Effects of Nanoparticle Size on the Thermal Decomposition Mechanisms of 3,5-Diamino-6-hydroxy-2-oxide-4-nitropyrimidone through ReaxFF Large-Scale Molecular Dynamics Simulations
Abstract
:1. Introduction
2. Results and Discussion
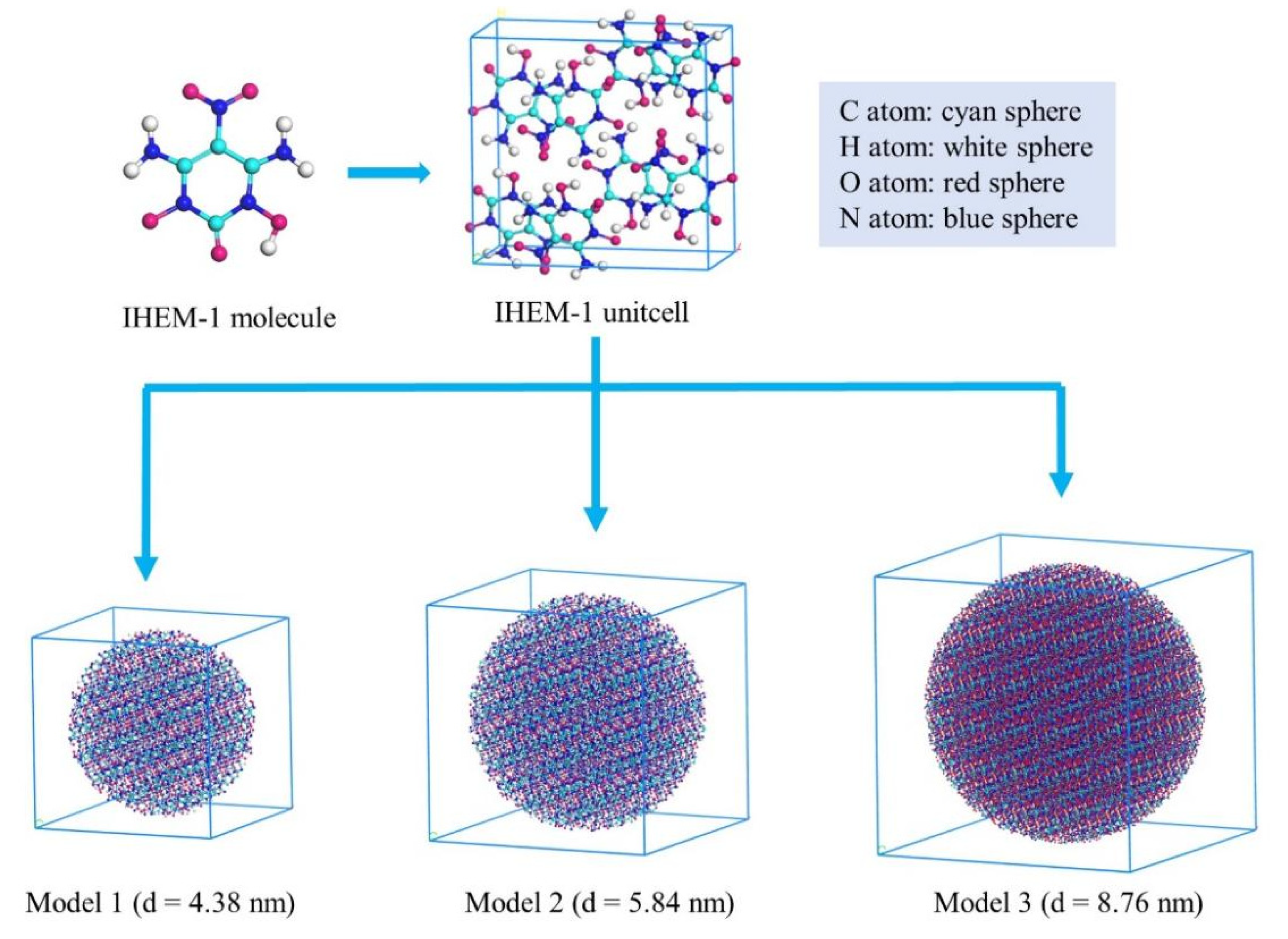
2.1. Early Evolution of the IHEM−1 Molecules
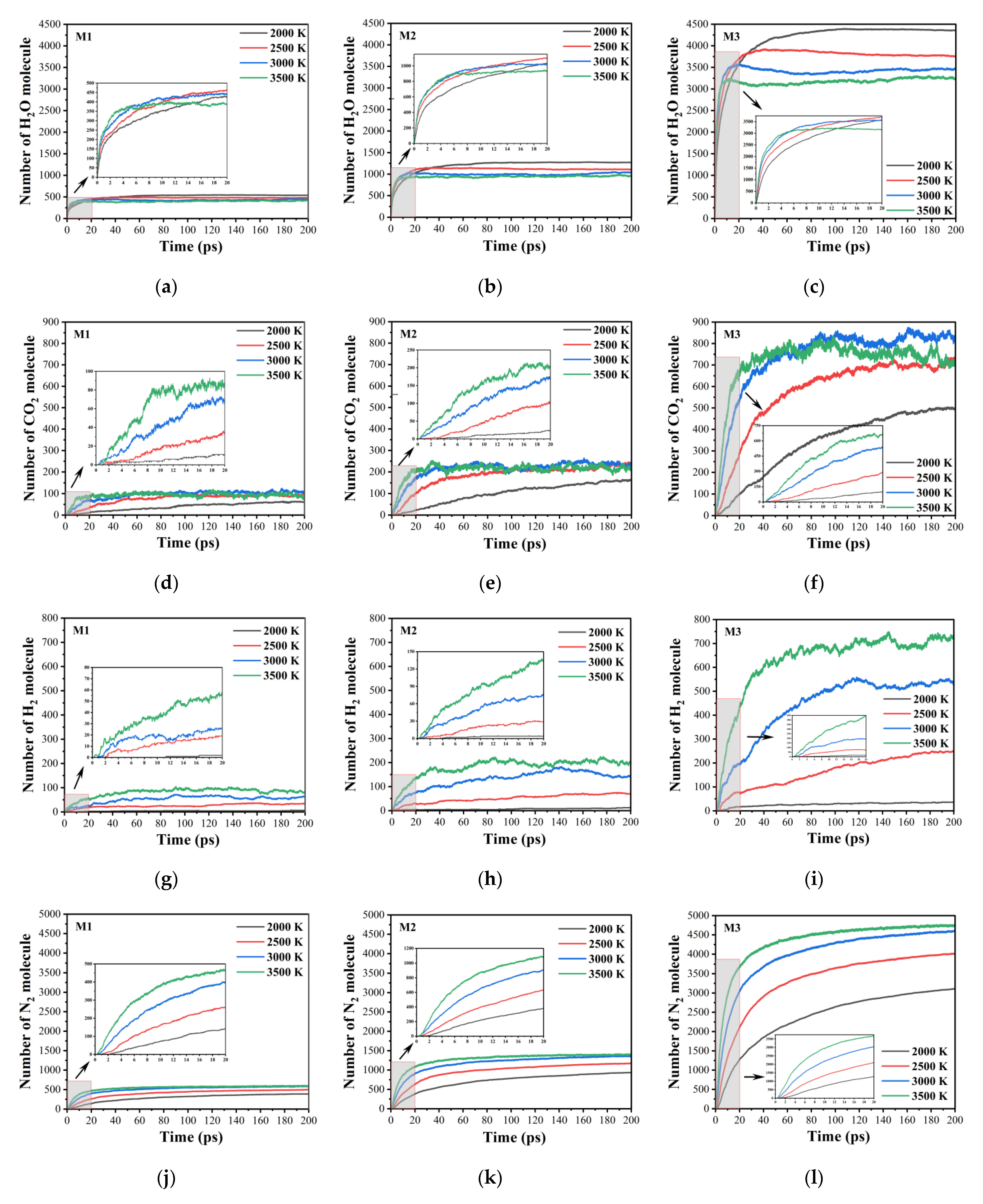
2.2. Evolution of Total Species
2.3. Initial Decomposition Pathway
2.4. Evolution of Main Intermediates
2.5. Evolution of Small Molecule Products
2.6. Evolution of Final Products
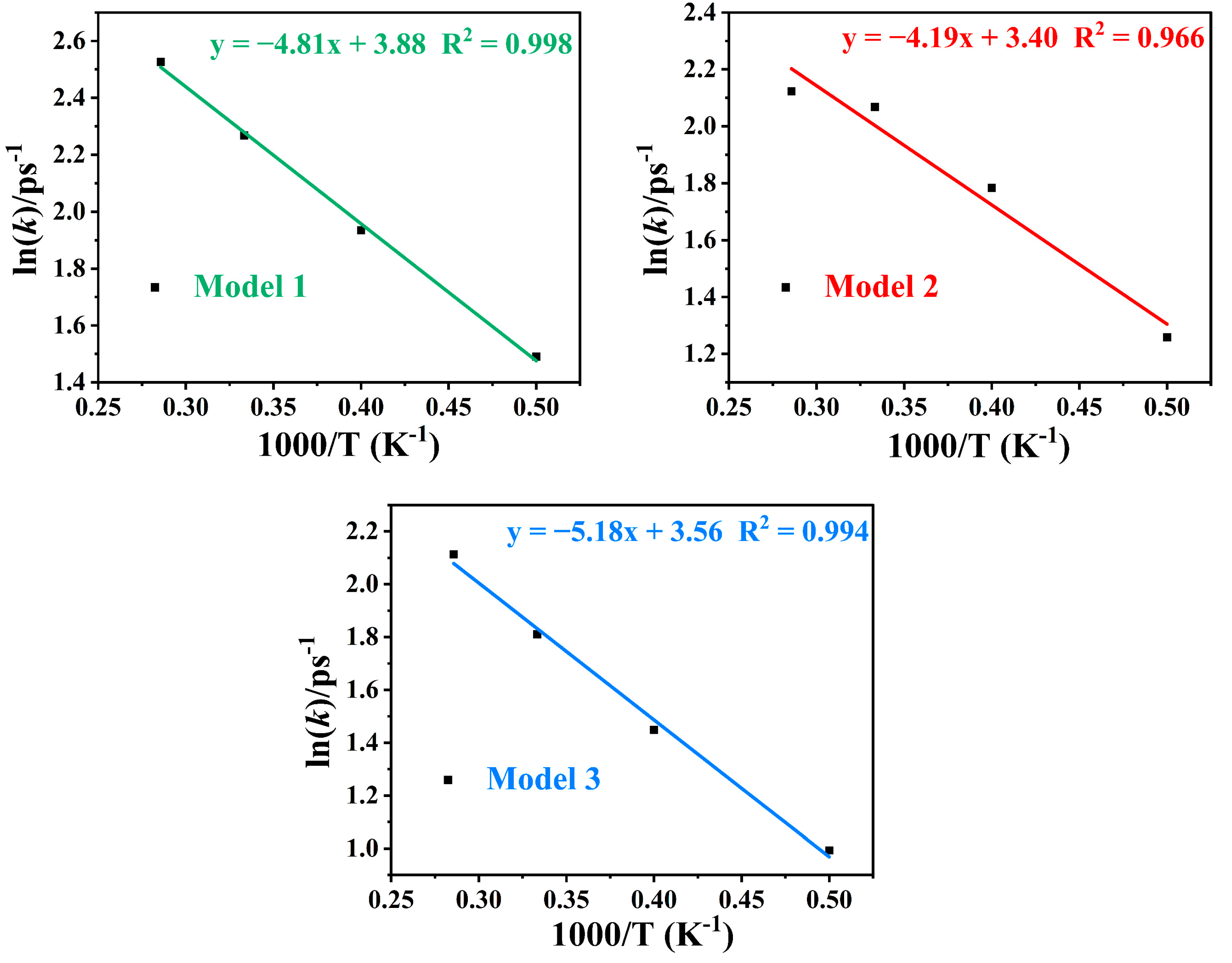
2.7. Decomposition Reaction Kinetics
3. Computational Methods
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhang, J.; Feng, Y.; Bo, Y.; Staples, R.J.; Zhang, J.; Shreeve, J.M. One Step Closer to an Ideal Insensitive Energetic Molecule: 3,5-Diamino-6-Hydroxy-2-Oxide-4-Nitropyrimidone and Its Derivatives. J. Am. Chem. Soc. 2021, 143, 12665–12674. [Google Scholar] [CrossRef] [PubMed]
- Jiang, J.; Wang, H.-R.; Zhao, F.-Q.; Xu, S.-Y.; Ju, X.-H. Decomposition Mechanism of 1,3,5-Trinitro-2,4,6-Trinitroaminobenzene under Thermal and Shock Stimuli Using ReaxFF Molecular Dynamics Simulations. Phys. Chem. Chem. Phys. 2023, 25, 3799–3805. [Google Scholar] [CrossRef] [PubMed]
- Khichar, M.; Patidar, L.; Thynell, S.T. Improvement and Validation of a Detailed Reaction Mechanism for Thermal Decomposition of RDX in Liquid Phase. Combust. Flame 2018, 198, 455–465. [Google Scholar] [CrossRef]
- Zhang, X.-Q.; Chen, X.-R.; Kaliamurthi, S.; Selvaraj, G.; Ji, G.-F.; Wei, D.-Q. Initial Decomposition of the Co-Crystal of CL-20/TNT: Sensitivity Decrease under Shock Loading. J. Phys. Chem. C 2018, 122, 24270–24278. [Google Scholar] [CrossRef]
- Zhang, Z.; Ye, L.; Wang, X.; Wu, X.; Gao, W.; Li, J.; Bi, M. Unraveling the Reaction Mechanism on Pyrolysis of 1,3,5-Trinitro-1,3,5-Triazinane (RDX). Combust. Flame 2022, 242, 112220. [Google Scholar] [CrossRef]
- Zhang, H.-R.; Xue, Z.-H.; Fu, X.; Liu, J.-P.; Qi, X.; Yan, Q.-L. Detailed High Temperature Pyrolysis Mechanisms of Stabilized Hybrid HMX Crystals by Intercalation of 2D Energetic Polymer. Fuel 2022, 324, 124646. [Google Scholar] [CrossRef]
