than 50 g/kg and 1.67 g/kg, respectively [44]. In our previous oral acute toxicity experiments, we observed high safety when mice were administered L. bulbifera via gavage (2000 g/kg), as no mouse deaths occurred within 24 h [27].
8. Discussion
Firstly, this manuscript provides a comprehensive overview of the chemical composition of L. bulbifera, a traditional ethnomedicine. The analysis reveals that L. bulbifera is abundant in flavonoids and fatty acids, two crucial phytochemicals known for their potent antioxidant properties. These compounds exhibit the ability to neutralize free radicals, thereby mitigating cellular damage caused by oxidative stress [28,42]. Moreover, they also possess significant anti-inflammatory effects by effectively suppressing the release of inflammatory pathways and cytokines [29,56]. In fact, studies have found that flavonoids and phenolics could effectively ameliorate rheumatoid arthritis, a chronic inflammatory disorder [57]. Additionally, evidence suggests that fatty acids play a vital role in the prevention and treatment of rheumatoid arthritis [58]. Therefore, considering the aforementioned findings, it could be inferred that the therapeutic effects of L. bulbifera in mitigating rheumatic arthritis, fractures, and falling injuries are primarily attributed to its rich content of flavonoids and fatty acids.
Additionally, two important issues related to quality control need to be addressed. Firstly, there is variation in the methods used to determine the chemical components in L. bulbifera. Different compounds, such as β-sitosterol [43], flavonoids (isorhamnetin-3-O-α-L-rhamnopyranosyl-(1-2)-β-galactopyranoside (47), rutin (48) [26], and catechins [25]), and flavonoids in combination with phenylpropanoids [46], have been measured to assess the quality of L. bulbifera. However, these research studies lack systematicity, making it unclear which components truly reflect the quality of L. bulbifera. Secondly, the established indicators for quality control of L. bulbifera have not been based on their pharmacological substance basis and quality markers. As a result, the exclusive analysis of active ingredients is lacking, compromising the ability to accurately reflect and evaluate the quality of L. bulbifera. Given the increasing market demand for L. bulbifera, ensuring its safety and effectiveness from the source is crucial. To achieve this, researchers should explore the anti-inflammatory material basis of L. bulbifera, clarify its mechanism of action, and establish the relationship between its anti-inflammatory spectrum and effects. It is also important to screen and identify quality biomarkers that can faithfully represent the quality of L. bulbifera. Addressing these issues is vital in maintaining the stable and reliable quality of L. bulbifera, thus meeting the growing demand for this medicinal plant.
Furthermore, coumarins and flavonoids have been identified as significant components in the treatment of arthritis and inflammation, respectively [1,11,16]. These two phytochemicals exhibit distinct active properties, indicating that they play different roles in the treatment process. Therefore, we believe that the origin and specific medicinal parts of L. bulbifera represent the primary influencing factors. It is well-known that numerous environmental elements, including growth conditions, geographical location, and habitat, can result in variations in plant composition. Factors like plant growth environment, soil quality, climate conditions, and light intensity may vary across different regions, leading to diverse chemical compositions and contents in the same plant species. Consequently, medicinal plants grown in different habitats may exhibit dissimilar ingredient profiles and quantities, potentially resulting in varied pharmacological and clinical effects within different regions. Additionally, the medicinal parts utilized can significantly impact the therapeutic outcomes. Our previous investigations, supported by the literature, have demonstrated that the roots possess superior antioxidant capacity compared to the aerial parts [27]. However, previous studies have employed a variety of medicinal parts, including roots [1], aerial parts [11], and the whole herb [25], contributing to disparate findings.
