A Comprehensive Review of the Classification, Sources, Phytochemistry, and Pharmacology of Norditerpenes
Abstract
:1. Introduction
2. Chemical Constituents of C19 Norditerpenes
2.1. Labdane
2.2. Clerodane
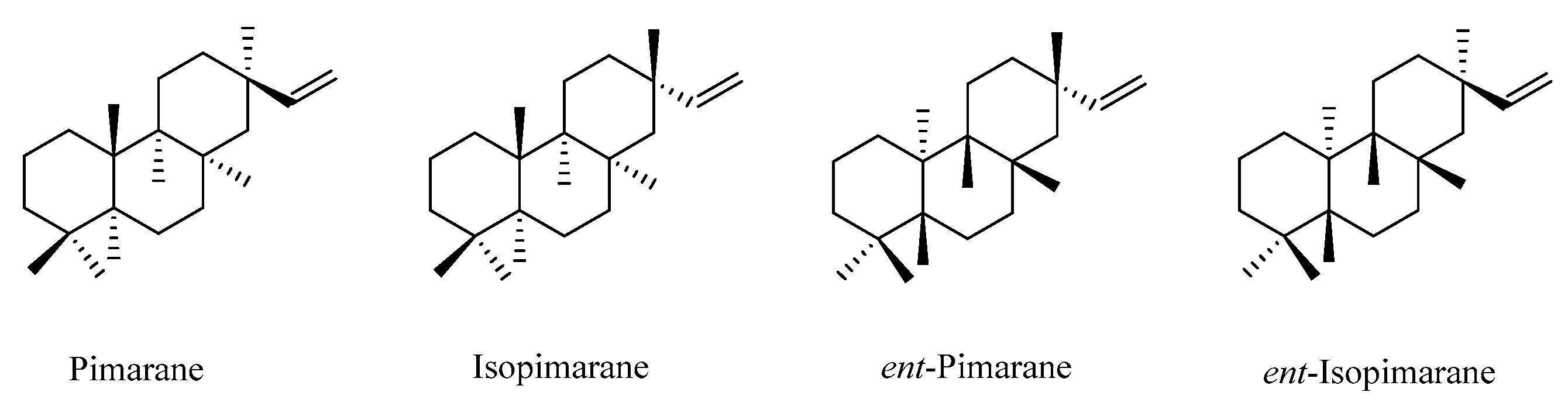
2.3. Pimarane
2.3.1. Pimarane
2.3.2. Isopimarane
2.3.3. Ent-Pimarane
2.3.4. Ent-Isopimarane
2.4. Abietane
2.5. Kaurane
2.6. Cephalotane
2.7. Cembranoid
2.8. Others
3. Chemical Constituents of C18 Norditerpenes
3.1. Abietane
3.2. Podocarpane
3.3. Other Compounds
4. Chemical Constituents of C17 Norditerpenes
4.1. Abietane
4.2. Podocarpane
4.3. Others
5. Chemical Constituents of C16 Norditerpenes
5.1. Labdane
5.2. Others
6. Pharmacological Activities
6.1. Cytotoxic activity
6.2. Antimicrobial Activity
6.2.1. Antibacterial Activity
6.2.2. Antifungal Activity
6.2.3. Antiviral Activity
6.3. Anti-Inflammatory
6.4. Antioxidative Activity
6.5. α-Glucosidase Inhibitory Activity
6.6. Cell Proliferation Activity
6.7. Other Activities
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| A549 | Human non-small cell lung cancer cells |
| AGS | Human gastric adenocarcinoma cells |
| ARE | Antioxidant response element |
| BGC-823 | Human gastric adenocarcinoma cells |
| BMDCs | Bone marrow-derived cells |
| COXs | Cyclooxygenases |
| Con A | Knife-beetle protein A |
| ED50 | Median effective dose |
| GlmU | GlcNAc-P uridyltransferase |
| HepG2 | Human hepatocellular carcinoma cells |
| Hela | Human cervical carcinoma cells |
| Huh-7 | Human hepatocellular carcinoma cells |
| HL-60 | Human promyelocytic leukemia cells |
| HT-29 | Human colon cancer cells |
| HCTs | Human colon cancer cells |
| HAECs | Human aortic endothelial cells |
| iNOS | Inducible nitric oxide synthase |
| IC50 | 50% inhibiting concentration |
| ICAM-1 | Intercellular adhesion molecule-1 |
| ILs | Interleukins |
| JNK | c-Jun NH2-terminal kinase |
| KBs | Human oral epidermoid carcinoma cells |
| LPS | Lipopolysaccharide |
| MIC | Minimum inhibitory concentration |
| MCF-7 | Human breast cancer cells |
| MDA-MB | Triple-negative breast cancer cells |
| MG132 | A proteasome inhibitor |
| NF-κB | Nuclear factor kappa B |
| NO | Nitric oxide |
| NGF | Nerve growth factor |
| PANC-1 | Human pancreatic cancer cells |
| PDTC | Pyrrolidinedithiocarbamate |
| SMMC-7721 | Human hepatocarcinoma cells |
| SW-480 | Human colon cancer cells |
| SGC-7901 | Human stomach cancer cells |
| SK-BR-3 | Human breast cancer cells |
| SKOV3 | Human ovarian cancer cells |
| STAT | Signal transducers and activators of transcription |
| T-47D | Human breast duct cancer cells |
| TNF-α | Tumor necrosis factor-α |
| TCM | Traditional Chinese medicine |
| VCAM-1 | Vascular cell adhesion molecule-1 |
References
- Shen, Y.; Liang, W.J.; Shi, Y.N.; Kennelly, E.J.; Zhao, D.K. Structural diversity, bioactivities, and biosynthesis of natural diterpenoid alkaloids. Nat. Prod. Rep. 2020, 37, 763–796. [Google Scholar] [CrossRef] [PubMed]
- Bian, X.Q.; Bai, J.; Hu, X.L.; Wu, X.; Xue, C.M.; Han, A.H.; Su, G.Y.; Hua, H.M.; Pei, Y.H. Penioxalicin, a novel 3-nor-2,3-seco-labdane type diterpene from the fungus Penicillium oxalicum TW01-1. Tetrahedron Lett. 2015, 56, 5013–5016. [Google Scholar] [CrossRef]
- Li, Y.L.; Liu, W.; Han, S.Y.; Zhang, J.; Xu, W.; Li, Q.; Cheng, Z.B. Penitholabene, a rare 19-nor labdane-type diterpenoid from the deep-sea-derived fungus Penicillium thomii YPGA3. Fitoterapia 2020, 146, 104691. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.L.; Yang, X.Y.; Zhang, X.K.; Song, Z.T.; Liu, F.; Liang, Y.; Zhang, J.; Jin, D.Q.; Xu, J.; Lee, D.; et al. Bioactive terpenoids from Euonymus verrucosus var. pauciflorus showing no inhibitory activities. Bioorg. Chem. 2019, 87, 447–456. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Linuma, M.; Wang, J.S.; Oyama, M.; Ito, T.; Kong, L.Y. Terpenoids from Chloranthus serratus and their anti-inflammatory activities. J. Nat. Prod. 2012, 75, 694–698. [Google Scholar] [CrossRef]
- Li, Y.; Wang, T.T.; Zhao, J.; Shi, X.; Hu, S.C.; Gao, K. Norditerpenoids from Agathis macrophylla. Food Chem. 2012, 131, 972–976. [Google Scholar] [CrossRef]
- Kim, T.H.; Li, H.; Wu, Q.; Lee, H.J.; Ryu, J.H. A new labdane diterpenoid with anti-inflammatory activity from Thuja orientalis. J. Ethnopharmacol. 2013, 146, 760–767. [Google Scholar] [CrossRef]
- Balbinot, R.B.; Oliveira, J.A.M.D.; Bernardi, D.I.; Melo, U.Z.; Zanqueta, E.B.; Endo, E.H.; Ribeiro, F.M.; Volpato, H.; Figueiredo, M.C.; Back, D.F.; et al. Structural characterization and biological evaluation of 18-nor-ent-labdane diterpenoids from Grazielia gaudichaudeana. Chem. Biodivers. 2019, 16, e1800644. [Google Scholar] [CrossRef]
- Yoshinori, S.; Sachie, M.; Suyatno, S.; Motoo, T. Nine new norlabdane diterpenoids from the leaves of Austroeupatorium inulifolium. Helv. Chim. Acta 2011, 94, 313–326. [Google Scholar] [CrossRef]
- Chacon-Morales, P.A.; Amaro-Luis, J.M.; Fermin, L.B.R.; Peixoto, P.A.; Deffieux, D.; Pouysegu, L.; Quideau, S. Preparation and bactericidal activity of oxidation derivatives of austroeupatol, an ent-nor-furano diterpenoid of the labdane series from Austroeupatorium inulifolium. Phytochem. Lett. 2019, 29, 47–52. [Google Scholar] [CrossRef]
- Yin, H.; Luo, J.G.; Shan, S.M.; Wang, X.B.; Luo, J.; Yang, M.H.; Kong, L.Y. Amomaxins A and B, two unprecedented rearranged labdane norditerpenoids with a nine-membered ring from Amomum maximum. Org. Lett. 2013, 15, 1572–1575. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Q.; Xiao, L.G.; Bi, L.S.; Si, Y.; Zhang, X.M.; Chen, J.H.; Liu, H.Y. Hedychins E and F: Labdane-type norditerpenoids with anti-Inflammatory activity from the rhizomes of Hedychium forrestii. Org. Lett. 2022, 24, 6936–6939. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.N.; Zhang, J.Z.; Li, X.; Wang, X.N.; Xie, C.F.; Zhou, J.C.; Lou, H.X. Pallambins A and B, unprecedented hexacyclic 19-nor-secolabdane diterpenoids from the Chinese liverwort Pallavicinia ambigua. Org. Lett. 2012, 14, 1102–1105. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.M.; Jiang, W.Q.; Feng, Q.; Lu, H.; Zhou, Y.P.; Liao, J.; Wang, Q.T.; Sheng, G.Y. Identification of 15-nor-cleroda-3,12-diene in a Dominican amber. Org. Geochem. 2017, 113, 90–96. [Google Scholar] [CrossRef]
- Bi, D.W.; Xiong, F.; Cheng, B.; Zhou, Y.L.; Zeb, M.A.; Tang, P.; Pang, W.H.; Zhang, R.H.; Li, X.L.; Zhang, X.J.; et al. Callintegers A and B, unusual tricyclo[4.4.0.09,10]tetradecane clerodane diterpenoids from Callicarpa integerrima with inhibitory effects on NLRP3 inflammasome activation. J. Nat. Prod. 2022, 85, 2675–2681. [Google Scholar] [CrossRef] [PubMed]
- Soares, B.A.; Firme, C.L.; Maciel, M.A.M.; Kaiser, C.R.; Schilling, E.; Bortoluzzi, A.J. Experimental and NMR theoretical methodology applied to geometric analysis of the bioactive clerodane trans-dehydrocrotonin. J. Braz. Chem. Soc. 2014, 25, 629–638. [Google Scholar] [CrossRef]
- Zou, M.F.; Pan, Y.H.; Hu, R.; Yuan, F.Y.; Huang, D.; Tang, G.H.; Li, W.; Yin, S. Highly modified nor-clerodane diterpenoids from Croton yanhuii. Fitoterapia 2021, 153, 104979. [Google Scholar] [CrossRef]
- Pan, Z.H.; Ning, D.S.; Wu, X.D.; Huang, S.S.; Li, D.P.; Lv, S.H. New clerodane diterpenoids from the twigs and leaves of Croton euryphyllus. Bioorg. Med. Chem. Lett. 2015, 25, 1329–1332. [Google Scholar] [CrossRef]
- Zou, M.F.; Hu, R.; Liu, Y.X.; Fan, R.Z.; Xie, X.L.; Yin, S. Two highly oxygenated nor-clerodane diterpenoids from Croton caudatus. J. Asian. Nat. Prod. Res. 2020, 22, 927–934. [Google Scholar] [CrossRef]
- Wang, B.; Zhang, P.L.; Zhou, M.X.; Shen, T.; Zou, Y.X.; Lou, H.X.; Wang, X.N. New nor-clerodane-type furanoditerpenoids from the rhizomes of Tinospora capillipes. Phytochem. Lett. 2016, 15, 225–229. [Google Scholar] [CrossRef]
- Ye, G.H.; Xue, J.J.; Liang, W.L.; Yang, S.J. Three new bioactive diterpenoids from the roots of Croton crassifolius. Nat. Prod. Res. 2021, 35, 1421–1427. [Google Scholar] [CrossRef] [PubMed]
- Lv, H.W.; Luo, J.G.; Zhu, M.D.; Shan, S.M.; Kong, L.Y. Teucvisins A-E, five new neo-clerodane diterpenes from Teucrium viscidum. Chem. Pharm. Bull. 2014, 62, 472–476. [Google Scholar] [CrossRef] [PubMed]
- Huang, D.; Luo, X.K.; Yin, Z.Y.; Xu, J.; Gu, Q. Diterpenoids from the aerial parts of Flueggea acicularis and their activity against RANKL-induced osteoclastogenesis. Bioorg. Chem. 2020, 94, 103453. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Guo, P.Y.; Liu, A.J.; Sui, S.Y.; Shi, S.; Guo, S.X.; Dai, J.G. Aquilariaenes A–H, eight new diterpenoids from Chinese eaglewood. Fitoterapia 2019, 133, 180–185. [Google Scholar] [CrossRef] [PubMed]
- Shin, B.; Kim, B.Y.; Cho, E.J.; Oh, K.B.; Shin, J.H.; Goodfellow, M.; Oh, D.C. Actinomadurol, an antibacterial norditerpenoid from a rare actinomycete, Actinomadura sp. KC 191. J. Nat. Prod. 2016, 79, 1886–1890. [Google Scholar] [CrossRef] [PubMed]
- Guo, B.; Zhao, M.; Wu, Z.L.; Onakpa, M.M.; Burdette, J.E.; Che, C.T. 19-nor-pimaranes from Icacina trichantha. Fitoterapia 2020, 144, 104612. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.M.; Zhou, J.F.; Zeng, L.P.; Xu, J.C.; Onakpa, M.M.; Duan, J.A.; Che, C.T.; Bi, H.K.; Zhao, M. Pimarane-derived diterpenoids with anti-Helicobacter pylori activity from the tuber of Icacina trichantha. Org. Chem. Front. 2021, 8, 3014–3022. [Google Scholar] [CrossRef]
- Zhao, M.; Onakpa, M.M.; Chen, W.L.; Santarsiero, B.D.; Swanson, S.M.; Burdette, J.E.; Asuzu, I.U.; Che, C.T. 17-Norpimaranes and (9βH)-17-norpimaranes from the tuber of Icacina trichantha. J. Nat. Prod. 2015, 78, 789–796. [Google Scholar] [CrossRef]
- Li, C.X.; Li, B.; Ye, J.; Zhang, W.D.; Shen, Y.H.; Yin, J. A new norditerpenoid from Euonymus grandiflorus Wall. Nat. Prod. Res. 2013, 27, 1716–1721. [Google Scholar] [CrossRef]
- Joy, M.; Chakraborty, K. An unprecedented antioxidative isopimarane norditerpenoid from bivalve clam, Paphia malabarica with anti-cyclooxygenase and lipoxygenase potential. Pharm. Biol. 2017, 55, 819–824. [Google Scholar] [CrossRef]
- Wang, X.N.; Bashyal, B.P.; Wijeratne, E.M.K.; U'Ren, J.M.; Liu, M.P.; Gunatilaka, M.K.; Arnold, A.E.; Gunatilaka, A.A.L. Smardaesidins A-G, isopimarane and 20-nor-isopimarane diterpenoids from Smardaea sp., a fungal endophyte of the moss Ceratodon purpureus. J. Nat. Prod. 2011, 74, 2052–2061. [Google Scholar] [CrossRef] [PubMed]
- Di Lecce, R.; Masi, M.; Linaldeddu, B.T.; Pescitelli, G.; Maddau, L.; Evidente, A. Bioactive specialized metabolites produced by the emerging pathogen Diplodia olivarum. Beilstein. Archi. 2020, 2020, 105. [Google Scholar] [CrossRef]
- Kato, H.; Sebe, M.; Nagaki, M.; Eguchi, K.; Kagiyama, I.; Hitora, Y.; Frisvad, J.C.; Williams, R.M.; Tsukamoto, S. Taichunins A-D, Norditerpenes from Aspergillus taichungensis. J. Nat. Prod. 2019, 82, 1377–1381. [Google Scholar] [CrossRef] [PubMed]
- Li, X.D.; Li, X.M.; Li, X.; Xu, G.M.; Liu, Y.; Wang, B.G. Aspewentins D–H, 20-nor-isopimarane derivatives from the deep sea sediment-derived fungus Aspergillus wentii SD-310. J. Nat. Prod. 2016, 79, 1347–1353. [Google Scholar] [CrossRef] [PubMed]
- Li, X.D.; Lin, X.; Li, X.M.; Xu, G.M.; Liu, Y.; Wang, B.G. 20-nor-isopimarane epimers produced by Aspergillus wentii SD-310, a fungal strain obtained from deep sea sediment. Mar. Drugs 2018, 16, 440. [Google Scholar] [CrossRef] [PubMed]
- Miao, F.P.; Liang, X.R.; Liu, X.H.; Ji, N.Y. Aspewentins A–C, norditerpenes from a cryptic pathway in an algicolous strain of Aspergillus wentii. J. Nat. Prod. 2014, 77, 429–432. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.P.; Li, J.; Zhao, Z.Z.; Li, X.Y.; Liu, S.L.; Wang, Q.Y.; Liu, J.K. Diterpenes with bicyclo[2.2.2]octane moieties from the fungicolous fungus Xylaria longipes HFG1018. Org. Biomol. Chem. 2020, 18, 2410–2415. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Li, X.M.; Li, X.D.; Xu, G.M.; Liu, Y.; Wang, B.G. 20-Nor-isopimarane cycloethers from the deep-sea sediment-derived fungus Aspergillus wentii SD-310. RSC Adv. 2016, 79, 75981–75987. [Google Scholar] [CrossRef]
- Li, H.; Zhao, J.J.; Chen, J.L.; Zhu, L.P.; Wang, D.M.; Jiang, L.; Yao, D.P.; Zhao, Z.M. Diterpenoids from aerial parts of Flickingeria fimbriata and their nuclear factor-kappaB inhibitory activities. Phytochemistry 2015, 117, 400–409. [Google Scholar] [CrossRef]