- Liu, L.; Liu, P.; Hu, S.; He, G. Ab Initio Calculations of the N-N Bond Dissociation for the Gas-Phase RDX and HMX. Sci. Rep. 2017, 7, 40630. [Google Scholar] [CrossRef] [PubMed]
- Qian, W.; Xue, X.; Liu, J.; Zhang, C. Molecular Forcefield Methods for Describing Energetic Molecular Crystals: A Review. Molecules 2022, 27, 1611. [Google Scholar] [CrossRef]
- van Duin, A.C.T.; Dasgupta, S.; Lorant, F.; Goddard, W.A. ReaxFF: A Reactive Force Field for Hydrocarbons. J. Phys. Chem. A 2001, 105, 9396–9409. [Google Scholar] [CrossRef]
- Strachan, A.; Kober, E.M.; Van Duin, A.C.T.; Oxgaard, J.; Goddard, W.A. Thermal Decomposition of RDX from Reactive Molecular Dynamics. J. Chem. Phys. 2005, 122, 054502. [Google Scholar] [CrossRef]
- Ren, C.; Li, X.; Guo, L. Reaction Mechanisms in the Thermal Decomposition of CL-20 Revealed by ReaxFF Molecular Dynamics Simulations. Acta Phys.-Chim. Sin. 2018, 34, 1151–1162. [Google Scholar] [CrossRef]
- Wen, Y.; Xue, X.; Zhou, X.; Guo, F.; Long, X.; Zhou, Y.; Li, H.; Zhang, C. Twin Induced Sensitivity Enhancement of HMX versus Shock: A Molecular Reactive Force Field Simulation. J. Phys. Chem. C 2013, 117, 24368–24374. [Google Scholar] [CrossRef]
- Zhang, L.; Zybin, S.V.; Van Duin, A.C.T.; Dasgupta, S.; Goddard, W.A.; Kober, E.M. Carbon Cluster Formation during Thermal Decomposition of Octahydro-1,3,5,7-Tetranitro-1,3,5,7-Tetrazocine and 1,3,5-Triamino-2,4,6-Trinitrobenzene High Explosives from ReaxFF Reactive Molecular Dynamics Simulations. J. Phys. Chem. A 2009, 113, 10619–10640. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wang, T.; He, Y. Initial Response of Pentaerythritol Tetranitrate (PETN) under the Coupling Effect of Preheating, Shock and Defect via the Molecular Dynamics Simulations with the Multiscale Shock Technique Method. Molecules 2023, 28, 2911. [Google Scholar] [CrossRef] [PubMed]
