Synthesis, Characterization and Antimicrobial and Anticancer Evaluations of Some Novel Heteroannulated Difuro[3,2-c:3′,2′-g]Chromenes
Abstract
1. Introduction
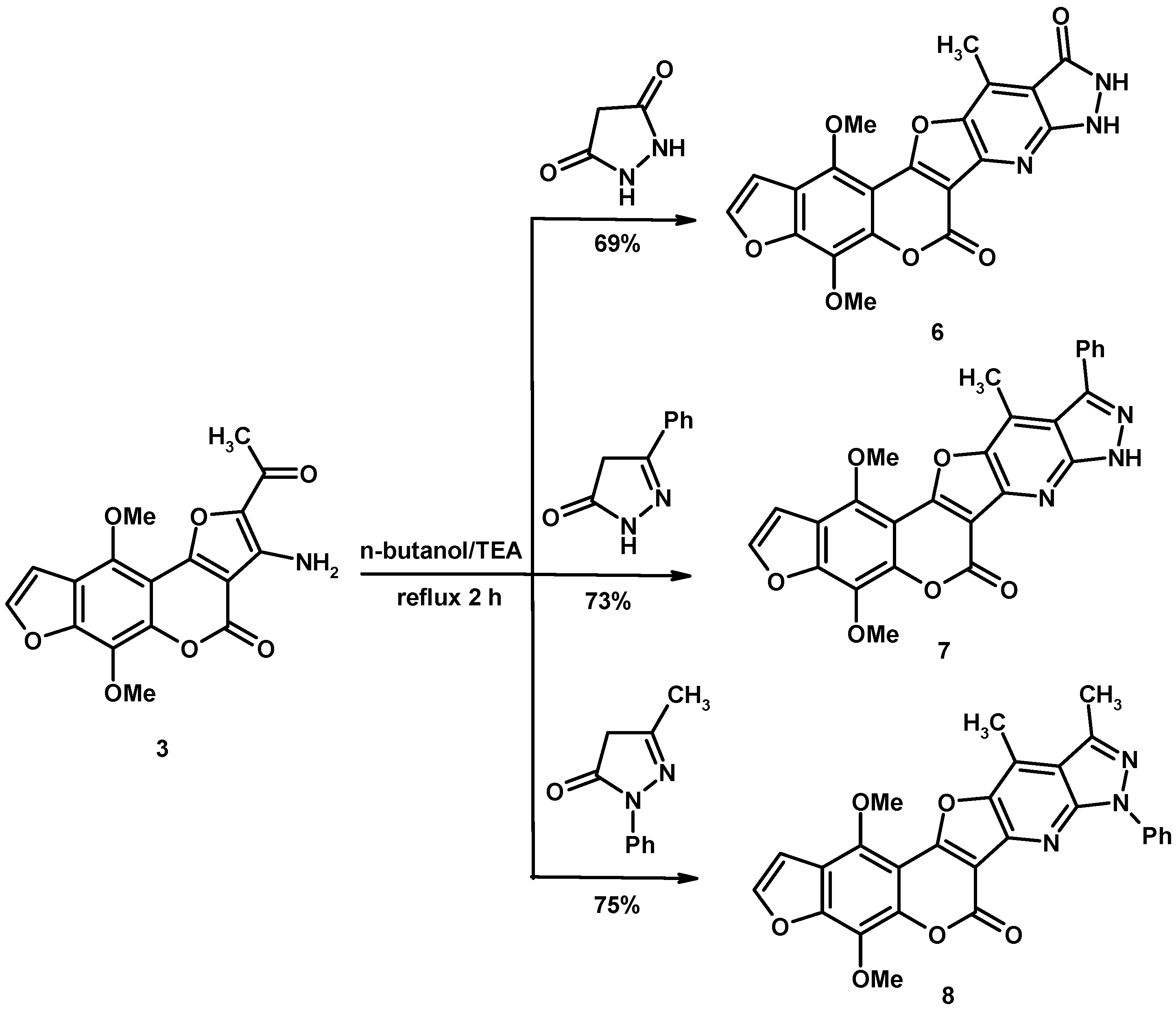
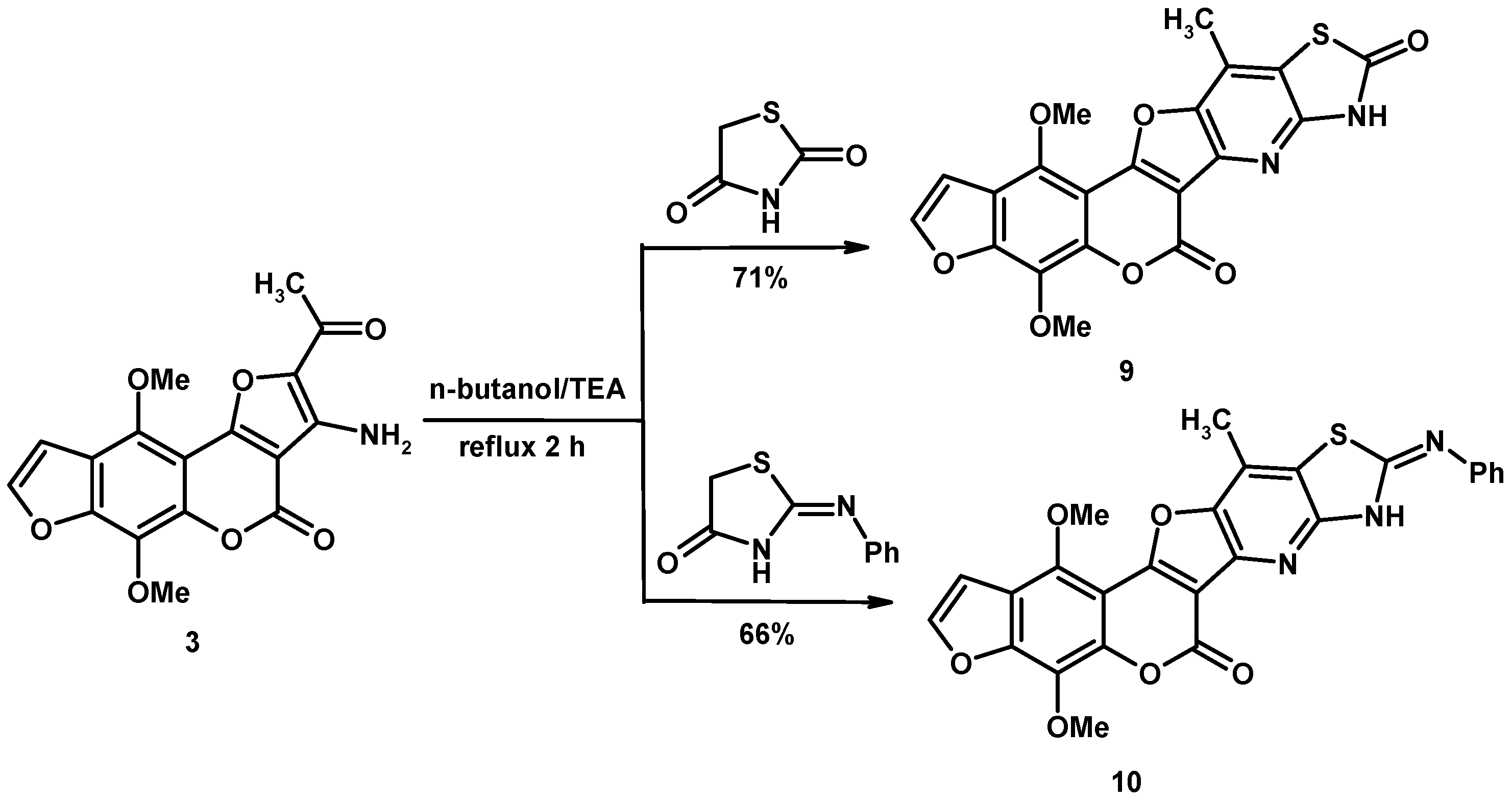
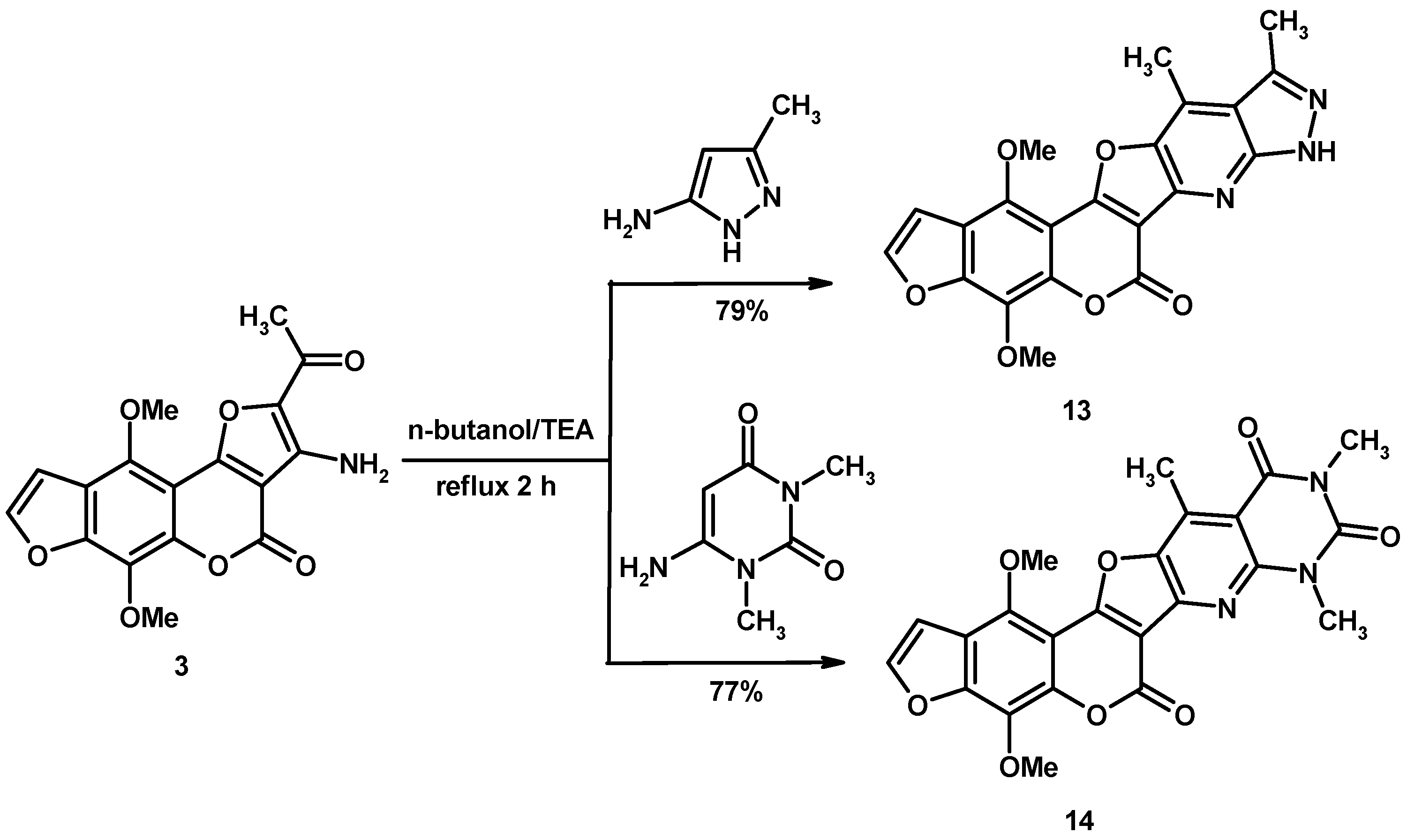
2. Results and Discussion
2.1. Characterization of the Synthesized Compounds
2.2. Antimicrobial Evaluation
2.3. Anticancer Evaluation
3. Materials and Methods
3.1. General Information
3.2. General Procedure for Synthesis of Heteroannulated Compounds 4–14
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Balbaa, S.I.; Zaki, A.Y.; Abdel-Wahab, S.M. A micro-method for the estimation of khellin in presence of other constituents of Ammi visnaga fruits. Planta Med. 1968, 16, 329–334. [Google Scholar] [CrossRef] [PubMed]
- Adımcılar, V.; Beyazit, N.; Erim, F.B. Khellin and visnagin in different organs of Ammi visnaga and Ammi majus. Nat. Prod. Res. 2023, 37, 164–166. [Google Scholar] [CrossRef] [PubMed]
- Abu-Hashem, A.A.; El-Shazly, M. Synthesis, reactions and biological activities of furochromones: A review. Eur. J. Med. Chem. 2015, 90, 633–665. [Google Scholar] [CrossRef] [PubMed]
- Vanachayangkul, P.; Byer, K.; Khan, S.; Butterweck, V. An aqueous extract of Ammi visnaga fruits and its constituents khellin and visnagin prevent cell damage caused by oxalate in renal epithelial cells. Phytomedicine 2010, 17, 653–658. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Aal, E.A.; Daosukho, S.; El-Shall, H. Effect of supersaturation ratio and Khella extract on nucleation and morphology of kidney stones. J. Cryst. Growth 2009, 311, 2673–2681. [Google Scholar] [CrossRef]
