Lactic Acid Bacteria-Derived Postbiotics as Adjunctive Agents in Breast Cancer Treatment to Boost the Antineoplastic Effect of a Conventional Therapeutic Comprising Tamoxifen and a New Drug Candidate: An Aziridine–Hydrazide Hydrazone Derivative
Abstract
1. Introduction
2. Results
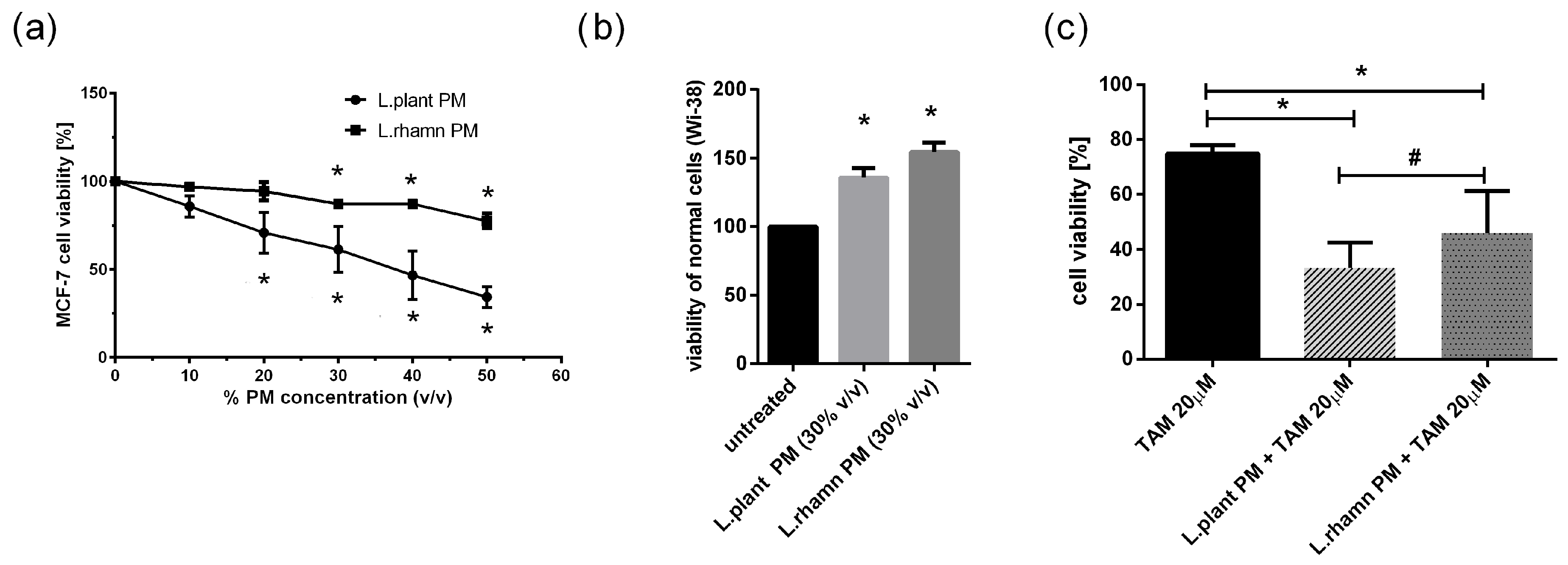
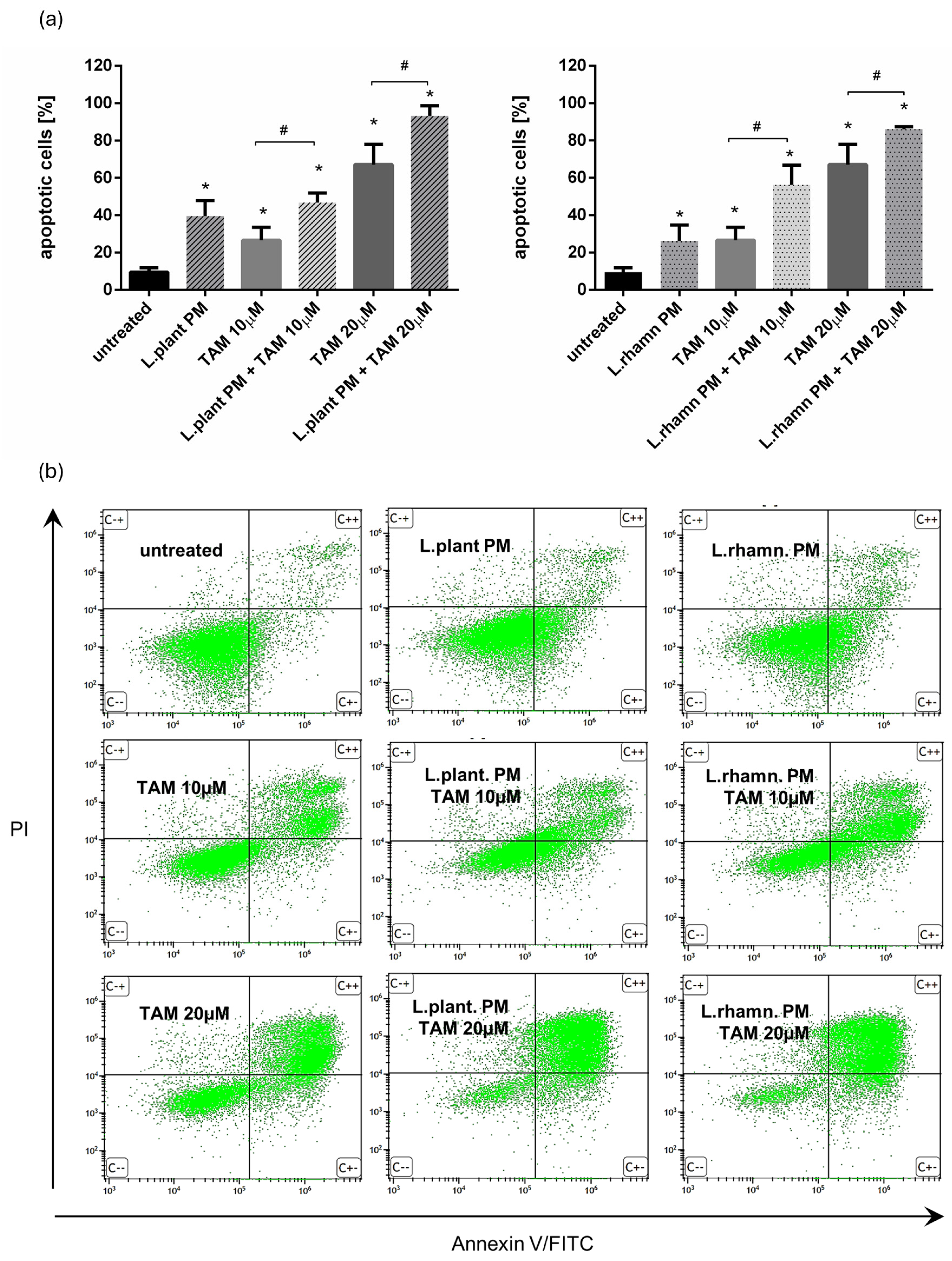
2.1. LAB Postbiotics Enhance the Cytotoxic Effect of Classic Tamoxifen Treatment
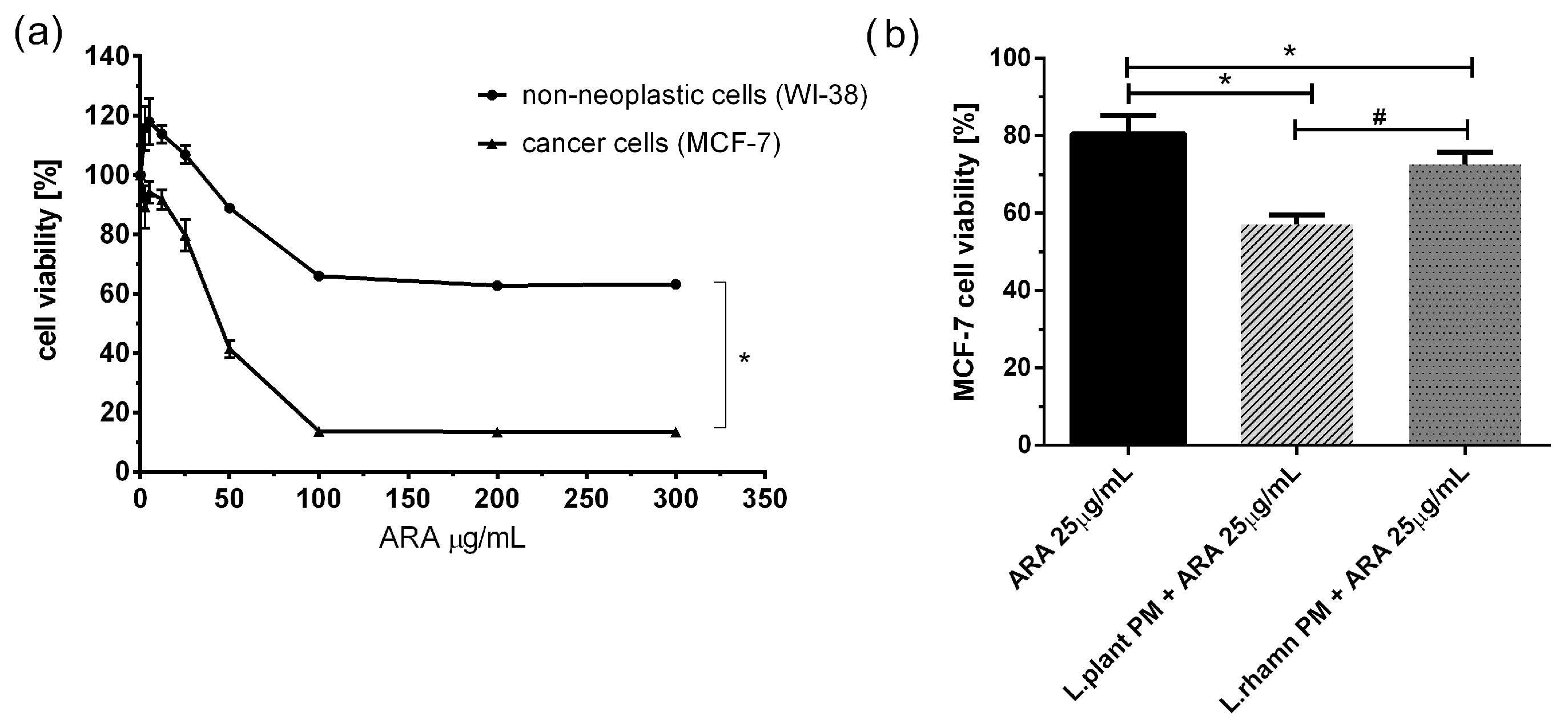
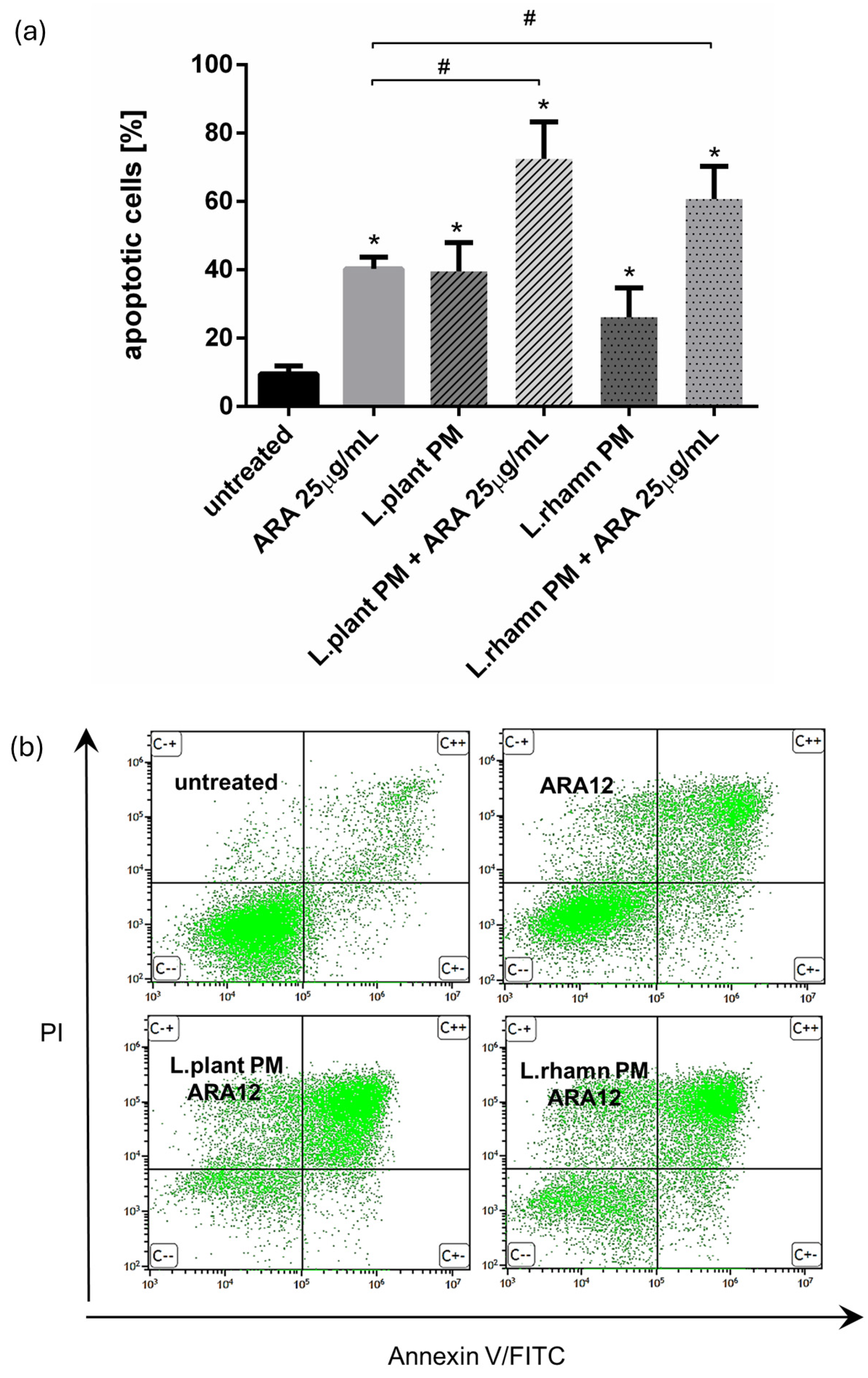
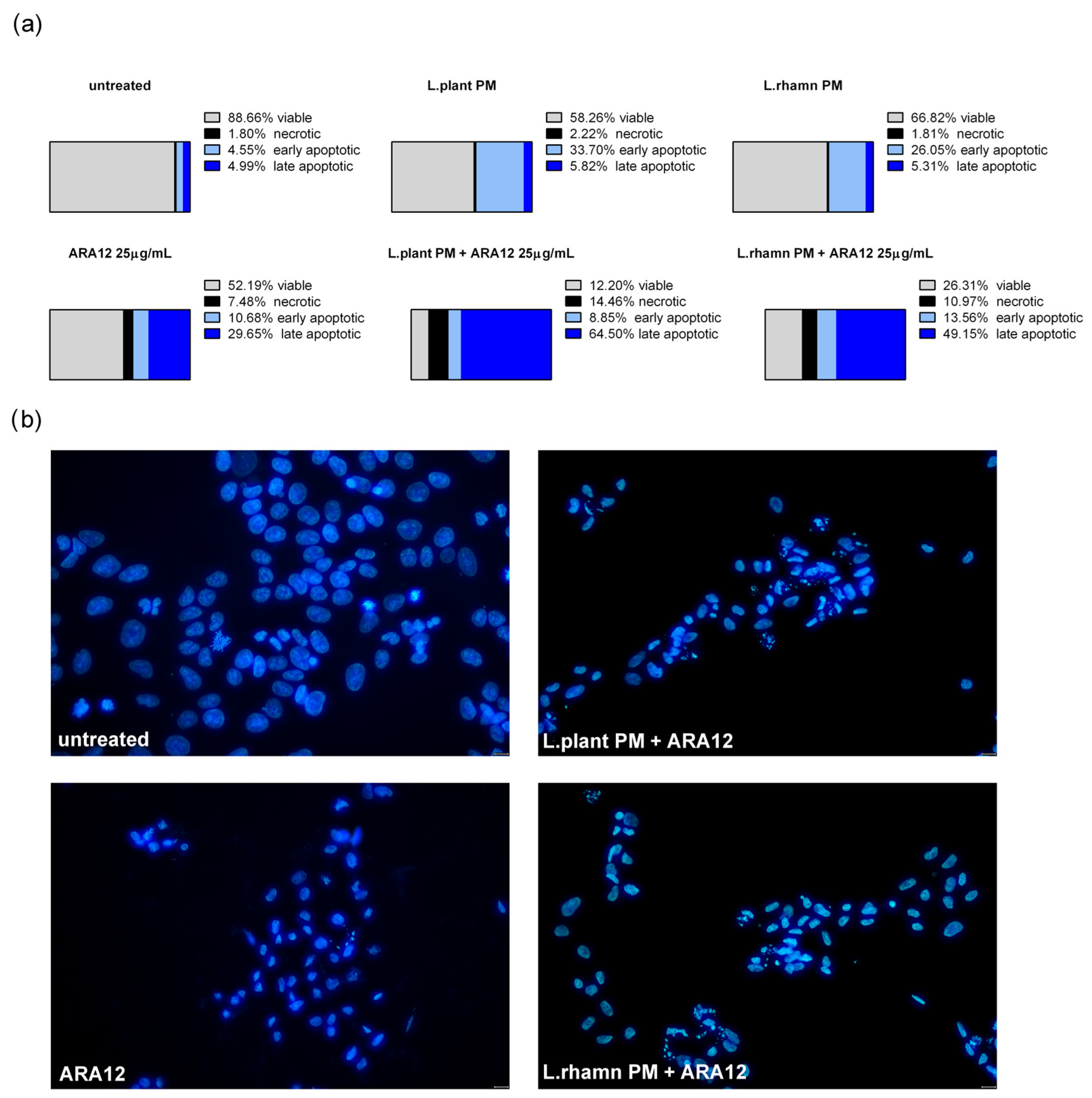
2.2. Antineoplastic Effect of New Candidate Compound ARA12 Is Increased by LAB Postbiotics
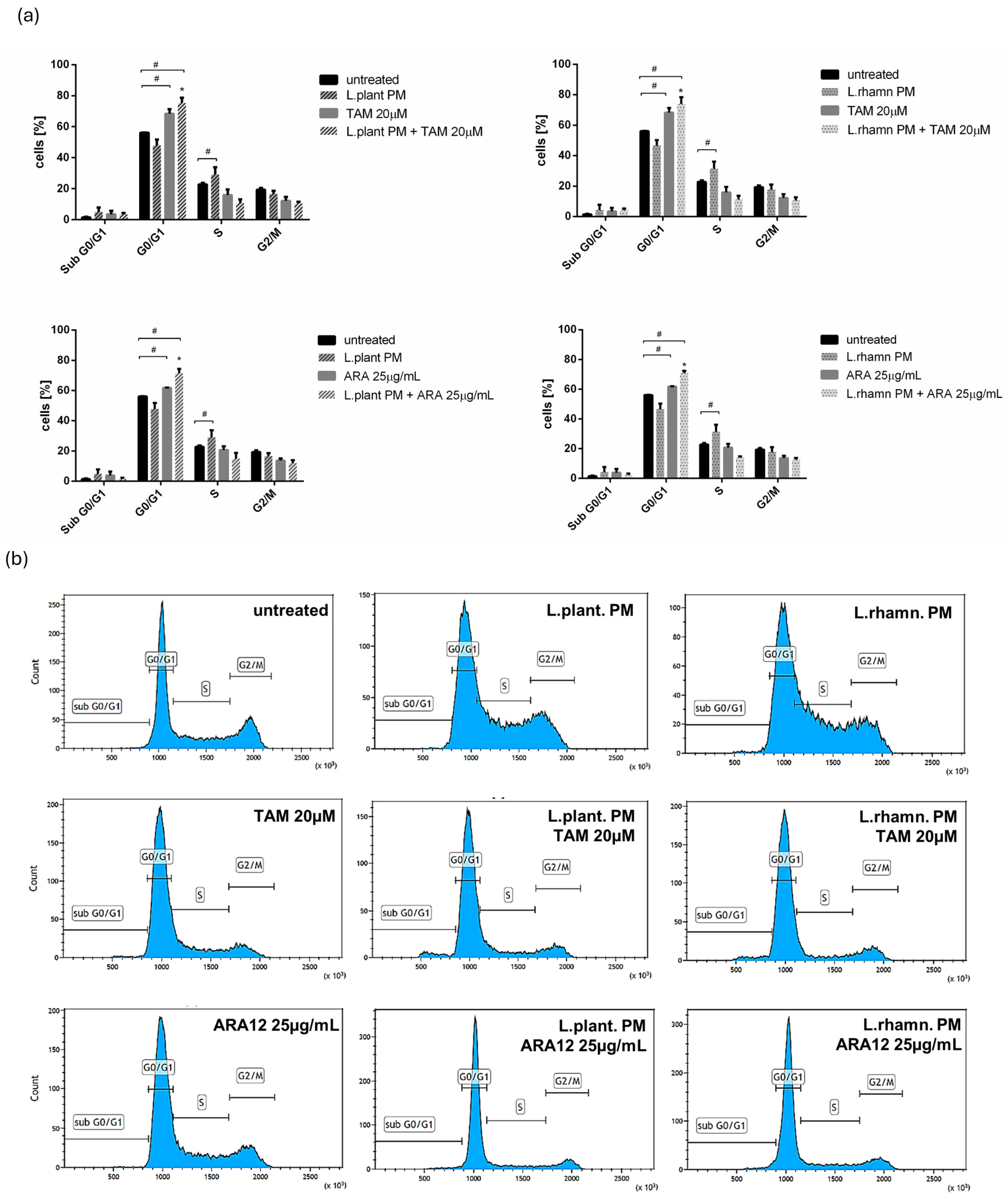
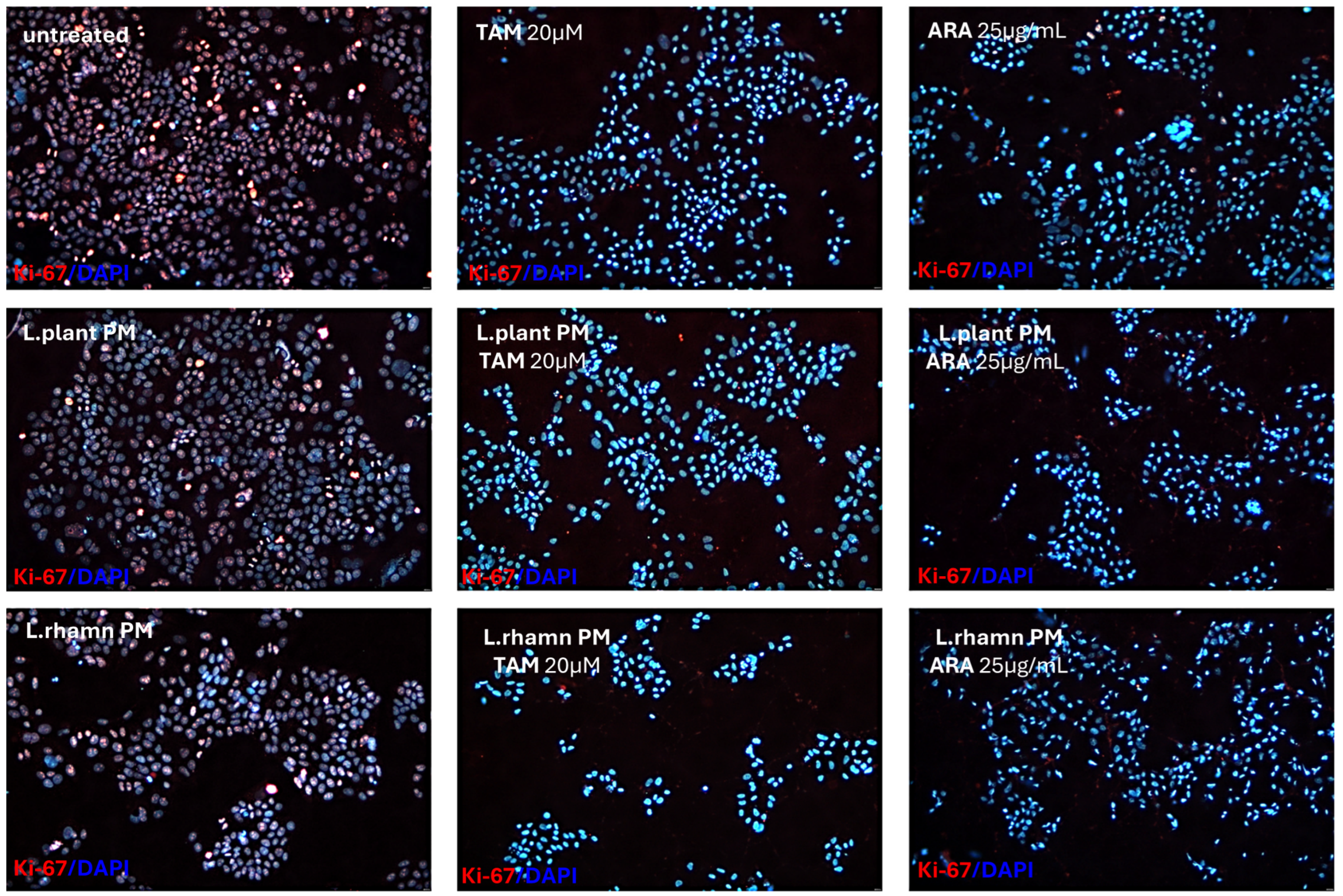
2.3. Influence of LAB-Derived Postbiotics on Cell Cycle and Proliferation of Cancer Cells
3. Discussion
| LAB | Postbiotics | Anticancer Activity | Cancer Cell Line | Ref. |
|---|---|---|---|---|
| L. plantarum, L. brevis, L. casei | Butyric, propionic acid | Induction of apoptosis, decreased cell proliferation, ROS generation | Caco-2 (colorectal cancer) HeLa (cervical cancer) | [24] |
| L. plantarum | Bacteriocin | Decreased cell proliferation | MCF-7 (breast cancer) HT-29 (colorectal cancer) | [7] |
| L. plantarum | Bacteriocin (plantaricin) | Reduction of cancerogenic cell viability | E705 (colorectal cancer) | [25] |
| L. plantarum | Lipoteichoic acid | Anti-inflammatory effect | MDA-MB-231 (breast cancer) | [26] |
| L. plantarum L. rhamnosus L. brevis | Exopolysaccharides | Induction of apoptosis (Bax, Casp-3, Casp-9 overexpression) | HT-29 (colorectal cancer) | [27] |
