Advancements in Thermal Insulation through Ceramic Micro-Nanofiber Materials
Abstract
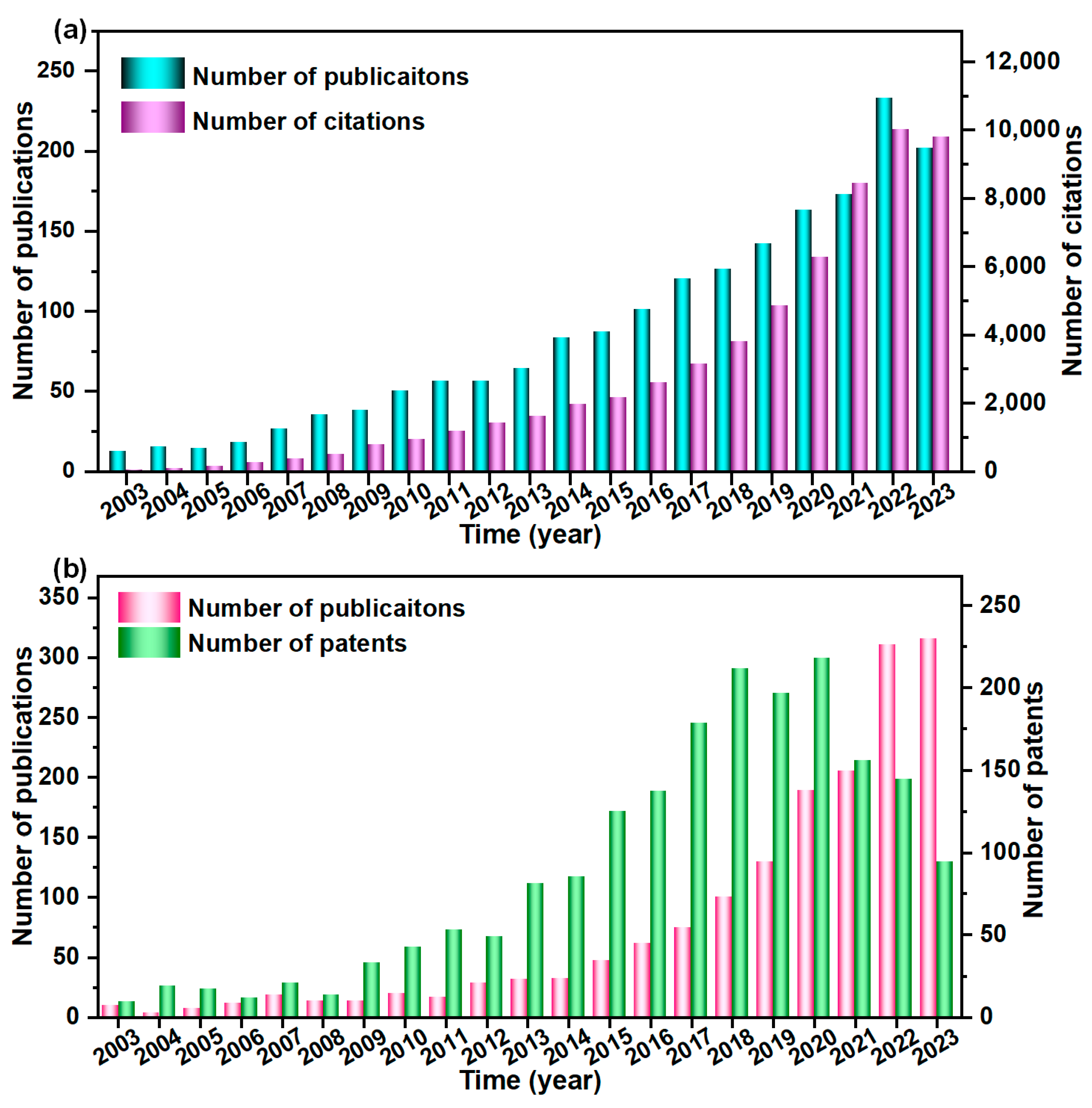
:1. Introduction
2. Heat Transfer in Ceramic Fiber Materials
2.1. Heat Conduction

2.2. Heat Convection
2.3. Heat Radiation
2.4. Thermal Conductivity Modeling of Ceramic Fibrous Materials
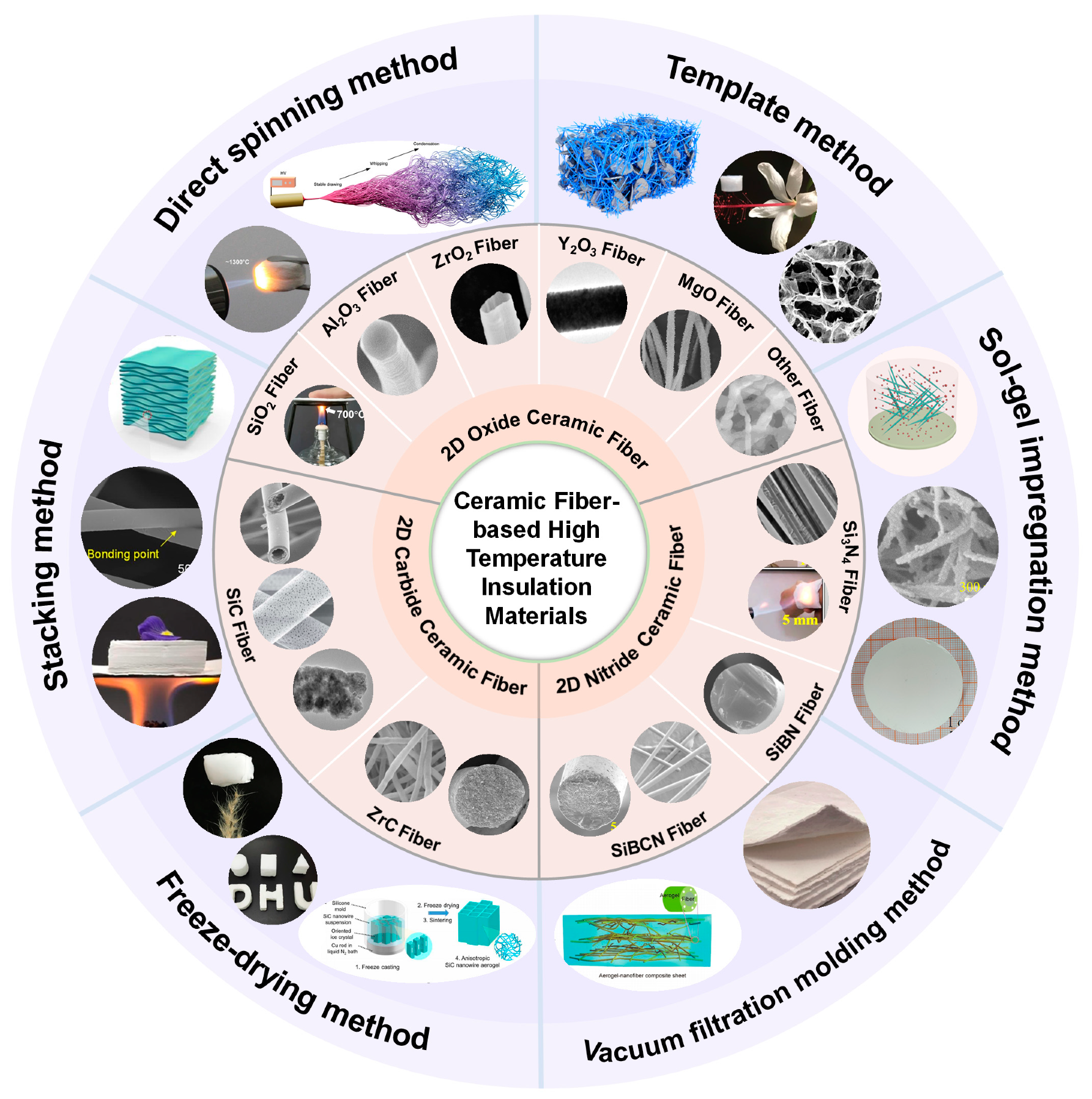
3. 2D Ceramic Fiber Membranes
3.1. Oxide Ceramic Fiber Insulation Material
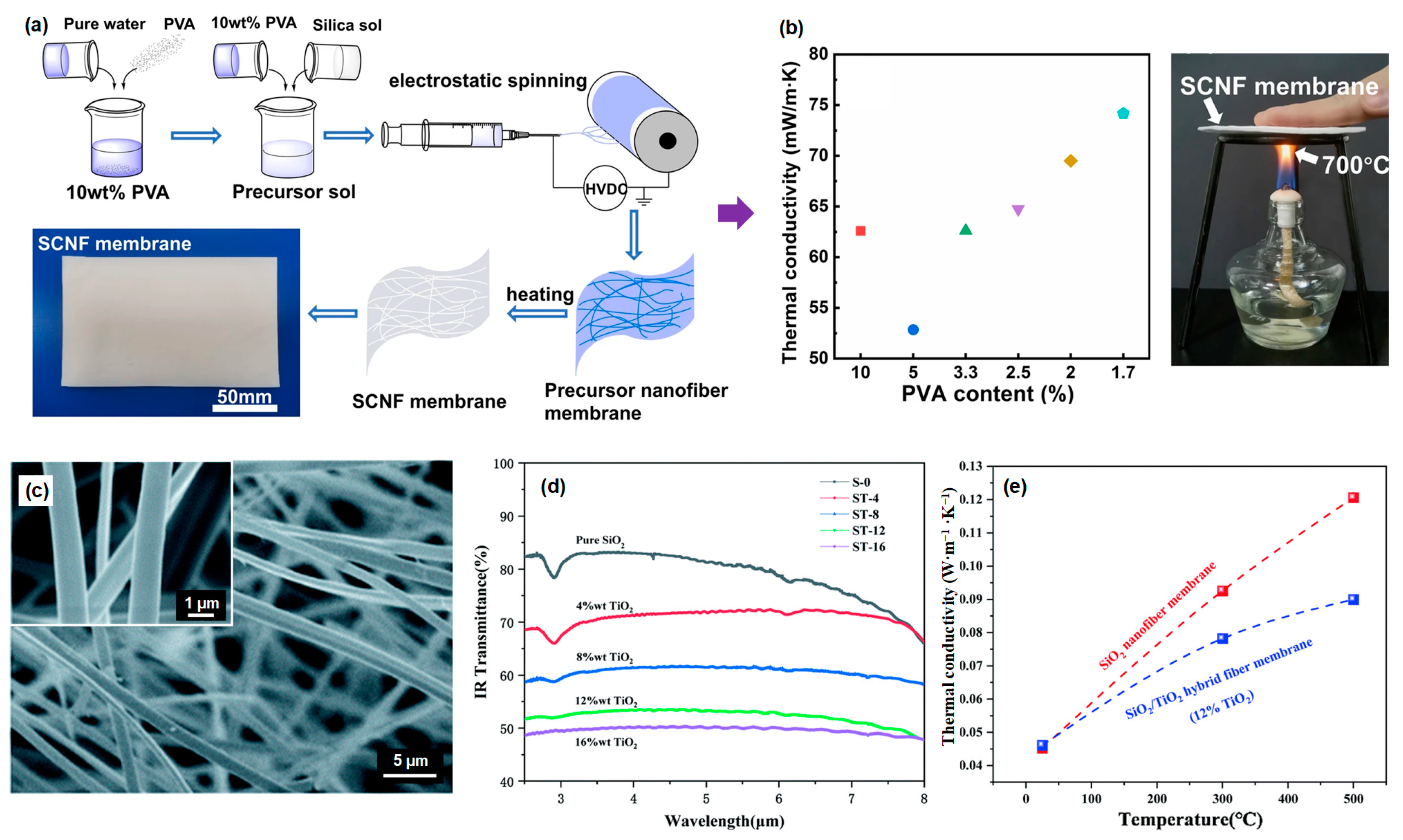
3.1.1. SiO2 Fiber
3.1.2. Al2O3 Fiber
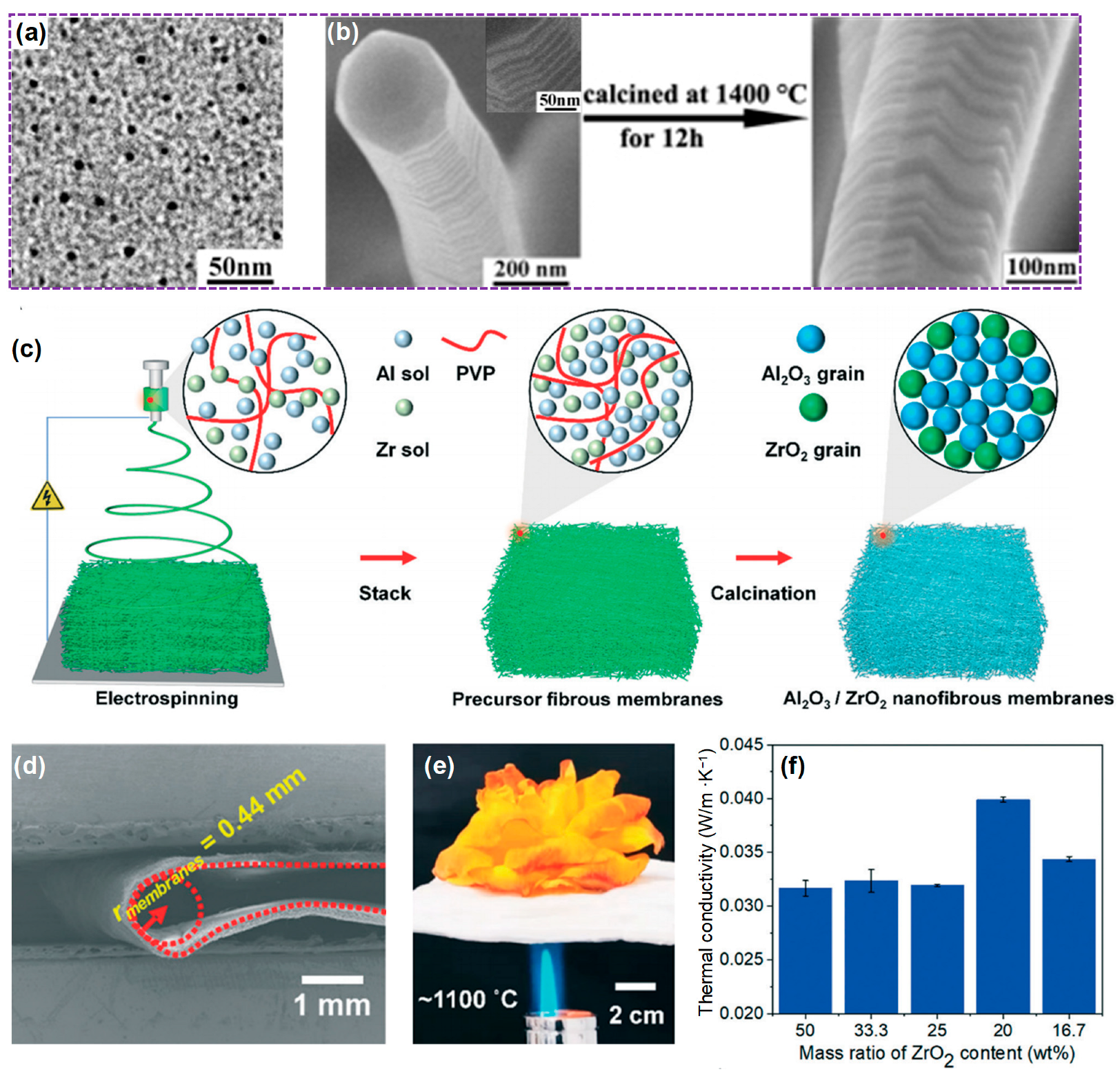
3.1.3. ZrO2 Fiber
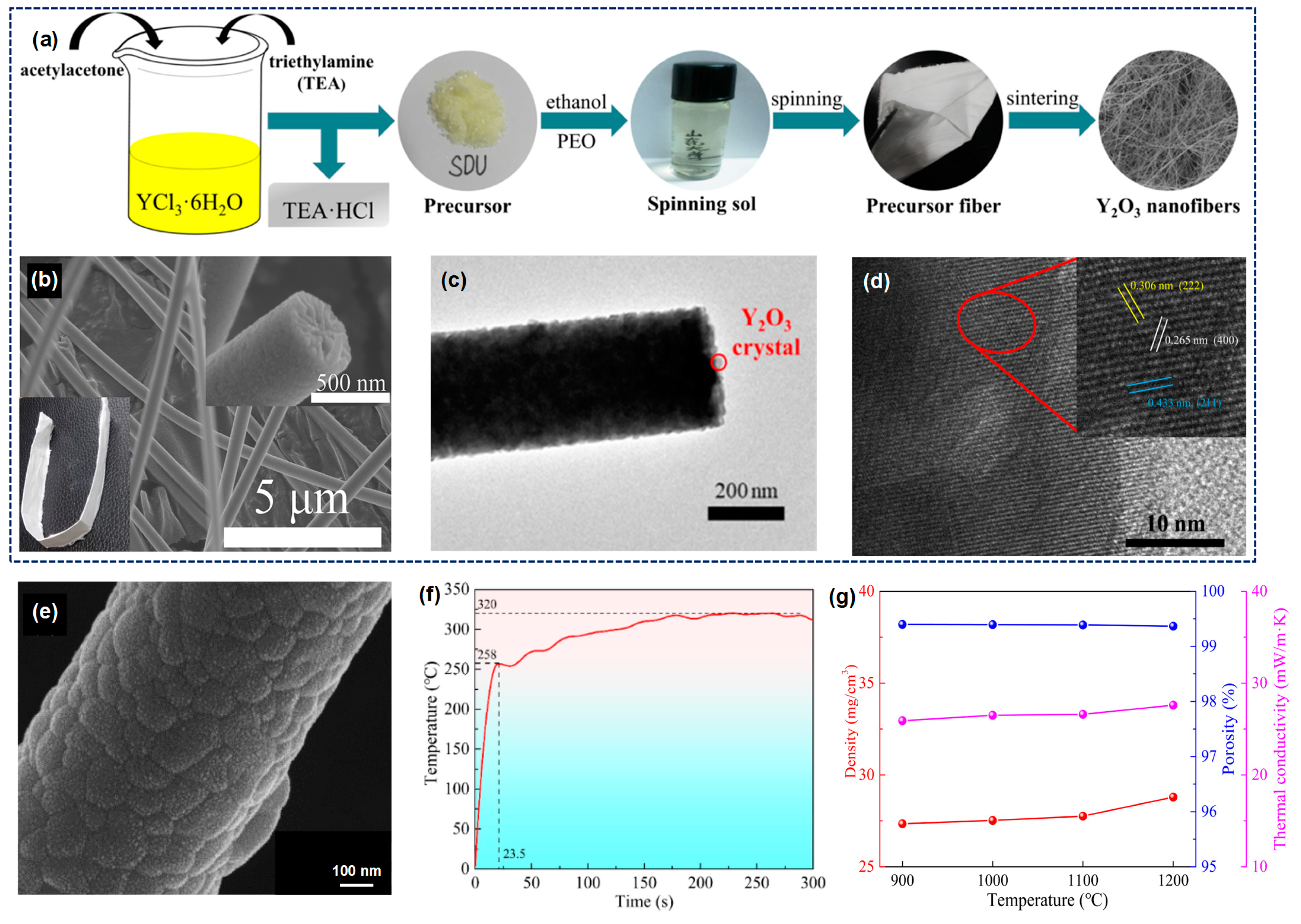
3.1.4. Y2O3 Fiber
3.1.5. MgO Fiber
3.1.6. Other Oxide Ceramic Fibers
3.2. Nitride Ceramic Fiber Insulation Material
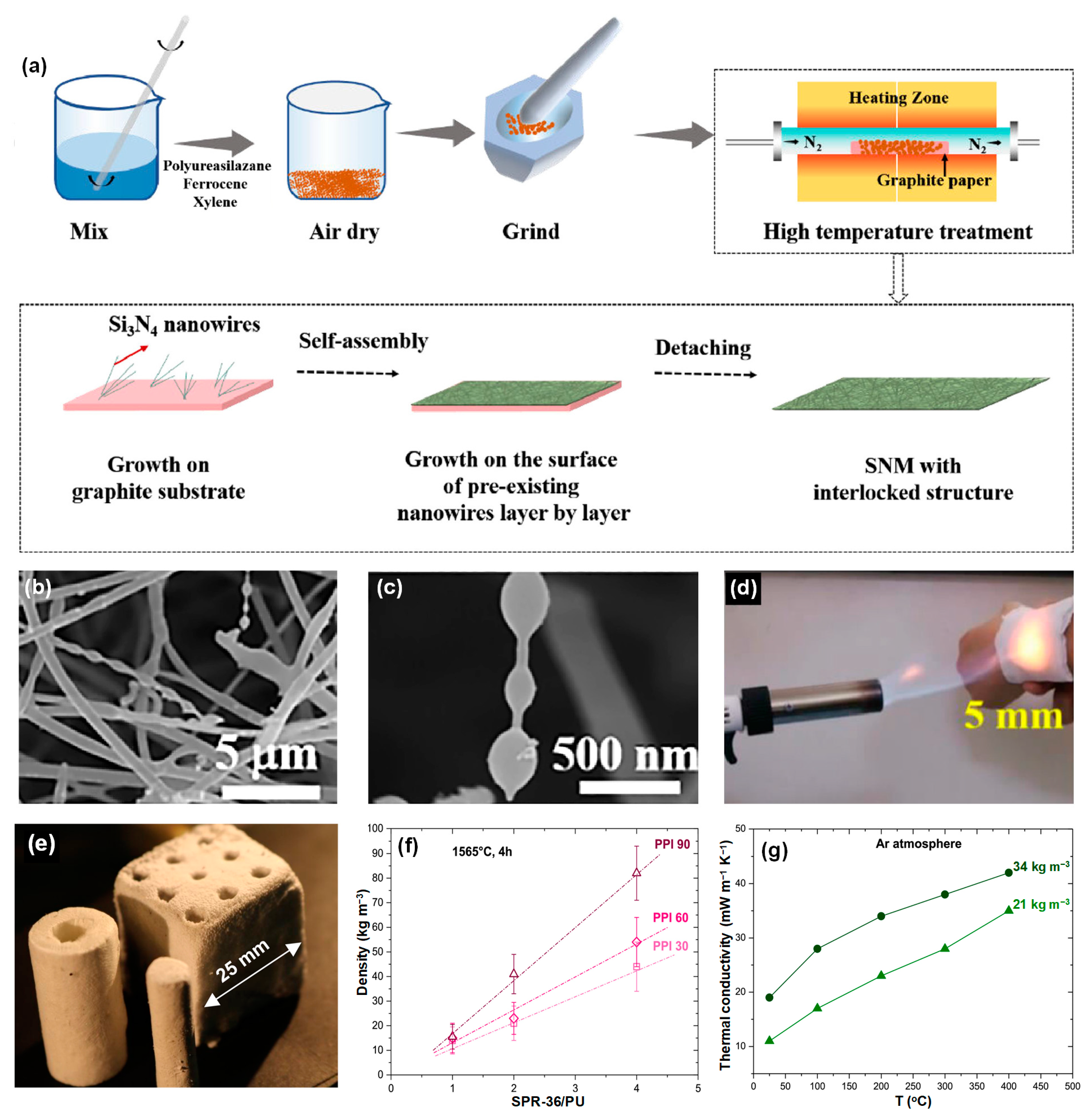
3.2.1. Si3N4 Fiber
3.2.2. SiBN Fiber
3.2.3. SiBCN Fiber
3.3. Carbide Ceramic Fiber Insulation Material
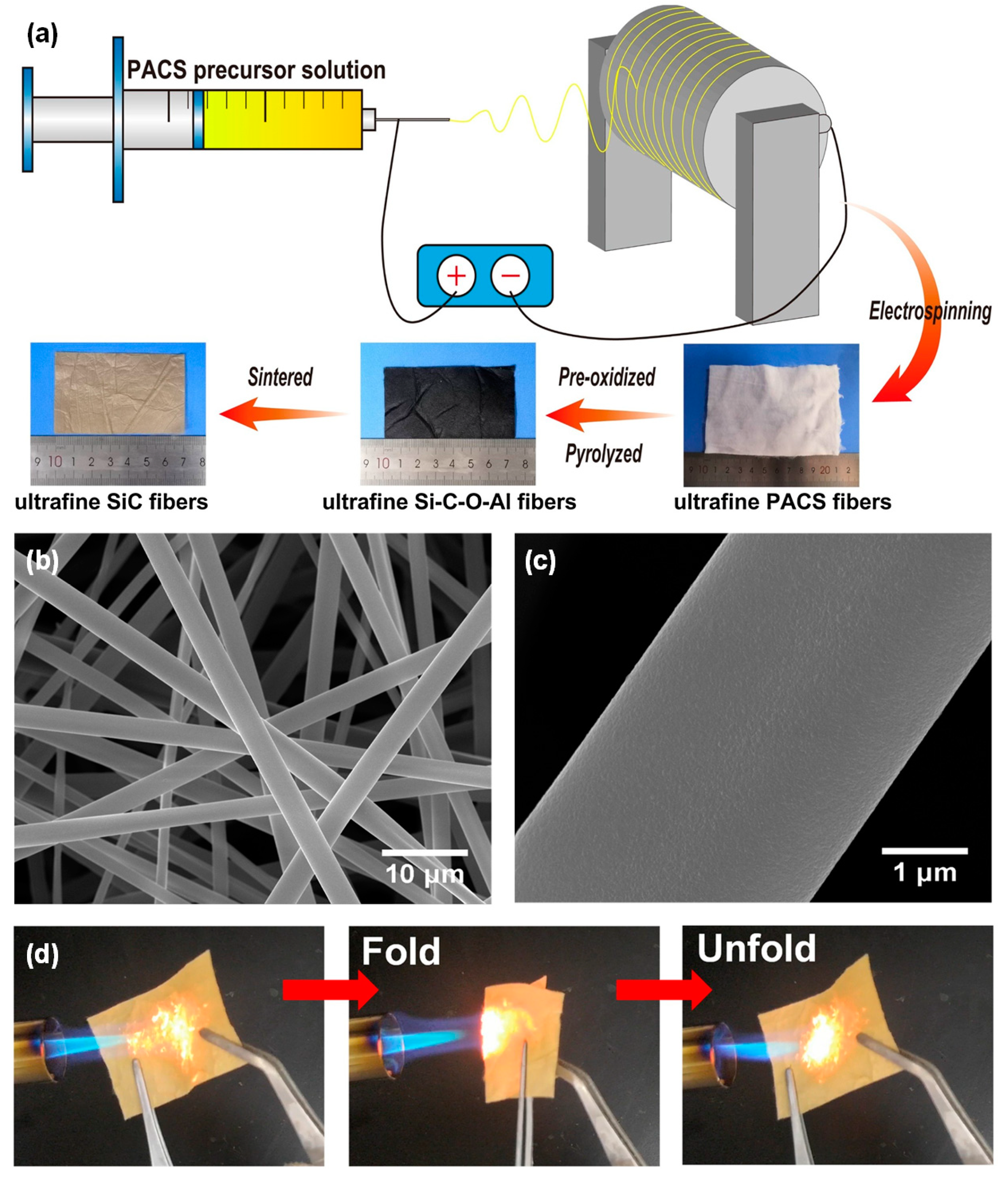
