Ameliorative Effect of Ginsenoside Rg6 in Periodontal Tissue Inflammation and Recovering Damaged Alveolar Bone
Abstract
:1. Introduction
2. Results
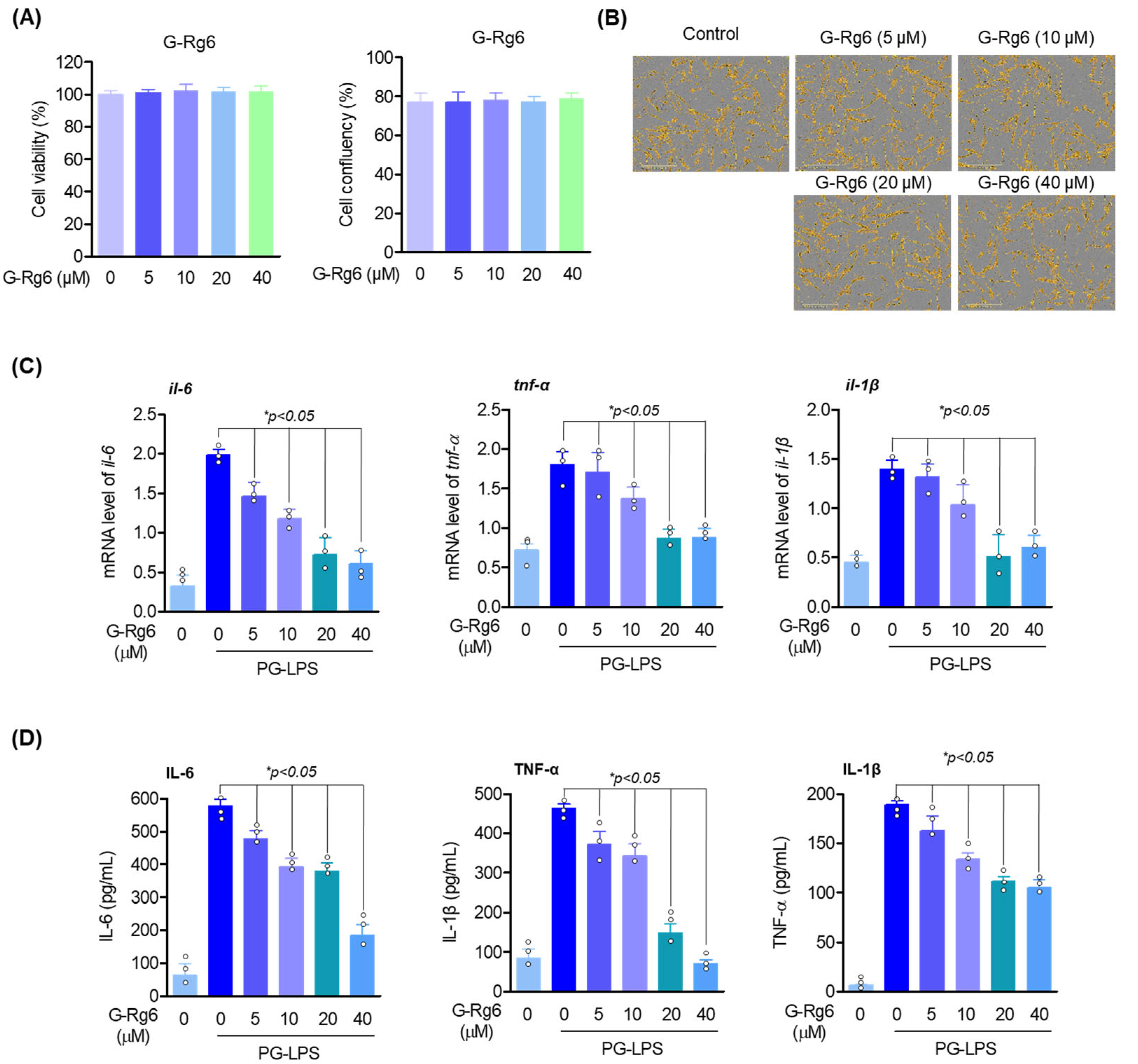
2.1. Cytotoxicity Assay and Analysis of Inflammatory Factors in Inflammatory Cytokines
2.2. Exploration of Osteoblast Differentiation-Inducing Effects of G-Rg6
2.3. Exploring the Antioxidant Effects of G-Rg6
2.4. An Antibacterial Effects of P. gingivalis on G-Rg6
2.5. Inhibitory Effect of G-Rg6 on Periodontitis in a Ligature-Induced In Vivo Model
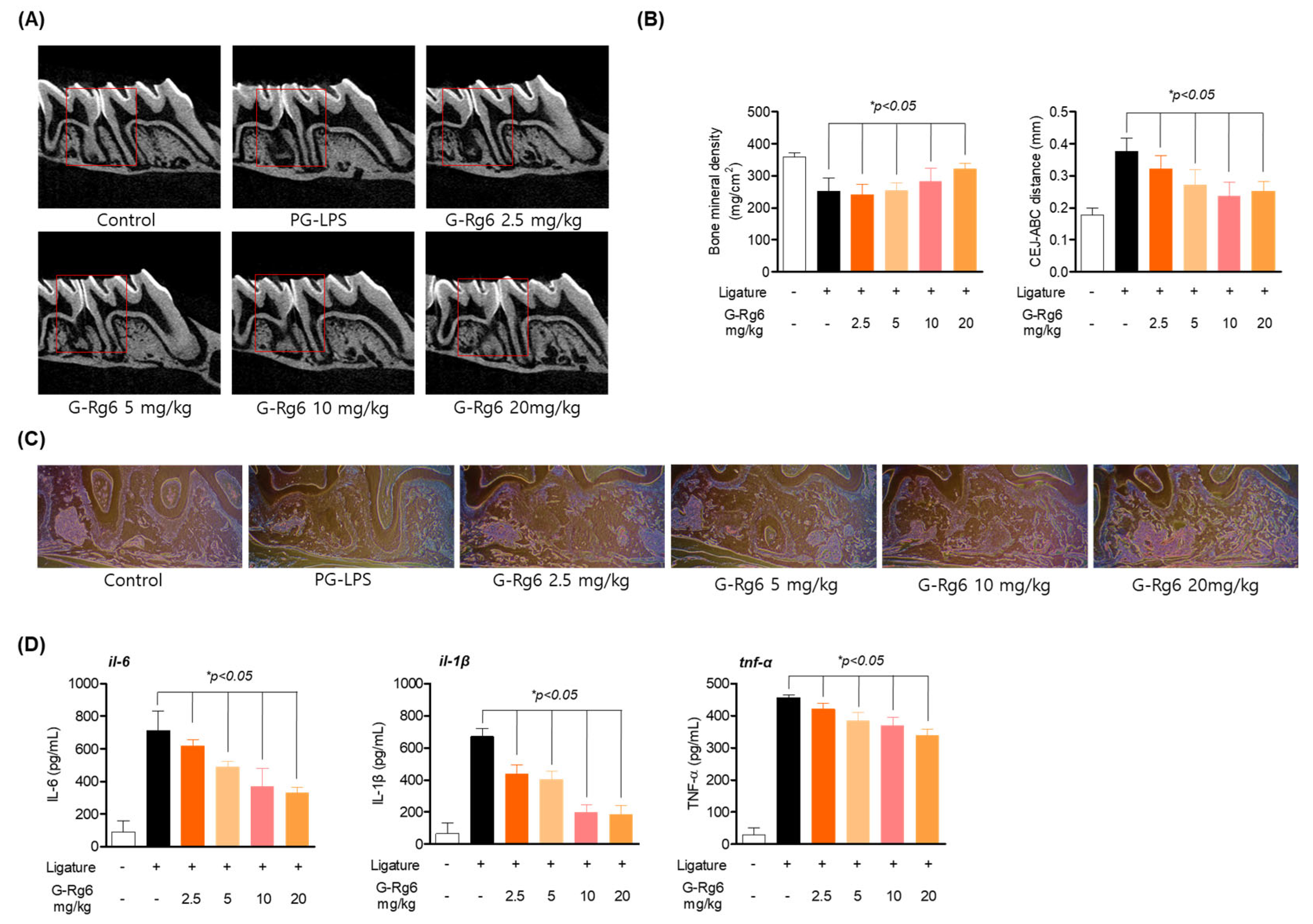
2.6. Inhibitory Effect of G-Rg6 on Periodontitis in a PG-LPS-Induced Periodontitis In Vivo Model
3. Discussion
4. Conclusions
5. Materials and Methods
5.1. Chemicals and Reagents
5.2. Cell Culture
5.3. Plant Materials
5.4. Preparation of G-Rg6
5.5. Cell Viability and Live Cell Analysis
5.6. ELISA Assay
5.7. Alizarin Red Staining
5.8. Determination of Intracellular Reactive Oxygen Species (ROS)
5.9. Western Blot Analysis
5.10. RT-qPCR Analysis
5.11. Determination of the Minimal Inhibition Concentration (MIC) and the Minimal Bactericidal Concentration (MBC)
5.12. Animals
5.13. Ligature-Induced Periodontitis Model
5.14. PG-LPS-Induced Periodontitis Model
5.15. Micro-CT Imaging and Analysis
5.16. Histological Staining
5.17. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pihlstrom, B.L.; Michalowicz, B.S.; Johnson, N.W. Periodontal diseases. Lancet 2005, 366, 1809–1820. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.X.; Zhong, Y.J.; Dong, Q.Q.; Wong, H.M.; Wen, Y.F. Global, regional, and national burden of severe periodontitis, 1990-2019: An analysis of the Global Burden of Disease Study 2019. J. Clin. Periodontol. 2021, 48, 1165–1188. [Google Scholar] [CrossRef] [PubMed]
- Williams, R.C. Periodontal disease. N. Engl. J. Med. 1990, 322, 373–382. [Google Scholar] [CrossRef] [PubMed]
- Lovegrove, J.M. Dental plaque revisited: Bacteria associated with periodontal disease. J. N. Z. Soc. Periodontol. 2004, 87, 7–21. [Google Scholar]