- Yang, K.; Chen, L.; Liu, D.; Lu, J.; Xiao, Y.; Geng, D.; Wu, J. Anisotropic Initial Reaction Mechanism and Sensitivity Characterization of the Layered Crystal Structure Explosive ICM-102 under Shock Loading. J. Phys. Chem. C 2020, 124, 10367–10375. [Google Scholar] [CrossRef]
- Wang, E.; Ding, J.; Qu, Z.; Han, K. Development of a Reactive Force Field for Hydrocarbons and Application to Iso-Octane Thermal Decomposition. Energy Fuels 2018, 32, 901–907. [Google Scholar] [CrossRef]
- Sultan, M.; Wu, J.; Haq, I.U.; Mudassar, M.; Yang, L.; Wu, J.; Lu, J.; Chen, L. A Complete Thermal Decomposition Mechanism Study of an Energetic-energetic CL-20/DNT Cocrystal at Different Extreme Temperatures by Using ReaxFF Reactive Molecular Dynamics Simulations. J. Mol. Struct. 2022, 1269, 133691. [Google Scholar] [CrossRef]
- Liu, L.; Liu, Y.; Zybin, S.V.; Sun, H.; Goddard, W.A. ReaxFF-lg: Correction of the ReaxFF Reactive Force Field for London Dispersion, with Applications to the Equations of State for Energetic Materials. J. Phys. Chem. A 2011, 115, 11016–11022. [Google Scholar] [CrossRef]
- Wang, F.; Chen, L.; Geng, D.; Wu, J.; Lu, J.; Wang, C. Thermal Decomposition Mechanism of CL-20 at Different Temperatures by ReaxFF Reactive Molecular Dynamics Simulations. J. Phys. Chem. A 2018, 122, 3971–3979. [Google Scholar] [CrossRef]
- Hai, L.; Xiao, D.; Yuan-Hang, H. Reactive Molecular Dynamics Simulations of Carbon-Containing Clusters Formation during Pyrolysis of TNT. Acta Phys.-Chim. Sin. 2014, 30, 232–240. [Google Scholar] [CrossRef]
- Wen, Y.; Xue, X.; Long, X.; Zhang, C. Cluster Evolution at Early Stages of 1,3,5-Triamino-2,4,6-Trinitrobenzene under Various Heating Conditions: A Molecular Reactive Force Field Study. J. Phys. Chem. A 2016, 120, 3929–3937. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Qiao, Z.; Dai, X.; Zhang, K.; Li, M.; Pei, G.; Wen, Y. Effects of Different Types of Defects on Ignition Mechanisms in Shocked β-Cyclotetramethylene Tetranitramine Crystals: A Molecular Dynamics Study Based on ReaxFF-lg Force Field. J. Appl. Phys. 2019, 125, 195101. [Google Scholar] [CrossRef]