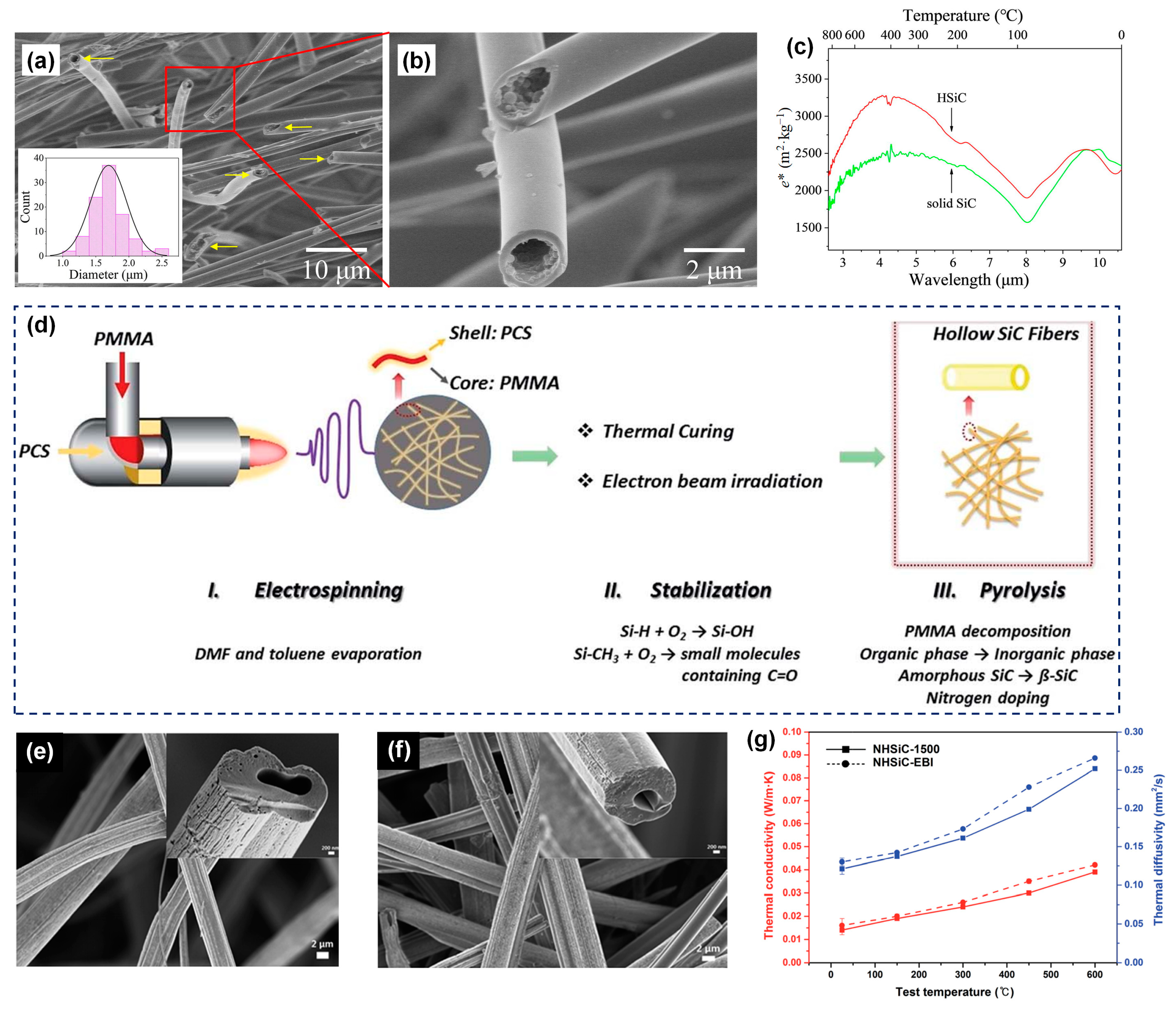
3.3.1. SiC Fiber
3.3.2. ZrC Fiber
4. 3D Ceramic Fiber Aerogels
4.1. Direct Spinning Method
4.2. Template Method
4.3. Sol-Gel Impregnation Method
4.4. Vacuum Filtration Molding Method
4.5. Freeze-Drying Method
4.6. Stacking Method
5. Challenges and Future Trends
- Although some ceramic micro-nanofibers can be transformed from brittle to flexible by adjusting the sol-gel process, calcination process parameters and ion doping, there is still no flexible mechanism to explain this transformation process. Therefore, in situ mechanical devices can be used to study the structural transformation of ceramic micro-nanofibers during the stress process. With the help of molecular dynamics and finite element simulation, the models of single fiber and fiber assembly are constructed to simulate the stress process of ceramic fiber materials, so as to provide a scientific explanation of the flexible mechanism of ceramic fiber, and to provide guidance for the preparation of other kinds of high-temperature resistant flexible ceramic micro-nanofiber materials.
- At present, the mechanical strength of ceramic micro-nanofibrous materials is relatively low, which makes it challenging to fulfill the needs of practical applications. Therefore, it is of vital importance to further improve the mechanical properties of ceramic micro-nanofibrous materials. The tensile strength of a single fiber can be enhanced by various means, such as controlling the crystallinity of the ceramic fiber, changing the direction of grain orientation, reducing micro defects in the fiber, and densifying the fiber. In addition, the mechanical properties of fiber aggregates can be improved by creating adhesion points between fibers, changing the direction of fiber accumulation, and increasing the degree of entanglement between fibers.