- Mysak, J.; Podzimek, S.; Sommerova, P.; Lyuya-Mi, Y.; Bartova, J.; Janatova, T.; Prochazkova, J.; Duskova, J. Porphyromonas gingivalis: Major periodontopathic pathogen overview. J. Immunol. Res. 2014, 2014, 476068. [Google Scholar] [CrossRef] [PubMed]
- Chiang, C.Y.; Kyritsis, G.; Graves, D.T.; Amar, S. Interleukin-1 and tumor necrosis factor activities partially account for calvarial bone resorption induced by local injection of lipopolysaccharide. Infect. Immun. 1999, 67, 4231–4236. [Google Scholar] [CrossRef] [PubMed]
- Graves, D.T.; Oates, T.; Garlet, G.P. Review of osteoimmunology and the host response in endodontic and periodontal lesions. J. Oral Microbiol. 2011, 3, 5304. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Zhang, Q.; Qin, X.; Li, J.; Zhao, Y.; Xia, Y. Superparamagnetic Iron Oxide Nanoparticles Protect Human Gingival Fibroblasts from Porphyromonas gingivalis Invasion and Inflammatory Stimulation. Int. J. Nanomed. 2022, 17, 45–60. [Google Scholar] [CrossRef]
- Zhang, Z.; Song, J.; Kwon, S.H.; Wang, Z.; Park, S.G.; Piao, X.; Ryu, J.H.; Kim, N.; Kim, O.S.; Kim, S.H.; et al. Pirfenidone Inhibits Alveolar Bone Loss in Ligature-Induced Periodontitis by Suppressing the NF-κB Signaling Pathway in Mice. Int. J. Mol. Sci. 2023, 24, 8682. [Google Scholar] [CrossRef]
- Kızıldağa, A.; Alpanb, A.L.; Özdedec, M.; Aydınd, T.; Özmene, Ö. Therapeutic effects of diosgenin on alveolar bone loss and apoptosis in diabetic rats with experimental periodontitis. Iran. J. Basic Med. Sci. 2023, 26, 785–790. [Google Scholar] [CrossRef]
- Lee, B.A.; Lee, H.S.; Jung, Y.S.; Kim, S.W.; Lee, Y.W.; Chang, S.H.; Chung, H.J.; Kim, O.S.; Kim, Y.J. The effects of a novel botanical agent on lipopolysaccharide-induced alveolar bone loss in rats. J. Periodontol. 2013, 84, 1221–1229. [Google Scholar] [CrossRef] [PubMed]
- Herrera, D.; Sanz, M.; Jepsen, S.; Needleman, I.; Roldán, S. A systematic review on the effect of systemic antimicrobials as an adjunct to scaling and root planing in periodontitis patients. J. Clin. Periodontol. 2002, 29, 136–159. [Google Scholar] [CrossRef]
