Optimization Conditions of Malachite Green Adsorption onto Almond Shell Carbon Waste Using Process Design
Abstract
:1. Introduction
2. Results and Discussion
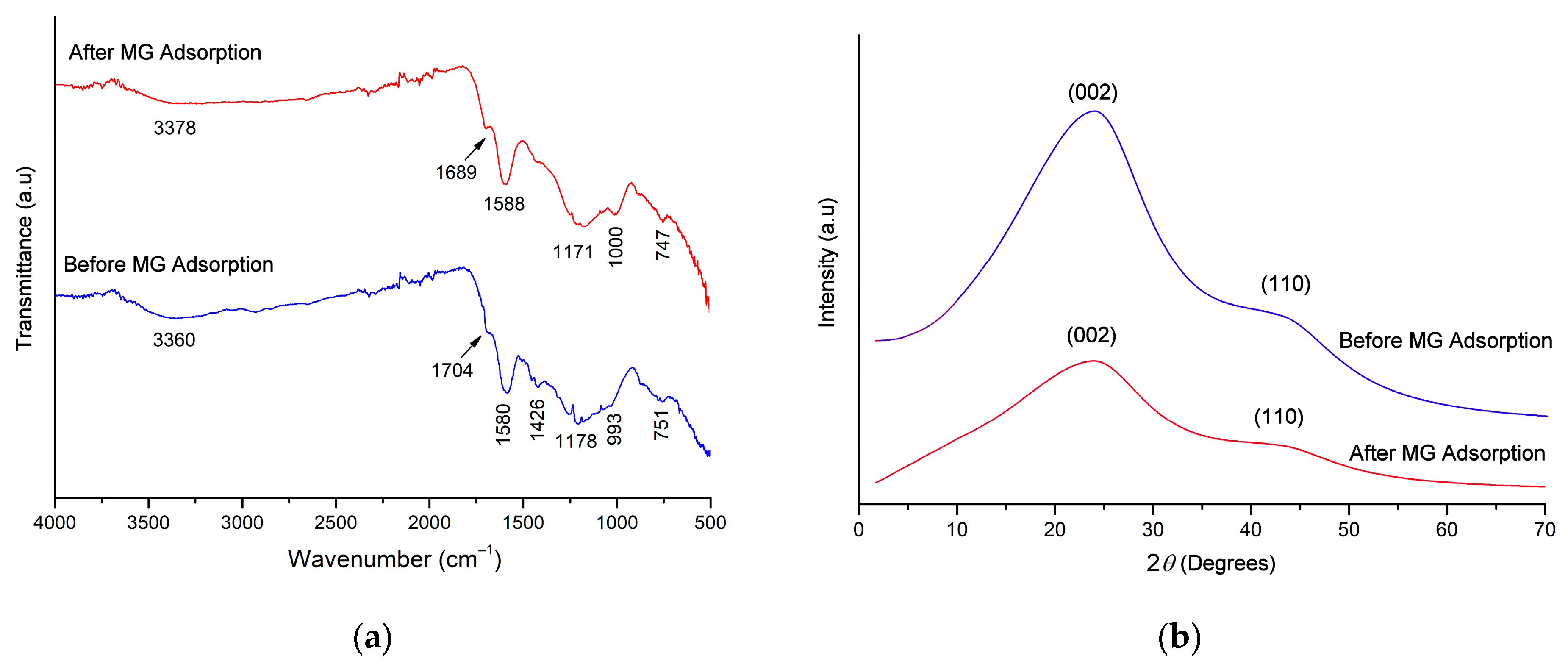
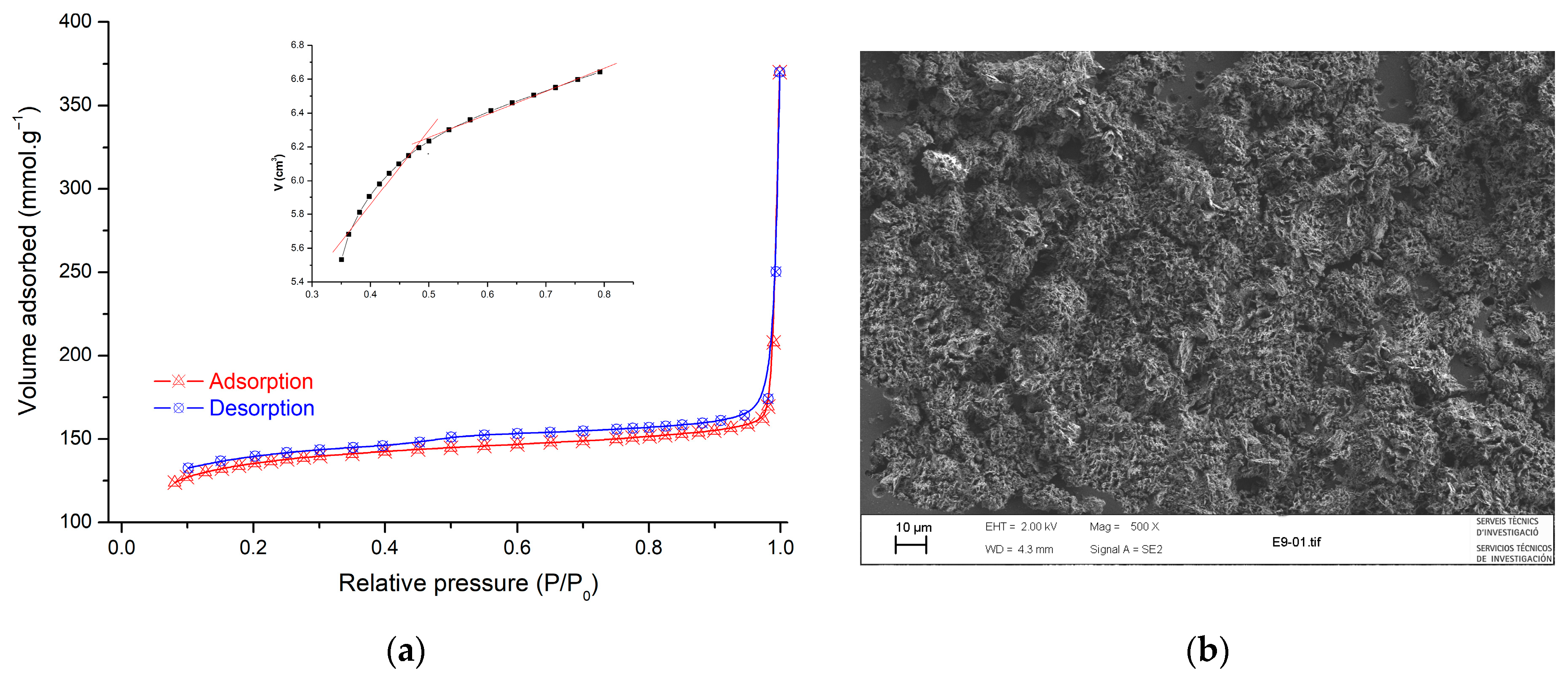
2.1. Adsorbent Characterization
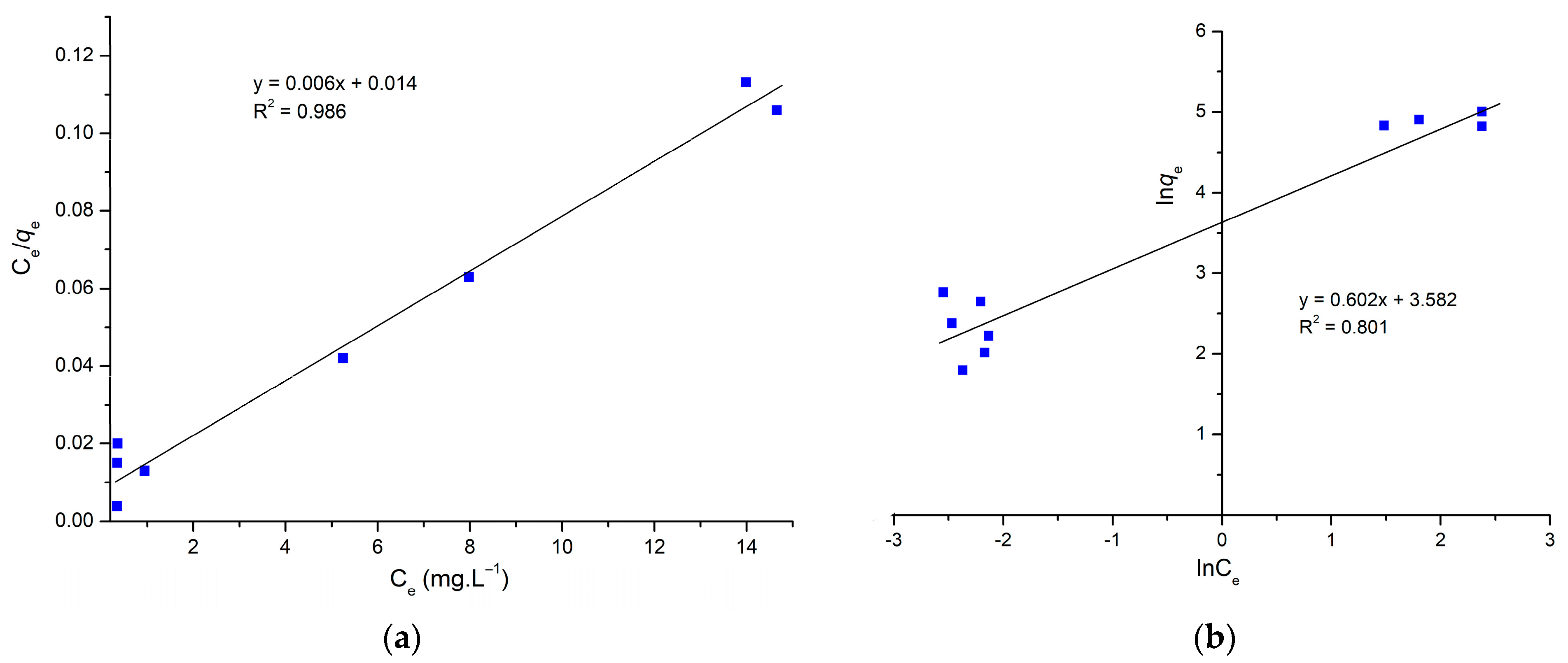
2.2. Batch Adsorption Studies
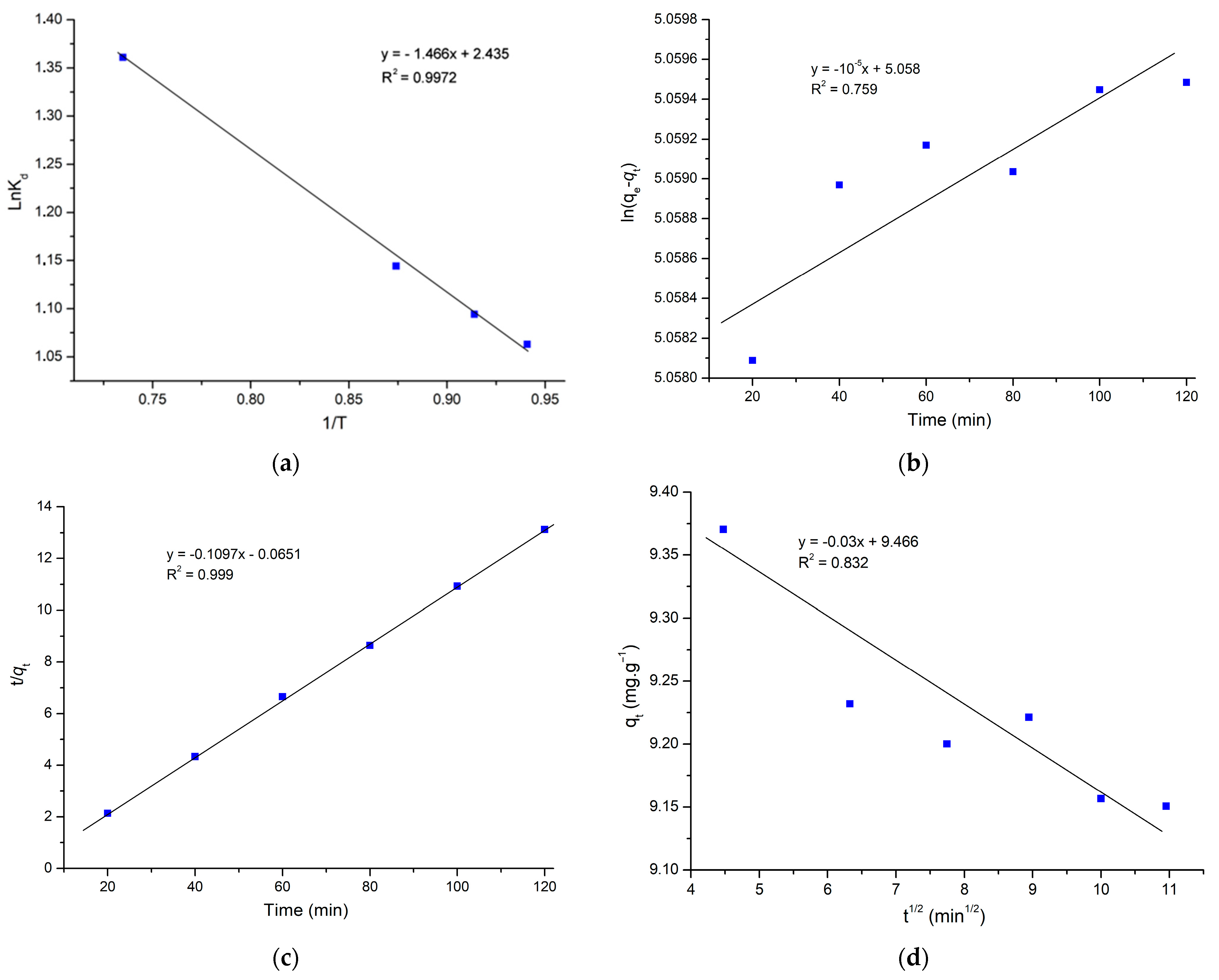
2.3. Thermodynamic Investigations
2.4. Adsorption Kinetic Study
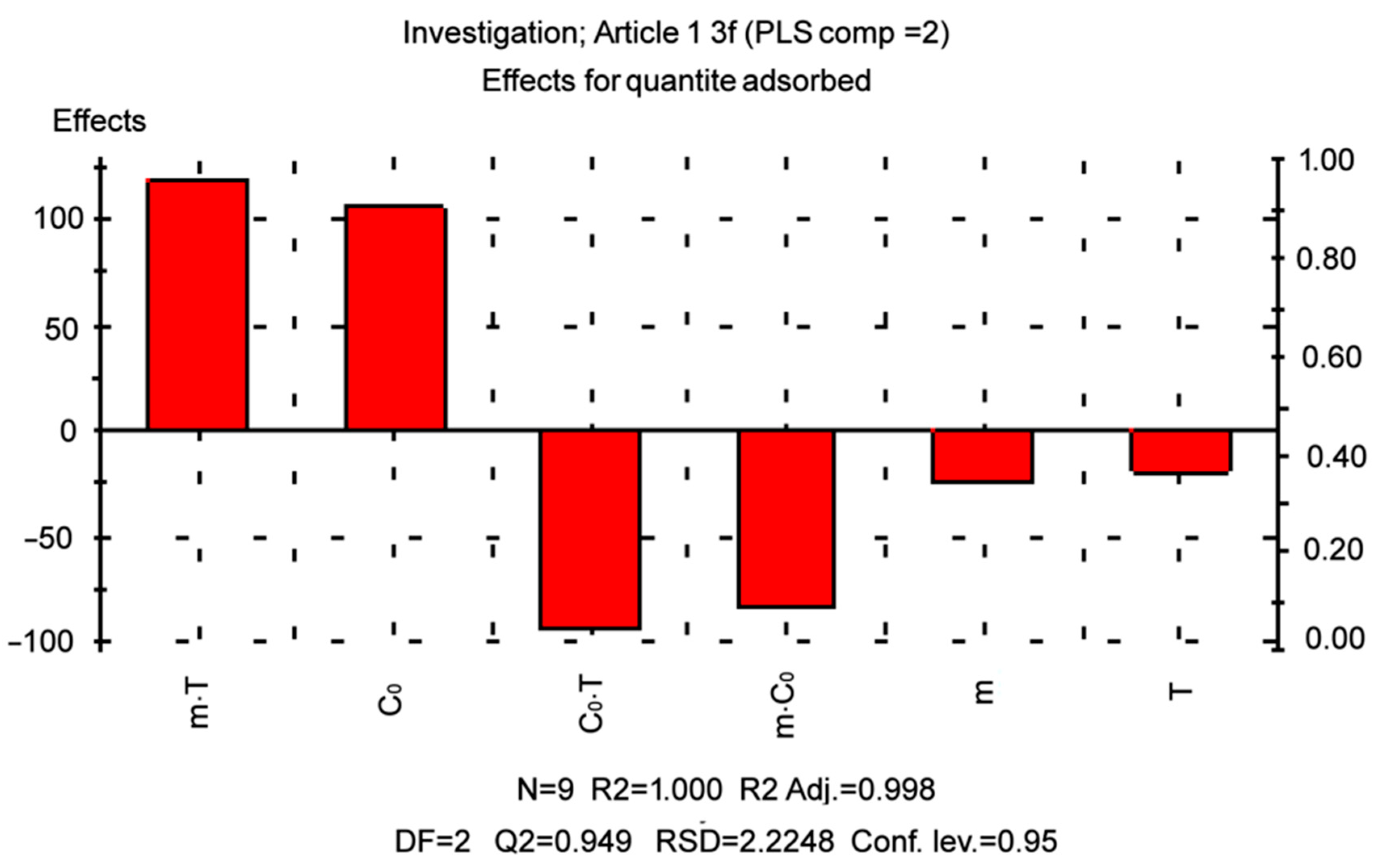
2.5. D-Optimal Designs for 3 Parameters
3. Materials and Methods
3.1. Materials
3.2. Physical Modification of the Natural Material
3.3. Characterization of the Adsorbent
3.4. Investigation of Parameters via Adsorption Procedure
3.5. Adsorption Isotherms
3.6. D-Optimal Design of Experiments
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Liu, Y.; Zheng, Y.; Wang, A. Enhanced Adsorption of Methylene Blue from aqueous Solution by chitosan-g-poly (acrylic acid)/Vermiculite Hydrogel Composites. J. Environ. Sci. 2010, 22, 486–493. [Google Scholar] [CrossRef] [PubMed]
- Wong, P.W.; Teng, T.T.; Norulaini, N.A.R.N. Efficiency of the Coagulation-Flocculation Method for the Treatment of Dye Mixtures Containing Disperse and Reactive Dye. Water Qual. Res. J. 2007, 42, 54–62. [Google Scholar] [CrossRef]
- Sintayehu, Y.D.; Gemeta, A.B.; Berehe, S.G. Optical Photocatalytic Degradation of Methylene Blue Using Lignocellulose Modified TiO2. Am. J. Opt. Photonics 2017, 5, 55–58. [Google Scholar] [CrossRef]
- Thamaraiselvan, C.; Noel, M. Membrane Processes for Dye Waste water Treatment: Recent Progress in Fouling Control. Crit. Rev. Environ. Sci. Technol. 2015, 45, 1007–1040. [Google Scholar] [CrossRef]
- Toumi, I.; Djelad, H.; Chouli, F.; Benyoucef, A. Synthesis of PANI@ZnO Hybrid Material and Evaluations in Adsorption of Congo Red and Methylene Blue Dyes: Structural Characterization and Adsorption Performance. J. Inorg. Organomet. Polym. Mater. 2022, 32, 112–121. [Google Scholar] [CrossRef]
- Ying, W.; Zhang, W.; Chang, Q.; Jiang, W.; Lib, G. Improved Methods for Carbon Adsorption Studies for Water and Wastewater Treatment. Environ. Prog. 2006, 25, 110–120. [Google Scholar] [CrossRef]
- Chandra, T.C.; Mirna, M.M.; Sunarso, J.; Sudaryanto, Y.; Ismadji, I. Activated carbon from durian shell: Preparation and characterization. J. Taiwan Inst. Chem. Eng. 2009, 40, 457–462. [Google Scholar] [CrossRef]