| L. rhamnosus GG L. casei | Protein, polysaccharide | Anti-inflammatory effect; antimetastasis | HCT-116 c (colorectal cancer) | [28] |
| L. rhamnosus GG | Butyric acid | Inhibition of tumor growth, immunomodulation | Caco-2 (colorectal cancer) | [29] |
| L. acidophilus, L. casei | Polysaccharide fraction | Induction of apoptosis, antioxidative activity | HT-29 (colorectal cancer) | [30] |
4. Materials and Methods
4.1. Bacterial Culture and Cell-Free Supernatant Preparation
4.2. Cell Culture
4.3. Natural and Synthetic Compounds
4.4. Cell Viability Assay
4.5. Annexin V/PI Staining for Cell Death Assay
4.6. Cell Cycle Analysis
4.7. Ki-67 Immunofluorescence Detection
4.8. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chang, M.-S. Tamoxifen Resistance in Breast Cancer. Biomol. Ther. 2012, 20, 256–267. [Google Scholar] [CrossRef] [PubMed]
- Yen, C.; Zhao, F.; Yu, Z.; Zhu, X.; Li, C.G. Interactions Between Natural Products and Tamoxifen in Breast Cancer: A Comprehensive Literature Review. Front. Pharmacol. 2022, 13, 847113. [Google Scholar] [CrossRef]
- Sabahi, S.; Homayouni Rad, A.; Aghebati-Maleki, L.; Sangtarash, N.; Ozma, M.A.; Karimi, A.; Hosseini, H.; Abbasi, A. Postbiotics as the New Frontier in Food and Pharmaceutical Research. Crit. Rev. Food Sci. Nutr. 2023, 63, 8375–8402. [Google Scholar] [CrossRef] [PubMed]
- Kunisawa, J. Future Prospects for Food Research in the Post-Microbiome Era. J. Nutr. Sci. Vitaminol. 2022, 68, S23–S25. [Google Scholar] [CrossRef] [PubMed]
- Scott, E.; De Paepe, K.; Van de Wiele, T. Postbiotics and Their Health Modulatory Biomolecules. Biomolecules 2022, 12, 1640. [Google Scholar] [CrossRef] [PubMed]