- Presently, the thermal conductivity of the ceramic micro-nanofiber insulation material has been reduced to some extent. However, the material still exhibits high thermal conductivity at high temperatures. In addition, the currently manufactured ceramic oxide nanofibers are typically polycrystalline. When used under high temperature conditions, there is secondary growth of grains, which further reduces the strength of the material. Therefore, it is necessary to improve the high-temperature insulation performance and resistance of the material. By directly processing ceramic materials with low solid thermal conductivity and high infrared reflectivity into 2D flexible ceramic micro-nanofibrous membranes or 3D aerogels. Alternatively, high reflectivity or high infrared shielding coatings are sprayed onto the surface of existing ceramic fiber materials. These approaches can be helpful in improving the high-temperature thermal insulation performance of materials.
- Electrospinning and air-jet spinning technologies are the primary methods for producing ceramic micro-nanofibers, and industrial equipment is now available. Despite these advances, the efficiency and yield of these technologies remain lower than those of conventional ceramic fibers, requiring further improvements in the stability of material morphology and structure. In addition, the development of ceramic fiber aerogels is still in the experimental phase, with a relatively long process flow, indicating the need for optimization in the production process. Therefore, it is necessary to optimize the production process and transform the production equipment based on the existing research and existing equipment to realize the industrial production of ceramic micro-nanofibrous materials.
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chen, Y.; Wang, N.N.; Ola, O.; Xia, Y.D.; Zhu, Y.Q. Porous ceramics: Light in weight but heavy in energy and environment technologies. Mater. Sci. Eng. R 2021, 143, 100589. [Google Scholar] [CrossRef]
- Ohji, T.; Fukushima, M. Macro-porous ceramics: Processing and properties. Int. Mater. Rev. 2012, 57, 115–131. [Google Scholar] [CrossRef]
- Hammel, E.C.; Ighodaro, O.L.R.; Okoli, O.I. Processing and properties of advanced porous ceramics: An application based review. Ceram. Int. 2014, 40, 15351–15370. [Google Scholar] [CrossRef]
- Rashad, A.M. Vermiculite as a construction material—A short guide for civil engineer. Constr. Build. Mater. 2016, 125, 53–62. [Google Scholar] [CrossRef]
- Wang, L.; Li, Z.; Jing, Q.; Liu, P. Synthesis of composite insulation materials-expanded perlite filled with silica aerogel. J. Porous Mater. 2018, 25, 373–382. [Google Scholar] [CrossRef]
- Kehal, M.; Reinert, L.; Duclaux, L. Characterization and boron adsorption capacity of vermiculite modified by thermal shock or H2O2 reaction and/or sonication. Appl. Clay Sci. 2010, 48, 561–568. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, X.; Wang, L.; Zhang, J.; Liu, R.; Sun, Q.; Ye, X.; Ma, X. Fabrication and applications of ceramic-based nanofiber materials service in high-temperature harsh conditions—A review. Gels 2023, 9, 208. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Wang, B.; Wu, N.; Han, C.; Wu, C.; Wang, Y. Micro-nano ceramic fibers for high temperature thermal insulation. J. Inorg. Mater. 2021, 36, 245–256. [Google Scholar] [CrossRef]
- Jia, C.; Xu, Z.; Luo, D.; Xiang, H.; Zhu, M. Flexible ceramic fibers: Recent development in preparation and application. Adv. Fiber Mater. 2022, 4, 573–603. [Google Scholar] [CrossRef]
- Esfahani, H.; Jose, R.; Ramakrishna, S. Electrospun ceramic nanofiber mats today: Synthesis, properties, and applications. Materials 2017, 10, 1238. [Google Scholar] [CrossRef]
- Yu, Y.; Huang, Y.; Li, L.; Huang, L.; Zhang, S. Silica ceramic nanofiber membrane with ultra-softness and high temperature insulation. J. Mater. Sci. 2022, 57, 4080–4091. [Google Scholar] [CrossRef]
- Zhang, P.; Chen, D.; Jiao, X. Fabrication of flexible α-alumina fibers composed of nanosheets. Eur. J. Inorg. Chem. 2012, 2012, 4167–4173. [Google Scholar] [CrossRef]
- Wang, T.; Zhang, Z.; Dai, C.; Li, Q.; Li, Y.; Kong, J.; Wong, C. Amorphous silicon and silicates-stabilized ZrO2 hollow fiber with low thermal conductivity and high phase stability derived from a cogon template. Ceram. Int. 2019, 45, 7120–7126. [Google Scholar] [CrossRef]
- Xie, Y.; Wang, L.; Liu, B.; Zhu, L.; Shi, S.; Wang, X. Flexible, controllable, and high-strength near-infrared reflective Y2O3 nanofiber membrane by electrospinning a polyacetylacetone-yttrium precursor. Mater. Des. 2018, 160, 918–925. [Google Scholar] [CrossRef]
- Xu, C.; Yuan, K.; Jin, X.; Yu, Z.; Zheng, L.; Lü, Y.; Wang, X.; Zhu, L.; Zhang, G.; Xu, D. High-temperature stable electrospun MgO nanofibers, formation mechanism and thermal properties. Ceram. Int. 2017, 43, 16210–16216. [Google Scholar] [CrossRef]
- Wei, H.; Li, H.; Cui, Y.; Sang, R.; Wang, H.; Wang, P.; Bu, J.; Dong, G. Synthesis of flexible mullite nanofibres by electrospinning based on nonhydrolytic sol–gel method. J. Sol-Gel Sci. Technol. 2017, 82, 718–727. [Google Scholar] [CrossRef]
- Wang, J.; Li, X.; Jin, Z.; Ji, H. Non-catalytic vapor synthesis of millimeter-scale α-Si3N4 nanowires from oxidized silicon powders. Mater. Lett. 2014, 124, 249–252. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, L.; Zhang, R.; Shao, S.; Sun, L.; Wan, X.; Wang, T. Thermal insulating and fire-retardant Si3N4 nanowire membranes resistant to high temperatures up to 1300 °C. J. Mater. Sci. Technol. 2023, 155, 82–88. [Google Scholar] [CrossRef]
- Long, X.; Shao, C.; Wang, Y. Effects of boron content on the microwave-transparent property and high-temperature stability of continuous SiBN fibers. J. Am. Ceram. Soc. 2020, 103, 4436–4444. [Google Scholar] [CrossRef]
- Ji, X.; Gao, H.; Zhang, S.; Jia, Y.; Ji, M.-S.; Zhou, X.; Shao, C. Fine-diameter Si–B–C–N ceramic fibers enabled by polyborosilazanes with N-methyl pendant group. J. Eur. Ceram. Soc. 2021, 41, 5016–5025. [Google Scholar] [CrossRef]
- Tian, Y.; Long, X.; Shao, C.; Wang, Y. SiC nanograins stabilized Si–C–B–N fibers with ultrahigh-temperature resistance. J. Am. Ceram. Soc. 2023, 106, 1981–1992. [Google Scholar] [CrossRef]
- Wang, K.; Meng, Q.; Zhao, K.; Li, X.; Bai, Q.; Jiao, H.; Tang, Y. A facile method to prepare ZrC ceramic fibers by centrifugal melt-spinning using zirconium-containing polymeric precursors. Mater. Lett. 2022, 312, 131693. [Google Scholar] [CrossRef]
- Ghelich, R.; Mehdinavaz Aghdam, R.; Torknik, F.S.; Jahannama, M.R. Low temperature carbothermal reduction synthesis of ZrC nanofibers via cyclized electrospun PVP/Zr(OPr)4 hybrid. Int. J. Appl. Ceram. Technol. 2016, 13, 352–358. [Google Scholar] [CrossRef]
- Wang, B.; Sun, L.; Wu, N.; Wang, Y. Combined synthesis of aligned SiC nanofibers via electrospinning and carbothermal reduction. Ceram. Int. 2017, 43, 10619–10623. [Google Scholar] [CrossRef]
- Wang, B.; Wang, Y.; Lei, Y.; Wu, N.; Gou, Y.; Han, C.; Fang, D. Hierarchically porous SiC ultrathin fibers mat with enhanced mass transport, amphipathic property and high-temperature erosion resistance. J. Mater. Chem. A 2014, 2, 20873–20881. [Google Scholar] [CrossRef]
- Tian, Q.; Wu, N.; Wang, B.; Wang, Y. Fabrication of hollow SiC ultrafine fibers by single-nozzle electrospinning for high-temperature thermal insulation application. Mater. Lett. 2019, 239, 109–112. [Google Scholar] [CrossRef]