- Mebrek, O.R.; Derriche, Z. Removal of Furfural from Aqueous Solutions by Adsorption Using Organobentonite: Isotherm and Kinetic Studies. J. Adsorpt. Sci. Technol. 2010, 28, 533–545. [Google Scholar] [CrossRef]
- Lahreche, S.; Moulefera, I.; El Kebir, A.; Sabantina, L.; Kaid, M.; Benyoucef, A. Application of Activated Carbon Adsorbents Prepared from Prickly Pear Fruit Seeds and a Conductive Polymer Matrix to Remove Congo Red from Aqueous Solutions. Fibers 2022, 10, 7. [Google Scholar] [CrossRef]
- Bekhoukh, A.; Moulefera, I.; Zeggai, F.Z.; Benyoucef, A.; Bachari, K. Anionic Methyl Orange Removal from Aqueous Solutions by Activated Carbon Reinforced Conducting Polyaniline as Adsorbent: Synthesis, Characterization, Adsorption Behavior, Regeneration and Kinetics Study. J. Polym. Environ. 2022, 30, 886–895. [Google Scholar] [CrossRef]
- Bedia, J.; Peñas-Garzón, M.; Gómez-Avilés, A.; Rodriguez, J.J.; Belver, C. Review on Activated Carbons by Chemical Activation with FeCl3. C 2020, 6, 21. [Google Scholar] [CrossRef]
- Kiełbasa, K.; Bayar, S.; Varol, E.A.; Nazzal, J.S.; Bosacka, M.; Michalkiewicz, B. Thermochemical conversion of lignocellulosic biomass-olive pomace-into activated biocarbon for CO2 adsorption. Ind. Crops Prod. 2022, 187, 115416. [Google Scholar] [CrossRef]
- Mahi, O.; Khaldi, K.; Belardja, M.S.; Belmokhtar, A.; Benyoucef, A. Development of a New Hybrid Adsorbent from Opuntia Ficus Indica NaOH Activated with PANI Reinforced and Its Potential Use in Orange G Dye Removal. J. Inorg. Organomet. Polym. Mater. 2021, 31, 2095–2104. [Google Scholar] [CrossRef]
- Muttil, N.; Jagadeesan, S.; Chanda, A.; Duke, M.; Singh, S.K. Production, Types, and Applications of Activated Carbon Derived from Waste Tyres: An Overview. Appl. Sci. 2023, 13, 257. [Google Scholar] [CrossRef]
- Hassan, S.S.; Al-Ghouti, M.A.; Abu-Dieyeh, M.; McKay, G. Novel bioadsorbents based on date pits for organophosphorus pesticide remediation from water. J. Environ. Chem. Eng. 2020, 8, 103593. [Google Scholar] [CrossRef]
- Shaheen, J.F.; Eniola, J.O.; Sizirici, B. Adsorption of ibuprofen from aqueous solution by modified date palm biochar: Performance, optimization, and life cycle assessment. Bioresour. Technol. Rep. 2024, 25, 101696. [Google Scholar] [CrossRef]