- Abu Shady, H.A.; Hassib, S.T.; Mikhael, A.N.; El Ansary, S.L. Synthesis of some chromones and angular furochromones of expected pharmacological activities. Egypt J. Pharm. Sci. 1988, 29, 393–407. [Google Scholar]
- Anrep, G.V.; Kenawy, M.R.; Barsoum, G.S. The coronary vasodilator action of khellin. Am. Heart J. 1949, 37, 531–542. [Google Scholar] [CrossRef] [PubMed]
- Mgamat, F.; Benaddou, M.; Haida, S.; Oubihi, A.; El Ibaoui, H.; Zouhair, R.; Kribii, A.; El Ayadi, R.; Hmoun, D. Phytochemical evaluation, antioxidant, and antibacterial activity of extracts of Ammi visnaga Lam. Chem. Data Collect. 2023, 48, 101096. [Google Scholar] [CrossRef]
- Askari, V.R.; Najafi, Z.; Rahimi, V.B.; Boskabady, M.H. Comparative study on the impacts of visnagin and its methoxy derivative khellin on human lymphocyte proliferation and Th1/Th2 balance. Pharmacol. Rep. 2023, 75, 411–422. [Google Scholar] [CrossRef]
- Abu-Hashem, A.A.; Youssef, M.M. Synthesis of new visnagen and khellin furochromone pyrimidine derivatives and their anti-Inflammatory and analgesic activity. Molecules 2011, 16, 1956–1972. [Google Scholar] [CrossRef]
- Harb, N.; Sarhan, A.G.; El Dougdoug, K.A.; Gomaa, H.H.A. Ammi-visnaga extract; a novel phyto-antiviral agent against bovine rotavirus. Virus Disease 2023, 34, 76–87. [Google Scholar] [CrossRef] [PubMed]
- Ragab, F.A.; El-Sayed, N.A.; Eissa, A.A.M.; El Kerdawy, A.M. Synthesis and anticonvulsant activity of certain substituted furochromone, benzofuran and flavone derivatives. Chem. Pharm. Bull. 2010, 58, 1148–1156. [Google Scholar] [CrossRef] [PubMed]
- Trabalzini, L.; Martelli, P.; Bovalini, L.; Dall’Acqua, F.; Sage, E. Photosensitization of DNA of defined sequence by furochromones, khellin and visnagin. J. Photochem. Photobiol. B 1990, 7, 317–336. [Google Scholar] [CrossRef] [PubMed]