- Cheng, X.; Liu, Y.-T.; Si, Y.; Yu, J.; Ding, B. Direct synthesis of highly stretchable ceramic nanofibrous aerogels via 3D reaction electrospinning. Nat. Commun. 2022, 13, 2637. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Zhang, X.; Wang, N.; Li, Y.; Feng, X.; Huang, Y.; Zhao, C.; Liu, Z.; Fang, M.; Ou, G.; et al. Ultralight, scalable, and high-temperature–resilient ceramic nanofiber sponges. Sci. Adv. 2017, 3, e1603170. [Google Scholar] [CrossRef] [PubMed]
- Guo, C.; Ye, F.; Cheng, L. In situ growth of single crystal Si3N4 nanowire foam with good wave-transparency and heat insulation performance. Compos. Part B-Eng. 2021, 224, 109129. [Google Scholar] [CrossRef]
- Xu, X.; Zhang, Q.; Hao, M.; Hu, Y.; Lin, Z.; Peng, L.; Wang, T.; Ren, X.; Wang, C.; Zhao, Z.; et al. Double-negative-index ceramic aerogels for thermal superinsulation. Science 2019, 363, 723–727. [Google Scholar] [CrossRef]
- Zhang, E.; Zhang, W.; Lv, T.; Li, J.; Dai, J.; Zhang, F.; Zhao, Y.; Yang, J.; Li, W.; Zhang, H. Insulating and robust ceramic nanorod aerogels with high-temperature resistance over 1400 °C. ACS Appl. Mater. Interfaces 2021, 13, 20548–20558. [Google Scholar] [CrossRef]
- An, L.; Wang, J.; Petit, D.; Armstrong, J.N.; Hanson, K.; Hamilton, J.; Souza, M.; Zhao, D.; Li, C.; Liu, Y.; et al. An all-ceramic, anisotropic, and flexible aerogel insulation material. Nano Lett. 2020, 20, 3828–3835. [Google Scholar] [CrossRef] [PubMed]
- Su, L.; Wang, H.; Niu, M.; Dai, S.; Cai, Z.; Yang, B.; Huyan, H.; Pan, X. Anisotropic and hierarchical SiC@SiO2 nanowire aerogel with exceptional stiffness and stability for thermal superinsulation. Sci. Adv. 2020, 6, eaay6689. [Google Scholar] [CrossRef] [PubMed]
- Si, Y.; Wang, X.; Dou, L.; Yu, J.; Ding, B. Ultralight and fire-resistant ceramic nanofibrous aerogels with temperature-invariant superelasticity. Sci. Adv. 2018, 4, eaas8925. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Cheng, X.; Si, Y.; Yu, J.; Ding, B. Elastic and highly fatigue resistant ZrO2-SiO2 nanofibrous aerogel with low energy dissipation for thermal insulation. Chem. Eng. J. 2022, 433, 133628. [Google Scholar] [CrossRef]
- Shin, S.; Wang, Q.; Luo, J.; Chen, R. Advanced materials for high-temperature thermal transport. Adv. Funct. Mater. 2020, 30, 1904815. [Google Scholar] [CrossRef]
- Flynn, D.R. Thermal conductivity of ceramics. In Mechanical and Thermal Properties of Ceramics; Wachtman, J.B., Ed.; Special Publication; National Bureau of Standards: Gaithersburg, MD, USA, 1969; Volume 303, pp. 63–123. [Google Scholar]
- Crossno, J.; Shi, J.; Wang, K.; Liu, X.; Harzheim, A.; Lucas, A.; Sachdev, S.; Kim, P.; Taniguchi, T.; Watanabe, K.; et al. Observation of the Dirac fluid and the breakdown of the Wiedemann-Franz law in graphene. Science 2016, 351, 6277. [Google Scholar] [CrossRef]
- Apostolopoulou-Kalkavoura, V.; Munier, P.; Bergstrom, L. Thermally insulating nanocellulose-based materials. Adv. Mater. 2021, 33, e2001839. [Google Scholar] [CrossRef]
- Tychanicz-Kwiecien, M.; Wilk, J.; Gil, P. Review of high-temperature thermal insulation materials. J. Thermophys. Heat Transf. 2019, 33, 271–284. [Google Scholar] [CrossRef]
- Kudo, K.; Kato, T.; Chida, H.; Takagi, S.; Tsui, N. Modelling of combined forced- and natural-convection heat transfer over upward-facing horizontal heated flat plates. Int. J. Energy Res. 2003, 27, 327–335. [Google Scholar] [CrossRef]
- Stark, C.; Fricke, J. Improved heat-transfer models for fibrous insulations. Int. J. Heat Mass Transf. 1993, 17, 617–625. [Google Scholar] [CrossRef]
- Daryabeigi, K. Heat transfer in high-temperature fibrous insulation. J. Thermophys. Heat Transf. 2003, 17, 10–20. [Google Scholar] [CrossRef]
- Montambaux, G. Generalized Stefan-Boltzmann law. Found. Phys. 2018, 48, 395–410. [Google Scholar] [CrossRef]
- Zhang, Y.; Long, E.; Li, Y.; Li, P. Solar radiation reflective coating material on building envelopes: Heat transfer analysis and cooling energy saving. Energy Explor. Exploit. 2017, 35, 748–766. [Google Scholar] [CrossRef]
- Gan, X.; Yu, Z.; Yuan, K.; Xu, C.; Wang, X.; Zhu, L.; Zhang, G.; Xu, D. Preparation of a CeO2-nanoparticle thermal radiation shield coating on ZrO2 fibers via a hydrothermal method. Ceram. Int. 2017, 43, 14183–14191. [Google Scholar] [CrossRef]
- Bogaty, H.; Hollies, N.R.S.; Harris, M. Some thermal properties of fabrics: Part I: The effect of fiber arrangement. Text. Res. J. 1957, 27, 445–449. [Google Scholar] [CrossRef]
- Zimmerman, R.W. Thermal conductivity of fluid-saturated rocks. J. Petrol. Sci. Eng. 1989, 3, 219–227. [Google Scholar] [CrossRef]
- Tavman, I.H. Effective thermal conductivity of isotropic polymer composites. Int. Commun. Heat Mass 1998, 25, 723–732. [Google Scholar] [CrossRef]
- Daryabeigi, K. Thermal analysis and design optimization of multilayer insulation for reentry aerodynamic heating. J. Spacecr. Rocket. 2002, 39, 509–514. [Google Scholar] [CrossRef]
- Carson, J.K.; Lovatt, S.J.; Tanner, D.J.; Cleland, A.C. Thermal conductivity bounds for isotropic, porous materials. Int. J. Heat Mass Transf. 2005, 48, 2150–2158. [Google Scholar] [CrossRef]
- Zhao, S.; Zhang, B.; He, X. Temperature and pressure dependent effective thermal conductivity of fibrous insulation. Int. J. Therm. Sci. 2009, 48, 440–448. [Google Scholar] [CrossRef]
- Agarwal, S.; Khan, M.M.K.; Gupta, R.K. Thermal conductivity of polymer nanocomposites made with carbon nanofibers. Polym. Eng. Sci. 2008, 48, 2474–2481. [Google Scholar] [CrossRef]
- Zhao, S.; Zhang, B.; Du, S.; He, X. Inverse identification of thermal properties of fibrous insulation from transient temperature measurements. Int. J. Thermophys. 2009, 30, 2021–2035. [Google Scholar] [CrossRef]
- Islam, S.; Bhat, G. A model for predicting thermal conductivity of porous composite materials. Heat Mass Transf. 2023, 59, 2023–2034. [Google Scholar] [CrossRef]
- Jagjiwanram; Singh, R. Effective thermal conductivity of highly porous two-phase systems. Appl. Therm. Eng. 2004, 24, 2727–2735. [Google Scholar] [CrossRef]
- Xie, T.; He, Y.; Hu, Z. Theoretical study on thermal conductivities of silica aerogel composite insulating material. Int. J. Heat Mass Transf. 2013, 58, 540–552. [Google Scholar] [CrossRef]
- Bankvall, C. Heat transfer in fibrous materials. J. Test. Eval. 1973, 1, 235–243. [Google Scholar] [CrossRef]
- Pawel, R.E.; McElroy, D.L.; Weaver, F.J.; Graves, R.S. High temperature thermal conductivity of a fibrous alumina ceramic. In Thermal Conductivity; Yarbrough, D.W., Ed.; Springer: New York, NY, USA, 1985; Volume 19, pp. 301–313. [Google Scholar]
- Daryabeigi, K. Analysis and testing of high temperature fibrous insulation for reusable launch vehicles. In Proceedings of the 37th AIAA Aerospace Sciences Meeting and Exhibit, AIAA, Reno, NV, USA, 11–14 January 1999; p. 1044. [Google Scholar]
- Wang, M.; Pan, N. Predictions of effective physical properties of complex multiphase materials. Mater. Sci. Eng. R Rep. 2008, 63, 1–30. [Google Scholar] [CrossRef]
- Gong, L.; Wang, Y.; Cheng, X.; Zhang, R.; Zhang, H. A novel effective medium theory for modelling the thermal conductivity of porous materials. Int. J. Heat Mass Transf. 2014, 68, 295–298. [Google Scholar] [CrossRef]
- Hamilton, R.L.; Crosser, O.K. Thermal conductivity of heterogeneous two-component systems. Ind. Eng. Chem. Fundam. 1962, 1, 187–191. [Google Scholar] [CrossRef]