- Chen, J.L.; Zhao, Z.M.; Xue, X.; Tang, G.H.; Zhu, L.P.; Yang, D.P.; Jiang, L. Bioactive norditerpenoids from Flickingeria fimbriata. RSC Adv. 2014, 4, 14447–14456. [Google Scholar] [CrossRef]
- Li, C.J.; Dai, W.F.; Liu, D.; Jiang, M.Y.; Zhang, Z.J.; Chen, X.Q.; Chen, C.H.; Li, R.T.; Li, H.M. Bioactive ent-isopimarane diterpenoids from Euphorbia neriifolia. Phytochemistry 2020, 175, 112373. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.X.; Xie, C.F.; An, L.J.; Yang, X.Y.; Xi, Y.R.; Yuan, S.; Zhang, C.Y.; Tuerhong, M.; Jin, D.Q.; Lee, D.H.; et al. Bioactive diterpenoids from the stems of Euphorbia royleana. J. Nat. Prod. 2019, 82, 183–193. [Google Scholar] [CrossRef] [PubMed]
- Kadir, A.; Zheng, G.J.; Zheng, X.F.; Jin, P.F.; Maiwulanjiang, M.; Gao, B.; Aisa, H.A.; Yao, G.M. Structurally diverse diterpenoids from the roots of Salvia deserta based on nine different skeletal types. J. Nat. Prod. 2021, 84, 1442–1452. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.W.; Liu, X.; Xie, D.; Chen, R.D.; Tao, X.Y.; Zou, J.H.; Dai, J.G. Two new diterpenoids from cell cultures of Salvia miltiorrhiza. Chem. Pharm. Bull. 2013, 61, 576–580. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.; Li, L.; Wang, D.D.; Wang, H.Q.; Liu, C.; Li, B.M.; Yan, Y.; Fang, L.H.; Du, G.H.; Chen, R.Y. Isolation and bioactivity of diterpenoids from the roots of Salvia grandifolia. Phytochemistry 2015, 116, 337–348. [Google Scholar] [CrossRef]
- Hussain, A.; Adhikari, A.; Choudhary, M.I.; Ayatollahi, S.A.; Atta-Ur-Rahman. New adduct of abietane-type diterpene from Salvia leriifolia Benth. Nat. Prod. Res. 2016, 30, 1511–1516. [Google Scholar] [CrossRef]
- Eghtesadi, F.; Farimani, M.M.; Hazeri, N.; Valizadeh, J. Abietane and nor-abitane diterpenoids from the roots of Salvia rhytidea. SpringerPlus 2016, 5, 1068. [Google Scholar] [CrossRef]
- Alizadeh, Z.; Farimani, M.M.; Parisi, V.; Marzocco, S.; Ebrahimi, S.N.; Tommasi, N.D. Nor-abietane diterpenoids from Perovskia abrotanoides roots with anti-inflammatory potential. J. Nat. Prod. 2021, 84, 1185–1197. [Google Scholar] [CrossRef]
- Cao, Q.; Wang, S.X.; Chen, Y.X. Abietane diterpenoids with potent cytotoxic activities from the resins of Populus euphratica. Nat. Prod. Commune 2019, 14, 1934578X19850029. [Google Scholar] [CrossRef]
- Pan, Z.H.; Li, Y.; Wu, X.D.; He, J.; Chen, X.Q.; Xu, G.; Peng, L.Y.; Zhao, Q.S. Norditerpenoids from Salvia castanea Diels f. pubescens. Fitoterapia 2012, 83, 1072–1075. [Google Scholar] [CrossRef]
- Li, J.Y.; Peng, Y.; Li, L.Z.; Gao, P.Y.; Gao, C.; Xia, S.X.; Song, S.J. Two new abietane diterpenoids from the roots of Tripterygium wilfordii Hook. f. Helv. Chim. Acta 2013, 96, 313–319. [Google Scholar] [CrossRef]
- Yan, X.L.; Zou, M.F.; Chen, B.L.; Yuan, F.Y.; Zhu, Q.F.; Zhang, X.; Lin, Y.; Long, Q.D.; Liu, W.L.; Liao, S.G. Euphorane C, an unusual C17-norabietane diterpenoid from Euphorbia dracunculoides induces cell cycle arrest and apoptosis in human leukemia K562 cells. Arab. J. Chem. 2022, 15, 104203. [Google Scholar] [CrossRef]
- Zhao, H.M.; Li, H.L.; Huang, G.L.; Chen, Y.G. A new abietane mono-norditerpenoid from Podocarpus nagi. Nat. Prod. Res. 2017, 31, 844–848. [Google Scholar] [CrossRef] [PubMed]
- Lusarczyk, S.; Senol Deniz, F.S.; Abel, R.; Pecio, Ł.; Pérez-Sánchez, H.; Cerón-Carrasco, J.P.; Den-Haan, H.; Banerjee, P.; Preissner, R.; Krzyżak, E.; et al. Norditerpenoids with selective anti-cholinesterase activity from the roots of Perovskia atriplicifolia Benth. Int. J. Mol. Sci. 2020, 21, 4475. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.L.; Wang, X.Z.; Xiao, J.; Luo, X.H.; Yao, X.J.; Zhao, Y.Y.; Chen, Y.J.; Crews, P.; Wu, Q.X. New abietane diterpenoids from the roots of Salvia przewalskii. Tetrahedron 2013, 69, 6687–6692. [Google Scholar] [CrossRef]
- Cheng, Q.Q.; He, Y.F.; Li, G.; Liu, Y.J.; Gao, W.; Huang, L.Q. Effects of combined Eelicitors on tanshinone metabolic profiling and SmCPS expression in Salvia miltiorrhiza hairy root culture. Molecules 2013, 18, 7473–7485. [Google Scholar] [CrossRef] [PubMed]
- He, K.; Zou, J.; Wang, Y.X.; Zhao, C.L.; Ye, J.H.; Zhang, J.J.; Pan, L.T.; Zhang, H.J. Rubesanolides F and G: Two novel lactone-type norditerpenoids from Isodon rubescens. Molecules 2021, 26, 3865. [Google Scholar] [CrossRef]
- Yu, P.; Zhang, S.D.; Li, Y.L.; Yang, X.W.; Zeng, H.W.; Li, H.L.; Zhang, W.D. Abieseconordines A and B, two novel norditerpenoids with a 18-nor-5,10: 9,10-disecoabietane skeleton from Abies forrestii. Helv. Chim. Acta 2012, 95, 415–422. [Google Scholar] [CrossRef]
- Wang, L.J.; Xiong, J.; Liu, S.T.; Pan, L.L.; Hu, J.F. ent-Abietane-type and related seco-/nor-diterpenoids from the rare chloranthaceae plant Chloranthus sessilifolius and their nntineuroinflammatory activities. J. Nat. Prod. 2015, 78, 1635–1646. [Google Scholar] [CrossRef]
- Xie, T.T.; Ma, S.L.; Lou, H.X.; Zhu, R.X.; Sun, L.R. Two novel abietane norditerpenoids with anti-inflammatory properties from the roots of Salvia miltiorrhiza var. alba. Tetrahedron Lett. 2014, 55, 7106–7109. [Google Scholar] [CrossRef]
- Wang, W.G.; Yan, B.C.; Li, X.N.; Du, X.; Wu, H.Y.; Zhan, R.; Li, Y.; Pu, J.X.; Sun, H.D. 6,7-Seco-ent-kaurane-type diterpenoids from Isodon eriocalyx var. laxiflora. Tetrahedron 2014, 70, 7445–7453. [Google Scholar] [CrossRef]
- Zou, J.; Du, X.; Pang, G.; Shi, Y.M.; Wang, W.G.; Zhan, R.; Kong, L.M.; Li, X.N.; Li, Y.; Pu, J.X. Ternifolide A, a new diterpenoid possessing a rare macrolide motif from Isodon ternifolius. Org. Lett. 2012, 14, 3210–3213. [Google Scholar] [CrossRef] [PubMed]
- Ueno, A.K.; Barcellos, A.F.; Costa-Silva, T.A.; Mesquita, J.T.; Ferreira, D.D.; Tempone, A.G.; Romoff, P.; Antar, G.M.; Lago, J.H.G. Antitrypanosomal activity and evaluation of the mechanism of action of diterpenes from aerial parts of Baccharis retusa. Fitoterapia 2018, 125, 55–58. [Google Scholar] [CrossRef] [PubMed]
- Faiella, L.; Piaz, F.D.; Bader, A.; Braca, A. Diterpenes and phenolic compounds from Sideritis pullulans. Phytochemistry 2014, 106, 164–170. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Liang, Y.R.; Chen, S.X.; Wang, W.X.; Zou, Y.K.; Nuryyeva, S.; Houk, K.N.; Xiong, J.; Hu, J.F. Amentotaxins C–V, Structurally diverse diterpenoids from the leaves and twigs of the vulnerable conifer Amentotaxus argotaenia and their cytotoxic effects. J. Nat. Prod. 2020, 83, 2129–2144. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, M.L.; Llanos, G.G.; Castanys, S.; Gamarro, F.; Bazzocchi, I.L.; Jimenez, I.A. Terpenoids from Maytenus species and assessment of their reversal activity against a multidrug-resistant Leishmania tropica line. Chem. Biodivers. 2011, 8, 2291–2298. [Google Scholar] [CrossRef] [PubMed]
- Ren, H.; Xu, Q.L.; Luo, Y.; Zhang, M.; Zhou, Z.Y.; Dong, L.M.; Tan, J.W. Two new ent-kaurane diterpenoids from Wedelia trilobata (L.) Hitchc. Phytochem. Lett. 2015, 11, 260–263. [Google Scholar] [CrossRef]
- Isyaka, S.M.; Mas-Claret, E.; Langat, M.K.; Hodges, T.; Selway, B.; Mbala, B.M.; Mvingu, B.K.; Mulholland, D.A. Cytotoxic diterpenoids from the leaves and stem bark of Croton haumanianus (Euphorbiaceae). Phytochemistry 2020, 178, 112455. [Google Scholar] [CrossRef]
- Zhou, X.Q.; Li, S.Q.; Liao, C.C.; Dai, W.F.; Rao, K.R.; Ma, X.R.; Li, R.T.; Chen, X.Q. Structurally diversified ent-kaurane and abietane diterpenoids from the stems of Tripterygium wilfordii and their anti-inflammatory activity. Bioorg. Chem. 2021, 115, 105178. [Google Scholar] [CrossRef]
- Zhou, C.X.; Sun, L.R.; Feng, F.; Mo, J.X.; Zhu, H.; Yang, B.; He, Q.J.; Gan, L.S. Cytotoxic diterpenoids from the stem bark of Annona squamosa L. Helv. Chim. Acta 2013, 96, 656–662. [Google Scholar] [CrossRef]
- Yang, J.; Wang, W.G.; Wu, H.Y.; Liu, M.; Jiang, H.Y.; Du, X.; Li, Y.; Pu, J.X.; Sun, H.D. ent-Kaurene diterpenoids from Isodon phyllostachys. Tetrahedron Lett. 2017, 58, 349–351. [Google Scholar] [CrossRef]
- Zhan, R.; Li, X.N.; Du, X.; Wang, W.G.; Dong, K.; Su, J.; Li, Y.; Pu, J.X.; Sun, H.D. Bioactive ent-kaurane diterpenoids from Isodon rosthornii. J. Nat. Prod. 2013, 76, 1267–1277. [Google Scholar] [CrossRef]
- Xu, J.B.; Fan, Y.Y.; Gan, L.S.; Zhou, Y.B.; Li, J.; Yue, J.M. Cephalotanins A–D, four norditerpenoids represent three highly rigid carbon skeletons from Cephalotaxus sinensis. Chemistry 2016, 22, 14648–14654. [Google Scholar] [CrossRef]
- Ge, Z.P.; Zhou, B.; Zimbres, F.M.; Cassera, M.B.; Zhao, J.X.; Yue, J.M. Cephalotane-type norditerpenoids from Cephalotaxus fortunei var. alpine. Chin. J. Chem. 2022, 40, 1177–1184. [Google Scholar] [CrossRef]
- Fan, Y.Y.; Xu, J.B.; Liu, H.C.; Gan, L.S.; Ding, J.; Yue, J.M. Cephanolides A–J, cephalotane-type diterpenoids from Cephalotaxus sinensis. J. Nat. Prod. 2017, 80, 3159–3166. [Google Scholar] [CrossRef]
- Ge, Z.P.; Liu, H.C.; Wang, G.C.; Liu, Q.F.; Xu, C.H.; Ding, J.; Fan, Y.Y.; Yue, J.M. 17-nor-Cephalotane-type diterpenoids from Cephalotaxus fortunei. J. Nat. Prod. 2019, 82, 1565–1575. [Google Scholar] [CrossRef]
- Zhao, J.X.; Fan, Y.Y.; Xu, J.B.; Gan, L.S.; Xu, C.H.; Ding, J.; Yue, J.M. Diterpenoids and lignans from Cephalotaxus fortune. J. Nat. Prod. 2017, 80, 356–362. [Google Scholar] [CrossRef]
- Ni, L.; Zhong, X.H.; Chen, X.J.; Zhang, B.J.; Bao, M.F.; Cai, X.H. Bioactive norditerpenoids from Cephalotaxus fortunei var. alpina and C. lanceolata. Phytochemistry 2018, 151, 50–60. [Google Scholar] [CrossRef]
- Ni, G.; Zhang, H.; Fan, Y.; Liu, H.; Ding, J.; Yue, J.M. Mannolides A–C with an intact diterpenoid skeleton providing insights on the biosynthesis of antitumor Cephalotaxus Troponoids. Org. Lett. 2016, 18, 1880–1883. [Google Scholar] [CrossRef]
- Ge, Z.P.; Fan, Y.Y.; Deng, W.D.; Zheng, C.Y.; Li, T.; Yue, J.M. Cephalodiones A-D: Compound characterization and semisynthesis by [6+6] cycloaddition. Angew. Chem. Int. Ed. Engl. 2021, 60, 9374–9378. [Google Scholar] [CrossRef]
- Cui, W.X.; Yang, M.; Li, H.; Li, S.W.; Yao, L.G.; Li, G.; Tang, W.; Wang, C.H.; Liang, L.F.; Guo, Y.W. Polycyclic furanobutenolide-derived norditerpenoids from the South China Sea soft corals Sinularia scabra and Sinularia polydactyla with immunosuppressive activity. Bioorg. Chem. 2020, 94, 103350. [Google Scholar] [CrossRef]
- Nguyen, P.T.; Nguyen, H.N.; Nguyen, X.C.; Quang, T.H.; Tung, P.T.; Dat, L.D.; Chae, D.; Kim, S.; Koh, Y.S.; Kiem, P.V. Anti-inflammatory norditerpenoids from the soft coral Sinularia maxima. Bioorg. Med. Chem. Lett. 2013, 23, 228–231. [Google Scholar] [CrossRef]
- Wang, C.L.; Jin, T.Y.; Liu, X.H.; Zhang, J.R.; Shi, X.; Wang, M.F.; Huang, R.F.; Zhang, Y.; Liu, K.C.; Li, G.Q. Sinudenoids A–E, C19-norcembranoid diterpenes with unusual scaffolds from the soft coral Sinularia densa. Org. Lett. 2022, 24, 9007–9011. [Google Scholar] [CrossRef]
- Craig, R.A.; Smith, R.C.; Roizen, J.L.; Jones, A.C.; Virgil, S.C.; Stoltz, B.M. Development of a unified enantioselective, convergent synthetic Aapproach toward the furanobutenolide-derived polycyclic norcembranoid diterpenes: Asymmetric formation of the polycyclic norditerpenoid carbocyclic core by tandem annulation cascade. J. Org. Chem. 2018, 83, 3467–3485. [Google Scholar] [CrossRef]
- Thomas, S.A.L.; von Salm, J.L.; Clark, S.; Ferlita, S.; Nemani, P.; Azhari, A.; Rice, C.A.; Wilson, N.G.; Kyle, D.E.; Baker, B.J. Keikipukalides, furanocembrane diterpenes from the antarctic deep sea octocoral Plumarella delicatissima. J. Nat. Prod. 2018, 81, 117–123. [Google Scholar] [CrossRef]
- Cheng, W.; Ji, M.; Li, X.D.; Ren, J.W.; Yin, F.L.; van Ofwegen, L.; Yu, S.W.; Chen, X.G.; Lin, W.H. Fragilolides A–Q, norditerpenoid and briarane diterpenoids from the gorgonian coral Junceella fragilis. Tetrahedron 2017, 73, 2518–2528. [Google Scholar] [CrossRef]
- Liu, J.; Tang, Q.; Huang, J.; Li, T.; Ouyang, H.; Lin, W.H.; Yan, X.J.; Yan, X.; He, S. Sinuscalide A: An antiviral norcembranoid with an 8/8-fused carbon scaffold from the South China Sea soft coral Sinularia scabra. J. Org. Chem. 2022, 87, 9806–9814. [Google Scholar] [CrossRef]
- Liu, W.W.; Zhang, Y.; Yuan, C.M.; Yu, C.; Ding, J.Y.; Li, X.X.; Hao, X.J.; Wang, Q.; Li, S.L. Japodagricanones A and B, novel diterpenoids from Jatropha podagrica. Fitoterapia 2014, 98, 156–159. [Google Scholar] [CrossRef]