- Kirschneck, C.; Wolf, F.; Cieplik, F.; Blanck-Lubarsch, M.; Proff, P.; Schröder, A. Impact of NSAID etoricoxib on side effects of orthodontic tooth movement. Ann. Anat. 2020, 232, 151585. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.; Mirza, M.A.; Naseef, P.P.; Kuruniyan, M.S.; Zakir, F.; Aggarwal, G. Exploring the Potential of Natural Product-Based Nanomedicine for Maintaining Oral Health. Molecules 2022, 27, 1725. [Google Scholar] [CrossRef] [PubMed]
- Chu, K.; Zhang, Z.; Chu, Y.; Xu, Y.; Yang, W.; Guo, L. Ginsenoside Rg1 alleviates lipopolysaccharide-induced pyroptosis in human periodontal ligament cells via inhibiting Drp1-mediated mitochondrial fission. Arch. Oral Biol. 2023, 147, 105632. [Google Scholar] [CrossRef] [PubMed]
- Zhou, S.; Ji, Y.; Yao, H.; Guo, H.; Zhang, Z.; Wang, Z.; Du, M. Application of Ginsenoside Rd in Periodontitis with Inhibitory Effects on Pathogenicity, Inflammation, and Bone Resorption. Front. Cell Infect. Microbiol. 2022, 12, 813953. [Google Scholar] [CrossRef] [PubMed]
- Paik, S.; Choe, J.H.; Choi, G.E.; Kim, J.E.; Kim, J.M.; Song, G.Y.; Jo, E.K. Rg6, a rare ginsenoside, inhibits systemic inflammation through the induction of interleukin-10 and microRNA-146a. Sci. Rep. 2019, 9, 4342. [Google Scholar] [CrossRef] [PubMed]
- Rokot, N.T.; Kairupan, T.S.; Cheng, K.C.; Runtuwene, J.; Kapantow, N.H.; Amitani, M.; Morinaga, A.; Amitani, H.; Asakawa, A.; Inui, A. A Role of Ginseng and Its Constituents in the Treatment of Central Nervous System Disorders. Evid. Based Complement. Alternat. Med. 2016, 2016, 2614742. [Google Scholar] [CrossRef]
- Christensen, L.P. Ginsenosides chemistry, biosynthesis, analysis, and potential health effects. Adv. Food Nutr. Res. 2009, 55, 1–99. [Google Scholar] [CrossRef]
- Wu, W.; Sun, L.; Zhang, Z.; Guo, Y.; Liu, S. Profiling and multivariate statistical analysis of Panax ginseng based on ultra-high-performance liquid chromatography coupled with quadrupole-time-of-flight mass spectrometry. J. Pharm. Biomed. Anal. 2015, 107, 141–150. [Google Scholar] [CrossRef]
- Shin, B.K.; Kwon, S.W.; Park, J.H. Chemical diversity of ginseng saponins from Panax ginseng. J. Ginseng Res. 2015, 39, 287–298. [Google Scholar] [CrossRef] [PubMed]