- Bhattacharyya, R.K. Heat-transfer model for fibrous insulations. In Thermal Insulation Performance; ASTM STP 718; MeElroy, D.L., Tye, R.P., Eds.; American Society for Testing and Materials: West Conshohocken, PA, USA, 1980; pp. 272–286. [Google Scholar]
- Tugnoli, A.; Moricone, R.; Scarponi, G.E.; Cozzani, V. Effective thermal conductivity of fibrous fireproofing materials. Int. J. Therm. Sci. 2019, 136, 107–120. [Google Scholar] [CrossRef]
- Song, Y.; Zhao, F.; Li, Z.; Cheng, Z.; Huang, H.; Yang, M. Electrospinning preparation and anti-infrared radiation performance of silica/titanium dioxide composite nanofiber membrane. RSC Adv. 2021, 11, 23901–23907. [Google Scholar] [CrossRef] [PubMed]
- Si, Y.; Mao, X.; Zheng, H.; Yu, J.; Ding, B. Silica nanofibrous membranes with ultra-softness and enhanced tensile strength for thermal insulation. RSC Adv. 2015, 5, 6027–6032. [Google Scholar] [CrossRef]
- Li, X.; Su, X.; Xiao, H.; Chen, L.; Li, S.; Tang, M. Continuous α-Al2O3 fibers grown by seeding with in-situ suspension. Ceram. Int. 2020, 46, 15638–15645. [Google Scholar] [CrossRef]
- Wang, N.; Xie, Y.; Lv, J.; Zhang, J.; Zhu, L.; Jia, Z.; Tao, X. Preparation of ultrafine flexible alumina fiber for heat insulation by the electrospinning method. Ceram. Int. 2022, 48, 19460–19466. [Google Scholar] [CrossRef]
- Li, S.; Wu, F.; Zhang, X.; Han, G.; Si, Y.; Yu, J.; Ding, B. Flexible Al2O3/ZrO2 nanofibrous membranes for thermal insulation. CrystEngComm 2022, 24, 1859–1865. [Google Scholar] [CrossRef]
- Li, C.; Zhao, Z.; Liu, Y.; He, D.; Shen, X. Preparation and characterization of Al2O3/SiO2 composite nanofibers by using electrostatic spinning method. Inorg. Nano-Metal Chem. 2017, 47, 1275–1278. [Google Scholar] [CrossRef]
- Xu, Z.; Wang, F.; Yin, X.; Cheng, L.; Yu, J.; Liu, Y.; Ding, B. Recent advances in ZrO2 nanofibers: From structural design to emerging applications. Sci. China Mater. 2023, 66, 421–440. [Google Scholar] [CrossRef]
- Mao, X.; Hong, J.; Wu, Y.; Zhang, Q.; Liu, J.; Zhao, L.; Li, H.; Wang, Y.; Zhang, K. An efficient strategy for reinforcing flexible ceramic membranes. Nano Lett. 2021, 21, 9419–9425. [Google Scholar] [CrossRef]
- Mao, X.; Zhao, L.; Zhang, K.; Wang, Y.; Ding, B. Highly flexible ceramic nanofibrous membranes for superior thermal insulation and fire retardancy. Nano Res. 2022, 15, 2592–2598. [Google Scholar] [CrossRef]
- Peng, Y.; Xie, Y.; Wang, L.; Liu, L.; Zhu, S.; Ma, D.; Zhu, L.; Zhang, G.; Wang, X. High-temperature flexible, strength and hydrophobic YSZ/SiO2 nanofibrous membranes with excellent thermal insulation. J. Eur. Ceram. Soc. 2021, 41, 1471–1480. [Google Scholar] [CrossRef]
- Peng, Y.; Xie, Y.; Deng, Z.; Ma, D.; Liu, B.; Wang, X.; Zhang, G.; Zhu, L. Dual-phasic, well-aligned, and strong flexible hydrophobic ceramic membranes for efficient thermal insulation in extreme conditions. ACS Appl. Mater. Interfaces 2023, 15, 14835–14845. [Google Scholar] [CrossRef]
- Xie, Y.; Peng, Y.; Ma, D.; Liu, W.; Deng, Z.; Zhu, L.; Zhang, G.; Wang, X. Lightweight, high-strength, flexible YAG fibrous membrane for efficient heat insulation. J. Alloys Compd. 2021, 876, 159978. [Google Scholar] [CrossRef]
- Shao, C.; Guan, H.; Liu, Y.; Mu, R. MgO nanofibres via an electrospinning technique. J. Mater. Sci. 2006, 41, 3821–3824. [Google Scholar] [CrossRef]
- Zheng, L.; Yu, Z.; Yuan, K.; Jin, X.; Feng, C.; Lin, X.; Wang, X.; Zhu, L.; Zhang, G.; Xu, D. Fabrication, heat-treatment and formation mechanism of MgO fiber using propionic acid as ligand. Ceram. Int. 2017, 43, 2004–2011. [Google Scholar] [CrossRef]
- Yuan, K.; Feng, C.; Gan, X.; Yu, Z.; Wang, X.; Zhu, L.; Zhang, G.; Xu, D. Fabrication of La2Zr2O7 ceramic fibers via electrospinning method using different La2O3 precursors. Ceram. Int. 2016, 42, 16633–16639. [Google Scholar] [CrossRef]
- Xie, Y.; Wang, L.; Peng, Y.; Ma, D.; Zhu, L.; Zhang, G.; Wang, X. High temperature and high strength Y2Zr2O7 flexible fibrous membrane for efficient heat insulation and acoustic absorption. Chem. Eng. J. 2021, 416, 128994. [Google Scholar] [CrossRef]
- Chen, J.; Wang, X.; Xie, Y.; Shi, S.; Zhang, G.; Zhu, L. Preparation and fine thermal insulation performance of Gd2Zr2O7/ZrO2 composite fibers. Ceram. Int. 2020, 46, 1615–1620. [Google Scholar] [CrossRef]
- Jiang, J.; Ni, N.; Zhao, X.; Guo, F.; Fan, X.; Xiao, P. Flexible and robust YAG-Al2O3 composite nanofibrous membranes enabled by a hybrid nanocrystalline-amorphous structure. J. Eur. Ceram. Soc. 2020, 40, 2463–2469. [Google Scholar] [CrossRef]
- Liu, W.; Xie, Y.; Deng, Z.; Peng, Y.; Dong, J.; Zhu, Z.; Ma, D.; Zhu, L.; Zhang, G.; Wang, X. Modification of YSZ fiber composites by Al2TiO5 fibers for high thermal shock resistance. J. Adv. Ceram. 2022, 11, 922–934. [Google Scholar] [CrossRef]
- Wang, J.; Yao, S.; Yu, S.; Huang, Y.; Liu, W.; Zhu, M. Polymer templates effects on microstructure and mechanical properties of electrospun mullite nanofibers. Ceram. Int. 2022, 48, 5787–5794. [Google Scholar] [CrossRef]
- Wang, K.; Wang, H.; Cheng, Y. Large-scale synthesis of α-Si3N4 nanofibers and nanobelts from mesoporous silica-carbon nanocomposites. J. Ceram. Sci. Technol. 2017, 8, 259–264. [Google Scholar]
- Biesuz, M.; Zera, E.; Tomasi, M.; Jana, P.; Ersen, O.; Baaziz, W.; Lindemann, A.; Sorarù, G.D. Polymer-derived Si3N4 nanofelts for flexible, high temperature, lightweight and easy-manufacturable super-thermal insulators. Appl. Mater. Today 2020, 20, 100648. [Google Scholar] [CrossRef]
- Tang, Y.; Wang, J.; Li, X.; Xie, Z.; Wang, H.; Li, W.; Wang, X. Polymer-derived SiBN fiber for high-temperature structural/functional applications. Chem.—A Eur. J. 2010, 16, 6458–6462. [Google Scholar] [CrossRef] [PubMed]
- Long, X.; Wu, Z.; Shao, C.; Wang, X.; Wang, Y. High-temperature oxidation behavior of SiBN fibers in air. J. Adv. Ceram. 2021, 10, 768–777. [Google Scholar] [CrossRef]
- Wilfert, J.; von Hagen, R.; Fiz, R.; Jansen, M.; Mathur, S. Electrospinning of preceramic polymers for the preparation of SiBNC felts and their modification with semiconductor nanowires. J. Mater. Chem. 2012, 22, 2099–2104. [Google Scholar] [CrossRef]
- Chen, Q.; Jia, D.; Liang, B.; Yang, Z.; Zhou, Y.; Li, D.; Riedel, R.; Zhang, T.; Gao, C. Electrospinning of pure polymer-derived SiBCN nanofibers with high yield. Ceram. Int. 2021, 47, 10958–10964. [Google Scholar] [CrossRef]
- Xie, F.; Mo, G.; He, L.; Huang, Q.; Huang, Z. Fabrication of SiC ultrafine fiber with low oxygen content by electrospinning, thermal curing and pyrolysis of cyano-polycarbosilane. Silicon 2023, 15, 6421–6429. [Google Scholar] [CrossRef]
- Xie, F.; Duan, Y.; Mo, G.; Huang, Q.; Huang, Z. Structural evolution of polyaluminocarbosilane during the polymer–ceramic conversion process. Materials 2023, 16, 4172. [Google Scholar] [CrossRef]
- Chen, D.; Mo, G.; Qian, J.; He, L.; Huang, Q.; Huang, Z. Synthesis of cyano-polycarbosilane and investigation of its pyrolysis process. J. Eur. Ceram. Soc. 2020, 40, 5226–5237. [Google Scholar] [CrossRef]
- Shan, J.; Mo, G.; Sun, Q.; Huang, J.; Ouyang, Q.; Huang, Q. Fabrication of SiC(OAl) and SiC(Al) fibers by melt-spinning, UV curing, thermolysis and sintering of photo-sensitive polyvinylaluminocarbosilane. J. Eur. Ceram. Soc. 2024, 44, 3501–3513. [Google Scholar] [CrossRef]
