Advancements in Nanosystems for Ocular Drug Delivery: A Focus on Pediatric Retinoblastoma
Abstract
:1. Introduction
2. Overview of Retinoblastoma
2.1. Epidemiology
2.2. Etiological Insights and Risk Factors
2.3. Diagnostic Approaches and Clinical Presentation
2.3.1. Initial Assessment and Clinical Signs
2.3.2. Development and Variants
2.3.3. Diagnostic Imaging and Evaluation
2.3.4. Metastasis and Extraocular Extension
2.3.5. Classification
2.4. Prognosis and Management Strategies
3. Challenges in Treating Retinoblastoma
3.1. Enucleation
3.2. Laser and Cryotherapy
3.3. Radiotherapy
3.4. Chemotherapy
4. Nano-Based Strategies
4.1. Inorganic Nanoparticles
4.1.1. Gold Nanoparticles (GNPs)
4.1.2. Mesoporous Silica Nanoparticles (MSNPs)
4.1.3. Iron Nanoparticles
4.1.4. Silver Nanoparticles
4.2. Organic Nanoparticles
4.2.1. Polymeric Nanoparticles
4.2.2. Lipid Nanoparticles
4.2.3. Protein Nanoparticles
4.2.4. Nanomicelles
5. Clinical Barriers and Future Perspectives
5.1. Trends
5.1.1. Smart Release of Nanoparticle Contents
5.1.2. Surface Receptor Targeting Nanoparticles
5.1.3. Multi-Functional Nanoparticles
5.2. Barriers of Nanoparticles in Retinoblastoma
5.2.1. Long-Term Toxicity and Lack of Clinical Studies
5.2.2. Exploration of Common Variables
5.3. Future Perspectives
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mattosinho, C.C.D.S.; Moura, A.T.M.S.; Oigman, G.; Ferman, S.E.; Grigorovski, N. Time to Diagnosis of Retinoblastoma in Latin America: A Systematic Review. Pediatr. Hematol. Oncol. 2019, 36, 55–72. [Google Scholar] [CrossRef]
- Alkatan, H.M.; Al Marek, F.; Elkhamary, S. Demographics of Pediatric Orbital Lesions: A Tertiary Eye Center Experience in Saudi Arabia. J. Epidemiol. Glob. Health 2019, 9, 3–10. [Google Scholar] [CrossRef]
- Zahn, J.; Chan, M.P.; Wang, G.; Patel, R.M.; Andea, A.A.; Bresler, S.C.; Harms, P.W. Altered Rb, P16, and P53 Expression Is Specific for Porocarcinoma Relative to Poroma. J. Cutan. Pathol. 2019, 46, 659–664. [Google Scholar] [CrossRef] [PubMed]
- House, R.J.; Hsu, S.T.; Thomas, A.S.; Finn, A.P.; Toth, C.A.; Materin, M.A.; Vajzovic, L. Vascular Findings in a Small Retinoblastoma Tumor Using OCT Angiography. Ophthalmol. Retin. 2019, 3, 194–195. [Google Scholar] [CrossRef] [PubMed]
- Kletke, S.N.; Feng, Z.X.; Hazrati, L.-N.; Gallie, B.L.; Soliman, S.E. Clinical Predictors at Diagnosis of Low-Risk Histopathology in Unilateral Advanced Retinoblastoma. Ophthalmology 2019, 126, 1306–1314. [Google Scholar] [CrossRef] [PubMed]
- Ishaq, H.; Patel, B.C. Retinoblastoma. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2024. [Google Scholar]
- Retinoblastoma: Diagnosis and Management—The UK Perspective—PubMed. Available online: https://pubmed.ncbi.nlm.nih.gov/25940424/ (accessed on 20 March 2024).
- Darwich, R.; Ghazawi, F.M.; Rahme, E.; Alghazawi, N.; Burnier, J.V.; Sasseville, D.; Burnier, M.N.; Litvinov, I.V. Retinoblastoma Incidence Trends in Canada: A National Comprehensive Population-Based Study. J. Pediatr. Ophthalmol. Strabismus 2019, 56, 124–130. [Google Scholar] [CrossRef] [PubMed]
- Fabian, I.D.; Reddy, A.; Sagoo, M.S. Classification and Staging of Retinoblastoma. Community Eye Health 2018, 31, 11. [Google Scholar] [PubMed]
- Schefler, A.C.; Kim, R.S. Recent Advancements in the Management of Retinoblastoma and Uveal Melanoma. F1000Research 2018, 7, 476. [Google Scholar] [CrossRef] [PubMed]
- Pelton, R.W.; Patel, B.C. Superomedial Lid Crease Approach to the Medial Intraconal Space: A New Technique for Access to the Optic Nerve and Central Space. Ophthalmic Plast. Reconstr. Surg. 2001, 17, 241–253. [Google Scholar] [CrossRef]
- Chawla, B.; Jain, A.; Azad, R. Conservative Treatment Modalities in Retinoblastoma. Indian J. Ophthalmol. 2013, 61, 479–485. [Google Scholar] [CrossRef]