- Chakraborty, K.; Krishnan, S.; Joy, M. Antioxidative oxygenated terpenoids with bioactivities against pro-inflammatory inducible enzymes from Indian squid, Uroteuthis (Photololigo) duvaucelii. Nat. Prod. Res. 2021, 35, 909–920. [Google Scholar] [CrossRef]
- Li, L.L.; Chen, L.; Li, Y.H.; Sun, S.K.; Ma, S.G.; Li, Y.; Qu, J. Cassane and nor-cassane diterpenoids from the roots of Erythrophleum fordii. Phytochemistry 2020, 174, 112343. [Google Scholar] [CrossRef]
- Kamikawa, S.; Oshimo, S.; Ohta, E.; Nehira, T.; Omura, H.; Ohta, S. Cassane diterpenoids from the roots of Caesalpinia decapetala var. japonica and structure revision of caesaljapin. Phytochemistry 2016, 121, 50–57. [Google Scholar] [CrossRef]
- Sun, P.; Cao, D.H.; Xiao, Y.D.; Zhang, Z.Y.; Wang, J.N.; Shi, X.C.; Xiao, C.F.; Hu, H.B.; Xu, Y.K. Aspidoptoids A–D: Four new diterpenoids from Aspidopterys obcordata vine. Molecules 2020, 25, 529. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.Q.; Lv, J.J.; Wang, Y.M.; Xu, M.; Zhu, H.T.; Wang, D.; Yang, C.R.; Wang, Y.F.; Zhang, Y.J. Phyllanflexoid C: First example of phenylacetylene-bearing 18-nor-diterpenoid glycoside from the roots of Phyllanthus flexuosus. Tetrahedron Lett. 2013, 54, 4670–4674. [Google Scholar] [CrossRef]
- Li, F.L.; Lin, S.; Zhang, S.T.; Pan, L.F.; Chai, C.W.; Su, J.C.; Yang, B.Y.; Liu, J.J.; Wang, J.P.; Hu, Z.X. Modified fusicoccane-type diterpenoids from Alternaria brassicicola. J. Nat. Prod. 2020, 83, 1931–1938. [Google Scholar] [CrossRef]
- Wu, J.; Zhang, H.; He, L.M.; Xue, Y.Q.; Jia, J.; Wang, S.B.; Zhu, K.K.; Hong, K.; Cai, Y.S. A new fusicoccane-type norditerpene and a new indone from the marine-derived fungus Aspergillus aculeatinus WHUF0198. Chem. Biodivers. 2021, 18, e2100562. [Google Scholar] [CrossRef]
- Bie, Q.; Chen, C.M.; Yu, M.Y.; Guo, J.R.; Wang, J.P.; Liu, J.J.; Zhou, Y.; Zhu, H.C.; Zhang, Y.H. Dongtingnoids A–G: Fusicoccane diterpenoids from a Penicillium Species. J. Nat. Prod. 2019, 82, 80–86. [Google Scholar] [CrossRef] [PubMed]
- Kawakami, S.; Toyoda, H.; Harinantenaina, L.; Matsunami, K.; Otsuka, H.; Shinzato, T.; Takeda, Y.; Kawahata, M.; Yamaguchi, K. Eight new diterpenoids and two new nor-diterpenoids from the stems of Croton cascarilloides. Chem. Pharm. Bull. 2013, 61, 411–418. [Google Scholar] [CrossRef] [PubMed]
- Liang, W.J.; Geng, C.A.; Zhang, X.M.; Chen, H.; Yang, C.Y.; Rong, G.Q.; Zhao, Y.; Xu, H.B.; Wang, H.; Zhou, N.J. (±)-Paeoveitol, a pair of new norditerpene enantiomers from Paeonia veitchii. Org. Lett. 2014, 16, 424–427. [Google Scholar] [CrossRef]
- Zhao, J.X.; Liu, C.P.; Qi, W.Y.; Han, M.L.; Han, Y.S.; Wainberg, M.A.; Yue, J.M. Eurifoloids A–R, structurally diverse diterpenoids from Euphorbia neriifolia. J. Nat. Prod. 2014, 77, 2224–2233. [Google Scholar] [CrossRef]
- Oanh, V.T.K.; Ha, N.T.T.; Duc, H.V.; Thuc, D.N.; Hang, N.T.M.; Thanh, L.N. New triterpene and nor-diterpene derivatives from the leaves of Adinandra poilanei. Phytochem. Lett. 2021, 45, 110–113. [Google Scholar] [CrossRef]
- Liang, X.R.; Miao, F.P.; Song, Y.P.; Liu, X.H.; Ji, N.Y. Citrinovirin with a new norditerpene skeleton from the marine algicolous fungus Trichoderma citrinoviride. Bioorg. Med. Chem. Lett. 2016, 26, 5029–5031. [Google Scholar] [CrossRef]
- White, A.M.; Pierens, G.K.; Forster, L.C.; Winters, A.E.; Cheney, K.L.; Garson, M.J. Rearranged diterpenes and norditerpenes from three Australian Goniobranchus Mollusks. J. Nat. Prod. 2016, 79, 447–483. [Google Scholar] [CrossRef]
- Han, G.Y.; Sun, D.Y.; Liang, L.F.; Yao, L.G.; Chen, K.X.; Guo, Y.W. Spongian diterpenes from Chinese marine sponge Spongia officinalis. Fitoterapia 2018, 127, 159–165. [Google Scholar] [CrossRef]
- Zhang, L.Q.; Zhao, Y.Y.; Huang, C.; Chen, K.X.; Li, Y.M. Scrodentoids F-I, four C19-norditerpenoids from Scrophularia dentate. Tetrahedron 2016, 72, 8031–8035. [Google Scholar] [CrossRef]
- Mao, G.H.; Sun, L.Q.; Xu, J.W.; Li, Y.M.; Dunzhu, C.; Zhang, L.Q.; Qian, F. Scrodentoids H and I, a pair of natural epimerides from Scrophularia dentata, inhibit inflammation through JNK-STAT3 axis in THP-1 cells. Evid. Based Complement. Alternat. Med. 2020, 2020, 1842347. [Google Scholar] [CrossRef]
- San-Martin, A.; Bacho, M.; Nunez, S.; Rovirosa, J.; Soler, A.; Blanc, V.; Leon, R.; Olea, A.F. A novel normulinane isolated from Azorella compacta and assessment of its antibacterial activity. J. Chil. Chem. Soc. 2018, 63, 4082–4085. [Google Scholar] [CrossRef]
- Lam, Y.T.H.; Palfner, G.; Lima, C.; Porzel, A.; Brandt, W.; Frolov, A.; Sultani, H.; Franke, K.; Wagner, C.; Merzweiler, K. Nor-guanacastepene pigments from the Chilean mushroom Cortinarius pyromyxa. Phytochemistry 2019, 165, 112048. [Google Scholar] [CrossRef]
- Nidhal, N.; Zhou, X.M.; Chen, G.Y.; Zhang, B.; Han, C.R.; Song, X.P. Chemical constituents of Leucas zeylanica and their chemotaxonomic significance. Biochem. Syst. Ecol. 2020, 89, 104006. [Google Scholar] [CrossRef]
- Chen, Z.H.; Li, W.S.; Zhang, Z.Y.; Luo, H.; Wang, J.R.; Zhang, H.Y.; Zeng, Z.R.; Chen, B.; Li, X.W.; Guo, Y.W. Sinusiaetone A, an anti-inflammatory norditerpenoid with a bicyclo[11.3.0]hexadecane nucleus from the Hainan soft coral Sinularia siaesensis. Org. Lett. 2021, 23, 5621–5625. [Google Scholar] [CrossRef]
- Wang, S.S.; Cheng, Y.B.; Lin, Y.C.; Liaw, C.C.; Chang, J.Y.; Kuo, Y.H.; Shen, Y.C. Nitrogen-containing diterpenoids, sesquiterpenoids, and nor-diterpenoids from Cespitularia taeniata. Mar. Drugs 2015, 13, 5796–5814. [Google Scholar] [CrossRef]
- Lin, Y.C.; Lin, C.C.; Chu, Y.C.; Fu, C.W.; Sheu, J.H. Bioactive diterpenes, norditerpenes, and sesquiterpenes from a Formosan soft coral Cespitularia sp. Pharmaceuticals 2021, 14, 1252. [Google Scholar] [CrossRef]
- Gao, P.Y.; Li, L.Z.; Liu, K.C.; Sun, C.; Sun, X.; Wu, Y.N.; Song, S.J. Natural terpenoid glycosides with in vitro/vivo antithrombotic profiles from the leaves of Crataegus pinnatifida. RSC Adv. 2017, 7, 48466–48474. [Google Scholar] [CrossRef]
- Liu, Z.G.; Li, Z.L.; Bai, J.; Meng, D.L.; Li, N.; Pei, Y.H.; Zhao, F.; Hua, H.M. Anti-inflammatory diterpenoids from the roots of Euphorbia ebracteolata. J. Nat. Prod. 2014, 77, 792–799. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, Y.B.; Li, Y.; Li, L.; Ma, S.G.; Qu, J.; Jiang, J.D.; Chen, X.G.; Zhang, D.; Yu, S.S. Terpenoids from the roots of Alangium chinense. J. Asian Nat. Prod. Res. 2015, 17, 1025–1038. [Google Scholar] [CrossRef]
- Li, L.Z.; Liang, X.; Sun, X.; Qi, X.L.; Wang, J.; Zhao, Q.C.; Song, S.J. Bioactive norditerpenoids and neolignans from the roots of Salvia miltiorrhiza. Org. Biomol. Chem. 2016, 14, 10050–10057. [Google Scholar] [CrossRef]
- Rusman, Y.; Wilson, M.B.; Williams, J.M.; Held, B.W.; Blanchette, R.A.; Anderson, B.N.; Lupfer, C.R.; Salomon, C.E. Antifungal norditerpene oidiolactones from the fungus Oidiodendron truncatum, a potential biocontrol agent for whitenose syndrome in bats. J. Nat. Prod. 2020, 83, 344–353. [Google Scholar] [CrossRef]
- Olivon, F.; Retailleau, P.; Desrat, S.; Touboul, D.; Roussi, F.; Apel, C.; Litaudon, M. Isolation of picrotoxanes from Austrobuxus carunculatus using taxonomy-based molecular networking. J. Nat. Prod. 2020, 83, 3069–3079. [Google Scholar] [CrossRef]
- Olivon, F.; Retailleau, P.; Desrat, S.; Touboul, D.; Roussi, F.; Apel, C.; Litaudon, M. Sinuhirtone A, an uncommon 17, 19-dinorxeniaphyllanoid, and nine related new terpenoids from the Hainan soft coral Sinularia hirta. Mar. Drugs 2022, 20, 272. [Google Scholar] [CrossRef]
- Prieto, I.M.; Paola, A.; Perez, M.; Garcia, M.; Blustein, G.; Schejter, L.; Palermo, J.A. Antifouling Diterpenoids from the Sponge Dendrilla Antarctica. Chem. Biodivers. 2022, 19, e202100618. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.L.; Wang, C.; Cheng, Z.B.; Tian, X.G.; Jia, J.M.; Cui, Y.L.; Feng, L.; Sun, C.P.; Zhang, B.J.; Ma, X.C. Diterpenoids isolated from Euphorbia ebracteolata roots and their inhibitory effects on α-Glucosidase. J. Nat. Prod. 2017, 80, 3218–3223. [Google Scholar] [CrossRef]
- Wu, C.Y.; Liao, Y.; Yang, Z.G.; Yang, X.W.; Shen, X.L.; Li, R.T.; Xu, G. Cytotoxic diterpenoids from Salvia yunnanensis. Phytochemistry 2014, 106, 171–177. [Google Scholar] [CrossRef]
- Xu, G.; Yang, J.; Wang, Y.Y.; Peng, L.Y.; Yang, X.W.; Pan, Z.H.; Liu, E.D.; Li, Y.; Zhao, Q.S. Diterpenoid constituents of the roots of Salvia digitaloides. J. Agric. Food Chem. 2010, 58, 12157–12161. [Google Scholar] [CrossRef]
- Yao, F.; Zhang, D.W.; Qu, G.W.; Li, G.S.; Dai, S.J. New abietane norditerpenoid from Salvia miltiorrhiza with cytotoxic activities. J. Asian. Nat. Prod. Res. 2012, 14, 913–917. [Google Scholar] [CrossRef]
- Zhu, Q.; Tang, C.P.; Ke, C.Q.; Li, X.Q.; Liu, J.; Gan, L.S.; Weiss, H.; Gesing, E.; Ye, Y. Constituents of Trigonostemon chinensis. J. Nat. Prod. 2010, 73, 40–44. [Google Scholar] [CrossRef]
- Jiang, Z.Y.; Zhou, J.; Huang, C.G.; Hu, Q.F.; Huang, X.Z.; Wang, W.; Zhang, L.Z.; Li, G.P.; Xia, F.T. Two novel antiviral terpenoids from the cultured Perovskia atriplicifolia. Tetrahedron 2015, 71, 3844–3849. [Google Scholar] [CrossRef]
- Zhu, Q.; Tang, C.P.; Mandi, A.; Kurtan, T.; Ye, Y. Trigonostemons G and H, dinorditerpenoid dimers with axially chiral biaryl linkage from Trigonostemon chinensis. Chirality 2020, 32, 265–272. [Google Scholar] [CrossRef]
- Wang, X.F.; Liu, F.F.; Zhu, Z.D.; Fang, Q.Q.; Qu, S.J.; Zhu, W.L.; Yang, L.; Zuo, J.P.; Tan, C.H. Flueggenoids A–E, new dinorditerpenoids from Flueggea virosa. Fitoterapia 2019, 133, 96–101. [Google Scholar] [CrossRef]
- Chao, C.H.; Cheng, J.C.; Shen, D.Y.; Wu, T.S. Anti-hepatitis C virus dinorditerpenes from the roots of Flueggea virosa. J. Nat. Prod. 2014, 77, 22–28. [Google Scholar] [CrossRef]
- Zhu, J.Y.; Zhang, C.Y.; Dai, J.J.; Rahman, K.; Zhang, H. Diterpenoids with thioredoxin reductase inhibitory activities from Jatropha multifida. Nat. Prod. Res. 2017, 31, 2753–2758. [Google Scholar] [CrossRef] [PubMed]
- Chang, F.R.; Wang, S.W.; Chen, S.R.; Lee, C.Y.; Sheu, J.H.; Cheng, Y.B. Aleuritin, a novel dinor-diterpenoid from the twigs of Aleurites moluccanus with an anti-lymphangiogenic effect. Org. Biomol. Chem. 2020, 18, 7892–7898. [Google Scholar] [CrossRef] [PubMed]
- Qi, Y.Y.; Su, J.; Zhang, Z.J.; Li, L.W.; Fan, M.; Zhu, Y.; Wu, X.D.; Zhao, Q.S. Two new anti-proliferative C18-norditerpenes from the roots of Podocarpus macrophyllus. Chem. Biodivers. 2018, 15, e1800043. [Google Scholar] [CrossRef] [PubMed]
- Huang, T.; Ying, S.H.; Li, J.Y.; Chen, H.W.; Zang, Y.; Wang, W.X.; Li, J.; Xiong, J.; Hu, J.F. Phytochemical and biological studies on rare and endangered plants endemic to China. Part XV. Structurally diverse diterpenoids and sesquiterpenoids from the vulnerable conifer Pseudotsuga sinensis. Phytochemistry 2020, 169, 112184. [Google Scholar] [CrossRef] [PubMed]
- Mirzania, F.; Farimani, M.M.; Sarrafi, Y.; Ebrahimi, S.N.; Troppmair, J.; Kwiatkowski, M.; Stuppner, H.; Alilou, M. New sesterterpenoids from Salvia mirzayanii Rech.f. and Esfand. stereochemical characterization by computational electronic circular dichroism. Front. Chem. 2021, 9, 783292. [Google Scholar] [CrossRef] [PubMed]
- Cheng, B.; Fang, F.; Zhou, Q.T.; Li, Y.; Wu, X.W.; Zhao, X.R.; Bi, D.W.; Zhang, X.J.; Zhang, R.H.; Ji, X. Highly oxygenated labdane diterpenoids from Stevia rebaudiana and their anti-atherosclerosis activities. Chem. Biodivers. 2023, 20, e202200999. [Google Scholar] [CrossRef] [PubMed]
- Lv, X.J.; Li, Y.; Ma, S.G.; Qu, J.; Liu, Y.B.; Li, L.; Wang, R.B.; Yu, S.S. Isopimarane and nor-diterpene glucosides from the twigs and leaves of Lyonia ovalifolia. Tetrahedron 2017, 73, 776–784. [Google Scholar] [CrossRef]
- Ge, Y.Z.; Zhang, H.; Liu, H.C.; Dong, L.; Ding, J.; Yue, J.M. Cytotoxic dinorditerpenoids from Drypetes perreticulata. Phytochemistry 2014, 100, 120–125. [Google Scholar] [CrossRef]
- Chen, J.L.; Zhong, W.J.; Tang, G.H.; Li, J.; Zhao, Z.M.; Yang, D.P.; Jiang, L. Norditerpenoids from Flickingeria fimbriata and their inhibitory activities on nitric oxide and tumor necrosis factor-α production in mouse macrophages. Molecules 2014, 19, 5863–5875. [Google Scholar] [CrossRef]
- Dade, J.M.E.; Kablan, L.A.; Okpekon, T.A.; Say, M.; Yapo, K.D.; Komlaga, G.; Boti, J.B.; Koffi, A.P.; Guei, L.E.; Djakoure, L.A. Cassane diterpenoids from stem bark of Erythrophleum suaveolens. Phytochem. Lett. 2015, 12, 224–231. [Google Scholar] [CrossRef]