- Wang, F.; Chen, L.; Geng, D.; Lu, J.; Wu, J. Effect of Density on the Thermal Decomposition Mechanism of ε-CL-20: A ReaxFF Reactive Molecular Dynamics Simulation Study. Phys. Chem. Chem. Phys. 2018, 20, 22600–22609. [Google Scholar] [CrossRef] [PubMed]
- Du, L.; Jin, S.; Nie, P.; She, C.; Wang, J. Initial Decomposition Mechanism of 3-Nitro-1,2,4-Triazol-5-One (NTO) under Shock Loading: ReaxFF Parameterization and Molecular Dynamic Study. Molecules 2021, 26, 4808. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.-J.; Li, H.; Zhu, W. Reactive Molecular Dynamics Simulations on the Decomposition Process of 1,3,5-Trinitro-1,3,5-Triazine Crystal under High Temperatures and Pressure. J. Mol. Model. 2023, 29, 292. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Wu, Y.; Wang, X.; Huang, F.; Huang, X.; Wen, Y. Shock-Induced Energy Localization and Reaction Growth Considering Chemical-Inclusions Effects for Crystalline Explosives. Def. Technol. 2023, S2214914723000405. [Google Scholar] [CrossRef]
- Badran, H.M.; Eid, K.M.; Ammar, H.Y. A DFT Study on the Effect of the External Electric Field on Ammonia Interaction with Boron Nitride Nano-Cage. J. Phys. Chem. Solids 2020, 141, 109399. [Google Scholar] [CrossRef]
- Hu, F.; Wang, L.; Liu, Y.; Hessien, M.M.; Azab, I.H.E.; Jing, S.; Elnaggar, A.Y.; El-Bahy, S.M.; Huang, M.; Zhang, R. Molecular Dynamics Simulation and Experimental Study of 3,5-Difluoro-2,4,6-Trinitroanisole/2,4,6,8,10,12-Hexanitrohexaazaisowurtzitane Mixed Components. Adv. Compos. Hybrid Mater. 2022, 5, 1307–1318. [Google Scholar] [CrossRef]
- Yang, M.; Liao, C.; Tang, C.; Xu, S.; Li, H.; Huang, Z. The Auto-Ignition Behaviors of HMX/NC/NG Stimulated by Heating in a Rapid Compression Machine. Fuel 2021, 288, 119693. [Google Scholar] [CrossRef]
- Yuan, J.; Liu, J.; Zhou, Y.; Zhang, Y.; Cen, K. Thermal Decomposition and Combustion Characteristics of Al/AP/HTPB Propellant. J. Therm. Anal. Calorim. 2021, 143, 3935–3944. [Google Scholar] [CrossRef]
- Xue, X.; Wen, Y.; Long, X.; Li, J.; Zhang, C. Influence of Dislocations on the Shock Sensitivity of RDX: Molecular Dynamics Simulations by Reactive Force Field. J. Phys. Chem. C 2015, 119, 13735–13742. [Google Scholar] [CrossRef]