- Abramson, D.H.; Ellsworth, R.M.; Rozakis, G.W. Cryotherapy for Retinoblastoma. Arch. Ophthalmol. 1982, 100, 1253–1256. [Google Scholar] [CrossRef] [PubMed]
- Wong, F.L. Cancer Incidence after Retinoblastoma. Radiation Dose and Sarcoma Risk. JAMA J. Am. Med. Assoc. 1997, 278, 1262–1267. [Google Scholar] [CrossRef] [PubMed]
- Abramson, D.H.; Frank, C.M. Second Nonocular Tumors in Survivors of Bilateral Retinoblastoma. Ophthalmology 1998, 105, 573–580. [Google Scholar] [CrossRef] [PubMed]
- Yousef, Y.A.; Mohammad, M.; Jaradat, I.; Shatnawi, R.; Banat, S.; Mehyar, M.; Al-Nawaiseh, I. The Role of External Beam Radiation Therapy for Retinoblastoma after Failure of Combined Chemoreduction and Focal Consolidation Therapy. Ophthalmic Genet. 2020, 41, 20–25. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.-Y.; Park, Y. Treatment of Retinoblastoma: The Role of External Beam Radiotherapy. Yonsei Med. J. 2015, 56, 1478. [Google Scholar] [CrossRef] [PubMed]
- Rizzuti, A.E. The Adverse Events of Chemotherapy for Retinoblastoma: What Are They? Do We Know? Arch. Ophthalmol. 2008, 126, 862. [Google Scholar] [CrossRef] [PubMed]
- Manjandavida, F.; Stathopoulos, C.; Zhang, J.; Honavar, S.; Shields, C. Intra-Arterial Chemotherapy in Retinoblastoma—A Paradigm Change. Indian J. Ophthalmol. 2019, 67, 740. [Google Scholar] [CrossRef] [PubMed]
- Steinle, J.J.; Zhang, Q.; Thompson, K.E.; Toutounchian, J.; Yates, C.R.; Soderland, C.; Wang, F.; Stewart, C.F.; Haik, B.G.; Williams, J.S.; et al. Intra-Ophthalmic Artery Chemotherapy Triggers Vascular Toxicity through Endothelial Cell Inflammation and Leukostasis. Investig. Ophthalmol. Vis. Sci. 2012, 53, 2439. [Google Scholar] [CrossRef] [PubMed]
- Tse, B.C.; Steinle, J.J.; Johnson, D.; Haik, B.G.; Wilson, M.W. Superselective Intraophthalmic Artery Chemotherapy in a Nonhuman Primate Model: Histopathologic Findings. JAMA Ophthalmol. 2013, 131, 903. [Google Scholar] [CrossRef]
- Kalita, D.; Shome, D.; Jain, V.G.; Chadha, K.; Bellare, J.R. In Vivo Intraocular Distribution and Safety of Periocular Nanoparticle Carboplatin for Treatment of Advanced Retinoblastoma in Humans. Am. J. Ophthalmol. 2014, 157, 1109–1115. [Google Scholar] [CrossRef]
- Sims, L.B.; Tyo, K.M.; Stocke, S.; Mahmoud, M.Y.; Ramasubramanian, A.; Steinbach-Rankins, J.M. Surface-Modified Melphalan Nanoparticles for Intravitreal Chemotherapy of Retinoblastoma. Investig. Ophthalmol. Vis. Sci. 2019, 60, 1696. [Google Scholar] [CrossRef] [PubMed]
- Yousef, Y.A.; Al Jboor, M.; Mohammad, M.; Mehyar, M.; Toro, M.D.; Nazzal, R.; Alzureikat, Q.H.; Rejdak, M.; Elfalah, M.; Sultan, I.; et al. Safety and Efficacy of Intravitreal Chemotherapy (Melphalan) to Treat Vitreous Seeds in Retinoblastoma. Front. Pharmacol. 2021, 12, 696787. [Google Scholar] [CrossRef] [PubMed]
- Hammami, I.; Alabdallah, N.M.; Jomaa, A.A.; Kamoun, M. Gold Nanoparticles: Synthesis Properties and Applications. J. King Saud. Univ.-Sci. 2021, 33, 101560. [Google Scholar] [CrossRef]
- Boedtkjer, E.; Pedersen, S.F. The Acidic Tumor Microenvironment as a Driver of Cancer. Annu. Rev. Physiol. 2020, 82, 103–126. [Google Scholar] [CrossRef] [PubMed]
- Yan, S.; Yan, J.; Liu, D.; Li, X.; Kang, Q.; You, W.; Zhang, J.; Wang, L.; Tian, Z.; Lu, W.; et al. A Nano-Predator of Pathological MDMX Construct by Clearable Supramolecular Gold(I)-Thiol-Peptide Complexes Achieves Safe and Potent Anti-Tumor Activity. Theranostics 2021, 11, 6833–6846. [Google Scholar] [CrossRef] [PubMed]
- Kalmodia, S.; Parameswaran, S.; Ganapathy, K.; Yang, W.; Barrow, C.J.; Kanwar, J.R.; Roy, K.; Vasudevan, M.; Kulkarni, K.; Elchuri, S.V.; et al. Characterization and Molecular Mechanism of Peptide-Conjugated Gold Nanoparticle Inhibiting P53-HDM2 Interaction in Retinoblastoma. Mol. Ther.-Nucleic Acids 2017, 9, 349–364. [Google Scholar] [CrossRef] [PubMed]