- Lu, K.; Dong, S.; Wu, X.; Jin, R.; Chen, H. Probiotics in Cancer. Front. Oncol. 2021, 11, 638148. [Google Scholar] [CrossRef]
- Chuah, L.-O.; Foo, H.L.; Loh, T.C.; Mohammed Alitheen, N.B.; Yeap, S.K.; Abdul Mutalib, N.E.; Abdul Rahim, R.; Yusoff, K. Postbiotic Metabolites Produced by Lactobacillus Plantarum Strains Exert Selective Cytotoxicity Effects on Cancer Cells. BMC Complement. Altern. Med. 2019, 19, 114. [Google Scholar] [CrossRef] [PubMed]
- Nordström, E.A.; Teixeira, C.; Montelius, C.; Jeppsson, B.; Larsson, N. Lactiplantibacillus Plantarum 299v (LP299V®): Three Decades of Research. Benef. Microbes 2021, 12, 441–465. [Google Scholar] [CrossRef] [PubMed]
- Capurso, L. Thirty Years of Lactobacillus Rhamnosus GG: A Review. J. Clin. Gastroenterol. 2019, 53 (Suppl. S1), S1–S41. [Google Scholar] [CrossRef]
- Salemi, R.; Vivarelli, S.; Ricci, D.; Scillato, M.; Santagati, M.; Gattuso, G.; Falzone, L.; Libra, M. Lactobacillus Rhamnosus GG Cell-Free Supernatant as a Novel Anti-Cancer Adjuvant. J. Transl. Med. 2023, 21, 195. [Google Scholar] [CrossRef]
- Witusik-Perkowska, M.; Głowacka, P.; Pieczonka, A.M.; Świderska, E.; Pudlarz, A.; Rachwalski, M.; Szymańska, J.; Zakrzewska, M.; Jaskólski, D.J.; Szemraj, J. Autophagy Inhibition with Chloroquine Increased Pro-Apoptotic Potential of New Aziridine-Hydrazide Hydrazone Derivatives against Glioblastoma Cells. Cells 2023, 12, 1906. [Google Scholar] [CrossRef]
- Sui, Y.; Wu, J.; Chen, J. The Role of Gut Microbial β-Glucuronidase in Estrogen Reactivation and Breast Cancer. Front. Cell Dev. Biol. 2021, 9, 631552. [Google Scholar] [CrossRef] [PubMed]