- Wang, N.; Peng, J.; Pang, A.; He, T.; Du, F.; Jaramillo-Botero, A. Thermodynamic Simulation of the RDX–Aluminum Interface Using ReaxFF Molecular Dynamics. J. Phys. Chem. C 2017, 121, 14597–14610. [Google Scholar] [CrossRef]
- Zhong, K.; Liu, J.; Wang, L.; Zhang, C. Influence of Atmospheres on the Initial Thermal Decomposition of 1,3,5-Trinitro-1,3,5-Triazinane: Reactive Molecular Dynamics Simulation. J. Phys. Chem. C 2019, 123, 1483–1493. [Google Scholar] [CrossRef]
- Larentzos, J.P.; Rice, B.M. Transferable Reactive Force Fields: Extensions of ReaxFF-lg to Nitromethane. J. Phys. Chem. A 2017, 121, 2001–2013. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Wang, H.; Wang, F.; Geng, D.; Wu, J.; Lu, J. Thermal Decomposition Mechanism of 2,2′,4,4′,6,6′-Hexanitrostilbene by ReaxFF Reactive Molecular Dynamics Simulations. J. Phys. Chem. C 2018, 122, 19309–19318. [Google Scholar] [CrossRef]
- Meng, J.; Zhang, S.; Gou, R.; Chen, Y.; Li, Y.; Chen, M.; Li, Z. The Thermal Decomposition Process of Composition B by ReaxFF/lg Force Field. J. Mol. Model. 2020, 26, 245. [Google Scholar] [CrossRef]
- Lan, Q.; Zhang, H.; Ni, Y.; Chen, J.; Wang, H. Thermal Decomposition Mechanisms of LLM-105/HTPB Plastic-Bonded Explosive: ReaxFF-lg Molecular Dynamics Simulations. J. Energetic Mater. 2023, 41, 269–290. [Google Scholar] [CrossRef]
- Han, Q.; Zhu, W. Effect of Particle Size on the Thermal Decomposition of Nano ε-CL-20 by ReaxFF-lg Molecular Dynamics Simulations. Chem. Phys. Lett. 2020, 761, 138067. [Google Scholar] [CrossRef]
- Zheng, K.; Wen, Y.; Huang, B.; Wang, J.; Chen, J.; Xie, G.; Lv, G.; Liu, J.; Qiao, Z.; Yang, G. The Solid Phase Thermal Decomposition and Nanocrystal Effect of Hexahydro-1,3,5-Trinitro-1,3,5-Triazine (RDX) via ReaxFF Large-Scale Molecular Dynamics Simulation. Phys. Chem. Chem. Phys. 2019, 21, 17240–17252. [Google Scholar] [CrossRef]
- Huang, B.; Qiao, Z.; Nie, F.; Cao, M.; Su, J.; Huang, H.; Hu, C. Fabrication of FOX-7 Quasi-Three-Dimensional Grids of One-Dimensional Nanostructures via a Spray Freeze-Drying Technique and Size-Dependence of Thermal Properties. J. Hazard. Mater. 2010, 184, 561–566. [Google Scholar] [CrossRef]