- Wu, C.; Liu, J.; Wang, Y.; Zhao, Y.; Li, G.; Zhang, G. KCl-assisted activation of macadamia nut shell-derived carbon: Unveiling enhanced pore structure, adsorption and supercapacitor performance. Sep. Purif. Technol. 2024, 329, 125188. [Google Scholar] [CrossRef]
- Saghir, S.; Pu, C.; Fu, E.; Wang, Y.; Xiao, Z. Synthesis of high surface area porous biochar obtained from pistachio shells for the efficient adsorption of organic dyes from polluted water. Surf. Interfaces 2022, 34, 102357. [Google Scholar] [CrossRef]
- He, S.; Sun, J.; Jin, X.; Chen, Q.; Wu, X.; Tian, F.; Zhang, X.; Li, P.; Sheng, H. Adsorption enhancement of Congo red dye from wastewater based on edamame shell originated activated carbon by the cations: Experimental and theoretical studies. Diam. Relat. Mater. 2023, 136, 109930. [Google Scholar] [CrossRef]
- Campos, N.F.; Sales, D.C.S.; Díaz, J.M.R.; Barbosa, C.M.B.M.; Duarte, M.M.M.B. Adsorption of naphthenic acids on peanut shell activated carbon: Batch and fixed-bed column study of the process. Chem. Eng. Res. Des. 2022, 188, 633–644. [Google Scholar] [CrossRef]
- Hsini, A.; Essekri, A.; Aarab, N.; Laabd, M.; AitAddi, A.; Lakhmiri, R.; Albourine, A. Elaboration of novel polyaniline@Almond shell biocomposite for effective removal of hexavalent chromium ions and Orange G dye from aqueous solutions. Environ. Sci. Pollut. Res. 2020, 27, 15245–15258. [Google Scholar] [CrossRef] [PubMed]
- Jabar, J.M.; Owokotomo, I.A.; Ayinde, Y.T.; Alafabusuyi, A.M.; Olagunju, G.O.; Mobolaji, V.O. Characterization of prepared eco-friendly biochar from almond (Terminalia catappa L) leaf for sequestration of bromophenol blue (BPB) from aqueous solution. Carbon Lett. 2021, 31, 1001–1014. [Google Scholar] [CrossRef]
- Kali, A.; Amar, A.; Loulidi, I.; Hadey, C.; Jabri, M.; Alrashdi, A.A.; Lgaz, H.; Sadoq, M.; El-kordy, A.; Boukhlifi, F. Efficient Adsorption Removal of an Anionic Azo Dye by Lignocellulosic Waste Material and Sludge Recycling into Combustible Briquettes. Colloids Interfaces 2022, 6, 22. [Google Scholar] [CrossRef]
- Mehrasbi, M.R.; Farahmandkia, Z.; Taghibeigloo, B.; Taromi, A. Adsorption of Lead and Cadmium from Aqueous Solution by Using Almond Shells. Water Air Soil Pollut. 2009, 199, 343–351. [Google Scholar] [CrossRef]
- Yildiz, S. Kinetic and isotherm analysis of Cu(II) adsorption Onto almond shell (Prunus dulcis). J. Ecol. Chem. Eng. 2017, 24, 87–106. [Google Scholar] [CrossRef]