- Yazdi, M.H.; Dallal, M.M.S.; Hassan, Z.M.; Holakuyee, M.; Amiri, S.A.; Abolhassani, M.; Mahdavi, M. Oral Administration of Lactobacillus Acidophilus Induces IL-12 Production in Spleen Cell Culture of BALB/c Mice Bearing Transplanted Breast Tumour. Br. J. Nutr. 2010, 104, 227–232. [Google Scholar] [CrossRef] [PubMed]
- Fernández, M.F.; Reina-Pérez, I.; Astorga, J.M.; Rodríguez-Carrillo, A.; Plaza-Díaz, J.; Fontana, L. Breast Cancer and Its Relationship with the Microbiota. Int. J. Environ. Res. Public Health 2018, 15, 1747. [Google Scholar] [CrossRef]
- Donnet-Hughes, A.; Perez, P.F.; Doré, J.; Leclerc, M.; Levenez, F.; Benyacoub, J.; Serrant, P.; Segura-Roggero, I.; Schiffrin, E.J. Potential Role of the Intestinal Microbiota of the Mother in Neonatal Immune Education. Proc. Nutr. Soc. 2010, 69, 407–415. [Google Scholar] [CrossRef] [PubMed]
- Mendoza, L. Potential Effect of Probiotics in the Treatment of Breast Cancer. Oncol. Rev. 2019, 13, 422. [Google Scholar] [CrossRef] [PubMed]
- De Moreno de LeBlanc, A.; Matar, C.; Thériault, C.; Perdigón, G. Effects of Milk Fermented by Lactobacillus Helveticus R389 on Immune Cells Associated to Mammary Glands in Normal and a Breast Cancer Model. Immunobiology 2005, 210, 349–358. [Google Scholar] [CrossRef]
- Asseri, A.H.; Bakhsh, T.; Abuzahrah, S.S.; Ali, S.; Rather, I.A. The Gut Dysbiosis-Cancer Axis: Illuminating Novel Insights and Implications for Clinical Practice. Front. Pharmacol. 2023, 14, 1208044. [Google Scholar] [CrossRef]
- Li, H.; Gao, X.; Chen, Y.; Wang, M.; Xu, C.; Yu, Q.; Jin, Y.; Song, J.; Zhu, Q. Potential Risk of Tamoxifen: Gut Microbiota and Inflammation in Mice with Breast Cancer. Front. Oncol. 2023, 13, 1121471. [Google Scholar] [CrossRef]