- Ez-zahir, A.; Lahna, A.; Marnissi, F.; Oudghiri, M.; Naya, A. Immuno-Modulatory, Anti-Psoriatic Effects and Furanochromone (Khellin and Visnagin) Contents of Ammi visnaga (L.) Hydroethanolic extract. Biomed. Pharmac. J. 2022, 15, 1623–1635. [Google Scholar] [CrossRef]
- Esentürk-Güzel, I.; Topuzoğlu, S.; Abdo, L.; Gürer, E.S.; Yapar, E.A. Ammi visnaga L. and Nanocarrier Approaches in the Treatment of Skin Diseases. J. Res. Pharm. 2022, 26, 820–827. [Google Scholar] [CrossRef]
- Zaher, A.; Aslam, R.; Lee, H.-S.; Khafouri, A.; Boufellous, M.; Alrashdi, A.A.; El aoufir, Y.; Lgaz, H.; Ouhssine, M. A combined computational & electrochemical exploration of the Ammi visnaga L. extract as a green corrosion inhibitor for carbon steel in HCl solution. Arab. J. Chem. 2022, 15, 103573. [Google Scholar] [CrossRef]
- Abdel Halim, S.; Ibrahim, M.A. Quantum computational, Spectroscopic investigations on benzofuranylcarbonylpyrazolopyridine by DFT/TD-DFT: Synthesis, Structure, NBO and NLO research. J. Mol. Struct. 2023, 1293, 136201. [Google Scholar] [CrossRef]
- Ibrahim, M.A.; Badran, A.; El-Gohary, N.M.; Abdel-fatah, N.A. Chemical reactions of furochromones, visnagin and khellin. Heterocycles 2017, 94, 389–440. [Google Scholar] [CrossRef]
- Ibrahim, M.A.; Al-Harbi, S.A.; Allehyani, E.S. Chemical transformations with 4,9-dimethoxy-5-oxo-5H-furo[3,2-g] chromene-6-carbonitrile: Construction and antimicrobial evaluation of the novel heteroannulated furochromenopyridines. Heterocycles 2020, 100, 1172–1188. [Google Scholar] [CrossRef]