- Xuemin, L.; Yinan, L.; Jianxiu, H.; Weihong, W. Study of Almond Shell Characteristics. Materials 2018, 11, 1782. [Google Scholar] [CrossRef]
- Song, Y.; Ding, S.; Chen, S.; Xu, H.; Mei, Y.; Ren, J. Removal of malachite green in aqueous solution by adsorption on sawdust. Korean J. Chem. Eng. 2015, 32, 2443–2448. [Google Scholar] [CrossRef]
- Raval, N.P.; Shah, P.U.; Shah, N.K. Malachite green “a cationic dye” and its removal from aqueous solution by adsorption. J. Appl. Water Sci. 2016, 7, 3407–3445. [Google Scholar] [CrossRef]
- Czajka, A.M.K.; Dziejarski, B. Linear and Non-Linear Regression Analysis for the Adsorption Kinetics of SO2 in a Fixed Carbon Bed Reactor—A Case Study. Energies 2022, 15, 633. [Google Scholar] [CrossRef]
- Aitcheson, S.J.; Arnett, J.; Murray, K.R.; Zhang, J. Removal of aquaculture therapeutants by carbon adsorption: 2: Multicomponent adsorption and the equilibrium behaviour of mixtures. Aquaculture 2001, 192, 249–264. [Google Scholar] [CrossRef]
- Ahmad, A.A.M.; Ahmad, M.Z.; Yahaya, N.K.E.M.; Jamilah Karim, J. Adsorption of malachite green by activated carbon derived from gasified Hevea brasiliensis root. Arab. J. Chem. 2021, 14, 103104. [Google Scholar] [CrossRef]
- Yusop, M.F.M.; Abdullah, A.Z.; Ahmad, M.A. Malachite green dye adsorption by jackfruit based activated carbon: Optimization, mass transfer simulation and surface area prediction. Diam. Relat. Mater. 2023, 136, 109991. [Google Scholar] [CrossRef]
- Ahmad, M.A.; Ahmad, N.; Bello, O.S. Adsorptive removal of malachite green dye using durian seed-based activated carbon. Water Air Soil Pollut. 2014, 225, 2057–2064. [Google Scholar] [CrossRef]
- Akar, E.; Altinişik, A.; Seki, Y. Using of activated carbon produced from spent tea leaves for the removal of malachite green from aqueous solution. Ecol. Eng. 2013, 52, 19–27. [Google Scholar] [CrossRef]
- Basirun, A.A.; Othman, A.R.; Yasid, N.A.; Abd Shukor, M.Y.; Khayat, M.E. A Green Approach of Utilising Banana Peel (Musa paradisiaca) as Adsorbent Precursor for an Anionic Dye Removal: Kinetic, Isotherm and Thermodynamics Analysis. Processes 2023, 11, 1611. [Google Scholar] [CrossRef]
- Yadav, J.; Sahu, O. Malachite green dye purification from effluent using synthesized ceramic clay: Characterisation; optimization and scale up. J. Ceram Int. 2023, 49, 24831–24851. [Google Scholar] [CrossRef]