- Guo, Z.K.; Wang, R.; Huang, W.; Li, X.N.; Jiang, R.; Tan, R.X.; Ge, H.M. Aspergiloid I, an unprecedented spirolactone norditerpenoid from the plant-derived endophytic fungus Aspergillus sp. YXf3. Beilstein J. Org. Chem. 2014, 10, 2677–2682. [Google Scholar] [CrossRef]
- Su, X.D.; Wu, Y.C.; Wu, M.F.; Lu, J.F.; Jia, S.J.; He, X.; Liu, S.N.; Zhou, Y.Y.; Xing, H.; Xue, Y.B. Regioisomers Salviprolin A and B, unprecedented rosmarinic acid conjugated dinorditerpenoids from Salvia przewalskii Maxim. Molecules 2021, 26, 6955. [Google Scholar] [CrossRef]
- Dong, L.; Cheng, L.Z.; Yan, Y.M.; Wang, S.M.; Cheng, Y.X. Commiphoranes A-D, carbon skeletal terpenoids from Resina commiphora. Org. Lett. 2017, 19, 286–289. [Google Scholar] [CrossRef] [PubMed]
- Zhu, S.S.; Liu, J.W.; Yan, Y.M.; Liu, Y.; Mao, Z.; Cheng, Y.X. Terpenoids from Resina Commiphora regulating lipid metabolism via activating PPARα and CPT1 expression. Org. Lett. 2020, 22, 3428–3432. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.H.; Wang, D.W.; Yan, Y.M.; Jiao, Y.B.; Cheng, Y.X.; Wang, F. Commiphoranes K-O, new terpenoids from Resina Commiphora and their anti-inflammatory activities. Chem. Biodivers. 2021, 18, e2100265. [Google Scholar] [CrossRef] [PubMed]
- Feng, Z.L.; Zhang, L.L.; Zheng, Y.D.; Liu, Q.Y.; Liu, J.X.; Feng, L.; Huang, L.; Zhang, Q.W.; Lu, J.J.; Lin, L.G. Norditerpenoids and dinorditerpenoids from the seeds of Podocarpus nagi as cytotoxic agents and autophagy inducers. J. Nat. Prod. 2017, 80, 2110–2117. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.Y.; He, H.B.; Gao, S.H. Asymmetric total synthesis of cephanolide B. Org. Chem. Front. 2021, 8, 55–559. [Google Scholar] [CrossRef]
- Chen, S.S.; Tong, X.; Liu, X.Y.; Zheng, C.Y.; Zhou, J.S.; Fan, Y.Y.; He, S.J.; Zhou, B.; Yue, J.M. Baccaramiones A–D, four highly oxygenated and rearranged trinorditerpenoids from Baccaurea ramiflora. J. Org. Chem. 2023, 88, 455–461. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.F.; Yang, B.C.; Song, Z.M.; Qiao, L.Q.; Peng, R.; Feng, W.S.; Cheng, Y.X.; Wang, Y.Z. Seven diterpenoids from the resin of Pinus yunnanensis Franch and their anti-inflammatory activity. Fitoterapia 2023, 165, 105396. [Google Scholar] [CrossRef] [PubMed]
- Chao, C.H.; Cheng, J.C.; Hwang, T.L.; Shen, D.Y.; Wu, T.S. Trinorditerpenes from the roots of Flueggea virosa. Bioorg. Med. Chem. Lett. 2014, 24, 447–449. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.X.; Jin, H.; Li, H.L.; Zhang, W.D. Podocarpane trinorditerpenes from Celastrus angulatus and their biological activities. Fitoterapia 2018, 130, 156–162. [Google Scholar] [CrossRef]
- Jang, H.J.; Kim, K.H.; Park, E.J.; Kang, J.A.; Yun, B.S.; Lee, S.J.; Park, C.S.; Lee, S.; Lee, S.W.; Rho, M.C. Anti-inflammatory activity of diterpenoids from Celastrus orbiculatus in lipopolysaccharide-stimulated RAW264.7 cells. J. Immunol. Res. 2020, 2020, 7207354. [Google Scholar] [CrossRef]
- Wang, L.Y.; Wu, J.; Yang, Z.; Wang, X.J.; Fu, Y.; Liu, S.Z.; Wang, H.M.; Zhu, W.L.; Zhang, H.Y.; Zhao, W.M. (M)- and (P)-Bicelaphanol A, dimeric trinorditerpenes with promising neuroprotective activity from Celastrus orbiculatus. J. Nat. Prod. 2013, 76, 745–749. [Google Scholar] [CrossRef] [PubMed]
- Georges, P.; Legault, J.; Lavoie, S.; Grenon, C.; Pichette, A. Diterpenoids from the buds of Pinus banksiana Lamb. Molecules 2012, 17, 9716–9727. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.J.; Xiong, J.; Lau, C.; Pan, L.L.; Hu, J.F. Sesquiterpenoids and further diterpenoids from the rare Chloranthaceae plant Chloranthus sessilifolius. J. Asian Nat. Prod. Res. 2015, 17, 1220–1230. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.J.; Wang, L.Y.; Fu, Y.; Wu, J.; Tang, X.C.; Zhao, W.M.; Zhang, H.Y. Promising effects on ameliorating mitochondrial function and enhancing Akt signaling in SH-SY5Y cells by (M)-bicelaphanol A, a novel dimeric podocarpane type. Phytomedicine 2013, 20, 1064–1070. [Google Scholar] [CrossRef] [PubMed]
- Guo, M.L.; Xu, H.T.; Yang, J.J.; Chou, G.X. Diterpenoid glycosides from the flower of Trollius chinensis Bunge and their nitric oxide inhibitory activities. Bioorg. Chem. 2021, 116, 105312. [Google Scholar] [CrossRef] [PubMed]
- Thomas, S.A.L.; Sanchez, A.; Kee, Y.; Wilson, N.G.; Baker, B.J. Bathyptilones: Terpenoids from an antarctic sea pen, Anthoptilum grandiflorum (Verrill, 1879). Mar. Drugs 2019, 17, 513. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Zhang, S.W.; Xuan, L.J. Trinorcassane and cassane diterpenoids from the seeds of Caesalpinia minax. Fitoterapia 2015, 102, 177–181. [Google Scholar] [CrossRef]
- Liu, K.X.; Zhu, Y.X.; Yan, Y.M.; Zeng, Y.; Jiao, Y.B.; Qin, F.Y.; Liu, J.W.; Zhang, Y.Y.; Cheng, Y.X. Discovery of populusone, a skeletal stimulator of umbilical cord mesenchymal stem cells from Populus euphratica exudates. Org. Lett. 2019, 21, 1837–1840. [Google Scholar] [CrossRef]
- Hu, Z.X.; Xu, H.C.; Hu, K.; Liu, M.; Li, X.N.; Li, X.R.; Du, X.; Zhang, Y.H.; Puno, P.; Sun, H.D. Structurally diverse diterpenoids from Isodon pharicus. Org. Chem. Front. 2018, 5, 2379–2389. [Google Scholar] [CrossRef]
- Zhang, X.W.; Li, P.L.; Qin, G.F.; Li, S.Y.; de Voogd, N.J.; Tang, X.L.; Li, G.Q. Isolation and absolute configurations of diversiform C17, C21 and C25 terpenoids from the marine sponge Cacospongia sp. Mar. Drugs 2019, 17, 14. [Google Scholar] [CrossRef]
- Chen, Y.M.; Yang, Y.H.; Li, X.N.; Zou, C.; Zhao, P.J. Diterpenoids from the endophytic fungus Botryosphaeria sp. P483 of the Chinese herbal medicine Huperzia serrate. Molecules 2015, 20, 16924–16932. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.F.; Li, X.M.; Meng, L.; Cui, C.M.; Gao, S.S.; Li, C.S.; Huang, C.G.; Wang, B.G. Asperolides A–C, tetranorlabdane diterpenoids from the marine alga-derived endophytic fungus Aspergillus wentii EN-48. J. Nat. Prod. 2012, 75, 148–152. [Google Scholar] [CrossRef] [PubMed]
- Srivedavyasasri, R.; White, M.B.; Kustova, T.S.; Gemejiyeva, N.G.; Cantrell, C.L.; Ross, S.A. New tetranorlabdanoic acid from aerial parts of Salvia aethiopis. Nat. Prod. Res. 2018, 32, 14–17. [Google Scholar] [CrossRef] [PubMed]
- Yin, H.; Dan, W.J.; Fan, B.Y.; Guo, C.; Wu, K.; Li, D.; Xian, K.F.; Pescitelli, G.; Gao, J. M Anti-inflammatory and α-glucosidase inhibitory activities of labdane and norlabdane diterpenoids from the rhizomes of Amomum villosum. J. Nat. Prod. 2019, 82, 2963–2971. [Google Scholar] [CrossRef] [PubMed]
- Liang, S.; Luo, J.G.; Wang, Z.; Wang, X.B.; Kong, L. New tetranorlabdane diterpenoids from the fruits of Elettaria cardamomum Maton. Phytochem. Lett. 2017, 20, 295–299. [Google Scholar] [CrossRef]
- Li, X.D.; Li, X.; Li, X.M.; Xu, G.M.; Zhang, P.; Meng, L.H.; Wang, B.G. Tetranorlabdane diterpenoids from the deep sea sediment-derived fungus Aspergillus wentii SD-310. Planta Med. 2016, 82, 877–881. [Google Scholar] [CrossRef]
- Afolabi, S.; Olorundare, O.; Ninomiya, M.; Babatunde, A.; Mukhtar, H.; Koketsu, M. Comparative antileukemic activity of a tetranorditerpene isolated from Polyalthia longifolia leaves and the derivative against human leukemia HL-60 cells. J. Oleo Sci. 2017, 66, 1169–1174. [Google Scholar] [CrossRef]
- Hsu, F.Y.; Wang, S.K.; Duh, C.Y. Xeniaphyllane-derived terpenoids from soft coral Sinularia nanolobata. Mar. Drugs 2018, 16, 40. [Google Scholar] [CrossRef]
- Zhang, Z.X.; Li, H.H.; Zhi, D.J.; Wu, P.Q.; Hu, Q.L.; Yu, Y.F.; Zhao, Y.; Yu, C.X.; Fei, D.Q. Norcrocrassinone: A novel tetranorditerpenoid possessing a 6/6/5 fused ring system from Croton crassifolius. Tetrahedron Lett. 2018, 59, 4028–4030. [Google Scholar] [CrossRef]
- Zhang, Z.X.; Wu, P.Q.; Li, H.H.; Qi, F.M.; Fei, D.Q.; Hu, Q.L.; Liu, Y.H.; Huang, X.L. Norcrassin A, a novel C16 tetranorditerpenoid, and bicrotonol A, an unusual dimeric labdane-type diterpenoid, from the roots of Croton crassifolius. Org. Biomol. Chem. 2018, 16, 1745–1750. [Google Scholar] [CrossRef]
- Mofidi Tabatabaei, S.; Salehi, P.; Moridi Farimani, M.; Neuburger, M.; De Mieri, M.; Hamburger, M.; Nejad-Ebrahimi, S. A nor-diterpene from Salvia sahendica leaves. Nat. Prod. Res. 2017, 31, 1758–1765. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.Q.; Deng, Y.Y.; Li, T.Z.; Han, Q.; Li, Y.; Qiu, M.H. Three new tetranorditerpenes from aerial parts of acerola cherry (Malpighia emarginata). Molecules 2014, 19, 2629–2636. [Google Scholar] [CrossRef] [PubMed]
- Tang, G.H.; Zhang, Y.; Gu, Y.C.; Li, S.F.; Di, Y.T.; Wang, Y.H.; Yang, C.X.; Zuo, G.Y.; Li, S.L.; He, H.P. Trigoflavidols A–C, degraded diterpenoids with antimicrobial activity, from Trigonostemon flavidus. J. Nat. Prod. 2012, 75, 996–1000. [Google Scholar] [CrossRef] [PubMed]
- Li, S.F.; Yu, X.Q.; Li, Y.L.; Bai, M.; Lin, B.; Yao, G.D.; Song, S.J. Vibsane-type diterpenoids from Viburnum odoratissimum and their cytotoxic activities. Bioorg. Chem. 2021, 106, 104498. [Google Scholar] [CrossRef]









| Family | Genus | Species | Quantity of Norditerpenes |
|---|---|---|---|
| Lamiaceae (143) | Callicarpa | Callicarpa integerrima | 2 |
| Teucrium | Teucrium viscidum | 3 | |
| Sideritis | Sideritis pullulans | 2 | |
| Leucas | Leucas zeylanica | 2 | |
| Isodon | Isodon rubescens | 4 | |
| Isodon eriocalyx | 1 | ||
| Isodon pharicus | 1 | ||
| Isodon phyllostachys | 2 | ||
| Isodon rosthornii | 1 | ||
| Isodon ternifolius | 1 | ||
| Salvia | Salvia aethiopis | 1 | |
| Salvia deserta | 5 | ||
| Salvia miltiorrhiza | 26 | ||
| Salvia mirzayanii | 1 | ||
| Salvia grandifolia | 4 | ||
| Salvia leriifolia | 1 | ||
| Salvia yunnanensis | 3 | ||
| Salvia digitaloides | 17 | ||
| Salvia rhytidea | 3 | ||
| Salvia sahendica | 1 | ||
| Salvia castanea | 4 | ||
| Salvia przewalskii | 5 | ||
| Perovskia | Perovskia abrotanoides | 17 | |
| Perovskia atriplicifolia | 6 | ||
| Euphorbiaceae (75) | Croton | Croton cajucara | 1 |
| Croton haumanianus | 1 | ||
| Croton yanhuii | 4 | ||
| Croton euryphyllus | 7 | ||
| Croton caudatus | 2 | ||
| Croton cascarilloides | 2 | ||
| Croton crassifolius | 4 | ||
| Flueggea | Flueggea acicularis | 5 | |
| Flueggea virosa | 2 | ||
| Baccaurea | Baccaurea ramiflora | 4 | |
| Jatropha | Jatropha podagrica | 2 | |
| Trigonostemon | Trigonostemon chinensis | 11 | |
| Trigonostemon flavidus | 4 | ||
| Trigonostemon howii | 1 | ||
| Malpighia | Malpighia emarginata | 3 | |
| Phyllanthus | Phyllanthus flexuosus | 1 | |
| Drypetes | Drypetes perreticulata | 4 | |
| Aleurites | Aleurites moluccanus | 2 | |
| Euphorbia | Euphorbia neriifolia | 6 | |
| Euphorbia royleana | 1 | ||
| Euphorbia ebracteolata | 4 | ||
| Euphorbia dracunculoides | 2 | ||
| Cephalotaxaceae (66) | Cephalotaxus | Cephalotaxus sinensis | 8 |
| Cephalotaxus fortunei | 56 | ||
| Cephalotaxus mannii | 2 | ||
| Celastraceae (20) | Euonymus L. | Euonymus verrucosus var. pauciflorus | 3 |
| Euonymus grandiflflorus Wall | 1 | ||
| Maytenus | Maytenus senegalensis | 1 | |
| Celastrus | Celastrus angulatus | 9 | |
| Celastrus orbiculatus | 4 | ||
| Tripterygium | Tripterygium wilfordii | 2 | |
| Icacinaceae (16) | Icacina | Icacina trichantha | 16 |
| Orchidaceae (16) | Flickingeria | Flickingeria fimbriata | 16 |
| Podocarpaceae (14) | Podocarpus | Podocarpus nagi | 9 |
| Podocarpus macrophyllus | 5 | ||
| Pinaceae (13) | Abies | Abies forrestii | 2 |
| Pinus | Pinus yunnanensis | 1 | |
| Pinus banksiana Lamb | 1 | ||
| Pseudotsuga | Pseudotsuga sinensis | 9 | |
| Picrodendraceae (12) | Austrobuxus | Austrobuxus carunculatus | 12 |
| Taxaceae (11) | Amentotaxus | Amentotaxus argotaenia | 11 |
| Compositae (10) | Eupatorium | Austroeupatorium inulifolium | 10 |
| Chloranthaceae (9) | Chloranthus | Chloranthus serratus | 2 |
| Chloranthus sessilifolius | 7 | ||
| Asteraceae (8) | Grazielia | Grazielia gaudichaudeana | 3 |
| Wedelia | Wedelia trilobata | 1 | |
| Stevia | Stevia rebaudiana | 3 | |
| Baccharis | Baccharis retusa | 1 | |
| Zingiberaceae (8) | Amomum | Amomum maximum | 2 |
| Amomum villosum | 1 | ||
| Hedychium | Hedychium forrestii | 3 | |
| Elettaria | Elettaria cardamomum | 2 | |
| Salicaceae (7) | Populus | Populus euphratica | 7 |
| Araucariaceae (5) | Agathis | Agathis macrophylla | 5 |
| Leguminosae (5) | Erythrophleum | Erythrophleum fordii | 2 |
| Erythrophleum suaveolens | 1 | ||
| Caesalpinia | Caesalpinia decapetala var. japonica | 1 | |
| Caesalpinia minax | 1 | ||
| Menispermaceae (5) | Tinospora | Tinospora capillipes | 5 |