- Im, D.S. Pro-Resolving Effect of Ginsenosides as an Anti-Inflammatory Mechanism of Panax ginseng. Biomolecules 2020, 10, 444. [Google Scholar] [CrossRef] [PubMed]
- Heta, S.; Robo, I. The Side Effects of the Most Commonly Used Group of Antibiotics in Periodontal Treatments. Med. Sci. 2018, 6, 6. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Yang, B.; Dong, W.; Yu, W.; Jia, M.; Wang, J. DNA damage-inducible transcript 3 deficiency promotes bone resorption in murine periodontitis models. J. Periodontal Res. 2023, 58, 841–851. [Google Scholar] [CrossRef] [PubMed]
- Socransky, S.S.; Haffajee, A.D.; Cugini, M.A.; Smith, C.; Kent, R.L., Jr. Microbial complexes in subgingival plaque. J. Clin. Periodontol. 1998, 25, 134–144. [Google Scholar] [CrossRef]
- Wu, C.; Xia, L.; Zhang, B.; Bai, Z.; Yuan, L.; Xu, D. Astragaloside reduces toxic effect of periodontal ligament fibroblasts induced by lipopolysaccharide. Arch. Biochem. Biophys. 2023, 744, 109693. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhang, Y.; Cai, Y.; Li, D.; He, J.; Feng, Z.; Xu, Q. NAT10 regulates the LPS-induced inflammatory response via the NOX2-ROS-NF-κB pathway in macrophages. Biochim. Biophys. Acta Mol. Cell Res. 2023, 1870, 119521. [Google Scholar] [CrossRef]
- Ogawa, T.; Asai, Y.; Makimura, Y.; Tamai, R. Chemical structure and immunobiological activity of Porphyromonas gingivalis lipid A. Front. Biosci. 2007, 12, 3795–3812. [Google Scholar] [CrossRef]
- Fernández, A.; Herrera, D.; Hoare, A.; Hernández, M.; Torres, V.A. Lipopolysaccharides from Porphyromonas endodontalis and Porphyromonas gingivalis promote angiogenesis via Toll-like-receptors 2 and 4 pathways in vitro. Int. Endod. J. 2023, 56, 1270–1283. [Google Scholar] [CrossRef]
- Olsen, I.; Singhrao, S.K. Importance of heterogeneity in Porhyromonas gingivalis lipopolysaccharide lipid A in tissue specific inflammatory signalling. J. Oral Microbiol. 2018, 10, 1440128. [Google Scholar] [CrossRef]