- Hille, M.; Ouden, J.D. Charcoal and activated carbon as adsorbate of phytotoxic compounds, a comparative study. Oikos 2005, 108, 202–207. [Google Scholar] [CrossRef]
- Ahsaine, H.A.; Zbair, M.; Anfar, Z.; Naciri, Y.; El haouti, R.; El Alem, N.; Ezahri, M. Cationic dyes adsorption onto high surface area ‘almond shell’ activated carbon: Kinetics, equilibrium isotherms and surface statistical modeling. Mater. Today Chem. 2018, 8, 121–132. [Google Scholar] [CrossRef]
- Zhang, F.; Chen, X.; Zhang, W.; Ji, Y. Dual-functionalized strontium phosphate hybrid nanopowder for effective removal of Pb2+ and malachite green from aqueous solution. Powder Technol. 2017, 318, 128–134. [Google Scholar] [CrossRef]
- Bicil, Z.; Doğan, M. Characterization of Activated Carbons Prepared from Almond Shells and Their Hydrogen Storage Properties. Energy Fuels 2021, 35, 10227–10240. [Google Scholar] [CrossRef]
- Mennas, N.; Lahreche, S.; Chouli, F.; Sabantina, L.; Benyoucef, A. Adsorption of Methylene Blue Dye by Cetyltrimethylammonium Bromide Intercalated Polyaniline-Functionalized Montmorillonite Clay Nanocomposite: Kinetics, Isotherms, and Mechanism Study. Polymers 2023, 15, 3518. [Google Scholar] [CrossRef]
- Lee, J.; Choi, Y. Pore Structure Characteristics of Foam Composite with Active Carbon. J. Mater. 2020, 13, 4038. [Google Scholar] [CrossRef]
- Li, H.; Kong, J.; Zhang, H.; Gao, J.; Fang, Y.; Shi, J.; Ge, T.; Fang, T.; Shi, Y.; Zhang, R.; et al. Mechanisms and adsorption capacities of ball milled biomass fly ash/biochar composites for the adsorption of methylene blue dye from aqueous solution. J. Water Process Eng. 2023, 53, 103713. [Google Scholar] [CrossRef]
- Ben Arfi, R.; Karoui, S.; Mougin, K.; Ghorbal, A. Adsorptive removal of cationic and anionic dyes from aqueous solution by utilizing almond shell as bioadsorbent. Euro-Mediterr. J. Environ. Integr. 2017, 2, 20. [Google Scholar] [CrossRef]
- Ozdes, D.; Gundogdu, A.; Duran, C.; Senturk, H.B. Evaluation of Adsorption Characteristics of Malachite Green onto Almond Shell (Prunus dulcis). Sep. Sci. Technol. 2010, 45, 2076–2085. [Google Scholar] [CrossRef]
- Dahri, M.K.; Kooh, M.R.R.; Lim, L.B.L. Water remediation using low cost adsorbent walnut shell for removal of malachite green: Equilibrium, kinetics, thermodynamic and regeneration studies. J. Environ. Chem. Eng. 2014, 2, 1434–1444. [Google Scholar] [CrossRef]