| Annonaceae (4) | Annona | Annona squamosa L. | 2 |
| Polyalthia | Polyalthia longifolia | 2 | |
| Dipterocarpaceae (4) | Resina | Resina Commiphora | 4 |
| Pallaviciniaceae (4) | Pallavicinia | Pallavicinia ambigua | 4 |
| Scrophulariaceae (4) | Scrophularia | Scrophularia dentata | 4 |
| Alangiaceae (2) | Alangium | Alangium chinense (Lour.) Harms | 2 |
| Botryosphaeriaceae (2) | Diplodia | Diplodia olivarum | 2 |
| Caprifoliaceae (2) | Viburnum | Viburnum odoratissimum | 2 |
| Ditrichaceae (2) | Ceratodon | Ceratodon purpureus | 2 |
| Malpighiaceae (2) | Aspidopterys | Aspidopterys obcordata | 2 |
| Paeoniaceae (2) | Paeonia | Paeonia veitchii | 2 |
| Rosaceae (2) | Crataegus | Crataegus pinnatifida | 2 |
| Thymelaeaceae (2) | Aquilaria | Chinese eaglewood | 2 |
| Apiaceae (1) | Azorella | Azorella compacta | 1 |
| Cupressaceae (1) | Thuja | Thuja orientalis | 1 |
| Ericaceae (1) | Lyonia | Lyonia ovalifolia | 1 |
| Pentaphylacaceae (1) | Adinandra | Adinandra poilanei | 1 |
| Ranunculaceae (1) | Trollius | Trollius chinensis | 1 |
| Eurotiaceae (35) | Penicillium | Penicillium oxalicum | 1 |
| Penicillium sp. DT10 | 1 | ||
| Penicillium thomii | 1 | ||
| Aspergillus | Aspergillus taichungensis | 4 | |
| Aspergillus aculeatinus | 1 | ||
| Aspergillus sp. YXf3 | 1 | ||
| Aspergillus wentii EN-48 | 26 | ||
| Discellaceae (14) | Oidiodendron | Oidiodendron truncatum | 14 |
| Dematiaceae (3) | Alternaria | Alternaria brassicicola | 3 |
| Thermomonosporaceae (2) | Actinomadura | Actinomadura sp. KC 191 | 2 |
| Carboniaceae (1) | Xylaria | Xylaria longipes | 1 |
| Moniliaceae (1) | Trichoderma | Trichoderma citrinoviride | 1 |
| Cortinariaceae (1) | Cortinarius | Cortinarius pyromyxa | 1 |
| Alcyonidae (30) | Sinularia | Sinularia scabra | 16 |
| Sinularia maxima | 3 | ||
| Sinularia nanolobata | 1 | ||
| Sinularia siaesensis | 1 | ||
| Sinularia hirta | 2 | ||
| Sinularia densa | 7 | ||
| Choiidae (9) | Spongia | Spongia officinalis | 2 |
| Dendrilla | Dendrilla antarctica | 2 | |
| Cacospongia | Cacospongia sp. | 5 | |
| Xeniidae (5) | Cespitularia | Cespitularia taeniata | 2 |
| Cespitularia sp. | 3 | ||
| Chromodorididae (3) | Goniobranchus | Goniobranchus Mollusks | 3 |
| Gorgoniaceae (1) | Junceella | Junceella fragilis | 1 |
| Anthoptilidae (1) | Anthoptilum | Anthoptilum grandiflflorum | 1 |
| Loliginidae (1) | Uroteuthis | Uroteuthis (Photololigo) duvaucelii | 1 |
| Veneridae (1) | Paphia | Paphia malabarica | 1 |
| Amber (1) | - | Dominican amber | 1 |
| No. | Name | Plant Source | Plant Organ | Ref. |
|---|---|---|---|---|
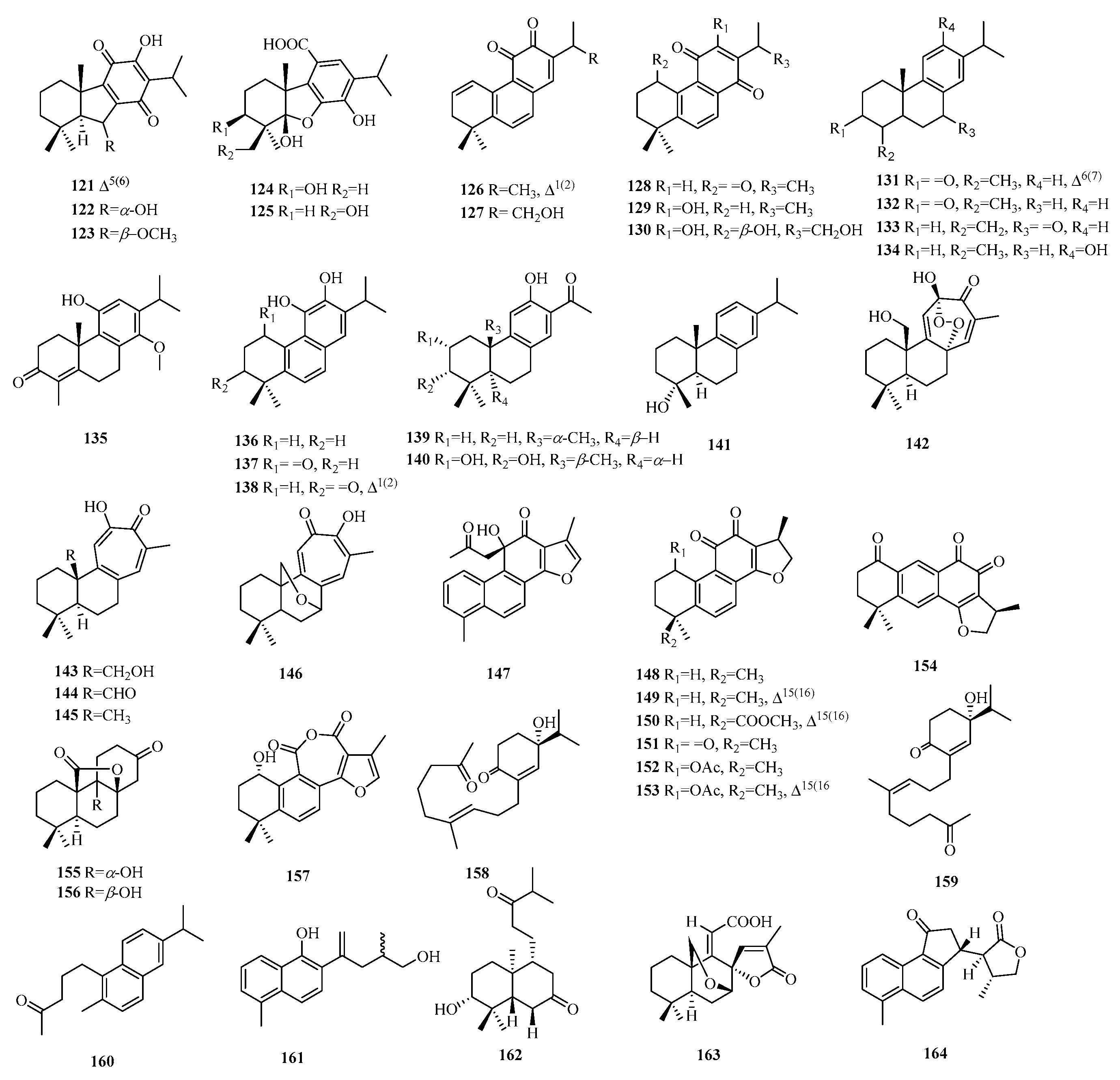
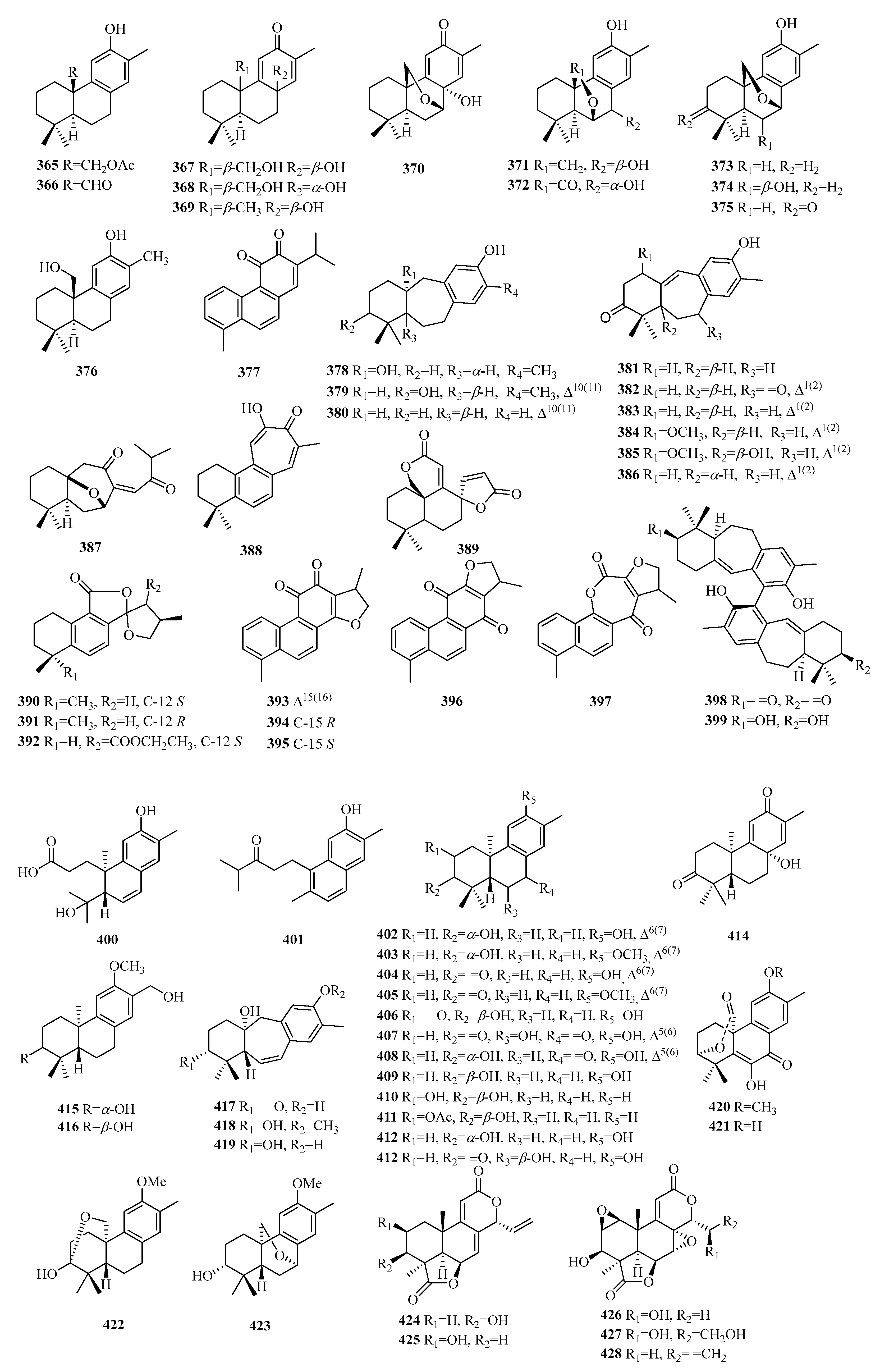
| 1 | penioxalicin | P. oxalicum | - | [2] |
| 2 | penitholabene | P. thomii | - | [3] |
| 3 | euonymupene C | E. verrucosus | twigs | [4] |
| 4 | euonymupene A | E. verrucosus | twigs | [4] |
| 5 | 3β-hydroxy-15-nor-14-oxo-8(17),12-labdadien-14-al | C. serratus | whole plants | [5] |
| 6 | 3β,6β-dihydroxy-15-nor-14-oxo-8(17),12-labdadien-14-al | C. serratus | whole plants | [5] |
| 7 | 15-nor-14-oxolabda-8(17),12E-dien-19-oic acid | A. macrophylla | aerial parts | [6] |
| 8 | 15-nor-14-oxolabda-8(17),13(16)-dien-19-oic acid | T. orientalis | leaves and stems | [7] |
| 9 | grazielabdane A | G. gaudichaudeana | aerial parts | [8] |
| 10 | grazielabdane B | G. gaudichaudeana | aerial parts | [8] |
| 11 | grazielabdane C | G. gaudichaudeana | aerial parts | [8] |
| 12 | austroeupatol | A. inulifolium | aerial parts | [9,10] |
| 13 | inulifolinone A | A. inulifolium | leaves | [9,10] |
| 14 | inulifolinone B | A. inulifolium | leaves | [9,10] |
| 15 | inulifolinone C | A. inulifolium | leaves | [9,10] |
| 16 | inulifolinone D | A. inulifolium | leaves | [9,10] |
| 17 | inulifolinone E | A. inulifolium | leaves | [9,10] |
| 18 | inulifolinone F | A. inulifolium | leaves | [9,10] |
| 19 | inulifolinone I | A. inulifolium | leaves | [9,10] |
| 20 | inulifolinone G | A. inulifolium | leaves | [9,10] |
| 21 | inulifolinone H | A. inulifolium | leaves | [9,10] |
| 22 | amomaxin B | A. maximum | roots | [11] |
| 23 | amomaxin A | A. maximum | roots | [11] |
| 24 | hedychin E | H. forrestii | rhizomes | [12] |
| 25 | pallambin A | P. ambigua | - | [13] |
| 26 | pallambin B | P. ambigua | - | [13] |
| 27 | pallambin C | P. ambigua | - | [13] |
| 28 | pallambin D | P. ambigua | - | [13] |
| No. | Name | Plant Source | Plant Organ | Ref. |
|---|---|---|---|---|
| 29 | 15-nor-cleroda-3,12-diene | D. amber | - | [14] |
| 30 | callinteger A | C. integerrima | twigs and leaves | [15] |
| 31 | callinteger B | C. integerrima | twigs and leaves | [15] |
| 32 | trans-dehydrocrotonin | C. cajucara | tree | [16] |
| 33 | croyanoid B | C. yanhuii | twigs and leaves | [17] |
| 34 | crotoeurin B | C. euryphyllus | twigs and leaves | [18] |
| 35 | crocleropene B | C. caudatus | twigs and leaves | [19] |
| 36 | crocleropene A | C. caudatus | twigs and leaves | [19] |
| 37 | croyanoid C | C. yanhuii | twigs and leaves | [17] |
| 38 | tinocapillin A | T. capillipes | rhizomes | [20] |
| 39 | tinocallone A | T. capillipes | rhizomes | [20] |
| 40 | tinocallone C | T. capillipes | rhizomes | [20] |
| 41 | tinocapillin B | T. capillipes | rhizomes | [20] |
| 42 | tinocapillin C | T. capillipes | rhizomes | [20] |
| 43 | crotoeurin C | C. euryphyllus | twigs and leaves | [18] |
| 44 | 6-epi-crotoeurin C | C. crassifolius | roots | [21] |
| 45 | teucvidin | C. euryphyllus | twigs and leaves | [18] |
| 46 | isoteucvin | C. euryphyllus | twigs and leaves | [18] |
| 47 | teucvin | C. euryphyllus | twigs and leaves | [17,18] |
| 48 | isocrotocaudin | C. euryphyllus | twigs and leaves | [17,18] |
| 49 | crotocaudin | C. crassifolius | roots | [21] |
| 50 | croyanoid D | C. yanhuii | twigs and leaves | [17] |
| 51 | teucvisin C | T. viscidum | whole plants | [22] |
| 52 | teucvisin D | T. viscidum | whole plants | [22] |
| 53 | teucvisin E | T. viscidum | whole plants | [22] |
| 54 | croyanoid A | C. yanhuii | twigs and leaves | [17] |
| 55 | crotoeurin A | C. euryphyllus | twigs and leaves | [17,18] |
| No. | Name | Plant Source | Plant Organ | Ref. |
|---|---|---|---|---|
| 56 | fluacinoid D | F. acicularis | aerial parts | [23] |
| 57 | fluacinoid E | F. acicularis | aerial parts | [23] |
| 58 | fluacinoid F | F. acicularis | aerial parts | [23] |
| 59 | fluacinoid G | F. acicularis | aerial parts | [23] |
| 60 | fluacinoid H | F. acicularis | aerial parts | [23] |
| 61 | aquilariaene F | C. eaglewood | - | [24] |
| 62 | aquilariaene H | C. eaglewood | - | [24] |
| 63 | jbir-65 | Actinomadura sp. KC 191 | - | [25] |
| 64 | actinomadurol | Actinomadura sp. KC 191 | - | [25] |
| 65 | icatrichanone | I. trichantha | tubers | [26] |
| 66 | 14-hydroxyicatrichanone | I. trichantha | tubers | [26] |
| 67 | 3-O-methylicatrichanone | I. trichantha | tubers | [26] |
| 68 | 3-O-methyl-14-hydroxyicatrichanone | I. trichantha | tubers | [26] |
| 69 | 7α-hydroxyicacenone | I. trichantha | tubers | [27] |
| 70 | icacenone | I. trichantha | tubers | [27] |
| 71 | 12-hydroxy-icacinlactone | I. trichantha | tubers | [27] |
| 72 | icacinlactone H | I. trichantha | tubers | [27] |
| 73 | icacinlactone A | I. trichantha | tubers | [28] |
| 74 | icacinlactone B | I. trichantha | tubers | [27,28] |
| 75 | icacinlactone E | I. trichantha | tubers | [28] |
| 76 | icacinlactone F | I. trichantha | tubers | [28] |
| 77 | icacinlactone G | I. trichantha | tubers | [28] |
| 78 | icacinlactone C | I. trichantha | tubers | [28] |
| 79 | icacinlactone D | I. trichantha | tubers | [27,28] |
| 80 | trichanthol B | I. trichantha | tubers | [27] |
| 81 | 8β-hydroxy-18-nor-4(5),15-isopimaradien-3-one | E. grandiflflorus Wall. | branches and leaves | [29] |
| 82 | 18(4→14), 19(4→8)-bis-abeo-nor-isopimarane-1,5-diene-3-yl-3β-methoxy propyl pentanoate | P. malabarica | edible portion | [30] |
| 83 | smardaesidin F | C. purpureus | - | [31] |
| 84 | smardaesidin G | C. purpureus | - | [31] |
| 85 | sphaeropsidin G | D. olivarum | - | [32] |
| 86 | taichunin D | A. taichungensis | - | [33] |
| 87 | aspewentin D | A. wentii EN-48 | - | [34] |
| 88 | aspewentin E | A. wentii EN-48 | - | [34] |
| 89 | aspewentin F | A. wentii EN-48 | - | [34] |
| 90 | aspewentin I | A. wentii EN-48 | - | [35] |
| 91 | aspewentin J | A. wentii EN-48 | - | [35] |
| 92 | aspewentin K | A. wentii EN-48 | - | [35] |
| 93 | aspewentin M | A. wentii EN-48 | - | [35] |
| 94 | aspewentin L | A. wentii EN-48 | - | [35] |
| 95 | aspewentin C | A. wentii EN-48 | - | [36] |
| 96 | aspewentin A | A. wentii EN-48 | - | [36] |
| 97 | aspewentin B | A. wentii EN-48 | - | [36] |
| 98 | aspewentin G | A. wentii EN-48 | - | [34] |
| 99 | aspewentin H | A. wentii EN-48 | - | [34] |
| 100 | xylarilongipin A | A. wentii EN-48 | - | [37] |
| 101 | asperether A | A. wentii EN-48 | - | [38] |
| 102 | asperether B | A. wentii EN-48 | - | [38] |
| 103 | asperether C | A. wentii EN-48 | - | [38] |
| 104 | asperether D | A. wentii EN-48 | - | [38] |
| 105 | asperether E | A. wentii EN-48 | - | [38] |
| 106 | (2S,3S,5S,9S,10S,13S)-2,3-dihydroxy-16-nor-ent-pimar-8(14)-en-15-oic acid | F. fimbriata | aerial parts | [39] |
| 107 | (3R,5S,9S,10S,13S)-2-hydroxy-16-nor-ent-pimar-8(14)-en-15-oic acid | F. fimbriata | aerial parts | [39] |
| 108 | (2S,3R,5S,9S,10S,13S)-2-acetoxy-3-hydroxy-16-nor-ent-pimar-8(14)-en-15-oic acid | F. fimbriata | aerial parts | [39] |
| 109 | norflickinflimiod A | F. fimbriata | aerial parts | [39,40] |
| 110 | (2S,3R,5S,9S,10S,13S)-2-O-E-cinnamoyl-3-hydroxy-16-nor-ent-pimar-8(14)-en-15-oic acid | F. fimbriata | aerial parts | [39] |
| 111 | 3α,14β-diacetoxy-16-nor-ent-pimar-15α,8-olide | F. fimbriata | aerial parts | [39] |
| 112 | norflickinflimiod B | F. fimbriata | aerial parts | [39,40] |
| 113 | norflickinflimiod C | F. fimbriata | aerial parts | [40] |
| 114 | norflickinflimoside | F. fimbriata | aerial parts | [40] |
| 115 | norflickinflimiod D | F. fimbriata | aerial parts | [40] |
| 116 | eupneria K | E. neriifolia | stem bark | [41] |
| 117 | eupneria L | E. neriifolia | stem bark | [41] |
| 118 | eupneria J | E. neriifolia | stem bark | [41] |
| 119 | eupneria M | E. neriifolia | stem bark | [41] |
| 120 | (3R,4R,5S,9R,10S,12S,13S)-ent-18-nor-8(14),15-isopimaradiene-3β,12β,4α-triol | E. neriifolia | stems | [42] |
| No. | Name | Plant Source | Plant Organ | Ref. |
|---|---|---|---|---|
| 121 | dichroanone | S. deserta | roots | [43] |
| 122 | salviadesertin A | S. deserta | roots | [43] |
| 123 | salviadesertin B | S. deserta | roots | [43] |
| 124 | salviadesertin E | S. deserta | roots | [43] |
| 125 | salviadesertin F | S. deserta | roots | [43] |
| 126 | dehydromiltirone | S. miltiorrhiza | cell | [44] |
| 127 | grandifolia D | S. grandifolia | roots | [45] |
| 128 | 2-isopropyl-8,8-dimethyl-7,8-dihydroph-enanthrene-1,4,5(6H)-trione | S. leriifolia | whole plants | [46] |
| 129 | deoxyneocryptotanshinone | S. rhytidea | roots | [47] |
| 130 | (1R,15R)-1β-hydroxyneocryptotanshinone. | P. abrotanoides | roots | [48] |
| 131 | 19-nor-abieta-4,6,8,11,13-tetraen-3-one | P. euphratica | resins | [49] |
| 132 | 4,4α,9,10-tetrahydro-1,4α-dimethyl-7-isopropyl-2(3H)-phenanthrone | P. euphratica | resins | [49] |
| 133 | 19-nor-abieta-4(18),8,11,13-tetraen-7-one | P. euphratica | resins | [49] |
| 134 | castanol B | S. castanea | whole plants | [50] |
| 135 | triptobenzene S | T. wilfordii | roots | [51] |
| 136 | 1-deoxo aurocadiol | S. rhytidea | roots | [47] |
| 137 | arucadiol | S. rhytidea | roots | [47] |
| 138 | 1-deoxy-1,2-dien-3-oxoarucadiol. | P. abrotanoides | roots | [48] |
| 139 | euphorane C | E. dracunculoides | powder | [52] |
| 140 | nagiol A | P. nagi | leaves | [53] |
| 141 | 18-norabieta-8,11,13-4-ol | P. euphratica | resins | [49] |
| 142 | grandifolia A | S. grandifolia | roots | [45] |
| 143 | isograndifoliol | P. atriplicifolia | roots | [45,54] |
| 144 | grandifolia B | S. grandifolia | roots | [45] |
| 145 | przewalskin Y-1 | P. abrotanoides | roots | [48] |
| 146 | miltipolone | S. grandifolia | roots | [45] |
| 147 | dehydrodanshenol A | S. przewalskii | roots | [55] |
| 148 | cryptotanshinone | S. miltiorrhiza | seeds | [44,56] |
| 149 | tanshinone IIA | S. miltiorrhiza | seeds | [56] |