- Pan, W.; Wang, Q.; Chen, Q. The cytokine network involved in the host immune response to periodontitis. Int. J. Oral Sci. 2019, 11, 30. [Google Scholar] [CrossRef] [PubMed]
- Kook, K.E.; Kim, C.; Kang, W.; Hwang, J.K. Inhibitory Effect of Standardized Curcuma xanthorrhiza Supercritical Extract on LPS-Induced Periodontitis in Rats. J. Microbiol. Biotechnol. 2018, 28, 1614–1625. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Luo, W.; Zhang, G.; Li, H.; Zhou, F.; Wang, D.; Feng, X.; Xiong, Y.; Wu, Y. FoxO1 knockdown inhibits RANKL-induced osteoclastogenesis by blocking NLRP3 inflammasome activation. Oral Dis. 2023, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Jönsson, D.; Nebel, D.; Bratthall, G.; Nilsson, B.O. The human periodontal ligament cell: A fibroblast-like cell acting as an immune cell. J. Periodontal Res. 2011, 46, 153–157. [Google Scholar] [CrossRef] [PubMed]
- Park, E.K.; Shin, Y.W.; Lee, H.U.; Kim, S.S.; Lee, Y.C.; Lee, B.Y.; Kim, D.H. Inhibitory effect of ginsenoside Rb1 and compound K on NO and prostaglandin E2 biosyntheses of RAW264.7 cells induced by lipopolysaccharide. Biol. Pharm. Bull. 2005, 28, 652–656. [Google Scholar] [CrossRef] [PubMed]
- Du, J.; Cheng, B.; Zhu, X.; Ling, C. Ginsenoside Rg1, a novel glucocorticoid receptor agonist of plant origin, maintains glucocorticoid efficacy with reduced side effects. J. Immunol. 2011, 187, 942–950. [Google Scholar] [CrossRef] [PubMed]
- Park, E.K.; Choo, M.K.; Han, M.J.; Kim, D.H. Ginsenoside Rh1 possesses antiallergic and anti-inflammatory activities. Int. Arch. Allergy Immunol. 2004, 133, 113–120. [Google Scholar] [CrossRef]
- Balli, U.; Cetinkaya, B.O.; Keles, G.C.; Keles, Z.P.; Guler, S.; Sogut, M.U.; Erisgin, Z. Assessment of MMP-1, MMP-8 and TIMP-2 in experimental periodontitis treated with kaempferol. J. Periodontal Implant. Sci. 2016, 46, 84–95. [Google Scholar] [CrossRef]
- Jang, W.Y.; Hwang, J.Y.; Cho, J.Y. Ginsenosides from Panax ginseng as Key Modulators of NF-κB Signaling Are Powerful Anti-Inflammatory and Anticancer Agents. Int. J. Mol. Sci. 2023, 24, 6119. [Google Scholar] [CrossRef]