- Jadhav, G.R.; Medhane, V.J.; Deshmukh, D.G.; Gaikwad, S.S. New synthetic strategy for Friedlander condensation of 4-amino-2-oxo-2H-chromene-3-carbaldehyde by heterogeneous catalysis. J. Heterocycl. Chem. 2021, 58, 1775–1783. [Google Scholar] [CrossRef]
- Ghosh, S.; Krishnan, J.; Karthik, V.; Rana, A.; Dhakshinamoorthy, A.; Biswas, S. Friedlander condensation reaction catalyzed by hafnium-based metal-organic framework. Mol. Catal. 2022, 533, 112748. [Google Scholar] [CrossRef]
- Kargar, H.; Fallah-Mehrjardi, M.; Munawar, K.S. Dioxovanadium(V) Complex Incorporating Tridentate ONO Donor Aminobenzohydrazone Ligand: Synthesis, Spectral Characterization and Application as a Homogeneous Lewis Acid Catalyst in the Friedländer Synthesis of Substituted Quinolines. Polycycl. Aromat. Compd. 2022, 42, 6485–6500. [Google Scholar] [CrossRef]
- El-Gohary, N.M.; Ibrahim, M.A.; El-Sawy, E.R.; Abdel-fatah, N.A. Chemical reactivity of 4,9-dimethoxy-5-oxo-5H-furo[3,2-g] chromene-6-carboxaldehyde toward some nucleophilic reagents. J. Heterocycl. Chem. 2017, 54, 1467–1478. [Google Scholar] [CrossRef]
- Allehyani, E.S. Design, characterization and antimicrobial efficiency of novel annulated furo[3″,2″:6′,7′]chromeno[3′,4′:4,5] furo[3,2-b]pyridines. Polycycl. Arom. Compds. 2023, in press. [CrossRef]
- Irgashev, R.A.; Demina, N.S.; Kazin, N.A.; Rusinov, G.L. Construction of new heteroacenes based on benzo[b]thieno [2,3-d] thiophene/quinoline or 1,8-naphthyridine systems using the Friedländer reaction. Tetrahedron Lett. 2019, 60, 1135–1138. [Google Scholar] [CrossRef]
- Hussain, Z.; Ibrahim, M.A.; El-Gohary, N.M.; Badran, A. Synthesis, characterization, DFT, QSAR, antimicrobial, and antitumor studies of some novel pyridopyrimidines. J. Mol. Struct. 2022, 1269, 133870. [Google Scholar] [CrossRef]