- Darviot, C.; Hardy, P.; Meunier, M. Laser-induced Plasmon-mediated Treatment of Retinoblastoma in Viscous Vitreous Phantom. J. Biophotonics 2019, 12, e201900193. [Google Scholar] [CrossRef] [PubMed]
- Moradi, S.; Mokhtari-Dizaji, M.; Ghassemi, F.; Sheibani, S.; Amoli, F.A. The Effect of Ultrasound Hyperthermia with Gold Nanoparticles on Retinoblastoma Y79 Cells. Gold Bull. 2020, 53, 111–120. [Google Scholar] [CrossRef]
- Moradi, S.; Mokhtari-Dizaji, M.; Ghassemi, F.; Sheibani, S.; Asadi Amoli, F. Increasing the Efficiency of the Retinoblastoma Brachytherapy Protocol with Ultrasonic Hyperthermia and Gold Nanoparticles: A Rabbit Model. Int. J. Radiat. Biol. 2020, 96, 1614–1627. [Google Scholar] [CrossRef]
- Niculescu, V.-C. Mesoporous Silica Nanoparticles for Bio-Applications. Front. Mater. 2020, 7, 36. [Google Scholar] [CrossRef]
- Leamon, C. Folate-Targeted Chemotherapy. Adv. Drug Deliv. Rev. 2004, 56, 1127–1141. [Google Scholar] [CrossRef] [PubMed]
- Qu, W.; Meng, B.; Yu, Y.; Wang, S. Folic Acid-Conjugated Mesoporous Silica Nanoparticles for Enhanced Therapeutic Efficacy of Topotecan in Retina Cancers. Int. J. Nanomed. 2018, 13, 4379–4389. [Google Scholar] [CrossRef] [PubMed]
- Mitra, M.; Kandalam, M.; Verma, R.S.; UmaMaheswari, K.; Krishnakumar, S. Genome-Wide Changes Accompanying the Knockdown of Ep-CAM in Retinoblastoma. Mol. Vis. 2010, 16, 828–842. [Google Scholar] [PubMed]
- Qu, W.; Meng, B.; Yu, Y.; Wang, S. EpCAM Antibody-Conjugated Mesoporous Silica Nanoparticles to Enhance the Anticancer Efficacy of Carboplatin in Retinoblastoma. Mater. Sci. Eng. C 2017, 76, 646–651. [Google Scholar] [CrossRef]
- Sodagar Taleghani, A.; Ebrahimnejad, P.; Heidarinasab, A.; Akbarzadeh, A. Sugar-Conjugated Dendritic Mesoporous Silica Nanoparticles as pH-Responsive Nanocarriers for Tumor Targeting and Controlled Release of Deferasirox. Mater. Sci. Eng. C 2019, 98, 358–368. [Google Scholar] [CrossRef] [PubMed]
- Pucci, C.; Degl’Innocenti, A.; Belenli Gümüş, M.; Ciofani, G. Superparamagnetic Iron Oxide Nanoparticles for Magnetic Hyperthermia: Recent Advancements, Molecular Effects, and Future Directions in the Omics Era. Biomater. Sci. 2022, 10, 2103–2121. [Google Scholar] [CrossRef] [PubMed]
- Demirci, H.; Slimani, N.; Pawar, M.; Kumon, R.E.; Vaishnava, P.; Besirli, C.G. Magnetic Hyperthermia in Y79 Retinoblastoma and ARPE-19 Retinal Epithelial Cells: Tumor Selective Apoptotic Activity of Iron Oxide Nanoparticle. Trans. Vis. Sci. Technol. 2019, 8, 18. [Google Scholar] [CrossRef] [PubMed]
- Seydi, E.; Tahmasebi, G.; Arjmand, A.; Pourahmad, J. Toxicity of Superparamagnetic Iron Oxide Nanoparticles on Retinoblastoma Mitochondria. Cutan. Ocul. Toxicol. 2023, 43, 69–74. [Google Scholar] [CrossRef]
- Ali, A.; Zafar, H.; Zia, M.; Ul Haq, I.; Phull, A.R.; Ali, J.S.; Hussain, A. Synthesis, Characterization, Applications, and Challenges of Iron Oxide Nanoparticles. Nanotechnol. Sci. Appl. 2016, 9, 49–67. [Google Scholar] [CrossRef]
- Kuda, T.; Yano, T.; Matsuda, N.; Nishizawa, M. Inhibitory Effects of Laminaran and Low Molecular Alginate against the Putrefactive Compounds Produced by Intestinal Microflora in Vitro and in Rats. Food Chem. 2005, 91, 745–749. [Google Scholar] [CrossRef]
- Kim, K.-H.; Kim, Y.-W.; Kim, H.B.; Lee, B.J.; Lee, D.S. Anti-Apoptotic Activity of Laminarin Polysaccharides and Their Enzymatically Hydrolyzed Oligosaccharides from Laminaria Japonica. Biotechnol. Lett. 2006, 28, 439–446. [Google Scholar] [CrossRef] [PubMed]
- Barwal, I.; Ranjan, P.; Kateriya, S.; Yadav, S.C. Cellular Oxido-Reductive Proteins of Chlamydomonas Reinhardtii Control the Biosynthesis of Silver Nanoparticles. J. Nanobiotechnol. 2011, 9, 56. [Google Scholar] [CrossRef] [PubMed]