| 150 | methyltanshinoate | S. castanea | whole plants | [50] |
| 151 | 1-oxocryptotanshinone | P. atriplicifolia | roots | [48] |
| 152 | (1R,15R)-1-acetoxycryptotanshinone | P. atriplicifolia | roots | [54] |
| 153 | (1R)-1-acetoxytanshinone IIA | P. atriplicifolia | roots | [54] |
| 154 | (15R)-1-oxoaegyptinone A | P. atriplicifolia | roots | [54] |
| 155 | rubesanolide F | I. rubescens | leaves | [57] |
| 156 | rubesanolide G | I. rubescens | leaves | [57] |
| 157 | castanol A | S. castanea | whole plants | [50] |
| 158 | abieseconordine A | A. forrestii | twigs | [58] |
| 159 | abieseconordine B | A. forrestii | twigs | [58] |
| 160 | 5-(6-isopropyl-2-methylnaphthalen-1-yl)pentan-2-one | P. euphratica | resins | [49] |
| 161 | deacetylsalvianonol | S. przewalskii | roots | [55] |
| 162 | sessilifol O | C. sessilifolius | whole plants | [59] |
| 163 | salvialba acid | S. miltiorrhiza | roots | [60] |
| 164 | salprzelactone | S. przewalskii | roots | [55] |
| No. | Name | Plant Source | Plant Organ | Ref. |
|---|---|---|---|---|
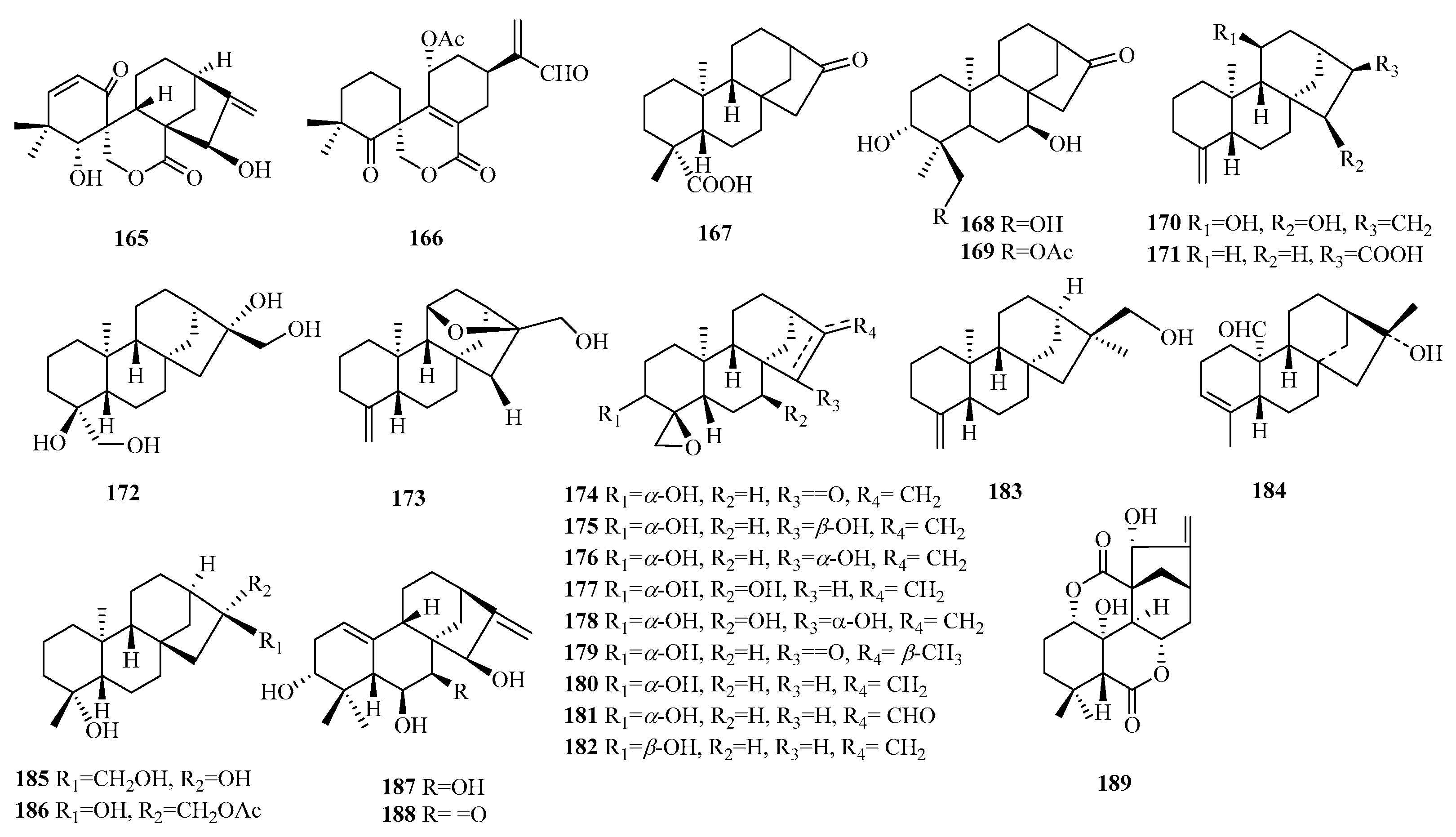
| 165 | laxiflorolide M | I. eriocalyx | leaves | [61] |
| 166 | ternifolide B | I. ternifolius | - | [62] |
| 167 | ent-16-oxo-17-nor-kauran-19-oic | B. retusa | aerial parts | [63] |
| 168 | sideritone A | S. pullulans | aerial parts and roots | [64] |
| 169 | sideritone B | S. pullulans | aerial parts and roots | [64] |
| 170 | amentotaxin L | A. argotaenia | twigs and leaves | [65] |
| 171 | 18-nor-ent-kaur-4(19)-en-17-oic acid | M. senegalensis | root bark | [66] |
| 172 | 16α,17,19-trihydroxy-18-nor-ent-kauran-4β-ol | W. trilobata | whole plants | [67] |
| 173 | amentotaxin M | A. argotaenia | twigs and leaves | [65] |
| 174 | amentotaxin C | A. argotaenia | twigs and leaves | [65] |
| 175 | amentotaxin D | A. argotaenia | twigs and leaves | [65] |
| 176 | amentotaxin E | A. argotaenia | twigs and leaves | [65] |
| 177 | amentotaxin F | A. argotaenia | twigs and leaves | [65] |
| 178 | amentotaxin G | A. argotaenia | twigs and leaves | [65] |
| 179 | amentotaxin H | A. argotaenia | twigs and leaves | [65] |
| 180 | amentotaxin I | A. argotaenia | twigs and leaves | [65] |
| 181 | amentotaxin J | A. argotaenia | twigs and leaves | [65] |
| 182 | amentotaxin K | A. argotaenia | twigs and leaves | [65] |
| 183 | 19-nor-16,17-dihydroxy-ent-kaur-4(18)-ene | C. haumanianus | leaves and stem bark | [68] |
| 184 | wilkaunoid D | T. wilfordii | stems | [69] |
| 185 | (4α)-19-nor-ent-kaurane-4,16,17-triol | A. squamosa L. | stem bark | [70] |
| 186 | (4α,16α)-17-(acetyloxy)-19-nor-ent-kaurane-4,16-diol | A. squamosa L. | stem bark | [70] |
| 187 | phyllostachysin N | I. phyllostachys | aerial parts | [71] |
| 188 | phyllostachysin O | I. phyllostachys | aerial parts | [71] |
| 189 | isorosthin A | I. rosthornii | aerial parts | [72] |
| No. | Name | Plant Source | Plant Organ | Ref. |
|---|---|---|---|---|
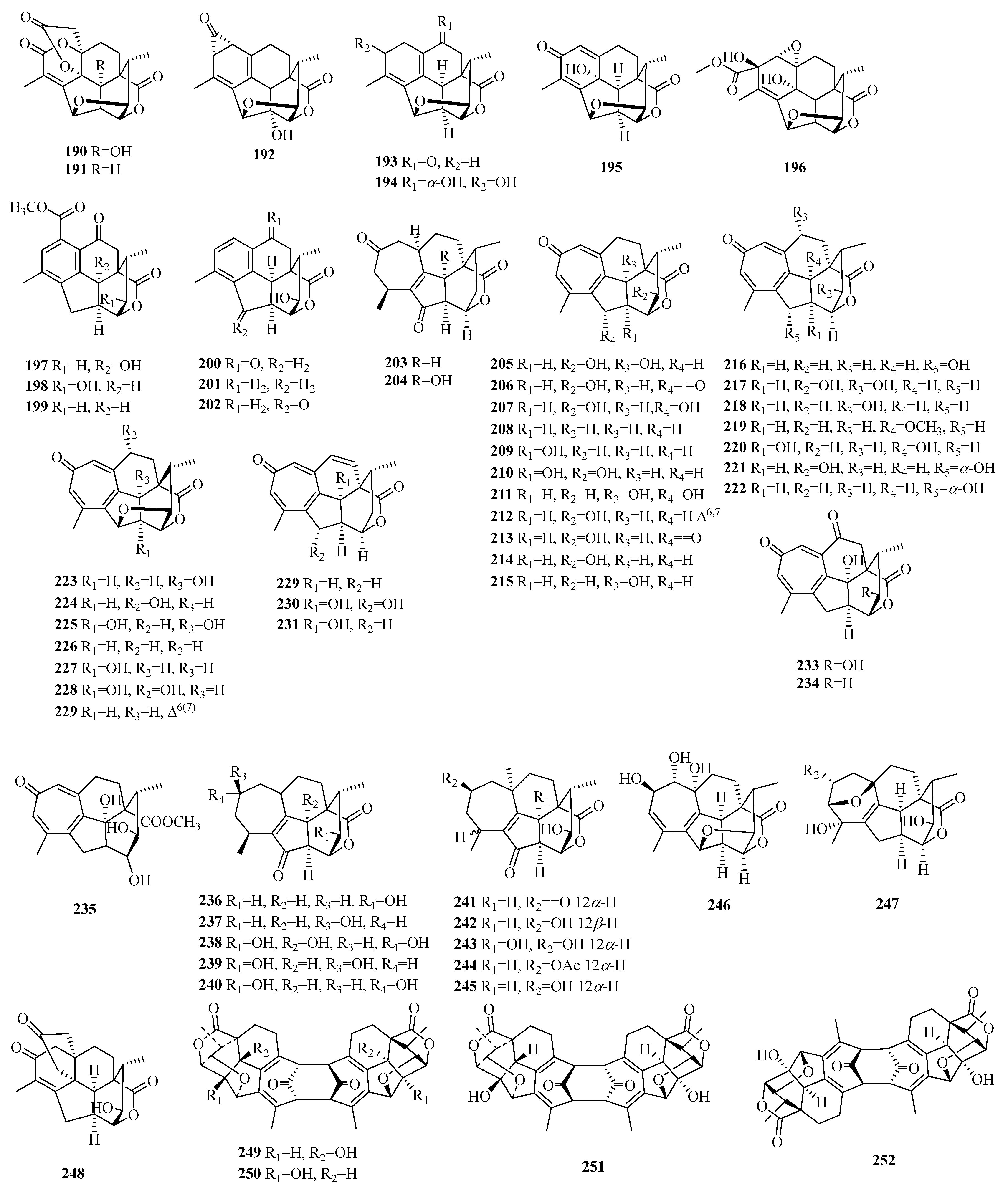
| 190 | cephalotanin A | C. sinensis | leaves | [73] |
| 191 | cephalotanin B | C. sinensis | leaves | [73] |
| 192 | cephalotanin C | C. sinensis | leaves | [73] |
| 193 | ceforalide F | C. fortunei | seeds | [74] |
| 194 | ceforalide G | C. fortunei | seeds | [74] |
| 195 | ceforalide H | C. fortunei | seeds | [74] |
| 196 | cephalotanin D | C. sinensis | leaves | [73] |
| 197 | cephanolide D | C. sinensis | twigs and leaves | [75] |
| 198 | ceforalide A | C. fortunei | seeds | [74] |
| 199 | ceforalide B | C. fortunei | seeds | [74] |
| 200 | ceforalide C | C. fortunei | seeds | [74] |
| 201 | ceforalide D | C. fortunei | seeds | [74] |
| 202 | ceforalide E | C. fortunei | seeds | [74] |
| 203 | fortalpinoid P | C. fortunei | seeds | [76] |
| 204 | fortalpinoid Q | C. fortunei | seeds | [76] |
| 205 | 20-oxohainanolidol | C. fortunei | twigs and leaves | [77] |
| 206 | 20α-hydroxyhainanolidol | C. fortunei | twigs and leaves | [77] |
| 207 | 10-hydroxyhainanolidol | C. fortunei | twigs and leaves | [77] |
| 208 | cephinoid H | C. fortunei | twigs and leaves | [78] |
| 209 | cephinoid I | C. fortunei | twigs and leaves | [78] |
| 210 | cephinoid J | C. fortunei | twigs and leaves | [78] |
| 211 | cephinoid K | C. fortunei | twigs and leaves | [78] |
| 212 | cephinoid L | C. fortunei | twigs and leaves | [78] |
| 213 | cephinoid M | C. fortunei | twigs and leaves | [78] |
| 214 | hainanolidol | C. fortunei | twigs and leaves | [78] |
| 215 | fortunolide A | C. fortunei | twigs and leaves | [78] |
| 216 | fortalpinoid A | C. fortunei | seeds | [76] |
| 217 | fortalpinoid B | C. fortunei | seeds | [76] |
| 218 | fortalpinoid C | C. fortunei | seeds | [76] |
| 219 | fortalpinoid D | C. fortunei | seeds | [76] |
| 220 | fortalpinoid E | C. fortunei | seeds | [76] |
| 221 | fortalpinoid F | C. fortunei | seeds | [76] |
| 222 | 3-deoxyfortalpinoid F | C. fortunei | seeds | [76] |
| 223 | 10-hydroxyharringtonolide | C. mannii | twigs and leaves | [78,79] |
| 224 | cephinoid F | C. fortunei | twigs and leaves | [78] |
| 225 | cephinoid G | C. fortunei | twigs and leaves | [78] |
| 226 | harringtonolide | C. fortunei | twigs and leaves | [78] |
| 227 | fortunolide B | C. fortunei | twigs and leaves | [78] |
| 228 | fortalpinoid J | C. fortunei | seeds | [76] |
| 229 | 6-en-harringtonolide | C. mannii | twigs and leaves | [78,79] |
| 230 | fortalpinoid H | C. fortunei | seeds | [76] |
| 231 | fortalpinoid I | C. fortunei | seeds | [76] |
| 232 | cephanolide J | C. fortunei | seeds | [76] |
| 233 | fortalpinoid G | C. fortunei | seeds | [76] |
| 234 | cephinoid N | C. fortunei | twigs and leaves | [78] |
| 235 | cephinoid O | C. fortunei | twigs and leaves | [78] |
| 236 | fortalpinoid M | C. fortunei | seeds | [76] |
| 237 | fortalpinoid N | C. fortunei | seeds | [76] |
| 238 | fortalpinoid O | C. fortunei | seeds | [76] |
| 239 | cephafortoid A | C. fortunei | twigs and leaves | [77] |
| 240 | 14-epi-cephafortoid A | C. fortunei | twigs and leaves | [77] |
| 241 | cephinoid P | C. fortunei | twigs and leaves | [78] |
| 242 | cephinoid Q | C. fortunei | twigs and leaves | [78] |
| 243 | cephinoid R | C. fortunei | twigs and leaves | [78] |
| 244 | cephinoid S | C. fortunei | twigs and leaves | [78] |
| 245 | gongshanolide | C. fortunei | twigs and leaves | [78] |
| 246 | fortalpinoid K | C. fortunei | seeds | [76] |
| 247 | fortalpinoid L | C. fortunei | seeds | [76] |
| 248 | ceforalide I | C. fortunei | seeds | [74] |
| 249 | cephalodione A | C. fortunei | seeds | [80] |
| 250 | cephalodione B | C. fortunei | seeds | [80] |
| 251 | cephalodione C | C. fortunei | seeds | [80] |
| 252 | cephalodione D | C. fortunei | seeds | [80] |
| No. | Name | Plant Source | Plant Organ | Ref. |
|---|---|---|---|---|
| 253 | xiguscabrolide H | S. scabra | - | [81] |
| 254 | 10-epi-gyrosanolide E | S. scabra | - | [81] |
| 255 | 5-epi-sinuleptolide | S. scabra | - | [81] |
| 256 | norcembrene 5 | S. scabra | - | [81] |
| 257 | scabrolide D | S. scabra | - | [81] |
| 258 | scabrolide G | S. scabra | - | [81] |
| 259 | sinularcasbane O | S. scabra | - | [81] |
| 260 | gyrosanolide F | S. scabra | - | [81] |
| 261 | sinuleptolide | S. scabra | - | [81] |
| 262 | 5-epi-norcembrene | S. maxima | - | [82] |
| 263 | 13-epi-scabrolide C | S. maxima | - | [82] |
| 264 | sinudenoid A | S. densa | - | [83] |
| 265 | sinudenoid B | S. densa | - | [83] |
| 266 | sinudenoid C | S. densa | - | [83] |
| 267 | sinudenoid D | S. densa | - | [83] |
| 268 | ineleganolide | S. maxima | - | [81,82,84,85] |
| 269 | fragilolide A | J. fragilis | - | [86] |
| 270 | sinuscalide C | S. scabra | - | [87] |
| 271 | sinuscalide D | S. scabra | - | [87] |
| 272 | sinuscalide B | S. scabra | - | [87] |
| 273 | scabrolide B | S. densa | - | [83] |
| 274 | scabrolide A | S. densa | - | [83] |
| 275 | sinudenoid E | S. densa | - | [83] |
| 276 | sinuscalide A | S. scabra | - | [87] |
| No. | Name | Plant Source | Plant Organ | Ref. |
|---|---|---|---|---|
| 277 | japodagricanone A | J. podagrica | twigs and leaves | [88] |
| 278 | japodagricanone B | J. podagrica | twigs and leaves | [88] |
| 279 | 6-((E)-12-(furan-13-yl)-10-methylpent-10-en-9-yl)-6,7,8,8atetrahydro-3H-isochromen-1-(5H)-one | U. (Photololigo) duvaucelii | - | [89] |
| 280 | erythro-norcassanoid A | E. fordii | roots | [90] |
| 281 | erythro-norcassanoid B | E. fordii | roots | [90] |
| 282 | caesalpinone | C. decapetala var. japonica | roots | [91] |
| 283 | aspidoptoid A | A. obcordata | vine | [92] |
| 284 | aspidoptoid B | A. obcordata | vine | [92] |
| 285 | phyllanflexoid C | P. flexuosus | roots | [93] |
| 286 | olicleistanone | D. olivarum | - | [32] |
| 287 | alterbrassicicene B | A. brassicicola | - | [94] |
| 288 | 1β-hydroxy-brassicicene Q | A. brassicicola | - | [94] |
| 289 | 3-ketobrassicicene W | A. brassicicola | - | [94] |
| 290 | aculeaterpene A | A. aculeatinus | - | [95] |
| 291 | dongtingnoid C | P. sp. DT10 | - | [96] |
| 292 | crotocascarin α | C. cascarilloides | stems | [97] |
| 293 | crotocascarin β | C. cascarilloides | stems | [97] |
| 294 | (+)-paeoveitol | P. veitchii | roots | [98] |
| 295 | (−)-paeoveitol | P. veitchii | roots | [98] |
| 296 | eurifoloid M | E. neriifolia | twigs and leaves | [99] |
| 297 | eurifoloid P | E. neriifolia | twigs and leaves | [99] |
| 298 | adipoiloside | A. poilanei | leaves | [100] |
| 299 | citrinovirin | T. citrinoviride | - | [101] |
| 300 | 16-deacetoxy-9,11-dihydrogracilin A | G. Mollusks | - | [102] |
| 301 | 15,16-deacetoxy-15-hydroxy-9,11-dihydrogracilin A | G. Mollusks | - | [102] |
| 302 | verrielactone | G. Mollusks | - | [102] |
| 303 | 3-nor-spongiolide A | S. officinalis | - | [103] |
| 304 | 3-nor-spongiolide B | S. officinalis | - | [103] |
| 305 | scrodentoid F | S. dentata | whole plants | [104] |
| 306 | scrodentoid G | S. dentata | whole plants | [104] |
| 307 | scrodentoid H | S. dentata | whole plants | [104,105] |
| 308 | scrodentoid I | S. dentata | whole plants | [104,105] |
| 309 | normulin-11-en-13-oxo-20-oic acid | A. compacta | aerial part | [106] |
| 310 | pyromyxone D | C. pyromyxa | fruiting bodies | [107] |
| 311 | adenica | L. zeylanica | whole plants | [108] |
| 312 | 6β-acetoxy-9α,13-epoxy-16-norlabd-13E-en-15-al | L. zeylanica | whole plants | [108] |
| 313 | scabrolide A | S. scabra | - | [81] |
| 314 | yonarolide | S. scabra | - | [81] |
| 315 | 12-hydroxy-scabrolide A | S. scabra | - | [81] |
| 316 | sinusiaetone A | S. siaesensis | - | [109] |
| 317 | cespitaenin A | C. taeniata | - | [110] |
| 318 | cespitaenin B | C. taeniata | - | [110] |
| 319 | cespitulin Q | Cespitularia sp. | - | [111] |
| 320 | cespitulin R | Cespitularia sp. | - | [111] |
| 321 | cespitulin P | Cespitularia sp. | - | [111] |
| 322 | norhawthornoid A | C. pinnatifida | leaves | [112] |
| 323 | norhawthornoid B | C. pinnatifida | leaves | [112] |
| 324 | ebractenoid A | E. ebracteolata | roots | [113] |
| 325 | ebractenoid B | E. ebracteolata | roots | [113] |
| 326 | (2S,7S,11S)-(8E,12Z)-2, 10-dihydroxy-pellialactone | A. chinense | roots | [114] |
| 327 | (2S,4S,7S,11S)-(8E,12Z)-2,4,10-trihydroxy-pellialactone | A. chinense | roots | [114] |
| 328 | miltiolactone A | S. miltiorrhiza | roots | [115] |
| 329 | miltiolactone B | S. miltiorrhiza | roots | [115] |
| 330 | taichunin A | A. taichungensis | - | [33] |
| 331 | taichunin B | A. taichungensis | - | [33] |
| 332 | taichunin C | A. taichungensis | - | [33] |
| 333 | PR 1388 | O. truncatum | - | [116] |
| 334 | oidiolactone D | O. truncatum | - | [116] |
| 335 | oidiolactone C | O. truncatum | - | [116] |
| 336 | oidiolactone G | O. truncatum | - | [116] |
| 337 | epi-oidiolactone G | O. truncatum | - | [116] |
| 338 | oidiolactone H | O. truncatum | - | [116] |
| 339 | oidiolactone I | O. truncatum | - | [116] |
| 340 | oidiodendronic acid | O. truncatum | - | [116] |
| 341 | oidiolactone E | O. truncatum | - | [116] |
| 342 | LL-Z1271α | O. truncatum | - | [116] |
| 343 | oidiolactone J | O. truncatum | - | [116] |
| 344 | oidiolactone K | O. truncatum | - | [116] |
| 345 | oidiolactone L | O. truncatum | - | [116] |
| 346 | LL-Z1271β | O. truncatum | - | [116] |
| 347 | 16-epi-pretoxin | A. carunculatus | fruits | [117] |
| 348 | austrobuxusin F | A. carunculatus | fruits | [117] |