- Diot, C.; García-González, A.P.; Vieira, A.F.; Walker, M.; Honeywell, M.; Doyle, H.; Ponomarova, O.; Rivera, Y.; Na, H.; Zhang, H.; et al. Bacterial Diet Modulates Tamoxifen-Induced Death via Host Fatty Acid Metabolism. Nat. Commun. 2022, 13, 5595. [Google Scholar] [CrossRef]
- Garbacz, K. Anticancer Activity of Lactic Acid Bacteria. Semin. Cancer Biol. 2022, 86, 356–366. [Google Scholar] [CrossRef] [PubMed]
- Bedada, T.L.; Feto, T.K.; Awoke, K.S.; Garedew, A.D.; Yifat, F.T.; Birri, D.J. Probiotics for Cancer Alternative Prevention and Treatment. Biomed. Pharmacother. 2020, 129, 110409. [Google Scholar] [CrossRef] [PubMed]
- Górska, A.; Przystupski, D.; Niemczura, M.J.; Kulbacka, J. Probiotic Bacteria: A Promising Tool in Cancer Prevention and Therapy. Curr. Microbiol. 2019, 76, 939–949. [Google Scholar] [CrossRef] [PubMed]
- Nowak, A.; Zakłos-Szyda, M.; Rosicka-Kaczmarek, J.; Motyl, I. Anticancer Potential of Post-Fermentation Media and Cell Extracts of Probiotic Strains: An In Vitro Study. Cancers 2022, 14, 1853. [Google Scholar] [CrossRef] [PubMed]
- De Giani, A.; Bovio, F.; Forcella, M.; Fusi, P.; Sello, G.; Di Gennaro, P. Identification of a Bacteriocin-like Compound from Lactobacillus Plantarum with Antimicrobial Activity and Effects on Normal and Cancerogenic Human Intestinal Cells. AMB Express 2019, 9, 88. [Google Scholar] [CrossRef] [PubMed]
- Ahn, J.E.; Kim, H.; Chung, D.K. Lipoteichoic Acid Isolated from Lactobacillus Plantarum Maintains Inflammatory Homeostasis through Regulation of Th1- and Th2-Induced Cytokines. J. Microbiol. Biotechnol. 2019, 29, 151–159. [Google Scholar] [CrossRef] [PubMed]