- Buzea, C.; Pacheco, I.I.; Robbie, K. Nanomaterials and Nanoparticles: Sources and Toxicity. Biointerphases 2007, 2, MR17–MR71. [Google Scholar] [CrossRef] [PubMed]
- Remya, R.R.; Radhika Rajasree, S.R.; Aranganathan, L.; Suman, T.Y.; Gayathri, S. Enhanced Cytotoxic Activity of AgNPs on Retinoblastoma Y79 Cell Lines Synthesised Using Marine Seaweed Turbinaria ornata. IET Nanobiotechnol. 2017, 11, 18–23. [Google Scholar] [CrossRef] [PubMed]
- Remya, R.R.; Rajasree, S.R.R.; Suman, T.Y.; Aranganathan, L.; Gayathri, S.; Gobalakrishnan, M.; Karthih, M.G. Laminarin Based AgNPs Using Brown Seaweed Turbinaria ornata and Its Induction of Apoptosis in Human Retinoblastoma Y79 Cancer Cell Lines. Mater. Res. Express 2018, 5, 035403. [Google Scholar] [CrossRef]
- Davis, T.A.; Volesky, B.; Mucci, A. A Review of the Biochemistry of Heavy Metal Biosorption by Brown Algae. Water Res. 2003, 37, 4311–4330. [Google Scholar] [CrossRef] [PubMed]
- Zielińska, A.; Carreiró, F.; Oliveira, A.M.; Neves, A.; Pires, B.; Venkatesh, D.N.; Durazzo, A.; Lucarini, M.; Eder, P.; Silva, A.M.; et al. Polymeric Nanoparticles: Production, Characterization, Toxicology and Ecotoxicology. Molecules 2020, 25, 3731. [Google Scholar] [CrossRef] [PubMed]
- Nagati, V.; Tenugu, S.; Pasupulati, A.K. Stability of Therapeutic Nano-Drugs during Storage and Transportation as Well as after Ingestion in the Human Body. In Advances in Nanotechnology-Based Drug Delivery Systems; Elsevier: Amsterdam, The Netherlands, 2022; pp. 83–102. ISBN 978-0-323-88450-1. [Google Scholar]
- Godse, R.; Rathod, M.; De, A.; Shinde, U. Intravitreal Galactose Conjugated Polymeric Nanoparticles of Etoposide for Retinoblastoma. J. Drug Deliv. Sci. Technol. 2021, 61, 102259. [Google Scholar] [CrossRef]
- Silva, A.M.; Alvarado, H.L.; Abrego, G.; Martins-Gomes, C.; Garduño-Ramirez, M.L.; García, M.L.; Calpena, A.C.; Souto, E.B. In Vitro Cytotoxicity of Oleanolic/Ursolic Acids-Loaded in PLGA Nanoparticles in Different Cell Lines. Pharmaceutics 2019, 11, 362. [Google Scholar] [CrossRef]
- Zhuang, H.; Xu, Y.-N.; Zheng, H.-H.; Huan, Y.-R.; Zheng, N.-X.; Lin, L.; Zhang, W.-Z.; Xu, W. Carboplatin-Loaded Surface Modified-PLGA Nanoparticles Confer Sustained Inhibitory Effect against Retinoblastoma Cell In Vitro. Arq. Bras. Oftalmol. 2021, 85, 450–458. [Google Scholar] [CrossRef]
- Delrish, E.; Jabbarvand, M.; Ghassemi, F.; Amoli, F.A.; Atyabi, F.; Lashay, A.; Soleimani, M.; Aghajanpour, L.; Dinarvand, R. Efficacy of Topotecan Nanoparticles for Intravitreal Chemotherapy of Retinoblastoma. Exp. Eye Res. 2021, 204, 108423. [Google Scholar] [CrossRef] [PubMed]
- Delrish, E.; Ghassemi, F.; Jabbarvand, M.; Lashay, A.; Atyabi, F.; Soleimani, M.; Dinarvand, R. Biodistribution of Cy5-Labeled Thiolated and Methylated Chitosan-Carboxymethyl Dextran Nanoparticles in an Animal Model of Retinoblastoma. J. Ophthalmic Vis. Res. 2022, 17, 58–68. [Google Scholar] [CrossRef] [PubMed]
- Ghassemi, F.; Delrish, E.; Jabbarvand, M.; Lashay, A.; Asadi Amoli, F.; Atyabi, F.; Sadat Mirzazadeh Tekie, F.; Dinarvand, R.; Heidari Keshel, S.; Soleimani, M. The Antitumor Effect of Topotecan Loaded Thiolated Chitosan-Dextran Nanoparticles for Intravitreal Chemotherapy: A Xenograft Retinoblastoma Model. J. Ophthalmic Vis. Res. 2023, 18, 68–80. [Google Scholar] [CrossRef] [PubMed]
- Viegas, C.; Patrício, A.B.; Prata, J.M.; Nadhman, A.; Chintamaneni, P.K.; Fonte, P. Solid Lipid Nanoparticles vs. Nanostructured Lipid Carriers: A Comparative Review. Pharmaceutics 2023, 15, 1593. [Google Scholar] [CrossRef]
- Ahmad, I.; Pandit, J.; Sultana, Y.; Mishra, A.K.; Hazari, P.P.; Aqil, M. Optimization by Design of Etoposide Loaded Solid Lipid Nanoparticles for Ocular Delivery: Characterization, Pharmacokinetic and Deposition Study. Mater. Sci. Eng. C 2019, 100, 959–970. [Google Scholar] [CrossRef] [PubMed]