- Gould, J.C.; Bowie, J.M. The Determination of Bacterial Sensitivity to Antibiotics. Edinb. Med. J. 1952, 59, 178–199. [Google Scholar] [PubMed]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]



| No. | Zone Diameter (mm) * | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Gram-Positive Bacteria | Gram-Negative Bacteria | Yeasts and Fungi | ||||||||||
| Compd. No. | Staphylococcus aureus | Bacillus subtilis | Salmonella typhimurium | Escherichia coli | Candida albicans | Asperigillus fumigatus | ||||||
| 1000 μg/mL | 500 μg/mL | 1000 μg/mL | 500 μg/mL | 1000 μg/mL | 500 μg/mL | 1000 μg/mL | 500 μg/mL | 1000 μg/mL | 500 μg/mL | 1000 μg/mL | 500 μg/mL | |
| 3 | 26 H | 19 H | 24 H | 18 H | 26 H | 19 H | 28 H | 20 H | 27 H | 22 H | 27 H | 18 H |
| 4 | 18 I | 12 I | 21 I | 14 I | 19 I | 14 I | 17 I | 13 I | 15 I | 12 I | 19 I | 14 I |
| 5 | 17 I | 13 I | 17 I | 11 I | 14 I | 10 I | 15 I | 11 I | 18 I | 15 I | 18 I | 12 I |
| 6 | 16 I | 10 I | 15 I | 12 I | 27 H | 21 H | 26 H | 18 H | 27 H | 21 H | 26 H | 19 H |
| 7 | 19 I | 12 I | 13 I | 9 I | 25 H | 20 H | 28 H | 22 H | 24 H | 19 H | 25 H | 18 H |
| 8 | 18 I | 13 I | 15 I | 10 I | 26 H | 20 H | 25 H | 19 H | 27 H | 20 H | 28 H | 20 H |
| 9 | 20 I | 13 I | 14 I | 11 I | 25 H | 18 I | 27 H | 20 H | 22 I | 18 I | 19 I | 16 I |
| 10 | 14 I | 10 I | 16 I | 11 I | 28 H | 21 H | 25 H | 18 H | 20 I | 16 I | 17 I | 12 I |
| 11 | 25 H | 18 H | 27 H | 20 H | 20 I | 15 I | 17 I | 12 I | 17 I | 13 I | 19 I | 14 I |
| 12 | 29 H | 21 H | 30 H | 21 H | 18 I | 13 I | 16 I | 11 I | 16 I | 12 I | 14 I | 10 I |
| 13 | 17 I | 13 I | 20 I | 16 I | 27 H | 20 H | 30 H | 22 H | 26 H | 19 H | 27 H | 21 H |
| 14 | 26 H | 20 H | 25 H | 19 H | 20 I | 15 I | 16 I | 10 I | 17 I | 12 I | 17 I | 13 I |