- Chen, J.; Zhang, Y.; Yan, D.; Gou, Y. Flexible ultrafine nearly stoichiometric polycrystalline SiC fibers with excellent oxidation resistance and superior thermal stability up to 1900 °C. J. Eur. Ceram. Soc. 2022, 42, 1938–1946. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, Y.; Choi, W.C.; Chae, S.; Lee, J.; Kim, B.-S.; Park, M.; Kim, H.Y. Highly flexible, erosion resistant and nitrogen doped hollow SiC fibrous mats for high temperature thermal insulators. J. Mater. Chem. A 2017, 5, 2664–2672. [Google Scholar] [CrossRef]
- Zhang, B.; Tong, Z.; Yu, H.; Xu, H.; Chen, Z.; Li, X.; Ji, H. Flexible and high-temperature resistant ZrO2/SiC-based nanofiber membranes for high temperature thermal insulation. J. Alloys Compd. 2021, 872, 159618. [Google Scholar] [CrossRef]
- Zhang, B.; Wang, W.; Tong, Z.; Xu, H.; Li, X.; Ji, H. Design and preparation of hollow SiO2 nanospheres/SiC-based nanocomposite fiber membrane for high temperature thermal insulators. Microporous Mesoporous Mater. 2022, 329, 111551. [Google Scholar] [CrossRef]
- Tao, X.; Zhou, S.; Ma, J.; Xiang, Z.; Hou, R.; Wang, J.; Li, X. A facile method to prepare ZrC nanofibers by electrospinning and pyrolysis of polymeric precursors. Ceram. Int. 2017, 43, 3910–3914. [Google Scholar] [CrossRef]
- Cui, X.M.; Nam, Y.S.; Lee, J.Y.; Park, W.H. Fabrication of zirconium carbide (ZrC) ultra-thin fibers by electrospinning. Mater. Lett. 2008, 62, 1961–1964. [Google Scholar] [CrossRef]
- Li, F.; Kang, Z.; Huang, X.; Zhang, G. Fabrication of zirconium carbide nanofibers by electrospinning. Ceram. Int. 2014, 40, 10137–10141. [Google Scholar] [CrossRef]
- Ge, M.; Lv, X.; Zhang, H.; Yu, S.; Lu, Z.; Zhang, W. Microstructures of a SiC–ZrC ceramic fiber derived from a polymeric precursor. Materials 2020, 13, 2142. [Google Scholar] [CrossRef]
- Zhang, X.; Liu, C.; Zhang, X.; Si, Y.; Yu, J.; Ding, B. Super strong, shear resistant, and highly elastic lamellar structured ceramic nanofibrous aerogels for thermal insulation. J. Mater. Chem. A 2021, 9, 27415–27423. [Google Scholar] [CrossRef]
- An, Z.; Hou, X.; Zhou, P.; Zhang, R.; Fang, D. A novel flexible, layered, recoverable SiO2 fiber skeleton and aerogel composites material prepared by papermaking process. Ceram. Int. 2021, 47, 12963–12969. [Google Scholar] [CrossRef]
- Shafi, S.; Navik, R.; Ding, X.; Zhao, Y. Improved heat insulation and mechanical properties of silica aerogel/glass fiber composite by impregnating silica gel. J. Non-Cryst. Solids 2019, 503–504, 78–83. [Google Scholar] [CrossRef]
- Xu, C.; Wang, H.; Song, J.; Bai, X.; Liu, Z.; Fang, M.; Yuan, Y.; Sheng, J.; Li, X.; Wang, N.; et al. Ultralight and resilient Al2O3 nanotube aerogels with low thermal conductivity. J. Am. Ceram. Soc. 2018, 101, 1677–1683. [Google Scholar] [CrossRef]
- Yu, H.; Tong, Z.; Zhang, B.; Chen, Z.; Li, X.; Su, D.; Ji, H. Thermal radiation shielded, high strength, fire resistant fiber/nanorod/aerogel composites fabricated by in-situ growth of TiO2 nanorods for thermal insulation. Chem. Eng. J. 2021, 418, 129342. [Google Scholar] [CrossRef]
- Xian, L.; Zhang, Y.; Wu, Y.; Zhang, X.; Dong, X.; Liu, J.; Guo, A. Microstructural evolution of mullite nanofibrous aerogels with different ice crystal growth inhibitors. Ceram. Int. 2020, 46, 1869–1875. [Google Scholar] [CrossRef]
- Zhang, R.; Ye, C.; Hou, X.; Li, S.; Wang, B. Microstructure and properties of lightweight fibrous porous mullite ceramics prepared by vacuum squeeze moulding technique. Ceram. Int. 2016, 42, 14843–14848. [Google Scholar] [CrossRef]
- Peng, F.; Jiang, Y.; Feng, J.; Cai, H.; Feng, J.; Li, L. Thermally insulating, fiber-reinforced alumina–silica aerogel composites with ultra-low shrinkage up to 1500 °C. Chem. Eng. J. 2021, 411, 128402. [Google Scholar] [CrossRef]
- Zhu, J.; Zhu, R.; Hu, Y.; Wang, Z. Low-cost and temperature-resistant mullite fiber sponges with superior thermal insulation and high-temperature pm filtration. Sep. Purif. Technol. 2023, 305, 122445. [Google Scholar] [CrossRef]
- Zhang, X.; Cheng, X.; Si, Y.; Yu, J.; Ding, B. All-ceramic and elastic aerogels with nanofibrous-granular binary synergistic structure for thermal superinsulation. ACS Nano 2022, 16, 5487–5495. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, F.; Dou, L.; Cheng, X.; Si, Y.; Yu, J.; Ding, B. Ultrastrong, superelastic, and lamellar multiarch structured ZrO2–Al2O3 nanofibrous aerogels with high-temperature resistance over 1300 °C. ACS Nano 2020, 14, 15616–15625. [Google Scholar] [CrossRef]
- Su, L.; Li, M.; Wang, H.; Niu, M.; Lu, D.; Cai, Z. Resilient Si3N4 nanobelt aerogel as fire-resistant and electromagnetic wave-transparent thermal insulator. ACS Appl. Mater. Interfaces 2019, 11, 15795–15803. [Google Scholar] [CrossRef] [PubMed]
- Tong, Z.; Zhang, B.; Yu, H.; Yan, X.; Xu, H.; Li, X.; Ji, H. Si3N4 nanofibrous aerogel with in situ growth of SiOx coating and nanowires for oil/water separation and thermal insulation. ACS Appl. Mater. Interfaces 2021, 13, 22765–22773. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Zhu, M.; Gong, W.; Du, R.; Eychmüller, A.; Li, T.; Lv, W.; Zhang, X. Boron nitride aerogels with super-flexibility ranging from liquid nitrogen temperature to 1000 °C. Adv. Funct. Mater. 2019, 29, 1900188. [Google Scholar] [CrossRef]
- Lu, D.; Su, L.; Wang, H.; Niu, M.; Xu, L.; Ma, M.; Gao, H.; Cai, Z.; Fan, X. Scalable fabrication of resilient SiC nanowires aerogels with exceptional high-temperature stability. ACS Appl. Mater. Interfaces 2019, 11, 45338–45344. [Google Scholar] [CrossRef] [PubMed]
- Su, K.; Wang, Y.; Hu, K.; Fang, X.; Yao, J.; Li, Q.; Yang, J. Ultralight and high-strength SiCnw@SiC foam with highly efficient microwave absorption and heat insulation properties. ACS Appl. Mater. Interfaces 2021, 13, 22017–22030. [Google Scholar] [CrossRef] [PubMed]
- Huo, Y.; Zhao, K.; Xu, Z.; Li, F.; Zhao, X.; Meng, Q.; Tang, C.; Tang, Y. Ultralight and superelastic polyvinyl alcohol/SiC nanofiber/reduced graphene oxide hybrid foams with excellent thermal insulation and microwave absorption properties. Ceram. Int. 2021, 47, 25986–25996. [Google Scholar] [CrossRef]
- Dong, J.; Xie, Y.; Liu, L.; Deng, Z.; Liu, W.; Zhu, L.; Wang, X.; Xu, D.; Zhang, G. Lightweight and resilient ZrO2–TiO2 fiber sponges with layered structure for thermal insulation. Adv. Eng. Mater. 2022, 24, 2101603. [Google Scholar] [CrossRef]
- He, J.; Li, X.; Su, D.; Ji, H.; Wang, X. Ultra-low thermal conductivity and high strength of aerogels/fibrous ceramic composites. J. Eur. Ceram. Soc. 2016, 36, 1487–1493. [Google Scholar] [CrossRef]
- He, J.; Zhao, H.; Li, X.; Su, D.; Ji, H.; Yu, H.; Hu, Z. Large-scale and ultra-low thermal conductivity of ZrO2 fibrofelt/ZrO2-SiO2 aerogels composites for thermal insulation. Ceram. Int. 2018, 44, 8742–8748. [Google Scholar] [CrossRef]
- Zhang, R.; Hou, X.; Ye, C.; Wang, B. Enhanced mechanical and thermal properties of anisotropic fibrous porous mullite–zirconia composites produced using sol-gel impregnation. J. Alloys Compd. 2017, 699, 511–516. [Google Scholar] [CrossRef]
- Dong, X.; Sui, G.; Yun, Z.; Wang, M.; Guo, A.; Zhang, J.; Liu, J. Effect of temperature on the mechanical behavior of mullite fibrous ceramics with a 3D skeleton structure prepared by molding method. Mater. Des. 2016, 90, 942–948. [Google Scholar] [CrossRef]
- Wang, F.; Dou, L.; Dai, J.; Li, Y.; Huang, L.; Si, Y.; Yu, J.; Ding, B. In situ synthesis of biomimetic silica nanofibrous aerogels with temperature-invariant superelasticity over one million compressions. Angew. Chem. Int. Ed. 2020, 132, 8285–8292. [Google Scholar] [CrossRef] [PubMed]