- Marathe, S.; Shadambikar, G.; Mehraj, T.; Sulochana, S.P.; Dudhipala, N.; Majumdar, S. Development of α-Tocopherol Succinate-Based Nanostructured Lipid Carriers for Delivery of Paclitaxel. Pharmaceutics 2022, 14, 1034. [Google Scholar] [CrossRef] [PubMed]
- Tabatabaei, S.N.; Derbali, R.M.; Yang, C.; Superstein, R.; Hamel, P.; Chain, J.L.; Hardy, P. Co-Delivery of miR-181a and Melphalan by Lipid Nanoparticles for Treatment of Seeded Retinoblastoma. J. Control. Release 2019, 298, 177–185. [Google Scholar] [CrossRef] [PubMed]
- Passos Gibson, V.; Derbali, R.M.; Phan, H.T.; Tahiri, H.; Allen, C.; Hardy, P.; Chain, J.L. Survivin Silencing Improved the Cytotoxicity of Carboplatin and Melphalan in Y79 and Primary Retinoblastoma Cells. Int. J. Pharm. 2020, 589, 119824. [Google Scholar] [CrossRef] [PubMed]
- He, C.; Ruan, F.; Jiang, S.; Zeng, J.; Yin, H.; Liu, R.; Zhang, Y.; Huang, L.; Wang, C.; Ma, S.; et al. Black Phosphorus Quantum Dots Cause Nephrotoxicity in Organoids, Mice, and Human Cells. Small 2020, 16, 2001371. [Google Scholar] [CrossRef]
- Wang, S.; Wei, P.; Zhang, Y.; Zhang, S. Lipid-Nanoparticles-Based Co-Delivery of Black Phosphorus Quantum Dots and Melphalan by Photothermal Therapy Combined with Chemotherapy for Retinoblastoma. Polym. Test. 2023, 129, 108292. [Google Scholar] [CrossRef]
- Hong, S.; Choi, D.W.; Kim, H.N.; Park, C.G.; Lee, W.; Park, H.H. Protein-Based Nanoparticles as Drug Delivery Systems. Pharmaceutics 2020, 12, 604. [Google Scholar] [CrossRef] [PubMed]
- Narayana, R.V.L.; Jana, P.; Tomar, N.; Prabhu, V.; Nair, R.M.; Manukonda, R.; Kaliki, S.; Coupland, S.E.; Alexander, J.; Kalirai, H.; et al. Carboplatin- and Etoposide-Loaded Lactoferrin Protein Nanoparticles for Targeting Cancer Stem Cells in Retinoblastoma In Vitro. Investig. Ophthalmol. Vis. Sci. 2021, 62, 13. [Google Scholar] [CrossRef] [PubMed]
- Nair, R.M.; Balla, M.M.; Khan, I.; Kalathur, R.K.R.; Kondaiah, P.; Vemuganti, G.K. In Vitro Characterization of CD133lo Cancer Stem Cells in Retinoblastoma Y79 Cell Line. BMC Cancer 2017, 17, 779. [Google Scholar] [CrossRef] [PubMed]
- Balla, M.M.S.; Vemuganti, G.K.; Kannabiran, C.; Honavar, S.G.; Murthy, R. Phenotypic Characterization of Retinoblastoma for the Presence of Putative Cancer Stem-like Cell Markers by Flow Cytometry. Investig. Ophthalmol. Vis. Sci. 2009, 50, 1506. [Google Scholar] [CrossRef] [PubMed]
- Zeuner, A. The Secret Life of Quiescent Cancer Stem Cells. Mol. Cell. Oncol. 2015, 2, e968067. [Google Scholar] [CrossRef]
- Abdullah, L.N.; Chow, E.K. Mechanisms of Chemoresistance in Cancer Stem Cells. Clin. Transl. Med. 2013, 2, e3. [Google Scholar] [CrossRef] [PubMed]
- Trinh, H.M.; Joseph, M.; Cholkar, K.; Mitra, R.; Mitra, A.K. Nanomicelles in Diagnosis and Drug Delivery. In Emerging Nanotechnologies for Diagnostics, Drug Delivery and Medical Devices; Elsevier: Amsterdam, The Netherlands, 2017; pp. 45–58. ISBN 978-0-323-42978-8. [Google Scholar]
- Anand, P.; Kunnumakkara, A.B.; Newman, R.A.; Aggarwal, B.B. Bioavailability of Curcumin: Problems and Promises. Mol. Pharm. 2007, 4, 807–818. [Google Scholar] [CrossRef] [PubMed]
- Alsaab, H.; Alzhrani, R.; Kesharwani, P.; Sau, S.; Boddu, S.; Iyer, A. Folate Decorated Nanomicelles Loaded with a Potent Curcumin Analogue for Targeting Retinoblastoma. Pharmaceutics 2017, 9, 15. [Google Scholar] [CrossRef]
- Cascão, R.; Fonseca, J.E.; Moita, L.F. Celastrol: A Spectrum of Treatment Opportunities in Chronic Diseases. Front. Med. 2017, 4, 69. [Google Scholar] [CrossRef]
- Li, Z.; Guo, Z.; Chu, D.; Feng, H.; Zhang, J.; Zhu, L.; Li, J. Effectively Suppressed Angiogenesis-Mediated Retinoblastoma Growth Using Celastrol Nanomicelles. Drug Deliv. 2020, 27, 358–366. [Google Scholar] [CrossRef]