- López-Valverde, N.; López-Valverde, A.; Montero, J.; Rodríguez, C.; Macedo de Sousa, B.; Aragoneses, J.M. Antioxidant, anti-inflammatory and antimicrobial activity of natural products in periodontal disease: A comprehensive review. Front. Bioeng. Biotechnol. 2023, 11, 1226907. [Google Scholar] [CrossRef]




| Target Gene | Sequence (5′→3′) | |
|---|---|---|
| IL-6 | Forward | AGTGAGGAACAAGCCAGAGC |
| Reverse | GTCAGGGGTGGTTATTGCAT | |
| IL-1Β | Forward | AACCTCTTCGAGGCACAAGG |
| Reverse | GTCCTGGAAGGAGCACTTCAT | |
| TNF-A | Forward | GCCTCTTCTCCTTCCTGATCGT |
| Reverse | TGAGGGTTTGCTACAACATGGG | |
| ALP | Forward | TGCAGTACGAGCTGAACAGG |
| Reverse | GTCAATTCTGCCTCCTTCCA | |
| MMP-1 | Forward | ATGAAGCAGCCCAGATGTGGAG |
| Reverse | TGGTCCACATCTGCTCTTGGCA | |
| OCN | Forward | CGCTACCTGTATCAATGGCTGG |
| Reverse | CTCCTGAAAGCCGATGTGGTCA | |
| GAPDH | Forward | TGTTCGTCATGGGTGTGAAC |
| Reverse | GTCTTCTGGGTGGCAGTGAT | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, W.-J.; Kim, E.-N.; Trang, N.M.; Lee, J.-H.; Cho, S.-H.; Choi, H.-J.; Song, G.-Y.; Jeong, G.-S. Ameliorative Effect of Ginsenoside Rg6 in Periodontal Tissue Inflammation and Recovering Damaged Alveolar Bone. Molecules 2024, 29, 46. https://doi.org/10.3390/molecules29010046
Lee W-J, Kim E-N, Trang NM, Lee J-H, Cho S-H, Choi H-J, Song G-Y, Jeong G-S. Ameliorative Effect of Ginsenoside Rg6 in Periodontal Tissue Inflammation and Recovering Damaged Alveolar Bone. Molecules. 2024; 29(1):46. https://doi.org/10.3390/molecules29010046
Chicago/Turabian StyleLee, Won-Jin, Eun-Nam Kim, Nguyen Minh Trang, Jee-Hyun Lee, Soo-Hyun Cho, Hui-Ji Choi, Gyu-Yong Song, and Gil-Saeng Jeong. 2024. "Ameliorative Effect of Ginsenoside Rg6 in Periodontal Tissue Inflammation and Recovering Damaged Alveolar Bone" Molecules 29, no. 1: 46. https://doi.org/10.3390/molecules29010046
APA StyleLee, W.-J., Kim, E.-N., Trang, N. M., Lee, J.-H., Cho, S.-H., Choi, H.-J., Song, G.-Y., & Jeong, G.-S. (2024). Ameliorative Effect of Ginsenoside Rg6 in Periodontal Tissue Inflammation and Recovering Damaged Alveolar Bone. Molecules, 29(1), 46. https://doi.org/10.3390/molecules29010046