- Tukenmez, U.; Aktas, B.; Aslim, B.; Yavuz, S. The Relationship between the Structural Characteristics of Lactobacilli-EPS and Its Ability to Induce Apoptosis in Colon Cancer Cells in Vitro. Sci. Rep. 2019, 9, 8268. [Google Scholar] [CrossRef] [PubMed]
- Escamilla, J.; Lane, M.A.; Maitin, V. Cell-Free Supernatants from Probiotic Lactobacillus Casei and Lactobacillus Rhamnosus GG Decrease Colon Cancer Cell Invasion in Vitro. Nutr. Cancer 2012, 64, 871–878. [Google Scholar] [CrossRef] [PubMed]
- Thananimit, S.; Pahumunto, N.; Teanpaisan, R. Characterization of Short Chain Fatty Acids Produced by Selected Potential Probiotic Lactobacillus Strains. Biomolecules 2022, 12, 1829. [Google Scholar] [CrossRef]
- Choi, S.S.; Kim, Y.; Han, K.S.; You, S.; Oh, S.; Kim, S.H. Effects of Lactobacillus Strains on Cancer Cell Proliferation and Oxidative Stress in Vitro. Lett. Appl. Microbiol. 2006, 42, 452–458. [Google Scholar] [CrossRef]
- Magryś, A.; Pawlik, M. Postbiotic Fractions of Probiotics Lactobacillus Plantarum 299v and Lactobacillus Rhamnosus GG Show Immune-Modulating Effects. Cells 2023, 12, 2538. [Google Scholar] [CrossRef]
- Fattahi, Y.; Heidari, H.R.; Khosroushahi, A.Y. Review of Short-Chain Fatty Acids Effects on the Immune System and Cancer. Food Biosci. 2020, 38, 100793. [Google Scholar] [CrossRef]
- Licciardi, P.V.; Ververis, K.; Karagiannis, T.C. Histone Deacetylase Inhibition and Dietary Short-Chain Fatty Acids. ISRN Allergy 2011, 2011, 869647. [Google Scholar] [CrossRef]
- Pattayil, L.; Balakrishnan-Saraswathi, H.-T. In Vitro Evaluation of Apoptotic Induction of Butyric Acid Derivatives in Colorectal Carcinoma Cells. Anticancer Res. 2019, 39, 3795–3801. [Google Scholar] [CrossRef] [PubMed]