- Banerjee, S.; Sharma, G.C.; Gautam, R.K.; Chattopadhyaya, M.C.; Upadhyay, S.N.; Sharma, Y.C. Removal of Malachite Green, a hazardous dye from aqueous solutions using Avena sativa (oat) hull as a potential adsorbent. J. Mol. Liq. 2016, 213, 162–172. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, Y.; Hou, C.; Liu, M. Mussel-inspired synthesis of magnetic polydopamine–chitosan nanoparticles as biosorbent for dyes and metals removal. J. Taiwan Inst. Chem. Eng. 2016, 61, 292–298. [Google Scholar] [CrossRef]
- Hameed, B.H.; El-Khaiary, M.I. Malachite green adsorption by rattan sawdust: Isotherm, kinetic and mechanism modeling. J. Hazard. Mater. 2008, 159, 574–579. [Google Scholar] [CrossRef] [PubMed]
- Abbas, M. Experimental investigation of activated carbon prepared from apricot stones material (ASM) adsorbent for removal of malachite green (MG) from aqueous solution. Adsorpt. Sci. Technol. 2020, 38, 24–45. [Google Scholar] [CrossRef]
- Ghosh, K.; Bar, N.; Roymahapatra, G.; Biswas, A.B.; Das, S.K. Adsorptive removal of toxic malachite green from its aqueous solution by Bambusa vulgaris leaves and its acid-treated form: DFT, MPR and GA modeling. J. Mol. Liq. 2022, 363, 119841. [Google Scholar] [CrossRef]
- Baek, M.H.; Ijagbemi, C.O.; Jin, O.S.; Kim, D.S. Removal of Malachite Green from aqueous solution using degreased coffee bean. J. Hazard. Mater. 2010, 176, 820–828. [Google Scholar] [CrossRef]
- Sartape, A.S.; Mandhare, A.M.; Jadhav, V.V.; Raut, P.D.; Anuse, M.A.; Kolekar, S.S. Removal of malachite green dye from aqueous solution with adsorption technique using Limonia acidissima (wood apple) shell as low cost adsorbent. Arab. J. Chem. 2017, 10, 3229–3238. [Google Scholar] [CrossRef]
- Borsato, D.; Galvan, D.; Pereira, J.L.; Orives, J.R.; Angilelli, K.G.; Coppo, R.L. Kinetic and Thermodynamic Parameters of Biodiesel Oxidation with Synthetic Antioxidants: Simplex Centroid Mixture Design. J. Braz. Chem. Soc. 2014, 251, 984–1992. [Google Scholar] [CrossRef]
- Miao, P.; Sang, Y.; Gao, J.; Han, X.; Zhao, Y.; Chen, T. Adsorption and Recognition Property of Tyrosine Molecularly Imprinted Polymer Prepared via Electron Beam Irradiation. Polymers 2023, 15, 4048. [Google Scholar] [CrossRef] [PubMed]
- Atasoy, A.D.; Bilgic, B. Adsorption of Copper and Zinc Ions from Aqueous Solutions Using Montmorillonite and Bauxite as Low-Cost Adsorbents. Mine Water Environ. 2018, 37, 205–210. [Google Scholar] [CrossRef]