| 349 | austrobuxusin G | A. carunculatus | fruits | [117] |
| 350 | austrobuxusin H | A. carunculatus | fruits | [117] |
| 351 | 16-epi-austrobuxusin H | A. carunculatus | fruits | [117] |
| 352 | 16-epi-austrobuxusin G | A. carunculatus | fruits | [117] |
| 353 | austrobuxusin I | A. carunculatus | fruits | [117] |
| 354 | austrobuxusin J | A. carunculatus | fruits | [117] |
| 355 | 16-epi-austrobuxusin B | A. carunculatus | fruits | [117] |
| 356 | austrobuxusin K | A. carunculatus | fruits | [117] |
| 357 | austrobuxusin L | A. carunculatus | fruits | [117] |
| 358 | austrobuxusin M | A. carunculatus | fruits | [117] |
| 359 | methyl-13-acetyl-podocarpa-8,11,13-trien-18-oate | P. euphratica | resins | [49] |
| 360 | sinuhirtone B | S. hirta | - | [118] |
| 361 | 9,11-dihydrogracilin A | D. antarctica | - | [119] |
| 362 | 9,11-dihydrogracillinone A | D. antarctica | - | [119] |
| 363 | eupractenoid A | E. ebracteolata | roots | [120] |
| 364 | eupractenoid B | E. ebracteolata | roots | [120] |
| No. | Name | Plant Source | Plant Organ | Ref. |
|---|---|---|---|---|
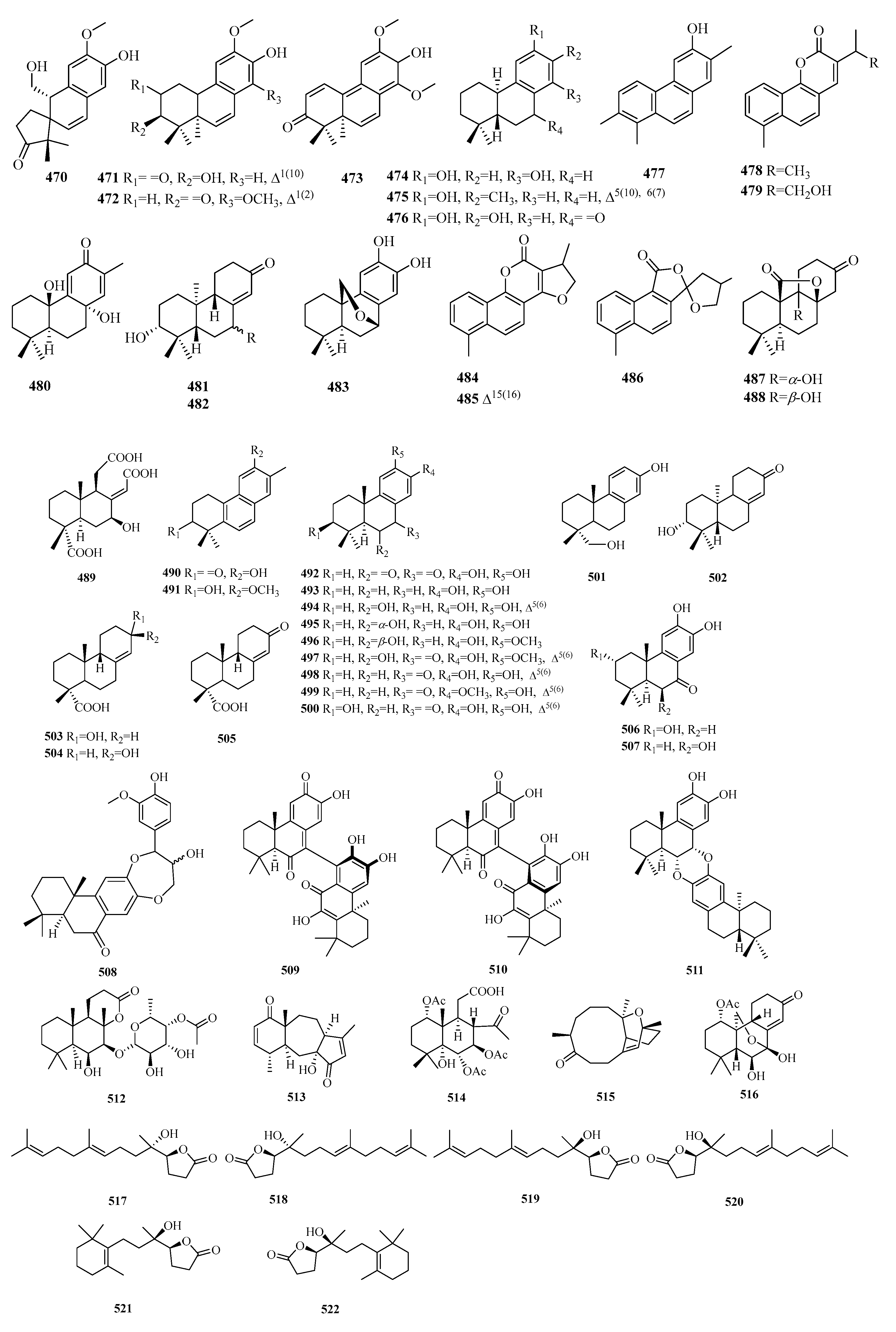
| 365 | salyunnanin F | S. yunnanensis | roots | [121] |
| 366 | 16,17-dinorpisferal A | S. digitaloides | roots | [122] |
| 367 | militibetin A | S. miltiorrhiza | dry roots | [48] |
| 368 | (5S,8S,10R)-militibetinA | P. abrotanoides | roots | [48] |
| 369 | normiltioane | S. miltiorrhiza | cell cultures | [44] |
| 370 | yunnannin A | S. miltiorrhiza | dry roots | [123] |
| 371 | (5S,6S,7R,10R)-16,17-bis-nor-7α-hydroxy-18,6-epoxyferruginol | P. abrotanoides | roots | [48] |
| 372 | (5S,6S,7S)-16,17-bis-nor-7α-hydroxyferruginol-18,6-olide | P. abrotanoides | roots | [48] |
| 373 | przewalskin | S. digitaloides | roots | [122] |
| 374 | (5S,6S,7R,10R)-16,17-bis-nor-6β-hydroxy-18,7-epoxyferruginol | P. abrotanoides | roots | [48] |
| 375 | (5R,7S,10R)-3-oxoprzewalskin | P. abrotanoides | roots | [48] |
| 376 | grandifolia C | P. abrotanoides | roots | [48] |
| 377 | 2-isopropyl-8-methylphenan-threne-3,4-dione | S. miltiorrhiza | cell cultures | [44] |
| 378 | (5S,10R)-16,17-bis-nor-pisiferanol | P. abrotanoides | roots | [48] |
| 379 | 5S-1,2-dihydroheudelotinol | T. chinensis | stem bark and wood | [124] |
| 380 | deoxofaveline | P. abrotanoides | roots | [48] |
| 381 | trigonostemon A | T. chinensis | stem bark and wood | [124] |
| 382 | trigonostemon B | T. chinensis | stem bark and wood | [124] |
| 383 | 5S-heudelotinone | T. chinensis | stem bark and wood | [124] |
| 384 | trigonostemon C | T. chinensis | stem bark and wood | [124] |
| 385 | trigonostemon D | T. chinensis | stem bark and wood | [124] |
| 386 | heudelotinone | T. howii | stems and leaves | [124] |
| 387 | norperovskatone | P. atriplicifolia | flowers | [125] |
| 388 | salviolone | P. abrotanoides | roots | [48] |
| 389 | epi-castanolide | P. abrotanoides | roots | [48] |
| 390 | cryptoacetalide | S. miltiorrhiza | cell cultures | [44] |
| 391 | epicryptoacetalide | S. miltiorrhiza | cell cultures | [44] |
| 392 | epi-danshenspiroketallactone A | S. miltiorrhiza | cell cultures | [44] |
| 393 | tanshinone I | S. digitaloides | cell cultures | [56,122] |
| 394 | dihydrotanshinone I | S. miltiorrhiza | cell cultures | [56] |
| 395 | dihydrotanshinone | S. digitaloides | roots | [122] |
| 396 | dihydroisotanshinone I | S. miltiorrhiza | cell cultures | [44] |
| 397 | tanshinketolactone | S. miltiorrhiza | cell cultures | [44] |
| 398 | trigonostemon G | T. chinensis | stem bark | [126] |
| 399 | trigonostemon H | T. chinensis | stem bark | [126] |
| 400 | flueggenoid E | S. miltiorrhiza | roots and rhizomes | [127] |
| 401 | 12-hydroxy-20(10→5)-abeo-4,5-seco-podocarpa-5(10),6,8,11,13-pentaen-3-one | S. miltiorrhiza | roots and rhizomes | [127] |
| 402 | 3β,12-dihydroxy-13-methylpodocarpa-6,8,11,13-tetraene | S. digitaloides | roots | [128] |
| 403 | 3α-hydroxy-12-methoxy-13-methyl-ent-podocar-6,8,11,13-tetraene | S. digitaloides | roots | [128] |
| 404 | 12-hydroxy-13-methyl-ent-podocarp-6,8,11,13-tetraen-3-one | S. digitaloides | roots | [128] |
| 405 | 12-methoxy-13-methyl-ent-podocarp-6,8,11,13-tetraen-3-one | S. digitaloides | roots | [128] |
| 406 | jatromulone A | T. chinensis | stem bark and wood | [129] |
| 407 | gossweilone | T. chinensis | stem bark and wood | [129] |
| 408 | flueggenoid C | S. miltiorrhiza | roots and rhizomes | [127] |
| 409 | 3β,12-dihydroxy-13-methylpodocarpane-8,10,13-triene | T. chinensis | stem bark and wood | [129] |
| 410 | 3β,12-dihydroxy-13-methylpodocarpa-8,11,13-triene | S. digitaloides | roots | [128] |
| 411 | 3α-hydroxy-12-methoxy-13-methyl-ent-podocarpa-6,8,11,13-tetraene | S. miltiorrhiza | roots and rhizomes | [127] |
| 412 | 3α,12-dihydroxy-13-methyl-ent-podocarpa-6,8,11,13-tetraene | S. miltiorrhiza | roots and rhizomes | [127] |
| 413 | 6β,12-dihydroxy-13-methyl-ent-podocarp-8,11,13-trien-3-one | S. digitaloides | roots | [128] |
| 414 | rel-(5β,8α,10α)-8-hydroxy-13-methylpodocarpa-9(11),13-diene-3,12-dione | A. moluccanus | twigs | [130] |
| 415 | 3α-hydroxy-13-hydroxymethyl-12-methoxy-ent-podocarp-6,8,11,13-tetraene | S. digitaloides | roots | [128] |
| 416 | 3β-hydroxy-13-hydroxymethyl-12-methoxy-ent-podocarp-6,8,11,13-tetraene | S. digitaloides | roots | [128] |
| 417 | 10α,12-dihydroxy-13-methyl-9(10→20)-abeo-ent-podocarpa-6,8,11,13-tetraen-3-one | S. miltiorrhiza | roots and rhizomes | [127] |
| 418 | flueggenoid A | S. miltiorrhiza | roots and rhizomes | [127] |
| 419 | flueggenoid.B | S. miltiorrhiza | roots and rhizomes | [127] |
| 420 | flueggenoid D | S. miltiorrhiza | roots and rhizomes | [127] |
| 421 | 6,12-dihydroxy-13-methyl-7-oxo-ent-podocarpa-5,8,11,13-tetraeno-20,3α-lactone | S. miltiorrhiza | roots and rhizomes | [127] |
| 422 | 3α,20-epoxy-3β-hydroxy-12-methoxy-13-methyl-ent-podocarp-8,11,13-triene | S. digitaloides | roots | [128] |
| 423 | 7α,20-epoxy-3α-hydroxy-12-methoxy-13-methyl-ent-podocarp-8,11,13-triene | S. digitaloides | roots | [128] |
| 424 | 3β-hydroxymakilactone A | P. macrophyllus | roots | [131] |
| 425 | 2β-hydroxymakilactone A | P. macrophyllus | roots | [131] |
| 426 | inumakilactone A | P. macrophyllus | roots | [131] |
| 427 | makilactone M | P. macrophyllus | roots | [131] |
| 428 | inumakilactone B | P. macrophyllus | roots | [131] |
| 429 | (4S,5R,9S,10R)-methyl-19-hydroxy-15,16-dinorlabda-8(17),11E-dien-13-oxo-18-oate | A. macrophylla | - | [6] |
| 430 | pseudosinin C | P. sinensis | needles and twigs | [132] |
| 431 | pseudosinin B | P. sinensis | needles and twigs | [132] |
| 432 | 8α-hydroxy-11(E)-en-13-oxo-14,15-dinorlabdan-18-oic | S. mirzayanii | - | [133] |
| 433 | austroinulin | S. rebaudiana | aerial parts | [134] |
| 434 | sterebin A | S. rebaudiana | aerial parts | [134] |
| 435 | sterebin D | S. rebaudiana | aerial parts | [134] |
| 436 | hedychin A | H. forrestii | rhizomes | [12] |
| 437 | hedychin F | H. forrestii | rhizomes | [12] |
| 438 | lyonivaloside I | L. ovalifolia | twigs and leaves | [135] |
| 439 | dryperrein A | D. perreticulata | twigs and leaves | [136] |
| 440 | dryperrein B | D. perreticulata | twigs and leaves | [136] |
| 441 | dryperrein C | D. perreticulata | twigs and leaves | [136] |
| 442 | dryperrein D | D. perreticulata | twigs and leaves | [136] |
| 443 | (2S,3R,5S,10R)-2,3-dihydroxy-15,16-dinor-ent-pimar-8,11,13-triene | F. fimbriata | aerial parts | [39] |
| 444 | (2S,3R,5S,10R)-2-acetoxy-3-hydroxy-15,16-dinor-ent-pimar-8,11,13-triene | F. fimbriata | aerial parts | [39] |
| 445 | flickinflimilin B | F. fimbriata | leaves | [137] |
| 446 | flickinflimilin A | F. fimbriata | leaves | [137] |
| 447 | norflickinflimiod E | F. fimbriata | stems | [40] |
| 448 | norflickinflimiod F | F. fimbriata | stems | [40] |
| 449 | 4β-carbometoxy-14-methyltotarol | E. suaveolens | stems | [138] |
| 450 | aspergiloid I | Aspergillus sp. | culture broth | [139] |
| 451 | aleuritin | A. moluccanus | twigs | [130] |
| 452 | salviprolin A | S. przewalskii | roots | [140] |
| 453 | salviprolin B | S. przewalskii | roots | [140] |
| 454 | commiphorane A | R. Commiphora | - | [141] |
| 455 | commiphorane B | R. Commiphora | - | [141] |
| 456 | commiphoranoid C | R. Commiphora | - | [142] |
| 457 | commiphorane K | R. Commiphora | - | [143] |
| 458 | 7β-hydroxynagilactone D | P. nagi | seeds | [144] |
| 459 | 3-epi-15-hydroxynagilactoneD | P. nagi | seeds | [144] |
| 460 | nagilactone K | P. nagi | seeds | [144] |
| 461 | nagilactone L | P. nagi | seeds | [144] |
| 462 | 3β-hydroxynagilactone L | P. nagi | seeds | [144] |
| 463 | 2β-hydroxynagilactone L | P. nagi | seeds | [144] |
| 464 | 1α-chloro-2β,3β,15-trihydroxynagilactone | P. nagi | seeds | [144] |
| 465 | 15-hydroxynagilactone L | P. nagi | seeds | [144] |
| 466 | cephanolide A | C. sinensis | - | [75,145] |
| 467 | cephanolide B | C. sinensis | - | [75,145] |
| 468 | cephanolide C | C. sinensis | - | [75,145] |
| 469 | sinuhirtone A | S. hirta | - | [118] |
| No. | Name | Plant Source | Plant Organ | Ref. |
|---|---|---|---|---|
| 470 | baccaramione A | B. ramiflora | twigs | [146] |
| 471 | baccaramione B | B. ramiflora | twigs | [146] |
| 472 | baccaramione C | B. ramiflora | twigs | [146] |
| 473 | baccaramione D | B. ramiflora | twigs | [146] |
| 474 | euphorane B | E. dracunculoides | whole plants | [52] |
| 475 | 12-hydroxy-16,17-bis-nor-simonellite | P. abrotanoides | roots | [48] |
| 476 | nimbidiol | P. abrotanoides | roots | [48] |
| 477 | 16,17-bis-nor-multicauline | P. abrotanoides | roots | [48] |
| 478 | salyunnanin D | S. yunnanensis | roots | [121] |
| 479 | salyunnanin E | S. yunnanensis | roots | [121] |
| 480 | (5S,8R,10S)-20-nor-militibetin A | P. abrotanoides | roots | [48] |
| 481 | sessilifol P | C. sessilifolius | - | [59] |
| 482 | sessilifol Q | C. sessilifolius | - | [59] |
| 483 | (5S,7S,10R)-7-deoxy-7,18-epoxynimbidiol | C. sessilifolius | - | [48] |
| 484 | dihydroneotanshinlactone | S. digitaloides | roots | [122] |
| 485 | neotanshinlactone | S. digitaloides | roots | [122] |
| 486 | danshenspiroketallactone | S. digitaloides | roots | [122] |
| 487 | rubesanolides F | I. rubescens | leaves | [57] |
| 488 | rubesanolide G | I. rubescens | leaves | [57] |
| 489 | pinuyunnanacid O | P. yunnanensis | resins | [147] |
| 490 | flueggrene A | F. virosa | roots | [148] |
| 491 | flueggrene B | F. virosa | roots | [148] |
| 492 | epi-6-oxonimbidiol | C. angulatus | root bark and leaves | [149] |
| 493 | (+)-7-deoxynimbidiol | C. sessilifolius | - | [150] |
| 494 | celaphanol A | C. orbiculatus | - | [150,151] |
| 495 | angulatusphenol C | C. angulatus | root bark and leaves | [149] |
| 496 | angulatusphenol D | C. angulatus | root bark and leaves | [149] |
| 497 | angulatusphenol E | C. angulatus | root bark and leaves | [149] |
| 498 | △5-nimbidiol | C. angulatus | root bark and leaves | [149] |
| 499 | margosolone | C. angulatus | root bark and leaves | [149] |
| 500 | demethylnimbionol | C. angulatus | root bark and leaves | [149] |
| 501 | 13,15-dihydroxypodocarpa-8,11,13-triene | P. banksiana Lamb | buds | [152] |
| 502 | (3R,5S,9R,10S)-3-hydroxy-ent-podocarpa-8(14)-ene-13-one | C. sessilifolius | - | [153] |
| 503 | (4R,5R,9R,10R,13S)-13-hydroxypodocarp-8(14)-en-19-oic acid | A. macrophylla | - | [6] |
| 504 | (4R,5R,9R,10R,13R)-13-hydroxypodocarp-8(14)-en-19-oic acid | A. macrophylla | - | [6] |
| 505 | 13-oxo-podocarp-8(14)-en-19-oic acid | A. macrophylla | - | [6] |
| 506 | angulatusphenol A | C. angulatus | root bark and leaves | [149] |
| 507 | angulatusphenol B | C. angulatus | root bark and leaves | [149] |
| 508 | 7′,8′-threo-guaiacylglycerol-α,γ-O-nimbidiol diether | C. sessilifolius | - | [150] |
| 509 | (M)-bicelaphanol A | C. orbiculatus | root bark | [151,154] |
| 510 | (P)-bicelaphanol A | C. orbiculatus | root bark | [151,154] |
| 511 | angulatusdiphenol A | C. angulatus | root bark and leaves | [149] |
| 512 | trolliusditerpenoside N | T. chinensis | flowers | [155] |
| 513 | enbepeanone A | A. grandiflflorum | - | [156] |
| 514 | caesalminaxin M | C. minax | seeds | [157] |
| 515 | populusone | P. euphratica | exudates | [158] |
| 516 | pharicusin B | I. pharicus | aerial parts | [159] |
| 517 | (±)-8,13-secoepicavernosine | Cacospongia sp. | - | [160] |