Moving forward, several crucial avenues of research should be pursued regarding L. bulbifera. Firstly, a more extensive exploration of its chemical composition is warranted to elucidate the specific substances responsible for its pharmacological effects. Secondly, a comprehensive analysis of its pharmacological mechanisms should be conducted to offer theoretical guidance and technical support for drug development and clinical application. Subsequently, quality control measures must be implemented to ensure the consistency and reliability of therapeutic effects. Finally, it is essential to systematically validate and optimize traditional applications of L. bulbifera, harnessing its full potential and broadening its prospects for practical use.
9. Conclusions
However, there is currently a lack of comprehensive and detailed documentation on the ethnomedicinal uses, geographical distribution, botanical description, phytochemistry, pharmacology, and quality control of L. bulbifera. Consequently, the primary objective of this review is to comprehensively explore existing research on L. bulbifera by examining multiple databases and addressing these aforementioned aspects. Furthermore, this review will identify potential areas for future research, such as isolating and identifying additional compounds found in L. bulbifera, conducting more extensive pharmacological evaluations, elucidating its mechanisms of action, and ultimately establishing a more robust quality control system. The outcomes of this research will serve as a solid basis for the quality control, product development, and clinical application of L. bulbifera.
Author Contributions
Conceptualization and original draft preparation: L.L., J.F., G.X. and J.Z.; reviewing and editing: H.Z.; supervision: L.L. and H.Z. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Project of The Education Department of Jilin Province (No. JJKH20240652KJ).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are contained within the article.
Acknowledgments
We thank Junlin Yu from Tonghua Normal University for providing pictures of Laportea bulbifera.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Wang, M.M.; Li, Y.N.; Ming, W.K.; Wu, P.F.; Yi, P.; Gong, Z.P.; Hao, X.J.; Yuan, C.M. Bioassay-guided isolation of human carboxylesterase 2 inhibitory and antioxidant constituents from Laportea bulbifera: Inhibition interactions and molecular mechanism. Arab. J. Chem. 2022, 15, 103723. [Google Scholar] [CrossRef]
- Zhang, Y.; Lu, X.; Li, B.; Yu, D.Y.; Feng, B.M. Flavonoids from Laportea bulbifera and their anti-N1 neuraminidase activities. J. Shenyang Pharm. Univ. 2018, 35, 931–935+942. [Google Scholar]
- Yang, M.C.; Choi, S.Z.; Lee, S.O.; Chung, A.K.; Nam, J.H.; Lee, K.H.; Lee, K.R. Flavonoid constituents and their antioxidant activity of Laportea bulbifera Weddell. Korean J. Pharmacogn. 2003, 34, 18–24. [Google Scholar]
- Hou, W.R.; Su, Z.Q.; Pi, H.F.; Yao, G.M.; Zhang, P.; Luo, X.; Xie, S.N.; Xiang, M. Immunosuppressive constituents from Urtica dentata Hand. J. Asian Nat. Prod. 2010, 12, 707–713. [Google Scholar] [CrossRef] [PubMed]