- Son, M.-Y.; Cho, H.-S. Anticancer Effects of Gut Microbiota-Derived Short-Chain Fatty Acids in Cancers. J. Microbiol. Biotechnol. 2023, 33, 849–856. [Google Scholar] [CrossRef]
- Kaur, S.; Kaur, S. Bacteriocins as Potential Anticancer Agents. Front. Pharmacol. 2015, 6, 272. [Google Scholar] [CrossRef]
- El-Deeb, N.M.; Yassin, A.M.; Al-Madboly, L.A.; El-Hawiet, A. A Novel Purified Lactobacillus Acidophilus 20079 Exopolysaccharide, LA-EPS-20079, Molecularly Regulates Both Apoptotic and NF-κB Inflammatory Pathways in Human Colon Cancer. Microb. Cell Fact. 2018, 17, 29. [Google Scholar] [CrossRef] [PubMed]
- Rad, A.H.; Maleki, L.A.; Kafil, H.S.; Zavoshti, H.F.; Abbasi, A. Postbiotics as Promising Tools for Cancer Adjuvant Therapy. Adv. Pharm. Bull. 2021, 11, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Shi, X.; Xu, Y.; Wan, J.; Wei, S.; Zhu, R. Tamoxifen Promotes Apoptosis and Inhibits Invasion in Estrogen-positive Breast Cancer MCF-7 Cells. Mol. Med. Rep. 2017, 16, 478–484. [Google Scholar] [CrossRef]
- Ziółkowska, S.; Czarny, P.; Szemraj, J.; (Department of Medical Biochemistry, Medical University of Lodz, 92-215 Lodz, Poland); Kołodziej, Ł.; (Laboratory of Medical Genetics, Faculty of Biology and Environmental Protection, University of Lodz, 90-236 Lodz, Poland; Bio-Med-Chem Doctoral School of University, University of Lodz, 90-236 Lodz, Poland); Pieczonka, A.; (Department of Organic and Applied Chemistry, Faculty of Chemistry, University of Lodz, Tamka 12, 91-403 Lodz, Poland). Manuscript in preparation.