- Yang, G.; Nie, F.; Huang, H.; Zhao, L.; Pang, W. Preparation and Characterization of Nano-TATB Explosive. Propellants Explos. Pyrotech. 2006, 31, 390–394. [Google Scholar] [CrossRef]
- Yang, G.; Nie, F.; Li, J.; Guo, Q.; Qiao, Z. Preparation and Characterization of Nano-NTO Explosive. J. Energetic Mater. 2007, 25, 35–47. [Google Scholar] [CrossRef]
- Fathollahi, M.; Mohammadi, B.; Mohammadi, J. Kinetic Investigation on Thermal Decomposition of Hexahydro-1,3,5-Trinitro-1,3,5-Triazine (RDX) Nanoparticles. Fuel 2013, 104, 95–100. [Google Scholar] [CrossRef]
- Zeng, J.; Cao, L.; Chin, C.-H.; Ren, H.; Zhang, J.Z.H.; Zhu, T. ReacNetGenerator: An Automatic Reaction Network Generator for Reactive Molecular Dynamics Simulations. Phys. Chem. Chem. Phys. 2020, 22, 683–691. [Google Scholar] [CrossRef] [PubMed]
- Ji, J.; Zhu, W. Thermal Decomposition Mechanisms of Benzotrifuroxan:2,4,6-Trinitrotoluene Cocrystal Using Quantum Molecular Dynamics Simulations. Chem. Phys. Lett. 2021, 778, 138820. [Google Scholar] [CrossRef]
- Zhu, S.; Yang, W.; Gan, Q.; Cheng, N.; Feng, C. Early Thermal Decay of Energetic Hydrogen- and Nitro-Free Furoxan Compounds: The Case of DNTF and BTF. Phys. Chem. Chem. Phys. 2022, 24, 1520–1531. [Google Scholar] [CrossRef]
- Tang, Y.; He, C.; Imler, G.H.; Parrish, D.A.; Shreeve, J.M. Aminonitro Groups Surrounding a Fused Pyrazolotriazine Ring: A Superior Thermally Stable and Insensitive Energetic Material. ACS Appl. Energy Mater. 2019, 2, 2263–2267. [Google Scholar] [CrossRef]
- Thompson, A.P.; Aktulga, H.M.; Berger, R.; Bolintineanu, D.S.; Brown, W.M.; Crozier, P.S.; In ’T Veld, P.J.; Kohlmeyer, A.; Moore, S.G.; Nguyen, T.D.; et al. LAMMPS—A Flexible Simulation Tool for Particle-Based Materials Modeling at the Atomic, Meso, and Continuum Scales. Comput. Phys. Commun. 2022, 271, 108171. [Google Scholar] [CrossRef]
- Stukowski, A. Visualization and Analysis of Atomistic Simulation Data with OVITO–the Open Visualization Tool. Model. Simul. Mater. Sci. Eng. 2010, 18, 015012. [Google Scholar] [CrossRef]