- Shi, Q.X. Study on the Mechanism of Anti-Hyperlipidemia Effects and Its Pharmacodynamics Substantial Foundation of Laportea bulbifera. Master’s Thesis, Hubei University of Chinese Medicine, Wuhan, China, 2018. [Google Scholar]
- Wang, Y.M.; Wen, H.C.; Dou, D.Q. Chemical constituents from Laportea bulbifera, a Zhuang medicine. Chin. Pharm. J. 2019, 54, 773–776. [Google Scholar]
- Tang, J.; Wu, D.; Chen, S.Y.; Li, Y.; Li, J.; Gong, Z.P.; Li, W.W.; Lan, Y.Y.; Wang, Y.L. Identification of chemical compositions in Laportea bulbifera by UPLC-ESI-Q-TOF-MS. Chin. J. Exp. Tradit. Med. Formulae. 2018, 24, 67–72. [Google Scholar] [CrossRef]
- Han, H.Y. Study on the Anti-Inflammatory Material Basis and Preliminary Metabolism In Vivo of the Ethnic Medicine Laportea bulbifera. Master’s Thesis, Beijing University of Chinese Medicine, Beijing, China, 2018. [Google Scholar]
- Zhu, Z.; Ma, L.; Zhu, H.Y.; Yang, X.S.; Hao, X.J. Studies on the chemical constituents of Laportea bulbifera. Chin. Med. Mat. 2011, 34, 223–225. [Google Scholar] [CrossRef]
- Xu, L.J. Study on Chemical Constituents and Bioactivities of Two National Medicinal Plants. Master’s Thesis, China State Institute of Pharmaceutical Industry, Shanghai, China, 2018. [Google Scholar]
- Lu, X.; Zhao, Y.; Li, B.; Feng, W.; Qi, J.; Feng, B. Phytochemical, chemotaxonomic and bioinformatics study on Laportea bulbifera (Urticaceae). Chem. Biodivers. 2022, 19, 202200070. [Google Scholar] [CrossRef]
- Lu, J.L.; Li, W.J.; Hou, W.R.; Lan, Y.; Zhou, H.; Yi, L.J.; Zeng, Y.; Xiang, M. Study on effect of total coumarins from Urtica dentata on dextran sulfate sodium-induced colitis in mice. China J. Chin. Mater. Med. 2012, 37, 3316–3320. [Google Scholar] [CrossRef]
- Chen, Y.R.; Zou, S.H.; Xu, W.F.; Sun, Q.W.; Yun, L. Spectrum–effect relationship of antioxidant and antiinflammatory activities of Laportea bulbifera based on multivariate statistical analysis. Biomed. Chromatogr. 2019, 34, e4734. [Google Scholar] [CrossRef]
- Xiang, M.; Hou, W.R.; Xie, S.N.; Zhang, W.D.; Wang, X. Immunosuppressive effects of an ethyl acetate extract from Urtica dentata Hand on skin allograft rejection. J. Ethnopharmacol. 2009, 126, 57–63. [Google Scholar] [CrossRef] [PubMed]
- Su, Z.Q.; Zhao, Z.Y.; Xie, S.N.; Hou, W.R.; Tao, E.; Xiang, M. Effects of analgesia, anti-inflammation and immunosuppression of acetic ether extract of Chinese medicine honghuoma. Chin. Pharmacol. Bull. 2009, 25, 559–560. [Google Scholar]
- Luo, X.; Li, L.L.; Zhang, S.S.; Lu, J.L.; Zeng, Y.; Zhang, H.Y.; Xiang, M. Therapeutic effects of total coumarins from Urtica dentata Hand on collagen-induced arthritis in Balb/c mice. J. Ethnopharmacol. 2011, 138, 523–529. [Google Scholar] [CrossRef] [PubMed]
- Hu, H.J.; Tang, J.; Chen, S.Y.; Wu, D.; Xiao, H.Q.; Lan, Y.Y.; Wang, A.M.; Xi, X.L.; Gong, Z.P. Study on quality control of Miao medicine Laportea bulbifera based on fingerprint analysis and quantitative analysis of multi-components. Chin. Tradit. Herb. Drugs 2020, 51, 4325–4330. [Google Scholar]
- Yang, H.J.; Li, G. Report on the Scientific and Technological Competitiveness of Large Varieties of Traditional Chinese Medicine; The People’s Medical Publishing House: Beijing, China, 2019. [Google Scholar]