- Izquierdo, M.T.; de Yuso, A.M.; Rubio, B.; Pino, M.R. Conversion of almond shell to activated carbons: Methodical study of the chemical activation based on an experimental design and relationship with their characteristics. Biomass Bioenergy 2011, 35, 1235–1244. [Google Scholar] [CrossRef]
- Castañeda, L.F.; Walsh, F.C.; Nava, J.L.; de León, C.P. Graphite felt as a versatile electrode material: Properties, reaction environment, performance and applications. Electrochim. Acta 2017, 258, 1115–1139. [Google Scholar] [CrossRef]


| Parameters | |
|---|---|
| BET surface area (m2.g−1) | 120.21 |
| Pore volume (cm3.g−1) | 0.572 |
| Pore diameter (nm) | 9.263 |
| Micropore volume (cm3.g−1) | 0.213 |
| Isotherm Model | Parameters | Values |
|---|---|---|
| Langmuir | qm | 166.66 (mg.g−1) |
| KL | 428 (L.g−1) | |
| R2 | 0.986 | |
| RL | 0.0034 | |
| Freundlich | n | 1.661 |
| KF | 35.945 (mg.g−1) | |
| R2 | 0.801 |
| Adsorbents | qm (mg.g−1) | pH | C0 (mg.L−1) | References |
|---|---|---|---|---|
| Tunisian almond shell | 126.90 | 6.0 | 200 | [44] |
| Almond shell (P. dulcis) | 22.30 | // | 600 | [45] |
| Walnut shell | 90.80 | 3.8 | 400 | [46] |
| Avena sativa (oat) hull | 51.42 | 8.0 | 200 | [47] |
| Polydopamine–chitosan nanoparticles | 60.97 | 8.0 | 300 | [48] |
| Rattan sawdust | 62.71 | 10.0 | 300 | [49] |
| Apricot stone (ASAC) | 23.80 | 10.0 | 10 | [50] |
| Bamboo leaves | 98.00 | 6.0 | 100 | [51] |
| Coffee bean | 16.07 | 4.0 | 100 | [52] |
| Wood apple shell | 34.56 | 7.5 | 100 | [53] |
| Almond shell treatment at 400 °C | 166.66 | 5.0 | 600 | This study |
| ∆G (KJ.mol−1) | ∆S (J.mol−1K−1) | ∆H (J.mol−1) | |||
|---|---|---|---|---|---|
| 298 K | 323 K | 348 K | 373 K | 20.24 | 12.19 |
| −0.76 | −0.16 | −0.26 | −0.41 | ||
| Model | Parameter | Value |
|---|---|---|
| Pseudo-first-order | K1 (min−1) | −10−5 |
| qe (mg.g−1) | 157.27 | |
| R2 | 0.759 | |
| Pseudo-second-order | qe (mg.g−1) | 9.17 |
| K2 (g.mg−1.min−1) | 0.183 | |
| R2 | 0.999 | |
| Intraparticle diffusion | Kid (mg.g−1.min½) | −0.03 |
| C (mg.g−1) | 9.466 | |
| R2 | 0.832 |
| Term | Effect | Coef | SE Coef | P |
|---|---|---|---|---|
| Without interactions | ||||
| Constant | 43.5778 | 0.4579 | 2.4290 × 10−9 | |
| m | −6.6792 | −2.2264 | 0.5064 | 0.0070 |