- Strachan, A.; Van Duin, A.C.T.; Chakraborty, D.; Dasgupta, S.; Goddard, W.A. Shock Waves in High-Energy Materials: The Initial Chemical Events in Nitramine RDX. Phys. Rev. Lett. 2003, 91, 098301. [Google Scholar] [CrossRef]
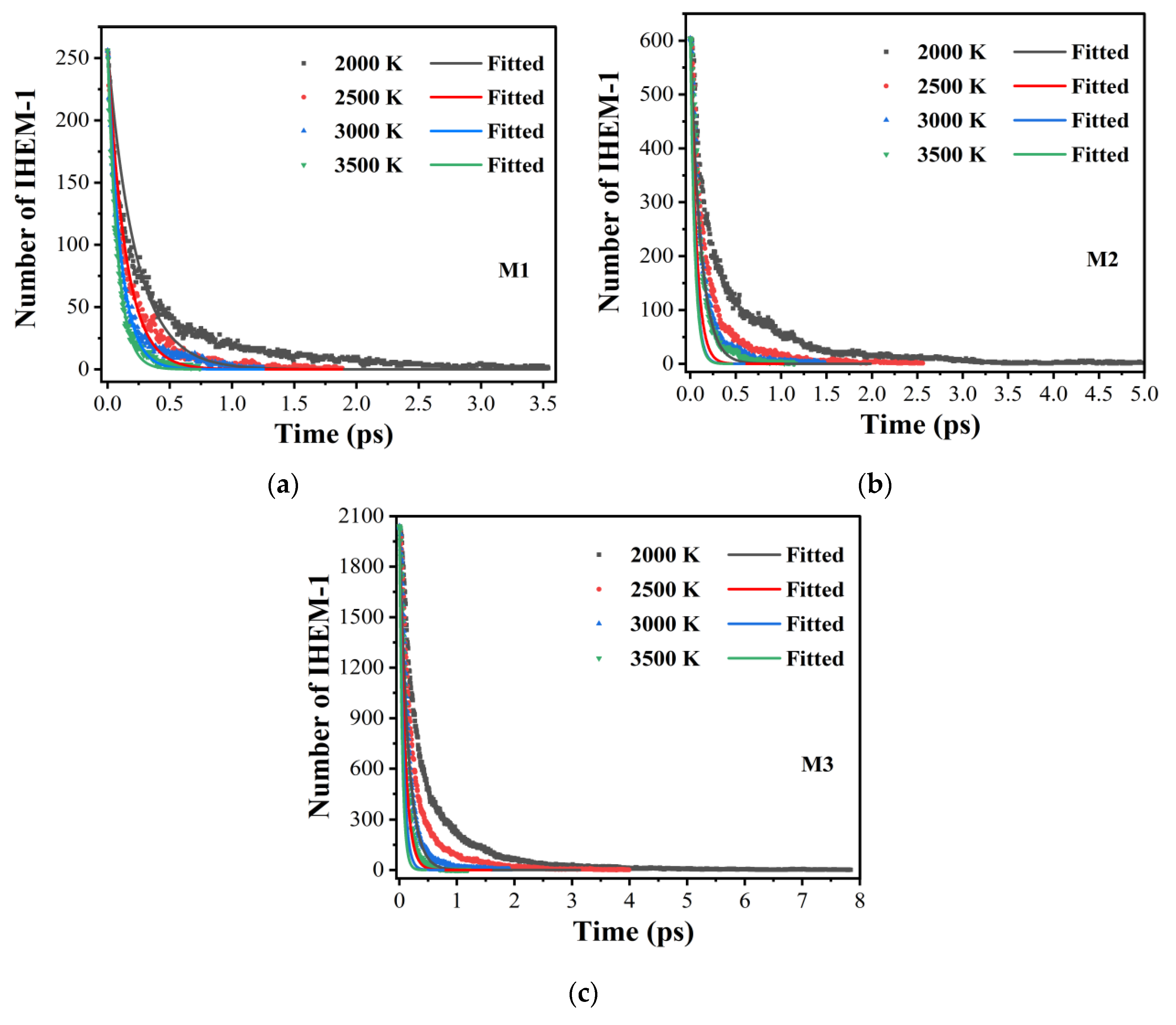

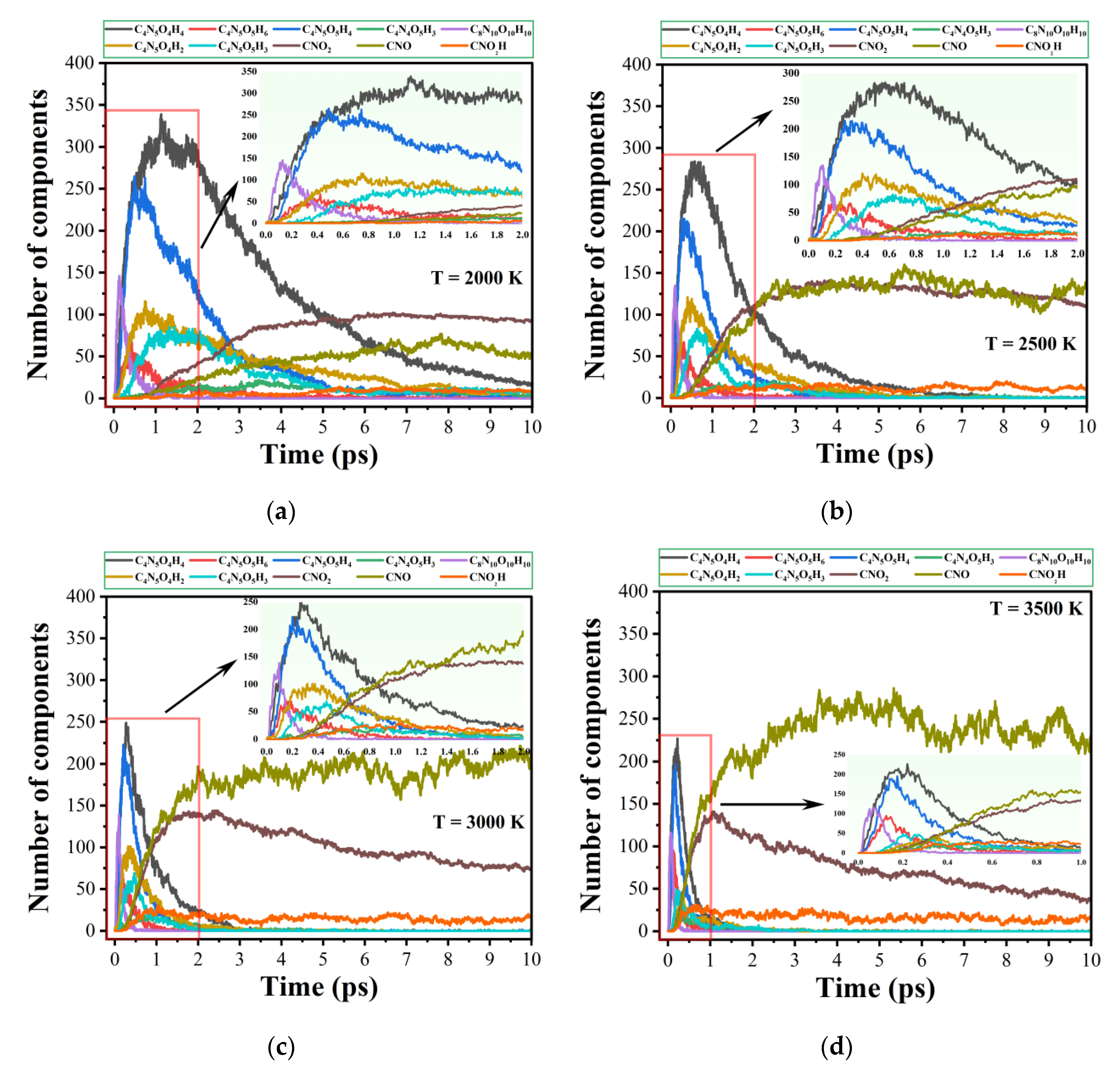
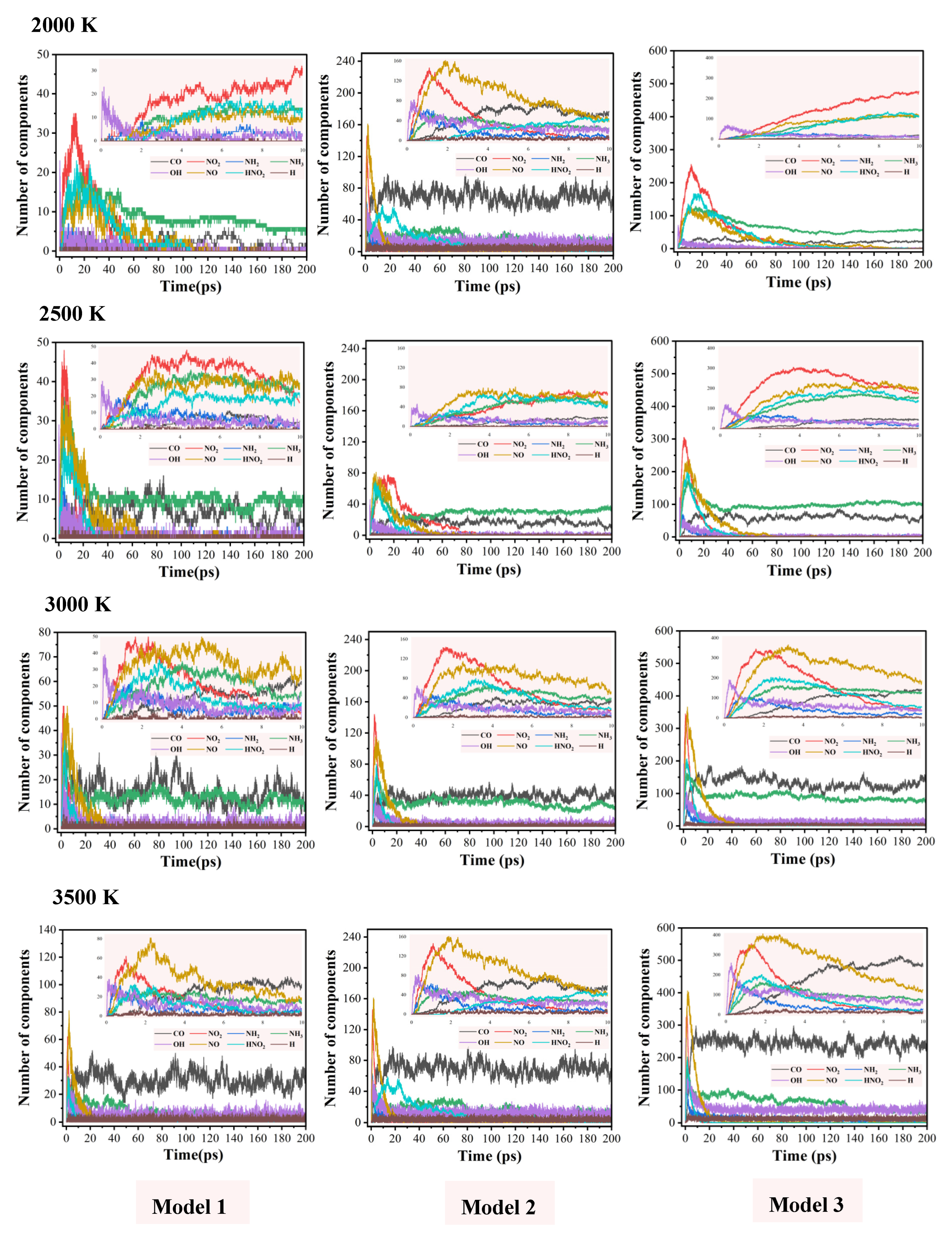
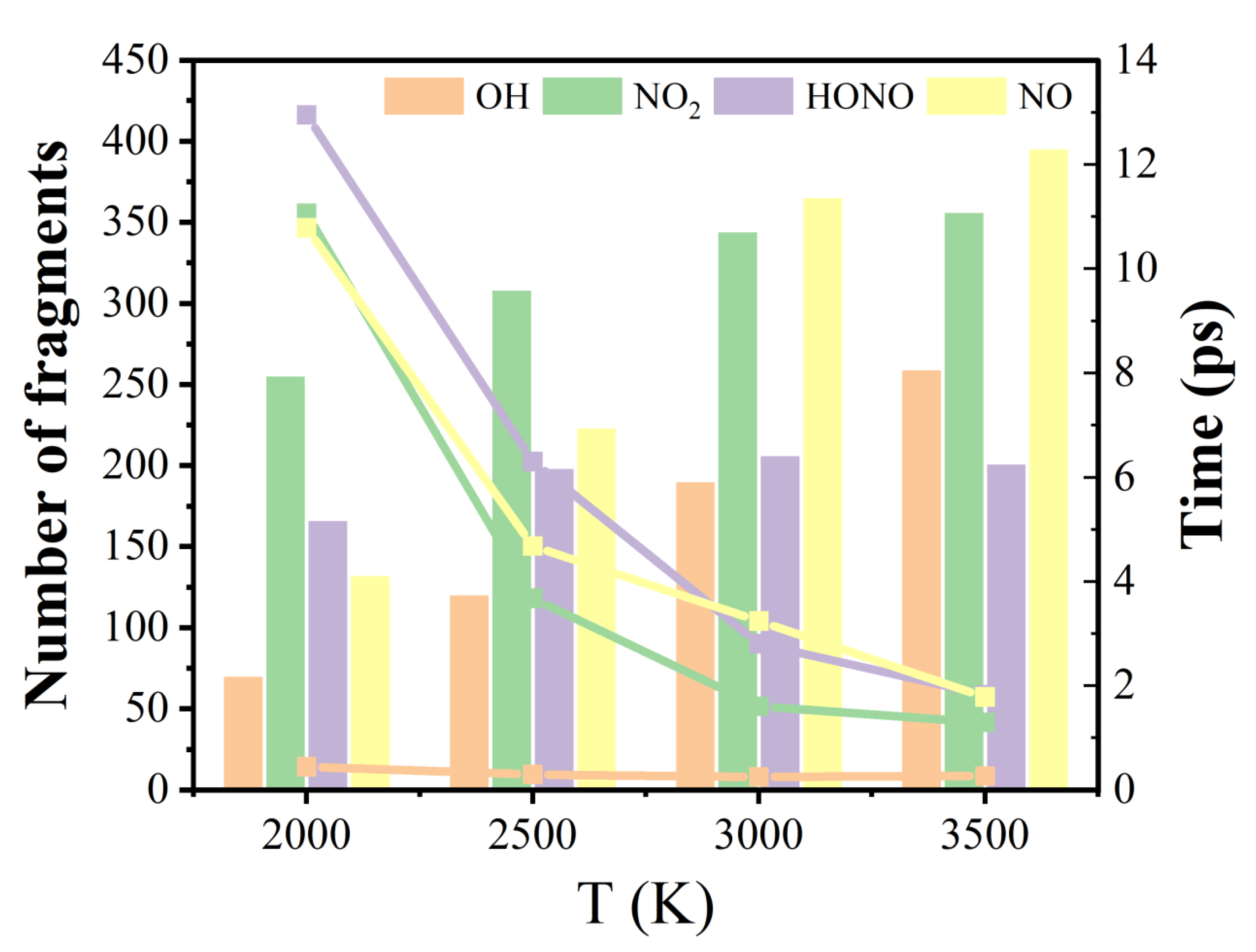
| T (K) | Model 1 | Model 2 | Model 3 |
|---|---|---|---|
| 2000 | 3.54 | 4.96 | 7.785 |
| 2500 | 1.885 | 2.56 | 3.765 |
| 3000 | 1.255 | 1.485 | 1.915 |
| 3500 | 0.745 | 1.15 | 1.195 |
| Path No. | Type | Reactant | Product |
|---|---|---|---|
| Path 1 | Intramolecular H transfer |  | 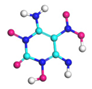 |
| Path 2 | Intramolecular H transfer | 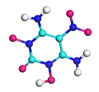 | 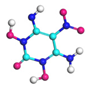 |
| Path 3 | Intermolecular H transfer | 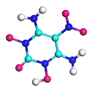 | 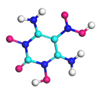 |
| Path 4 | Intermolecular H transfer | 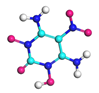 | 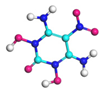 |
| Path 5 | Small moleculeformation (OH) | 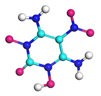 | 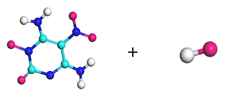 |
| Path 6 | Small moleculeFormation (NH2) | 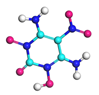 | 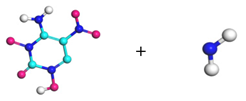 |
| Path 7 | Small moleculeformation (NO2) |  | 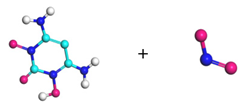 |
| Path 8 | Small moleculeformation (NH2) | 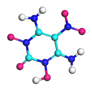 | 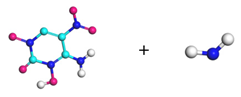 |
| Path 9 | Small moleculeformation (H) | 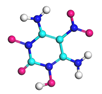 |  |
| Path 10 | Small moleculeformation (H) |  |  |
| Path 11 | Small moleculeformation (H2O) | 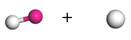 | 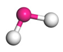 |
| Path 12 | Ring breakage (C-N) |  | 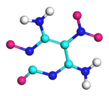 |
| Path 13 | Ring breakage (C-N) | 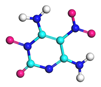 |  |