- Flora of China Editorial Committee of Chinese Academy of Sciences. The Flora of China; Science Press: Beijing, China, 1995; Volume 23, pp. 31–32. [Google Scholar]
- Xie, A.; Su, Y.J.; Luo, G.; Wu, Q.; Wen, H.C. Research progress on chemical constituents and pharmacological effects of Laportea bulbifera. Agric. Biotechnol. 2021, 10, 101–104. [Google Scholar] [CrossRef]
- Editorial Committee of Chinese Materia Medica, State Administration of TCM. Chinese Materia Medica (Zhonghua Bencao). In Miao’s Material Medica; Guizhou Science and Technology Publishing House: Guiyang, China, 2005. [Google Scholar]
- Fu, L.G.; Chen, T.Q.; Lang, K.Y.; Hong, T.; Lin, Q. Higher Plants of China; Qingdao Publishing House: Qingdao, China, 2000; Volume 4. [Google Scholar]
- Institute of Botany, the Chinese Academy of Sciences. Iconographia Cormophytorum Sinicorum; Science Press: Beijing, China, 1972; Volume 1. [Google Scholar]
- Li, B.; Lu, X.; Feng, B.M.; Yu, D.Y.; Wang, H.G.; Shi, L.Y.; Yu, Z.X.; Qin, H.H. Flavonoids isolated from Laportea bulbifera. In Proceedings of the 11th National Conference on Natural Organic Chemistry of the Chinese Chemical Society, Shanghai, China, 25–28 September 2016; Chinese Chemical Society: Beijing, China, 2016; Volume 3. [Google Scholar]
- Wang, S.L. Material Basis for the Anti-Inflammatory and Analgesic Effects of Laportea bulbifera and Its Quality Control. Master’s Thesis, Guiyang Medical College, Guiyang, China, 2015. [Google Scholar]
- Zou, S.H. Preliminary Study on Quality Control and Spectral Effect Relationship of Antioxidant and Anti-Inflammatory Activities of Medicinal Materials of Miao Medicine Honghema. Master’s Thesis, Guiyang College of Traditional Chinese Medicine, Guiyang, China, 2017. [Google Scholar]
- Feng, J.X.; Sun, Y.; Wei, Z.B.; Sun, H.; Li, L.; Zhu, J.Y.; Xia, G.Q.; Zang, H. Screening the extract of Laportea bulbifera (Sieb. et Zucc.) Wedd. based on active component content, its antioxidant capacity and exploration of hepatoprotective activity in rats. Molecules 2023, 28, 6256. [Google Scholar] [CrossRef] [PubMed]
- Shen, N.; Wang, T.; Gan, Q.; Liu, S.; Wang, L.; Jin, B. Plant flavonoids: Classification, distribution, biosynthesis, and antioxidant activity. Food Chem. 2022, 383, 132531. [Google Scholar] [CrossRef] [PubMed]
- Al-Khayri, J.M.; Sahana, G.R.; Nagella, P.; Joseph, B.V.; Alessa, F.M.; Al-Mssallem, M.Q. Flavonoids as potential anti-inflammatory molecules: A review. Molecules 2022, 27, 2901. [Google Scholar] [CrossRef]
- Han, H.Y.; Suo, Y.R.; Liu, X.; Wu, Y.Q.; Dai, Y.H.; Ni, Y.Y.; Yang, R.R.; Qiao, Y.H.; Ma, Z.Q.; Lin, R.C. Screening of active components of Urtica dentata Hand by RAW264.7 anti-inflammatory cell model and chemical constituents. Glob. Tradit. Chin. Med. 2018, 11, 651–655. [Google Scholar] [CrossRef]
- de Lima Cherubim, D.J.; Buzanello Martins, C.V.; Oliveira Fariña, L.; da Silva de Lucca, R.A. Polyphenols as natural antioxidants in cosmetics applications. J. Cosmet. Dermatol. 2020, 19, 33–37. [Google Scholar] [CrossRef]