- Guo, Z.; Shi, L.; Feng, H.; Yang, F.; Li, Z.; Zhang, J.; Jin, L.; Li, J. Reduction-Sensitive Nanomicelles: Delivery Celastrol for Retinoblastoma Cells Effective Apoptosis. Chin. Chem. Lett. 2021, 32, 1046–1050. [Google Scholar] [CrossRef]
- Long, K.; Yang, Y.; Lv, W.; Jiang, K.; Li, Y.; Lo, A.C.Y.; Lam, W.C.; Zhan, C.; Wang, W. Green Light-Triggered Intraocular Drug Release for Intravenous Chemotherapy of Retinoblastoma. Adv. Sci. 2021, 8, 2101754. [Google Scholar] [CrossRef] [PubMed]
- Kanwar, J.R.; Samarasinghe, R.M.; Sehgal, R. Nano-Lactoferrin in Diagnostic, Imaging and Targeted Delivery for Cancer and Infectious Diseases. J. Cancer Sci. Ther. 2012, 4, 31–42. [Google Scholar] [CrossRef]
- Sadri, E.; Khoee, S.; Moayeri, S.; Haji Ali, B.; Pirhajati Mahabadi, V.; Shirvalilou, S.; Khoei, S. Enhanced Anti-Tumor Activity of Transferrin/Folate Dual-Targeting Magnetic Nanoparticles Using Chemo-Thermo Therapy on Retinoblastoma Cancer Cells Y79. Sci. Rep. 2023, 13, 22358. [Google Scholar] [CrossRef] [PubMed]
- Griegel, S.; Rajewsky, M.F.; Ciesiolka, T.; Gabius, H.J. Endogenous Sugar Receptor (Lectin) Profiles of Human Retinoblastoma and Retinoblast Cell Lines Analyzed by Cytological Markers, Affinity Chromatography and Neoglycoprotein-Targeted Photolysis. Anticancer Res. 1989, 9, 723–730. [Google Scholar] [PubMed]
- Zou, H.; Li, M.; Li, X.; Zheng, W.; Kuang, H.; Wang, M.; Zhang, W.; Ran, H.; Ma, H.; Zhou, X. Multimodal Imaging and Photothermal Synergistic Immunotherapy of Retinoblastoma with Tuftsin-Loaded Carbonized MOF Nanoparticles. Drug Deliv. 2022, 29, 1785–1799. [Google Scholar] [CrossRef] [PubMed]
- Zheng, W.; Li, X.; Zou, H.; Xu, Y.; Li, P.; Zhou, X.; Wu, M. Dual-Target Multifunctional Superparamagnetic Cationic Nanoliposomes for Multimodal Imaging-Guided Synergistic Photothermal/Photodynamic Therapy of Retinoblastoma. Int. J. Nanomed. 2022, 17, 3217–3237. [Google Scholar] [CrossRef] [PubMed]
- Mudigunda, S.V.; Pemmaraju, D.B.; Paradkar, S.; Puppala, E.R.; Gawali, B.; Upadhyayula, S.M.; Vegi Gangamodi, N.; Rengan, A.K. Multifunctional Polymeric Nanoparticles for Chemo/Phototheranostics of Retinoblastoma. ACS Biomater. Sci. Eng. 2022, 8, 151–160. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.; Xiong, H.; Zou, H.; Li, M.; Li, P.; Zhou, Y.; Xu, Y.; Jian, J.; Liu, F.; Zhao, H.; et al. A Laser-Activated Multifunctional Targeted Nanoagent for Imaging and Gene Therapy in a Mouse Xenograft Model with Retinoblastoma Y79 Cells. Acta Biomater. 2018, 70, 211–226. [Google Scholar] [CrossRef]
- Mudigunda, S.V.; Pemmaraju, D.B.; Sankaranarayanan, S.A.; Rengan, A.K. Bioactive Polymeric Nanoparticles of Moringa Oleifera Induced Phyto-Photothermal Sensitization for the Enhanced Therapy of Retinoblastoma. Pharmaceutics 2023, 15, 475. [Google Scholar] [CrossRef]
- Wang, M.; Yang, Q.; Li, M.; Zou, H.; Wang, Z.; Ran, H.; Zheng, Y.; Jian, J.; Zhou, Y.; Luo, Y.; et al. Multifunctional Nanoparticles for Multimodal Imaging-Guided Low-Intensity Focused Ultrasound/Immunosynergistic Retinoblastoma Therapy. ACS Appl. Mater. Interfaces 2020, 12, 5642–5657. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Bian, X.; Chen, X.; Fan, N.; Zou, H.; Bao, Y.; Zhou, Y. Multifunctional Liposome for Photoacoustic/Ultrasound Imaging-Guided Chemo/Photothermal Retinoblastoma Therapy. Drug Deliv. 2022, 29, 519–533. [Google Scholar] [CrossRef] [PubMed]
- Overchuk, M.; Zheng, G. Overcoming Obstacles in the Tumor Microenvironment: Recent Advancements in Nanoparticle Delivery for Cancer Theranostics. Biomaterials 2018, 156, 217–237. [Google Scholar] [CrossRef] [PubMed]
- Bodratti, A.M.; Alexandridis, P. Amphiphilic Block Copolymers in Drug Delivery: Advances in Formulation Structure and Performance. Expert Opin. Drug Deliv. 2018, 15, 1085–1104. [Google Scholar] [CrossRef] [PubMed]