- Da Silva Sabo, S.; Vitolo, M.; González, J.M.D.; Oliveira, R.P.d.S. Overview of Lactobacillus Plantarum as a Promising Bacteriocin Producer among Lactic Acid Bacteria. Food Res. Int. 2014, 64, 527–536. [Google Scholar] [CrossRef]
- Goh, K.; Ng, Z.J.; Halim, M.; Oslan, S.; Oslan, S.N.; Tan, J.S. A Comprehensive Review on the Anticancer Potential of Bacteriocin: Preclinical and Clinical Studies. Int. J. Pept. Res. Ther. 2022, 28, 75. [Google Scholar] [CrossRef]
- Molujin, A.M.; Abbasiliasi, S.; Nurdin, A.; Lee, P.-C.; Gansau, J.A.; Jawan, R. Bacteriocins as Potential Therapeutic Approaches in the Treatment of Various Cancers: A Review of In Vitro Studies. Cancers 2022, 14, 4758. [Google Scholar] [CrossRef] [PubMed]
- Chakrobarty, S.; Garai, S.; Ghosh, A.; Mukerjee, N.; Das, D. Bioactive Plantaricins as Potent Anti-Cancer Drug Candidates: Double Docking, Molecular Dynamics Simulation and in Vitro Cytotoxicity Analysis. J. Biomol. Struct. Dyn. 2023, 41, 13605–13615. [Google Scholar] [CrossRef]
- Avand, A.; Akbari, V.; Shafizadegan, S. In Vitro Cytotoxic Activity of a Lactococcus Lactis Antimicrobial Peptide Against Breast Cancer Cells. Iran. J. Biotechnol. 2018, 16, e1867. [Google Scholar] [CrossRef]
- Hu, J.; Wang, C.; Ye, L.; Yang, W.; Huang, H.; Meng, F.; Shi, S.; Ding, Z. Anti-Tumour Immune Effect of Oral Administration of Lactobacillus Plantarum to CT26 Tumour-Bearing Mice. J. Biosci. 2015, 40, 269–279. [Google Scholar] [CrossRef] [PubMed]
- Mirzadeh, M.A.; Eslami, M.; Ghanbari, A.; Zarbakhsh, S.; Yosefi, S.; Pakdel, A. Coadministration of Doxorubicin with Vitamin D3, Lactobacillus Acidophilus, and Lactobacillus Casei in the 4T1 Mouse Model of Breast Cancer: Anticancer and Enteroprotective Effects. Med. Oncol. 2024, 41, 111. [Google Scholar] [CrossRef]
- Hashemi-Khah, M.-S.; Arbab-Soleimani, N.; Forghanifard, M.-M.; Gholami, O.; Taheri, S.; Amoueian, S. An In Vivo Study of Lactobacillus Rhamnosus (PTCC 1637) as a New Therapeutic Candidate in Esophageal Cancer. BioMed Res. Int. 2022, 2022, 7607470. [Google Scholar] [CrossRef] [PubMed]
- Mokriani, S.; Tukmechi, A.; Harzandi, N.; Jabalameli, L. In Vivo Murine Breast Cancer Targeting by Magnetic Iron Nanoparticles Involving, L. GG Cytoplasmic Fraction. Iran. J. Basic Med. Sci. 2021, 24, 682–689. [Google Scholar] [CrossRef]
- Rana, K.; Sharma, R.; Preet, S. Augmented Therapeutic Efficacy of 5-Fluorouracil in Conjunction with Lantibiotic Nisin against Skin Cancer. Biochem. Biophys. Res. Commun. 2019, 520, 551–559. [Google Scholar] [CrossRef]
- Rana, K.; Pandey, S.K.; Chauhan, S.; Preet, S. Anticancer Therapeutic Potential of 5-Fluorouracil and Nisin Co-Loaded Chitosan Coated Silver Nanoparticles against Murine Skin Cancer. Int. J. Pharm. 2022, 620, 121744. [Google Scholar] [CrossRef]
- Fu, W.; Kapila, Y.L. Nisin, A Probiotic Bacteriocin, Combined with Limited Chemoradiation Therapy in Head and Neck Cancer. Arch. Clin. Med. Case Rep. 2021, 5, 531–536. [Google Scholar] [CrossRef]
- Al-Qadami, G.H.; Secombe, K.R.; Subramaniam, C.B.; Wardill, H.R.; Bowen, J.M. Gut Microbiota-Derived Short-Chain Fatty Acids: Impact on Cancer Treatment Response and Toxicities. Microorganisms 2022, 10, 2048. [Google Scholar] [CrossRef] [PubMed]
- Nasiri, Z.; Montazeri, H.; Akbari, N.; Mirfazli, S.S.; Tarighi, P. Synergistic Cytotoxic and Apoptotic Effects of Local Probiotic Lactobacillus Brevis Isolated from Regional Dairy Products in Combination with Tamoxifen. Nutr. Cancer 2021, 73, 290–299. [Google Scholar] [CrossRef] [PubMed]
- Gurunathan, S.; Thangaraj, P.; Kim, J.-H. Postbiotics: Functional Food Materials and Therapeutic Agents for Cancer, Diabetes, and Inflammatory Diseases. Foods 2024, 13, 89. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wasiak, J.; Głowacka, P.; Pudlarz, A.; Pieczonka, A.M.; Dzitko, K.; Szemraj, J.; Witusik-Perkowska, M. Lactic Acid Bacteria-Derived Postbiotics as Adjunctive Agents in Breast Cancer Treatment to Boost the Antineoplastic Effect of a Conventional Therapeutic Comprising Tamoxifen and a New Drug Candidate: An Aziridine–Hydrazide Hydrazone Derivative. Molecules 2024, 29, 2292. https://doi.org/10.3390/molecules29102292
Wasiak J, Głowacka P, Pudlarz A, Pieczonka AM, Dzitko K, Szemraj J, Witusik-Perkowska M. Lactic Acid Bacteria-Derived Postbiotics as Adjunctive Agents in Breast Cancer Treatment to Boost the Antineoplastic Effect of a Conventional Therapeutic Comprising Tamoxifen and a New Drug Candidate: An Aziridine–Hydrazide Hydrazone Derivative. Molecules. 2024; 29(10):2292. https://doi.org/10.3390/molecules29102292
Chicago/Turabian StyleWasiak, Joanna, Pola Głowacka, Agnieszka Pudlarz, Adam M. Pieczonka, Katarzyna Dzitko, Janusz Szemraj, and Monika Witusik-Perkowska. 2024. "Lactic Acid Bacteria-Derived Postbiotics as Adjunctive Agents in Breast Cancer Treatment to Boost the Antineoplastic Effect of a Conventional Therapeutic Comprising Tamoxifen and a New Drug Candidate: An Aziridine–Hydrazide Hydrazone Derivative" Molecules 29, no. 10: 2292. https://doi.org/10.3390/molecules29102292
APA StyleWasiak, J., Głowacka, P., Pudlarz, A., Pieczonka, A. M., Dzitko, K., Szemraj, J., & Witusik-Perkowska, M. (2024). Lactic Acid Bacteria-Derived Postbiotics as Adjunctive Agents in Breast Cancer Treatment to Boost the Antineoplastic Effect of a Conventional Therapeutic Comprising Tamoxifen and a New Drug Candidate: An Aziridine–Hydrazide Hydrazone Derivative. Molecules, 29(10), 2292. https://doi.org/10.3390/molecules29102292