| Path 14 | Ring breakage (C-N) | 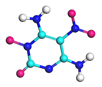 |  |
| Path 15 | Ring breakage (C-N) | 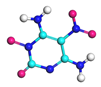 | 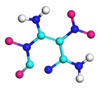 |
| Path 16 | Rearrangement of –NO2 | 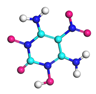 | 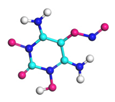 |
| Path 17 | Bimolecular polymerization | 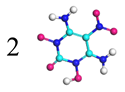 | 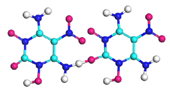 |
| T (K) | kM1/ps−1 | kM2/ps−1 | kM3/ps−1 |
|---|---|---|---|
| 2100 | 4.44 | 3.52 | 2.70 |
| 2400 | 6.92 | 5.95 | 4.26 |
| 2700 | 9.66 | 7.91 | 6.11 |
| 3000 | 12.51 | 8.36 | 8.27 |
| Ea (kJ·mol−1) | 40.03 | 34.81 | 43.06 |
| lnA (ps−1) | 3.88 | 3.40 | 3.56 |
| Model | Atoms | Molecules | Length of Box (Å) | Particle Diameter (nm) |
|---|---|---|---|---|
| 1 | 4864 | 256 | 43.883 | 4.38 |
| 2 | 11,476 | 604 | 58.511 | 5.84 |
| 3 | 38,760 | 2040 | 87.767 | 8.76 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, Z.; Ji, J.; Zhu, W. Effects of Nanoparticle Size on the Thermal Decomposition Mechanisms of 3,5-Diamino-6-hydroxy-2-oxide-4-nitropyrimidone through ReaxFF Large-Scale Molecular Dynamics Simulations. Molecules 2024, 29, 56. https://doi.org/10.3390/molecules29010056
Sun Z, Ji J, Zhu W. Effects of Nanoparticle Size on the Thermal Decomposition Mechanisms of 3,5-Diamino-6-hydroxy-2-oxide-4-nitropyrimidone through ReaxFF Large-Scale Molecular Dynamics Simulations. Molecules. 2024; 29(1):56. https://doi.org/10.3390/molecules29010056
Chicago/Turabian StyleSun, Zijian, Jincheng Ji, and Weihua Zhu. 2024. "Effects of Nanoparticle Size on the Thermal Decomposition Mechanisms of 3,5-Diamino-6-hydroxy-2-oxide-4-nitropyrimidone through ReaxFF Large-Scale Molecular Dynamics Simulations" Molecules 29, no. 1: 56. https://doi.org/10.3390/molecules29010056
APA StyleSun, Z., Ji, J., & Zhu, W. (2024). Effects of Nanoparticle Size on the Thermal Decomposition Mechanisms of 3,5-Diamino-6-hydroxy-2-oxide-4-nitropyrimidone through ReaxFF Large-Scale Molecular Dynamics Simulations. Molecules, 29(1), 56. https://doi.org/10.3390/molecules29010056