- Finamore, A.; Palmery, M.; Bensehaila, S.; Peluso, I. Antioxidant, immunomodulating, and microbial-modulating activities of the sustainable and ecofriendly spirulina. Oxid. Med. Cell. Longev. 2017, 2017, 3247528. [Google Scholar] [CrossRef] [PubMed]
- Babu, S.; Jayaraman, S. An update on β-sitosterol: A potential herbal nutraceutical for diabetic management. Biomed. Pharmacother. 2020, 131, 110702. [Google Scholar] [CrossRef] [PubMed]
- Antika, L.D.; Tasfiyati, A.N.; Hikmat, H.; Septama, A.W. Scopoletin: A review of its source, biosynthesis, methods of extraction, and pharmacological activities. Z. Naturforsch. C J. Biosci. 2022, 77, 303–316. [Google Scholar] [CrossRef] [PubMed]
- Hui, Y.; Wang, X.; Yu, Z.; Fan, X.; Cui, B.; Zhao, T.; Mao, L.; Feng, H.; Lin, L.; Yu, Q.; et al. Scoparone as a therapeutic drug in liver diseases: Pharmacology, pharmacokinetics and molecular mechanisms of action. Pharmacol. Res. 2020, 160, 105170. [Google Scholar] [CrossRef] [PubMed]
- Naveed, M.; Hejazi, V.; Abbas, M.; Kamboh, A.A.; Khan, G.J.; Shumzaid, M.; Ahmad, F.; Babazadeh, D.; Xia, F.F.; Modarresi-Ghazani, F.; et al. Chlorogenic acid (CGA): A pharmacological review and call for further research. Biomed. Pharmacother. 2018, 97, 67–74. [Google Scholar] [CrossRef] [PubMed]
- Muhammad Abdul Kadar, N.N.; Ahmad, F.; Teoh, S.L.; Yahaya, M.F. Caffeic acid on metabolic syndrome: A review. Molecules 2021, 26, 5490. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Zhang, Q.; Liu, G.; Zhang, N. Therapeutic potentials and mechanisms of the Chinese traditional medicine danshensu. Eur. J. Pharmacol. 2019, 864, 172710. [Google Scholar] [CrossRef]
- Calder, P.C. Functional roles of fatty acids and their effects on human health. JPEN J. Parenter. Enter. Nutr. 2015, 39, 18S–32S. [Google Scholar] [CrossRef]
- Calder, P.C.; Willemsen, L.E.M. Immunopharmacology of fatty acids. Eur. J. Pharmacol. 2016, 785, 1. [Google Scholar] [CrossRef]
- Marangoni, F.; Agostoni, C.; Borghi, C.; Catapano, A.L.; Cena, H.; Ghiselli, A.; La Vecchia, C.; Lercker, G.; Manzato, E.; Pirillo, A.; et al. Dietary linoleic acid and human health: Focus on cardiovascular and cardiometabolic effects. Atherosclerosis 2020, 292, 90–98. [Google Scholar] [CrossRef]
- Yuan, Q.; Xie, F.; Huang, W.; Hu, M.; Yan, Q.; Chen, Z.; Zheng, Y.; Liu, L. The review of alpha-linolenic acid: Sources, metabolism, and pharmacology. Phytother. Res. 2022, 36, 164–188. [Google Scholar] [CrossRef] [PubMed]
- Guizhou Medical Products Administration. Quality Standards for Traditional Chinese Medicine and Ethnomedicine in Guizhou Province (2019 Edition); China Medical Science Press: Beijing, China, 2022; Volume 2. [Google Scholar]
- Wan, D.R.; Feng, S.Q.; Li, A.J. Pharmacognostic identification of ethnic medicine honghuoma and huoma. Chin. Tradit. Herb. Drugs 1989, 20, 34–36. [Google Scholar]
- Sun, Q.W.; Xu, W.F.; Qi, W.N.; Bai, C.H.; Wei, S.H. Study on the pharmacognostic identification of Miao herbs honghema and confusion varieties aima. J. Chin. Med. Mater. 2015, 38, 1862–1867. [Google Scholar] [CrossRef]