| 518 | (+)-8,13-secocavernosine | Cacospongia sp. | - | [160] |
| 519 | (−)-8,13-secocavernosine | Cacospongia sp. | - | [160] |
| 520 | (+)-cavernosine | Cacospongia sp. | - | [160] |
| 521 | (−)-cavernosine | Cacospongia sp. | - | [160] |
| No. | Name | Plant Source | Plant Organ | Ref. |
|---|---|---|---|---|
| 522 | acrostalic acid | P. sinensis | needles and twigs | [132,161] |
| 523 | LL-Z1271-β | A. wentii EN-48 | - | [161,162] |
| 524 | 13,14,15,16-tetranorlabda-8(17)-en-12-carboxylic acid | E. verrucosus | - | [4] |
| 525 | 3α-hydroxy-8α-acetoxy-13,14,15,16-tetranorlabdan-12-oeic acid | S. aethiopis | aerial parts | [163] |
| 526 | avxanthin A | A. villosum | rhizomes | [164] |
| 527 | elettarin A | E. cardamomum | fruits | [165] |
| 528 | elettarin B | E. cardamomum | fruits | [165] |
| 529 | asperolide C | A. wentii EN-48 | - | [162] |
| 530 | botryosphaerin B | P. sinensis | needles and twigs | [162] |
| 531 | asperolide A | A. wentii EN-48 | - | [162,166] |
| 532 | asperolide B | A. wentii EN-48 | - | [162,166] |
| 533 | asperolide E | A. wentii EN-48 | culture extract | [166] |
| 534 | tetranorditerpenoid derivative | A. wentii EN-48 | - | [162] |
| 535 | wentilactone A | A. wentii EN-48 | - | [162] |
| 536 | wentilactone B | A. wentii EN-48 | culture extract | [162] |
| 537 | 13,14,15,16-tetranorlabd-7-en-19,6β:12,17-diolide | P. sinensis | needles and twigs | [161] |
| 538 | botryosphaerin G | P. sinensis | needles and twigs | [161] |
| 539 | botryosphaerin H | P. sinensis | needles and twigs | [161] |
| 540 | 3α,10β-dimethyl-1,2,3,3a,5a,7,10b,10c-octahydro-5,8-dioxa-acephenanthrylene-4,9-dione | P. sinensis | needles and twigs | [161] |
| 541 | botryosphaerin A | P. sinensis | needles and twigs | [161] |
| 542 | 1-naphthaleneacetic-7-oxo-1,2,3,4,4a,7,8,8a-octahydro1,2,4a,5-tetramethyl acid | P. longifolia | leaves | [167] |
| 543 | methyl-7-oxo-1,2,3,4,4a,7,8,8a-octahydro-1,2,4a,5-tetramethyl-1-naph-thaleneacetate | P. longifolia | leaves | [167] |
| 544 | castanol C | S. castanea | flowers | [50] |
| 545 | sinubatin A | S. nanolobata | - | [168] |
| 546 | norcrocrassinone | C. crassifolius | roots | [169] |
| 547 | norcrassin A | C. crassifolius | roots | [170] |
| 548 | (3aR,5S,5aR,6R,9aS,9bR)-methyl5-hydroxy-3α,6,9α-trimethyl-2oxododecahydronaphtho[21-b]furan-6-carboxylate | S. sahendica | leaves | [171] |
| 549 | acerolanin A | M. emarginata | aerial parts | [172] |
| 550 | acerolanin B | M. emarginata | aerial parts | [172] |
| 551 | acerolanin C | M. emarginata | aerial parts | [172] |
| 552 | trigonochinene E | T. flavidus | stems | [173] |
| 553 | vibsanolide F | V. odoratissimum | leaves | [174] |
| 554 | vibsanolide G | V. odoratissimum | leaves | [174] |
| 555 | trigoflavidol A | T. flavidus | stems | [173] |
| 556 | trigoflavidol B | T. flavidus | stems | [173] |
| 557 | neoboutomannin | T. flavidus | stems | [173] |
| Name | Extraction Solvent | Cancer Types | Cancer Cells | Activities (IC50) | Ref. |
|---|---|---|---|---|---|
| tinocapillin A (38) | 95% EtOH | liver cancer cervical cancer | HepG-2 Hela | 9.9 ± 1.5 μM 9.7 ± 3.4 μM | [20] |
| tinocallone C (40) | 95% EtOH | lung cancer | A549 | 14.0 ± 0.9 μM | [20] |
| tinocapillin B (41) | 95% EtOH | lung cancer liver cancer cervical cancer | A549 HepG-2 Hela | 9.6 ± 1.2 μM 10.1 ± 1.1 μM 12.0 ± 1.0 μM | [20] |
| icacinlactone F (76) | 80% aqueous MeOH | breast cancer | MDA-MB-435 MDA-MB-231 | 6.16 μM 8.94 μM | [28] |
| asperether A (101) | EtOAc | breast duct cancer | T-47D | 10 μM | [38] |
| asperether B (102) | EtOAc | breast cancer liver cancer | MCF-7 SMMC-7721 | 14 μM 12 μM | [38] |
| cryptotanshinone (148) | 80% EtOH | colon cancer gastric cancer lung cancer | HCT-8 BGC-823 A549 | 3.9 μM 8.3 μM 2.6 μM | [44] |
| euphorane C (139) | 95% EtOH | liver cancer | HepG-2 | 6.95 μM | [52] |
| methyltanshinoate (150) | acetone | liver cancer lung cancer breast cancer colon cancer | SMMC-7721 A549 MCF-7 SW-480 | 4.07 μM 5.26 μM 3.44 μM 6.35 μM | [50] |
| amentotaxin C (174) | MeOH | cervical cancer lung cancer breast cancer ovarian cancer liver cancer colon cancer | HeLa A549 MDA-MB-231 SKOV3 Huh-7 HCT-116 | 5.1 μM 9.8 μM 6.8 μM 2.9 μM 4.1 μM 1.9 μM | [65] |
| 20-oxohainanolidol (205) | 95% EtOH | leukemia lung cancer | HL-60 A549 | 0.77 ± 0.05 μM 1.129 ± 0.057 μM | [77] |
| cephinoid H (208) | MeOH | lung cancer cervical cancer gastric cancer | A549 HeLa SGC-7901 | 0.10 μM 0.13 μM 0.14 μM | [78] |
| 10-hydroxyharringtonolide (223) | - | lung cancer oral epidermoid cancer leukemia colon cancer | A549 KB HL-60 HT-29 | 3.683 ± 0.947 μM 2.325 ± 0.040 μM 1.038 ± 0.002 μM 2.108 ± 0.108 μM | [79] |
| 6-en-harringtonolide (229) | - | lung cancer oral epidermoid cancer leukemia colon cancer | A549 KB HL-60 HT-29 | 7.804 ± 3.797 μM 5.115 ± 0.148 μM 2.319 ± 0.247 μM 4.890 ± 0.622 μM | [79] |
| tanshinone I (393) | acetone | leukemia lung cancer breast cancer pancreatic cancer | HL-60 A549 SK-BR-3 PANC-1 | 3.19 μM 6.64 μM 3.40 μM 3.67 μM | [56,122] |
| dihydroisotanshinone I (394) | 80% EtOH | lung cancer | A549 | 2.7 μM | [44] |
| dihydrotanshinone (395) | acetone | leukemia liver cancer lung cancer breast cancer pancreatic cancer | HL-60 SMMC-7721 A549 SK-BR-3 PANC-1 | 2.36 μM 3.03 μM 5.15 μM 3.97 μM 2.75 μM | [122] |
| 3β-hydroxymakilactone A (424) | acetone | gastric cancer breast cancer liver cancer pancreatic cancer | AGS MDA-MB-231 HepG-2 PANC-1 | 0.88 ± 0.01 μM 5.46 ± 1.12 μM 5.56 ± 1.73 μM 1.35 ± 0.08 μM | [131] |
| 2β-hydroxymakilactone A (425) | MeOH | cervical cancer gastric cancer breast cancer | Hela AGS MDA-MB-231 | 0.87 ± 0.04 μM 0.38 ± 0.03 μM 4.23 ± 2.06 μM | [131] |
| inumakilactone A (426) | MeOH | cervical cancer gastric cancer breast cancer | Hela AGS MDA-MB-231 | 1.77 ± 0.69 μM 1.33 ± 0.05 μM 2.98 ± 1.06 μM | [131] |
| inumakilactone B (428) | MeOH | cervical cancer gastric cancer breast cancer liver cancer pancreatic cancer | Hela AGS MDA-MB-231 HepG-2 PANC-1 | 0.62 ± 0.18 μM 0.55 ± 0.21 μM 0.66 ± 0.15 μM 3.54 ± 1.45 μM 8.51 ± 2.65 μM | [131] |
| dryperrein C (441) | 95% EtOH | lung cancer leukemia | A549 HL-60 | 8.50 μM 1.95 μM | [136] |
| dryperrein D (442) | 95% EtOH | leukemia | HL-60 | 1.37 μM | [136] |
| salyunnanin E (479) | acetone | cervical cancer | HeLa | 0.86 μM | [121] |
| neotanshinlactone (485) | acetone | breast cancer | SK-BR-3 | 4.07 μM | [122] |
| Name | Pathways | Activities (IC50) | Ref. |
|---|---|---|---|
| callinteger B (31) | Inhibited IL-1β secretion and maturations of caspase-1 in a dose-dependent manner | 9.9 ± 1.5 μM | [15] |
| 18(4→14), 19(4→8)-bis-abeo-nor-isopimarane-1,5-diene-3-yl-3β-methoxy propyl pentanoate (82) | Inhibited of pro-inflammatory cyclooxygenases (COX-2, COX-1) and 5-lipoxygenase (5-LOX) enzymes | 0.75 mg/mL | [30] |
| 6-((E)-12-(furan-13-yl)-10-methylpent-10-en-9-yl)-6,7,8,8atetrahydro-3H-isochromen-1-(5H)-one (279) | Inhibited of 5-LOX enzymes | 0.92 mg/mL | [89] |
| (2S,3R,5S,9S,10S,13S)-2-O-E-cinnamoyl-3-hydroxy-16-nor-ent-pimar-8(14)-en-15-oic acid (110) | Inhibited the NF-κB pathway in LPS-stimulated RAW264.7 cells | 14.7 ± 1.8 μM | [39] |
| cephalotanin A (190) | Evaluated in an NF-kB pathway luciferase assay for inhibitory effects | 4.12 ± 0.61 µM | [73] |
| salvialba acid (163) | Lowered the levels of ICAM-1 and VCAM-1 in HAECs induced by TNF-α | 20 µM caused significant reductions in cell viability; 0.05, 0.5, 5, and 10 µM did not affect cell viability | [60] |
| cephinoid H (208) | Inhibited TNF-α-induced NF-κB activation | 0.10 μM | [78] |
| 5-epi-sinuleptolide (255) | Activated ARE expression and inhibited NO production and NF-κB expression in RAW264.7 macrophage cells | 5.6 ± 0.2 µM 57.9 ± 0.4 µM 28.6 ± 0.2 µM | [82,86] |
| sinuleptolide (261) | Activated ARE expression and inhibited NO production and NF-κB expression in RAW264.7 macrophage cells | 3.6 ± 0.3 µM 56.0 ± 0.3 µM 25.1 ± 0.5 µM | [82,86] |
| fragilolide A (269) | Activated ARE expression and inhibited NO production and NF-κB expression in RAW264.7 macrophage cells | 1.2 ± 0.2 µM 27.8 ± 0.6 µM 12.5 ± 0.2 µM | [82,86] |
| celaphanol A (494) | Demonstrated moderate inhibitory activities against NF-κB activation in RAW264.7 macrophages | 15 μM | [149,150,151] |
| angulatusphenol C (495) | Demonstrated moderate inhibitory activities against NF-κB activation in RAW264.7 macrophages | 25 μM | [149,150,151] |
| angulatusphenol D (496) | Demonstrated moderate inhibitory activities against NF-κB activation in RAW264.7 macrophages | 19 μM | [149,150,151] |
| demethylnimbionol (500) | Demonstrated moderate inhibitory activities against NF-κB activation in RAW264.7 macrophages | 4 μM | [149,150,151] |
| 15-nor-14-oxolabda-8(17),13(16)-dien-19-oic acid (8) | Inhibited LPS-induced nitric oxide (NO) production. Attenuated the expression of iNOS and COX-2 at both mRNA and protein levels by inhibiting the LPS-induced degradation of I-κBα and the activation of NF-κB, as well as reducing ERK phosphorylation | 3.56 μM | [7] |
| ebractenoid A (324) | Inhibited the production of NO in LPS-induced macrophages | 7.50 μM | [113] |
| ebractenoid B (325) | Inhibited the production of NO in LPS-induced macrophages | 6.49 μM | [113] |
| hedychin F (437) | Inhibited the production of NO in LPS-induced macrophages | 21.0 μM | [12] |
| flickinflimilin B (445) | Inhibited the production of NO and TNF-α in LPS-induced macrophages | <25.0 μM | [40,137] |
| flickinflimilin A (446) | Inhibited the production of NO and TNF-α in LPS-induced macrophages | <25.0 μM | [40,137] |
| norflickinflimiod E (447) | Inhibited the production of NO and TNF-α in LPS-induced macrophages | <25.0 μM | [40,137] |
| norflickinflimiod F (448) | Inhibited the production of NO and TNF-α in LPS-induced macrophages | <25.0 μM | [40,137] |
| przewalskin (373) | Inhibited iNOS expression in J774A.1 macrophages stimulated with LPS | - | [48] |
| (5S,6S,7R,10R)-16,17-bis-nor-6β-hydroxy-18,7-epoxyferruginol (374) | Inhibited iNOS expression in J774A.1 macrophages stimulated with LPS | - | [48] |
| (5S,10R)-16,17-bis-nor-pisiferanol (378) | Inhibited iNOS expression in J774A.1 macrophages stimulated with LPS | - | [48] |
| (5S,8R,10S)-20-nor-militibetin A (480) | Inhibited iNOS expression in J774A.1 macrophages stimulated with LPS | - | [48] |
| (+)-7-deoxynimbidiol (493) | Inhibited LPS-stimulated NO releases and pro-inflammatory mediators and suppressed iNOS and COX-2 expressions to prevent NO production | 4.9 μM | [139] |
| 7′,8′-threo-guaiacylglycerol-α,γ-O-nimbidiol diether (508) | Inhibited LPS-stimulated NO releases and pro-inflammatory mediators, suppressed iNOS and COX-2 expressions to prevent NO production | 12.6 μM | [139] |
| 13-epi-scabrolide C (263) | Inhibited the production of IL-12 and IL-6 in LPS-stimulated BMDCs | 5.30 ± 0.21 μM 13.12 ± 0.64 μM | [82] |
| scrodentoid H (307) | Reduced LPS-induced inflammation and inhibited the JNK/STAT3 pathway in macrophages | - | [105] |
| scrodentoid I (308) | Reduced LPS-induced inflammation and inhibited the JNK/STAT3 pathway in macrophages | - | [105] |
| sinusiaetone A (316) | Inhibited LPS-induced inflammation in BV-2 microglia at a concentration of 20 μM and decreased the mRNA levels of pro-inflammatory cytokines IL-6 and IL-1β | - | [109] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zeng, N.; Zhang, Q.; Yao, Q.; Fu, G.; Su, W.; Wang, W.; Li, B. A Comprehensive Review of the Classification, Sources, Phytochemistry, and Pharmacology of Norditerpenes. Molecules 2024, 29, 60. https://doi.org/10.3390/molecules29010060
Zeng N, Zhang Q, Yao Q, Fu G, Su W, Wang W, Li B. A Comprehensive Review of the Classification, Sources, Phytochemistry, and Pharmacology of Norditerpenes. Molecules. 2024; 29(1):60. https://doi.org/10.3390/molecules29010060
Chicago/Turabian StyleZeng, Ni, Qiongdan Zhang, Qingying Yao, Gang Fu, Wei Su, Wei Wang, and Bin Li. 2024. "A Comprehensive Review of the Classification, Sources, Phytochemistry, and Pharmacology of Norditerpenes" Molecules 29, no. 1: 60. https://doi.org/10.3390/molecules29010060
APA StyleZeng, N., Zhang, Q., Yao, Q., Fu, G., Su, W., Wang, W., & Li, B. (2024). A Comprehensive Review of the Classification, Sources, Phytochemistry, and Pharmacology of Norditerpenes. Molecules, 29(1), 60. https://doi.org/10.3390/molecules29010060