| C0 | 142.609 | 55.0073 | 0.5146 | 1.3589 × 10−9 |
| T | −1.6715 | −0.5259 | 0.5042 | 0.3447 |
| R2 | 0.999 | |||
| DF | 5 | |||
| 2-way interactions | ||||
| Constant | 37.3984 | |||
| m | −24.720 | −8.2400 | ||
| C0 | 105.606 | 40.7344 | ||
| T | −18.838 | −5.9278 | ||
| M × C0 | −84.981 | −21.852 | ||
| M × T | 118.225 | 24.8007 | ||
| C0 × T | −93.860 | −22.784 | ||
| R2 | 0.998 | |||
| DF | 2 | |||
| m | C0 | T | Yi |
|---|---|---|---|
| 0.2985 | 20.0045 | 70.8051 | 137.333 |
| 0.1 | 600 | 25 | 158.015 |
| 0.2399 | 20.0245 | 100 | 167.043 |
| 0.3 | 108.771 | 89.7654 | 173.242 |
| 0.3 | 20.0001 | 88.75 | 197.009 |
| 0.1 | 599.994 | 34.2698 | 135.901 |
| 0.1 | 599.99 | 25.0117 | 157.982 |
| 0.1 | 600 | 30 | 146.089 |
| Experiments | m | C0 | T | Y |
|---|---|---|---|---|
| 1 | 0.3 | 50 | 30 | 3.99 |
| 2 | 0.1 | 50 | 25 | 9.95 |
| 3 | 0.1 | 50 | 100 | 9.48 |
| 4 | 0.1 | 20 | 30 | 4.96 |
| 5 | 0.1 | 60 | 30 | 14.98 |
| 6 | 0.1 | 300 | 30 | 71.75 |
| 7 | 0.1 | 500 | 30 | 121.49 |
| 8 | 0.1 | 600 | 30 | 146.45 |
| 9 | 0.1 | 50 | 30 | 9.15 |
| Factors | Low Factor Level | High Factor Level |
|---|---|---|
| −1 | +1 | |
| Adsorbent mass, m (g) | 0.1 | 0.3 |
| Initial concentration of the colorant, C0 (mg.L−1) | 50 | 600 |
| Temperature during adsorption, T (°C) | 25 | 100 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chouli, F.; Ezzat, A.O.; Sabantina, L.; Benyoucef, A.; Zehhaf, A. Optimization Conditions of Malachite Green Adsorption onto Almond Shell Carbon Waste Using Process Design. Molecules 2024, 29, 54. https://doi.org/10.3390/molecules29010054
Chouli F, Ezzat AO, Sabantina L, Benyoucef A, Zehhaf A. Optimization Conditions of Malachite Green Adsorption onto Almond Shell Carbon Waste Using Process Design. Molecules. 2024; 29(1):54. https://doi.org/10.3390/molecules29010054
Chicago/Turabian StyleChouli, Faiza, Abdelrahman Osama Ezzat, Lilia Sabantina, Abdelghani Benyoucef, and Abdelhafid Zehhaf. 2024. "Optimization Conditions of Malachite Green Adsorption onto Almond Shell Carbon Waste Using Process Design" Molecules 29, no. 1: 54. https://doi.org/10.3390/molecules29010054
APA StyleChouli, F., Ezzat, A. O., Sabantina, L., Benyoucef, A., & Zehhaf, A. (2024). Optimization Conditions of Malachite Green Adsorption onto Almond Shell Carbon Waste Using Process Design. Molecules, 29(1), 54. https://doi.org/10.3390/molecules29010054