- Wu, D.; Tang, J.; Li, Y.; Li, J.; Chen, S.Y.; Gong, Z.P.; Li, Y.J.; Wang, A.M.; Li, W.W.; Wang, Y.L.; et al. Simultaneous determination of 11 constituents in Laportea bulbifera of Miao medicine by UPLC-ESI-MS. Chin. J. Pharm. Anal. 2019, 39, 1425–1432. [Google Scholar] [CrossRef]
- Zou, S.H.; Yang, Y.; Wen, D.; Chen, Y.R.; Xu, W.F.; Wei, S.H. Correlation analysis on polysaccharide content of medicinal material of Miao National Herbs Laportea bulbifera germplasm with environmental factors. Seed 2016, 35, 60–65. [Google Scholar] [CrossRef]
- Xiang, M.; Lu, J.; Zhang, C.; Lan, Y.; Zhou, H.; Li, X.; Peng, W. Identification and quantification of total coumarins from Urtica dentata Hand and its roles in promoting immune tolerance via TLR4-mediated dendritic cell immaturation. Biosci. Biotechnol. Biochem. 2013, 77, 1200–1206. [Google Scholar] [CrossRef]
- Zhu, F.; Du, B.; Xu, B. Anti-inflammatory effects of phytochemicals from fruits, vegetables, and food legumes: A review. Crit. Rev. Food Sci. Nutr. 2018, 58, 1260–1270. [Google Scholar] [CrossRef]
- Tang, J.; Zhang, Q.; Wu, D.; Chen, S.Y.; Chen, Y.; Li, Y.T.; Zheng, L.; Huang, Y.; Lan, Y.Y.; Wang, Y.; et al. Potential pharmacodynamic substances of Laportea bulbifera in treatment of rheumatoid arthritis based on serum pharmacochemistry and pharmacology. China J. Chin. Mater. Med. 2022, 47, 4755–4764. [Google Scholar] [CrossRef]
- Li, Y.; Chen, S.Y.; Gong, Z.P.; Kang, N.F.; Wu, D.; Tang, J.; Li, Y.T.; Pan, J.; Huang, Y.; Zheng, L.; et al. Differences in intestinal absorption characteristics of Laportea bulbifera extract in normal and rheumatoid arthritis model rats by isolated everted intestine model. China J. Chin. Mater. Med. 2020, 45, 405–411. [Google Scholar] [CrossRef]
- Yao, Y. Study on Effects and Mechanism of the Active Fraction of Honghuoma in Rheumatoid Arthritis. Master’s Thesis, Huazhong University of Science and Technology, Wuhan, China, 2008. [Google Scholar]
- Wang, J.; Lu, J.; Lan, Y.; Zhou, H.; Li, W.; Xiang, M. Total coumarins from Urtica dentata Hand prevent murine autoimmune diabetes via suppression of the TLR4-signaling pathways. J. Ethnopharmacol. 2013, 146, 379–392. [Google Scholar] [CrossRef]
- Zhao, Z.Y. Preparation and Anti-T2DM-IR Effects of Flavonoid Rich Extract from Urtica dentata Hand. Master’s Thesis, Huazhong University of Science and Technology, Wuhan, China, 2012. [Google Scholar]
- Hubei Provincial Revolutionary Committee Health Bureau. Hubei Chinese Herbal Medicine Record (II); Hubei People’s Press: Wuhan, China, 1982. [Google Scholar]
- Calder, P.C. Omega-3 fatty acids and inflammatory processes: From molecules to man. Biochem. Soc. Trans. 2017, 45, 1105–1115. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Chen, S.; Du, K.; Liang, C.; Wang, S.; Owusu Boadi, E.; Li, J.; Pang, X.; He, J.; Chang, Y.X. Traditional herbal medicine: Therapeutic potential in rheumatoid arthritis. J. Ethnopharmacol. 2021, 279, 114368. [Google Scholar] [CrossRef] [PubMed]
- Yi, W. Exploration of the Effects of N-3 Polyunsaturated Fatty Acids on the Prevention and Treatment of Rheumatoid Arthritis and Related Mechanisms. Master’s Thesis, Ningbo University, Ningbo, China, 2023. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).