- Lin, D.; Shen, Y.; Liang, T. Oncolytic Virotherapy: Basic Principles, Recent Advances and Future Directions. Signal Transduct. Target. Ther. 2023, 8, 156. [Google Scholar] [CrossRef] [PubMed]
- Pascual-Pasto, G.; Bazan-Peregrino, M.; Olaciregui, N.G.; Restrepo-Perdomo, C.A.; Mato-Berciano, A.; Ottaviani, D.; Weber, K.; Correa, G.; Paco, S.; Vila-Ubach, M.; et al. Therapeutic Targeting of the RB1 Pathway in Retinoblastoma with the Oncolytic Adenovirus VCN-01. Sci. Transl. Med. 2019, 11, eaat9321. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Ji, X.; Ruan, M.; Liu, W.; Song, R.; Dai, J.; Xue, W. Worm-Like Biomimetic Nanoerythrocyte Carrying siRNA for Melanoma Gene Therapy. Small 2018, 14, 1803002. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Fan, X.; Jiang, K.; Hu, Y.; Liu, Y.; Lu, W.; Wei, G. Intraocular siRNA Delivery Mediated by Penetratin Derivative to Silence Orthotopic Retinoblastoma Gene. Pharmaceutics 2023, 15, 745. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.; Siegwart, D.J.; Anderson, D.G. Strategies, Design, and Chemistry in siRNA Delivery Systems. Adv. Drug Deliv. Rev. 2019, 144, 133–147. [Google Scholar] [CrossRef]
- Wang, S.; Zhao, Y.; Yao, F.; Wei, P.; Ma, L.; Zhang, S. An Anti-GD2 Aptamer-Based Bifunctional Spherical Nucleic Acid Nanoplatform for Synergistic Therapy Targeting MDM2 for Retinoblastoma. Biomed. Pharmacother. 2024, 174, 116437. [Google Scholar] [CrossRef]
- Su, J.; Sun, C.; Du, J.; Xing, X.; Wang, F.; Dong, H. RNA-Cleaving DNAzyme-Based Amplification Strategies for Biosensing and Therapy. Adv. Healthc. Mater. 2023, 12, 2300367. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Zhang, D.; Huang, L.; Zeng, Z.; Su, Y.; Chen, S.; Lin, X.; Hong, S. Construction of a Functional Nucleic Acid-Based Artificial Vesicle-Encapsulated Composite Nanoparticle and Its Application in Retinoblastoma-Targeted Theranostics. ACS Biomater. Sci. Eng. 2024, 10, 1830–1842. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Luo, Y.; Dai, J.; Yang, L.; Huang, L.; Wang, R.; Chen, W.; Huang, Y.; Sun, S.; Cao, J.; et al. Monitoring Retinoblastoma by Machine Learning of Aqueous Humor Metabolic Fingerprinting. Small Methods 2022, 6, 2101220. [Google Scholar] [CrossRef] [PubMed]
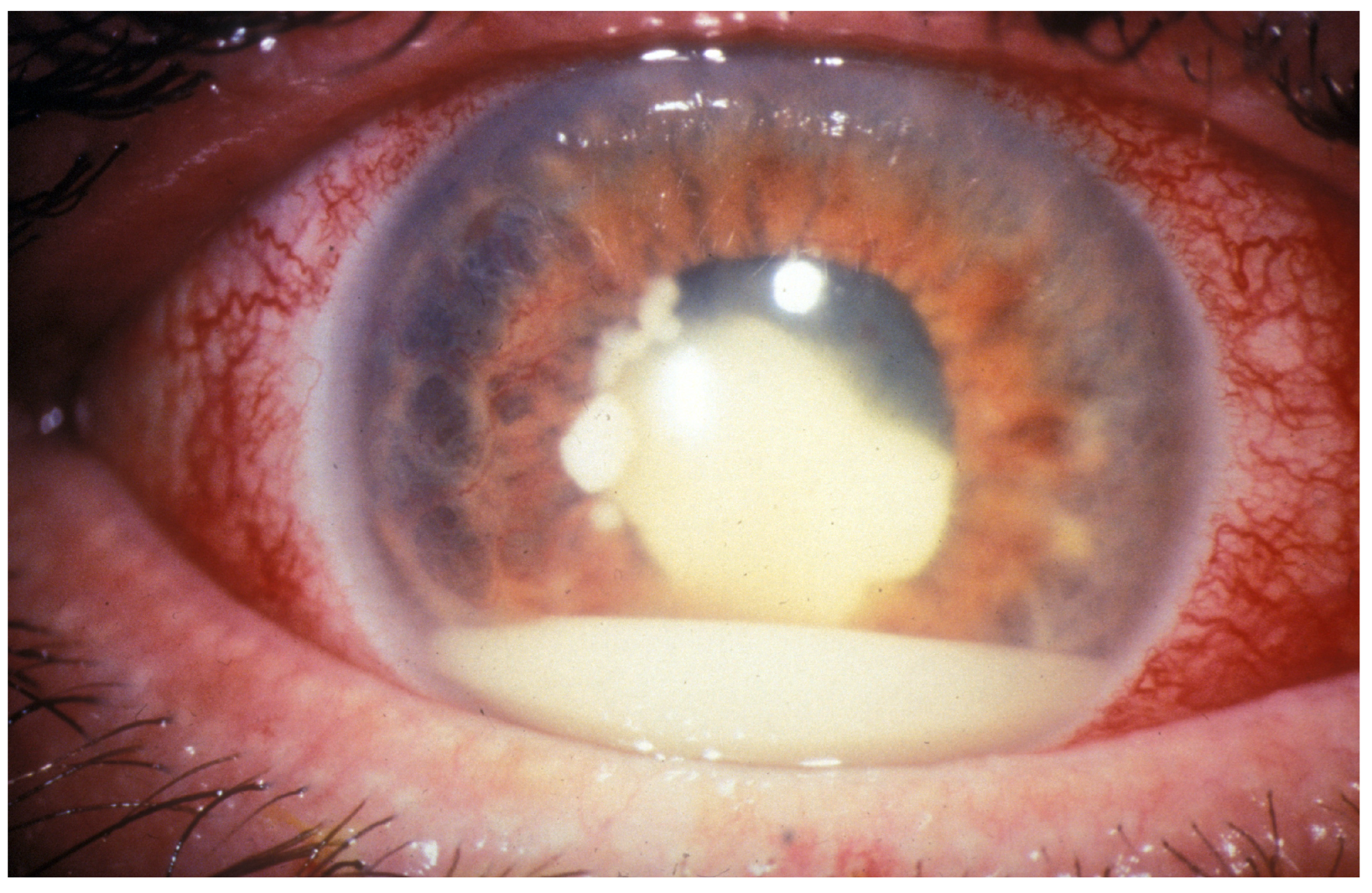
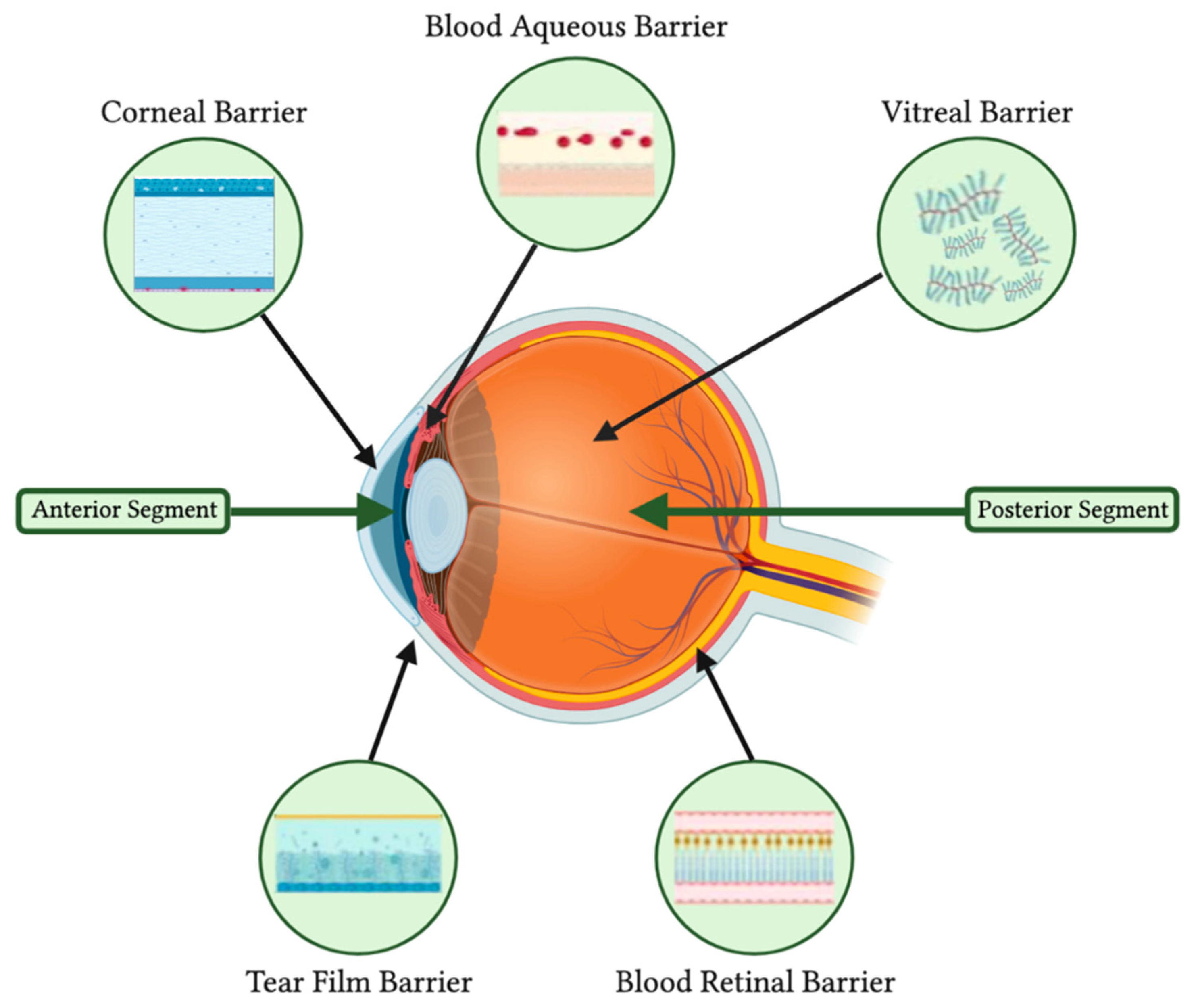
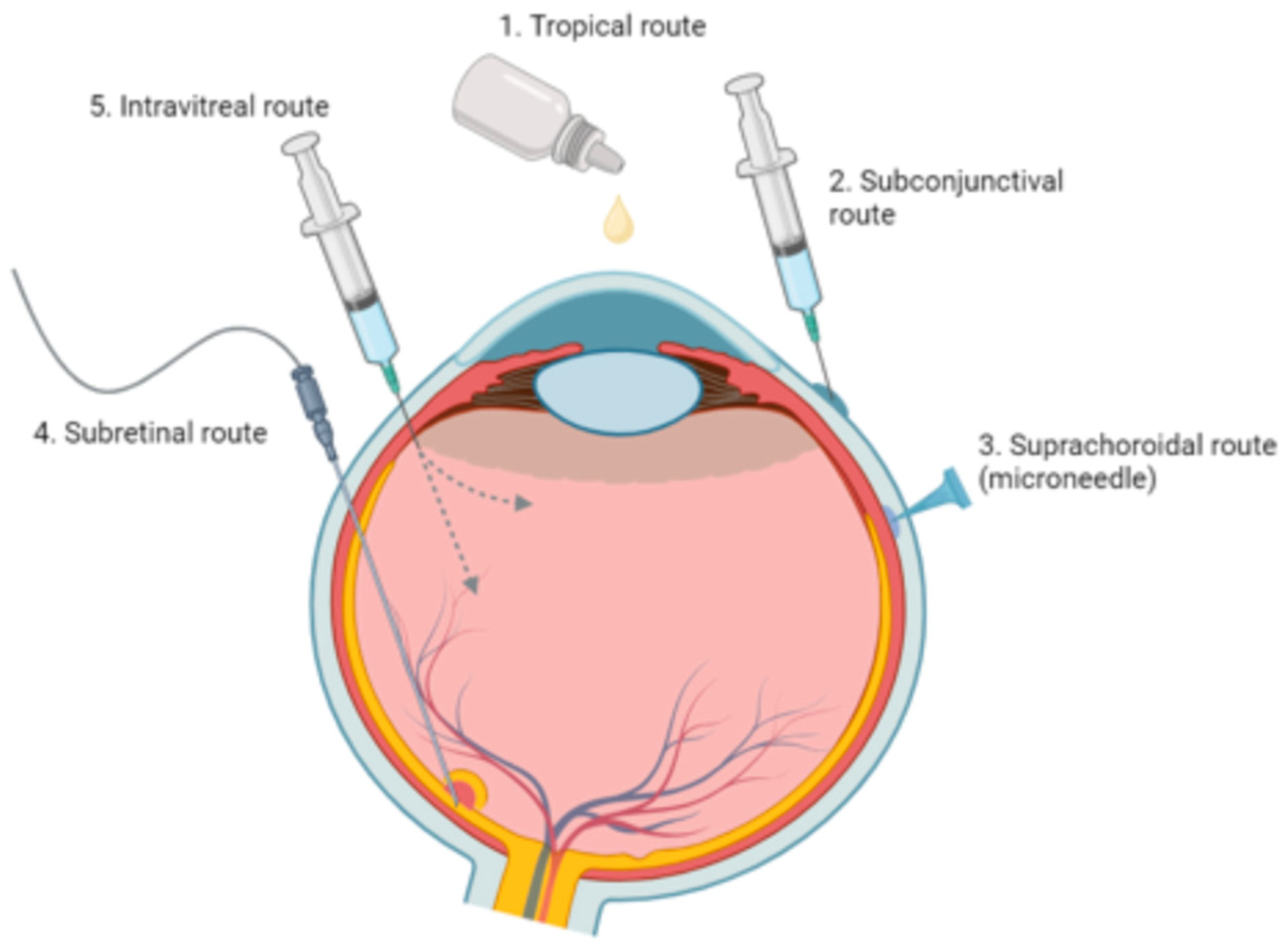
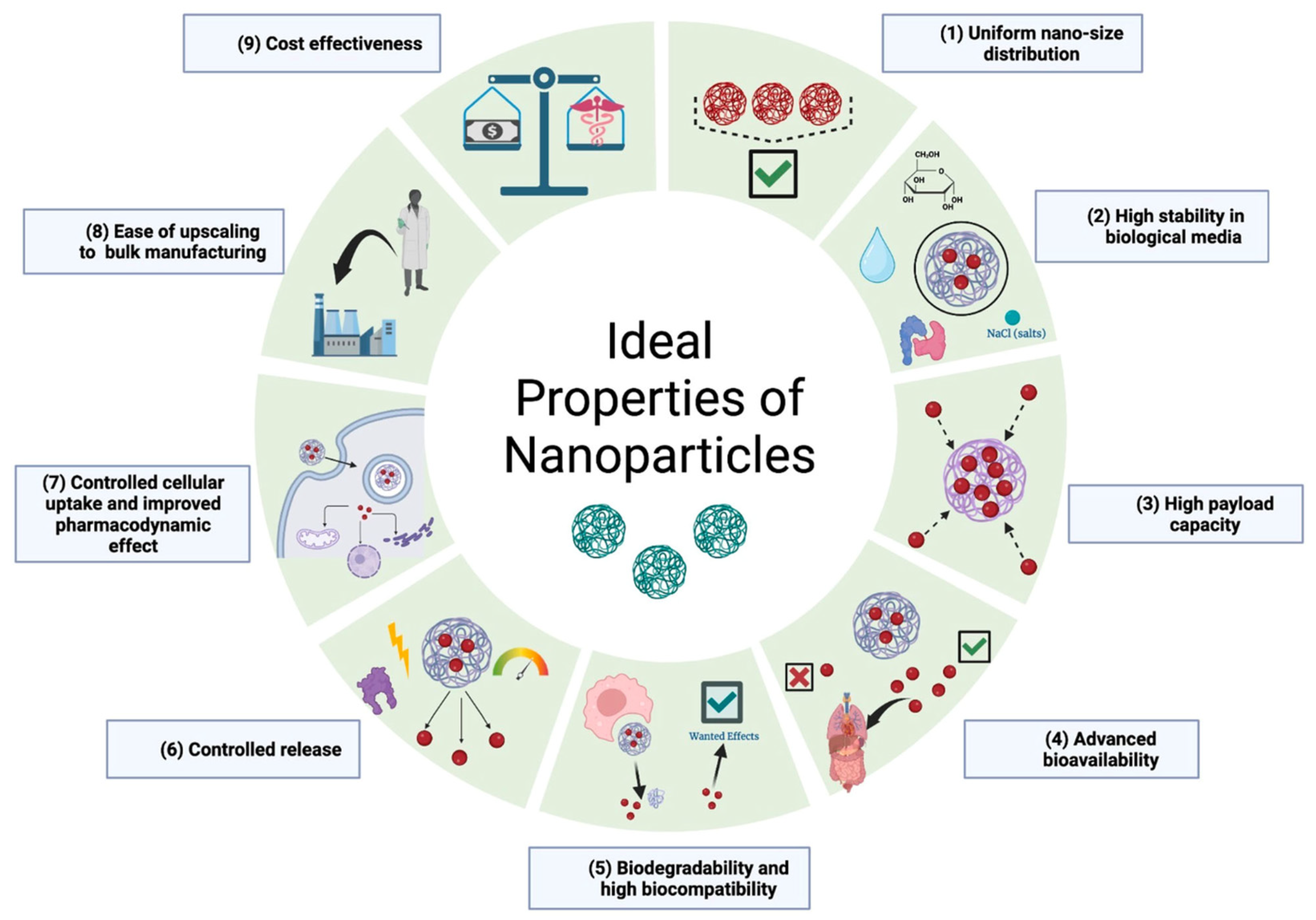
| Treatment | Modality | Challenges | References |
|---|---|---|---|
| EBRT | N/A |
| [14,15,16,17] |
| Chemotherapy | Systemic |
| [18] |
| Intra-arterial |
| [19,20,21,22,23] | |
| Intravitreal |
| [24] |
| Nanocarrier | Function | Characteristics | Observations | Stage | Reference |
|---|---|---|---|---|---|
| GNPs | Target MDMX degradation |
|
| WERI-RB-1 cells in vitro, intravitreal orthotopic xenograft retinoblastoma mouse model in vivo, PDX model of pancreatic carcinoma in vivo | [27] |
| Inhibit p53-HDM2 |
|
| Y79 cells in vitro | [28] | |
| Laser-induced cell disruption | N/A |
| Y79 cells in vitro and vitreous phantom | [29] | |
| Ultrasonic hyperthermia | N/A |
| Y79 cells in vitro | [30] | |
| Ultrasound hyperthermia |
|
| RB-induced rabbit cells in vivo | [31] | |
| Imaging, LIFU, and immunotherapy |
|
| Y79 and ARPE-19 cells in vivo, Y79-tumor-bearing mice in flanks in vivo | [85] | |
| MSNPs | Enhance topotecan |
|
| Y79 cells in vitro, Y79-cell-bearing xenograft tumor model in vivo | [34] |
| Target malignant cells |
|
| Y79 in vitro | [36] | |
| N/A |
|
| Y79 in vitro | [37] | |
| Iron NPs | Magnetic hyperthermia using iron NPs |
|
| Y79 and ARPE-19 cells in vitro | [39] |
| SPIONs | Target mitochondria | N/A |
| Y79 cells in vitro | [40] |
| Chemo-hyperthermia therapy |