| S | 35 | 26 | 35 | 25 | 36 | 28 | 38 | 27 | 35 | 28 | 37 | 26 |
| Compound No. | IC50 (µg/mL) * | |
|---|---|---|
| HCT-116 | HepG2 | |
| 3 | 31.70 ± 1.25 | 29.53 ± 1.02 |
| 4 | 17.36 ± 0.91 | 18.76 ± 0.84 |
| 5 | 19.27 ± 0.65 | 14.37 ± 0.65 |
| 6 | 18.83 ± 0.63 | 16.94 ± 0.75 |
| 7 | 14.26 ± 0.39 | 15.16 ± 0.32 |
| 8 | 19.21 ± 0.84 | 17.09 ± 0.65 |
| 9 | 8.44 ± 0.38 | 6.76 ± 0.21 |
| 10 | 9.30 ± 0.57 | 8.52 ± 0.37 |
| 11 | 9.15 ± 0.46 | 9.41 ± 0.61 |
| 12 | 8.17 ± 0.43 | 7.87 ± 0.52 |
| 13 | 17.53 ± 0.48 | 13.82 ± 0.96 |
| 14 | 7.56 ± 0.67 | 5.96 ± 0.74 |
| Doxorubicin | 4.22 ± 0.18 | 4.58 ± 0.13 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alshaye, N.A.; Ibrahim, M.A.; Badran, A.-S. Synthesis, Characterization and Antimicrobial and Anticancer Evaluations of Some Novel Heteroannulated Difuro[3,2-c:3′,2′-g]Chromenes. Molecules 2024, 29, 2319. https://doi.org/10.3390/molecules29102319
Alshaye NA, Ibrahim MA, Badran A-S. Synthesis, Characterization and Antimicrobial and Anticancer Evaluations of Some Novel Heteroannulated Difuro[3,2-c:3′,2′-g]Chromenes. Molecules. 2024; 29(10):2319. https://doi.org/10.3390/molecules29102319
Chicago/Turabian StyleAlshaye, Najla A., Magdy A. Ibrahim, and Al-Shimaa Badran. 2024. "Synthesis, Characterization and Antimicrobial and Anticancer Evaluations of Some Novel Heteroannulated Difuro[3,2-c:3′,2′-g]Chromenes" Molecules 29, no. 10: 2319. https://doi.org/10.3390/molecules29102319
APA StyleAlshaye, N. A., Ibrahim, M. A., & Badran, A.-S. (2024). Synthesis, Characterization and Antimicrobial and Anticancer Evaluations of Some Novel Heteroannulated Difuro[3,2-c:3′,2′-g]Chromenes. Molecules, 29(10), 2319. https://doi.org/10.3390/molecules29102319