- Dou, L.; Zhang, X.; Cheng, X.; Ma, Z.; Wang, X.; Si, Y.; Yu, J.; Ding, B. Hierarchical cellular structured ceramic nanofibrous aerogels with temperature-invariant superelasticity for thermal insulation. ACS Appl. Mater. Interfaces 2019, 11, 29056–29064. [Google Scholar] [CrossRef] [PubMed]



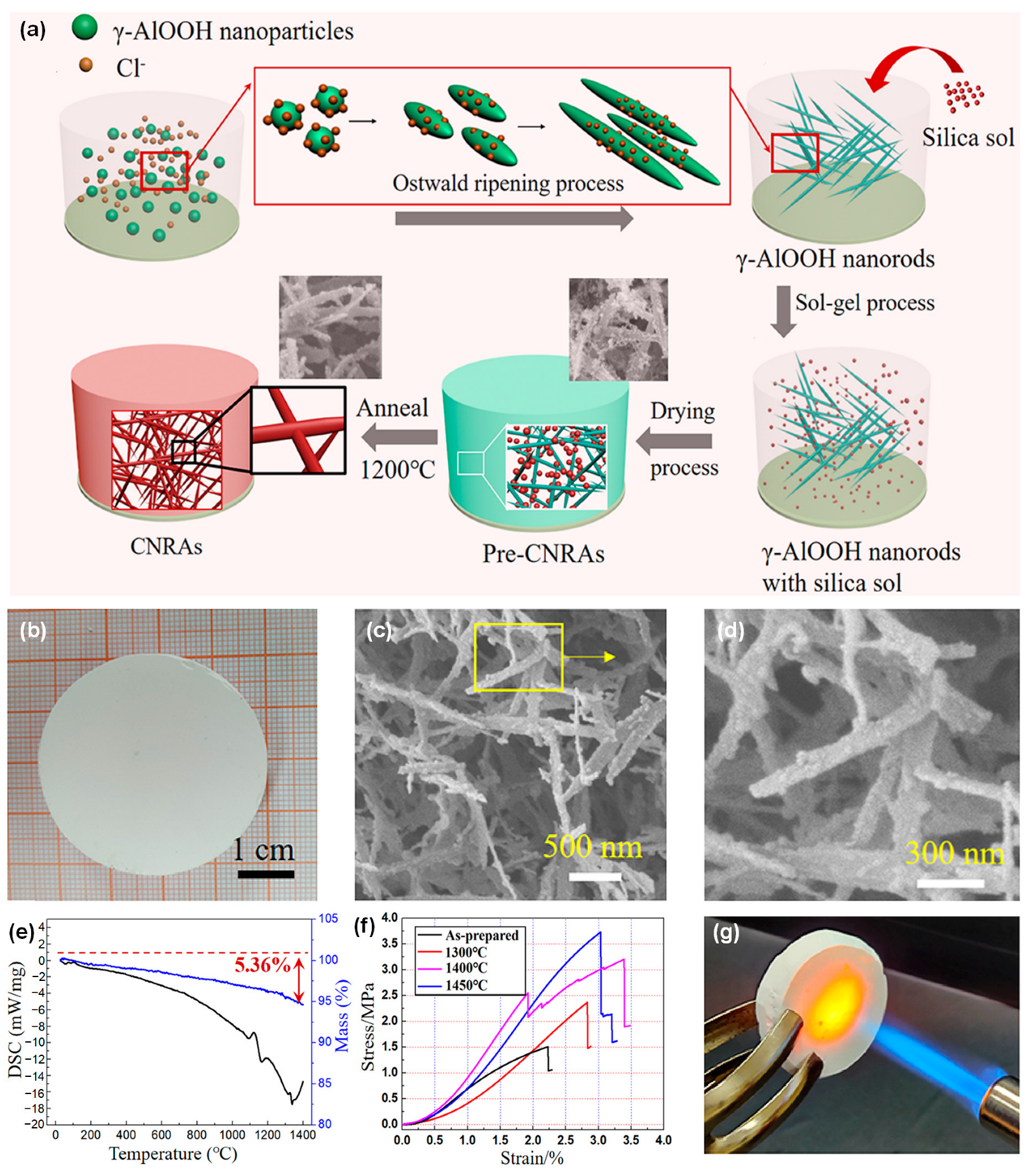
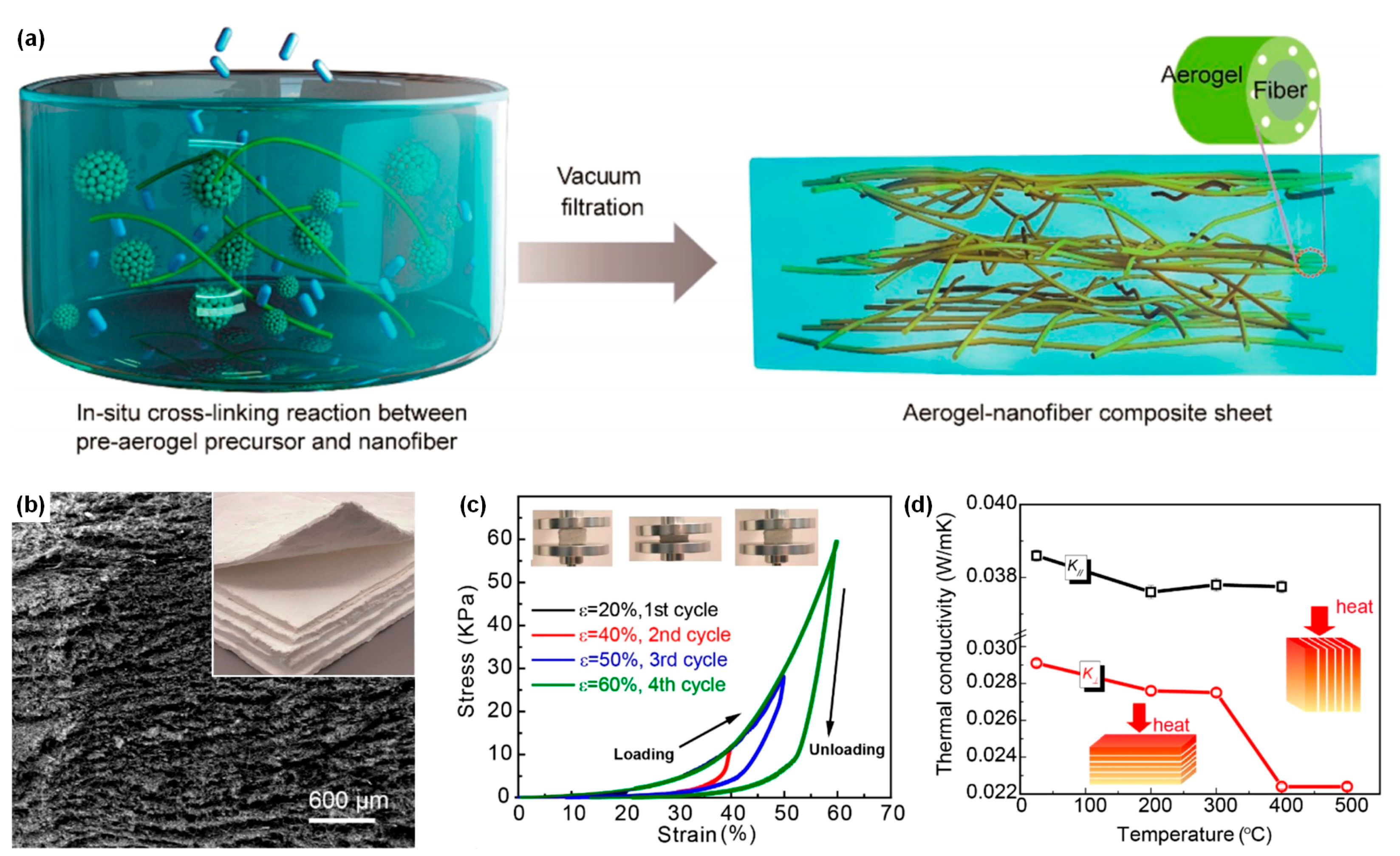


| Model | Structure Schematic | Equations | Ref. |
|---|---|---|---|
| Parallel model |  | [43,47,48,49,50,51,52] | |
| where λc is thermal conductivity due to solid and gas conductions, λg is the gas thermal conductivity, λs is the solid thermal conductivity, and ω is the volume fraction of solid. | |||
| Series model | 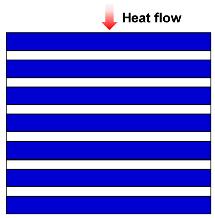 | [47,53,54,55] | |
| where λc is thermal conductivity due to solid and gas conductions, λg is the gas thermal conductivity, λs is the solid thermal conductivity, and ω is the volume fraction of solid. | |||
| Singh model | 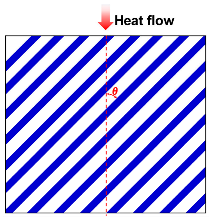 | [56,57] | |
| where λc is thermal conductivity due to solid and gas conductions, λg is the gas thermal conductivity, λs is the solid thermal conductivity, ω is the volume fraction of solid, and θ is the angle between fiber orientation and heat flow direction. | |||
| Bankvall model | 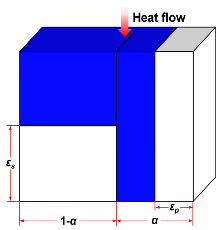 | [54,58,59,60] | |
| where λc is thermal conductivity due to solid and gas conductions, λg is the gas thermal conductivity, λs is the solid thermal conductivity, α is the proportion of fibers parallel to heat flow, and εp and εs represent structural parameters, relating to parallel and series heat transfer mechanisms, respectively. The total porosity (ε) of the fibrous materials could be expressed as ε = α·εp + (1 − α)·εs. | |||
| Maxwell–Eucken model | 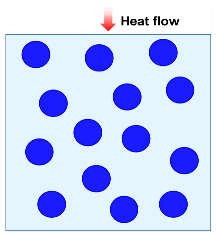 | [55] | |
| where λc is thermal conductivity due to solid and gas conductions, λg is the gas thermal conductivity, λs is the solid thermal conductivity, and ω is the volume fraction of solid. | |||
| EMT model | 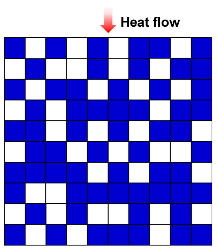 | [51,61,62] | |
| where λc is thermal conductivity due to solid and gas conductions, λg is the gas thermal conductivity, λs is the solid thermal conductivity, and ω is the volume fraction of solid. | |||
| Hamilton model | 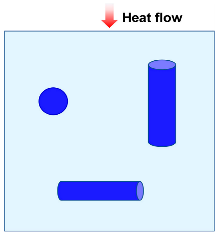 | [53,61,63] | |
| where λc is thermal conductivity due to solid and gas conductions, λg is the gas thermal conductivity, λs is the solid thermal conductivity, and ω is the volume fraction of solid. n is an empirical shape factor. For spherical particles, n = 3; for infinitely long cylinders, n = 6. | |||
| Bhattacharyya model |  | [43,64,65] | |
| where λc is thermal conductivity due to solid and gas conductions, λg is the gas thermal conductivity, λs is the solid thermal conductivity, and ω is the volume fraction of solid. |
| Category | Preparation Method | Calcination Parameters | Diameter (μm) | Density (mg·cm−3) | Porosity (%) | Thermal Conductivity Test Method | Thermal Conductivity (W·m−1·K−1) | Ref. |
|---|---|---|---|---|---|---|---|---|
| SiO2 | Direct spinning method | 900 °C, 60 min, Air | 0.343 | – | >96 | Hot Disk instrument | 0.0389 (25 °C) | [104] |
| Vacuum filtration molding method | – | ~3 | 80–220 | 90–96.4 | Hot Disk method | 0.023–0.037 (room temperature) | [105] | |
| Sol-gel impregnation method | – | 8–12 | 226–271 | 87.2–91.3 | Transient hot-wire method | 0.0179–0.0283 (25 °C) | [106] | |
| Freeze-drying method | 900 °C, 30 min, Air | 0.206 | >0.15 | 99.993 | Hot Disk instrument | 0.025–0.032 (room temperature) | [34] | |
| Al2O3 | Direct spinning method | 900 °C, 2 h; 1200 °C, 2 h, Air | – | 2 | >99.9 | Hot Disk instrument | 0.022 (room temperature) | [107] |
| Sol-gel impregnation method | 1200 °C, 1 h, Air | 0.15 | 146 | 96 | 25 °C: Heat flow method 200–1200: Heat flow meter apparatus | 0.026 (25 °C); 0.089 (1200 °C) | [31] | |
| ZrO2 | Direct spinning method | 800 °C, 200 min, Air | – | – | – | – | 0.027 (room temperature) | [28] |
| TiO2 | Sol-gel impregnation method | 600 °C, 6 h, Air | 0.01–0.02 | 304 | – | Transient hot-wire technique | 0.071 (1100 °C) | [108] |
| Mullite | Freeze-drying method | 1400 °C, 2 h, Air | 0.73 | 49.13–82.21 | 97.07–98.25 | Hot Disk instrument | 0.038–0.058 (room temperature) | [109] |
| Vacuum filtration molding method | 1400 °C, 1 h, Air | 6 | 410–650 | 79.4–87.3 | Heat flow test method | 0.095 (room temperature) | [110] | |
| Sol-gel impregnation method | 800 °C, 24 h, Air | 4 | 360 | – | Water flow plate method | 0.082 (1200 °C) | [111] | |
| Direct spinning method | 1000–1500 °C, 1 h, Air | 2 | 15 | – | Transient plane source method | 0.0265 (40 °C) | [112] | |
| Direct spinning method | 1100–1500 °C, 1 h, Air | 0.29 ± 0.03 | 1.5 | 99.95 | Hot Disk instrument | 0.0228 (room temperature) | [27] | |
| ZrO2-SiO2 | Stacking method | 1000 °C, Air | 0.5 | 23 | 99.58 | Hot Disk instrument | 0.024 (room temperature) | [113] |
| Stacking method | 1000 °C, 2 h, Air | 0.4–0.8 | – | – | Hot Disk instrument | 0.0268 (25 °C); 0.11 (900 °C) | [35] | |
| ZrO2-Al2O3 | Stacking method | 800 °C, 2 h, Air | 0.38–0.74 | – | >98 | Hot Disk instrument | 0.0322 (room temperature) | [114] |
| Si3N4 | Stacking method | Partial-hot-pressing at 1200 °C, 30 min, Air | 0.5–1.6 | 1.8–9.6 | ~99.94 | Transient hot-wire method | 0.029 (room temperature) | [115] |
| Freeze-drying method | – | ~0.05 | 10 | >99 | Transient hot-wire method | 0.0157 (room temperature) | [116] | |
| Template method | 1300 °C, 2 h, N2; 700 °C, 4–7 h, Air | ~0.08 | 8.9 | 96.6674 | Hot Disk instrument | 0.03–0.11 (room temperature) | [29] | |
| BN | Freeze-drying method | 1200 °C, 3 h, NH3 | 0.6–1.8 | 15.5 | – | – | 0.0346 ± 0.0015 | [117] |
| Template method | 90 °C, 1 h, 500 °C, 1 h, 1500 °C, 3 h, Air | – | 0.1 | 99.99 | Homemade steady-state device | 0.02 | [30] | |
| SiC | Vacuum filtration molding method | 700 °C, 3 h, Air | 0.03–0.28 | 3–19 | >99 | Hot Disk instrument | 0.025 (room temperature) | [118] |
| Template method | 800 °C, 10 h, Air | 0.3 | 26–206 | 84.34–98.01 | Laser thermal conductivity meter | 0.304 (1200 °C) | [119] | |
| Freeze-drying method | 1000 °C, 30 min, Air | 0.02–0.05 | 6.5 | 98 | Laser flash apparatus | 0.014 | [33] | |
| Freeze-drying method | – | 0.2 | 10 | – | Hot Disk instrument | 0.053 (50 °C) | [120] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, W.; Fu, Q.; Ge, J.; Xu, S.; Liu, Q.; Zhang, J.; Shan, H. Advancements in Thermal Insulation through Ceramic Micro-Nanofiber Materials. Molecules 2024, 29, 2279. https://doi.org/10.3390/molecules29102279
Wang W, Fu Q, Ge J, Xu S, Liu Q, Zhang J, Shan H. Advancements in Thermal Insulation through Ceramic Micro-Nanofiber Materials. Molecules. 2024; 29(10):2279. https://doi.org/10.3390/molecules29102279
Chicago/Turabian StyleWang, Wenqiang, Qiuxia Fu, Jianlong Ge, Sijun Xu, Qixia Liu, Junxiong Zhang, and Haoru Shan. 2024. "Advancements in Thermal Insulation through Ceramic Micro-Nanofiber Materials" Molecules 29, no. 10: 2279. https://doi.org/10.3390/molecules29102279
APA StyleWang, W., Fu, Q., Ge, J., Xu, S., Liu, Q., Zhang, J., & Shan, H. (2024). Advancements in Thermal Insulation through Ceramic Micro-Nanofiber Materials. Molecules, 29(10), 2279. https://doi.org/10.3390/molecules29102279