|
| Y79 and ARPE-19 cells in vitro | [78] | |
| AgNPs | N/A |
|
| Y79 cells in vitro | [47] |
| N/A |
|
| Y79 cells in vitro | [46] | |
| PLGA NPs | Treatment with galactose conjugated ENPs |
|
| Y79 cells in vitro | [51] |
| Evaluate difference in NP loading vs. free |
|
| Y79, HepG2, Caco-2 cells in vitro | [52] | |
| Effects of these NPs on RB |
|
| Y79 cells | [53] | |
| PLGA NPs | Compare various surface-modified PLGAs |
|
| Y79 cells in vitro | [23] |
| PCL NPs | Photothermal potential |
|
| Y79 cells in vitro | [84] |
| TMC and TC Nps | Biodistribution of intravitreal injection |
|
| Y79 cells in vitro | [55] |
| Delivery of TPH |
|
| BSS and FBS media in vitro, in vitro RB xenograft mouse model in vivo | [54] | |
| TC-NPs | Intravitreal delivery |
|
| Y79 cells in vitro, xenograft RB rabbit models in vivo | [56] |
| SLN | Posterior eye delivery optimization |
|
| In vitro and rat eye in vivo | [58] |
| NLC | Stable delivery of PTX |
|
| In vitro | [59] |
| LNPs | PTT |
|
| RB cells in vitro, orthotopic xenograft of WERIRB-1 cells in mice in vivo | [63] |
| Switchable LNPs | Combination therapy |
|
| Primary RB cells and Y79 in vitro, xenograft RB models in rat in vivo | [60] |
| Sensitize cancer cells to chemotherapy |
|
| 13 cell lines, including Y79 RB cells in vitro | [61] | |
| LFNPs | Evaluate efficacy of novel NP in chemoresistance |
|
| Y79 CSCs in vitro | [65] |
| Nanomicelles | Evaluate the potential of CDF in RB therapy |
|
| Y79, WERI-RB, ARPE-19 cells in vitro | [72] |
| CNMs | Evaluate Celastrol potential in angiogenesis |
|
| HUVECs and SO-RB 50 in vitro and xenograft mouse model in vivo | [74] |
| RSNMs | N/A |
|
| Y79 cells in vitro | [75] |
| CMT NPs | Dual-mode image-guided laser/immune co-therapy |
|
| Y79, ARPE-19, Ana-1 in vitro, Y79-tumor-bearing mice in vivo | [80] |
| LNPs | Dual-target cationic |
|
| Y79 and ARPE-19 cells in vitro, Y79-tumor-bearing mice in vivo | [81] |
| Multi-functional LNPs |
|
| Y79 and HUVECs in vitro, xenograft mouse models in vivo | [86] | |
| Multi-functionality, including gene therapy |
|
| Y79 cells in vitro, nude mouse limbs in vivo | [83] | |
| PNPs | PCB and PTT combination therapy |
|
| Y79 cells in vivo, mouse models in vivo | [82] |
| Clathrin-like NPs | Overcome BRB |
|
| HUVECs and WERI-RB-1 cells in vitro, and orthotopic retinoblastoma tumor mouse model in vivo | [76] |
| N/A | VCN-01 therapy | N/A |
| Y79 and primary RB cultures in vitro, mouse, rabbit, and human in vivo | [90] |
| PAMAM | Non-viral gene therapy |
|
| ARPE-19 and WERI-RB-1 cells in vitro | [92] |
| SNA NPs | siRNA delivery |
|
| Y79 and WERI-RB-1 cells in vitro and in vivo in nude mouse xenograft models | [94] |
| MNO2 and DNAzymes NPs | Dual-gene therapy | Aptamer surface modified |
| ARPE-19, Y79 cells, Y79-tumor-bearing mice | [96] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, K.Y.; Wang, X.C.; Anderson, M.; Tran, S.D. Advancements in Nanosystems for Ocular Drug Delivery: A Focus on Pediatric Retinoblastoma. Molecules 2024, 29, 2263. https://doi.org/10.3390/molecules29102263
Wu KY, Wang XC, Anderson M, Tran SD. Advancements in Nanosystems for Ocular Drug Delivery: A Focus on Pediatric Retinoblastoma. Molecules. 2024; 29(10):2263. https://doi.org/10.3390/molecules29102263
Chicago/Turabian StyleWu, Kevin Y., Xingao C. Wang, Maude Anderson, and Simon D. Tran. 2024. "Advancements in Nanosystems for Ocular Drug Delivery: A Focus on Pediatric Retinoblastoma" Molecules 29, no. 10: 2263. https://doi.org/10.3390/molecules29102263
APA StyleWu, K. Y., Wang, X. C., Anderson, M., & Tran, S. D. (2024). Advancements in Nanosystems for Ocular Drug Delivery: A Focus on Pediatric Retinoblastoma. Molecules, 29(10), 2263. https://doi.org/10.3390/molecules29102263






